Abstract
Reactive oxygen species (ROS) scavengers have been shown to relieve persistent pain; however, the mechanism is not clearly understood. Superoxide produced from mitochondrial oxidative phosphorylation is considered the major source of ROS in neurons during excitation where mitochondrial superoxide levels are normally controlled by superoxide dismutase (SOD-2). The present study hypothesizes that capsaicin-induced secondary hyperalgesia is a consequence of superoxide build-up in spinal dorsal horn neurons and SOD-2 is a major determinant. To test this hypothesis, the spinal levels of SOD-2 activity, inactivated SOD-2 proteins, and mitochondrial superoxide were measured and correlated to the levels of capsaicin-induced secondary hyperalgesia in mice with and without SOD-2 manipulations. The data suggest that superoxide accumulation is a culprit in the abnormal sensory processing in the spinal cord in capsaicin-induced secondary hyperalgesia. Our studies also support the notion that SOD-2 nitration is a critical mechanism that maintains elevated superoxide levels in the spinal cord after capsaicin treatment. Finally, our findings suggest a therapeutic potential for the manipulation of spinal SOD-2 activity in pain conditions.
Keywords: oxidative stress, persistent pain, central sensitization, SOD-2, superoxide, mitochondria
Introduction
Reactive oxygen species (ROS) are highly reactive oxygen containing molecules that exert a cytotoxic effect in neurodegenerative diseases, stroke, and the normal aging process (Jenner, 1994). Cellular ROS levels are under the tight control of antioxidant systems. The first and most crucial antioxidant is mitochondrial superoxide dismutase (MnSOD; SOD-2), which neutralizes superoxide by converting it into hydrogen peroxide (H2O2). Hydrogen peroxides are normally further metabolized into molecular oxygen and water by either catalase or glutathione peroxidase (Fridovich, 1995). Superoxide (O2●−) generated from mitochondrial oxidative phosphorylation is a major source of neuronal ROS (Jenner, 1994; Fridovich, 1995). Superoxide has not received much consideration in persistent pain, but increased ROS production is observed in injured peripheral nerve and inflamed tissue (Khalil et al., 1999; Levy et al., 1999; Cízková et al., 2002; Twining et al., 2004; Park et al., 2006), and antioxidants produce analgesia in both neuropathic (Tal, 1996; Khalil and Khodr, 2001; Kim et al., 2004) and inflammation (Haley et al., 1992; Wang et al., 1996; Coderre et al., 2004; Lee et al., 2007) pain. However, the underlying mechanism by which ROS reduction alleviates pain is not well understood.
The first step of establishing a clear understanding of ROS mechanism in persistent pain is an identification of the major action site: peripheral tissue, the spinal cord, etc. In the capsaicin-induced pain model, an intradermal injection of capsaicin produces two distinctive pain components: primary and secondary hyperalgesia. The primary hyperalgesia is caused by peripheral sensitization and the secondary hyperalgesia is caused by central sensitization (Willis, 2001). Therefore, the capsaicin-induced pain model is an excellent model with which to identify the ROS action site. In our earlier study in the rat, a significant analgesic effect of ROS scavengers was observed in capsaicin-induced secondary hyperalgesia (Lee et al., 2007), suggesting ROS involvement in the spinal cord. Furthermore, ROS accumulation was observed primarily in the mitochondria of dorsal horn neurons after capsaicin treatment in mice (Schwartz et al., 2008). These results suggest that elevated mitochondrial ROS may be an important mechanism of capsaicin-induced hyperalgesia. Thus, we hypothesize that the mitochondrial antioxidant SOD-2 is a critical determinant for mitochondrial superoxide accumulation and subsequent capsaicin-induced secondary hyperalgesia. The importance of SOD-2 has been suggested in a chronic inflammatory pain model (Wang et al., 2004) and a NMDA-induced hyperalgesia (Muscoli et al. 2004). To test this hypothesis, the spinal levels of SOD-2 protein, SOD-2 activity, and inactivated SOD-2 protein were measured in mice after a capsaicin injection to a hind foot with and without ROS scavenger treatments. Capsaicin-induced secondary hyperalgesia was also examined in mice in which SOD levels were manipulated pharmacologically or genetically. Our results show that the spinal SOD-2 activity levels correlate with levels of capsaicin-induced secondary hyperalgesia. Moreover, elevated mitochondrial superoxide accumulation after capsaicin treatment is, in part, attributable to inactivation of SOD-2 proteins. Thus, these data suggest that endogenous levels of mitochondrial SOD-2 determine the levels of spinal superoxide, which in turn determine the levels of central sensitization and, thus, capsaicin-induced secondary hyperalgesia.
Materials and Methods
Experimental animals
Young adult male mice (20–25 g of body weight) of various stains were used for this study. Wild-type FVB/NJ mice and heterozygous SOD-2 knock-out (KO) mice [B6.129S7-Sod2tm1Leb/J] in the C57BL/6 background were purchased from Jackson Laboratory, and heterozygous SOD-2 transgenic mice (TG) in the C57BL/6 background originated from Ho et al. (1998). All heterozygous mice (SOD-2 KO and SOD-2 TG) were compared with wild-type littermates for all experiments. Animals were housed in groups of four to five in plastic cages with soft bedding and ad libitum access to food and water under a 12 h light/dark cycle. All animals were acclimated for 1 week before any experimental procedures. All experimental protocols were approved by the Animal Care and Use Committee at the University of Texas Medical Branch and are in accordance with the National Institutes of Health's Guide for the Care and Use of Laboratory Animals.
Capsaicin injection
For injection of capsaicin, each mouse was anesthetized with isoflurane (2.0% for induction and 1.5% for maintenance) in a flow of O2 and placed in a prone position. Capsaicin solution (0.5%) was made by dissolving 5 mg of capsaicin (Sigma) in 1 ml of vehicle containing 20% alcohol and 7% Tween 80 in saline, immediately before injection. For behavioral testing, 5 μl of capsaicin solution was injected intradermally using a 30 gauge needle attached to a Hamilton syringe. The needle was inserted near the heel of the left hind foot and advanced to the middle of the plantar surface as shown in Figure 1a. A successful injection was noted by the formation of a “bleb” ∼2 mm in diameter. The insertion site was pressed for 1 min to prevent leakage of the solution after removal of the needle. Anesthesia was discontinued and the mice were aroused within 5 min and then returned to their cages. For SOD protein and activity analysis and ROS imaging, 25 μl of 0.5% capsaicin was injected into multiple sites of both the plantar and dorsal surface of the left hind foot to increase receptive fields of capsaicin responsive afferents and, thus, to maximize the number of affected neurons in the spinal cord.
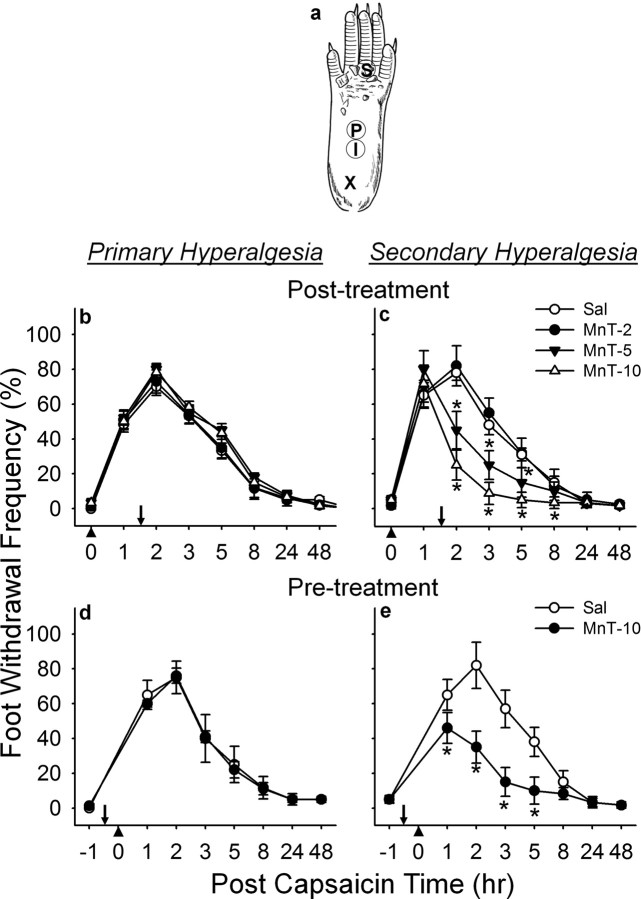
Figure 1.
Effect of a SOD mimetic, MnTBAP, on capsaicin-induced hyperalgesia in response to vF (#3.61). a, Sites of capsaicin injection and behavioral testing in the mouse hind foot. For capsaicin injection, a 30-gauge needle was inserted at the heel of the foot (X) and advanced to the injection site (I), and capsaicin (25 μg in 5 μl of vehicle) was injected intradermally. Foot withdrawal frequencies in response to von Frey stimuli were measured at site P for primary hyperalgesia and at site S for secondary hyperalgesia. b, c, Posttreatment effect; d, e, pretreatment effect. b–e,The mean ± SEM (n = 6/group) of primary (b, d) and secondary hyperalgesia (c, e) are plotted against the postcapsaicin treatment time. MnTBAP [or vehicle saline (Sal)] was injected systemically (intraperitoneally) 1.5 h after or 0.5 h before (indicated by downward arrows) capsaicin injection (indicated by upward arrowheads) for posttreatments or pretreatments, respectively. MnTBAP reduced capsaicin-induced secondary hyperalgesia in a dose-dependent manner but not primary hyperalgesia. *Values significantly different from corresponding values of the vehicle-treated group at p ≤ 0.05, by Duncan's post hoc test after two-way repeated ANOVA. The data indicate that removal of superoxides exogenously supplemented by a SOD mimetic, reduces capsaicin induced secondary hyperalgesia. MnTBAP(Mn-T) doses: 2, 5, or 10 mg/kg body weight.
Behavioral testing for assessment of mechanical sensitivity of the foot
Foot withdrawal frequencies in response to von Frey stimuli were measured and used as an indicator of mechanical sensitivity. Tests were conducted blindly and foot withdrawal responses were assessed before and 1, 2, 3, 5, 8, 24 and 48 h (sometimes 72 h) after intradermal capsaicin injection. For each test, the animal was placed in a plastic chamber on top of a mesh screen platform and was habituated for at least 10 min. Mechanical stimuli were applied from underneath to the plantar surface of the left hind foot. The mechanical sensitivity of the foot was determined by the frequency of positive foot withdrawal responses to 10 repetitive von Frey stimuli. The von Frey monofilaments of 2.48, 3.00, or 3.61 (vF#2.48, #3.0, or #3.61), which are equivalent to 0.03, 0.10, or 0.41 g force, respectively, were used for stimulation. For mechanical stimulation, a von Frey filament was applied perpendicular to the stimulation site with sufficient force to bend the filament for 2–3 s and then removed. An abrupt withdrawal with or without licking of the foot, during stimulation, or immediately after stimulus removal, was considered a positive response. Each test was composed of 10 stimuli for each area. The number of positive responses was converted into a percentage with 10 positive responses corresponding to 100%. Primary and secondary hyperalgesia were assessed as shown previously (Schwartz et al., 2008). In brief, primary hyperalgesia was assessed by applying von Frey filaments to site P in Figure 1a, which is 2 mm distal from the injection site (site I). For secondary hyperalgesia, a von Frey monofilament was applied at the base and/or proximal part of the third and fourth toes (site S). This area is at a sufficient distance from the capsaicin injection site (∼7 mm), and is thus not directly affected by capsaicin, but becomes extremely sensitive and thus is identified as a site of secondary hyperalgesia.
Direct transcutaneous intrathecal injection
For direct transcutaneous intrathecal injection, a modified method (Lee et al., 2007) of the original intrathecal injection method (Hylden and Wilcox, 1980) was used. Mice were anesthetized (2.0% for induction and 1.5% for maintenance) with isoflurane in a flow of O2, placed in a prone position, and the hair on their back was clipped. The caudal paralumbar region, just cranial to the iliac crests, was securely held by the thumb and middle fingers of the left hand, and the index finger was placed on the tip of sixth lumbar (L6) spinous process, the highest point of the vertebral column. A 1.5-inch-long 30 gauge needle connected to a Hamilton syringe was positioned underneath the index finger with the beveled side facing upward in a forward direction. The needle was inserted into the tissue at a 45° angle. The angle of the needle was maintained until the needle went through the fifth intervertebral space (L5–L6) and “slipped in” causing a sudden lateral movement of the tail. Solution was injected at a rate of 1 μl/s. The needle was held in position for 10 s and removed slowly to avoid any outflow of the solution. Anesthesia was discontinued and the mice were aroused within 5 min and then returned to their cages.
Administration of drugs
SOD mimetic, Mn (III) tetrakis(4-benzoic acid) porphyrin.
Mn (III) tetrakis(4-benzoic acid) porphyrin (MnTBAP; 5mg; Calbiochem) was dissolved in 2.5 ml of saline, and 100 μl of this solution was injected intraperitoneally per 20 g of body weight (10 mg/kg). The same volume of saline was used as a control. To determine the role of SOD in capsaicin-induced hyperalgesia, MnTBAP was administered either 0.5 h before (pretreatment) or 1.5 h after (posttreatment) capsaicin treatment. To examine the dose–response, one of three doses of MnTBAP, 2 mg, 5 mg, or 10 mg/kg of body weight, or saline was injected intraperitoneally, 1.5 h (posttreatment) after intradermal injection of capsaicin.
SOD inhibitor, diethylthiocarbamate.
Diethylthiocarbamate (DETC; 0.2 mg; Sigma) was dissolved in 4 ml of saline, and 5 μl of this solution was injected intrathecally (0.05 mg). The same volume of saline was used as a control. To determine the role of SOD in capsaicin-induced hyperalgesia, DETC was administered either 0.5 h before capsaicin treatment (pretreatment) or 1.5 h after (posttreatment). To examine the dose–response, one of three doses of DETC, 0.01, 0.02, or 0.05 mg, or saline was injected intrathecally, 1.5 h (posttreatment) after intradermal injection of capsaicin.
ROS scavengers: phenyl N-tert-butylnitrone; 4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl.
Either 0.2 mg of phenyl N-tert-butylnitrone (PBN) or 0.5 mg of 4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPOL; Sigma) was dissolved in 1 ml of saline, and 5 μl was injected intrathecally 15 min after capsaicin injection (200 μg of PBN; 500 μg of TEMPOL). The same volume of saline was used as a control.
Western blot analysis of SOD-2
At the time of tissue sampling, mice were anesthetized deeply with sodium pentobarbital (Nembutal, 75 mg/kg) and perfused through the aorta with cold saline for ∼1 min. The L4–5 spinal cord segments were removed after laminectomy and divided into right (contralateral) and left (ipsilateral to capsaicin injection) halves and frozen immediately on dry ice. The spinal cord segments were homogenized, using a bead beater, in 100 μl of lysis buffer (20 mm Tris-Base, 150 mm NaCL, 10% glycerol, 0.1% Triton X-100, 1% Chaps, 2 mm EGTA, 1% protease inhibitor mixture). Lysates were centrifuged at 12,500 × g for 30 min at 4°C. The supernatants were collected and protein concentration was determined using the bicinchoninic acid protein assay (BCA; Pierce). Protein (40 μg) was resuspended in 40 μl of Laemmli sample buffer and 5% 2-mercaptoethanol and heated for 10 min. The samples were loaded in 15% SDS polyacrylamide gels. After separation by SDS-PAGE, proteins were transferred electrophoretically to polyvinylidene fluoride (PVDF) membranes and blocked. For detection of SOD-2, blots were incubated with rabbit polyclonal anti-SOD-2 (1:3000 dilution; Upstate Cell Signaling) and then incubated with anti-rabbit horseradish peroxidase-conjugated secondary antibody (1:3000 dilution; Bio-Rad). The specific complex was detected by an enhanced chemiluminescence detection system. The density levels of SOD-2 bands were quantified by using MetaMorph software (Molecular Devices). Equal protein loading was determined using β-actin (1:10,000 dilution; Sigma) expression as a control.
Immunoprecipitation assay and Western blot analysis of N-SOD-2
The L4–5 spinal cord segments were sampled after cold saline perfusion as described in the previous section. The spinal cord segment was homogenized, using a bead beater, in 100 μl of lysis buffer. After homogenization, solubilized extracts were sonicated and centrifuged at 12,500 × g. The supernatants were collected and stored at −80°C immediately. Protein concentration was determined by BCA method, as described in Western blot analysis of SOD-2. Solubilized proteins (40 μg) were incubated with agarose-conjugated anti-nitrotyrosine antibody (Upstate Cell Signaling) overnight at 4°C. Agarose beads were collected by centrifugation at 12,500 × g, washed in PBS, and collected. The mixture of the beads-antibody and binding proteins complex was resuspended in 50 μl of Laemmli sample buffer (Bio-Rad) and 5% 2-mercaptoethanol (Bio-Rad), and heated. The samples were loaded in 15% SDS-PAGE minigels. After separation by SDS-PAGE, proteins were transferred electrophoretically to PVDF membranes (Bio-Rad), and Western blots were performed as above. Mouse brain treated with peroxynitrite for 10 min (Upstate Cell Signaling) was used as a positive control.
SOD-2 activity measurement and analysis
Tissue samples were homogenized in 50 mm sodium phosphate buffer, ph 7.4. The homogenates were centrifuged for 20 min at 12,500 × g; supernatant was collected and stored on ice. Protein concentration was determined by BCA method as above. SOD activity was measured using a microtiter plate assay for SOD using a water soluble tetrazolium (WST-1) salt (Peskin and Winterbourn, 2000). The reagent mixture for detection consisted of the following: assay buffer: 1 mm diethylenetriamine-pentaacetic acid (DTPA; Sigma), 1 mm hypoxanthine (Sigma), 50 mm phosphate buffer, pH 8.0; Catalase: 2 mg/ml (Sigma); WST-1: 50 μm (Dojindo Laboratories); and xanthine oxidase: 4.5 mU/ml (Sigma). Xanthine oxidase was added to the reaction mixture immediately before starting the assay. Samples were diluted 1:10, and each sample was pipetted in triplicates as well as standards. Reagent mixture (200 μl) per well was pipetted into each well and absorbance was read at 450 nm from time 0 min and every 10 min for 1 h on a microplate reader (model 680-UV, Bio-Rad). Bovine Cu/Zn SOD (Sigma) was used as a standard and final SOD concentrations of 0, 1, 3, 10, 30, and 100 ng/well were used for the standard curve. SOD1 activity was inhibited, by performing the assay in the presence of 2 mm NaCN 30 min before assay.
In vivo ROS imaging: mitochondrial superoxide using MitoSox
MitoSox Red (Invitrogen) was dissolved in a 1:1 mixture of dimethylsulfoxide (DMSO) and saline to a final concentration of 33 μm. Under isoflurane anesthesia, 10 μl of MitoSox was injected intrathecally by using a direct transcutaneous intrathecal injection method described previously. Approximately 23 h after MitoSox injection, mice received an intradermal injection of either capsaicin (0.5%, 25 μl) or the same volume of vehicle on both the plantar and dorsal surfaces of the left hind foot. Mice remained under 1.5% isoflurane anesthesia for 30 min to suppress any side effects induced by capsaicin. Mice were perfused through the aorta with saline followed by fixative containing 4% paraformaldehyde 1 h after capsaicin injection, and the L4–L5 spinal cord segments were removed. The cord was postfixed 4–15 h in the perfusion fixative, equilibrated in 30% sucrose, cryosectioned at 30 μm, and mounted on gelatin coated slides. The sections were examined under a fluorescent microscope with a rhodamine filter. Two different regions of the dorsal horn were photographed from 10 randomly selected sections from each animal: the lateral part of laminas I-II and laminas III-V. Photographs were taken with a Q-Imaging Retiga 2000R digital camera attached to an Olympus BX50 microscope using a 63× oil objective lens and saved as digital image files. The number of MitoSox-positive cellular profiles with distinctive nuclei (dark oval shaped space surrounded by red granules) was counted from these pictures.
ROS producing spinothalamic tract neurons: MitoSox and fluorogold double labeling
To identify spinothalamic tract (STT) neurons, a retrograde marker fluorogold (FG; Fluorochrome) was injected into the ventroposterior lateral thalamic nucleus (VPL). For FG injection, mice were anesthetized with sodium pentobarbital (75 mg/kg, i.p.) and placed in a stereotactic frame (Stoelting Company). A burr hole was made on the cranium at 1.5 mm posterior to the bregma and 1.62 mm lateral from the midline. A Hamilton syringe was inserted to 3.6 mm depth from the brain surface and 1 μl of FG (4% in d.w.) was injected into the VPL at a rate of 0.1 μl/min. Twenty minutes later, the needle was removed, the wounds were sutured, and the mice were returned to their cage after recovering from anesthesia. Three to four days later, mice were treated with MitoSox and capsaicin as described above and the spinal cord tissues were processed and examined. The spinal cord sections were examined under a fluorescent microscope with rhodamine and DAPI filters. FG labeled STT neurons were photographed for FG and MitoSox from 10 randomly selected sections from each animal. The number of double labeled, FG and MitoSox, STT neurons were counted in each animal and averaged in capsaicin (n = 5) and vehicle (n = 5) groups.
Statistical analysis
Data are presented as mean ± SEM and were analyzed using the statistical program SigmaStat (Version 3.1, Systat Software). Statistical analyses for differences in changes over time in multiple groups were performed using two-way ANOVA with one repeated factor (time), followed by Duncan's post hoc test. Differences of values in different groups were tested using the Student's t test (when comparing two groups) or the one-way ANOVA (when comparing more than two groups) followed by post hoc pairwise multiple comparisons. All statistical tests were evaluated at an α level of significance of 0.05.
Results
Confirmation of primary and secondary hyperalgesia
After an intradermal injection of capsaicin in mice, primary and secondary hyperalgesia were determined by applying a von Frey monofilament to sites P and S in Figure 1a, respectively. It is well known that after capsaicin injection only mechanical, but no heat, secondary hyperalgesia develops; whereas, both mechanical and heat components are present in primary hyperalgesia (Torebjörk et al., 1992; Willis, 2001). Using this feature, we attempted to confirm the sites testing for both primary and secondary hyperalgesia. Two hours after an intradermal capsaicin injection to the foot, thermal hyperalgesia developed, as shown by 44.5 ± 7.31% decrease in foot withdrawal latency (n = 6; p < 0.05 by paired Student's t test) from baseline values, at the capsaicin injection site (site P), but there was no statistical difference in heat response at site S (85.1 ± 6.72% from baseline value). However when mechanical sensitivity was measured in the same mice, mechanical hyperalgesia developed at both sites P and S similar to the results of all experiments in this study (Figs. 1–2). Therefore, the data confirm that these two tested sites P and S elicit primary and secondary hyperalgesia, respectively.
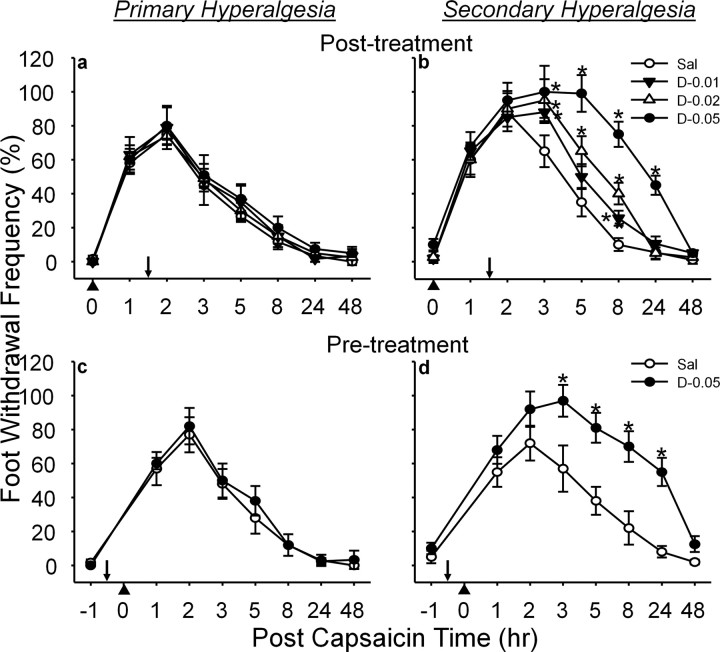
Figure 2.
Effect of a SOD inhibitor, DETC, on capsaicin-induced hyperalgesia in response to vF (#3.61). a, b, Posttreatment results; c, d, pretreatment results. The mean ± SEM (n = 6/group) of primary (a, c) and secondary hyperalgesia (b, d) are plotted against the postcapsaicin treatment time. DETC [or vehicle saline (Sal)] was injected intrathecally 1.5 h after or 0.5 h before (indicated by downward arrows) capsaicin injection (indicated by upward arrowheads) for posttreatments or pretreatments, respectively. DETC enhanced and prolonged capsaicin-induced secondary hyperalgesia in a dose-dependent manner but not primary hyperalgesia. *Values significantly different from corresponding values of the vehicle-treated group at p ≤ 0.05, by Duncan's post hoc test after two-way repeated ANOVA. The data indicate that superoxide accumulation caused by inhibition of SOD enhances and prolongs the capsaicin induced secondary hyperalgesia. DETC (D) doses: 0.01, 0.02, or 0.05 mg in 5 μl of saline.
Superoxide is involved in capsaicin-induced secondary hyperalgesia
To test whether superoxide is involved in capsaicin-induced hyperalgesia, the effect of a SOD mimetic, MnTBAP, and a SOD inhibitor, DETC, was examined on pain. Systemic (intraperitoneal) MnTBAP (10 mg/kg) treatment, 1.5 h after capsaicin injection, had no effect on the primary hyperalgesia (Fig. 1b). The same treatment, however, showed a significant reduction on the secondary hyperalgesia (Fig. 1c), which lasted ∼6 h. In a dose–response study, both 5 and 10 mg/kg of MnTBAP produced graded antihyperalgesic effect, whereas, 2 mg/kg had no effect compared with the saline-treated group. The fact that a SOD mimetic produced antihyperalgesia only on secondary hyperalgesia suggests that superoxide is involved in central sensitization.
Intrathecal injection of SOD inhibitor DETC (0.05 mg) 1.5 h after capsaicin treatment, in contrast, significantly enhanced and prolonged the secondary hyperalgesia but not the primary hyperalgesia (Fig. 2a,b). The enhanced hyperalgesia lasted up to 24 h with the highest dose tested (0.05 mg) but for 8 h with two lower doses (0.02 and 0.01 mg). In a dose–response study, all three doses showed graded enhanced hyperalgesia compared with the saline-treated group. Because DETC would block SOD activity and lead to superoxide accumulation, the enhancement of capsaicin-induced secondary hyperalgesia by DETC also supports the idea of superoxide involvement in central sensitization.
Although posttreatment of either MnTBAP or DETC suggests that superoxide is involved in the maintenance of secondary hyperalgesia, it is not known whether superoxide is also critical for the development of capsaicin-induced hyperalgesia. To address this issue, mice were treated with either MnTBAP or DETC 0.5 h before capsaicin treatment and mechanical hyperalgesia was measured. Pretreatment with MnTBAP (10 mg/kg) significantly reduced the capsaicin-induced secondary hyperalgesia (Fig. 1e), whereas DETC (0.05 mg) increased both the duration and the magnitude of capsaicin-induced secondary hyperalgesia (Fig. 2d). Neither MnTBAP nor DETC had any effect on the development of capsaicin-induced primary hyperalgesia (Figs. 1d, 2c). These results suggest that superoxide is critical for not only the development but also the maintenance of capsaicin-induced secondary hyperalgesia, thus suggesting a role of superoxide in central sensitization.
Spinal levels of SOD-2 proteins were unchanged but activities were decreased after capsaicin treatment
Previous findings have indicated that the levels of superoxide in the spinal cord are increased in response to intradermal capsaicin. We considered two possibilities that could explain the spinal superoxide elevation: a decrease in SOD-2 protein levels and/or a decrease in SOD-2 activity levels independent of a change in SOD-2 protein levels. First, the total amount of SOD-2 protein in the spinal cord was measured by Western blotting. As shown in Figure 3a, the levels of SOD-2 protein in the ipsilateral L4–5 spinal cord did not change significantly 1 h after capsaicin treatment compared with vehicle-treated mice. Thus, the data indicate that changes in the total SOD protein levels are not likely the cause of superoxide elevation.
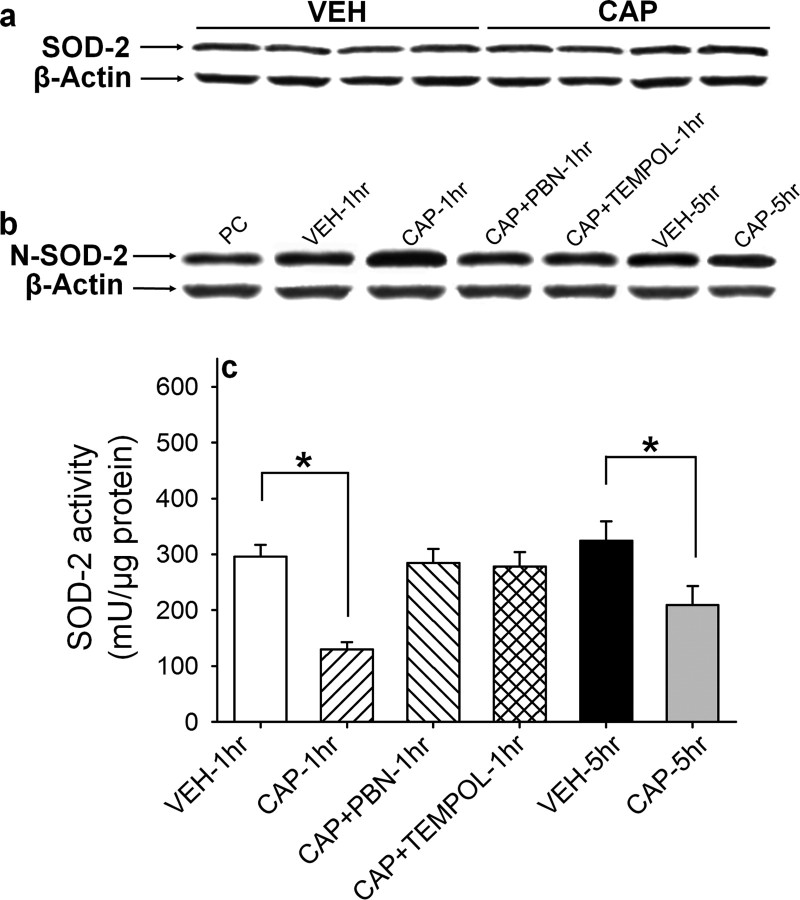
Figure 3.
Total SOD-2 protein, N-SOD-2 protein levels, and SOD-2 activity levels in the L4/5 spinal cord. a, Example of Western blot gel for SOD2 proteins. There is no change in the total amount of SOD2 proteins in the spinal cord 1 h after capsaicin injection into the paw compared with that of vehicle injection, and statistical analysis was done by nonpaired t test. b, Example of Western blot gel for N-SOD2 proteins. One hour after intradermal capsaicin treatment (CAP-1 h), the inactivated N-SOD2 levels increase approximately three times compared with vehicle treatment (VEH-1 h). Treatment with ROS scavengers (PBN: 100 mg/kg; TEMPOL: 300 mg/kg) 15 min after capsaicin injection (CAP+PBN-1 h and CAP+TEMPOL-1 h), completely reversed N-SOD2 levels to normal levels. Five hours after capsaicin treatment (CAP-5 h), N-SOD2 levels were slightly higher than vehicle-treated (VEH-5 h) control mice but not significantly different. The data indicate that ROS accumulation in the spinal cord might be caused, in part, by inactivation of SOD2 by nitration. c, The SOD2 activity levels were measured in six groups of mice (n = 12/group), and the average values (±SEM) are shown in c. CAP-1 h and CAP-5 h: 1 and 5 h after capsaicin; VEH-1 h and VEH-5 h: 1 and 5 h after vehicle; CAP+PBN-1 h and CAP+TEMPOL-1 h: 1 h after capsaicin plus PBN or TEMPOL treatment; PBN or TEMPOL was injected systemically (intraperitoneally) 15 min after capsaicin. SOD activity levels were significantly reduced 1 h after capsaicin, then moderately recovered at 5 h compared with that of vehicle treatment. Capsaicin-induced reduction on SOD2 activity was completely recovered by treatment with a ROS scavenger, PBN or TEMPOL. *Values significantly different from corresponding values in the vehicle-treated group at p ≤ 0.05 (n = 12), by Duncan's post hoc test after one-way repeated ANOVA. The data indicate that intradermal capsaicin induce the reduction in SOD2 activity in the spinal cord resulting from ROS elevation.
In the next experiment, levels of SOD-2 activity in the spinal cord were examined with a microtiter plate assay for SOD using a WST-1 salt (Peskin and Winterbourn, 2000). SOD-2 activity levels in the spinal cord after various manipulations in mice are shown in Figure 3c. One hour after intradermal capsaicin treatment (CAP-1 h), SOD-2 activity levels in the ipsilateral L4/5 spinal cord were greatly decreased compared with that of vehicle-treated mice. This decrease, however, was completely reversed when the mice were treated with ROS scavengers, PBN or TEMPOL, 15 min after capsaicin (CAP+PBN-1 h and CAP+TEMPOL-1 h). When spinal SOD-2 activity levels were measured 5 h after capsaicin treatment, the time mechanical hyperalgesia was significantly recovered from the peak response at 2 h after capsaicin treatment, SOD-2 activity levels also were significantly recovered from the lowest value at 1 h after treatment. The results show that spinal levels of SOD-2 activity, but not SOD-2 protein levels, were decreased after intradermal capsaicin and then recovered by ROS scavenger treatment, suggesting that ROS are involved in the reduction of SOD-2 activity. The data also indicate that the levels of SOD-2 activity are correlated with the amount of mechanical hypersensitivity. These findings suggest that decreased SOD-2 activity in the spinal cord, at least in part, results in elevation of superoxide and subsequently hyperalgesia.
Nitrated SOD-2 levels were increased in the spinal cord after capsaicin treatment
Because spinal SOD-2 activity levels were decreased without changes in protein levels, we speculated that the SOD-2 enzyme might have been inactivated. Inactivation of SOD can be caused via nitration by peroxinitrite under some oxidative stress conditions (Yamakura et al., 1998; Hylden and Wilcox, 1980; Salvemini et al., 2001; Wang et al., 2004; Muscoli et al., 2004). Therefore, the levels of nitrated SOD-2 (N-SOD-2) were measured in the L4/5 spinal cord of mice after various manipulations. The results of N-SOD-2 protein levels in the spinal cord are shown in Figure 3b. The levels of N-SOD-2 in the ipsilateral L4/5 spinal cord were significantly increased (∼3-fold) 1 h after capsaicin treatment (CAP-1 h), compared with that of vehicle-treated mice. Five hours after capsaicin treatment (CAP-5 h), spinal N-SOD-2 levels were significantly reduced from the peak levels but still slightly higher than, but not significantly different from, the vehicle-treated mice (VEH-5 h). In addition, PBN and TEMPOL treatment in combination with capsaicin (CAP+PBN-1 h and CAP+TEMPOL-1 h) did not alter the spinal N-SOD-2 protein levels compared with that of vehicle-treated mice (VEH-1 h). These results suggest that the decrease in SOD-2 activity in the spinal cord after capsaicin treatment is caused by inactivation of SOD-2 by nitration. The significant reduction of N-SOD-2 levels by ROS scavengers also support the notion that elevated levels of ROS are involved in nitration of SOD-2.
Capsaicin-induced hyperalgesia is correlated to endogenous SOD-2 activity levels
Previous findings indicate that spinal SOD activity levels are inversely correlated with the levels of the capsaicin-induced secondary hyperalgesia. Because mitochondria is speculated as the primary source of neuronal ROS and SOD-2 is a mitochondria-specific antioxidant, we determined whether SOD-2 could modulate hyperalgesia by measuring capsaicin-induced hyperalgesia in mice whose SOD-2 expression was modified genetically. Two genetically altered lines of mice were used: heterozygous SOD-2 overexpressing transgenic mice (SOD-2 TG) and heterozygous SOD-2 underexpressing knock-out (SOD-2 KO) mice. Capsaicin-induced mechanical hyperalgesia was determined as described previously by measuring foot withdrawal frequencies in response to vF filament stimuli. Mechanical sensitivity was measured before and various times after a capsaicin treatment, and results are shown in Figures 4 and 5.
Figure 4.
a–d, Capsaicin-induced primary (a) and secondary (b) hyperalgesia in SOD-2 KO mice and ROS scavenger effect on primary (c) and secondary (d) hyperalgesia. The average foot withdrawal frequencies (±SEM, n = 10 per group) in response to vF (#3.61) stimuli are plotted against the postcapsaicin treatment (indicated by upward arrowheads) time. The capsaicin-induced hyperalgesia, both primary and secondary, was significantly enhanced and prolonged in SOD-2 KO mice compared with that of wild-type littermates. Posttreatment with a ROS scavenger, TEMPOL [500 μg in 5 μl of saline (Sal); indicated by downward arrow], 1.5 h after capsaicin, significantly reduced secondary hyperalgesia but not primary hyperalgesia. Asterisks (*), Values are significantly different from corresponding values in the control groups (wild-type mice in a, b; saline group in c, d) at p ≤ 0.05 by Duncan's post hoc test after two-way repeated ANOVA.
Figure 5.
a, b, Capsaicin-induced primary (a) and secondary (b) hyperalgesia in SOD-2 TG mice in response to vF (#3.61). The capsaicin-induced hyperalgesia, both primary and secondary, were significantly reduced in SOD-2 TG mice compared with wild-type littermates. The effect is severer for secondary hyperalgesia than primary hyperalgesia; thus, secondary hyperalgesia is almost nonexistent. Asterisks (*), Values are significantly different from corresponding values in the wild-type littermates (Wt CAP) at p ≤ 0.05 (n = 10) by Duncan's post hoc test after two-way repeated ANOVA.
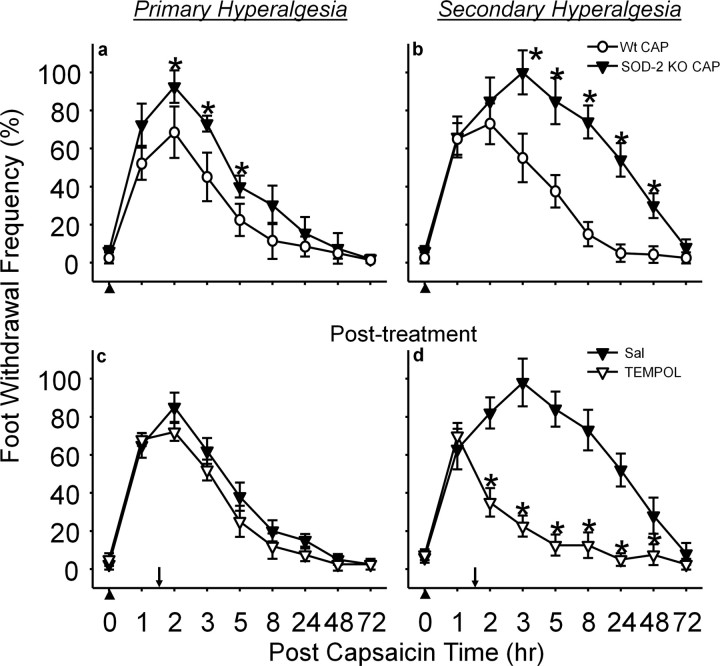
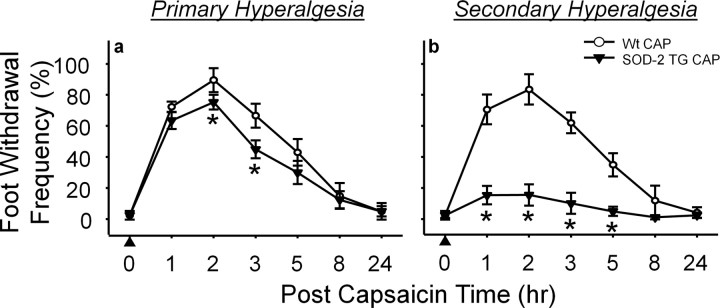
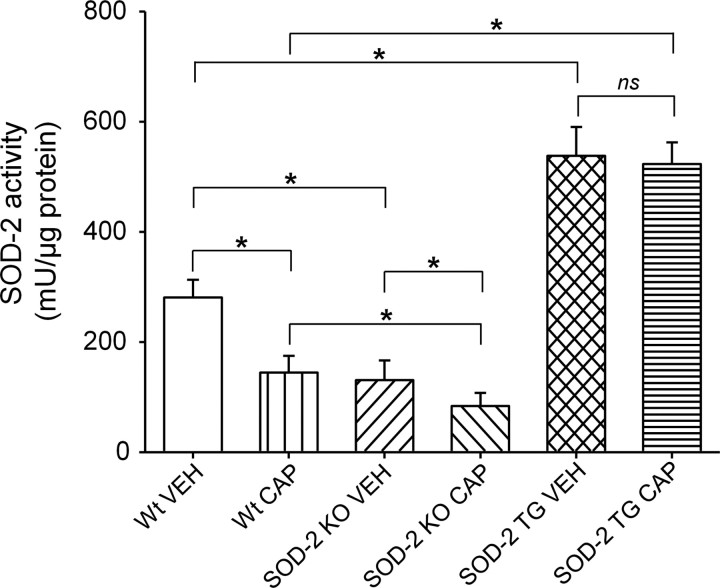
In SOD-2 KO mice, capsaicin injection produced significantly enhanced and prolonged (up to 48 h) secondary hyperalgesia compared with wild-type littermates (Fig. 4b). Primary hyperalgesia also was increased modestly (Fig. 4a). When SOD-2 KO mice were treated with TEMPOL, 1.5 h after capsaicin treatment, secondary hyperalgesia quickly subsided 30 min after TEMPOL treatment, returning to normal levels within 5 h after capsaicin treatment (Fig. 4d). These results suggest that reduced levels of SOD-2 in SOD-2 KO mice allowed increased superoxide accumulation and enhanced secondary hyperalgesia. To confirm whether the enhanced hyperalgesia is caused by reduced SOD-2 function in these mice, the levels of SOD-2 activity in the spinal cord were also measured after capsaicin treatment in SOD-2 KO mice. As shown in Figure 6, the basal endogenous levels of SOD-2 activity were significantly lower in SOD-2 KO mice (SOD-2 KO VEH) compared with their wild-type littermates (Wt VEH). One hour after capsaicin treatment, SOD-2 activity levels were further decreased in SOD-2 KO mice (SOD-2 KO CAP) and, thus, significantly lower than that of capsaicin-treated wild-type littermates (Wt CAP) or of vehicle-treated SOD-2 KO mice (SOD-2 KO VEH).
Figure 6.
SOD2 activity levels in the L4/5 spinal cord in SOD-2 KO and SOD-2 TG mice with and without intradermal capsaicin treatment. The endogenous SOD2 activity levels are significantly lower in SOD-2 KO mice (SOD-2 KOVEH) and significantly higher in SOD-2 TG mice (SOD-2 TGVEH) compared with wild type (Wt-VEH). Capsaicin treatment (CAP-1 h), SOD2 activity levels significantly decreased in Wt and SOD-2 KO mice but not in SOD-2 TG mice. *Values are significantly different between two linked groups at p ≤ 0.05 (n = 12) by Bonferroni's post hoc test after one-way repeated ANOVA. ns, Nonsignificant.
In SOD-2 TG mice, the levels of capsaicin-induced primary and secondary hyperalgesia were significantly reduced, especially the secondary hyperalgesia, compared with that of wild-type littermates (Fig. 5). Furthermore, the basal levels of SOD-2 activity were approximately twice in SOD-2 TG mice (SOD-2 TG VEH) compared with that of wild type (Wt VEH), and their levels did not decrease significantly after capsaicin treatment (Fig. 6). These results suggest that high levels of SOD-2 activity in SOD-2 TG mice do not allow superoxide accumulation beyond normal levels even after capsaicin treatment, and thus prevent the development of secondary hyperalgesia. These results are consistent with the notion that manipulating endogenous SOD-2 levels can either enhance or reduce superoxide accumulation in the spinal cord, which in turn will either increase or decrease central sensitization, and thus influence the levels of hyperalgesia.
Mitochondrial superoxide accumulation in response to capsaicin treatment is correlated to the levels of SOD-2 activity
Previous data indicate that superoxide accumulation in the spinal cord is correlated to the levels of endogenous SOD-2 activity. To determine whether mitochondrial superoxide levels in the spinal dorsal horn were modified by SOD-2 activity levels, the number of dorsal horn neurons showing increased superoxide was examined by using redox sensitive dye MitoSox. The reduced form of colorless MitoSox was injected intrathecally ∼24 h before sacrificing to allow dye to penetrate into the spinal cord and to sequester in the mitochondria. One day later, mice were treated with either capsaicin or vehicle and then killed by perfusion fixation 1 h after capsaicin treatment. This study was done in SOD-2 TG and SOD-2 KO mice and their wild-type littermates with and without capsaicin treatment, and the results were compared among the different groups.
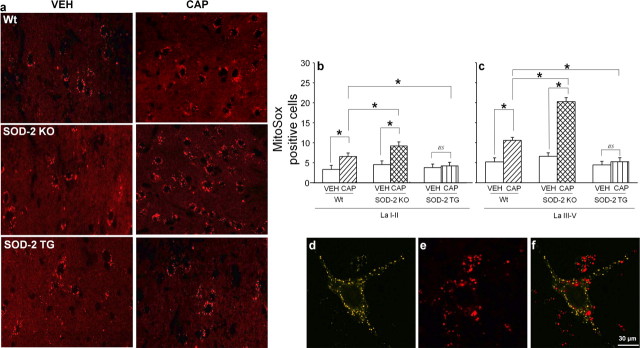
When the spinal cord sections were examined, oxidized MitoSox labeling was detected as red fluorescent granules scattered in the cytoplasm of cells (Fig. 7a), confirming our previous findings (Schwartz et al., 2008). From a glance, it was clear that the number of MitoSox-positive cells varied greatly among different tissues (Fig. 7a). Thus, quantification was done for the number of MitoSox-positive cells in the superficial (laminas I-II) and deep (laminas III-V) layers of the spinal dorsal horn. The results of these studies are shown in Figure 7, b and c. In addition, we also examined that the number of MitoSox-positive STT neurons (Fig. 7d–f), representing ascending pain projection neurons. MitoSox-positive STT neurons were increased significantly in capsaicin-treated mice (76% of total STT neurons) compared with that of vehicle-treated control group (52%).
Figure 7.
a, MitoSox-labeled L5 spinal dorsal horn (lamina V) sections in various groups. MitoSox labeling is identified as red fluorescent granules in the cytoplasm of cellular profiles. The round/oval-shaped black space, which is enclosed by red granules, represents the nucleus. The numbers of MitoSox-positive cells are increased after capsaicin treatment in both Wt and SOD-2 KO mice but not in SOD-2 TG mice compared with that of vehicle-treated mice. In addition, the numbers of MitoSox-labeled cells are more numerous in capsaicin-treated SOD-2 KO mice compared with capsaicin-treated Wt mice. The number of MitoSox-positive cells were counted in the superficial (laminas I-II) and deep (laminas III-V) dorsal horn of various groups of mice (n = 7/group), and the results are shown in b and c. MitoSox-positive cells are very small in VEH mice in all three groups (Wt, SOD-2 KO, and SOD-2 TG). After CAP treatment, the number of MitoSox-positive cells increases significantly in Wt and SODKO mice but not in SODTG mice. This increase is far greater in SODKO+/− mice compared with Wt mice. *Values are significantly different between two linked groups at p ≤ 0.05 by Bonferroni's post hoc test after one-way repeated ANOVA. d–f, MitoSox and fluorogold double-labeled ROS producing STT neuron. The STT cell identified by fluorogold label (d) also shows MitoSox label (e) as merged in f. The data show that some STT cells show enhanced ROS production in response to intradermal capsaicin. ns, Nonsignificant.
Intradermal capsaicin (CAP) induced a significant increase in the number of MitoSox-positive cells in the spinal dorsal horn compared with that of vehicle treatment (VEH). In SOD-2 KO mice, the number of MitoSox-positive cells is slightly higher, but not significantly different, than wild-type mice with vehicle treatment. However, the number of MitoSox-positive cells was increased 2–3-fold after capsaicin treatment (SOD-2 KO CAP) compared with that of vehicle treatment (SOD-2 KO VEH). This increase in SOD-2 KO mice is significantly higher than that in wild-type mice with capsaicin treatment. In SOD-2 TG mice, there was no difference in the number of MitoSox-positive cells after capsaicin treatment as well as basal levels compared with that of vehicle-treated wild-type littermates. The results confirmed that the levels of endogenous SOD-2 determine the amount of superoxide accumulated by the mitochondria, which in turn induce central sensitization and secondary hyperalgesia.
Discussion
Our previous study has shown that increased levels of spinal ROS are critical for the maintenance of capsaicin-induced secondary hyperalgesia (Schwartz et al., 2008), suggesting ROS involvement in central sensitization. The present study extends this work by showing that mitochondrial superoxide is one of the major sources of elevated spinal, but most likely not peripheral, ROS in capsaicin-induced hyperalgesia, and inactivation of SOD-2 by nitration contributes toward maintaining increased levels of mitochondrial superoxide. The fact that the level of capsaicin-induced secondary hyperalgesia is correlated with the level of SOD-2, further supports the idea that mitochondrial superoxide controls the level of central sensitization. Increased and decreased number of MitoSox-positive dorsal horn neurons, correlated with changes in capsaicin-induced hyperalgesia in SOD-2 KO and SOD-2 TG mice, is consistent with the hypothesis that decreased mitochondrial SOD-2 activity level significantly contributes to spinal superoxide increase and thus hyperalgesia.
Importance of SOD-2 and superoxide in a rat inflammatory pain model has been proposed by others (Muscoli et al., 2004; Wang et al., 2004), who suggested that SOD-2 in the spinal cord and superoxides in both the spinal cord and the peripheral tissue are important in the carrageenan-induced inflammatory thermal hyperalgesia in the rat. The present study extends this earlier proposal by showing that (1) spinal SOD-2 are important in capsaicin-induced hyperalgesia in mice; (2) determination of hyperalgesia by the levels of endogenous SOD-2 is clearly demonstrated in mice in which SOD-2 levels are manipulated genetically; (3) imaging data provide a direct evidence of decreased/increased accumulation of spinal superoxides because of increased/decreased levels of SOD-2 in relation to capsaicin-induced hyperalgesia in mice; and (4) the spinal cord seems to be the major action site of superoxides and SOD-2 in capsaicin-induced hyperalgesia, suggesting the role of superoxides and SOD-2 for the central sensitization process.
To demonstrate that elevation of superoxide levels in the spinal cord is a key for the development and maintenance of capsaicin-induced secondary hyperalgesia, we show that dismutation of superoxide by an exogenously supplied SOD mimetic, MnTBAP, reduces the secondary hyperalgesia, but not the primary hyperalgesia, in capsaicin-treated mice. Thus, the data suggest the main function of MnTBAP is the reduction of central sensitization by removing superoxide from the spinal cord. In contrast, the capsaicin-induced secondary hyperalgesia is significantly enhanced by the SOD inhibitor DETC. This result further supports the hypothesis that SOD determines the levels of superoxide in the spinal cord, which in turn causes central sensitization and thus secondary hyperalgesia. Thus, it is clear that pain modulation by a SOD mimetic or a SOD inhibitor is mainly achieved through the reduction or increase of superoxide in the spinal cord, respectively, in capsaicin-induced hyperalgesia. However, the possibility of ROS-induced sensitization of supraspinal regions, which in turn influence spinal sensitization, cannot be excluded because an intracerebroventricular injection of ROS scavengers produces a small but significant antihyperalgesic effect in capsaicin-induced secondary hyperalgesia (Lee et al., 2007).
Whereas the present study suggests that the spinal cord appears to be the main site of action of a SOD mimetic in capsaicin-induced hyperalgesia, another study has shown that the SOD mimetic, M40403, reduces carrageenan-induced inflammation and hyperalgesia through both peripheral and central mechanisms (Wang et al., 2004). We are not clear why these two studies show different results. One reason for the difference in models could be that the peripheral inflammatory factor is relatively strong as in carrageen-induced hyperalgesia (Wang et al., 2004), whereas it is small in acute capsaicin-induced hyperalgesia as shown in this study.
Another important point related to the MnTBAP and DETC study is that both drugs are nonspecific in terms of their subcellular action sites; thus, they will change superoxide levels not only in the mitochondria but also in the cytoplasm and extracellular space. This issue was further probed in this study by using genetically manipulated SOD-2 KO and SOD-2 TG mice to explore the role of mitochondrial SOD. One other issue on the use of MnTBAP is that it can also scavenge peroxynitrite (Hachmeister et al., 2006) in addition to superoxide; thus, it is possible that the reduction of secondary hyperalgesia by MnTBAP may be caused by the removal of peroxinitrite. Future studies are warranted by using more specific SOD mimetics when they become available.
The present study also shows that SOD-2 activity levels are altered in the spinal cord after intradermal capsaicin injection without reducing the total SOD-2 protein levels, thus suggesting that inactivation of SOD-2 is important rather than changes in protein levels. Nitration of SOD is one of the modes by which SOD is inactivated (Yamakura et al., 1998; Macmillan-Crow and Cruthirds, 2001; Echtay et al., 2002; Muscoli et al., 2004; Wang et al., 2004; Demicheli et al., 2007). SOD nitration can be reversed by removal of either superoxide or nitric oxide, inhibiting the formation of peroxynitrite and peroxynitrite-mediated protein nitration (Akizuki et al., 2000; Salvemini et al., 2001; Fries et al., 2003). In line with this idea, a previous study has shown that intraplantar injection of carrageenan induces thermal hyperalgesia (Salvemini et al., 1999; Wang et al., 2004) and increases nitration of spinal SOD-2 (Wang et al., 2004), and a SOD mimetic, M40403, reverses these effects (Salvemini et al., 1999; Wang et al., 2004). Increased spinal SOD-2 nitration is also found after intrathecal injection of NMDA, and this effect is reversed by a systemic administration of a SOD mimetic (Muscoli et al. 2004). The present study also confirms that both increased SOD-2 nitration and decreased SOD-2 activity in the spinal cord coincide with the capsaicin-induced secondary mechanical hyperalgesia. Thus, our data are in agreement with previous studies in which SOD-2 nitration in the cord is critical for hyperalgesia. The reversal of hyperalgesia and SOD-2 activity levels by a SOD mimetic is in agreement with the known reversal process of nitrated SOD-2 by removing superoxide from the spinal cord.
Previous studies (Muscoli et al. 2004; Wang et al. 2004) have suggested that mitochondrial SOD, SOD-2, is critical for hyperalgesia. This point is further illustrated by using genetically manipulated SOD-2 KO and SOD-2 TG mice in the present study. In SOD-2 KO mice, the SOD-2 protein and activity levels are significantly lower and the magnitude of capsaicin-induced secondary hyperalgesia is significantly greater compared with that of their wild-type littermates. The effects are opposite in SOD-2 TG mice, in which the level of SOD-2 activity is high in the spinal cord, and the capsaicin-induced secondary hyperalgesia is almost nonexistence. Thus, the data indicate that mitochondrial superoxide accumulation, resulting from the insufficiency of mitochondrial antioxidant SOD-2, is critical for hyperalgesia. It is important to note that in SOD-2 TG and SOD-2 KO mice, levels of primary hyperalgesia were also modestly affected. This suggests that SOD-2 manipulations change to some extent the physiological properties of the primary afferents that contribute to primary hyperalgesia. Furthermore, increased and decreased numbers of MitoSox-positive dorsal horn neurons in response to intradermal capsaicin in these SOD-2 KO and SOD-2 TG mice, respectively, confirm that mitochondrial superoxide generation is the major driver of ROS elevation in the spinal cord.
Although it is evident that elevated superoxide levels in the mitochondria of dorsal horn neurons are critical for secondary capsaicin-induced hyperalgesia, the exact mechanism of neuronal sensitization by mitochondrial superoxide is not known. Possibilities include deregulation of membrane potentials because of lipid peroxidation (Wang and Thayer, 2002; Choi et al., 2004) or modification of cellular signal transduction pathways (Sluka et al., 1997a; Palecek et al., 2002). Considering that hyperalgesia and SOD-2 nitration is reversible within 30 min after treatment with a SOD mimetic, it is likely that superoxide is involved in the modification of reversible cellular signaling process rather than permanent damage. It has been shown that central sensitization involves activation of ligand-gated ion channels, the substance-P receptor neurokinin-1 (NK1)-R (Sluka et al., 1997b; Palecek et al., 2002), metabotropic glutamate receptors (mGLUR) (Adwanikar et al., 2004; Park et al., 2006), and tyrosine kinase receptors (trkB and Eph) (Ren and Dubner 2007). Furthermore, ROS are considered to be small messenger molecules that modify cellular signaling pathways (Kamata and Hirata 1999; Maher and Schubert 2000). Thus, it is likely that an elevated level of ROS leads the redox status of dorsal horn neurons toward oxidation and, thus, changes the signal transduction pathways toward sensitization. In addition, functional loss of glutamate transporters and glutamine synthase (Trotti et al., 1996, 1999; Görg et al., 2005) can lead to increased glutamate levels in the synaptic cleft and overstimulation of the NMDA receptor. Thus, the functional loss of transporters and enzymes in the presence of excess ROS may also contribute toward central sensitization. Future studies are thus warranted to explore detailed redox-sensitive signal transduction pathways in the dorsal horn of the spinal cord.
In conclusion, our findings suggest that superoxide is particularly important in the sensory processing in the spinal cord in capsaicin-induced secondary hyperalgesia. Our studies also support that SOD-2 nitration is a critical mechanism that maintains elevated superoxide levels in the spinal cord after capsaicin treatment. Finally, our findings suggest a therapeutic potential for the manipulation of spinal SOD-2 activity in pain conditions.
Footnotes
This work was supported by National Institutes of Health Grants R01 NS31680 and P01 NS11255.
The authors declare no competing financial interests.
References
- Adwanikar H, Karim F, Gereau RW., 4th Inflammation persistently enhances nocifensive behaviors mediated by spinal group I mGluRs through sustained ERK activation. Pain. 2004;111:125–135. doi: 10.1016/j.pain.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Akizuki E, Akaike T, Okamoto S, Fujii S, Yamaguchi Y, Ogawa M, Maeda H. Role of nitric oxide and superoxide in acute cardiac allograft rejection in rats. Proc Soc Exp Biol Med. 2000;225:151–159. doi: 10.1046/j.1525-1373.2000.22519.x. [DOI] [PubMed] [Google Scholar]
- Choi J, Forster MJ, McDonald SR, Weintraub ST, Carroll CA, Gracy RW. Proteomic identification of specific oxidized proteins in ApoE-knockout mice: Relevance to Alzheimer's disease. Free Radic Biol Med. 2004;36:1155–1162. doi: 10.1016/j.freeradbiomed.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Cízková D, Lukácová N, Marsala M, Marsala J. Neuropathic pain is associated with alterations of nitric oxide synthase immunoreactivity and catalytic activity in dorsal root ganglia and spinal dorsal horn. Brain Res Bull. 2002;58:161–171. doi: 10.1016/s0361-9230(02)00761-x. [DOI] [PubMed] [Google Scholar]
- Coderre TJ, Xanthos DN, Francis L, Bennett GJ. Chronic post-ischemia pain (CPIP): a novel animal model of complex regional pain syndrome-Type I (CRPS-I; reflex sympathetic dystrophy) produced by prolonged hind paw ischemia and reperfusion in the rat. Pain. 2004;112:94–105. doi: 10.1016/j.pain.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Demicheli V, Quijano C, Alvarez B, Radi R. Inactivation and nitration of human superoxide dismutase (SOD) by fluxes of nitric oxide and superoxide. Free Radic Biol Med. 2007;42:1359–1368. doi: 10.1016/j.freeradbiomed.2007.01.034. [DOI] [PubMed] [Google Scholar]
- Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, Stuart JA, Harper JA, Roebuck SJ, Morrison A, Pickering S, Clapham JC, Brand MD. Superoxide activates mitochondrial uncoupling proteins. Nature. 2002;415:96–99. doi: 10.1038/415096a. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- Fries DM, Paxinou E, Themistocleous M, Swanberg E, Griendling KK, Salvemini D, Slot JW, Heijnen HF, Hazen SL, Ischiropoulos H. Expression of inducible nitric-oxide synthase and intracellular protein tyrosine nitration in vascular smooth muscle cells: role of reactive oxygen species. J Biol Chem. 2003;278:22901–22907. doi: 10.1074/jbc.M210806200. [DOI] [PubMed] [Google Scholar]
- Görg B, Wettstein M, Metzger S, Schliess F, Häussinger D. Lipopolysaccharide-induced tyrosine nitration and inactivation of hepatic glutamine synthetase in the rat. Hepatology. 2005;41:1065–1073. doi: 10.1002/hep.20662. [DOI] [PubMed] [Google Scholar]
- Hachmeister JE, Valluru L, Bao F, Liu D. Mn (III) tetrakis (4-benzoic acid) porphyrin administered into the intrathecal space reduces oxidative damage and neuron death after spinal cord injury: a comparison with methylprednisolone. J Neurotrauma. 2006;23:1766–1778. doi: 10.1089/neu.2006.23.1766. [DOI] [PubMed] [Google Scholar]
- Haley JE, Dickenson AH, Schachter M. Electrophysiological evidence for a role of nitric oxide in prolonged chemical nociception in the rat. Neuropharmacology. 1992;31:251–258. doi: 10.1016/0028-3908(92)90175-o. [DOI] [PubMed] [Google Scholar]
- Ho YS, Vincent R, Dey MS, Slot JW, Crapo JD. Transgenic models for the study of lung antioxidant defense: enhanced manganese-containing superoxide dismutase activity gives partial protection to B6C3 hybrid mice exposed to hyperoxia. Am J Respir Cell Mol Biol. 1998;18:538–547. doi: 10.1165/ajrcmb.18.4.2959. [DOI] [PubMed] [Google Scholar]
- Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- Jenner P. Oxidative damage in neurodegenerative disease. Lancet. 1994;344:796–798. doi: 10.1016/s0140-6736(94)92347-7. [DOI] [PubMed] [Google Scholar]
- Kamata H, Hirata H. Redox regulation of cellular signalling. Cell Signal. 1999;11:1–14. doi: 10.1016/s0898-6568(98)00037-0. [DOI] [PubMed] [Google Scholar]
- Khalil Z, Khodr B. A role for free radicals and nitric oxide in delayed recovery in aged rats with chronic constriction nerve injury. Free Radic Biol Med. 2001;31:430–439. doi: 10.1016/s0891-5849(01)00597-4. [DOI] [PubMed] [Google Scholar]
- Khalil Z, Liu T, Helme RD. Free radicals contribute to the reduction in peripheral vascular responses and the maintenance of thermal hyperalgesia in rats with chronic constriction injury. Pain. 1999;79:31–37. doi: 10.1016/S0304-3959(98)00143-2. [DOI] [PubMed] [Google Scholar]
- Kim HK, Park SK, Zhou JL, Taglialatela G, Chung K, Coggeshall RE, Chung JM. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain. 2004;111:116–124. doi: 10.1016/j.pain.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Lee I, Kim HK, Kim JH, Chung K, Chung JM. The role of reactive oxygen species in capsaicin-induced mechanical hyperalgesia and in the activities of dorsal horn neurons. Pain. 2007;133:9–17. doi: 10.1016/j.pain.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy MA, Tsai YH, Reaume A, Bray TM. Cellular response of antioxidant metalloproteins in Cu/Zn SOD transgenic mice exposed to hyperoxia. Am J Physiol Lung Cell Mol Physiol. 2001;281:172–182. doi: 10.1152/ajplung.2001.281.1.L172. [DOI] [PubMed] [Google Scholar]
- Macmillan-Crow LA, Cruthirds DL. Invited review: manganese superoxide dismutase in disease. Free Radic Res. 2001;34:325–336. doi: 10.1080/10715760100300281. [DOI] [PubMed] [Google Scholar]
- Maher P, Schubert D. Signaling by reactive oxygen species in the nervous system. Cell Mol Life Sci. 2000;57:1287–1305. doi: 10.1007/PL00000766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscoli C, Mollace V, Wheatley J, Masini E, Ndengele M, Wang ZQ, Salvemini D. Superoxide-mediated nitration of spinal manganese superoxide dismutase: a novel pathway in N-methyl-D-aspartate-mediated hyperalgesia. Pain. 2004;111:96–103. doi: 10.1016/j.pain.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Palecek J, Paleckova V, Willis WD. The roles of pathways in the spinal cord lateral and dorsal funiculi in signaling nociceptive somatic and visceral stimuli in rats. Pain. 2002;96:297–307. doi: 10.1016/S0304-3959(01)00459-6. [DOI] [PubMed] [Google Scholar]
- Park ES, Gao X, Chung JM, Chung K. Levels of mitochondrial reactive oxygen species increase in rat neuropathic spinal dorsal horn neurons. Neurosci Lett. 2006;391:108–111. doi: 10.1016/j.neulet.2005.08.055. [DOI] [PubMed] [Google Scholar]
- Peskin AV, Winterbourn CC. A microtiter plate assay for superoxide dismutase using a water-soluble tetrazolium salt (WST-1) Clin Chim Acta. 2000;293:157–166. doi: 10.1016/s0009-8981(99)00246-6. [DOI] [PubMed] [Google Scholar]
- Ren K, Dubner R. Pain facilitation and activity-dependent plasticity in pain modulatory circuitry: role of BDNF-TrkB signaling and NMDA receptors. Mol Neurobiol. 2007;35:224–235. doi: 10.1007/s12035-007-0028-8. [DOI] [PubMed] [Google Scholar]
- Salvemini D, Wang ZQ, Zweier JL, Samouilov A, Macarthur H, Misko TP, Currie MG, Cuzzocrea S, Sikorski JA, Riley DP. A nonpeptidyl mimic of superoxide dismutase with therapeutic activity in rats. Science. 1999;286:304–306. doi: 10.1126/science.286.5438.304. [DOI] [PubMed] [Google Scholar]
- Salvemini D, Mazzon E, Dugo L, Riley DP, Serraino I, Caputi AP, Cuzzocrea S. Pharmacological manipulation of the inflammatory cascade by the superoxide dismutase mimetic, M40403. Br J Pharmacol. 2001;132:815–827. doi: 10.1038/sj.bjp.0703841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz ES, Lee I, Chung K, Chung JM. Oxidative stress in the spinal cord is an important contributor in capsaicin-induced mechanical secondary hyperalgesia in mice. Pain. 2008;138:514–524. doi: 10.1016/j.pain.2008.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluka KA, Rees H, Chen PS, Tsuruoka M, Willis WD. Inhibitors of G-proteins and protein kinases reduce the sensitization to mechanical stimulation and the desensitization to heat of spinothalamic tract neurons induced by intradermal injection of capsaicin in the primate. Exp Brain Res. 1997a;115:15–24. doi: 10.1007/pl00005675. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Milton MA, Willis WD, Westlund KN. Differential roles of neurokinin 1 and neurokinin 2 receptors in the development and maintenance of heat hyperalgesia induced by acute inflammation. Br J Pharmacol. 1997b;120:1263–1273. doi: 10.1038/sj.bjp.0701044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal M. A novel antioxidant alleviates heat hyperalgesia in rats with an experimental painful peripheral neuropathy. Neuroreport. 1996;7:1382–1384. doi: 10.1097/00001756-199605310-00010. [DOI] [PubMed] [Google Scholar]
- Torebjörk HE, Lundberg LE, LaMotte RH. Central changes in processing of mechanoreceptive input in capsaicin-induced secondary hyperalgesia in humans. J Physiol. 1992;448:765–780. doi: 10.1113/jphysiol.1992.sp019069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotti D, Rossi D, Gjesdal O, Levy LM, Racagni G, Danbolt NC, Volterra A. Peroxynitrite inhibits glutamate transporter subtypes. J Biol Chem. 1996;271:5976–5979. doi: 10.1074/jbc.271.11.5976. [DOI] [PubMed] [Google Scholar]
- Trotti D, Rolfs A, Danbolt NC, Brown RH, Jr, Hediger MA. SOD1 mutants linked to amyotrophic lateral sclerosis selectively inactivate a glial glutamate transporter. 1999;2:848. doi: 10.1038/12227. [DOI] [PubMed] [Google Scholar]
- Twining CM, Sloane EM, Milligan ED, Chacur M, Martin D, Poole S, Marsh H, Maier SF, Watkins LR. Peri-sciatic proinflammatory cytokines, reactive oxygen species, and complement induce mirror-image neuropathic pain in rats. Pain. 2004;110:299–309. doi: 10.1016/j.pain.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Thayer SA. NMDA-induced calcium loads recycle across the mitochondrial inner membrane of hippocampal neurons in culture. J Neurophysiol. 2002;87:740–749. doi: 10.1152/jn.00345.2001. [DOI] [PubMed] [Google Scholar]
- Wang X, Culotta VC, Klee CB. Superoxide dismutase protects calcineurin from inactivation. Nature. 1996;383:434–437. doi: 10.1038/383434a0. [DOI] [PubMed] [Google Scholar]
- Wang ZQ, Porreca F, Cuzzocrea S, Galen K, Lightfoot R, Masini E, Muscoli C, Mollace V, Ndengele M, Ischiropoulos H, Salvemini D. A newly identified role for superoxide in inflammatory pain. J Pharmacol Exp Ther. 2004;309:869–878. doi: 10.1124/jpet.103.064154. [DOI] [PubMed] [Google Scholar]
- Willis WD. Role of neurotransmitters in sensitization of pain responses. Ann N Y Acad Sci. 2001;933:142–156. doi: 10.1111/j.1749-6632.2001.tb05821.x. [DOI] [PubMed] [Google Scholar]
- Yamakura F, Taka H, Fujimura T, Murayama K. Inactivation of human manganese-superoxide dismutase by peroxynitrite is caused by exclusive nitration of tyrosine 34 to 3-nitrotyrosine. J Biol Chem. 1998;273:14085–14089. doi: 10.1074/jbc.273.23.14085. [DOI] [PubMed] [Google Scholar]