Abstract
Many naturally occurring agents are believed to protect against UV-induced skin damage. In this study, we have investigated the effects of naringenin (NG), a naturally occurring citrus flavonone, on the removal of UVB-induced cyclobutane pyrimidine dimers (CPD) from the genome and apoptosis in immortalized p53-mutant human keratinocyte HaCaT cells. The colony-forming assay shows that treatment with NG significantly increases long-term cell survival after UVB irradiation. NG treatment also protects the cells from UVB-induced apoptosis, as indicated by the absence of the 180 base pair DNA ladders and the appearance of sub-G1 peak using agarose gel electrophoresis and flow cytometric analysis, respectively. The UVB-induced poly (ADP-ribose) polymerase-1 (PARP-1) cleavage, caspase activation and Bax/Bcl2 ratio were modulated following NG treatment, indicating an antiapoptotic effect of NG in UVB-damaged cells that occurs at least in part via caspase cascade pathway. Moreover, treatment of UVB-irradiated HaCaT cells with NG enhances the removal of CPD from the genome, as observed by both direct quantitation of CPD in genomic DNA and immuno-localization of the damage within the nuclei. The study provides a molecular basis for the action of NG as a promising natural flavonoid in preventing skin aging and carcinogenesis.
INTRODUCTION
Exposure to UV radiation induces genotoxic effects that contribute not only to skin photoaging but also to skin carcinogenesis (1). Although UVC (<280 nm) radiation is deemed physiologically irrelevant as it is absorbed by the atmospheric oxygen and ozone layer before reaching the earth, the longer wavelength UVB (280–315 nm) and UVA (315–400 nm) have significant effects on the living organisms (2). According to “World Cancer Report,” skin cancer is the most frequently diagnosed malignancy in Caucasians and accounts for ~30% of all diagnosed cancers in the world (3). Ninety percentage of skin cancer cases have been attributed to the solar UV radiation, particularly its UVB component that is greatly absorbed by cellular DNA (2,4). As a direct effect, UVB induces cyclobutane pyrimidine dimers (CPD) and 6–4 pyrimidine–pyrimidone photoproducts (6-4PP), which cause DNA mutation leading to tumor initiation, transcriptional modulation of genes involved in tumor promotion as well as activation of several signal transduction pathways (5,6). Also, UVB indirectly damages DNA through reactive oxygen species (ROS) formation, which facilitate the oxidation of DNA (7).
Living cells have developed a number of mechanisms to counteract the DNA damage caused by environmental stressors, including UV light. Upon DNA damage, many cellular proteins orchestrate to shut off the cell replication and DNA synthesis allowing extended time either for apoptosis or DNA repair. The apoptosis is set to eliminate the DNA-damaged cell, while the DNA repair machinery is to restore the normal structure of DNA (8).
Exposure to UV light leading to massive apoptosis could dangerously compromise the natural barrier functions of the skin and accelerate skin photoaging (9). Apoptosis is a highly regulated process that involves the activation of a series of cellular events leading to cell death. Apoptotic cell death is characterized by chromatin condensation and formation of apoptotic bodies (10,11). Signaling for apoptosis occurs through multiple pathways, initiated by diverse extracellular and/or intracellular signals. A family of cysteine proteases, known as caspases, play a pivotal role in the regulation and execution of apoptotic cell death. When caspases are activated, they cleave a number of key substrates, resulting in their activation or inactivation. These key substrates dictate the morphologic and biochemical features of apoptosis (12). The role of mitochondria, as the key cellular organelle modulating apoptosis, has been well established. It is known that the antiapoptotic protein Bcl2 localizes in the outer membrane of mitochondria (13). Mitochondria amplify and mediate extrinsic apoptotic pathways and play a central role in integrating and propagating death signals inside the cell. Most apoptosis-inducing stimuli involve disruption of the mitochondrial inner transmembrane potential as well as the permeability transition, resulting in release of the proapoptotic proteins from the mitochondrial inter-membrane space into the cytoplasm (14).
UV exposure also causes DNA damage including UV-induced CPD and 6-4PP and these adducts can be removed by nucleotide excision repair (NER). NER is a versatile DNA repair pathway that eliminates a wide variety of helix-distorting DNA lesions. Mammalian NER consists of two distinct subpathways: global genomic repair, which operates throughout the genome and transcription-coupled repair, which works on damage within transcribed DNA strands of transcriptionally active genes (15). Loss or impairment of NER is associated with three sun-sensitive, genetic disorders, e.g. Xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy (16). NER defective individuals have ~1000 times more risk of skin cancer in comparison to normal individuals (17).
In recent years, there has been a substantial interest in the use of naturally occurring agents such as flavonoids for the prevention and treatment of different kinds of skin cancer. Flavonoids are a group of polyphenolic compounds, which are widely discovered throughout the plant kingdom. They are classified as flavonols, flavonones, flavones, flavanols, flavan-3-ols and isoflavones according to the positions of the substitutes present on the parent molecule. Flavonoids of different classes have several pharmacological activities. They are potent antioxidants and have free radical scavenging abilities. Some of them provide protection against cardiovascular mortality through inhibition of apoptosis (18). They have also been shown to reduce tumor development in experimental animals and various cancer cell lines in vitro. Naringenin (NG) is one of the most abundant citrus flavonones found in citrus fruits such as lemon, orange, tangerine and grapefruit. NG has antioxidant and antitumor activity and has been reported to play a role in cancer, heart disease, hypertension, circulation and Alzheimer’s disease (19).
Several reports have shown the effectiveness of naturally occurring agents against UV-induced skin damage and nonmelanoma skin cancer (20–24). In most of the cases, such effects are attributed to the free radical scavenging potentials of those compounds (25,26). However, other effects beyond antioxidation could play an important role in determining the biological value of phytochemicals like flavonoids. These include effects on cell proliferation (27), angiogenesis (28), subcellular signaling (29) and DNA repair enzymes (30). Here, we have used immortalized human keratinocyte HaCaT cells to study the effect of NG on UVB-induced cellular apoptosis, removal of UVB-induced CPD and other crucial cell survival responses. We demonstrate that NG protects HaCaT cells from UVB induced apoptosis and enhances the removal of CPD from the genome.
MATERIALS AND METHODS
Chemicals and reagents
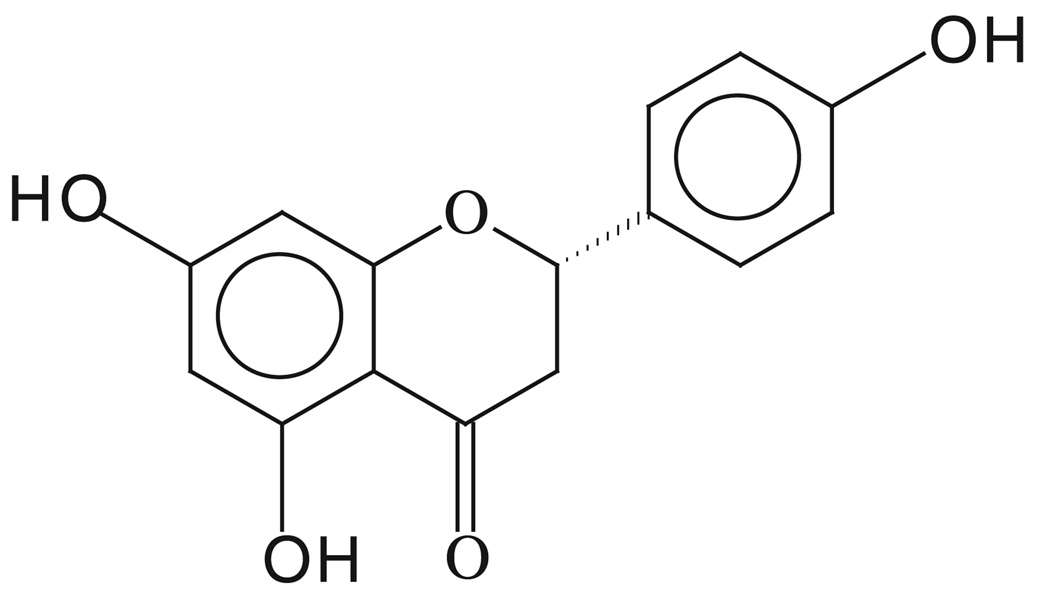
Naringenin (Fig. 1) and all other chemicals, except otherwise specified, were purchased from Sigma/Aldrich (St. Louis, MO). The 10 mm stock solution of NG was made in dimethyl sulfoxide and appropriate working concentrations were prepared in cell culture medium immediately before use. Cell culture supplies were obtained from Life Technologies (Grand Island, NY). Anti-xeroderma pigmentosum-C (XPC) antibody (XPC-2) was generated by immunizing rabbits with synthetic peptide KTKREKKAAASHLFPFEKL which matches to the C-terminus of human XPC protein. The antibody was affinity purified with the corresponding peptide (BioSource, Hopkinton, MA). Polyclonal anti-CPD was raised and characterized in our laboratory as previously described (31). Monoclonal anti-CPD antibody was purchased from MBL International Corporation (Woburn, MA). Monoclonal antibodies against actin and XPB were from Neomarkers (Fremont, CA) and Santa Cruz Biotechnology (Santa Cruz, CA), respectively. Fluorescent-conjugated antibodies (Alexa Fluora® 488-goat anti-mouse IgG2a and IgG1) were from Molecular Probes (Eugene, OR); Texas Red-conjugated goat anti-rabbit IgG and fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG were from Santa Cruz Biotechnology. Antibodies against poly (ADP-ribose) polymerase-1 (PARP-1), caspase 9, Bax and Bcl2 were bought from Upstate Biotechnology (Charlottesville, VA). Horseradish peroxidase (HRP)-conjugated secondary antibodies and protease inhibitor cocktail tablets were from Roche (Indianapolis, IN). Caspase colorimetric assay kits were purchased from R&D Systems (Minneapolis, MN). Chemiluminescence substrate was obtained from Pierce (Rockford, IL). The DC Bio-Rad protein quantitation reagents were from Bio-Rad (Hercules, CA).
Figure 1.
The chemical structure of naringenin (2, 3-dihydro-5,7-dihydroxy-2-(4-hydroxyphenyl)-4-benzopyrone; 4,5,7-Trihydroxyflavanone; 5,7-Dihydroxy-2-(4-hydroxyphenyl)chroman-4-one).
Cell culture and treatment
The immortalized human keratinocyte cell line HaCaT was cultured in low glucose Dulbecco’s modified Eagle’s media (DMEM) supplemented with 10% heat-inactivated fetal calf serum, 50 IU mL−1 penicillin, 50 mg mL−1 streptomycin and 2 mm glutamine at 37°C in a humidified 5% CO2 incubator. The cells were incubated in culture medium for at least 24 h, exposed to 15 or 30 mJ cm−2 of UVB or 20 J m−2 of UVC and then treated with NG at 5 or 10 µm for 6–8 h immediately after UV irradiation. For DNA repair assay, confluent cells were incubated in serum-free medium for at least 12 h prior to NG treatment and/or UV irradiation.
UVB irradiation
When HaCaT cells grew to 70% or 100% confluency, the medium was removed and the cells were washed twice with PBS. A thin layer of PBS was left in plates, and the cells were irradiated using FS24T12-UVB-HO sunlamps equipped with a UVB Spectra 305 Dosimeter (Daavlin Co., Bryan, OH), which emitted radiation in the range of 280–340 nm with a peak emission at 314 nm. The filtered UVB was monitored with a UVX digital radiometer connected to a UVX-31 sensor (UVP, Inc., Upland, CA).
Colony formation assay
Exponentially growing HaCaT cells were treated with different concentrations of NG for 6 h immediately following UVB irradiation at doses of 15 or 30 mJ cm−2. The cells were then trypsinized and plated in a six-well plate in fresh culture medium at a density of 1000 cells/well. After growing for 14 days in DMEM medium, the cell colonies were fixed with methanol and stained with crystal violet (5 mg mL−1 in 100% ethanol). The plates were then rinsed with water, and colonies were counted.
DNA fragmentation analysis
Exponentially growing cells were irradiated with UVB dose of 15 or 30 mJ cm−2, left untreated or treated with 5 or 10 µm of NG for 6 h. Cells were then centrifuged, washed once with PBS, resuspended in lysis buffer (Tris–EDTA [TE] pH 8 containing 1% SDS and 100 µg mL−1 proteinase K) and incubated at 56°C overnight. Samples were incubated for an additional 2 h at 37°C with 100 µg mL−1 ribonuclease A. DNA was precipitated with isopropanol, washed with 70% ethanol and dissolved in TE. DNA samples were separated by electrophoresis on 2% agarose gel, stained with ethidium bromide and visualized under UV light.
Caspase activity assay
The activity of caspases was determined by a caspase colorimetric assay kit, according to the manufacturer’s protocol. Briefly, cells were washed with ice-cold PBS and lysed in a lysis buffer. Cell lysates were tested for protease activity using a caspase-specific peptide, conjugated to the color reporter molecule p-nitroaniline. The chromophore p-nitroaniline, cleaved by caspases, was quantitated with a plate reader at a wavelength of 405 nm. Caspase enzymatic activity in cell lysate is directly proportional to the color reaction.
Western blot analysis
Exponentially growing cells were irradiated with either 15 or 30 mJ cm−2 of UVB and incubated in fresh medium with or without NG (5 or 10 µm) for 6 h. Cells were harvested, washed with PBS and lysed by boiling for 10 min in sample buffer (2% SDS, 10% glycerol, 1 mm dithiothreitol and a protease inhibitor cocktail in 62 mm Tris–HCl, pH 6.8), snap-frozen and kept at −20°C until further processing. After protein quantitation, equal amounts of protein were separated on a polyacrylamide gel and electrophoretically transferred to a polyvinylidene fluoride membrane. After blocking with 5% nonfat dry milk in tris-buffered saline/Tween-20 buffer, membranes were incubated with the primary antibodies at 4°C overnight, followed by incubation with an appropriate HRP-conjugated secondary antibody at 37°C for 1 h. Membranes were examined by chemiluminescence detection with a photographic film.
Flow cytometric analysis of cell cycle and apoptosis
Six hours following UVB irradiation and/or NG treatment, both adherent and floating cells were collected, washed with ice-cold PBS and fixed with 70% ice-cold ethanol overnight at 4°C. Fixed cells were washed twice with PBS and treated with 100 µg mL−1 RNase for 30 min at 37°C and then stained with 1 mg mL−1 propidium iodide in PBS containing 0.05% Nonidet-P40. Cells were then analyzed by FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ). From the analysis of DNA histograms, the percentages of cells in different cell cycle phases were evaluated. Cells with a sub-G0/G1 DNA (sub-G1) were taken as apoptotic cells.
Quantitation of cyclobutane pyrimidine dimers
HaCaT cells were maintained in serum-free medium for 12 h before exposure to 20 J m−2 dose of UVC irradiation and either left untreated or treated with 10 µm of NG. At the indicated post-UV time, the cells were recovered and genomic DNA was isolated for damage assessment. The initial CPD formation and that remaining in genomic DNA after cellular repair for varying times were quantitated using a noncompetitive immunoslotblot assay as described earlier (32). The damage levels were calculated by comparing the band intensities of the samples with UV-irradiated DNA standards run in parallel with all the blots. The total amount of DNA loaded on the nitrocellulose membrane was kept constant for each sample.
Local UVC irradiation and immunofluorescence assay
For local UVC irradiation, the cells were grown for 24 h on glass coverslips. The medium was aspirated and the cells were washed with PBS. Prior to UV irradiation, an isopore polycarbonate filter (Millipore, Bedford, MA) with a pore size of 3 µm diameter, was placed on top of the cell monolayer. The filter-covered cells were irradiated with 20 J m−2 of UVC using a germicidal lamp at a dose rate of 0.5 J m−2 s−1 as measured by a Kettering model 65 radiometer (Cole-Palmer Instrument Co., Vernon Hills, IL). The filter was then gently removed, and the cells were processed immediately or maintained in a suitable medium for the desired period and processed thereafter.
Immunofluorescence staining of the cells was conducted according to our published procedure (33). The UVC-irradiated cells, grown on coverslips, were washed twice with cold PBS, and then fixed with 2% p-formaldehyde in 0.5% Triton X-100/PBS at 4°C for 30 min, followed by three washes with PBS. For DNA denaturation, the cells were incubated in 2 N HCl for 10 min at 37°C. The coverslips were rinsed three time with PBS and blocked with 20% normal goat serum in washing buffer (0.1% Triton X-100/PBS) at room temperature for 30 min. Primary rabbit anti-XPC and anti-CPD, as well as fluorescent (FITC or Texas Red)-conjugated secondary antibodies were all prepared in washing buffer containing 1.5% normal goat serum and layered on the coverslips for 1 h at room temperature. Following each antibody incubation step, the cells were washed with 0.1% Tween-20/PBS four times for 5 min each. After fluorescent staining, the coverslips were mounted in VectaShield antifade containing medium with 1.5 µg mL−1 of 4′, 6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA) as a DNA counterstain. Fluorescence images were obtained with a Nikon fluorescence microscope E80i (Nikon, Tokyo, Japan) fitted with appropriate filters for FITC, Texas Red and DAPI. The digital images were then captured through automatic time exposures with a cooled CCD camera and processed with SPOT analysis software (Diagnostic Instruments, Sterling Heights, MI).
Statistical analysis
GraphPad InStat software, version 3.06 (GraphPad, San Diego, CA), was used to compute statistical data. Data are expressed as mean ± SD of three to five independent experiments. Statistical comparisons were performed using ANOVA test. The 0.05 level of probability was used as the criterion of significance.
RESULTS
NG protects HaCaT cells against UVB-induced cell growth inhibition
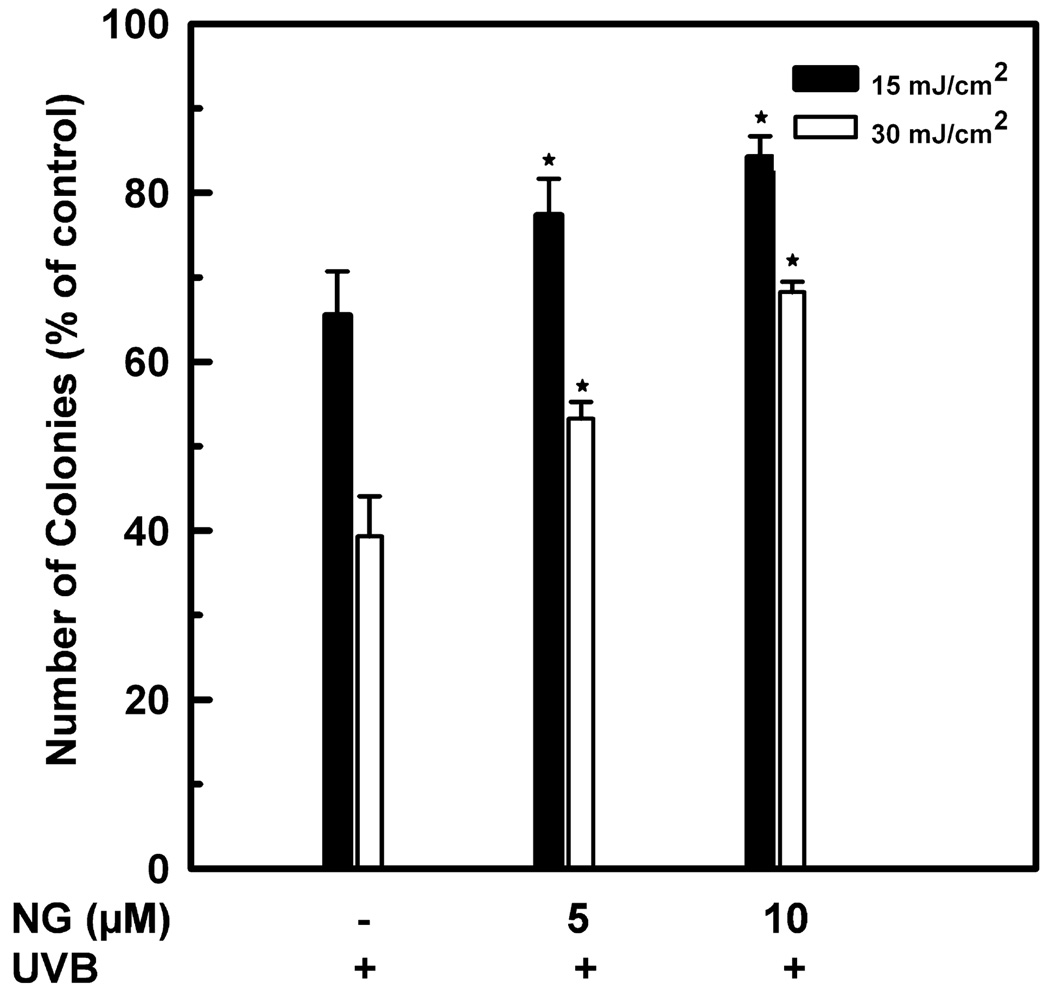
The effect of NG treatment on clonogenicity of HaCaT cells was assessed with the colony-forming assay. Compared to UVB-irradiated cells, an increase in the colony formation was seen in the cells exposed to UVB/NG (Fig. 2). For example, the percentage of colonies formed following 30 mJ cm−2 of UVB alone was ~39%. As a result of 5 or 10 µm NG treatment, the colony formation increased to 53% and 68%, respectively. No change was observed in NG-treated cells when compared with the corresponding untreated controls. These results indicate that NG increases long-term cell survival of HaCaT cell upon UVB-induced DNA damage.
Figure 2.
Enhancement of colony forming efficiency of UVB-irradiated HaCaT cells by NG. HaCaT cells were much-irradiated or irradiated with 15 or 30 mJ cm−2 of UVB. The cells were left untreated or treated with 5 or 10 µm NG. Six hours after NG treatment, cells were washed twice with PBS, trypsinized, reseeded and incubated in fresh medium for 14 days. Cell survival was assessed by counting the number of colonies following different treatments. *Shows significant difference from UV-treated cells at P < 0.05.
NG protects HaCaT cell from UVB-induced apoptosis
To assess the effect of NG on UVB-induced apoptosis, HaCaT cells were exposed to UVB (15 or 30 mJ cm−2) or treated with NG alone (5 or 10 µM) or with NG post-UVB irradiation. After a 6 h NG treatment, cellular apoptosis was examined by DNA fragmentation assay and flow cytometry. As expected, inter-nucleosomal fragmentation and the appearance of a sub-G1 DNA-containing cells (Fig. 3A and B), which are typical features of damage-induced apoptosis, were seen at 6 h post-irradiation. A prominent decrease in both DNA fragmentation and sub-G1 cell population was observed following NG treatment. This antiapoptotic effect appeared in a NG concentration-dependent manner. In UVB-irradiated cells, the percentage of sub-G1-containing cells was found to be 12% after 30 mJ cm−2 UVB irradiation. Upon 5 and 10 µm NG treatment, the sub-G1 population decreases to ~7% and 4%, respectively. This attenuated effect of NG on apoptosis was further confirmed by examination of the UVB-induced reduction of morphological changes, e.g. nuclear blebbing, fragmented nuclei and formation of apoptotic bodies (data not shown).
Figure 3.
NG protects HaCaT cells from UVB-induced apoptosis. Exponentially growing HaCaT cells were washed with PBS, irradiated with 15 or 30 mJ cm−2 of UVB, and then incubated for 6 h in culture medium with or without 5 or 10 µm NG. (A) Agarose gel analysis of fragmentation patterns of cellular DNA isolated from treated or untreated cells. (B) The percentage of cell populations at sub-G0/G1 (sub-G1) phase as measured by flow cytometry. *Shows significant difference from UV-treated cells at P < 0.05.
NG treatment affects caspase pathway in UVB-irradiated cells
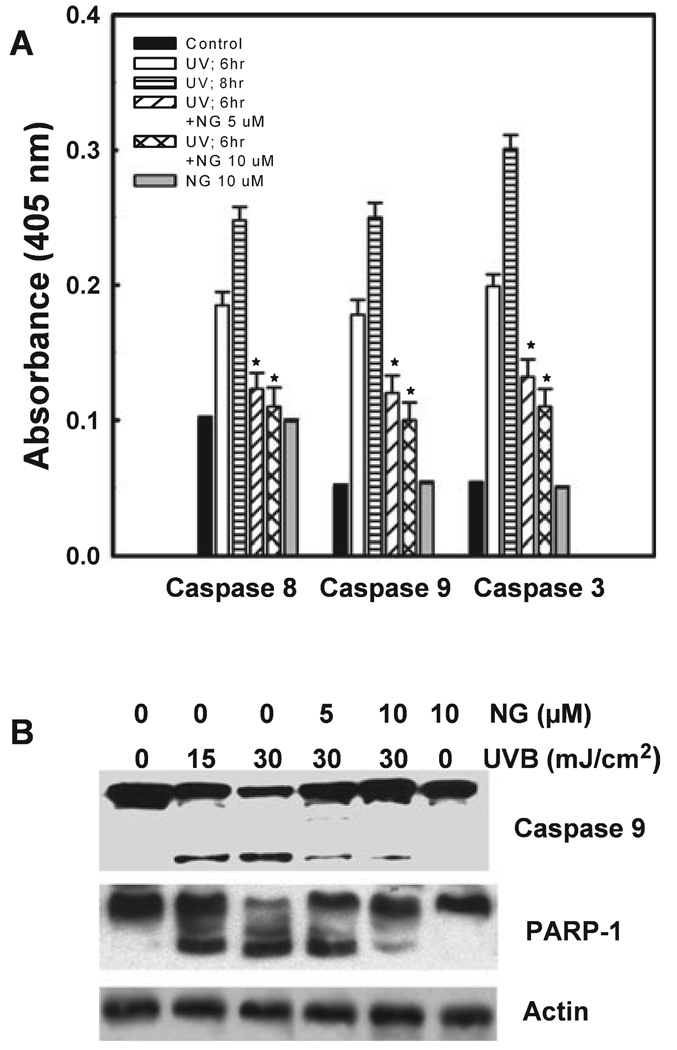
The involvement of the caspase pathway in UVB induced apoptosis has been documented earlier (34). We, therefore, asked whether the observed antiapoptotic effect of NG in HaCaT cells was mediated through an interference of caspase cascade. The relative extent and kinetics of caspases 3, 8 and 9 activation in response to UVB radiation were measured by colorimetric enzyme assay (Fig. 4A). The activation of all three caspases begins at 6–8 h after UVB exposure. Among the caspases tested, the effector caspase 3 was activated to the highest extent. Between the initiator caspases 8 and 9, the activity of caspase 9 was higher, suggesting that the intrinsic pathway plays a predominant role in UV-induced apoptosis. Interestingly, a dose-dependent decrease in all three caspase activities was found when the UV-irradiated cells were treated with NG (Fig. 4A). Consistent with this observation, the biochemical activities of caspases were supported by the western blot analysis of specific caspase and PARP-1 cleavage (Fig. 4B). UVB irradiation causes a dose-dependent cleavage of caspase 9 which was prevented by the treatment of increased concentration of NG. Examination of cleavage of PARP-1, a known substrate of caspase 3, showed an accumulation of an 85 kDa fragment and disappearance of the 116 kDa original PARP-1 protein band, indicating a dose-dependent proteolytic cleavage of PARP-1 upon UV irradiation. Again, UVB-induced PARP-1 cleavage was inhibited by NG treatment at both 5 and 10 µm concentrations. In conclusion, these results indicate that NG treatment protects HaCaT cells from UVB-induced apoptosis through inhibition of activation of caspases and their substrate cleavage.
Figure 4.
NG inhibits proteolytic activation of caspases and PARP-1 cleavage. Cells were either untreated or treated with 5 or 10 µm NG immediately after 30 mJ cm−2 UVB irradiation and incubated in culture medium containing NG for 6 or 8 h. The activity of caspases 8, 9 and 3 was quantitated colorimetrically (A). Six hours following different treatments, cell lysates were analyzed by western blotting for PARP-1 cleavage and caspase 9 activity (B). *Shows significant difference from UV-treated cells at P < 0.05.
NG modulates Bax/Bcl2 ratio in UVB-irradiated HaCaT cells
The Bcl2 family is the central regulator of caspase activation, and opposing actions of its anti- and proapoptotic members arbitrate the life-or-death decision for cells (35). Bcl2 and Bcl-XL can bind to Apaf-1, inhibiting its association with caspase 9 and thereby the activation of effector caspases (36). We assessed whether NG-mediated protection of HaCaT cells against UVB-caused apoptosis involves an alteration in the expression of Bcl2 and/or Bax. A dose-dependent decrease of Bcl2 band was seen upon 15 or 30 mJ cm−2 UVB irradiation (Fig. 5). NG treatment of UVB-irradiated HaCaT cells gradually returned to the normal level of the antiapoptotic protein Bcl2 expression. Similarly, UVB-irradiation caused a dose-dependent increase in the level of the proapoptotic protein Bax. However, NG treatment caused a dramatic dose-dependent decrease of Bax protein elevated by UV irradiation at 30 mJ cm−2. These results suggest that the antiapoptotic effect of NG in UVB-irradiated HaCaT cells involves the modulation of Bax/Bcl2 ratio.
Figure 5.
NG modulates expression of Bcl2 and Bax protein in HaCaT cells. UVB-exposed cells were either untreated or treated with NG and incubated in cultured medium for 6 h. The cell extracts were made and the cellular proteins were resolved by SDS–PAGE and immunoblotted for Bax, Bcl2 or XPB with corresponding antibodies. The XPB panel serves as loading control.
NG increases S-phase population in UVB-irradiated cells
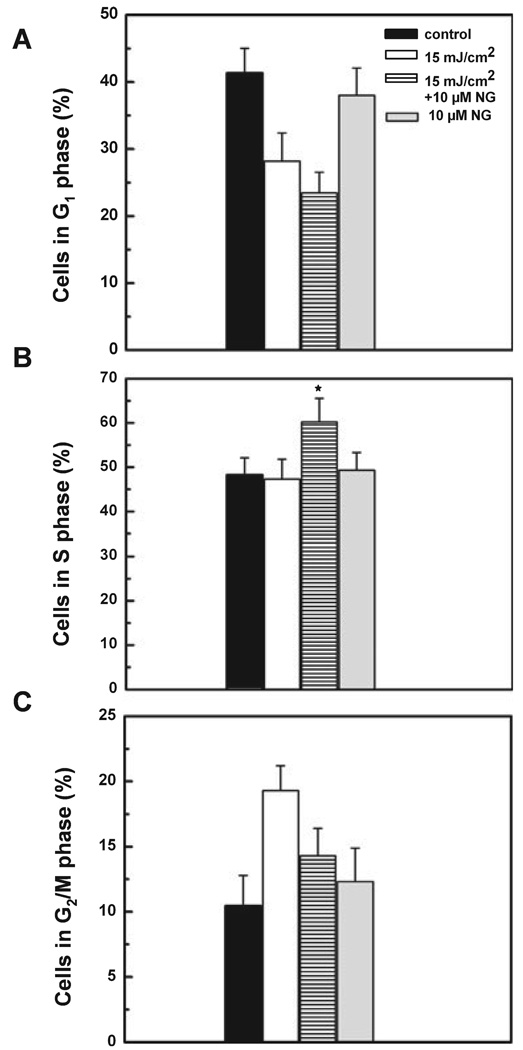
In response to DNA damage, eukaryotic cells cease to progress through the cell cycle and arrest at specific checkpoints which serve to maintain genomic integrity. We, therefore, examined the effect of NG in modulating cell cycle following UVB irradiation (Fig. 6). In non-irradiated control cells the percentage of G1, S and G2/M phases of the cell cycle was found at 41%, 48.22% and 10.45%, respectively. Upon exposure to 15 mJ cm−2, the G2/M population was significantly increased to 19.3% with a slight change in S-phase population at 6 h following irradiation. Treatment with 10 µm of NG resulted in a significant increase in S-phase population in UVB-irradiated cells. For example, the S-phase population in UV/NG-treated cells was found to be 60.2% when compared with 47.3% in UV-treated cells. These findings show that post-irradiation NG treatment resulted in cessation in cell division and accumulation of UVB-irradiated cells in S phase, suggesting that it allows more time for the cellular repair of DNA damage.
Figure 6.
Accumulation of HaCaT cells in S phase following NG treatment. Cells were grown to 60–70% confluency, unirradiated or irradiated with 15 mJ cm−2 UVB followed by incubation with or without 10 µm NG. Six hours later, cells were trypsinized, stained with propidium iodide and analyzed for DNA content using flow cytometry. The percentage of cells in the G1 (A), S (B), G2/M (C) phases was calculated using ModFit computer software. *Shows significant difference from UV-treated cells at P < 0.05.
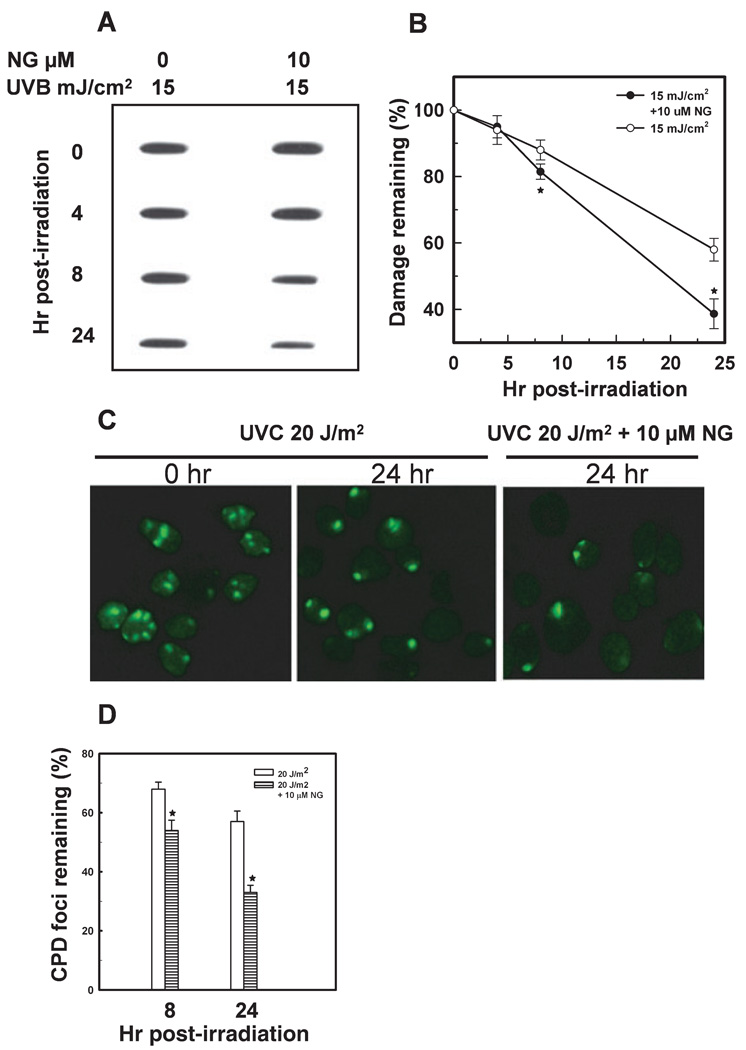
NG enhances CPD removal from the genome of HaCaT cells
We next assessed the effect of NG on the removal of UV-induced CPD from the genome of HaCaT cells. The CPD was directly measured in genomic DNA of HaCaT cells using immunoslot-blot method using dimer specific antibody. The results revealed that NG treatment enhanced the removal of CPD in cells exposed to 15 mJ cm−2 in a time-dependent manner. For example, the percentage of CPD remaining following 8 and 24 h of UVB exposure was found to be about 86% and 56%, respectively. These values were decreased to 80% and 38% as a result of NG treatment (Fig. 7A and B). These results were further substantiated by investigation of the CPD foci directly in the UVC-irradiated HaCaT cells. The UVC was delivered through an isopore polycarbonate filter to generate local DNA damage spots and the CPD were detected with immunofluorescent staining and microscopy. As shown in Fig. 7C and D, the UVC exposed cells treated with 10 µm NG show about 33% of CPD foci remaining at 24 h of irradiation, compared with 57% remaining in UVC-treated cells. An examination of the kinetics of XPC recruitment to the CPD damage sites showed no significant change between NG treated or untreated group (data not shown).
Figure 7.
NG enhances the removal of CPD from the genome of HaCaT cells. Cells were grown to 100% confluency in plates or on coverslips, left unirradiated or irradiated with UVB or UVC, and incubated in fresh medium with or without NG. The cells were allowed to repair the damage for the indicated times. The CPD in genomic DNA were quantitated by immunoslot-blot assay using CPD damage-specific antibodies. A representative plot and mean ± SD of five independent experiments are shown (A and B). For immunofluorescence detection of CPD foci, cells were irradiated with 20 J m−2 UVC through an isopore filter. The cells were allowed to repair the damage, fixed with 2% paraformaldehyde and immunostained with anti-CPD antibody (C and D). CPD foci in 200 individual cells from three different microscope fields were counted for calculating the percentage of remaining damage. *Shows significant difference from UV-treated cells at P < 0.05.
DISCUSSION
In the current study, we investigated the effect of NG on cellular response of the human immortalized keratinocyte HaCaT cell line to UVB-induced DNA damage. The exposure to solar UV radiation is the crucial factor implicated in several skin disorders (37). The UVB range of solar radiation can penetrate inside epidermis of the skin, inducing both direct and indirect DNA damaging effect. UV radiation depletes the cutaneous defense system and leads to the accumulation of DNA damage, excessive cell apoptosis, skin aging and impairs the epidermal integrity (38,39). In an attempt to use radiation dose relevant to cancer development, we have used low UVB dose that is approximately equivalent to five minimal erythemal dose which represent the irradiation reaching basal keratinocytes (40). We provide evidence that NG is capable of preventing the deleterious effects of UVB irradiation by enhancing the removal of CPD and inhibition of apoptosis. The fact that UVB doses used in this study fall within the physiological range of UVB exposure makes these effects valuable in their impact on human health. The ability of NG to inhibit apoptosis caused by UVB can be a useful effect, especially for people exposed to a daily physiological dose of sunlight, by preventing skin aging and maintaining the integrity and barrier function of the skin. Another great impact of NG effect on human health is the potential of such a compound in preventing the risk of skin cancer formation through its ability to enhance the removal of precancerous CPD lesions.
In recent years, there has been a considerable interest in the use of naturally occurring botanicals for the protection of human skin from UV-induced damage. As flavonoids and other phenolics are UVB-absorbent and they are produced in the upper epidermal cells of leaves, these compounds have been considered as a major class of protectants against UV-induced damage (41). NG belongs to natural flavonoids; therefore, we tested whether it could protect the human keratinocytes from UVB-induced photodamage. The HaCaT cells used in our study are spontaneously immortalized through mutations of p53 gene. Earlier studies with this cell line have argued for their appropriateness and as a closest model to normal keratinocytes (42). In fact, HaCaT cells have been used extensively as an in vitro model of epidermal skin to investigate the effects of UVB (43,44).
Mammalian cells have sophisticated mechanisms that enable them to engage in programmed cell death in response to a variety of physiological or pathological stimuli. In the present study, several characteristics of apoptosis were observed in HaCaT cells following UVB irradiation, including DNA ladder formation, morphological changes and the appearance of sub-G1 DNA containing cell population. Such effects have been established by several studies (45,46). Our observation of caspase activation following UVB exposure confirmed that UVB-induced apoptosis occur through caspase cascade. Typical kinetics and yet different magnitudes of activation for all tested caspases were observed. As the activity of caspase 3 is attributed to its function as an effecter caspase and the caspase 9 was activated more than caspase 8, it can be inferred that the UVB-induced apoptosis primarily occur through the intrinsic pathway triggered by DNA damage. In accordance with our observation, it has been shown that expression of dominant negative caspase 9 blocks UVB-induced apoptosis (36). In the present study, post-treatment of UVB-irradiated cells with NG showed significant inhibition of UVB-induced caspase activation, indicating that NG interferes with caspase pathway. This may be one of the mechanisms through which NG exhibits its antiapoptotic effect independent of p53. The protective effect of several naturally occurring botanicals, including flavonoids, against different apoptotic inducers and UV-induced damage has previously been demonstrated. Lee et al. (47) has reported that 3,4′dihydroxy flavone protects HaCaT cells from etoposide-induced apoptosis through different mechanisms, including caspase pathway. Recently, the flavonoid eriodictyol was shown to exert antiapoptotic effect in HaCaT cell line and normal human keratinocyte exposed to UV light and the effect was suggested to occur through modulation of caspase pathway and attenuation of ROS generation (48). Maalouf et al. (20) has reported the protective effect of vitamin E against UVB-induced damage in keratinocytes. More recently, delphinidin, a major anthocyanidin present in many pigmented fruits and vegetables, has been reported to protect HaCaT cells and mouse skin against UVB-induced damage and apoptosis (49).
The view that UVB-induced apoptosis occurs through the intrinsic pathway is suggested to be due to the ability of Bcl2 family proteins to inhibit the apoptosis following UV irradiation (50–52). The Bcl2 family consists of both apoptotic and antiapoptotic proteins and the balance between them turns the cellular apoptotic machinery on or off (53). Bcl2 localizes in the outer membrane of mitochondria and most apoptosis-inducing stimuli involve disruption of potential as well as the permeability transition of the mitochondrial inner transmembrane and results in release of the proapoptotic proteins from the mitochondrial inter-membrane space into the cytoplasm (14,54). Altered Bax/Bcl2 ratio leads to the release of cytochrome c from mitochondria, and thereby the initiation of caspase activation. In the present study, we observed that UVB-induced alteration in Bax/Bcl2 ratio was modulated upon NG treatment. The UVB-irradiated cells treated with NG retained the normal level of Bcl2 expression (Fig. 5) and displayed a gradual decrease in the level of proapoptotic protein Bax. This modulation is in accordance with the inhibitory effect of NG on caspase activation. It suggests that NG may protect the mitochondrial membrane and prevent DNA damage triggered apoptotic signal from propagating or being amplified through mitochondria.
Besides apoptotic pathway, normal cell cycle is also disrupted upon exposure to DNA damaging agents. Activation of the cell cycle checkpoint prevents cells from progressing through, at least, one of two points of cell cycle—either entry into S phase (G1/S) or entry into mitosis (G2/M). Arrest at these phases would allow time for DNA repair or initiation of cell death (55). As shown in Fig. 6, the lower dose of UVB irradiation (15 mJ cm−2) caused a G2/M arrest with a slight change in S-phase population in HaCaT cells. The absence of S-phase arrest is possibly attributed to the mutation of p53 in HaCaT cells (56,57). A similar response has been reported by other investigators when HaCaT cells were exposed to a similar dose of UVB (46). It is established that progression through the cell cycle is regulated by formation, activation and deactivation of a series of serine/threonine protein kinases. These structurally related enzymes consist of a regulatory and a catalytic subunit called cyclin and cyclin-dependent kinase (cdk), respectively (58). The activity of the cdk/cyclin complexes is regulated by post-translational modifications and different cdk inhibitors (59). The rapid inhibition of cyclin B-associated cdc2 kinase activity has been a key player in prolonged G2 arrest of human keratinocytes exposed to UVB (60). The fact that cyclin A-associated kinase activity is required for entry into S phase, completion of S phase and entry into M phase (61–63) suggests that cyclin/cdk complexes may have a role in DNA repair (64). In the present study, NG treatment leads to a significant accumulation of cells in S phase in UVB-treated cells, indicating that NG may play a role in an efficient repair process that eliminates UVB-induced damage. The ability of naturally occurring botanicals to modulate cell cycle distribution in human keratinocytes and the involvement of different cyclins as an underlying cause have been reported (45,46). A consequence of NG mediated cell cycle modulation could also enhance the removal of CPD from the genome. Our results show that NG treatment significantly enhanced the removal of CPD from the entire genome of UVB-irradiated cells (Fig. 7), but without affecting the recruitment of XPC protein to DNA damage sites. As XPC recruitment to damage is known to occur instantly, it could not be a factor in the NG mediated differences in CPD repair observed at about 8 h following irradiation. Consistent with our repair modulation by NG, lower level of CPD at the UVB irradiated sites has been shown upon topical application of green tea polyphenols to human skin (37). The combination of vitamins C and E has been reported to decrease the sunburn reaction and protect epidermal cells against the induction of CPD in human subjects irradiated with UVB light (65). In the same vein, it has also been shown that another naturally occurring compound silibinin inhibited UV-induced CPD in mouse skin (37,66). Furthermore, several naturally occurring agents, including flavonoids, have been shown to enhance the activity of enzymes involved in base excision repair pathway (29,67). We conclude that enhanced DNA repair may be one of the mechanisms for these naturally occurring botanicals to reduce DNA damage and prevent carcinogenesis. It will be interesting to know how NG enhances the removal of CPD from the genome of HaCaT cells. One possibility is that the cell cycle regulatory effect of NG makes significant contribution to enhanced DNA repair in NG-treated HaCaT cells. The study by Feng et al. (68) indicates that malondialdehyde (MDA), an oxidative stress lipid peroxidation product, sensitizes human cells to UV and benzopyrene diolepoxide-induced damage through inhibition of NER. Thus, the antioxidant ability of naturally occurring agents, including NG, to minimize MDA formation upon UV-irradiation could have a role in enhancing the removal of CPD from the genome.
In summary, our combined data suggest that NG could protect human skin from UVB-induced aging and carcinogenesis via an inhibition of excessive apoptosis and accelerated elimination of UVB-induced promutagenic and precarcinogenic CPD lesions.
Acknowledgements
We thank Haroon Haque, Rishi Dalsani and Annum Yasin for assisting with experimentation and manuscript editing. This work was supported by NIH grants CA93413, ES6074 and ES12991 to A.A.W.
REFERENCES
- 1.Pourzand C, Tyrrell RM. Apoptosis, the role of oxidative stress and the example of solar UV radiation. Photochem. Photobiol. 1999;70:380–390. [PubMed] [Google Scholar]
- 2.Madronich S, McKenzie RL, Bjorn LO, Caldwell MM. Changes in biologically active ultraviolet radiation reaching the Earth’s surface. J. Photochem. Photobiol. B. 1998;46:5–19. doi: 10.1016/s1011-1344(98)00182-1. [DOI] [PubMed] [Google Scholar]
- 3.Stewart BW, Kleihues P, Stewart BW, Kletter Y. World Cancer Report. Washington, DC: IARC Press; 2003. [Google Scholar]
- 4.Gailani MR, Leffell DJ, Ziegler A, Gross EG, Brash DE, Bale AE. Relationship between sunlight exposure and a key genetic alteration in basal cell carcinoma. J. Natl Cancer Inst. 1996;88:349–354. doi: 10.1093/jnci/88.6.349. [DOI] [PubMed] [Google Scholar]
- 5.Cleaver JE, Crowley E. UV damage, DNA repair and skin carcinogenesis. Front Biosci. 2002;7:d1024–d1043. doi: 10.2741/A829. [DOI] [PubMed] [Google Scholar]
- 6.Sarasin A. The molecular pathways of ultraviolet-induced carcinogenesis. Mutat. Res. 1999;428:5–10. doi: 10.1016/s1383-5742(99)00025-3. [DOI] [PubMed] [Google Scholar]
- 7.de Gruijl FR. Photocarcinogenesis: UVA vs. UVB radiation. Skin Pharmacol. Appl. Skin Physiol. 2002;15:316–320. doi: 10.1159/000064535. [DOI] [PubMed] [Google Scholar]
- 8.Assefa Z, Van LA, Garmyn M, Agostinis P. Ultraviolet radiation-induced apoptosis in keratinocytes: On the role of cytosolic factors. Biochim. Biophys. Acta. 2005;1755:90–106. doi: 10.1016/j.bbcan.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Matsumura Y, Ananthaswamy HN. Toxic effects of ultraviolet radiation on the skin. Toxicol. Appl. Pharmacol. 2004;195:298–308. doi: 10.1016/j.taap.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 10.Chen YC, Lin-Shiau SY, Lin JK. Involvement of reactive oxygen species and caspase 3 activation in arsenite-induced apoptosis. J. Cell. Physiol. 1998;177:324–333. doi: 10.1002/(SICI)1097-4652(199811)177:2<324::AID-JCP14>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 11.Chen YC, Shen SC, Lee WR, Hsu FL, Lin HY, Ko CH, Tseng SW. Emodin induces apoptosis in human promyeloleukemic HL-60 cells accompanied by activation of caspase 3 cascade but independent of reactive oxygen species production. Biochem. Pharmacol. 2002;64:1713–1724. doi: 10.1016/s0006-2952(02)01386-2. [DOI] [PubMed] [Google Scholar]
- 12.Bossy-Wetzel E, Green DR. Caspases induce cytochrome c release from mitochondria by activating cytosolic factors. J. Biol. Chem. 1999;274:17484–17490. doi: 10.1074/jbc.274.25.17484. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen M, Millar DG, Yong VW, Korsmeyer SJ, Shore GC. Targeting of Bcl-2 to the mitochondrial outer membrane by a COOH-terminal signal anchor sequence. J. Biol. Chem. 1993;268:25265–25268. [PubMed] [Google Scholar]
- 14.Loeffler M, Kroemer G. The mitochondrion in cell death control: Certainties and incognita. Exp. Cell Res. 2000;256:19–26. doi: 10.1006/excr.2000.4833. [DOI] [PubMed] [Google Scholar]
- 15.De Laat WL, Jaspers NG, Hoeijmakers JH. Molecular mechanism of nucleotide excision repair. Genes Dev. 1999;13:768–785. doi: 10.1101/gad.13.7.768. [DOI] [PubMed] [Google Scholar]
- 16.Lehmann AR. Nucleotide excision repair and the link with transcription. Trends Biochem. Sci. 1995;20:402–405. doi: 10.1016/s0968-0004(00)89088-x. [DOI] [PubMed] [Google Scholar]
- 17.Lindahl T, Wood RD. Quality control by DNA repair. Science. 1999;286:1897–1905. doi: 10.1126/science.286.5446.1897. [DOI] [PubMed] [Google Scholar]
- 18.Narayana KR, Reddy MS, Chaluvadi MR, Krishna DR. Bioflavonoids classification, pharmacological, biochemical effects and therapeutic potential. Indian J. Pharmacol. 2001;33:2–16. [Google Scholar]
- 19.Mori A, Nishinoc C, Enoki N, Tawata S. Cytotoxicity of plant flavonoids against Hela cells. Phytochemistry. 1988;27:1017–1020. [Google Scholar]
- 20.Maalouf S, El-Sabban M, Darwiche N, Gali-Muhtasib H. Protective effect of vitamin E on ultraviolet B light-induced damage in keratinocytes. Mol. Carcinog. 2002;34:121–130. doi: 10.1002/mc.10055. [DOI] [PubMed] [Google Scholar]
- 21.Gupta S, Mukhtar H. Chemoprevention of skin cancer: Current status and future prospects. Cancer Metastasis Rev. 2002;21:363–380. doi: 10.1023/a:1021275330385. [DOI] [PubMed] [Google Scholar]
- 22.Bode AM, Dong Z. Signal transduction pathways: Targets for chemoprevention of skin cancer. Lancet Oncol. 2000;1:181–188. doi: 10.1016/s1470-2045(00)00029-2. [DOI] [PubMed] [Google Scholar]
- 23.Afaq F, Adhami VM, Ahmad N, Mukhtar H. Botanical antioxidants for chemoprevention of photocarcinogenesis. Front Biosci. 2002;7:d784–d792. doi: 10.2741/afaq. [DOI] [PubMed] [Google Scholar]
- 24.Einspahr JG, Stratton SP, Bowden GT, Alberts DS. Chemoprevention of human skin cancer. Crit. Rev. Oncol. Hematol. 2002;41:269–285. doi: 10.1016/s1040-8428(01)00185-8. [DOI] [PubMed] [Google Scholar]
- 25.Valenzuela A, Guerra R, Videla LA. Antioxidant properties of the flavonoids silybin and (+)-cyanidanol-3: Comparison with butylated hydroxyanisole and butylated hydroxytoluene. Planta Med. 1986;52:438–440. doi: 10.1055/s-2007-969247. [DOI] [PubMed] [Google Scholar]
- 26.Katiyar SK, Korman NJ, Mukhtar H, Agarwal R. Protective effects of silymarin against photocarcinogenesis in a mouse skin model. J. Natl Cancer Inst. 1997;89:556–566. doi: 10.1093/jnci/89.8.556. [DOI] [PubMed] [Google Scholar]
- 27.Yang CS, Landau JM, Huang MT, Newmark HL. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu. Rev. Nutr. 2001;21:381–406. doi: 10.1146/annurev.nutr.21.1.381. [DOI] [PubMed] [Google Scholar]
- 28.Afaq F, Saleem M, Krueger CG, Reed JD, Mukhtar H. Anthocyanin- and hydrolyzable tannin-rich pomegranate fruit extract modulates MAPK and NF-kappaB pathways and inhibits skin tumorigenesis in CD-1 mice. Int. J. Cancer. 2005;113:423–433. doi: 10.1002/ijc.20587. [DOI] [PubMed] [Google Scholar]
- 29.Williams RJ, Spencer JP, Rice-Evans C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004;36:838–849. doi: 10.1016/j.freeradbiomed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 30.Morel I, Abalea V, Cillard P, Cillard J. Repair of oxidized DNA by the flavonoid myricetin. Methods Enzymol. 2001;335:308–316. doi: 10.1016/s0076-6879(01)35253-9. [DOI] [PubMed] [Google Scholar]
- 31.Wani AA, Gibson-D’Ambrosio RE, D’Ambrosio SM. Antibodies to UV irradiated DNA: The monitoring of DNA damage by ELISA and indirect immunofluorescence. Photochem. Photobiol. 1984;40:465–471. doi: 10.1111/j.1751-1097.1984.tb04619.x. [DOI] [PubMed] [Google Scholar]
- 32.Wani AA, D’Ambrosio SM, Alvi NK. Quantitation of pyrimidine dimers by immunoslot blot following sublethal UV-irradiation of human cells. Photochem. Photobiol. 1987;46:477–482. doi: 10.1111/j.1751-1097.1987.tb04798.x. [DOI] [PubMed] [Google Scholar]
- 33.Wang Q, Zhu Q, Wani MA, Wani G, Chen J, Wani AA. Tumor suppressor p53 dependent recruitment of nucleotide excision repair factors XPC and TFIIH to DNA damage. DNA Repair. 2003;2:483–499. doi: 10.1016/s1568-7864(03)00002-8. [DOI] [PubMed] [Google Scholar]
- 34.Sitailo LA, Tibudan SS, Denning MF. Activation of caspase-9 is required for UV-induced apoptosis of human keratinocytes. J. Biol. Chem. 2002;277:19346–19352. doi: 10.1074/jbc.M200401200. [DOI] [PubMed] [Google Scholar]
- 35.Cory S, Adams JM. The Bcl2 family: Regulators of the cellular life-or-death switch. Nat. Rev. Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 36.Hu Y, Benedict MA, Wu D, Inohara N, Nunez G. Bcl-XL interacts with Apaf-1 and inhibits Apaf-1-dependent caspase-9 activation. Proc. Natl Acad. Sci. USA. 1998;95:4386–4391. doi: 10.1073/pnas.95.8.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baliga MS, Katiyar SK. Chemoprevention of photocarcinogenesis by selected dietary botanicals. Photochem. Photobiol. Sci. 2006;5:243–253. doi: 10.1039/b505311k. [DOI] [PubMed] [Google Scholar]
- 38.Katiyar SK, Mukhtar H. Green tea polyphenol (−)-epigallocatechin-3-gallate treatment to mouse skin prevents UVB-induced infiltration of leukocytes, depletion of antigen-presenting cells, and oxidative stress. J. Leukoc. Biol. 2001;69:719–726. [PubMed] [Google Scholar]
- 39.Mittal A, Elmets CA, Katiyar SK. Dietary feeding of proanthocyanidins from grape seeds prevents photocarcinogenesis in SKH-1 hairless mice: Relationship to decreased fat and lipid peroxidation. Carcinogenesis. 2003;24:1379–1388. doi: 10.1093/carcin/bgg095. [DOI] [PubMed] [Google Scholar]
- 40.Herzinger T, Funk JO, Hillmer K, Eick D, Wolf DA, Kind P. Ultraviolet B irradiation-induced G2 cell cycle arrest in human keratinocytes by inhibitory phosphorylation of the cdc2 cell cycle kinase. Oncogene. 1995;11:2151–2156. [PubMed] [Google Scholar]
- 41.Caldwell MM, Robberecht R, Flint SD. Internal filters: Prospects for UV-acclimation in higher plants. Physiol. Plant. 1983;58:445–450. [Google Scholar]
- 42.Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988;106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Catani MV, Rossi A, Costanzo A, Sabatini S, Levrero M, Melino G, Avigliano L. Induction of gene expression via activator protein-1 in the ascorbate protection against UV-induced damage. Biochem. J. 2001;356:77–85. doi: 10.1042/0264-6021:3560077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Phillipson RP, Tobi SE, Morris JA, McMillan TJ. UV-A induces persistent genomic instability in human keratinocytes through an oxidative stress mechanism. Free Radic. Biol. Med. 2002;32:474–480. doi: 10.1016/s0891-5849(01)00829-2. [DOI] [PubMed] [Google Scholar]
- 45.Dhanalakshmi S, Mallikarjuna GU, Singh RP, Agarwal R. Dual efficacy of silibinin in protecting or enhancing ultra-violet B radiation-caused apoptosis in HaCaT human immortalized keratinocytes. Carcinogenesis. 2004;25:99–106. doi: 10.1093/carcin/bgg188. [DOI] [PubMed] [Google Scholar]
- 46.Reagan-Shaw S, Breur J, Ahmad N. Enhancement of UVB radiation-mediated apoptosis by sanguinarine in HaCaT human immortalized keratinocytes. Mol. Cancer Ther. 2006;5:418–429. doi: 10.1158/1535-7163.MCT-05-0250. [DOI] [PubMed] [Google Scholar]
- 47.Lee ER, Kang YJ, Kim JH, Lee HT, Cho SG. Modulation of apoptosis in HaCaT keratinocytes via differential regulation of ERK signaling pathway by flavonoids. J. Biol. Chem. 2005;280:31498–31507. doi: 10.1074/jbc.M505537200. [DOI] [PubMed] [Google Scholar]
- 48.Lee ER, Kim JH, Kang YJ, Cho SG. The anti-apoptotic and anti-oxidant effect of eriodictyol on UV-induced apoptosis in keratinocytes. Biol. Pharm. Bull. 2007;30:32–37. doi: 10.1248/bpb.30.32. [DOI] [PubMed] [Google Scholar]
- 49.Afaq F, Syed DN, Malik A, Hadi N, Sarfaraz S, Kweon MH, Khan N, Zaid MA, Mukhtar H. Delphinidin, an anthocyanidin in pigmented fruits and vegetables, protects human HaCaT keratinocytes and mouse skin against UVB-mediated oxidative stress and apoptosis. J. Invest. Dermatol. 2007;127:222–232. doi: 10.1038/sj.jid.5700510. [DOI] [PubMed] [Google Scholar]
- 50.Rodriguez-Villanueva J, Greenhalgh D, Wang XJ, Bundman D, Cho S, Delehedde M, Roop D, McDonnell TJ. Human keratin-1.bcl-2 transgenic mice aberrantly express keratin 6, exhibit reduced sensitivity to keratinocyte cell death induction, and are susceptible to skin tumor formation. Oncogene. 1998;16:853–863. doi: 10.1038/sj.onc.1201610. [DOI] [PubMed] [Google Scholar]
- 51.Pena JC, Fuchs E, Thompson CB. Bcl-x expression influences keratinocyte cell survival but not terminal differentiation. Cell Growth Differ. 1997;8:619–629. [PubMed] [Google Scholar]
- 52.Gillardon F, Moll I, Meyer M, Michaelidis TM. Alterations in cell death and cell cycle progression in the UV-irradiated epidermis of bcl-2-deficient mice. Cell Death Differ. 1999;6:55–60. doi: 10.1038/sj.cdd.4400455. [DOI] [PubMed] [Google Scholar]
- 53.Ricci JE, Gottlieb RA, Green DR. Caspase-mediated loss of mitochondrial function and generation of reactive oxygen species during apoptosis. J. Cell Biol. 2003;160:65–75. doi: 10.1083/jcb.200208089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bernardi R, Negri C, Donzelli M, Guano F, Torti M, Prosperi E, Scovassi AI. Activation of poly(ADP-ribose) polymerase in apoptotic human cells. Biochimie. 1995;77:378–384. doi: 10.1016/0300-9084(96)88150-8. [DOI] [PubMed] [Google Scholar]
- 55.de Laat A, van Tilburg M, van der Leun JC, van Vloten WA, de Gruijl FR. Cell cycle kinetics following UVA irradiation in comparison to UVB and UVC irradiation. Photochem. Photobiol. 1996;63:492–497. doi: 10.1111/j.1751-1097.1996.tb03075.x. [DOI] [PubMed] [Google Scholar]
- 56.Petrocelli T, Poon R, Drucker DJ, Slingerland JM, Rosen CF. UVB radiation induces p21Cip1/WAF1 and mediates G1 and S phase checkpoints. Oncogene. 1996;12:1387–1396. [PubMed] [Google Scholar]
- 57.Latonen L, Taya Y, Laiho M. UV-radiation induces dose-dependent regulation of p53 response and modulates p53-HDM2 interaction in human fibroblasts. Oncogene. 2001;20:6784–6793. doi: 10.1038/sj.onc.1204883. [DOI] [PubMed] [Google Scholar]
- 58.Murray AW. Creative blocks: Cell-cycle checkpoints and feedback controls. Nature. 1992;359:599–604. doi: 10.1038/359599a0. [DOI] [PubMed] [Google Scholar]
- 59.Morgan DO. Principles of CDK regulation. Nature. 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- 60.Herzinger T, Wolf DA, Eick D, Kind P. The pRb-related protein p130 is a possible effector of transforming growth factor beta 1 induced cell cycle arrest in keratinocytes. Oncogene. 1995;10:2079–2084. [PubMed] [Google Scholar]
- 61.Girard F, Strausfeld U, Fernandez A, Lamb NJ. Cyclin A is required for the onset of DNA replication in mammalian fibroblasts. Cell. 1991;67:1169–1179. doi: 10.1016/0092-8674(91)90293-8. [DOI] [PubMed] [Google Scholar]
- 62.Lehner CF, O’Farrell PH. Expression and function of Drosophila cyclin A during embryonic cell cycle progression. Cell. 1989;56:957–968. doi: 10.1016/0092-8674(89)90629-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walker DH, Maller JL. Role for cyclin A in the dependence of mitosis on completion of DNA replication. Nature. 1991;354:314–317. doi: 10.1038/354314a0. [DOI] [PubMed] [Google Scholar]
- 64.Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu. Rev. Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- 65.Placzek M, Gaube S, Kerkmann U, Gilbertz KP, Herzinger T, Haen E, Przybilla B. Ultraviolet B-induced DNA damage in human epidermis is modified by the antioxidants ascorbic acid and D-alpha-tocopherol. J. Invest. Dermatol. 2005;124:304–307. doi: 10.1111/j.0022-202X.2004.23560.x. [DOI] [PubMed] [Google Scholar]
- 66.Dhanalakshmi S, Mallikarjuna GU, Singh RP, Agarwal R. Silibinin prevents ultraviolet radiation-caused skin damages in SKH-1 hairless mice via a decrease in thymine dimer positive cells and an up-regulation of p53-p21/Cip1 in epidermis. Carcinogenesis. 2004;25:1459–1465. doi: 10.1093/carcin/bgh152. [DOI] [PubMed] [Google Scholar]
- 67.Park CH, Lee MJ, Kim JP, Yoo ID, Chung JH. Prevention of UV radiation-induced premature skin aging in hairless mice by the novel compound Melanocin A. Photochem. Photobiol. 2006;82:574–578. doi: 10.1562/2005-07-26-RA-623. [DOI] [PubMed] [Google Scholar]
- 68.Feng Z, Hu W, Marnett LJ, Tang MS. Malondialdehyde, a major endogenous lipid peroxidation product, sensitizes human cells to UV- and BPDE-induced killing and mutagenesis through inhibition of nucleotide excision repair. Mutat. Res. 2006;601:125–136. doi: 10.1016/j.mrfmmm.2006.06.003. [DOI] [PubMed] [Google Scholar]