Abstract
It has been reported that the phosphorylated form of histone variant H2AX (γH2AX) plays an important role in the recruitment of DNA repair and checkpoint proteins to sites of DNA damage, particularly at double strand breaks (DSBs). Using γH2AX foci formation as an indicator for DNA damage, several chemicals/stress factors were chosen to assess their ability to induce γH2AX foci in a 24 h time frame in a human amnion FL cell line. Two direct-acting genotoxins, methyl methanesulfonate (MMS) and N-ethyl-N-nitrosourea (ENU), can induce γH2AX foci formation in a time- and dose-dependent manner. Similarly, an indirect-acting genotoxin, benzo[a]pyrene (BP), also induced the formation of γH2AX foci in a time- and dose-dependent manner. Another indirect genotoxin, 2-acetyl-aminofluorene (AAF), did not induce γH2AX foci formation in FL cells; however, AAF can induce γH2AX foci formation in Chinese hamster CHL cells. Neutral comet assays also revealed the induction of DNA strand breaks by these agents. In contrast, epigenetic carcinogens azathioprine and cyclosporine A, as well as non-carcinogen dimethyl sulfoxide, did not induce γH2AX foci formation in FL cells. In addition, heat shock and hypertonic saline did not induce γH2AX foci. Cell survival analyses indicated that the induction of γH2AX is not correlated with the cytotoxic effects of these agents/factors. Taken together, these results suggest that γH2AX foci formation could be used for evaluating DNA damage; however, the different cell types used may play an important role in determining γH2AX foci formation induced by a specific agent.
Keywords: γH2AX, Genotoxin, Double strand breaks, Cytotoxicity
1. Introduction
Cancer is a major threat to human life, and chemical carcinogens are responsible for a significant portion of the occurrence of the disease [1]. Human health and safety are important issues, and substantial resources have been expanded in efforts to identify and classify human carcinogens. Over the years, many methods have been developed and are routinely used to evaluate the carcinogenicity of chemicals, such as the Ames test, chromosome aberrations and sister chromatid exchange in Chinese hamster ovary cells, and in vivo animal testing [1].
Recently, the phosphorylated form of histone variant H2AX (termed γH2AX) has gained attention for its relationship with DNA damage, particularly double strand breaks (DSBs) [2–4]. It has been shown that within minutes of exposure to ionizing radiation (IR) or other factors that induce DSBs, H2AX is phosphorylated by members of the phosphatidylinositol 3-kinase family (PI-3K) and forms localized “foci” at the sites of DSBs [5–9]. Additionally, it is responsible for the recruitment of many other repair or checkpoint proteins to the damaged sites, such as the Mre11/Rad50/Nbs1 (MRN) complex, BRCA1, 53BP1, etc. [10–13]. Rothkamm and Lobrich further demonstrated that the number of γH2AX foci detected by immunofluorescence is quantitatively the same as that of DSBs, suggesting that γH2AX may be used as an indicator for DSBs [14]. Some other genotoxic agents, such as cisplatin, camptothecin, or hexavalent chromium [Cr(VI)] compounds, which do not induce DSB directly but nonetheless cause DSB indirectly through either the replication or repair process, have also been shown to induce γH2AX foci formation [15–17]. Based on these findings, some researchers have tried to apply this property of γH2AX into practical use. For example, Banath and Olive found that γH2AX level can be a useful indicator of cell killing by drugs that create DSBs [18]. Taneja et al. also showed that γH2AX could be used as a reporter of tumor radiosensitivity and a potential target to enhance the effectiveness of radiation therapy [19]. γH2AX induction has also been used to show that tobacco smoke or smoke condensate can rapidly induce DSBs in cells [20]. Very recently, by combining the p53 assay and γH2AX assay, Gallmeier et al. showed that norethindrone, a commonly used drug for contraception and hormone replacement therapy, can induce DSBs [21].
All the above information points to the potential use of γH2AX in the evaluation of genotoxic damage, particularly DSBs. Therefore, a group of chemicals was evaluated for their ability to induce γH2AX, including direct-acting genotoxins, indirect-acting genotoxins, epigenetic carcinogens, as well as non-carcinogens. In addition, two stressors, heat shock and hypertonic stress, were also examined. As reported here, both direct- and indirect-acting genotoxins can induce γH2AX, while epigenetic carcinogens, as well as heat shock and hypertonic stress, did not induce γH2AX foci formation.
2. Materials and methods
2.1. Cell culture and treatment
Human amnion FL cells and Chinese hamster CHL cells were routinely subcultured in Eagle’s Minimum Essential Medium (EMEM, Gibco, Carlsbad, CA), containing 10% newborn calf serum (Gibco), 100 U/ml penicillin, 125 μg/ml streptomycin, and 0.03% glutamine. Methyl methanesulfonate (MMS), N-ethyl-N-nitrosourea (ENU), 2-acetyl-aminofluorene (AAF), benzo[a]pyrene (BP), azathioprine, cyclosporine A, MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide), dimethylsulfoxide (DMSO), 4,6 diamidino-2-phenylindole (DAPI) and wortmannin were purchased from Sigma (St. Louis, MO). Mouse monoclonal antibody against γH2AX was purchased from Upstate Technology (Lake Placid, NY). FITC-conjugated goat anti-mouse IgG and goat blocking serum were obtained from Beijing Zhongshan Biotechnology Co. Other chemical reagents used were all of analytical purity from commercial sources.
All the test chemicals were dissolved in DMSO and added directly to the culture plate. Time or dose–response experiments for each individual chemical were carried out as described before [22]. For heat shock experiments, cells were incubated at 45.5 °C for 20 min, then were further incubated at 37 °C for specified times before analysis. For hypertonic experiments, cells were incubated with 0.2, 0.5, 1, and 1.5 M NaCl for various time points.
2.2. Cytotoxicity assay
The cytotoxic effects of each chemical were examined by the MTT test. Briefly, cells were seeded into 96-well culture plate at a density of 1 × 104 cells/well. Twenty-four hours later, the medium was discarded, and new medium containing the test chemical was added to respective wells. At the end of each time point, 20 μl of MTT (5 mg/ml in PBS) were added to each well. Four hour later, the solution was discarded, and 150 μl DMSO was added to each well. After formazan was dissolved, the absorbance at 490 nm was read on a microtiter plate reader (BioTek, Winooski, VT). Relative survival was represented as the absorbance of treated sample/absorbance of control group.
2.3. Immunofluorescent microscopy and quantification of γ H2AX foci
Immunofluorescent microscopy was conducted basically the same as described earlier, with modifications [23]. In short, 1 × 105 cells were seeded into 6-well culture plate containing a glass cover slip in each well. After treatment, cells were fixed in 4% paraformaldehyde for 15 min, washed with PBS, and permeabilized in 0.2% Triton X-100. After blocked with blocking serum for 1.5 h, samples were incubated with a mouse monoclonal anti-γH2AX antibody (1:1000) for 2 h, followed with FITC-conjugated goat-anti-mouse secondary antibody (1:500) for 1 h. To stain the nuclei, DAPI was added to the cells and incubated for another 15 min. The cover slip was then removed from the plate and mounted on to a glass slide, and observed with an Olympus AX70 fluorescent microscope (Olympus, Tokyo, Japan).
To study the effects of wortmannin on the phosphorylation of H2AX, cells were either preincubated with 200 μM of wortmannin for 30 min before chemical treatment, or 200 μM wortmannin post-chemical treatment for 1.5 h to prevent the early decomposition of wortmannin [24,25]. The procedure for immunofluorescent microscopy was essentially the same as described above.
To prevent bias in selection of cells that display foci, all the cells were counted in the field of vision (at least 50 cells). Image Pro Plus (Media Cybernetics, Silver Spring, MD) was used to count the γH2AX foci in each cell. In addition, to exclude relatively weak foci and background spots we used a setting as a standard for quantification in all the cells selected for analysis [26].
2.4. Comet assay
The neutral comet assay was performed as described before with some modifications [27]. First, the fully frosted microscope slides were covered with 100 μl of 0.65% normal melting point agarose and immediately covered with a coverslip. Slides were placed on ice for 8 min to allow the agarose to solidify. Second, the coverslips were removed, and the first agarose layer was covered with the cell suspension (1 × 106 cells in 15 μl PBS were mixed with 75 μl of 0.65% low melting point agarose). After replacing the coverslip back, the slide was allowed to solidify on ice for 8 min. Third, another layer of agarose (75 μl of 0.65% low melting point agarose) was added as described above. Finally, the coverslip was removed, and the slides were immersed in the lysis buffer (2 M NaCl, 30 mM EDTA, 10 mM Tris, with 1% Triton X-100 and 10% DMSO added just before use, pH 8.2–8.5) for 2 h at 4 °C. The slides were removed from the lysis buffer, washed for 10 min in 0.5 × TBE, and transferred to an electrophoresis chamber. After equilibration in 0.5 × TBE for 20 min, electrophoresis was conducted at 25 V, 150 mA, for 20 min. The slides were then washed in a neutralization buffer (0.4 M Tris, pH 7.5) three times for 5 min. The slides were drained and stained with 1.0 μg/ml DAPI, and observed with a fluorescent microscope.
Single cell images were captured and analyzed using an Olympus AX70 immunofluorescent microscope, and tail length was measured by ImagePro Plus software.
2.5. Statistical analysis
Each experiment was conducted at least three times. Statistical analysis was performed with χ2 test and Student’s t-test. A probability level of P < 0.05 was considered significant. Data are presented as mean ± S.D.
3. Results
3.1. Direct-acting genotoxins induce γH2AX foci formation in a time- and dose-dependent manner in FL cells
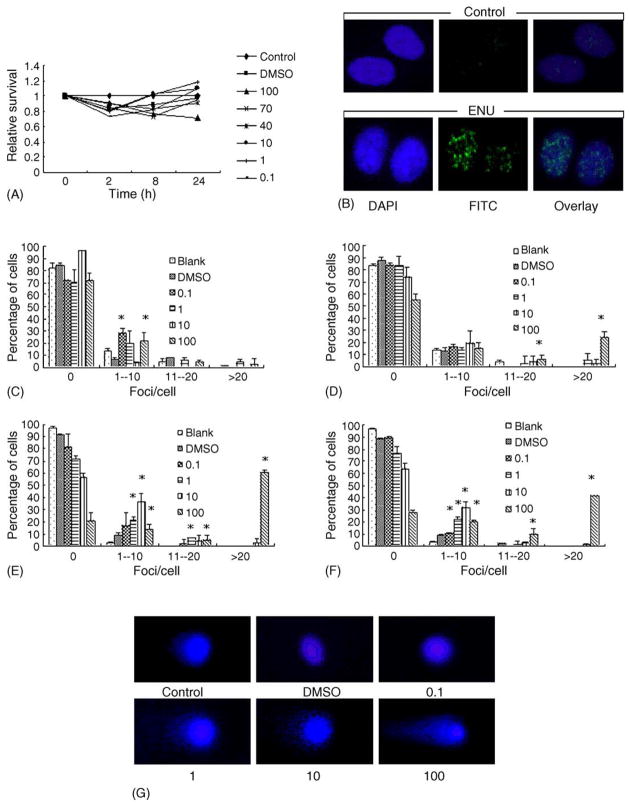
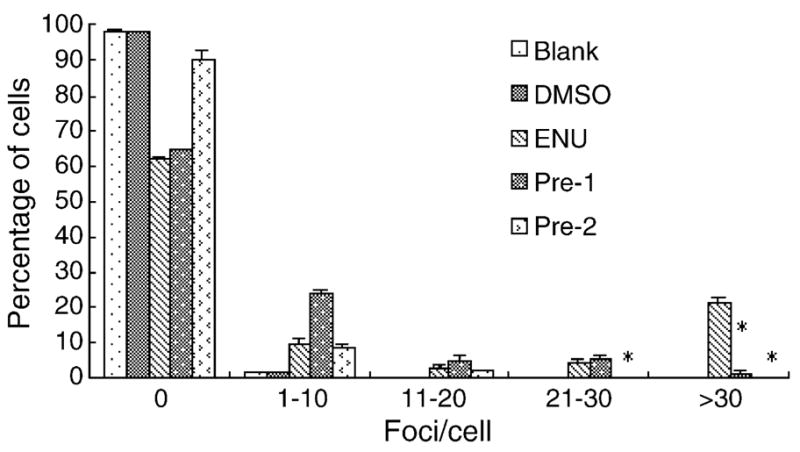
The length of time prior to the observation of γH2AX foci induced by different agents varies in different studies. Therefore, the dose and time–responses of two direct-acting genotoxins MMS and ENU, both of which are alkylating agents and are widely used in different bioassays for mutagenesis and carcinogenesis, were tested in human amnion FL cells. For ENU, only minor cytotoxicity was shown for the different concentrations (ranging from 0.1 to 100 μg/ml) tested, with the lowest survival ratio at around 80%; furthermore, no significant differences in cytotoxicity existed for the different concentrations tested except for 100 μg/ml at 24 h (Fig. 1A), therefore, four concentrations (0.1, 1, 10 and 100 μg/ml) were chosen for further analyses. Fig. 1B is a representative image showing that 100 μg/ml ENU induced γH2AX foci formation at 8 h. Detailed analyses (as shown in Fig. 1C–F) revealed that 80–90% of the control cells did not contain any foci, however, about 10–20% of the control cells did have 1–10 or even more foci at all times. Fifteen minutes after ENU exposure, no significant differences were found for the presence of foci in all groups tested. Nonetheless, 2 h later, about 20% of 100 μg/ml ENU-treated cells contained over 30 foci/cell, and this ratio climbed to over 50% at 8 h. The effects of 1 and 10 μg/ml ENU also became evident at this time, as more percentage of cells showing the presence of γH2AX foci (Fig. 1E). And these effects lasted at least 24 h (Fig. 1F), although the ratio of γH2AX-positive cells dropped slightly. The solvent control, DMSO, did not affect γH2AX foci formation (Fig. 1C–F). Neutral comet assay was also conducted, and as shown in Fig. 1G, ENU exposure can induce the formation of comet tail, indicating the presence of DNA strand breaks.
Fig. 1.
ENU induces γH2AX foci formation in FL cells in a time- and dose-dependent manner. (A) The cytotoxic effects of ENU (μg/ml) for a 24 h period. (B) Representative images showing γH2AX foci induced by 100 μg/ml ENU treatment at 8 h. (C–F) Quantitative analyses of γH2AX foci formation induced by different concentrations (μg/ml) of ENU. (C), 15 min; (D), 2 h; (E), 8 h; (F), 24 h. (G) Representative images from the neutral comet assay showing the effects of different concentrations of ENU on FL cells at 8 h. *P < 0.05.
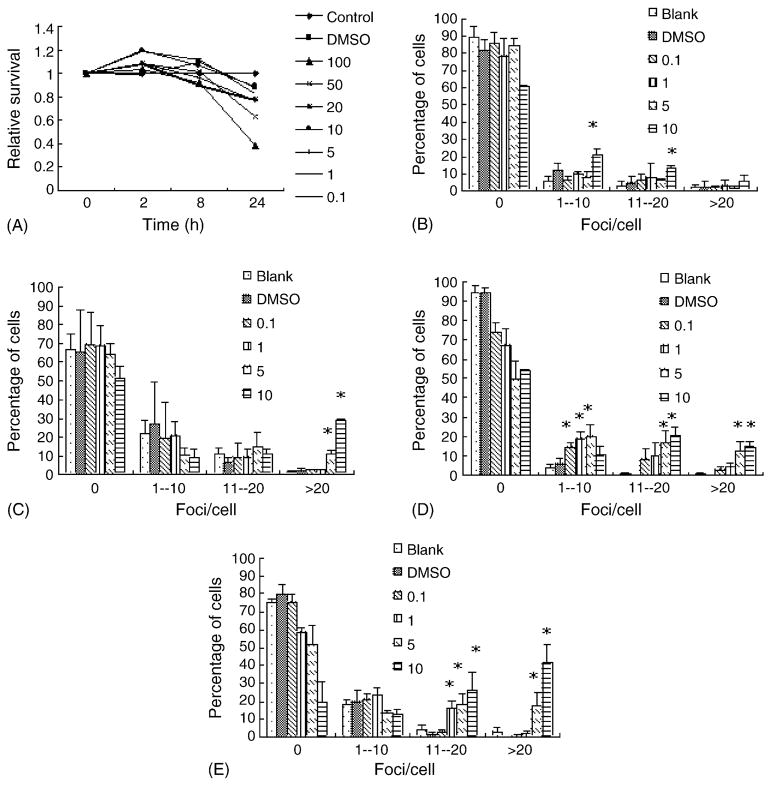
Compared to ENU, MMS exhibited strong cytotoxicity (Fig. 2A). At 24 h, less than 30% cells were still viable after 100 μg/ml MMS treatment, while other lower concentrations also induced various degree of cell death. Thus, only four concentrations were chosen for the γH2AX analyses, e.g., 0.1, 1, 5, and 10 μg/ml. Immunofluorescent microscopy data also revealed the presence of a time- and dose-dependent effect of MMS on the induction of γH2AX foci, particularly for the concentrations of 5 and 10 μg/ml (Fig. 2B–E). The neutral comet assay also revealed the presence of DNA damage in MMS exposed cells (data not shown).
Fig. 2.
MMS induces γH2AX foci formation in FL cells in a time- and dose-dependent manner. (A) The cytotoxic effects of MMS (μg/ml) for a 24 h period. (B–E) Quantitative analyses of γH2AX foci formation induced by different concentrations (μg/ml) of ENU. (B), 15 min; (C), 2 h; (D), 8 h; (E), 24 h. *P < 0.05.
3.2. Indirect genotoxin BP but not AAF induces γH2AX foci in FL cells
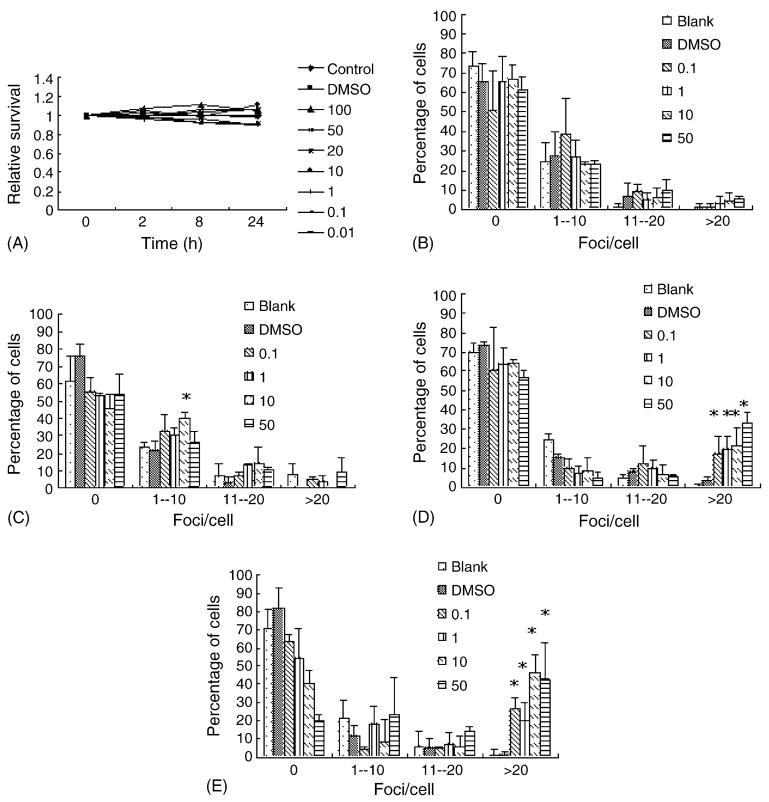
The time and dose–responses of two indirect genotoxins, BP and AAF, were also studied. No significant cytotoxic effects were observed for BP during the 24 h period (Fig. 3A). For the four concentrations tested (0.1, 1, 10, and 50 μM), BP showed a time-dependent manner in the induction of γH2AX foci, e.g., the percentage of cells with foci as well as the number of foci/cell increased with time, with higher concentrations having a stronger effect (Fig. 3B–E). The presence of DNA damage was also verified by neutral comet assay (data not shown). On the other hand, although AAF exhibited a dose-dependent cytotoxic effect for FL cells (data not shown), γH2AX foci were not observed in all the concentrations tested (0.1, 1, 10, and 20 μg/ml) for the 24 h period (data not shown). Furthermore, negative result was also obtained from neutral comet assay for AAF (data not shown), thus casting doubt on the ability of AAF to induce DNA damage in FL cells.
Fig. 3.
Indirect-acting genotoxin BP induces γH2AX foci formation in FL cells. (A) The cytotoxic effects of BP (μM) for a 24 h period. (B-E) Quantitative analyses of γH2AX foci formation induced by different concentrations (μM) of BP. (B), 15 min; (C), 2 h; (D), 8 h; (E), 24 h. *P < 0.05.
3.3. AAF induces γH2AX foci formation in CHL cells
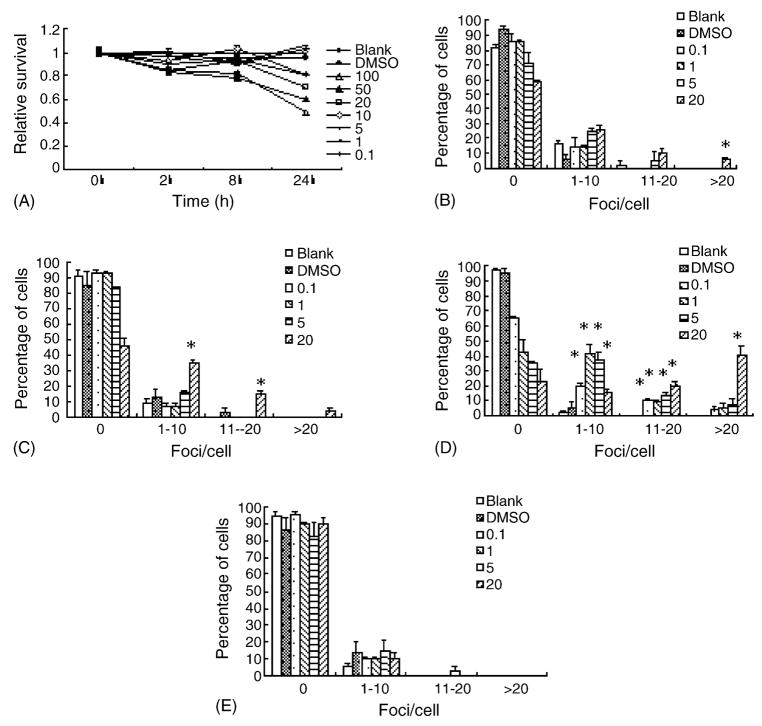
Since AAF did not induce γH2AX foci in FL cells, we then tested its ability to induce foci in CHL cells. As shown in Fig. 4A, AAF also exerted a dose-dependent cytotoxic effect on CHL cells. Interestingly, when these cells were examined for the formation of γH2AX foci, it was found that at 2 h, 20 μg/ml of AAF significantly increased the percentage of γH2AX-positive cells as well as the number of foci/cell (Fig. 4B–E). At 8 h, all four concentrations tested (0.1, 1, 5, 20 μg/ml) significantly increased γH2AX foci formation. However, this effect was transient as at 24 h no significant differences were found between treated groups and control group. Neutral comet assay revealed a similar pattern (data not shown). These results suggested that the ability of AAF to induce γH2AX foci depends on the cell type tested.
Fig. 4.
Indirect-acting genotoxin AAF induces γH2AX foci formation in CHL cells. (A) The cytotoxic effects of AAF (μg/ml) on CHL cells for a 24 h period. (B–E) Quantitative analyses of γH2AX foci formation induced by different concentrations (μg/ml) of AAF in CHL cells. (B), 15 min; (C), 2 h; (D), 8 h; (E), 24 h. *P < 0.05.
3.4. Non-genotoxic carcinogens azathioprine and cyclosporine A do not induce γH2AX foci formation in FL cells
Because γH2AX foci formation is considered to be related to DNA damage, epigenetic or non-genotoxic carcinogens and non-carcinogens are not expected to induce γH2AX foci. Therefore, two epigenetic carcinogens azathioprine and cyclosporine A were chosen to examine their effects on γH2AX foci formation. MTT test showed that at a concentration of 20 μg/ml, both azathioprine and cyclosporine A had significant cytotoxic effects on FL cells in a time-dependent manner (Fig. 5). However, neither was able to induce γH2AX foci formation during the 24 h period (data not shown). Neutral comet assay for both chemicals also showed negative results (data not shown). Higher concentrations of these two chemicals were further tested, with no significant effects observed in neither γH2AX foci formation nor comet assay (data not shown).
Fig. 5.
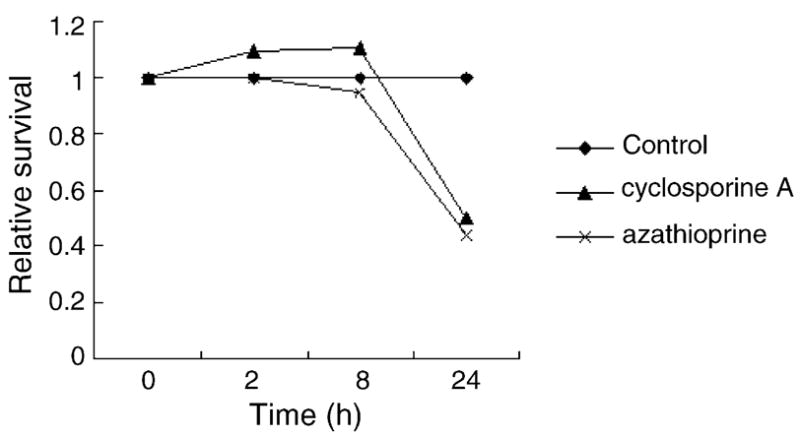
The cytotoxic effects of azathioprine (20 μg/ml) and cyclosporine A (20 μg/ml) on FL cell during a 24 h period.
3.5. Heat shock and hypertonic saline do not induce γ H2AX foci formation in FL cells
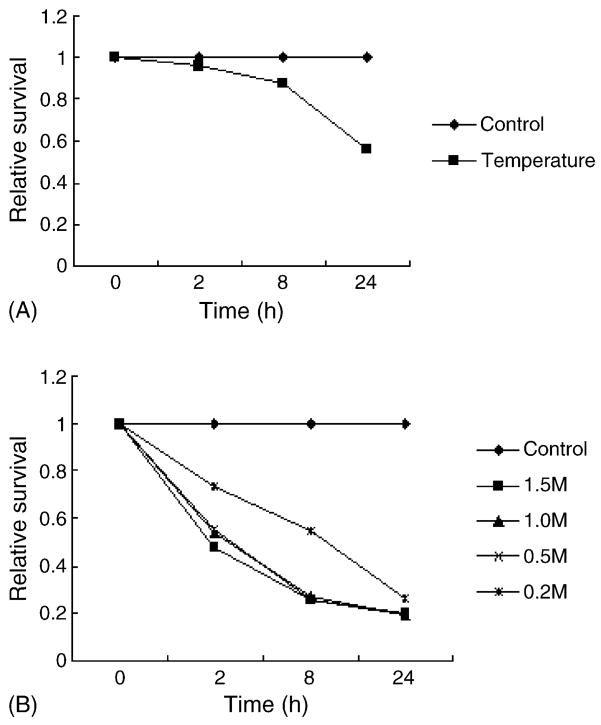
Recently, there are two reports showing that heat shock can induce γH2AX foci formation [28,29]. Hypertonic saline also has been shown to enhance the expression of γH2AX after irradiation, and DNA-PK is responsible for this phosphorylation [30,31]. Therefore, we tested the ability of heat shock and hypertonic saline in the induction of γH2AX foci in FL cells. FL cells exposed to 45.5 °C for 20 min resulted in a significant loss of cell viability, as 24 h after exposure only about 50% of the cells survived (Fig. 6A). Different concentrations of saline also exhibited various degrees of cytotoxic effects, with higher concentrations eliminated most cells within 8 h (Fig. 6B). However, no increase in γH2AX foci formation was detected in heat-treated FL cells during the 24 h period (data not shown). Similar results were also obtained from hypertonic saline-treated cells at 2 and 8 h (data not shown) (24 h was not tested as most cells were non-viable by that time). Neutral comet assay was further conducted for these cells with negative results (data not shown). Together, these data suggested that the ability of heat shock to induce γH2AX foci might be cell-type specific, and hypertonic saline itself may not induce significant DNA damage.
Fig. 6.
The cytotoxic effects of heat shock and hypertonic saline on FL cells. (A) FL cells were preincubated at 45.5 °C for 20 min, then were removed and continued to incubate at 37 °C for indicated times. (B) FL cells were exposed to various concentrations of NaCl (M), and cell viability was measured by MTT test.
3.6. Wortmannin inhibits γH2AX foci formation induced by both direct and indirect genotoxins
Wortmannin is an inhibitor for PI3K family members, which has been shown to inhibit the phosphorylation of H2AX by IR or other agents that induce DSBs [24,25]. Therefore, the effect of wortmannin on direct or indirect genotoxin-induced γH2AX foci formation was also examined. Cells were either preincubated with wortmannin for 30 min before genotoxin treatment, or with a second dose of wortmannin added 1.5 h after genotoxin treatment. Two hour later, the phosphorylation of H2AX was observed by immunofluorescent microscopy. As shown in Fig. 7, it was found that preincubation with wortmannin alone could greatly decrease the formation of γH2AX foci in FL cells and double treatment completely inhibited the formation of γH2AX foci induced by ENU. Similar results were also obtained for MMS, AAF and BP (data not shown). Therefore, the phosphorylation of H2AX in response to these genotoxic agents was dependent on PI3K family kinases.
Fig. 7.
Wortmannin inhibits ENU-induced γH2AX foci formation. FL cells were preincubated with wortmannin (200 μM) for 30 min, then treated with 100 μg/ml ENU for 8 h (Pre I); or a second dose of wortmannin was added 1.5 h after ENU treatment (Pre II), and the effects on γH2AX foci formation were measured at 8 h after ENU treatment. * P < 0.05.
4. Discussion
Previously, we have proposed the use of p53 induction as an indicator for genotoxic damage, which has gained support from other studies [22,32,33]. The recently identified γH2AX protein may serve as an additional marker, as H2AX is directly associated with DNA, and also is an early participant in the DNA damage response [3,13]. Therefore, we evaluated γH2AX foci formation induced by different categories of carcinogens and non-carcinogens. As reported here, direct-acting genotoxins ENU and MMS, as well as an indirect-acting genotoxin BP, all induced γH2AX foci formation in FL cells in a time- and dose-dependent manner; while another indirect-acting genotoxin AAF, could only induce γH2AX foci formation in CHL cells but not FL cells. Non-genotoxic carcinogens azathioprine and cyclosporine A, along with the solvent control DMSO, did not cause an increase in γH2AX foci formation. Taken together, these data suggest that γH2AX could be used as an indicator for DNA damage.
In this study, a time frame of 24 h was chosen based on the observations of many other studies, which show that different agents induce γH2AX foci formation at various times. For example, IR-induced γH2AX foci usually appeared within minutes after irradiation, reaching peak amount at 30 min to 1 h [5–7]. For topoisomerase I inhibitor topotecan and topoisomerase II inhibitor mitoxantrone, the increase in γH2AX foci peaked at 1.5 or 2 h; while for a cross-linking agent cisplatin, the γH2AX foci were less intense and peaked later (3 h) [17]. On the other hand, for another cross-linking agent mitomycin C, the peak time was around 12 h [34]. Thus, during the 24 h period, a majority of genotoxic agent-induced γH2AX foci formation should be detected. The difference in the induction time may also reflect the different DNA damaging mechanisms for genotoxic agents, as γH2AX is regarded as specific for DSBs, the most deleterious form of DNA damage. Thus, for those agents that can induce DSBs directly, such as IR, the time for the appearance of γH2AX foci would be earlier; while for those agents that do not induce DSBs directly, the time of γH2AX foci appearance would be delayed. For this group of genotoxic agents, the generation of DSBs may be due to the repair process or the replication of damaged DNA, which may explain why some agents induce γH2AX foci in a cell cycle dependent manner [12,16,17,35–37].
The inability of AAF to induce γH2AX foci in FL cells was surprising, as it is a well-known genotoxin. Since AAF is a weaker mutagen than BP in the Ames test, yet the concentrations investigated here (20 μg/ml) are lower than those of BP (50 μg/ml); consequently, this may explain the different responses with these two mutagens in FL cells. Another possibility is that AAF is an indirect-acting genotoxin, different cells may have different ability to metabolize AAF, and in this case, FL cells cannot metabolize AAF. Therefore, we further examined the ability of AAF to induce γH2AX foci in another cell line, the CHL cells. CHL cells were treated with AAF the same way as for FL cells, and γH2AX foci formation was measured by immunofluorescent microscopy. It was found that AAF can induce γH2AX foci formation at a concentration of as low as 0.1 μg/ml (Fig. 4). Similar results were obtained in comet assay (data not shown). Hence, it seems that there exists unique, cell-type specific γH2AX responses to a given indirect-acting genotoxic agent. And the reason for this could be attributed to the different metabolizing ability of these cells for these agents.
Heat shock and hypertonic stress are hardly regarded as carcinogenic even though they may be able to cause DNA damage. Heat shock can affect many nuclear functions associated with semi-conservative replication of DNA including the incorporation of radiolabelled precursors into acid-insoluble DNA, the initiation of new replicons, the elongation of the DNA fiber at the replication fork, the synthesis and deposition of new histones into chromatin, and the reorganization of nascent DNA into mature chromatin [28,38]. It has been used clinically to sensitize tumor cells to radiation treatment, for which the molecular mechanism has been attributed to a heat-dependent inhibition of the repair of radiation-induced DSBs [28,38]. However, its genotoxicity remains controversial. For example, there are some reports showing heat shock cannot induce DNA damage [39,40], while others showed the opposite [41]. And recently, using γH2AX foci formation as an indicator, two groups also reported the induction of DSBs by heat shock in several cell lines [28,29], supporting the notion that heat shock may be genotoxic. Nevertheless, in our experimental system, no γH2AX foci formation was detected after heat shock even though significant cell death was observed, thus conflicting with these reports. The most possible explanation may be the different cells used in these experiments, however, other factors cannot be ruled out.
Similarly, hypertonic stress has been reported to induce cell cycle arrest, the expression of tumor suppressor p53 and the growth arrest and DNA damage inducible proteins GADD45and GADD153, which are known to be involved in cellular responses to DNA damage [42,43]. It has also been shown that postirradiation hypertonic treatment increased the formation of chromosome aberrations and cell killing and reduced DNA synthesis, as well as an increase in γH2AX foci formation, which may result from the inhibition of DNA repair pathways activated by radiation [30,44,45]. Elevated NaCl itself also can induce DSBs in murine kidney cells, however, DSBs is not caused by hyperosmolality per se but by changes in ionic strength, cell volume, or macromolecular crowding [46]. In our experimental system, FL cells were quickly killed, however, no increase in γH2AX foci was observed. It should be noted that renal cells are normally exposed to extremely high NaCl concentrations, but remarkably, under these conditions, although the high NaCl causes DNA damage and inhibits its repair, the cells still survive and function both in cell culture and in vivo [47]. This may provide an answer to the obvious discrepancy between our results and Kultz’s results.
Taken together, in this study, we have evaluated γH2AX foci formation by different classes of chemical/physical stressors. From the limited agents tested, it was found that γH2AX foci formation can be used as an indicator for DNA damage. And the results correlated well with the neutral comet assay. However, because this method only detects DSBs, the alkaline comet offers greater sensitivity since it detects more types of DNA damage. In addition, choosing the right cell line may be vital as there seems to be a cell-type specific response for the induction of γH2AX foci for certain genotoxic agents, particularly indirect-acting genotoxins.
Acknowledgments
This work was supported in part by grants from the National Hi-Tech Research and Development Program (2004AA649120), Natural Science Foundation of China (30300277 and 30471956), the Initiative Fund for Returned Oversea Chinese Scholars, Ministry of Education, China, and the Y.C. TANG Disciplinary Development Fund, Zhejiang University to J. Yang; National Institute of Health (NIH CA95393-01 and NIH P50 grant CA112970) and National Aeronautics and Space Administration (NASA NNA04CA75I), The United States of America, to F.F. Chen. The authors gratefully thank the anonymous reviewers whose suggestions greatly improved this manuscript, and S. Schwartz for the critical reading of the manuscript.
References
- 1.Friedberg EC, Walker GC, Siede W. DNA repair and mutagenesis. ASM Press; Washington, DC: 1995. [Google Scholar]
- 2.Redon C, Pilch D, Rogakou E, Sedelnikova O, Newrock K, Bonner W. Histone H2A variants H2AX and H2AZ. Curr Opin Genet Dev. 2002;12:162–169. doi: 10.1016/s0959-437x(02)00282-4. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez-Capetillo O, Lee A, Nussenzweig M, Nussenzweig A. H2AX: the histone guardian of the genome. DNA Repair (Amst) 2004;3:959–967. doi: 10.1016/j.dnarep.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 4.Pilch DR, Sedelnikova OA, Redon C, Celeste A, Nussenzweig A, Bonner WM. Characteristics of gamma-H2AX foci at DNA double-strand breaks sites. Biochem Cell Biol. 2003;81:123–129. doi: 10.1139/o03-042. [DOI] [PubMed] [Google Scholar]
- 5.Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 6.Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol. 2000;10:886–895. doi: 10.1016/s0960-9822(00)00610-2. [DOI] [PubMed] [Google Scholar]
- 7.MacPhail SH, Banath JP, Yu TY, Chu EH, Lambur H, Olive PL. Expression of phosphorylated histone H2AX in cultured cell lines following exposure to X-rays. Int J Radiat Biol. 2003;79:351–358. doi: 10.1080/0955300032000093128. [DOI] [PubMed] [Google Scholar]
- 8.Stiff T, O’Driscoll M, Rief N, Iwabuchi K, Lobrich M, Jeggo PA. ATM and DNA-PK function redundantly to phosphorylate H2AX after exposure to ionizing radiation. Cancer Res. 2004;64:2390–2396. doi: 10.1158/0008-5472.can-03-3207. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Wang M, Bocker W, Iliakis G. Complex H2AX phosphorylation patterns by multiple kinases including ATM and DNA-PK in human cells exposed to ionizing radiation and treated with kinase inhibitors. J Cell Physiol. 2005;202:492–502. doi: 10.1002/jcp.20141. [DOI] [PubMed] [Google Scholar]
- 10.Ward IM, Minn K, Jorda KG, Chen J. Accumulation of checkpoint protein 53BP1 at DNA breaks involves its binding to phosphorylated histone H2AX. J Biol Chem. 2003;278:19579–19582. doi: 10.1074/jbc.C300117200. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez-Capetillo O, Chen HT, Celeste A, Ward I, Romanienko PJ, Morales JC, Naka K, Xia Z, Camerini-Otero RD, Motoyama N, Carpenter PB, Bonner WM, Chen J, Nussenzweig A. DNA damage-induced G2-M checkpoint activation by histone H2AX and 53BP1. Nat Cell Biol. 2002;4:993–997. doi: 10.1038/ncb884. [DOI] [PubMed] [Google Scholar]
- 12.Furuta T, Takemura H, Liao ZY, Aune GJ, Redon C, Sedelnikova OA, Pilch DR, Rogakou EP, Celeste A, Chen HT, Nussenzweig A, Aladjem MI, Bonner WM, Pommier Y. Phosphorylation of histone H2AX and activation of Mre11, Rad50, and Nbs1 in response to replication-dependent DNA double-strand breaks induced by mammalian DNA topoisomerase I cleavage complexes. J Biol Chem. 2003;278:20303–20312. doi: 10.1074/jbc.M300198200. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez-Capetillo O, Celeste A, Nussenzweig A. Focusing on foci: H2AX and the recruitment of DNA-damage response factors. Cell Cycle. 2003;2:426–427. [PubMed] [Google Scholar]
- 14.Rothkamm K, Lobrich M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low X-ray doses. Proc Natl Acad Sci USA. 2003;100:5057–5062. doi: 10.1073/pnas.0830918100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bosco EE, Mayhew CN, Hennigan RF, Sage J, Jacks T, Knudsen ES. RB signaling prevents replication-dependent DNA double-strand breaks following genotoxic insult. Nucleic Acids Res. 2004;32:25–34. doi: 10.1093/nar/gkg919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ha L, Ceryak S, Patierno SR. Generation of S phase-dependent DNA double-strand breaks by Cr(VI) exposure: involvement of ATM in Cr(VI) induction of gamma-H2AX. Carcinogenesis. 2004;25:2265–2274. doi: 10.1093/carcin/bgh242. [DOI] [PubMed] [Google Scholar]
- 17.Huang X, Okafuji M, Traganos F, Luther E, Holden E, Darzynkiewicz Z. Assessment of histone H2AX phosphorylation induced by DNA topoisomerase I and II inhibitors topotecan and mitoxantrone and by the DNA cross-linking agent cisplatin. Cytometry. 2004;58A:99–110. doi: 10.1002/cyto.a.20018. [DOI] [PubMed] [Google Scholar]
- 18.Banath JP, Olive PL. Expression of phosphorylated histone H2AX as a surrogate of cell killing by drugs that create DNA double-strand breaks. Cancer Res. 2003;63:4347–4350. [PubMed] [Google Scholar]
- 19.Taneja N, Davis M, Choy JS, Beckett MA, Singh R, Kron SJ, Weichselbaum RR. Histone H2AX phosphorylation as a predictor of radiosensitivity and target for radiotherapy. J Biol Chem. 2004;279:2273–2280. doi: 10.1074/jbc.M310030200. [DOI] [PubMed] [Google Scholar]
- 20.Albino AP, Huang X, Jorgensen E, Yang J, Gietl D, Traganos F, Darzynkiewicz Z. Induction of H2AX phosphorylation in pulmonary cells by tobacco smoke: a new assay for carcinogens. Cell Cycle. 2004;3:1062–1068. [PubMed] [Google Scholar]
- 21.Gallmeier E, Winter JM, Cunningham SC, Kahn SR, Kern SE. Novel genotoxicity assays identify norethindrone to activate p53 and phosphorylate H2AX. Carcinogenesis. 2005;26:1811–1820. doi: 10.1093/carcin/bgi132. [DOI] [PubMed] [Google Scholar]
- 22.Yang J, Duerksen-Hughes P. A new approach to identifying genotoxic carcinogens: p53 induction as an indicator of genotoxic damage. Carcinogenesis. 1998;19:1117–1125. doi: 10.1093/carcin/19.6.1117. [DOI] [PubMed] [Google Scholar]
- 23.Yang J, Hooper WC, Phillips DJ, Talkington DF. Regulation of proinflammatory cytokines in human lung epithelial cells infected with Mycoplasma pneumoniae. Infect Immun. 2002;70:3649–3655. doi: 10.1128/IAI.70.7.3649-3655.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ward IM, Chen J. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J Biol Chem. 2001;276:47759–47762. doi: 10.1074/jbc.C100569200. [DOI] [PubMed] [Google Scholar]
- 25.Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ. ATM phosphorylates histone H2AX in response to DNA double-strand breaks. J Biol Chem. 2001;276:42462–42467. doi: 10.1074/jbc.C100466200. [DOI] [PubMed] [Google Scholar]
- 26.Daniel R, Ramcharan J, Rogakou E, Taganov KD, Greger JG, Bonner W, Nussenzweig A, Katz RA, Skalka AM. Histone H2AX is phosphorylated at sites of retroviral DNA integration but is dispensable for postintegration repair. J Biol Chem. 2004;279:45810–45814. doi: 10.1074/jbc.M407886200. [DOI] [PubMed] [Google Scholar]
- 27.Vaughan AT, Milner AM, Gordon DG, Schwartz JL. Interaction between ionizing radiation and supercoiled DNA within human tumor cells. Cancer Res. 1991;51:3857–3861. [PubMed] [Google Scholar]
- 28.Kaneko H, Igarashi K, Kataoka K, Miura M. Heat shock induces phosphorylation of histone H2AX in mammalian cells. Biochem Biophys Res Commun. 2005;328:1101–1106. doi: 10.1016/j.bbrc.2005.01.073. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi A, Matsumoto H, Nagayama K, Kitano M, Hirose S, Tanaka H, Mori E, Yamakawa N, Yasumoto J, Yuki K, Ohnishi K, Ohnishi T. Evidence for the involvement of double-strand breaks in heat-induced cell killing. Cancer Res. 2004;64:8839–8845. doi: 10.1158/0008-5472.CAN-04-1876. [DOI] [PubMed] [Google Scholar]
- 30.Reitsema TJ, Banath JP, MacPhail SH, Olive PL. Hypertonic saline enhances expression of phosphorylated histone H2AX after irradiation. Radiat Res. 2004;161:402–408. doi: 10.1667/rr3153. [DOI] [PubMed] [Google Scholar]
- 31.Reitsema T, Klokov D, Banath JP, Olive PL. DNA-PK is responsible for enhanced phosphorylation of histone H2AX under hypertonic conditions. DNA Repair (Amst) 2005;4:1172–1181. doi: 10.1016/j.dnarep.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Quinones A, Rainov NG. Identification of genotoxic stress in human cells by fluorescent monitoring of p53 expression. Mutat Res. 2001;494:73–85. doi: 10.1016/s1383-5718(01)00179-6. [DOI] [PubMed] [Google Scholar]
- 33.Sohn TA, Bansal R, Su GH, Murphy KM, Kern SE. High-throughput measurement of the Tp53 response to anti-cancer drugs and random compounds using a stably integrated Tp53-responsive luciferase reporter. Carcinogenesis. 2002;23:949–957. doi: 10.1093/carcin/23.6.949. [DOI] [PubMed] [Google Scholar]
- 34.Niedernhofer LJ, Odijk H, Budzowska M, van Drunen E, Maas A, Theil AF, de Wit J, Jaspers NG, Beverloo HB, Hoeijmakers JH, Kanaar R. The structure-specific endonuclease Ercc1-Xpf is required to resolve DNA interstrand cross-link-induced double-strand breaks. Mol Cell Biol. 2004;24:5776–5787. doi: 10.1128/MCB.24.13.5776-5787.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halicka HD, Huang X, Traganos F, King MA, Dai W, Darzynkiewicz Z. Histone H2AX phosphorylation after cell irradiation with UV-B: relationship to cell cycle phase and induction of apoptosis. Cell Cycle. 2005;4:339–345. [PubMed] [Google Scholar]
- 36.Huang X, Traganos F, Darzynkiewicz Z. DNA damage induced by DNA topoisomerase I- and topoisomerase II-inhibitors detected by histone H2AX phosphorylation in relation to the cell cycle phase and apoptosis. Cell Cycle. 2003;2:614–619. [PubMed] [Google Scholar]
- 37.MacPhail SH, Banath JP, Yu Y, Chu E, Olive PL. Cell cycle-dependent expression of phosphorylated histone H2AX: reduced expression in unirradiated but not X-irradiated G1-phase cells. Radiat Res. 2003;159:759–767. doi: 10.1667/rr3003. [DOI] [PubMed] [Google Scholar]
- 38.Iliakis G, Krieg T, Guan J, Wang Y, Leeper D. Evidence for an S-phase checkpoint regulating DNA replication after heat shock: a review. Int J Hyperthermia. 2004;20:240–249. doi: 10.1080/02656730310001656379. [DOI] [PubMed] [Google Scholar]
- 39.Mittler S. Effects of hyperthermia on radiation-induced chromosome breakage and loss in excision repair deficient Drosophila melanogaster. Int J Radiat Biol Relat Stud Phys Chem Med. 1986;50:225–230. doi: 10.1080/09553008614550611. [DOI] [PubMed] [Google Scholar]
- 40.Iliakis G, Seaner R, Okayasu R. Effects of hyperthermia on the repair of radiation-induced DNA single- and double-strand breaks in DNA double-strand break repair-deficient and repair-proficient cell lines. Int J Hyperthermia. 1990;6:813–833. doi: 10.3109/02656739009140828. [DOI] [PubMed] [Google Scholar]
- 41.Anitha B, Chandra N, Gopinath PM, Durairaj G. Genotoxicity evaluation of heat shock in gold fish (Carassius auratus) Mutat Res. 2000;469:1–8. doi: 10.1016/s1383-5718(00)00029-2. [DOI] [PubMed] [Google Scholar]
- 42.Kultz D, Madhany S, Burg MB. Hyperosmolality causes growth arrest of murine kidney cells. Induction of GADD45 and GADD153 by osmosensing via stress-activated protein kinase 2. J Biol Chem. 1998;273:13645–13651. doi: 10.1074/jbc.273.22.13645. [DOI] [PubMed] [Google Scholar]
- 43.Dmitrieva N, Kultz D, Michea L, Ferraris J, Burg M. Protection of renal inner medullary epithelial cells from apoptosis by hypertonic stress-induced p53 activation. J Biol Chem. 2000;275:18243–18247. doi: 10.1074/jbc.M000522200. [DOI] [PubMed] [Google Scholar]
- 44.Okayasu R, Iliakis G. Ionizing radiation induces two forms of interphase chromosome breaks in Chinese hamster ovary cells that rejoin with different kinetics and show different sensitivity to treatment in hypertonic medium or beta-araA. Radiat Res. 1993;136:262–270. [PubMed] [Google Scholar]
- 45.Kosaka T, Kaneko I, Koide F. Correlation between non-repairable DNA lesions and fixation of cell damage by hypertonic solutions in Chinese hamster cells. Int J Radiat Biol. 1990;58:417–425. doi: 10.1080/09553009014551771. [DOI] [PubMed] [Google Scholar]
- 46.Kultz D, Chakravarty D. Hyperosmolality in the form of elevated NaCl but not urea causes DNA damage in murine kidney cells. Proc Natl Acad Sci USA. 2001;98:1999–2004. doi: 10.1073/pnas.98.4.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dmitrieva NI, Burg MB. Hypertonic stress response. Mutat Res. 2005;569:65–74. doi: 10.1016/j.mrfmmm.2004.06.053. [DOI] [PubMed] [Google Scholar]