Abstract
Comet assay is a useful technique in the detection of DNA damages, particularly DNA strand breaks; and it has been utilized to show that a potent carcinogen N-methyl-N′-nitro-N-nitrosoguanidine (MNNG), can induce such damages. Recently, γH2AX foci formation has been suggested as another sensitive way to detect DNA double strand breaks (DSBs). However, there is no systematic comparison being conducted to evaluate the consistency of these two methods. Using MNNG as a model chemical, the sensitivity of neutral comet assay and γH2AX foci formation in detecting MNNG-induced damage was studied. It was found that at concentrations of 0.1 and 1 μg/ml, both methods can detect MNNG-induced damage in human amnion FL cells. However, at 0.1 μg/ml, comet assay revealed more percentage of cells with DNA damage than γH2AX fluorescence revealed. On the other hand, while γH2AX foci were readily formed at very early times by 10 μg/ml MNNG treatment, neutral comet assay did not detect any significant DNA damage at the same time points. In addition, 10 μg/ml MNNG induced a distinct whole nuclei staining pattern of γH2AX, a type of DNA damage which was not detected by neutral comet assay but could be detected by alkaline comet assay. Therefore, γH2AX may be used as a sensitive indicator for DNA damage.
Keywords: Comet assay, H2AX, N-methyl-N′-nitro-N-nitrosoguanidine, Double strand breaks, Phosphatidylinositol 3-kinase family
1. Introduction
Cancer is the second leading cause of death in developed countries, and exposure to environmental carcinogen is a major contributing factor for the cancer epidemic. Most of the known environmental carcinogens are genotoxins, meaning these agents cause cancer by damaging DNA (Yang and Duerksen-Hughes, 1998; Yang and Duerksen-Hughes, 2001). There are many types of DNA damage, including base modification which covers chemical changes to bases altering their base pairing attributes and DNA adduction, single strand breaks (SSBs), double strand breaks (DSBs), intra- or inter-strand cross-links, among which DSBs are regarded as the most severe type of damage. Comet assay, a classical method for detecting DNA damage, is frequently used to measure DNA strand breaks in single cells. Comet assay can be divided into two classes: one is alkaline comet assay, in which alkaline conditions denature DNA thereby releasing a short strand when SSB occurs; another one is neutral comet assay, while under neutral conditions, there is no denaturation, the strands stay together and thus only become shorter when DSBs are induced. The general concept is that neutral comet assay may be specific for detecting DSBs in cells, while alkaline comet assay can be used to detect SSBs and DSBs or even base modification (Crumpton and Collins, 2004). Nonetheless, it is clear that comet assay is a powerful method for detecting DNA damages.
Recently, the relationship between DSBs and histone H2AX has been gradually recognized. The histone H2A family comprises of many variants. There are 10 genes in human encoding H2A1 variants, in which six are identical, and four vary in up to three of four positions. H2A2 is closely related to H2A1, with only a single amino acid change in sequence. Both are abundant in mammalian cells and thus are the major H2A variants, and they do not have any known differential functions. The other members, H2AX, H2AZ, macroH2AX1, macroH2AX2, and H2A–BbD, differ considerably from H2A1 and H2A2 in sequence and are present in small amount. However, they may have more important roles in chromatin metabolism (Pilch et al., 2003; Redon et al., 2002; Sedelnikova et al., 2003). As one of the variants of H2A, H2AX represents about 2–25% of total H2A (Rogakou et al., 1998). H2AX has a SQ motif with the serine (Ser 139) four residues from C-terminus, which is highly conserved during evolution. The serine residue in the SQ motif is phosphorylated (termed γH2AX) and γH2AX forms “foci” in response to DSBs induced by many stimuli, including ionizing radiation (IR), replication stresses, and cellular programmed DSBs (such as apoptosis, V(D)J recombination, and retroviral DNA integration) (Chen et al., 2000; Daniel et al., 2004; Rogakou et al., 1998; Ward and Chen, 2001). In an elegantly designed experiment, Rothkamm and Lobrich showed that the number of γH2AX foci detected by immunofluorescence is quantitatively the same as DSBs detected by pulsed-field gel electrophoresis (PFGE): first, the number of DSBs/cell was calculated based on the results obtained from PFGE; the number of γH2AX foci/cell was also counted; by comparison, the two numbers were strikingly close, suggesting that γH2AX may be used as an indicator for DSBs (Rothkamm and Lobrich, 2003). Furthermore, γH2AX seems to play an important role in the processing and repair of DSBs, which is crucial for the maintenance of genome integrity and stability, since H2AX−/− mice exhibited radiation sensitivity, growth retardation, immune deficiency, and infertilization in mutant males (Bassing et al., 2002; Celeste et al., 2002). The mechanisms for the phosphorylation of H2AX has also been investigated, and it was shown that members of the phosphatidylinositol 3-kinase family (PI-3K) such as ATM (ataxia telangiectasia mutated), ATR (ATM and Rad3-related), and DNA-PK (DNA-dependent protein kinase) can phosphorylate H2AX (Stiff et al., 2004; Wang et al., 2005; Yang et al., 2003).
Although γH2AX foci formation has been suggested as a sensitive way to detect DNA damages, it still needs to be carefully validated before being widely applied in research or other related areas. In addition, it is not clear how well it correlates with comet assay. The monofunctional alkylating agent N-methyl-N′-nitro-N-nitrosoguanidine (MNNG) is a potent carcinogen and mutagen which can be found in tobacco smoke, and it is involved in the development of gastric and colorectal cancer in both animals and humans (Yang and Duerksen-Hughes, 1998; Yang and Duerksen-Hughes, 2001). It generally targets DNA and proteins to generate adducts, and O6-alkyl guanine adducts are the predominant mutagenic lesions due to its mispairing properties, which can eventually lead to chromosomal aberrations, point mutations, and cell death (Jin et al., 2004). In addition, Roser et al. have demonstrated that MNNG can also induce DNA strand breaks by using comet assay (Roser et al., 2001). Therefore, using MNNG as the model chemical, both neutral comet assay and immunofluorescent microscopy for γH2AX foci formation were conducted to evaluate MNNG-induced DNA damage, and the results were systematically compared for their consistency. As reported here, although there are some discrepancies between the results of γH2AX and comet assay, γH2AX still is a useful indicator for DNA damages.
2. Materials and methods
2.1. Cell culture and treatment
Human amnion FL cells were routinely subcultured in Eagle’s Minimum Essential Medium (EMEM) (Invitrogen, Carlsbad, CA), containing 10% newborn calf serum, 100 U/ml penicillin, 125 μg/ml streptomycin, and 0.03% glutamine. MNNG, MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide), dimethylsulfoxide (DMSO), and 4,6 diamidino-2-phenylindole (DAPI) were purchased from Sigma (St. Louis, MO). MNNG was dissolved in DMSO as a 100 μg/ml stock. Mouse monoclonal antibody against γH2AX was purchased from Upstate Technology (Lake Placid, NY). FITC-conjugated goat anti-mouse IgG and goat blocking serum were obtained from Beijing Zhongshan Biotechnology Co., China.
2.2. Cytotoxicity assay
The cytotoxicity effects of MNNG on cells were examined by MTT test as described before (Zhu-Ge and Yu, 2004). Briefly, cells were seeded into 96-well culture plate at a density of 1 × 104 cells/well. Twenty four hours later, the medium was discarded, and new medium containing 0.1, 1, 5, 10, 20, 50, or 100 μg/ml of MNNG were added to respective wells. Cells were treated for 2, 8, 24 and 48 h, respectively. At the end of each time point, 20 μl of MTT (5 mg/ml in PBS) were added to each well. 4 h later, the solution was discarded, and 150 μl DMSO was added to each well. After formazan was dissolved, the absorbance at 490 nm was read on a microtiter plate reader (BioTek, Winooski, VT). Relative survival was represented as the absorbance of treated sample/absorbance of control group.
2.3. Immunofluorescent microscopy and quantification of γH2AX foci
Immunofluorescent microscopy was conducted basically the same as described before with modifications (Yang et al., 2002). In short, 1 × 105 cells were seeded into 6-well culture plate containing a glass cover slip in each well. After treatment, cells were fixed in 4% paraformaldehyde for 15 min, washed with PBS, and permeabilized in 0.2% Triton-X 100. After blocked with blocking serum for 1.5 h, samples were incubated with a mouse monoclonal anti-γH2AX antibody (1:1000) for 2 h, followed with FITC-conjugated goat-anti-mouse secondary antibody (1:500) for 1 h. To stain the nuclei, DAPI was added to the cells and incubated for another 15 min. The cover slip was then removed from the plate and mounted on to a glass slide, and observed with an Olympus AX70 fluorescent microscope (Olympus, Japan).
To prevent bias in selection of cells that display foci, all the cells were counted in the field of vision (at least 50 cells). Image Pro Plus (Media Cybernetics) was used to count the γH2AX foci in each cell. In addition, to exclude relatively weak foci and background spots we used a setting as a standard for quantification in all the cells selected for analysis (Daniel et al., 2004).
2.4. Comet assay
The neutral comet assay was performed as described before with some modifications (Vaughan et al., 1991). First, the fully frosted microscope slides were covered with 100 μl of 0.65% normal melting point agarose and immediately covered with a coverslip. Slides were placed on ice for 8 min to allow the agarose to solidify. Second, the coverslips were removed, and the first agarose layer was covered with cell suspension (1 × 106 cells in 15 μl PBS were mixed with 75 μl of 0.65% low melting point agarose). After putting the coverslip back, the slide was allowed to solidify on ice for 8 min. Third, another layer of agarose (75 μl of 0.65% low melting point agarose) was added as described above. Finally, the coverslip was removed, and the slides were immersed in the lysis buffer (2 M NaCl, 30 mM EDTA, 10 mM Tris, with 1% Triton X-100 and 10% DMSO added just before use, pH 8.2–8.5) for 2 h at 4 °C. The slides were removed from the lysis buffer, washed for 10 min in 0.5 X TBE, and transferred to an electrophoresis chamber. After equilibrated in the 0.5 X TBE for 20 min, electrophoresis was conducted at 25 V, 150 mA, for 20 min. The slides were then washed in a neutralization buffer (0.4 M Tris, pH 7.5) for 5 min for three times. The slides were drained and stained with 1.0 μg/ml DAPI, and observed with a fluorescent microscope.
The alkaline comet assay was performed basically the same as the neutral comet assay, except that cells were lysed in alkaline lysis buffer (2.5 M NaCl, 100 mM EDTA, 10 mM Tris base, 1% sodium lauryl sarcosinate, with 1% Triton X-100 and 10% DMSO added just before use, pH 10) for 2 h, and electrophoresed in a high pH electrophoresis buffer (300 mM NaOH, 1 mM EDTA) at 25 V, 300 mA for 20 min.
Single cell images were captured and analyzed using a Olympus AX70 immunofluorescent microscope (Olympus, Japan) and tail length was measure by ImagePro Plus software.
2.5. Statistical analysis
Statistical analysis was performed with Student’s t-test. Each experiment was conducted independently at least four times. Data are presented as mean ± SD. A probability level of P < 0.05 was considered significant.
3. Results
3.1. MNNG induces the formation of γH2AX foci in FL cells
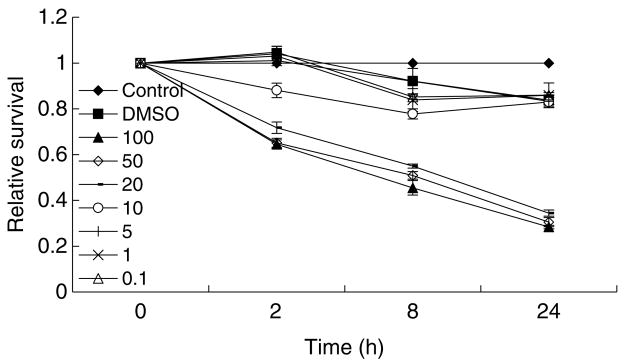
The cytotoxic effects of different concentrations of MNNG on FL cells were first examined by MTT test at 2, 8, 24, and 48 h post-treatment. It was shown that there was a clear dose- and time-dependent effect of MNNG on cell survival, with higher concentrations (10, 20, 50 and 100 μg/ml) exerted significant cytotoxic effects with time, while relatively lower concentrations (5, 1, and 0.1 μg/ml) showed marginal cytotoxic effects on FL cells (Fig. 1). Based on these results, three concentrations (10, 1, and 0.1 μg/ml) were chosen for further analyses for γH2AX foci formation.
Fig. 1. The cytotoxic effects of MNNG on FL cells.
FL cells were treated with different concentrations (μg/ml) of MNNG, and MTT test was conducted at indicated time points to measure cell viability.
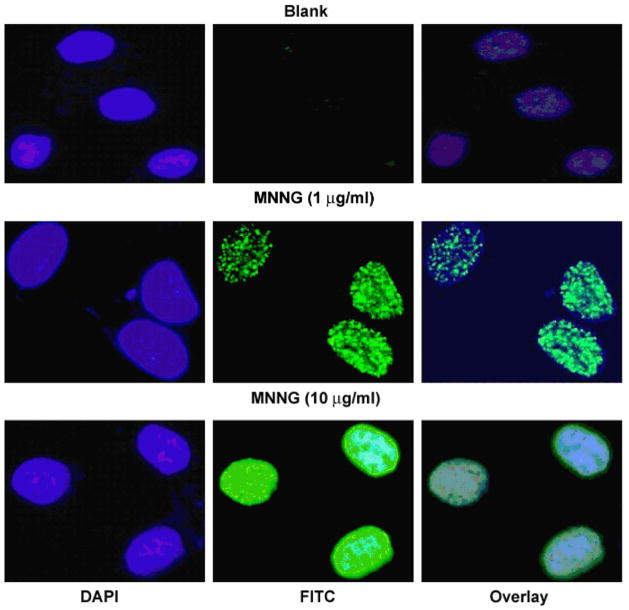
Fig. 2 is a representative immunofluorescent image of the microscopy analysis. It was found that although majority of the control and DMSO-treated cells had no γH2AX foci in the nuclei, there were still around 20% of cells contained between 1 and 10 foci, with even fewer percentages of cells contained more than 10 foci. On the other hand, various concentrations of MNNG all induced increases in foci formation, both in the percentage of cells containing foci and the number of foci per cell (see below). In addition, a distinct pattern of staining, in which the whole nucleus was stained by FITC-labeling (abbreviated as W), was also induced by MNNG treatment, particularly at 10 μg/ml (Fig. 2).
Fig. 2. MNNG induces γH2AX foci formation in FL cells.
After MNNG treatment for 8 h, cells were fixed and stained with anti-γH2AX antibody, and subjected to immunofluorescent microscopy. Shown are representative images from one of four independent experiments. Upper panel, control; middle and lower panel, MNNG treated cells. Blue, DAPI stain for nuclei; green, γH2AX. Notice that γH2AX all present in the nuclei. (For interpretation of the references in color in this figure legend, the reader is referred to the web version of this article.)
3.2. Different concentration of MNNG treatment exhibits distinct γH2AX foci formation kinetics
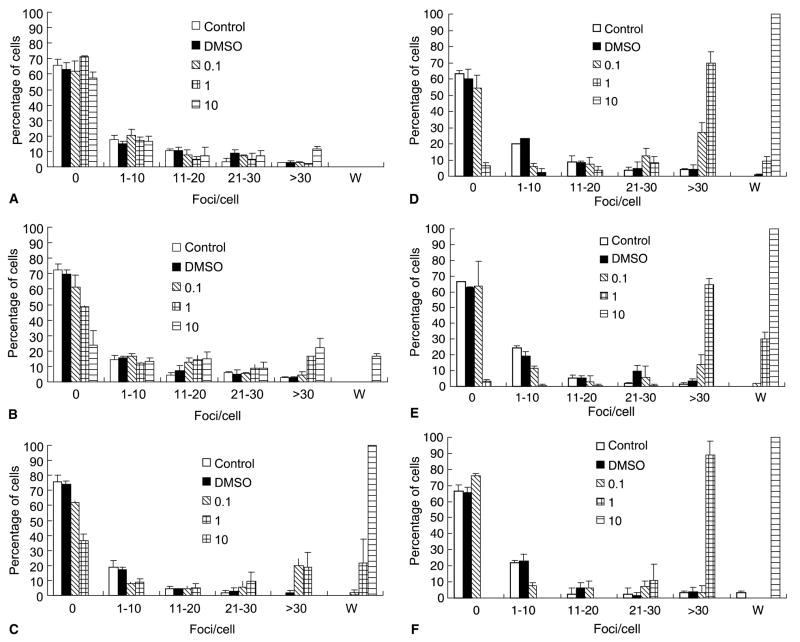
Ten minutes after MNNG treatment, most of the control, DMSO-, 0.1 and 1.0 μg/ml MNNG-treated cells had no foci (65%, 62%, 61% and 73%, respectively), with very few cells contained more than 30 foci/cell. However, although the majority of 10 μg/ml MNNG-treated cells had no foci (57%), around 11% of these cells had over 30 foci/cell (Fig. 3(A)). After 30 min, more than 25% of the 10 μg/ml MNNG-treated cells had over 30 foci/cell. In addition, about 20% of the cells showed the distinct whole nuclei staining pattern (Fig. 3(B)). At 2 h, almost all the cells exhibited the W staining pattern, and lasted to the end of the 48 h period examined (Fig. 3(C)–(F)). In contrast, 1 μg/ml of MNNG showed its effect on γH2AX foci formation at 30 min, with the percentage of cells containing 30 foci/cell or more increased to 20% (Fig. 3(B)). At 2 h, cells with W staining pattern also appeared (Fig. 3(C)). The percentage of cells containing over 30 foci/cell increased steadily, and at the end of the 48 h time frame, almost all cells had at least 30 foci/cell (Fig. 3(D)–(F)). On the other hand, the effect of 0.1 μg/ml MNNG on γH2AX foci formation only became evident at 2 h post-treatment, with about 19% of the cells contained over 30 foci/cell (Fig. 3(C)). At 8 h the percentage of cells containing 30 foci/cell or more increased to 29% (Fig. 3(D)); however, 24 h later, the percentage dropped to 18%, and at the end of the 48 h period, the distribution pattern of γH2AX foci in 0.1 μg/ml MNNG treated cells was almost the same as control or DMSO-treated cells (Fig. 3(E)–(F)). Taken together, these data showed that different concentrations of MNNG had distinct effects on γH2AX foci formation.
Fig. 3. Quantification of MNNG-induced γH2AX foci in FL cells.
After various MNNG treatments (μg/ml), the fluorescent images of γH2AX were captured and analyzed by Image-Pro software as described in Section 2. (A) 10 min, (B) 30 min, (C) 2 h, (D) 8 h, (E) 24 h and (F) 48 h.
3.3. Neutral comet assay demonstrates that MNNG induces DNA strand breaks
To confirm if MNNG indeed induced DNA strand breaks as implied by γH2AX foci formation, MNNG treated FL cells were further subjected neutral comet assay. As shown in Table 1, it was found that there were about 20% of control and DMSO-treated cells had tails throughout the 48 h period examined, with the average tail length less that 5 μm long. For 0.1 μg/ml of MNNG, there were no significant changes for neither the percentage of cells with tail nor the length of tail at 10 and 30 min. However, 2 h later, the percentage of cells with tail jumped to 73%, with an average tail length of 10 μm. This trend continued to 8 h, and then began to come down sometime after that. At 24 h, only 29% of cells still had tail, and the average tail length was decreased to about 5.49 ± 3.98 μm. At 48 h, both the percentage of cells with tail and the average tail length were comparable to those of controls. On the contrary, although 1 μg/ml MNNG also induced increases in both the percentage and tail length at 2 h, this trend continued and lasted to 48 h. In sharp contrast, neutral comet assay did not show any significant changes of 10 μg/ml MNNG treated cells at 8 h. Even more intriguing was that at 24 h, there was no cell with the comet tail, although at 48 h, about 33% cells showed the comet tail again.
Table 1.
Neutral comet assay of MNNG-treated FL cells
| Control | DMSO | 0.1 μg/ml | 1.0 μg/ml | 10 μg/ml | ||
|---|---|---|---|---|---|---|
| 10 min | Percentage (%) with tail | 20.28 ± 0.54 | 20.24 ± 5.05 | 19.79 ± 6.20 | 18.69 ± 3.81 | 16.15 ± 2.09 |
| Tail length (μm) | 2.30 ± 1.66 | 3.56 ± 1.73 | 2.99 ± 2.97 | 3.22 ± 1.44 | 1.01 ± 1.24 | |
| 30 min | Percentage (%) with tail | 16.89 ± 3.92 | 19.64 ± 3.57 | 21.11 ± 1.57 | 16.67 ± 0 | 18.75 ± 2.83 |
| Tail length (μm) | 4.43 ± 1.59 | 3.70 ± 1.67 | 2.72 ± 1.61 | 3.50 ± 1.82 | 2.22 ± 1.34 | |
| 2 h | Percentage (%) with tail | 28.29 ± 4.65 | 22.47 ± 1.48 | 73.74 ± 12.88 | 55.05 ± 1.69 | 29.17 ± 5.89 |
| Tail length (μm) | 1.56 ± 0.94 | 2.83 ± 1.69 | 10.81 ± 3.80 | 7.45 ± 4.10 | 2.67 ± 1.14 | |
| 8 h | Percentage (%) with tail | 15.04 ± 0.47 | 20 ± 0 | 75.64 ± 4.13 | 64.21 ± 5.95 | 15 ± 7.07 |
| Tail length (μm) | 1.69 ± 1.31 | 2.65 ± 1.41 | 14.01 ± 1.26 | 13.61 ± 3.7 | 4.53 ± 3.34 | |
| 24 h | Percentage (%) with tail | 19.44 ± 3.92 | 22.50 ± 3.89 | 29.32 ± 3.49 | 71.36 ± 1.92 | 0 |
| Tail length (μm) | 3.13 ± 2.08 | 1.78 ± 1.21 | 5.49 ± 3.98 | 9.71 ± 5.38 | 0 | |
| 48 h | Percentage (%) with tail | 18.33 ± 2.35 | 12.96 ± 2.61 | 20.19 ± 6.79 | 67.35 ± 3.74 | 33.33 ± 0 |
| Tail length (μm) | 2.84 ± 2.66 | 2.33 ± 1.65 | 3.36 ± 2.97 | 12.79 ± 4.67 | 4.34 ± 2.44 |
3.4. Alkaline comet assay reveals DNA strand breaks in whole nucleus staining cells
Although neutral comet assay did not detect the DSB type DNA damage in 10 μg/ml MNNG treated cells, most of these cells had the distinct whole nucleus staining pattern with γH2AX. These cells were further subjected to alkaline comet assay. As shown in Fig. 4, while neutral comet assay did not detect any strand breaks by 10 μg/ml MNNG in FL cells during a 24 h period, alkaline comet assay revealed the presence of DNA damage, probably including SSBs (Fig. 4). Therefore, γH2AX may be a better indicator for DNA damages than neutral comet assay under certain conditions.
Fig. 4. Alkaline comet assay reveals DNA damage induced by 10 μg/ml MNNG.
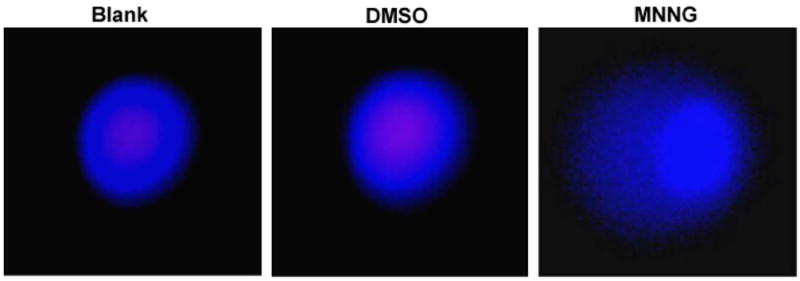
Shown are representative images of alkaline comet assay of FL cells after exposure to 10 μg/ml MNNG for 2 h. (For interpretation of the references in color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
A sensitive method to clearly identify DNA damage would greatly enhance our ability to understand the carcinogenesis mechanisms of many genotoxic agents. Ever since γH2AX was first reported, it has been considered a specific indicator for DNA damages, and has been applied in many studies to demonstrate the presence of DNA damages induced by various chemicals, viruses or even heat shock (Bosco et al., 2004; Daniel et al., 2004; Huang et al., 2004; Kaneko et al., 2005). In this study, we have shown that the presence of γH2AX foci could be indicative of DNA strand breaks, which can be confirmed by comet assay. Furthermore, at lower concentrations, it correlated well with comet assay for the general trend of DNA damages, although quantitatively it did not. For example, at 0.1 μg/ml of MNNG, even at its peak time, only slightly over 30% cells contained 30 foci/cell or more. At the same time point, neutral comet assay revealed that over 70% of cells had a tail length around 14 μm (Table 1). Therefore, more detailed studies are required to examine this apparent inconsistency.
One interesting aspect we observed during this study is the whole nucleus staining of γH2AX in 10 μg/ml MNNG treated cells (or longer treatment with 1 μg/ml) (Figs. 2 and 3), suggesting the presence of severe DNA damages. However, neutral comet assay showed no significant changes of this type of cells compared to controls, which was quite intriguing. When these cells were further subjected to alkaline comet assay, the appearance of comet tail clearly indicated the presence of DNA damages (Fig. 4). Thus, this observation suggested that γH2AX might detect certain type of DNA damage that neutral comet assay cannot. Even more intriguing is the 24 h neutral comet assay result, which showed no cells with tail (Table 1). Under light microscope observation all cells were round at this time point. Since it has been shown previously that 10 μg/ml MNNG can induce apoptosis (Yang and Duerksen-Hughes, 2001), therefore, one possible answer is that the cells were undergoing early stages of apoptosis, in which chromatin condensation might take place, thus the conditions used for neutral comet assay cannot separate the DNA fragments. However, the exact reason for this phenomenon is unknown. Also, the three concentrations of MNNG we chose here provided examples for three different types of DNA damage responses. At lower concentration (0.1 μg/ml), MNNG first induced the accumulation of γH2AX, followed by the gradually decrease of γH2AX. This could be explained by the activation of DNA repair systems, in which all the damages were processed and successfully removed. Similarly, medium concentration of MNNG may also activate the repair mechanisms. However, these more severe damages could not be successfully repaired, thus leading to the accumulation of unrepaired sites. And eventually for high concentration of MNNG, it might trigger the apoptosis mechanism instead of the repair machinery. These hypotheses are currently being investigated in our laboratory.
Taken together, in the present study, it was found that both γH2AX foci formation and neutral comet assay can be used to detect DNA damages using MNNG as a model chemical. In particular, high concentration of MNNG induced a special pattern of γH2AX formation, representing a type of DNA damage that cannot be detected by neutral comet assay but by alkaline comet assay.
Acknowledgments
This work was supported in part by Natural Science Foundation of China (Nos. 30300277 and 30471956, J. Yang), Hi-Tech Research and Development Program (2004AA649120, J. Yang), China. F.F. Chen is supported by NIH Grant CA95393-01, NASA Grant NNA04CA75I, and NIH P50 Grant CA112970.
References
- Bassing CH, Chua KF, Sekiguchi J, Suh H, Whitlow SR, Fleming JC, Monroe BC, Ciccone DN, Yan C, Vlasakova K, Livingston DM, Ferguson DO, Scully R, Alt FW. Increased ionizing radiation sensitivity and genomic instability in the absence of histone H2AX. Proc Natl Acad Sci Am. 2002;99:8173–8178. doi: 10.1073/pnas.122228699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco EE, Mayhew CN, Hennigan RF, Sage J, Jacks T, Knudsen ES. RB signaling prevents replication-dependent DNA double-strand breaks following genotoxic insult. Nucleic Acids Res. 2004;32:25–34. doi: 10.1093/nar/gkg919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celeste A, Petersen S, Romanienko PJ, Fernandez-Capetillo O, Chen HT, Sedelnikova OA, Reina-San-Martin B, Coppola V, Meffre E, Difilippantonio MJ, Redon C, Pilch DR, Olaru A, Eckhaus M, Camerini-Otero RD, Tessarollo L, Livak F, Manova K, Bonner WM, Nussenzweig MC, Nussenzweig A. Genomic instability in mice lacking histone H2AX. Science. 2002;296:922–927. doi: 10.1126/science.1069398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HT, Bhandoola A, Difilippantonio MJ, Zhu J, Brown MJ, Tai X, Rogakou EP, Brotz TM, Bonner WM, Ried T, Nussenzweig A. Response to RAG-mediated VDJ cleavage by NBS1 and γ-H2AX. Science. 2000;290:1962–1965. doi: 10.1126/science.290.5498.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumpton MJ, Collins AR. Are environmental electromagnetic fields genotoxic? DNA Repair (Amst) 2004;3:1385–1387. doi: 10.1016/j.dnarep.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Daniel R, Ramcharan J, Rogakou E, Taganov KD, Greger JG, Bonner W, Nussenzweig A, Katz RA, Skalka AM. Histone H2AX is phosphorylated at sites of retroviral DNA integration but is dispensable for postintegration repair. J Biol Chem. 2004;279:45810–45814. doi: 10.1074/jbc.M407886200. [DOI] [PubMed] [Google Scholar]
- Huang X, Okafuji M, Traganos F, Luther E, Holden E, Darzynkiewicz Z. Assessment of histone H2AX phosphorylation induced by DNA topoisomerase I and II inhibitors topotecan and mitoxantrone and by the DNA cross-linking agent cisplatin. Cytometry. 2004;58A:99–110. doi: 10.1002/cyto.a.20018. [DOI] [PubMed] [Google Scholar]
- Jin J, Yang J, Gao Z, Yu Y. Proteomic analysis of cellular responses to low concentration N-methyl-N′-nitro-N-nitrosoguanidine in human amnion FL cells. Environ Mol Mutagen. 2004;43:93–99. doi: 10.1002/em.20001. [DOI] [PubMed] [Google Scholar]
- Kaneko H, Igarashi K, Kataoka K, Miura M. Heat shock induces phosphorylation of histone H2AX in mammalian cells. Biochem Biophys Res Commun. 2005;328:1101–1106. doi: 10.1016/j.bbrc.2005.01.073. [DOI] [PubMed] [Google Scholar]
- Pilch DR, Sedelnikova OA, Redon C, Celeste A, Nussenzweig A, Bonner WM. Characteristics of gamma-H2AX foci at DNA double-strand breaks sites. Biochem Cell Biol. 2003;81:123–129. doi: 10.1139/o03-042. [DOI] [PubMed] [Google Scholar]
- Redon C, Pilch D, Rogakou E, Sedelnikova O, Newrock K, Bonner W. Histone H2A variants H2AX and H2AZ. Curr Opin Genet Dev. 2002;12:162–169. doi: 10.1016/s0959-437x(02)00282-4. [DOI] [PubMed] [Google Scholar]
- Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- Roser S, Pool-Zobel BL, Rechkemmer G. Contribution of apoptosis to responses in the comet assay. Mutat Res. 2001;497:169–175. doi: 10.1016/s1383-5718(01)00255-8. [DOI] [PubMed] [Google Scholar]
- Rothkamm K, Lobrich M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low X-ray doses. Proc Natl Acad Sci Am. 2003;100:5057–5062. doi: 10.1073/pnas.0830918100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedelnikova OA, Pilch DR, Redon C, Bonner WM. Histone H2AX in DNA damage and repair. Cancer Biol Ther. 2003;2:233–235. doi: 10.4161/cbt.2.3.373. [DOI] [PubMed] [Google Scholar]
- Stiff T, O’Driscoll M, Rief N, Iwabuchi K, Lobrich M, Jeggo PA. ATM and DNA-PK function redundantly to phosphorylate H2AX after exposure to ionizing radiation. Cancer Res. 2004;64:2390–2396. doi: 10.1158/0008-5472.can-03-3207. [DOI] [PubMed] [Google Scholar]
- Vaughan AT, Milner AM, Gordon DG, Schwartz JL. Interaction between ionizing radiation and supercoiled DNA within human tumor cells. Cancer Res. 1991;51:3857–3861. [PubMed] [Google Scholar]
- Wang H, Wang M, Bocker W, Iliakis G. Complex H2AX phosphorylation patterns by multiple kinases including ATM and DNA-PK in human cells exposed to ionizing radiation and treated with kinase inhibitors. J Cell Physiol. 2005;202:492–502. doi: 10.1002/jcp.20141. [DOI] [PubMed] [Google Scholar]
- Ward IM, Chen J. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J Biol Chem. 2001;276:47759–47762. doi: 10.1074/jbc.C100569200. [DOI] [PubMed] [Google Scholar]
- Yang J, Duerksen-Hughes P. A new approach to identifying genotoxic carcinogens: p53 induction as an indicator of genotoxic damage. Carcinogenesis. 1998;19:1117–1125. doi: 10.1093/carcin/19.6.1117. [DOI] [PubMed] [Google Scholar]
- Yang J, Duerksen-Hughes PJ. Activation of a p53-independent, sphingolipid-mediated cytolytic pathway in p53-negative mouse fibroblast cells treated with N-methyl-N-nitro-N-nitrosoguanidine. J Biol Chem. 2001;276:27129–27135. doi: 10.1074/jbc.M100729200. [DOI] [PubMed] [Google Scholar]
- Yang J, Hooper WC, Phillips DJ, Talkington DF. Regulation of proinflammatory cytokines in human lung epithelial cells infected with Mycoplasma pneumoniae. Infect Immun. 2002;70:3649–3655. doi: 10.1128/IAI.70.7.3649-3655.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Yu Y, Hamrick HE, Duerksen-Hughes PJ. ATM, ATR and DNA-PK: initiators of the cellular genotoxic stress responses. Carcinogenesis. 2003;24:1571–1580. doi: 10.1093/carcin/bgg137. [DOI] [PubMed] [Google Scholar]
- Zhu-Ge J, Yu YN. Enzyme activity analysis of CYP2C18 with exon 5 skipped. Acta Pharmacol Sinica. 2004;25:1065–1069. [PubMed] [Google Scholar]