Abstract
Background
The Epidemiology of Diabetes Interventions and Complications (EDIC) study, a prospective observational follow-up of the Diabetes Control and Complications Trial (DCCT) cohort, reported persistent benefit of prior intensive therapy on retinopathy and nephropathy in type 1 diabetes. We evaluated the effects of prior intensive insulin therapy on the prevalence and incidence of cardiac autonomic neuropathy (CAN) in former DCCT intensive (INT) and conventional (CONV) therapy subjects13-to-14 (13/14) years after DCCT closeout.
Methods and Results
DCCT autonomic measures (R-R variation with paced breathing, Valsalva ratio, postural blood pressure changes, and autonomic symptoms) were repeated in 1,226 EDIC subjects in EDIC year 13/14. Logistic regression models were used to calculate the odds of incident CAN by DCCT treatment group after adjusting for DCCT baseline covariates, duration in the DCCT, and quantitative autonomic measures at DCCT closeout. In EDIC year 13/14, the prevalence of CAN using the DCCT composite definition was significantly lower in former INT group vs. former CONV group (28.9 vs. 35.2%; P = 0.018). Adjusted R-R variation was significantly greater in former DCCT INT vs. former CONV group (29.9 vs.25.9, P < 0.001). Prior DCCT intensive therapy reduced the risks of incident CAN by 31% [odds ratio(95% CI) 0.69 (0.51–0.93)] and of incident abnormal R-R variation by 30% [odds ratio(95%CI) 0.70 (0.51–0.96)] in EDIC year 13/14.
Conclusions
Although CAN prevalence increased in both groups, the incidence was significantly lower in former INT group compared to former CONV group. The benefits of former intensive therapy extend to measures of CAN up to 14 years after DCCT closeout.
Keywords: type 1 diabetes mellitus, cardiac autonomic neuropathy, nervous system autonomic, intensive glucose control, metabolic memory
INTRODUCTION
Autonomic innervation is the primary extrinsic control mechanism regulating heart rate variability and cardiac performance. Chronic hyperglycemia promotes progressive autonomic neural dysfunction and cardiovascular autonomic neuropathy (CAN). Presence of CAN may be documented by abnormal heart rate variability. It may be the most overlooked complication of type 1 diabetes. CAN is associated with an increased prevalence of silent myocardial ischemia and is an independent predictor of increased cardiac mortality.1–4
The Diabetes Control and Complications Trial (DCCT) demonstrated that intensive insulin therapy for type 1 diabetes reduced the onset and progression of diabetic retinopathy, nephropathy and neuropathy 5–7, and reduced the incidence of CAN by 53% compared to conventional therapy.7 The Epidemiology of Diabetes Interventions and Complications (EDIC) is a prospective observational study of the DCCT cohort.8 Its goal is to describe the long-term effects of prior intensive therapy compared to conventional insulin therapy on the development and progression of microvascular complications and cardiovascular disease in type 1 diabetes. EDIC follow-up has shown that the differences in retinal and renal outcomes observed at the end of the DCCT between the former intensive and conventional treatment groups have persisted and even increased for as long as eight years, despite the loss of glycemic separation.8, 9 The persistent beneficial effect of past glucose control has been called “metabolic memory”.10 Preliminary results have suggested that metabolic memory may apply to peripheral neuropathy.11
Using data obtained during the 13th or 14th (13/14) year of EDIC follow-up, we evaluated CAN in EDIC participants and asked whether the former DCCT intensive treatment group (INT) continues to experience a lower prevalence and incidence of CAN compared to the former DCCT conventional treatment group (CONV) despite no differences in levels of glycemic control following the close of the DCCT.
METHODS
Study Design and Participants
The DCCT has been described elsewhere.5 Briefly, 1,441 subjects who had diabetes for 1–15 years with no (primary prevention cohort) or minimal diabetic retinopathy (secondary intervention cohort) were eligible to participate. Subjects were randomly assigned to either intensive treatment or conventional treatment and were followed for 3–9 years (mean 6.5 years).5 At the end of DCCT, intensive therapy was recommended for all subjects, and subjects in the conventional treatment group were trained in intensive therapy and returned to their own health care providers for diabetes care. Annual EDIC study examinations began in 1994, one year after completion of the DCCT; 1,375 (96%) agreed to participate in the follow-up evaluations. A detailed description of EDIC study procedures and baseline characteristics has been published.12 Clinical and biochemical endpoints were obtained annually using a standardized history, physical examination and laboratory testing protocol.12 Evaluation of glycemic control was based on measurements of hemoglobin A1c (HbA1c) using the same methods previously described for the DCCT.5
The DCCT and EDIC study procedures were approved by the institutional review boards of all participating centers, and all participants provided written informed consent.
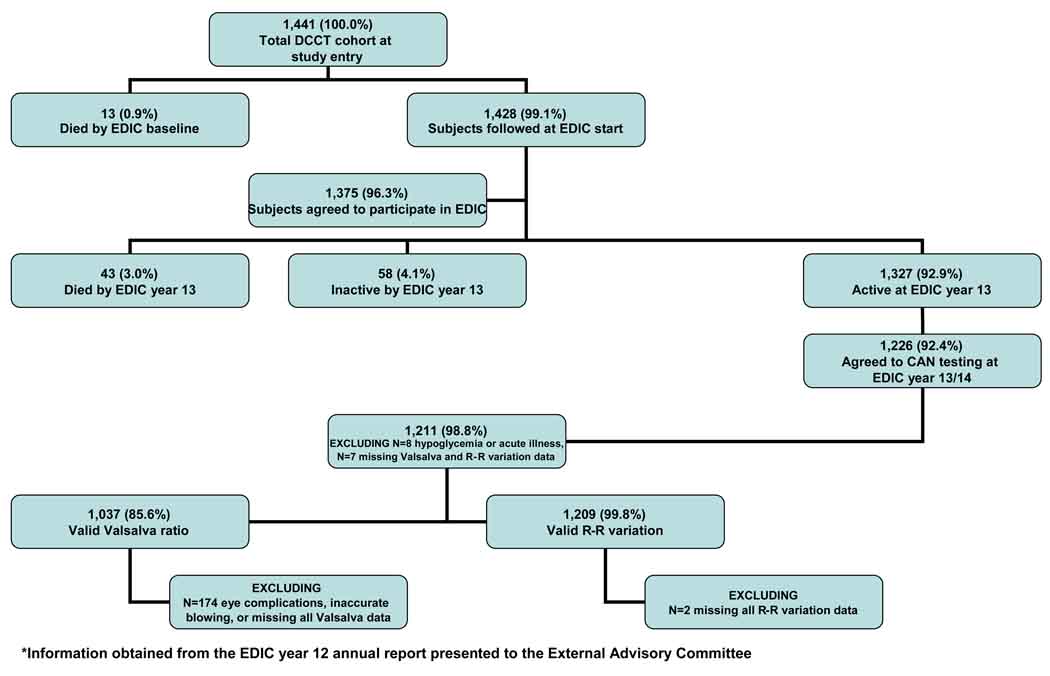
CAN testing (as performed during the DCCT) was repeated in 1,226 subjects one time during year 13/14 of EDIC follow-up (EDIC Neurology protocol or NeuroEDIC) (Figure 1).
Figure 1.
Flow diagram of NeuroEDIC participation
CAN Evaluations
During the DCCT, CAN was assessed at baseline and biennially with R-R response to paced breathing (R-R variation), Valsalva maneuver and postural changes in blood pressure.7 Reported autonomic symptoms (for postural hypotension, gastroparesis, diabetic diarrhea, colonic atony, genitourinary dysfunction, sudomotor abnormality and hypoglycemic unawareness) were also assessed.
During NeuroEDIC, the same CAN evaluations were performed once by EDIC nurse coordinators who were centrally trained and certified. The Autonomic Symptom Profile instrument 13 was used to assess autonomic symptoms.
Because autonomic function may be altered by a variety of factors, all subjects were required to fast and avoid caffeine and tobacco products, as well as prescription and over-the-counter medicines (except for their usual insulin regimen) for at least 8 hours before CAN testing.7 Subjects who experienced hypoglycemia after midnight (defined as a blood glucose ≤ 50mg/dl (2.775 mmol/L) and/or signs/symptoms of hypoglycemia, N=4) or subjects with acute illness 48 hours prior to testing (N=4) were excluded. Subjects with active proliferative retinopathy, history of laser therapy or vitrectomy, suspected (unconfirmed) proliferative retinopathy and/or no eye examination in the last 4 years, and those who could not accurately perform the required forced expiration (N=174) were excluded from performing the Valsalva maneuver (Figure 1).
Testing was performed with the Hokanson ANS 2000 device (Hokanson Inc, Bellevue, WA) and results were analyzed at a single reading center. All CAN measurements were reviewed for quality control purposes by a single masked investigator at the reading center (PAL), who decided if the technical quality of the recording and conditions of the test met study criteria. The R-R variation was certified as useable based on whether the test was performed according to protocol, whether cardiac arrhythmia interfered with interpretation of the test, and if the patient paced his/her breathing appropriately. Valsalva testing was done twice as previously described 14 and the average was calculated. Acceptable recordings were submitted for data entry and analysis. The postural change in blood pressure consisted of two supine measurements at least 6 min apart, followed by blood pressure measurements at 1, 2, 3, 4, 5, and 10 minutes after standing.
The reproducibility of the CAN measurements was evaluated at the reading center where a random sample of 10% of tests were re-read, demonstrating a coefficient of reliability between the primary and the repeat readings of 0.999.
Measures and Definition of CAN in the DCCT and EDIC
R-R variation is the measurement of the magnitude of cardiac sinus arrhythmia – predominantly a function of the parasympathetic nervous system.14, 15 It is computed as a dimensionless circular mean vector of R-R intervals.14 The Valsalva ratio evaluates cardiovagal function in response to a standardized increase in intrathoracic pressure and is influenced by both parasympathetic and sympathetic activity.14 Blood pressure response to standing reflects mainly sympathetic activity.
This battery of tests is well-suited to explore long term changes in the autonomic nervous system function, as they have been validated, shown to be reliable, reproducible and to have prognostic value,15–20 and have been recommended for assessment of autonomic neuropathy.3, 18, 20
In the DCCT, abnormal R-R variation was defined as < 15; abnormal Valsalva ratio as ≤ 1.5 and orthostatic hypotension as a postural decrease of > 10 mmHg in diastolic blood pressure.7 Presence of CAN was defined as either an R-R variation < 15 or an R-R variation between 15–19.9 in combination with a Valsalva ratio ≤ 1.5 or a decrease of >10 mmHg in diastolic blood pressure.7 Several studies published since the DCCT have demonstrated that heart rate variation is affected by age and possibly by gender.14, 17 Therefore, we have analyzed these data making adjustments for age, sex and other covariates as described in the statistical analysis. Orthostatic hypotension was defined in EDIC using both the DCCT criteria (without catecholamine measurements) as well as using consensus criteria.21
Outcome Measures
The primary outcome measures were the prevalence and incidence of CAN during EDIC. Secondary outcomes included changes in the continuous measures of R-R variation and Valsalva ratio during EDIC between the former intensive and conventional therapy cohorts, the prevalence of abnormal R-R variation and of abnormal Valsalva ratio at EDIC year 13/14 and the prevalence of autonomic symptoms.
Statistical Analyses
Demographic and clinical characteristics were compared between treatment groups using the Wilcoxon rank-sum test for ordinal or continuous variables and the contingency chi-square test for categorical variables. Normal errors linear models were used to assess treatment group differences in R-R variation and Valsalva ratio at each of the three time points separately (DCCT baseline, DCCT closeout, and EDIC year 13/14) adjusting for DCCT baseline age, sex, primary and secondary cohort and duration in the DCCT study.
Logistic regression models assessed the treatment group differences in the odds of incident CAN by a specific criterion (e.g. R-R variation < 15) at EDIC year 13/14 among those free of the condition at DCCT closeout. The percent reduction in odds of incident CAN with intensive therapy as compared to conventional therapy was computed as (1-OR)×100. Models for R-R variation < 15 were also adjusted for R-R variation at DCCT closeout, models for Valsalva ratio ≤ 1.5 adjusted for Valsalva ratio at DCCT closeout, and models for abnormal CAN function adjusted for both quantitative measures. P-values were calculated with likelihood-ratio tests. The proportion of the treatment group effect explained by the differences in the HbA1c between groups in DCCT and EDIC was calculated as the proportion reduction in the likelihood ratio test for treatment group after adjusting for both the mean HbA1c level during DCCT and during EDIC compared to that without adjustment. Statistical analyses were performed using the SAS V 8.2 statistical analysis software (Cary, NC) and statistical significance was defined as P < 0.05.
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
RESULTS
After all exclusions were made, we evaluated CAN in 1,211 EDIC participants. These included 304 INT and 306 CONV subjects in the primary prevention cohort and 316 INT and 285 CONV subjects in the former secondary intervention cohort (Figure 1). There were 1,209 valid tests of R-R variation (93% INT and 89% CONV subjects from the active and surviving DCCT cohort). Lower completion rates were observed for the Valsalva studies (1,037 valid tests), due to the restrictions on performing the Valsalva maneuver in subjects with proliferative retinopathy and those unable to sustain the required magnitude and duration of forced expiration (as described above). Thus, Valsalva studies were completed in 84% INT and 72% CONV subjects.
Table 1 summarizes the characteristics of these participants at DCCT baseline and closeout and EDIC year 13/14 by original treatment group. Data stratified by cohorts are presented in the appendix.
Table 1.
Characteristics of the 1,211 subjects with autonomic measurements at EDIC Year 13/14
| Characteristic | Group | N | DCCT Baseline | DCCT Closeout | EDIC Year 13/14 |
|---|---|---|---|---|---|
| Age, mean±SD, y | INT | 620 | 27.2 ± 7.1 | 33.9 ± 6.9a | 47.8 ± 7.0a |
| CONV | 591 | 26.5 ± 7.0 | 33.0 ± 6.9 | 47.0 ± 6.9 | |
| Female, No(%) | INT | 303 (49) | 303 (49) | 303 (49) | |
| CONV | 271 (46) | 271 (46) | 271 (46) | ||
| Body Mass Index, mean±SD, kg/m2 | INT | 23.3 ± 2.7 | 26.6 ± 4.3a | 28.6 ± 5.3 | |
| CONV | 23.4 ± 2.9 | 25.0 ± 3.0 | 27.9 ± 4.8 | ||
| Duration of diabetes, mean±SD, y | INT | 5.7 ± 4.2 | 12.3 ± 4.9 | 26.6 ± 4.9 | |
| CONV | 5.5 ± 4.1 | 11.9 ± 4.9 | 26.1 ± 4.9 | ||
| Hemoglobin A1c, mean±SD, % | INT | 9.1 ± 1.6 | 7.4 ± 1.0a | 7.9 ± 1.2 | |
| CONV | 9.0 ± 1.6 | 9.0 ± 1.5 | 7.8 ± 1.2 | ||
| Systolic BP, mean±SD, mm Hg | INT | 113.4 ± 11.5a | 116.4 ± 11.3 | 120.9 ± 14.4 | |
| CONV | 114.8 ± 11.7 | 116.2 ± 11.5 | 119.6 ± 13.8 | ||
| Diastolic BP, mean±SD, mm Hg | INT | 72.3 ± 8.9 | 74.8 ± 8.6 | 73.4 ± 9.1 | |
| CONV | 72.7 ± 8.8 | 74.1 ± 8.8 | 72.4 ± 8.7 | ||
| Total cholesterol, mean±SD, mg/dl | INT | 177.2± 33.2 | 180.5 ± 30.7 | 176.6 ± 36.1a | |
| CONV | 173.6± 32.3 | 182.3 ± 36.0 | 172.3 ± 35.1 | ||
| LDL cholesterol, mean±SD, mg/dl | INT | 110.5 ± 28.9 | 112.7 ± 27.2 | 103.2 ± 30.0 | |
| CONV | 107.8± 28.3 | 113.7 ± 30.6 | 100.2 ± 30.3 | ||
| Current smoker, No(%) | INT | 127 (20) | 139 (22) | 84 (14) | |
| CONV | 112 (19) | 118 (20) | 67 (11) | ||
| Any BP lowering medication, No(%)b | INT | b | b | 200 (32) | |
| CONV | b | b | 212 (36) | ||
| Beta blockers, No(%) b | INT | b | b | 31 (5)a | |
| CONV | b | b | 54 (9) | ||
| ACE, No(%) b | INT | b | b | 243 (39) | |
| CONV | b | b | 255 (43) | ||
| ARB, No(%) b | INT | b | b | 57 (9) | |
| CONV | b | b | 67 (11) | ||
| Lipid-lowering medications, No(%) b | INT | b | b | 316 (51) | |
| CONV | b | b | 318 (54) |
CAN Cardiac autonomic neuropathy, EDIC Epidemiology of Diabetes Interventions and Complications, DCCT Diabetes Control and Complications Trial, INT intensive, CONV conventional, BP blood pressure, LDL low density lipoprotein, ACE Angiotensin converting enzyme inhibitors, ARB Angiotensin receptor blockers.
P < 0.05 for treatment group differences by the Wilcoxon rank-sum test or chi-square test comparing INT and CONV treatment groups.
No medication data were collected in the DCCT. The use of blood pressure lowering medications was an exclusion criterion at DCCT baseline.
Participants in both former treatment groups (INT and CONV) were heavier in EDIC year 13/14 than at DCCT closeout (body mass index 28.3±5.0 vs. 25.9±3.8 kg/m2; P < 0.001). At EDIC year 13/14, participants from the former INT group were slightly older than the former CONV group (47.8±7.0 vs. 47.0±6.9 years; P = 0.040), and had higher total cholesterol levels (177±36 vs. 172 ±35 mg/dl P = 0.041). There were no significant differences in LDL cholesterol, blood pressure (systolic or diastolic) or smoking status. In EDIC year 13/14, the use of beta-blockers was significantly greater in the former CONV group (9.1% vs. 5.0%; P = 0.005). There were no differences in the use of any other drugs including angiotensin converting enzyme inhibitors, angiotensin receptor blockers, other blood pressure lowering agents or statins (Table 1).
There were no significant differences at EDIC year 13/14 in any of the characteristics presented in Table 1 between participants in the NeuroEDIC and subjects who were still actively participating in other EDIC evaluations but who did not participate in NeuroEDIC.
Glycemic control in EDIC year 13/14
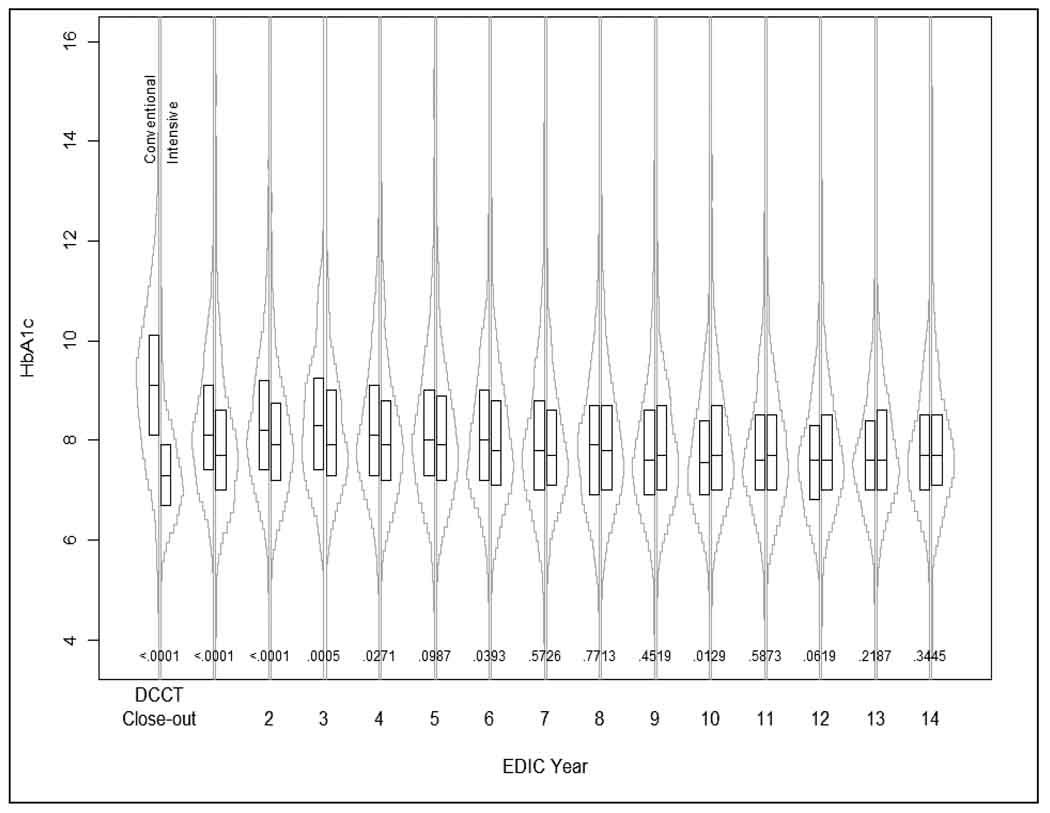
At DCCT completion, HbA1c was 7.4% in the INT group and 9.1% in the CONV group (P < 0.001). At the first EDIC study examination, HbA1c separation between DCCT INT and CONV groups narrowed substantially to 7.9% vs. 8.3% and by the 5th year of the EDIC study, the difference in HbA1c between groups was no longer statistically significant (8.1% vs. 8.2%, P = 0.099).8 The difference in HbA1c between the former INT and CONV groups continued to show no significant difference (P = 0.219 and P = 0.345 respectively) at the time of CAN testing in EDIC year 13 or 14 (Figure 2).
Figure 2.
HbA1c at DCCT closeout and through EDIC year 13/14. Boxplots: interquartile range; horizontal line: mean HbA1c
Changes in measures of CAN from DCCT to EDIC Year 13/14
The prevalence of CAN as assessed by heart rate variability was quite low at DCCT closeout in both treatment groups (4% INT vs. 9% CONV).7 Abnormalities were more prevalent in subjects from the secondary intervention cohort and among patients followed for longer periods.7
As shown in Table 2, in EDIC year 13/14, the cross-sectional prevalence of CAN using the DCCT composite definition (R-R < 15 or R-R < 20 and Valsalva ≤ 1.5 or a decrease of >10 mm/Hg in diastolic blood pressure) was significantly lower in the former INT group as compared to the CONV group (28.9% vs. 35.2%; P = 0.018) in pooled data from the primary prevention and secondary intervention cohorts. The prevalence of abnormal R-R variation (R-R < 15) increased during EDIC in both groups, but continued to be significantly higher in the former CONV group as compared with the former INT group (30.2% vs. 23.8%, P = 0.012), whereas the prevalence of an abnormal Valsalva ratio (<1.5) did not differ significantly between the former INT and CONV groups (Table 2).
Table 2.
CAN Outcomes in 1,211 subjects with EDIC year 13/14 autonomic measurements
| DCCT | DCCT | EDIC | ||
|---|---|---|---|---|
| Test | Group | Baseline | Closeout | Year 13/14 |
| CAN prevalence, No. (%)d | INT | 24 (3.9) | 43 (7.1) | 179 (28.9)b |
| CONV | 31 (5.3) | 57 (9.9) | 208 (35.2) | |
| R-R Variation < 15, No. (%) | INT | 20 (3.3) | 39 (6.6) | 147 (23.8)b |
| CONV | 25 (4.3) | 53 (9.5) | 178 (30.2) | |
| Valsalva Ratio ≤ 1.5, No. (%) | INT | 31 (5.2) | 42 (7.4) | 145 (26.0) |
| CONV | 30 (5.2) | 51 (9.3) | 146 (30.4) | |
| R-R Variation, mean ±SD | INT | 48.5 ± 22.6 | 41.4 ± 20.5 | 29.6 ± 18.9a |
| CONV | 47.4 ± 21.2 | 39.3 ± 20.1 | 26.1 ± 17.5 | |
| Adjusted R-R Variation, mean ±SD c | INT | 48.8 ± 21.3 | 41.8 ± 19.5b | 29.9 ± 17.4a |
| CONV | 47.0 ± 21.4 | 38.9 ± 19.4 | 25.6 ± 17.5 | |
| Valsalva Ratio, mean ±SD | INT | 2.1 ± 0.4 | 2.0 ± 0.4 | 1.8 ± 0.4 |
| CONV | 2.0 ± 0.4 | 2.0 ± 0.4 | 1.8 ± 0.4 | |
| Adjusted Valsalva Ratio, mean ±SD c | INT | 2.1 ± 0.5 | 2.0 ± 0.5 | 1.8 ± 0.2b |
| CONV | 2.0 ± 0.5 | 2.0 ± 0.5 | 1.7 ± 0.4 | |
| Postural hypotension, No. (%) | INT | 2 (0.3) | 3 (0.5) | 11 (1.8) |
| CONV | 3 (0.5) | 4 (0.7) | 9 (1.6) |
CAN Cardiac autonomic neuropathy, EDIC Epidemiology of Diabetes Interventions and Complications, DCCT Diabetes Control and Complications Trial, INT intensive, CONV conventional.
P < 0.01 and
P<0.05 for treatment group differences by the Wilcoxon rank-sum test or chi-square test comparing INT and CONV treatment groups.
Means adjusted for DCCT baseline age, sex, cohort assignment and duration in the DCCT study.
CAN prevalence is defined as any one of the following conditions: R-R variation < 15; R-R variation < 20 in combination with Valsalva ratio ≤ 1.5; or Postural hypotension.
Table 2 also presents the pooled analysis of the changes in the continuous R-R variation and Valsalva ratio with adjustment for DCCT baseline age, sex, cohort assignment and duration in the DCCT study. R-R variation was significantly higher within the former INT group as compared to the former CONV group in EDIC year 13/14, in both unadjusted and adjusted analysis (adjusted means 29.9 vs. 25.6, P < 0.001) (Table 2). Although there were no differences in the unadjusted Valsalva ratios between former DCCT groups in EDIC year 13/14, the adjusted means for the Valsalva ratio were higher in the former INT as compared to the former CONV group (P = 0.036). By EDIC year 13/14, only a very small number of subjects in both cohorts presented with postural hypotension (1.8% INT vs. 1.6% CONV; P = 0.736) (Table 2).
Metabolic memory
The effects of metabolic memory were assessed for the incidence of abnormal R-R variation, abnormal Valsalva ratio and abnormal CAN function during EDIC, in those subjects who were free of the condition at the end of the DCCT.
The primary endpoint (incidence of CAN using the composite definition) among those without CAN at DCCT closeout was significantly lower in the former INT group compared to the former CONV group (24.4% vs. 29.8%, P < 0.05) (Table 3).
Table 3.
Incidence of abnormal autonomic measurements at EDIC year 13/14 among subjects with intact function at DCCT closeout
| Characteristic | Group | Incident Abnormal Function, No. (%) |
Unadjusted Odds Ratio (95% CI) |
Adjusted Odds Ratio (95% CI)c |
HbA1c Adjusted Odds Ratio (95% CI)c |
|---|---|---|---|---|---|
| R-R Variation < 15 | INT | 109 (18.8) | 0.76 (0.57–1.02) | 0.70 (0.51–0.96) | 1.36 (0.84–2.19) |
| CONV | 125 (23.2) | ||||
| Valsalva Ratio ≤ 1.5 | INT | 113 (19.7) | 0.92 (0.69–1.23) | 0.85 (0.62–1.16) | 0.86 (0.54–1.36) |
| CONV | 112 (21.1) | ||||
| Abnormal CAN function b | INT | 141 (24.4)a | 0.76 (0.59–0.995) | 0.69 (0.51–0.93) | 1.31 (0.83–2.07) |
| CONV | 159 (29.8) |
CAN Cardiac autonomic neuropathy, EDIC Epidemiology of Diabetes Interventions and Complications, DCCT Diabetes Control and Complications Trial, INT intensive, CONV conventional
P < 0.05 for treatment group differences by the chi-square test comparing INT and CONV treatment groups.
Abnormal CAN function was defined as any one of the following conditions: R-R variation < 15; R-R variation < 20 in combination with Valsalva ratio ≤ 1.5; or Postural hypotension.
Logistic regression models were adjusted for DCCT baseline age, sex, cohort assignment and duration in the DCCT study. Models for R-R variation <15 were also adjusted for R-R variation at DCCT closeout, models for Valsalva ratio ≤ 1.5 adjusted for Valsalva ratio at DCCT closeout, and models for abnormal CAN function adjusted for both quantitative measures. HbA1c models include both the mean HbA1c level during DCCT and during EDIC.
Intensive insulin therapy during DCCT reduced the risk of incident CAN by 31% [odds ratio (95% CI) 0.69 (0.51–0.93)] and of abnormal R-R variation (<15) by 30% [odds ratio (95% CI) 0.70 (0.51–0.96)] after adjusting for DCCT baseline age, sex, cohort assignment, duration in the DCCT study and for the level of R-R variation at DCCT closeout. Results were not different with the inclusion of beta-blocker usage during EDIC. With the additional adjustment for the level of Valsalva ratio at DCCT closeout and beta-blocker usage during EDIC, the odds of incident CAN became barely insignificant [odds ratio (95% CI) 0.73 (0.54–1.004)].
An increased incidence of CAN using the composite definition was associated with higher mean HbA1c levels during the DCCT and also during EDIC. After adjusting for both the mean HbA1c levels during DCCT and during EDIC, treatment group differences were no longer significant [odds ratio (95% CI) 1.31 (0.83–2.07)]. The proportion of the DCCT treatment group effect explained by the group differences in HbA1c in DCCT and EDIC was 77.9%. Thus, virtually all of the difference between treatment groups in the incidence of CAN was explained by the differences in the HbA1c levels between groups. The same is true for the treatment group differences initially observed for abnormal R-R variation [odds ratio (95% CI) 1.36 (0.84–2.19)]. The proportion of the treatment group difference in R-R variation explained by the group differences in HbA1c was 68.6% (Table 3).
Symptoms
A small number of participants from both former treatment groups reported symptoms consistent with autonomic neuropathy in EDIC year 13/14 (Table 2 Appendix). Decreased adrenergic awareness of hypoglycemia (20% INT vs. 25% CONV), male impotence (23% INT vs. 30% CONV,P=0.039), and excessive postprandial epigastric fullness (8% INT vs. 8% CONV) were the most commonly reported symptoms. No significant treatment group differences were found for rest of the symptoms.
DICUSSION
During EDIC, CAN progressed substantially in both treatment groups, but the prevalence and incidence of CAN in EDIC year 13/14 remained significantly lower in the former INT group than in the former CONV group, despite similar levels of glycemic control.
Among patients with type 1 diabetes, the total exposure to hyperglycemia, as assessed by disease duration and degree of glycemic control, is the dominant determinant of risk of progression of microvascular complications, as documented during EDIC for nephropathy and retinopathy.8, 9 The development of CAN however, is a function of complex interactions among degree of glycemic control, disease duration, age-related neuronal attrition, and systolic and diastolic blood pressure.22, 23
The effects of age on measures of heart rate variability have been demonstrated in several independent cohorts of healthy subjects.14, 17, 24 The age-related declines in R-R variation and Valsalva ratio are presumably due to a progressive decline in vagal function and/or alterations in baroreceptor sensitivity and sympathetic adrenergic activity.14, 17, 24 For this reason, we adjusted for the effects of age and found that both the prevalence and incidence of CAN remained significantly lower in the former INT group compared with the former CONV group. In addition to age, the rate of forced respiration may also influence the R-R variation; however the respiration rate was paced to reduce the effects of hyperventilation.
Blood pressure significantly affects the interpretation of R-R variation. However, in EDIC year 13/14, we found no significant group difference in systolic or diastolic blood pressure among EDIC subjects with valid CAN measurements or in the use of any blood pressure lowering agents with the exception of beta-blockers. No patients used beta-blockers at DCCT baseline or at closeout. Treatment with beta-blockers was more common at EDIC year 13/14 in subjects from the former CONV group compared to former INT group, but in both groups use of beta-blockers was very low. Modulation of beta-adrenergic activity by beta-adrenergic blockade may positively influence heart rate variability. 15 Our analysis therefore adjusted for the difference in beta-blocker usage during EDIC, resulting in group differences barely losing significance.
The inconsistent group differences observed in the Valsalva ratio in EDIC year 13/14, suggests that R-R variation is a more sensitive test than the Valsalva maneuver. This may be because the major afferent and efferent pathways of the R-R variation with paced breathing are vagal.14 Other studies have shown that even during carefully standardized conditions, the Valsalva ratio can be an insensitive measure of CAN.14 Although the heart rate response to changes in blood pressure induced by the Valsalva maneuver is a parasympathetic response, the preceding blood pressure changes reflect complex autonomic functions including blood volume, venous capacitance, total systemic resistance tone and cardiac adrenergic tone.25, 26 It is therefore possible that the Valsalva ratio requires greater parasympathetic impairment before becoming abnormal. However, our ability to interpret the Valsalva ratio in our study was limited in that a substantial number of subjects were not eligible to perform the maneuver, due to presence of severe retinopathy. Selection bias may also explain these findings.
The DCCT cohort was young and generally healthy with a relatively short duration of type 1 diabetes at baseline. The EDIC cohort has a mean duration of diabetes of about 25 years. CAN studies in EDIC were done only in year 13/14, a substantially longer interval between tests compared to measures of retinopathy (every four years in EDIC) and nephropathy (every-other year assessments of urine albumin excretion and annual assessments of serum creatinine). While this may limit our understanding of how glycemic exposure affects measures of CAN, it is clear that the former INT group had significantly less abnormalities in R-R variation and a lower of incidence of CAN relative to the CONV group.
Even after adjusting for the level of autonomic measurements or presence of CAN at the end of the DCCT, there were persistent beneficial effects of INT versus CONV therapy after 13/14 years of follow-up in EDIC. Thus, a “metabolic memory” effect has occurred for measures of CAN as previously observed for retinopathy and nephropathy. Furthermore, differences between the original DCCT treatment groups in the level of the DCCT and EDIC mean HbA1c explain virtually all of the beneficial effect of intensive versus conventional therapy on risk of incident CAN.
The mechanism of this persistent beneficial effect of DCCT treatment on outcomes assessed during EDIC is unknown. It is possible that early intensive therapy had different impacts on the various pathogenic pathways associated with autonomic neuronal function including: formation of advanced glycation end products, increased oxidative/nitrosative stress with increased free radical production, activation of the polyol and protein kinase C pathways, activation of polyADP ribosylation and activation of genes involved in neuronal damage.3, 27 Such mechanisms could have had persistent effects, even though HbA1c levels did no longer differ during the EDIC study.
Another important clinical observation is that the prevalence and incidence of CAN from DCCT closeout to EDIC year 13/14 increased substantially in both the former CONV and INT groups. The prevalence of CAN in EDIC was higher than previously described in other cohorts of patients with type 1 diabetes.3 While variability among studies using different diagnostic methods, criteria and definitions of CAN are expected, this increase in CAN is important because CAN is associated with increased frequency of silent myocardial ischemia 3 and major cardiovascular events 2, and is a predictor of cardiovascular mortality. O’Brien and Ewing reported five-year mortality rates as high as 27% and 53% respectively in diabetic patients with CAN, with a high proportion attributed to sudden cardiac death.28, 29 In another population-based sample of individuals with type 1 diabetes, Orchard et al.30 found a four-fold higher mortality rate in individuals with CAN at baseline compared with individuals without. A meta-analysis of 15 studies which included 2,900 subjects with diabetes reported significantly higher relative risk of mortality in patients with CAN as compared with patients without CAN.1
Despite an increase in CAN prevalence in both treatment groups, only a relatively small number of participants reported symptoms consistent with autonomic neuropathy in EDIC year 13/14. Patient unawareness of cardiovascular dysfunction may increase the risks associated with CAN.
The high prevalence and incidence of CAN found in the EDIC cohort, 13-to14 years after the DCCT closeout, may have important prognostic consequences considering the still relatively young age of the EDIC cohort. Future prospective analyses will assess the predictive associations between CAN and the risk (hazard) of mortality and cardiovascular events in type 1 diabetes. These studies could also provide additional important information regarding the natural history and progression of CAN and would help better understand the long-term effects of intensive therapy in patients with type 1 diabetes.
In conclusion, our results suggest that the benefits of former intensive therapy extend to measures of CAN 13 to 14 years after the end of the DCCT, and support that intensive treatment of type 1 diabetes should be initiated as early as is safely possible in order to provide durable protection from the development and progression of diabetic complications.
Clinical Impact of the Paper
We present prospective findings of cardiovascular autonomic neuropathy (CAN) in patients with type 1 diabetes enrolled in the Diabetes Control and Complications Trial (DCCT) and followed annually through the Epidemiology of Diabetes Interventions and Complications (EDIC) for an additional 13 to 14 years after the DCCT closeout. CAN is associated with a high risk of cardiac arrhythmias and sudden death, possibly related to silent myocardial ischemia. Our study provides a comprehensive CAN assessment over 25 years in a well characterized cohort of patients with type 1 diabetes. The prevalence and incidence of CAN, as assessed by heart rate variability, increased substantially from DCCT closeout to EDIC year 13/14 despite the young age of the cohort, a very low prevalence of CAN at DCCT closeout and good glycemic control. Even after adjusting for the level or presence of CAN at DCCT closeout, there were persistent beneficial effects of prior intensive versus conventional therapy after 13/14 years of follow-up in EDIC. Treatment group differences in the mean level of HbA1c during DCCT and EDIC explained virtually all of the beneficial effects of intensive versus conventional therapy on risk of incident CAN. These findings support the recommendation that intensive treatment of type 1 diabetes be initiated as early as possible in order to provide durable protection from the development and progression of diabetic complications.
ACKNOWLEDGEMENTS
*A complete list of the individuals and institutions participating in the DCCT/EDIC research group appears in: Jacobson AM, Ryan CM, Cleary P, Waberski B, Burwood A, Weinger K et al. Long-term effects of diabetes and its treatment on cognitive function. N Engl J Med 2007; 356:1842–52.
FUNDING SOURCES:
Funding for this study was provided by grant 5 R01 DK062218-02 and contracts with the Division of Diabetes, Endocrinology and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Diseases and the General Clinical Research Centers Program, and the National Center for Research Resources.
Footnotes
Trial Registration: ClinicalTrials.gov number NCT00360893
DISCLOSURES
There are no potential, perceived, or real conflicts of interest to report for any of the authors.
REFERENCES
- 1.Maser RE, Mitchell BD, Vinik AI, Freeman R. The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: a meta-analysis. Diabetes Care. 2003;26:1895–1901. doi: 10.2337/diacare.26.6.1895. [DOI] [PubMed] [Google Scholar]
- 2.Valensi P, Sachs RN, Harfouche B, Lormeau B, Paries J, Cosson E, Paycha F, Leutenegger M, Attali JR. Predictive value of cardiac autonomic neuropathy in diabetic patients with or without silent myocardial ischemia. Diabetes Care. 2001;24:339–343. doi: 10.2337/diacare.24.2.339. [DOI] [PubMed] [Google Scholar]
- 3.Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care. 2003;26:1553–1579. doi: 10.2337/diacare.26.5.1553. [DOI] [PubMed] [Google Scholar]
- 4.Vinik AI, Ziegler D. Diabetic cardiovascular autonomic neuropathy. Circulation. 2007;115:387–397. doi: 10.1161/CIRCULATIONAHA.106.634949. [DOI] [PubMed] [Google Scholar]
- 5.DCCT. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 6.DCCT. Effect of intensive diabetes treatment on nerve conduction in the Diabetes Control and Complications Trial. Ann Neurol. 1995;38:869–880. doi: 10.1002/ana.410380607. [DOI] [PubMed] [Google Scholar]
- 7.DCCT. The effect of intensive diabetes therapy on measures of autonomic nervous system function in the Diabetes Control and Complications Trial (DCCT) Diabetologia. 1998;41:416–423. doi: 10.1007/s001250050924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DCCT. Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA. 2002;287:2563–2569. doi: 10.1001/jama.287.19.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DCCT/EDIC Writing Group. Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA. 2003;290:2159–2167. doi: 10.1001/jama.290.16.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin CL, Albers J, Herman WH, Cleary P, Waberski B, Greene DA, Stevens MJ, Feldman EL. Neuropathy among the diabetes control and complications trial cohort 8 years after trial completion. Diabetes Care. 2006;29:340–344. doi: 10.2337/diacare.29.02.06.dc05-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Epidemiology of Diabetes Interventions and Complications (EDIC) Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care. 1999;22:99–111. doi: 10.2337/diacare.22.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suarez GA, Opfer-Gehrking TL, Offord KP, Atkinson EJ, O'Brien PC, Low PA. The Autonomic Symptom Profile: a new instrument to assess autonomic symptoms. Neurology. 1999;52:523–528. doi: 10.1212/wnl.52.3.523. [DOI] [PubMed] [Google Scholar]
- 14.Low PA, Denq JC, Opfer-Gehrking TL, Dyck PJ, O'Brien PC, Slezak JM. Effect of age and gender on sudomotor and cardiovagal function and blood pressure response to tilt in normal subjects. Muscle Nerve. 1997;20:1561–1568. doi: 10.1002/(sici)1097-4598(199712)20:12<1561::aid-mus11>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 15.Pfeifer MA, Cook D, Brodsky J, Tice D, Reenan A, Swedine S, Halter JB, Porte D., Jr. Quantitative evaluation of cardiac parasympathetic activity in normal and diabetic man. Diabetes. 1982;31:339–345. doi: 10.2337/diab.31.4.339. [DOI] [PubMed] [Google Scholar]
- 16.Gelber DA, Pfeifer M, Schumer M. Reliability and comparison of methods to calculate heart rate variability. Diabetes. 1995;44:30A. [Google Scholar]
- 17.Gelber DA, Pfeifer M, Dawson B, Schumer M. Cardiovascular autonomic nervous system tests: determination of normative values and effect of confounding variables. J Auton Nerv Syst. 1997;62:40–44. doi: 10.1016/s0165-1838(96)00107-5. [DOI] [PubMed] [Google Scholar]
- 18.Pfeifer MA, Weinberg CR, Cook DL, Reenan A, Halter JB, Ensinck JW, Porte D., Jr. Autonomic neural dysfunction in recently diagnosed diabetic subjects. Diabetes Care. 1984;7:447–453. doi: 10.2337/diacare.7.5.447. [DOI] [PubMed] [Google Scholar]
- 19.Ziegler D. Diabetic cardiovascular autonomic neuropathy: prognosis, diagnosis and treatment. Diabetes Metab Rev. 1994;10:339–383. doi: 10.1002/dmr.5610100403. [DOI] [PubMed] [Google Scholar]
- 20.Assessment: Clinical autonomic testing report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 1996;46:873–880. [PubMed] [Google Scholar]
- 21.Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Neurology. 1996;46:1470. doi: 10.1212/wnl.46.5.1470. [DOI] [PubMed] [Google Scholar]
- 22.Stella P, Ellis D, Maser RE, Orchard TJ. Cardiovascular autonomic neuropathy (expiration and inspiration ratio) in type 1 diabetes. Incidence and predictors. J Diabetes Complications. 2000;14:1–6. doi: 10.1016/s1056-8727(00)00054-4. [DOI] [PubMed] [Google Scholar]
- 23.Witte DR, Tesfaye S, Chaturvedi N, Eaton SE, Kempler P, Fuller JH. Risk factors for cardiac autonomic neuropathy in type 1 diabetes mellitus. Diabetologia. 2005;48:164–171. doi: 10.1007/s00125-004-1617-y. [DOI] [PubMed] [Google Scholar]
- 24.Ziegler D, Laux G, Dannehl K, Spuler M, Muhlen H, Mayer P, Gries FA. Assessment of cardiovascular autonomic function: age-related normal ranges and reproducibility of spectral analysis, vector analysis, and standard tests of heart rate variation and blood pressure responses. Diabet Med. 1992;9:166–175. doi: 10.1111/j.1464-5491.1992.tb01754.x. [DOI] [PubMed] [Google Scholar]
- 25.Opfer-Gehrking TL, Low PA. Impaired respiratory sinus arrhythmia with paradoxically normal Valsalva ratio indicates combined cardiovagal and peripheral adrenergic failure. Clin Auton Res. 1993;3:169–173. doi: 10.1007/BF01826229. [DOI] [PubMed] [Google Scholar]
- 26.Sandroni P, Ahlskog JE, Fealey RD, Low PA. Autonomic involvement in extrapyramidal and cerebellar disorders. Clin Auton Res. 1991;1:147–155. doi: 10.1007/BF01826212. [DOI] [PubMed] [Google Scholar]
- 27.Pacher P, Liaudet L, Soriano FG, Mabley JG, Szabo E, Szabo C. The role of poly(ADP-ribose) polymerase activation in the development of myocardial and endothelial dysfunction in diabetes. Diabetes. 2002;51:514–521. doi: 10.2337/diabetes.51.2.514. [DOI] [PubMed] [Google Scholar]
- 28.Ewing DJ, Campbell IW, Clarke BF. The natural history of diabetic autonomic neuropathy. Q J Med. 1980;49:95–108. [PubMed] [Google Scholar]
- 29.O'Brien IA, McFadden JP, Corrall RJ. The influence of autonomic neuropathy on mortality in insulin-dependent diabetes. Q J Med. 1991;79:495–502. [PubMed] [Google Scholar]
- 30.Orchard TJ, CE LL, Maser RE, Kuller LH. Why does diabetic autonomic neuropathy predict IDDM mortality? An analysis from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Res Clin Pract. 1996;34 Suppl S:165–171. doi: 10.1016/s0168-8227(96)90025-x. [DOI] [PubMed] [Google Scholar]