Abstract
Photophobia, or painful oversensitivity to light, occurs in a number of clinical conditions, from superficial eye irritation to meningitis. In this case study, a healthy subject with transient photophobia (induced by the overuse of contact lenses) was examined using functional magnetic resonance imaging (fMRI). While being scanned in a darkened environment, the subject was presented with intermittent 6-second blocks of bright light. The subject was scanned twice, once during his photophobic state and once after recovery. The subject reported that the visual stimuli produced pain (pain intensity = 3/10, unpleasantness = 7/10) only during the photophobic state. During photophobia, specific activation patterns in the trigeminal system were seen at the level of the trigeminal ganglion, trigeminal nucleus caudalis, and ventroposteromedial thalamus. The anterior cingulate cortex, a brain structure associated with unpleasantness, was also active during photophobia. After recovery from photophobia, no significant activations were detected in these areas. This study may contribute to a better understanding of the pathways involved in photophobia in the human condition.
1. Introduction
Photophobia is a clinical term for painful oversensitivity to light and occurs in a number of conditions, including eye conditions (e.g., corneal abrasions, uveitis, cataracts) and intracranial diseases (e.g. migraine, meningeal inflammation/irritation/infection, tumors). The mechanisms underlying this symptom are not well understood, though convergence of the trigeminal nociceptive pathway with the visual afferent pathway has been proposed [1]. The trigeminal nerve has been linked to photophobia since the middle of the last century, when noxious stimulation of the eye surface was found to produce photophobia in human subjects [12]. However, functional activation within the human trigeminal system has not yet been recorded during photophobia.
Primary afferent nociceptive fibers innervate several structures of the mammalian eye, including the cornea and anterior uvea, which includes the iris and ciliary body [6,17,34]. Separate from the afferent visual pathway, afferent nociceptive innervation of the eye originates from the ophthalmic branch of the ipsilateral trigeminal ganglion [6,21,25]. Electrophysiological recordings in cats and rabbits indicate that these nociceptors can be divided into two functional categories [5,13,14,19,20,24]: polymodal unmyelinated C-fibers, which respond to chemical irritants as well as noxious mechanical and thermal stimuli, make up the majority (~70%) of the primary afferents in the cornea and uvea; and thinly myelinated A-delta fibers that respond specifically to noxious mechanical stimuli consist of the remainder of the nociceptive afferents. When the eye is damaged, these nociceptive afferents can become sensitized with the release of local inflammatory mediators such as calcitonin gene-related peptide (CGRP), substance P, and prostaglandins [36]. At least in the case of the rat, corneal nociceptive afferents project to the trigeminal nucleus caudalis [22,23,33] and light stimulation can result in Fos-like immunoreactivity in trigeminal brainstem neurons [28]. Though no evidence that we know of supports the theory that exposure to light itself can directly activate these afferents, the trigeminal nociceptive afferents to the eye provide a potential mechanistic basis for the induction of photophobia.
Functional magnetic resonance imaging (fMRI) may be able to contribute to a better understanding of the pathways involved in photophobia in patients. We hypothesized that bright light presented to a photophobic subject would produce activation in sensitized trigeminal pathways, which include the trigeminal ganglion (TG), trigeminal nucleus caudalis (spV), and ventroposteromedial thalamus (VPM) [10].
2. Case Report
A right-handed 54-year old male suffered from an acute onset of left-sided eye pain when exposed to bright light as a result of overuse of hard contact lenses. Bright light produced a sharp pain localized to the eye, along with associated autonomic responses that included lacrimation (tearing) and involuntary blinking. Besides photophobia, the subject was otherwise healthy. This study was approved by the McLean Hospital Institutional Review Board, and met the scientific and ethical guidelines for human research of the Helsinki Accord (http://ohsr.od.nih.gov/guidelines/helsinki.html). The subject provided written informed consent to participate in this study.
3. Methods
The subject participated in two fMRI scan sessions separated by nine days: the first during the photophobic state and the second after recovery. During each fMRI session, the subject was exposed to intermittent presentation of bright light in a darkened environment. At the end of each session, the subjects retrospectively rated light-evoked pain intensity and unpleasantness on a numerical rating scale (0–10). Both 1-hour sessions used the same experimental paradigm and acquisition parameters.
During fMRI scanning, the subject used a prism mirror to view a presentation projected on a screen just outside of the scanner. The presentation consisted of two types of slides: an OFF condition, which featured a white fixation cross on a black background (0.5 lux); and an ON condition, a featureless slide with a pure white background (65 lux). Using these slides during functional scanning, the subject was presented with nine episodes of sustained bright light, each lasting 6 sec. To avoid anticipatory processes, the interstimulus interval (ISI) was varied between 51–66 sec in 3 sec increments to match the fMRI acquisition. The scanner environment was kept dark during the entire experiment, with only the projector providing intermittent brief illumination.
Imaging was conducted using a 3T Siemens Trio scanner (Erlangen, Germany) with a phased array head coil. For anatomical scans, a sagittal three-dimensional T1-weighted scan (MPRAGE) was performed (TE/TR = 2.74/2100 ms; flip angle = 12°; field of view = 25.6 cm; slice thickness = contiguous 1.33 mm; in-plane resolution = 1.0 mm). For functional scans, a Gradient Echo (GE) echo planar imaging (EPI) sequence (TE/TR = 30/3000ms; flip angle = 90°; field of view = 22.4 cm; slice thickness = contiguous 3.5 mm; in-plane resolution = 3.5 mm) was performed, with 202 volumes (10 minutes and 6 seconds) captured for each scan. Each functional scan consisted of 49 slices oriented in an oblique plane to match the brainstem axis. This orientation of acquisition has proven useful for the functional imaging of brainstem structures [3,10,27].
Functional imaging datasets were processed and analyzed using FEAT (FMRI Expert Analysis Tool) Version 5.98, part of FSL 4.1.1 (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl) [32]. Pre-processing included: elimination of the first four acquired volumes to allow for signal equilibration; motion correction using MCFLIRT (Motion Correction using FMRIB’s Linear Image Registration Tool) [15]; non-brain removal using BET [Brain Extraction Tool] [31]; spatial smoothing using a Gaussian kernel of 5mm full-width half-maximum; grand-mean intensity normalization of the entire 4D dataset by a single multiplicative factor; and high pass temporal filtering (Gaussian-weighted least-squares straight line fitting, with sigma=50.0 sec). Time-series analysis was performed using FILM (FMRIB’s Improved Linear Model) with local autocorrelation correction [37]. The hemodynamic response was modeled using a gamma convolution (standard deviation = 3 sec, mean lag = 6 sec) of the subject’s report of light-evoked pain, which included 2 volumes during the stimulus and 2 volumes post-stimulus, as the subject reported that pain persisted for approximately 6 seconds after the light was turned off. Each activation map thus reflects a comparison of 9 stimulus events (totaling 36 volumes) vs. 162 volumes of baseline. A fixed effects contrast was performed between the first session (photophobic state) and second session (recovered state) using FLAME (fMRIB’s Local Analysis of Mixed Effects). The statistical parametric maps for each session and contrast maps were co-registered to the high-resolution anatomical scan from the first session using FLIRT (FMRIB’s Linear Image Registration Tool) [16]. Activation maps were thresholded to p<0.0001 (uncorrected for multiple comparisons).
4. Results
During the first session (“Affected”), the subject reported that bright light evoked pain intensity of 3/10 and unpleasantness of 7/10 at its peak. The subject noted that the pain was immediate in onset, but reached its peak within 2–3 seconds of the onset of the light and continued for 6–10 seconds after the light was turned off. The subject reported no pain intensity or unpleasantness during the second session (“Recovered”). Though not explicitly measured, the subject reported during debriefing that his rate of blinking increased to roughly 2 blinks/5 seconds during bright light. The subject did not notice a difference between the two sessions in this rate of blinking with bright light. However, the subject did report significant lacrimation in the affected eye in the first, but not the second, session.
The “Affected” scan session showed that bright light presented during photophobia produced significant activation (p<0.0001) within the trigeminal nociceptive pathway, in addition to other brain regions, with prominent activations within visual cortex and anterior cingulate cortex (Fig. 1; Fig. 2; Table 1). Specific trigeminal structures significantly activated were the ipsilateral trigeminal ganglion (to the photophobic eye), bilateral trigeminal nucleus caudalis, and contralateral ventroposteromedial thalamus.
Figure 1.
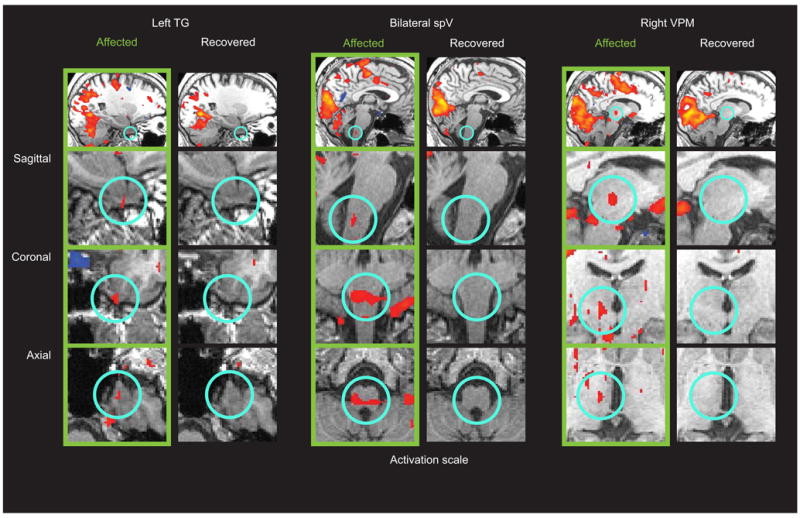
fMRI activation during photophobia across three levels of the trigeminal system. Scanning results from during the photophobic state (“Affected” in green) and after recovery (“Recovered”) are shown. Regions of interest (highlighted by light blue circles) are shown across three different viewing planes. Significant activation (p<0.0001, uncorrected) was detected in the “Affected” state in left trigeminal ganglion (TG), bilateral trigeminal nucleus caudalis (spV), and right ventroposteromedial thalamus (VPM). No significant activation in these regions was detected in the “Recovered” state. Other activations of note include the occipital gyrus and anterior cingulate cortex.
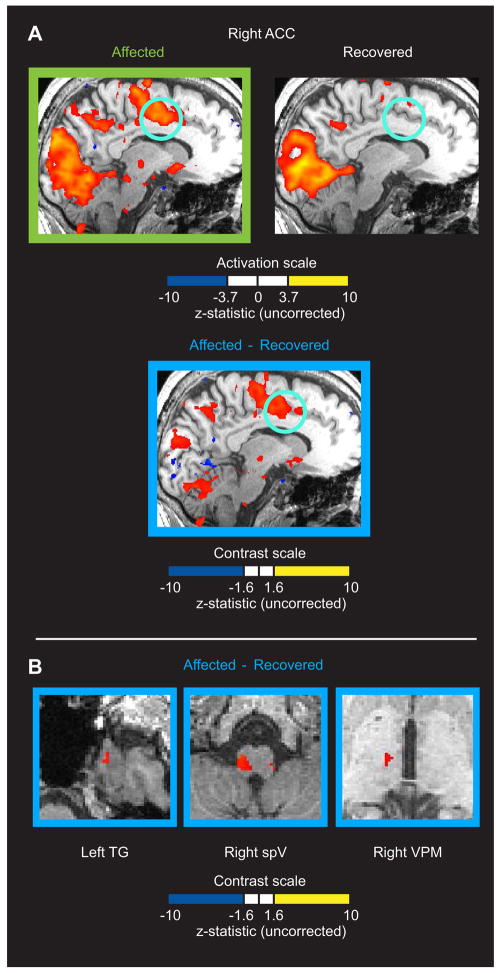
Figure 2.
A) fMRI activation of the right anterior cingulate cortex (ACC) with the presentation of light during the photophobic state, but not after recovery. The top row of sagittal images shows activation maps (p<0.0001, uncorrected) for the presentation of light during the “Affected” and “Recovered” state. The lower image shows areas with a significant contrast (p<0.05, uncorrected) for “Affected” vs. “Recovered” within areas that were active during the “Affected” state. Light blue circles highlight the ACC. The ACC activation was localized to the hemisphere contralateral to the affected eye. B) Region of interest-based contrast map of “Affected” vs. “Recovered” states reveals increased activation across the trigeminal pathway during photophobia. Significantly increased activation (p<0.05, uncorrected) was detected in the “Affected” state in left trigeminal ganglion (TG), right trigeminal nucleus caudalis (spV), and right ventroposteromedial thalamus (VPM). Regions of interest were defined by significant activation in these structures observed in the “Affected” state activation map (Figure 1). Note that the axial brain slices shown are the same as shown in Figure 1.
Table 1.
Activations elicited by bright light during photophobia and after recovery.
| Trigeminal nociceptive pathway |
MNI152 | ||
|---|---|---|---|
| Side | Max Z-statistic | x, y, z | |
| Trigeminal Ganglion | |||
| Photophobia | L | 4.1 | −22, 0, −48 |
| Recovery | - | (−0.1) | - |
| Trigeminal Nucleus Caudalis | |||
| Photophobia | B | 4.3 | 2, −40, −48 |
| Recovery | - | (0.6) | - |
| Thalamus, VPM | |||
| Photophobia | R | 4.7 | 8, −18, −4 |
| Recovery | - | (1.1) | - |
| Other areas (>4 active voxels in native space) | |||
| Visual Cortex | |||
| Photophobia | B | 9.7 | 20, −68, −2 |
| Recovery | B | 12.1 | 18, −72, 2 |
| Anterior Cingulate Cortex | |||
| Photophobia | B | 7.5 | 10, −2, 42 |
| Recovery | - | (1.5) | - |
| Anterior insula | |||
| Photophobia | B | 8.8 | 34, 18, 2 |
| Recovery | B | 5.4 | 34, 18, 0 |
| Parietal Operculum/Planum Temporale | |||
| Photophobia | B | 7.0 | 62, −28, 18 |
| Recovery | B | 5.3 | 60, −20, 18 |
| Middle frontal gyrus | |||
| Photophobia | B | 7.0 | 38, 30, 22 |
| Recovery | - | (1.8) | - |
| Superior parietal lobule | |||
| Photophobia | B | 7.6 | 30, −44, 36 |
| Recovery | B | 5.6 | −38, −50, 52 |
| Precentral gyrus | |||
| Photophobia | B | 9.5 | 58, −4, 28 |
| Recovery | B | 7.2 | 44, −8, 42 |
| Cerebellum | |||
| Photophobia | B | 8.4 | 22, −60, −24 |
| Recovery | B | 7.4 | −30, −62, −22 |
| Superior temporal gyrus | |||
| Photophobia | B | 8.3 | 68, −30, 14 |
| Recovery | B | 6.0 | −62, −34, 16 |
| Pons | |||
| Photophobia | C | 5.9 | 8, −28, −28 |
| Recovery | - | (1.2) | - |
Parentheses indicate that no activation cluster was found, and the number listed represents the Z-statistic in the voxel corresponding to peak activation in the photophobia condition.
The “Recovered” scan session showed that while significant activation (p<0.0001) of visual cortex was qualitatively similar with that observed in the “Affected” session, the trigeminal structures were not significantly active (Fig. 1). Anterior cingulate cortex was also not significantly active during the second session (Fig. 2A; Table 1). Contrast analysis revealed that activation in the trigeminal pathway was significantly increased during photophobia (Fig. 2B), as was activation in the anterior cingulate cortex (Fig. 2A).
5. Discussion
Using fMRI during photophobia in an otherwise healthy subject, we have observed specific activation patterns at the level of the ganglion, brainstem, thalamus, and the cortex. Furthermore, activation observed within the trigeminal system during photophobia was no longer detectable after recovery from the condition. This suggests that for this particular case of transient photophobia, triggered by an injury to the superficial eye, the trigeminal system plays a functional and perhaps driving role in the expression of photophobia.
In a subject with photophobia, presentation of bright light activated the human trigeminal nociceptive pathway in a manner similar to that seen with noxious stimuli applied to the area face innervated by the ophthalmic branch of the trigeminal ganglion [10]. The left trigeminal ganglion, ipsilateral to the photophobic eye, showed significant activation during the photophobic state, similar to how noxious heat activated the trigeminal ganglion ipsilateral to the stimulus. Trigeminal nucleus caudalis in the brainstem contains second-order neurons that receive nociceptive input from the trigeminal ganglion, and these brainstem neurons send projections to the ventroposteriomedial nucleus of the thalamus contralateral to the noxious stimulus. Brainstem activation observed during photophobia in this subject was localized to the same part of trigeminal nucleus caudalis that was activated with noxious thermal stimuli applied to the ophthalmic division of the face in a prior fMRI study [10]. Though contralateral primary somatosensory cortex (S1) is also part of this trigeminal nociceptive pathway, it was not found active during photophobia. Perhaps this is due to the relatively low pain intensity ratings reported by the subject, since S1 activation has previously been correlated with pain intensity [9,26]. However, the anterior cingulate cortex, which has been correlated with unpleasantness [30,35], did show activation specific to the photophobic state, when unpleasantness ratings were high. Other brain regions related to cutaneous experimental pain [2,29] were also found, including anterior insula, middle frontal gyrus, and parietal operculum (which includes secondary somatosensory cortex). However, the specificity of some of these regions to pain under these experimental conditions is ambiguous; anterior insula activation can be at least partially attributed to spatial attention [7], while the parietal operculum has been linked to visual attention to stimulus onset [18].
The activation of the ipsilateral trigeminal ganglion suggests that the nociceptive afferents that innervate the eye are activated by bright light. This transduction of light into a painful stimulus need not be direct; light is not required to activate nociceptors per se. Physiological processes associated with the sudden presentation of bright light, such as involuntary blinking and pupillary dilation, may trigger sensitized nociceptors that respond to noxious mechanical stimuli. With injury-related photophobia, reflexive blinking could activate sensitized corneal afferent nociceptors that are not normally affected by blinking. Mechanical nociceptors and polymodal nociceptors in the cornea are thought to be stimulated even by sliding of the eyelid over an abnormally dry eye [4]. Similarly, the mechanical action of pupillary dilation could stimulate sensitized afferent nociceptors in the iris and ciliary body. Thus, photophobia could be explained as a result of sensitization of mechano-sensitive primary nociceptive afferents due to peripheral injury to the eye. Since we are unaware of any studies that demonstrate that light alone can activate trigeminal nociceptive afferents, we favor this irritation mechanism as the source of trigeminal pathway activation in this specific case of photophobia. Activation of a trigeminal nociceptive afferent-based pathway supports the idea that in specific cases, the expression of photophobia (painful oversensitivity to light) could be based on pain arising from mechanical induced irritation of the cornea (perhaps due to pupillary reactivity), and may not require the transduction of light itself. Clearly, more research is required to address this hypothesis.
The generalizability of this mechanistic theory for all cases of photophobia is unknown, particularly regarding photophobic symptoms arising from intracranial pathophysiologies, such as migraine. For example, photophobia arising from damage to nociceptive afferents in the cornea may have an entirely different mechanism than photophobia from migraine, although some potential overlap is not out of the question. A mechanism for photophobia caused by meningitis and subarachnoid hemorrhage has been proposed that links these pathologies with the intracranial portion of the ophthalmic receptive field of the trigeminal ganglion [1]. In this case, nociceptive afferents in the ophthalmic division of the trigeminal ganglion may become sensitized by intracranial inflammation proximal to the internal carotid artery and middle cerebral artery. Neuroimaging may provide the means to address such hypotheses in future studies.
Blinking and lacrimation may account for some of the activations observed during photophobia, as well as after recovery. Blinking has previously been linked to activation in precentral gyrus [8], as well as the cerebellum [11]. Since the subject did not detect any changes in blink rate during photophobia, this may explain why these areas were found active in both conditions. Increased lacrimation during photophobia may explain activation in the pons that was specific to the photophobic state. The pons contains the several structures related to lacrimation, including the lacrimal nucleus of the facial nerve, and the superior salivatory nucleus. Although not easily addressed by this study, lacrimation may also be related to activation of the nucleus tractus solitarius near the trigeminal nucleus.
This case study is the first that we are aware of that directly demonstrates the functional activation of the trigeminal system during photophobia. It is also notable for presenting functional activation related to noxious stimulation of the human eye (cornea), albeit with an indirect stimulus (light). While the experimental paradigm could use refinement, particularly in regards to addressing scan order effects as well as the measurement of pupillary dilation and blinking, this paper presents a new entry point to study the mechanisms of photophobia.
Acknowledgments
This work was supported by an RO1 grant from NINDS (NS042721) and a K24 grant NINDS (NS064050) to DB. The authors declare no conflicts of interest regarding the contents of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amini A, Digre K, Couldwell WT. Photophobia in a blind patient: An alternate visual pathway. Case report J Neurosurg. 2006;105:765–768. doi: 10.3171/jns.2006.105.5.765. [DOI] [PubMed] [Google Scholar]
- 2.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Becerra L, Morris S, Bazes S, Gostic R, Sherman S, Gostic J, Pendse G, Moulton E, Scrivani S, Keith D, Chizh B, Borsook D. Trigeminal neuropathic pain alters responses in CNS circuits to mechanical (brush) and thermal (cold and heat) stimuli. J Neurosci. 2006;26:10646–10657. doi: 10.1523/JNEUROSCI.2305-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belmonte C, Acosta MC, Gallar J. Neural basis of sensation in intact and injured corneas. Exp Eye Res. 2004;78:513–525. doi: 10.1016/j.exer.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 5.Belmonte C, Gallar J, Pozo MA, Rebollo I. Excitation by irritant chemical substances of sensory afferent units in the cat’s cornea. J Physiol. 1991;437:709–725. doi: 10.1113/jphysiol.1991.sp018621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergmanson JP. The ophthalmic innervation of the uvea in monkeys. Exp Eye Res. 1977;24:225–240. doi: 10.1016/0014-4835(77)90160-9. [DOI] [PubMed] [Google Scholar]
- 7.Brooks JC, Nurmikko TJ, Bimson WE, Singh KD, Roberts N. fMRI of thermal pain: effects of stimulus laterality and attention. Neuroimage. 2002;15:293–301. doi: 10.1006/nimg.2001.0974. [DOI] [PubMed] [Google Scholar]
- 8.Chung JY, Yoon HW, Song MS, Park H. Event related fMRI studies of voluntary and inhibited eye blinking using a time marker of EOG. Neurosci Lett. 2006;395:196–200. doi: 10.1016/j.neulet.2005.10.094. [DOI] [PubMed] [Google Scholar]
- 9.Coghill RC, Sang CN, Maisog JM, Iadarola MJ. Pain intensity processing within the human brain: a bilateral, distributed mechanism. J Neurophysiol. 1999;82:1934–1943. doi: 10.1152/jn.1999.82.4.1934. [DOI] [PubMed] [Google Scholar]
- 10.DaSilva AF, Becerra L, Makris N, Strassman AM, Gonzalez RG, Geatrakis N, Borsook D. Somatotopic activation in the human trigeminal pain pathway. J Neurosci. 2002;22:8183–8192. doi: 10.1523/JNEUROSCI.22-18-08183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dimitrova A, Weber J, Maschke M, Elles HG, Kolb FP, Forsting M, Diener HC, Timmann D. Eyeblink-related areas in human cerebellum as shown by fMRI. Hum Brain Mapp. 2002;17:100–115. doi: 10.1002/hbm.10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eckhardt B, McClean JM, Goodell H. Experimental studies on headache: the genesis of pain from the eye. Proc Assoc Res Nerv Ment Dis. 1943;23:209–227. [Google Scholar]
- 13.Gallar J, Pozo MA, Tuckett RP, Belmonte C. Response of sensory units with unmyelinated fibres to mechanical, thermal and chemical stimulation of the cat’s cornea. J Physiol. 1993;468:609–622. doi: 10.1113/jphysiol.1993.sp019791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giraldez F, Geijo E, Belmonte C. Response characteristics of corneal sensory fibers to mechanical and thermal stimulation. Brain Res. 1979;177:571–576. doi: 10.1016/0006-8993(79)90475-x. [DOI] [PubMed] [Google Scholar]
- 15.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 16.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 17.Lele PP, Weddell G. Sensory nerves of the cornea and cutaneous sensibility. Exp Neurol. 1959;1:334–359. doi: 10.1016/0014-4886(59)90025-1. [DOI] [PubMed] [Google Scholar]
- 18.Lux S, Marshall JC, Ritzl A, Zilles K, Fink GR. Neural mechanisms associated with attention to temporal synchrony versus spatial orientation: an fMRI study. Neuroimage. 2003;20 (Suppl 1):S58–65. doi: 10.1016/j.neuroimage.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 19.MacIver MB, Tanelian DL. Free nerve ending terminal morphology is fiber type specific for A delta and C fibers innervating rabbit corneal epithelium. J Neurophysiol. 1993;69:1779–1783. doi: 10.1152/jn.1993.69.5.1779. [DOI] [PubMed] [Google Scholar]
- 20.MacIver MB, Tanelian DL. Structural and functional specialization of A delta and C fiber free nerve endings innervating rabbit corneal epithelium. J Neurosci. 1993;13:4511–4524. doi: 10.1523/JNEUROSCI.13-10-04511.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marfurt CF. The somatotopic organization of the cat trigeminal ganglion as determined by the horseradish peroxidase technique. Anat Rec. 1981;201:105–118. doi: 10.1002/ar.1092010113. [DOI] [PubMed] [Google Scholar]
- 22.Marfurt CF, Del Toro DR. Corneal sensory pathway in the rat: a horseradish peroxidase tracing study. J Comp Neurol. 1987;261:450–459. doi: 10.1002/cne.902610309. [DOI] [PubMed] [Google Scholar]
- 23.Meng ID, Bereiter DA. Differential distribution of Fos-like immunoreactivity in the spinal trigeminal nucleus after noxious and innocuous thermal and chemical stimulation of rat cornea. Neuroscience. 1996;72:243–254. doi: 10.1016/0306-4522(95)00541-2. [DOI] [PubMed] [Google Scholar]
- 24.Mintenig GM, Sanchez-Vives MV, Martin C, Gual A, Belmonte C. Sensory receptors in the anterior uvea of the cat’s eye. An in vitro study. Invest Ophthalmol Vis Sci. 1995;36:1615–1624. [PubMed] [Google Scholar]
- 25.Morgan CW, Nadelhaft I, de Groat WC. Anatomical localization of corneal afferent cells in the trigeminal ganglion. Neurosurgery. 1978;2:252–258. doi: 10.1227/00006123-197805000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Moulton EA, Keaser ML, Gullapalli RP, Greenspan JD. Regional intensive and temporal patterns of functional MRI activation distinguishing noxious and innocuous contact heat. J Neurophysiol. 2005;93:2183–2193. doi: 10.1152/jn.01025.2004. [DOI] [PubMed] [Google Scholar]
- 27.Moulton EA, Pendse G, Morris S, Strassman A, Aiello-Lammens M, Becerra L, Borsook D. Capsaicin-induced thermal hyperalgesia and sensitization in the human trigeminal nociceptive pathway: an fMRI study. Neuroimage. 2007;35:1586–1600. doi: 10.1016/j.neuroimage.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okamoto K, Thompson R, Tashiro A, Chang Z, Bereiter DA. Bright light produces Fos-positive neurons in caudal trigeminal brainstem. Neuroscience. 2009;160:858–64. doi: 10.1016/j.neuroscience.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000) Neurophysiol Clin. 2000;30:263–288. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- 30.Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- 31.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 (Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 33.Strassman AM, Vos BP. Somatotopic and laminar organization of fos-like immunoreactivity in the medullary and upper cervical dorsal horn induced by noxious facial stimulation in the rat. J Comp Neurol. 1993;331:495–516. doi: 10.1002/cne.903310406. [DOI] [PubMed] [Google Scholar]
- 34.Terenghi G, Polak JM, Ghatei MA, Mulderry PK, Butler JM, Unger WG, Bloom SR. Distribution and origin of calcitonin gene-related peptide (CGRP) immunoreactivity in the sensory innervation of the mammalian eye. J Comp Neurol. 1985;233:506–516. doi: 10.1002/cne.902330410. [DOI] [PubMed] [Google Scholar]
- 35.Tolle TR, Kaufmann T, Siessmeier T, Lautenbacher S, Berthele A, Munz F, Zieglgansberger W, Willoch F, Schwaiger M, Conrad B, Bartenstein P. Region-specific encoding of sensory and affective components of pain in the human brain: a positron emission tomography correlation analysis. Ann Neurol. 1999;45:40–47. doi: 10.1002/1531-8249(199901)45:1<40::aid-art8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 36.Unger WG. Mediation of the ocular response to injury and irritation: peptides versus prostaglandins. Prog Clin Biol Res. 1989;312:293–328. [PubMed] [Google Scholar]
- 37.Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]