Abstract
Purpose
Immunotherapeutic modalities are commonly used for treatment of patients with melanoma. The therapeutic success in pre-clinical models has not yielded the expected clinical results. To understand this discrepancy, we attempted to define immune homeostasis of 209 patients with melanoma across stages of disease relative to normal controls.
Patients and Methods
PBMC and plasma were collected from patients and healthy donors. PBMC were analyzed for frequencies of natural killer, dendritic, and T-cells, and their functional status. Matched plasma samples were analyzed for the concentrations of 27 cytokines, chemokines, and growth factors. RNA was isolated from 24 metastatic melanoma tumor biopsies and profiled by microarray analysis.
Results
The frequency of NK, T-cells, and DC in patients does not significantly change across stages of melanoma. However, plasma concentrations of Th2 cytokines (IL-4, IL-5, IL-10 and IL-13) in tumor bearing patients were significantly higher than those with resected melanoma. Expression array analysis of metastatic melanoma revealed that the malignant melanocytes were not the source of the Th2 cytokines, but did highly up-regulate VEGF transcripts, consistent with plasma VEGF concentrations. In vitro VEGF exposure of normal PBMC lead to re-polarization from Th1 to Th2 emulating the state of metastatic melanoma.
Conclusions
Patients with metastatic melanoma exist in a state of Th2 mediated “chronic inflammation” as a result of at least VEGF overproduction by malignant tumors. These data support prior observations regarding the impact of VEGF on immune cell function and suggests consideration of VEGF inhibitors in future cancer immunotherapy clinical studies in metastatic melanoma.
Keywords: Melanoma, Inflammation, Cytokines
INTRODUCTION
Immunotherapeutic approaches for the treatment of melanoma have been successfully tested in preclinical models (1). Similar therapeutic success in clinical testing remain unrealized (2). In particular, this has been the experience with melanoma cancer vaccines. Although immunization with tumor specific vaccines can frequently induce a measurable cytotoxic T lymphocyte (CTL) response in patients with metastatic melanoma (increased frequencies of tumor antigen specific CTL determined by tetramer or ELISPOT assay) clinical benefit remains anecdotal (3). This has not only been the experience of peptide based melanoma vaccines, but, other vaccination strategies as well: (a) tumor cell lysates (4); (b) irradiated whole cell tumor vaccines, (5); and (c) dendritic cell (DC) based vaccines (6,7). The increasingly accepted reason for the lack of therapeutic success of these interventions has been the demonstration of the immunosuppressive properties of the tumor micro-environment (8). In this model, fully functional, vaccine induced, anti-tumor immune cells lose their tumoricidal capacity upon entry into the tumor micro-environment thereby yielding tumor progression. Thus, most of the recent clinical efforts have focused on overcoming this immunosuppressive barrier by one of the following approaches: (a) generating greater numbers of tumor-specific CTL using in vitro expansion strategies (9,10); (b) non-specifically activating all endogenous CTL (anti-CTLA4) thereby expanding naturally developed tumor-specific CTL (11); or (c) blocking immunosuppressive signals at the tumor site by depletion of regulatory T (Treg) cells (12). These efforts are ongoing and have demonstrated anecdotal clinical success (13).
Faced with the discrepancy between effective anti-tumor immunization described with cancer vaccines (increased numbers of peripheral blood tumor specific CTL following cancer vaccination) and the associated lack of clinical benefit, we hypothesized that the quality of the vaccine generated anti-tumor immune response in patients with advanced cancer may not be adequate despite seemingly appropriate quantity; and that this may be a function not only of the therapy (poor cancer vaccine immunogenicity) but also of the patient’s immune system’s ability to respond to the vaccine i.e. the state of immune homeostasis. As most therapies in these clinical studies were performed in patients with advanced (metastatic) melanoma, we hypothesized that the increasingly recognized immunosuppressive properties of the tumor microenvironment may be affecting (“spilling over”) global immune homeostasis thereby hindering systemic immune responsiveness to cancer vaccination. Several groups have already suggested the existence of abnormalities in systemic immune competence (homeostasis) in patients with metastatic melanoma: (a) exhausted phenotype of tumor specific CTL(14); (b) circulating myeloid derived suppressor cells (15); (c) increased frequency of circulating Treg and Th2 in patients with advanced cancer versus normal controls (16,17); and (d) diminished capacity of circulating DC to present antigens (18). This would suggest that the barriers to the therapeutic success of cancer vaccines (or other immunotherapeutic strategies that rely on endogenous immune cells to achieve a therapeutic goal) may not be limited to the tumor microenvironment, but, also might include systemic immune dysfunction. In order to address this issue we systematically analyzed a broad range of parameters of immune homeostasis in 209 patients across all clinical stages of melanoma, patients with atypical/dysplastic nevi and normal volunteers. To define immune homeostasis in these patients we compared the following immune parameters: (1) peripheral blood frequencies of immune cell subsets and their functional status; (2) frequencies and functional status of tumor and recall antigen (e.g. CMV) specific CTL, (3) plasma concentrations of 27 cytokines, chemokines and growth factors in matched samples, and (4) microarray analysis of RNA isolated from matched tumor biopsies of metastatic melanoma. Herein, we describe the results of these comparisons.
PATIENTS AND METHODS
Patient Population
Blood samples collected from patients with early stage melanoma (melanoma in situ and melanoma stage I, II and III) and benign nevi (atypical/dysplastic nevi) were newly diagnosed patients with no previous treatment. All patients were tumor free at the time of peripheral blood collection. Samples from patients with metastatic melanoma (newly diagnosed, previously untreated) as well as healthy volunteers/controls were collected under a separate melanoma blood and tissue banking protocol. Both protocols were reviewed and approved by the Mayo Clinic Institutional Review Board for use in these studies. All biospecimens were collected, processed and stored in uniform fashion following established standard operating procedures in our laboratory. All patients signed an informed consent document approved by the Institutional Review Board at Mayo Clinic. Patient samples were collected between 2000 and 2007. The presented study describes data obtained from 113 men and 96 women ranging in age from 21 to 85 (Table 1).
Table 1.
Study patient population distributed by clinical category, age, sex and assayed immune parameters.
| Clinical category | Total Patients | Age mean±SD (range) | % female | Assayed immune parameters | ||||
|---|---|---|---|---|---|---|---|---|
| Cell Subset | Plasma Cytokines | Tetramer | T-cell Function Assay | RNA array | ||||
| Benign Nevi | 34 | 51±12 (21–71) | 68 | 26 | 34 | 7 | 2 | 0 |
| Atypical/Dysplastic | 25 | 52±16 (25–84) | 44 | 22 | 16 | 11 | 1 | 0 |
| In situ melanoma | 36 | 61±16 (26–84) | 36 | 30 | 35 | 16 | 3 | 0 |
| Stage I | 45 | 54±17 (21–82) | 44 | 36 | 44 | 16 | 4 | 0 |
| Stage II | 16 | 55±17 (22–81) | 44 | 11 | 12 | 9 | 0 | 0 |
| Stage III | 16 | 53±19 (23–83) | 44 | 14 | 16 | 6 | 1 | 0 |
| Stage IV | 37 | 56±14 (28–85) | 43 | 32 | 30 | 27 | 16 | 24 |
Collection of plasma and peripheral blood mononuclear cells
Peripheral venous blood (50–90mL) was drawn into heparinized Vacutainer tubes that were processed and separated into plasma and peripheral blood mononuclear cells (PBMC) following gradient centrifugation using Ficoll-Paque (GE Healthcare Uppsala, Sweden). Plasma was collected and immediately frozen at −80°C (1mL aliquots). PBMC were collected, washed in phosphate buffered saline (PBS), counted, diluted to 1 ×107/mL and viably frozen in 90% cosmic calf serum (Hyclone Inc. Logan, UT) and 10% DMSO (Sigma St. Louis, MO). All assays were batch-analyzed at the end of the study.
Immunophenotyping
The following anti-human monoclonal antibodies were used in PBMC immunophenotyping for flow cytometry: anti-CD3-APC, FITC and PE, anti-CD4-FITC, anti-CD8-PE, anti-CD16 PE, anti-CD56 PE, anti-CD62L APC, anti-CD69 FITC, anti-CD14 FITC, anti-CD16 FITC, anti-CD19 FITC, anti-CD11c APC, anti-CD80 PE, anti-CD83 PE, anti- CD86 PE, anti-CD40 APC, anti-HLA-DR PC5, anti-PD-1 (BD Pharmingen San Jose, CA). The human monoclonal antibodies anti-CD4 PC5 and anti-CD25 PE were purchased from Biolegend (San Diego, CA) and used in conjunction with anti-human FoxP3 for the enumeration of Treg cells. The following anti-human monoclonal antibodies were used for intracellular staining for flow cytometry: anti-IFNγ FITC, anti-IL-13 PE, anti-IL-4 PE (R and D Systems Minneapolis, MN) and anti-FoxP3 Alexaflour 488 (Biolegend San Diego, CA).
Previously frozen PBMC (0.5 – 1.0 × 106 cells/mL) were thawed and aliquoted into 96 well rounded bottom plates (100μL/well). The desired antibody or antibody pool was added at 5μL/well. The cells and antibodies were incubated for 30 minutes at 4°C and washed twice with 1x PBS (Cellgro Manassas, VA), 0.1% BSA and 0.05% sodium azide (Sigma St. Louis, MO). Four-color flow cytometry was performed on a LSRII flow cytometer (Becton Dickenson San Jose, CA) and Cellquest software (Becton Dickenson San Jose, CA) was utilized for data analysis. For dendritic cells, a gate was set on cells, which were HLA-DR+ and Lin− (CD3, CD14, CD16 and CD19). From this population the percentage of cells, which were CD11c+ and positive for costimulatory molecules (CD80, CD83 and CD86) was determined, as previously described (19). A panel of tumor associated antigen tetramers, MART-126-35, gp100264-272, gp100209-217, and tyrosinase369-377 (Beckman Coulter San Jose, CA) were used to enumerate the frequency of tumor antigen specific CD8 positive T-cells. Recall antigens, EBV280-288 and CMV495-503 (Beckman Coulter San Jose, CA) were used as positive controls. For tetramer frequencies, a gate was set on lymphocytes, which were CD8+ and negative for CD4, CD14 and CD19. Three-color flow cytometry was performed on a LSRII flow cytometer (Becton Dickenson San Jose, CA) and Cellquest software (Becton Dickenson San Jose, CA) was utilized for data analysis.
Functional enumeration of tumor antigen specific CTL was performed using an artificial antigen presenting cell method (aAPC) as previously reported (20). Briefly, frozen PBMC were thawed, labeled with the desired tumor antigen peptide/class I tetramers (Beckman Coulter Fullerton, CA) and stimulated for 6 hours with streptavidin coated microbeads (Invitrogen Oslo, Norway) loaded with HLA-A2 class I containing tumor antigen peptides of choice (MART-1, gp100 or tyrosinase) and anti-human CD28 in the presence of brefeldin A (Sigma, St. Louis, MO). After stimulation, the cells were fixed with 2% paraformaldehyde (Sigma, St. Louis, MO) and then permeabilized with 0.1% saponin (Sigma, St. Louis, MO) in PBS. Cells were then immunophenotyped with anti-human CD4-PC5 or CD8-APC and intracellular staining was done with anti-human IFNγ FITC or IL-4 PE. Four-color flow cytometry was performed with a FACSCaliber and Cellquest software (Becton Dickenson San Jose, CA) was utilized for data analysis.
Plasma cytokine, chemokines and growth factor concentrations
Protein levels for 27 cytokines, chemokines and growth factors, including IL-1β, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12p70, IL-13, IL-15, IL-17, Eotaxin, FGF basic, G-CSF, GM-CSF, IFN-γ, IP-10, MCP-1, MIP-1α, MIP-1β, PDGF, RANTES, TNF-α, and VEGF, were measured using the Bio-plex cytokine assay (Bio-rad, Hercules, CA) as per manufacturer’s instructions. Patient plasma was diluted 1:4 in dilution buffer and 50μL was added to washed, fluorescently dyed microspheres (beads) to which biomolecules of interest are bound. The beads and diluted patient plasma were incubated for 30 minutes at room temperature with agitation. After the incubation the beads were washed in Bio-plex wash buffer and placed in 25μL of detection antibody and incubated for 30′ as described above. After washing, the beads were placed in streptavidin-PE, incubated and washed a final time. The bound beads were resuspended in 125μL Bio-plex assay buffer and read with the Luminex plate reader (Bio-rad, Hercules, CA). Protein concentrations were determined using a standard curve generated using the high PMT concentrations with sensitivity from 10–1000 pg/mL.
VEGF mediated Th1/Th2 polarity
To determine the effect of VEGF on Th1 and Th2 polarity, PBMC from healthy donors were stimulated for 3 days with CD3/CD28 expander beads (Invitrogen Oslo, Norway) with and without increasing doses of recombinant VEGF (1–16pg/mL). Cells were also cultured with 10μg/mL recombinant human IL-12 (R and D Systems, Minneapolis, MN) or 8μg/mL of a monoclonal anti-human IL-12 (R and D Systems Minneapolis, MN clone # 24910). After the culture, the cells were harvested and restimulated with 50ng/mL PMA (Sigma, St. Louis, MO) and 1μg/mL ionomycin (Sigma, St. Louis, MO) in the presence of 10μg/mL brefeldin A for 4 hours. The cells were then stained with anti-human CD4, anti-human IFN-γ and anti-human IL-4 flow cytometry (see above).
Tumor tissue RNA extraction and microarray
Frozen tissue sections of melanoma biopsies, were examined, regions of pure tumor with little/no evidence of necrosis or stromal infiltration were outlined, scraped off the slides and used for RNA extraction. Total RNA was isolated from the excised tumor tissue using the Qiagen RNA extraction kit (Qiagen Valencia, CA). The quality of the RNA was evaluated by obtaining electropherograms on Agilent 2100 Bioanalyzer and RNA integrity number (RIN) using 2100 Expert software (Agilent Technologies, Inc. Palo Alto, CA). cDNA was prepared from a total of 10 μg of RNA. Samples werequantified using standard spectrophotometry using a Tecan spectrophotometer (Tecan US, Research Triangle Park, NC) and considered acceptable if the A260/280 reading was >1.7. The purified cDNA was used as a template for in vitro transcription reaction for the synthesis of biotinylated cRNA using RNA transcript labeling reagent (Affymetrix, Santa Clara, CA). Labeled cRNA was then fragmented and hybridized onto the U133 Plus 2.0 array. Appropriate amounts of fragmented cRNA and control oligonucleotide B2 wereadded along with control cRNA (BioB, BioC, and BioD), herring sperm DNA, and bovine serum albumin to the hybridization buffer. The hybridization mixture was heated at 99°C for 5 min followed by incubation at 45°C for 5 min before injecting the sample into the microarray. Then, the hybridization was carried out at 45°C for 16 hours with mixing on a rotisserie at 60 rpm. After hybridization, the solutions were removed and the arrays were washed and then stained with streptavidin-phycoerythrin (Molecular Probes, Eugene, OR). After washes, arrays were scanned using the GeneChip Scanner 3000 (Affymetrix, Santa Clara, CA). The quality of the fragmented biotinlabeled cRNA in each experiment was evaluated before hybridizingonto the U133A expression array by both obtaining electropherograms on Agilent 2100 Bioanalyzer and hybridizing a fraction of the sample onto test-3 array as a measure of quality control. GeneSpring GX 7.3 (Agilent Technologies, Inc. Santa Clara, CA) data analysis software was used to analyze the results of the microarray experiment. Gene expression values were normalized by the GCRMA algorithm. (21)
Statistical Analysis
The majority of samples analyzed in this report were randomly assigned to batches for each laboratory assay due to the fact that all samples were not collected/processed at the same time. The randomization was stratified to assure an even distribution across the stages of disease for each batch. The distributions of the results of each run were examined and those that did not appear to be normally distributed were transformed using either logarithmic or square root transformations. In order to look at differences in various parameters between stages of disease, analysis was performed utilizing analysis of covariance (ANCOVA), adjusting for age, gender, and batch effects. Results of this analysis are summarized by least square means and 95% confidence intervals for each stage of disease. The p-values presented are those from the overall ANCOVA, which compares the means levels of each parameter across all stages of disease. P-values <0.05 were considered to be statistically significant. Due to the magnitude of the cytokine data from the multiplex assay the data was processed using Partek 6.3 software (Partek Inc. St. Louis MO) and analyzed using a principal component analysis (PCA) approach. We utilized PCA in an effort to vector space transform a multidimensional data set representing 27 variables for each individual patient and group patients based on similar cytokine concentrations revealing the internal relationships of cytokines within patient groups (e.g. per stage of melanoma) in an unbiased way.
RESULTS
Plasma cytokine concentrations across stages of melanoma
Frozen aliquots of plasma collected from patients with benign/atypical nevi as well as all stages of melanoma (in situ, stage I, stage II, stage III and stage IV) were analyzed for the concentration of 27 cytokines/chemokines/growth factors. The large volume of data was analyzed per patient cohort looking for data groupings within clinical categories (stage of disease) using PCA (Figure 1). The data suggested that the cytokine profiles across stages of disease differed significantly only in patients with stage IV melanoma versus all other cohorts. Closer analysis comparing each of the 27 cytokines/chemokines/growth factors between patients with stage IV melanoma and all others revealed that the greatest difference of plasma cytokine concentrations in patients with stage IV melanoma was attributed to Th2 cytokines (Table 2). This was consistent with previous reports of increased numbers of peripheral blood Th2 cells in patients (and laboratory animals) with metastatic cancer (22,23).
Figure 1.
Principal component analysis of cytokine levels across all stages of disease. Plasma collected from patients diagnosed with atypical nevi (red), benign nevi (blue), melanoma in situ (green), stage I melanoma (purple), stage II melanoma (orange), stage III melanoma (yellow) and stage IV melanoma (brown) was tested in the 27-plex cytokine array to determine plasma levels of 27 cytokines. Using principal component analysis no difference in cytokine levels was noted among the early stages of disease (non-tumor bearing) patients, however cytokine levels were shown to be different in stage IV, tumor bearing patients, which is represented as separation of the brown spheres from all the others.
Table 2.
Results of statistical comparisons (Student’s t-test) of plasma levels of cytokines comparing early non-tumor bearing patient samples to late stage (stage IV) tumor bearing patient samples.
| Cytokine | p-value (stage IV vs all other) |
|---|---|
| IL-4 | 1.73×10−12 |
| RANTES (CCL5) | 6.17×10−06 |
| IL-10 | 5.29×10−05 |
| Eotaxin (CCL11) | 8.31×10−05 |
| IP-10 (CXCL10) | 0.0007 |
| IL-13 | 0.002 |
| IL-12p70 | 0.005 |
| IL-7, IL-9 | 0.009 |
| VEGF, MIB-1b (CCL4) | 0.02 |
| GM-CSF | 0.03 |
| IL-5 | 0.05 |
| IL-15, TNFa, MIP-1a, FGF, IL-2, G-CSF, IL-8, IL-6, IFN-g, MCP-1, IL-17, PDGF, IL-1ra, IL-1b | >0.05 |
Immune cell subsets across stages of melanoma
PBMC isolated from patients with benign nevi, atypical (including dysplastic) nevi, as well as patients with in situ, stage I, II, III or IV melanoma were analyzed by flow cytometry to determine the frequencies of T, NK and dendritic (DC) cell subsets. There were not significant differences in frequencies of T-cell among stages of melanoma as determined by numbers of CD3, CD4 or CD8 positive T-cells (Supplemental Table 1a). Similarly, no significant differences were found in activated T-cells (CD3/CD69), total NK cells (CD16/56+, CD3−) or most DC subset parameters. As patients with stage IV melanoma appeared to differ significantly from all others with regard to plasma cytokine profiles we proceeded to compare the cell subset analysis of patients with stage IV melanoma relative to all others. The analysis revealed no significant differences among most parameters (Table 3) with the following exceptions: (a) the frequency of naïve T-cells (CD3/CD62L+) as well as activated DC (CD11c/CD83+) were significantly less in patients with stage IV melanoma; and (b) the frequency of tetramer positive CTL for gp100 and tyrosinase (but not MART-1 or CMV and EBV) were increased in patients with stage IV melanoma. Due to lack of available biospecimens, Th1 and Th2 enumeration could not be performed. These data suggested that there appeared to be some level of “immune activation” in patients with metastatic melanoma that was different from all other cohorts and this was consistent with a state of Th2 mediated “chronic inflammation”.
Assessment of functional immunity in patients with stage IV melanoma
The emerging data seemed to suggest that patients with stage IV melanoma, unlike all other patients with earlier stages of melanoma (or healthy controls), existed in a state of systemic Th2 dominance with some evidence of cellular immune activation in peripheral blood (increased frequencies of tumor specific CTL and decreased frequencies of naïve T cells). This immune homeostasis profile resembled a state of Th2 dominant “chronic inflammation”, similar to chronic viral infection (24). A reflection of the chronic inflammatory state of chronic viral infection as well as metastatic melanoma is an increase in peripheral blood PD-1+ (exhausted) T-cells (25). We found the same to be true in our patient cohort of stage IV melanoma patients compared to healthy controls (Figure 2a). This was further supported by functional assessment of antigen specific CTL (20), revealing a significant reduction in the frequency of functional recall antigen (CMV495-503) specific CTL in patients with stage IV melanoma versus healthy volunteers (Figure 2b). Less than 5% of tumor antigen specific, PBMC derived, tetramer positive CTL (MART-1) were capable of intracellular IFN-γ synthesis suggesting immune tolerance.
Figure 2.
Assessment of T-cell phenotype and function in healthy donors and stage IV melanoma patients. The number of T-cells exhibiting the FoxP3 (Treg) or PD-1 phenotype in peripheral blood was determined in healthy donors and stage IV melanoma patients (A). The frequency of FoxP3 positive cells were measured by 3-color flow cytometry, CD4-PC5, CD25-PE and FoxP3-Alexa flour 488. The mean percent (+/− SD) of FoxP3 positive were determined from the CD4 and CD25 double positive population. The mean frequency (+/− SD) of PD-1+ cells was measured from the CD8+ population. The frequency (+/− SD) of tetramer positive (CMV or MART-1) CD8+ T cells was compared among normal volunteers and patients with stage IV melanoma (B).
Expression microarray analysis of metastatic (stage IV) melanoma
The greatest differences in systemic immune homeostasis in patients across the spectrum of different clinical stages of malignant melanoma were detected in patients that had extensive tumor burden as evidenced by metastatic melanoma (stage IV melanoma). In all other subgroups of melanoma (in situ, stage I, stage II and stage III), disease burden was minimal (stage I or II melanoma) or non-existent (blood collected after complete surgical resection). Therefore the presence of visible tumor bulk could be responsible for the plasma profile of Th2 cytokine dominance, i.e. the visible tumor may be the source of the Th2 cytokine overproduction. In an effort to understand this relationship and determine if the presence of tumor was responsible for the predominance of Th2 cytokines in plasma of patients with metastatic melanoma, we isolated RNA from 24 clinical specimens of metastatic melanoma (19 specimens were matched to our patient cohort of stage IV melanoma). Microscopically dissected fragments of non-necrotic, non-infiltrated tumor tissue (as determined by hematoxylin/eosin stain) were excised from slide-mounted frozen core biopsies, RNA was isolated and analyzed on Affymetrix Genechip microarray. We evaluated the expression of 23 cytokines (45 probes): IL1a and b, IL-1ra, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12, IL-13, IL-15, IL-17, IFN-γ, CCL5, CCL11, CSF-2, MCP-1, TNF-α, VEGF. The objective of the experiment was to determine whether or not the malignancy itself was the source of Th2 cytokines that were detected in plasma. The data revealed that many of the probed cytokines, chemokines and growth factors are up-regulated in tumor tissue (Figure 3a). However, there were no differences in expression of Th1 vs Th2 cytokines (IFN-g vs IL-4, IL-5, IL-10, and IL-13, Figure 3b) in tumor tissues suggesting that the observed Th2 cytokine predominance in plasma was not derived from the tumor. However, of the tested cytokines, the most highly/frequently up-regulated transcript in the tumor samples was VEGF (Figure 3b). Plasma VEGF levels were significantly higher in metastatic melanoma patients relative to healthy donors, (Figure 3c), consistent with published reports (26). Considering the described immune modulatory (down-regulatory) properties of VEGF (27), we postulated that tumor derived VEGF could be responsible for the Th2 polarization in patients with stage IV melanoma (away from the normal state of Th1 dominance).
Figure 3.
VEGF levels in patients with metastatic melanoma. (A) RNA expression of cytokines in human metastatic melanoma tissue. Twenty-four frozen biopsies of metastatic melanoma tumor tissues was used to extract RNA for expression array analysis. Illustrated are the RNA expression intensity profiles of 45 probes for 24 cytokines. (B) Comparison of expression intensities between genes coding for Th1 (IFN-γ and IL-2), Th2 (IL-4, IL-5, IL-10, and IL-13) cytokines and VEGF. There were no statistically significant differences when comparing Th1 vs Th2 cytokine expression levels (p=0.04); there was a statistically significant difference when comparing VEGF expression with Th1 or Th2 cytokines (p<0.001). Levels of significance were determined using the Wilcoxin signed-rank test. (C ) ELISA (mean concentration +/−SD) for VEGF-A was performed on plasma samples from healthy donors (n=30) and stage IV melanoma patients (n=40).
VEGF mediates Th2 bias
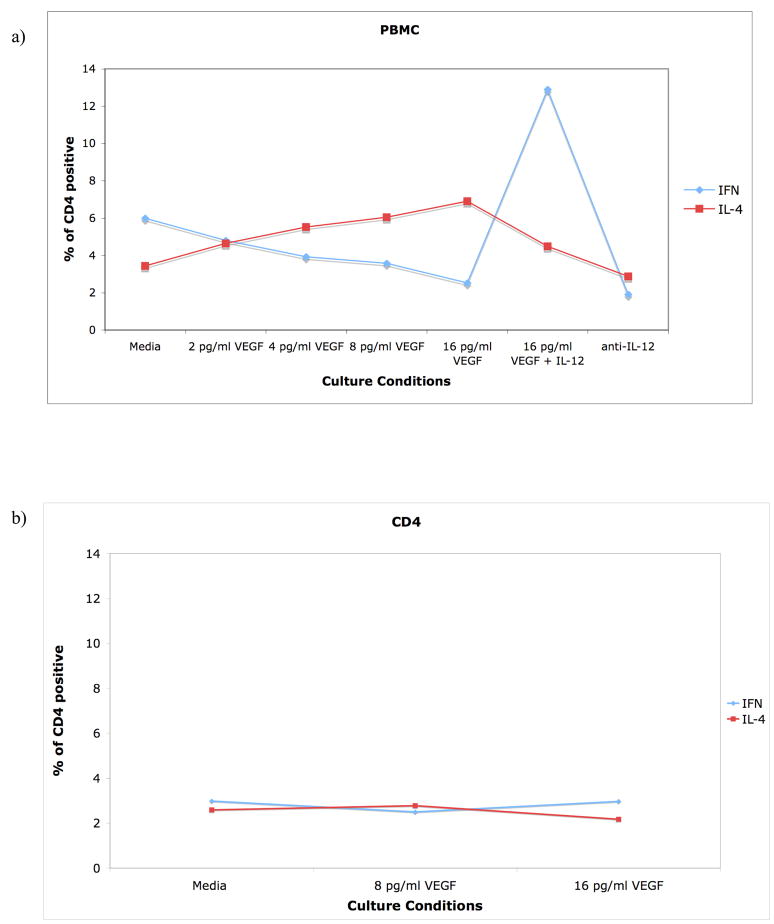
VEGF has been associated with Th2 bias in human disease (lung inflammation)(28). We hypothesized that elevated levels of plasma VEGF, derived at least in part from malignant melanocytes in patients with stage IV melanoma, could be responsible for the predominance of Th2 plasma cytokines. In order to test this hypothesis we stimulated healthy donor PBMC with CD3+/CD28+ expander micro-beads for 3 days with increasing concentrations (1pg/mL–16pg/mL) of recombinant VEGF and assessed intracellular cytokine production of IL-4 (Th2 cytokine) and IFN-γ (Th1 cytokine) in CD4+ T-cells at the end of in vitro culture (Figure 4a). The data clearly demonstrated that increasing concentrations of VEGF resulted in a dose-dependent reversal of the relative ratio of Th1 to Th2 cells in favor of Th2. Increased concentrations of VEGF were associated with a decrease in the number of Th1 cells (CD4+/IFNg+) with an associated reciprocal increase in Th2 cells (CD4+/IL-4+). The polarizing effects of VEGF were lost if the assay was performed with purified CD4 cells only (Figure 4b) suggesting that the observed Th polarization effect of VEGF is indirect, likely mediatd by other PBMC. The addition of 10μg/mL of IL-12 to the culture containing 16pg/mL of VEGF prevented the shift in T-helper polarity from Th1 to Th2; addition of anti-human IL-12 antibody to the stimulated PBMC mimicked the effect of VEGF (Figure 4a). These data suggest a possible role for monocyte/macrophages in the PBMC preparation as the mediators of the VEGF induced Th polarization.
Figure 4.
Co-culture with recombinant human VEGF shifts T-helper polarity from Th1 (IFN-γ) to Th2 (IL-4) predominance. PBMC (A) isolated from healthy donors were stimulated with PMA and ionomycin in the presence of brefeldin-A, permeabolized and intracellularly stained for human IFN-γ (FITC) and human IL-4 (PE). PBMC were exposed to increasing concentrations of VEGF (0–16pg/mL) without/with IL-12. All cells were immunostained with PC5 anti-human CD4. Purified CD4+ T-cells (B) were negatively isolated using Miltenyi beads, cultured and stained in the same fashion as PBMC (A). Similar results were observed in 5 different experiments.
DISCUSSION
The presented study is a description of the state of immune homeostasis of select parameters among 209 patients (and healthy volunteers) across the clinical spectrum of malignant melanoma from patients with benign nevi arousing clinical suspicion of melanoma, through patients with widely disseminated metastatic melanoma. Our data demonstrated: (a) Th2 cytokine dominant systemic environment in patients with metastatic (stage IV) melanoma, not present in any other clinical categories; (b) no significant differences in the relative concentrations of peripheral blood T, NK and DC subsets (and selected functional subsets) across clinical categories (with few noted exceptions correlating with systemic Th2 bias); and (c) possible role for tumor derived VEGF as a relevant mediator of the Th2 bias of systemic immune homeostasis.
The main observation from the presented data is that patients with visible metastatic melanoma (stage IV) exist in a state of systemic Th2 dominant immune homeostasis resembling a state of “chronic inflammation” that could at least in part be the result of tumor derived VEGF. This is different from healthy controls or patients with completely surgically resected melanoma exhibiting Th1 biased immune homeostasis (acute inflammation). Our data suggests that the malignancy may play an active role in “reprogramming” systemic immunity towards Th2 dominance/chronic inflammation that may be permissive to tumor progression/metastases. These data are consistent with clinical reports of associations of global parameters of chronic inflammation and outcomes in patients with advanced cancer (e.g. C-reactive protein elevation in the setting of metastatic cancer) (29) as well as specific tumor antigen associated Th2 cell bias (e.g. MAGE-6) identified in peripheral blood of patients with metastatic melanoma and renal cell carcinoma(16). In the latter example, Tatsumi et al describe the dominance of MAGE-6 peptide specific Th2 cells in patients with clinically evident metastatic melanoma/renal cell carcinoma, absent in healthy volunteers and patients with no detectable cancer. The authors conclude that future immunotherapeutic interventions will likely have to overcome systemic Th2-dominated, tumor-reactive CD4+ T cell responses in order to achieve optimal clinical benefit. It is noteworthy that the Th2 bias was described in both metastatic melanoma and renal cell carcinoma, two malignancies characterized by high levels of VEGF in plasma.
The notion that inflammation plays a role in tumor progression is not new. The association of inflammation and neoplastic tissue progression was originally suggested by Virchow et al the latter part of the 19th century (30). Progression of a number of malignancies (including melanoma) has been associated with “chronic inflammation” at the level of the tumor microenvironment (30–33). Tumor microenvironment associated immune dysfunction (suppression) has clearly been associated with poor clinical outcomes revealing several potential, clinically relevant, mechanisms of immune escape (18,34–36). One such example is that of Th2 cytokine producing tumor associated macrophages (TAM) that recognize tumor antigens but cannot properly mature in the tumor microenvironment thereby yielding a state of T-cell immune tolerance (36). Additionally, TAM down-regulate T cell receptor expression on tumor infiltrating lymphocytes (TIL) (37) and produce IL-4 and IL-5 (Th2) rather than IFN-γ(Th1) (38). Tumor infiltration by Treg has also been associated with poor prognosis in patients with o varian cancer (39), non-Hodgkin’s lymphoma (40), basal cell carcinoma (41) and squamous cell carcinoma of the head and neck (42). Thus, dysfunctional (suppressed) immunity in the tumor microenvironment appears clinically relevant, is associated with Th2 bias and leads to poor clinical outcome. Therefore, evidence of systemic reprogramming of Th immunity from a normal state of Th1 dominance to a state of Th2 biased (cytokine and cellular re-polarization) systemic immune homeostasis may be an extension of the immunological changes in the tumor microenvironment and contribute to the observed poor clinical results of immunotherapy (specifically cancer vaccines) in patients with stage IV melanoma.
Systemic Th2 immune bias of chronic inflammation is a state of immune homeostasis present in both pathologic and physiologic states in humans. Similar pathologic states of Th2 driven systemic chronic inflammation have already been described in the setting of chronic viral infections (43,44). Additionally, there are also examples of physiologic (normal) Th2 mediated “chronic inflammation” that are organ-specific (the mucosa of the gut and lungs (45) ) as well as systemic (normal pregnancy (46)). In both settings, local/systemic Th2 polarization seems to allow a symbiotic coexistence of the immunocompetent “host” with another organism (bacteria in the gut and lung; fetus in the uterus) by dampening robust immune Th1 acute inflammatory responses in the microenvironment (gut/lung) or both microenvironment and systemic immunity (pregnancy). Maternal systemic Th2 polarization of pregnancy may explain the existence of peripheral blood microchimerism (small numbers of persisting fetal blood cells surviving in maternal blood) in parous women. Thus, similar to the placental microenvironment leading to systemic Th2 polarization (at least in part mediated by placental growth factor, PLGF), the immunosuppressive tumor microenvironment may lead to systemic Th2 polarization via VEGF overproduction (a PLGF analog)(47). High plasma VEGF levels have been associated with poor clinical outcomes in patients with metastatic melanoma (48). Thus, in the context of the immunomodulatory properties of VEGF and its structural homology to PLGF, we postulated that systemic VEGF overproduction by tumors could be responsible for systemic Th2 immune polarization favoring tumor progression. As illustrated in figure 4, non-specific stimulation of CD4+ Th cells from healthy PBMC donors in the presence of recombinant VEGF results in a dose dependent increase in the numbers of Th2 cells with a reciprocal decrease in Th1 cells emulating the findings in peripheral blood of patients with stage IV melanoma. The specific mechanisms of this re-programming remain under ongoing investigation.
In summary, the presented data suggest that the sate of systemic immune homeostasis in patients with metastatic melanoma resembles Th2 mediated chronic inflammation and appears at least in part mediated by tumor derived VEGF. These data expand on published reports of the increased frequency of Th2 (over Th1) tumor antigen specific CD4+ T cells in the setting of advanced melanoma/renal cell carcinoma (16) and in part support the observed improved efficacy of human adoptive transfer experiments of tumor specific CTL delivered following “lymphodepletion” (depletion of systemic Th2 immune homeostasis) (49) when compared to adoptive transfer alone (50). These data may also offer some insight into the unrealized therapeutic benefit of non-specific systemic T cell activation (e.g. high-dose IL-2, or anti-CTLA-4) which may have augmented a pre-existing “dysfunctional” systemic immune response. Perhaps, therapeutic combinations adding inhibitors of angiogenesis (anti-VEGF) to systemic lymphodepletion (removing established Th2 bias) followed by attempts at immune reconstitution (e.g. anti-CTLA4, adoptive transfer, etc) may yield desirable immune recovery and beneficial clinical outcomes.
Supplementary Material
Acknowledgments
This work was supported by CA095670 as well as a grant by the Cancer Research Institute; the authors acknowledge the administrative support of Mrs. LuRaye Eischens in the completion of this manuscript.
Footnotes
STATEMENT OF TRANSLATIONAL RELEVANCE
The study presented in this manuscript is a comprehensive look at immune homeostasis among individuals with progressive stages of melanoma (benign, atypical, dysplastic, in situ and stage I–IV melanoma). In summary, we found elevated levels of Th2 cytokines exist in plasma of stage IV patients. Additionally using RNA micoarrays we found that the tumors express soluble factor(s), including VEGF-A, which mediate the production of Th2 cytokines by T-helper cells, and thereby render the immune system incapable of preventing tumor growth. This is important in the clinical setting because it potentially explains the limited success of immunotherapeutic strategies (cancer vaccines, DC and adoptive therapy) employed to date. This data suggests that a multi-modal treatment option including immunoablative chemotherapy to reset the immune system in conjunction with inhibitors of angiogenesis (anti-VEGF) followed by immune reconstitution with anti-CTL4 or adoptive transfer, for example, may improve clinical outcomes of these patients.
References
- 1.Finkelstein SE, Heimann DM, Klebanoff CA, et al. Bedside to bench and back again: how animal models are guiding the development of new immunotherapies for cancer. J Leukoc Biol. 2004;76:333–7. doi: 10.1189/jlb.0304120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riker AI, Jove R, Daud AI. Immunotherapy as part of a multidisciplinary approach to melanoma treatment. Front Biosci. 2006;11:1–14. doi: 10.2741/1775. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg SA, Sherry RM, Morton KE, et al. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J Immunol. 2005;175:6169–76. doi: 10.4049/jimmunol.175.9.6169. [DOI] [PubMed] [Google Scholar]
- 4.Vaishampayan U, Abrams J, Darrah D, Jones V, Mitchell MS. Active immunotherapy of metastatic melanoma with allogeneic melanoma lysates and interferon alpha. Clin Cancer Res. 2002;8:3696–701. [PubMed] [Google Scholar]
- 5.Osanto S, Schiphorst PP, Weijl NI, et al. Vaccination of melanoma patients with an allogeneic, genetically modified interleukin 2-producing melanoma cell line. Hum Gene Ther. 2000;11:739–50. doi: 10.1089/10430340050015635. [DOI] [PubMed] [Google Scholar]
- 6.Palucka AK, Dhodapkar MV, Paczesny S, et al. Single injection of CD34+ progenitor-derived dendritic cell vaccine can lead to induction of T-cell immunity in patients with stage IV melanoma. J Immunother (1997) 2003;26:432–9. doi: 10.1097/00002371-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Banchereau J, Ueno H, Dhodapkar M, et al. Immune and clinical outcomes in patients with stage IV melanoma vaccinated with peptide-pulsed dendritic cells derived from CD34+ progenitors and activated with type I interferon. J Immunother (1997) 2005;28:505–16. doi: 10.1097/01.cji.0000171292.79663.cb. [DOI] [PubMed] [Google Scholar]
- 8.Gajewski TF, Meng Y, Blank C, et al. Immune resistance orchestrated by the tumor microenvironment. Immunological reviews. 2006;213:131–45. doi: 10.1111/j.1600-065X.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- 9.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science (New York, NY) 2002;298:850–4. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kershaw MH, Westwood JA, Parker LL, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12:6106–15. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ribas A, Camacho LH, Lopez-Berestein G, et al. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J Clin Oncol. 2005;23:8968–77. doi: 10.1200/JCO.2005.01.109. [DOI] [PubMed] [Google Scholar]
- 12.Rasku MA, Clem AL, Telang S, et al. Transient T cell depletion causes regression of melanoma metastases. Journal of translational medicine. 2008;6:12. doi: 10.1186/1479-5876-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunder NN, Wallen H, Cao J, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. The New England journal of medicine. 2008;358:2698–703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Terheyden P, Schrama D, Pedersen LO, et al. Longitudinal analysis of MART-1/HLA-A2-reactive T cells over the course of melanoma progression. Scandinavian journal of immunology. 2003;58:566–71. doi: 10.1046/j.1365-3083.2003.01324.x. [DOI] [PubMed] [Google Scholar]
- 15.Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007;67:10019–26. doi: 10.1158/0008-5472.CAN-07-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tatsumi T, Kierstead LS, Ranieri E, et al. Disease-associated bias in T helper type 1 (Th1)/Th2 CD4(+) T cell responses against MAGE-6 in HLA-DRB10401(+) patients with renal cell carcinoma or melanoma. J Exp Med. 2002;196:619–28. doi: 10.1084/jem.20012142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bose A, Chakraborty T, Chakraborty K, Pal S, Baral R. Dysregulation in immune functions is reflected in tumor cell cytotoxicity by peripheral blood mononuclear cells from head and neck squamous cell carcinoma patients. Cancer Immun. 2008;8:10. [PMC free article] [PubMed] [Google Scholar]
- 18.Almand B, Clark JI, Nikitina E, et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–89. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 19.Fricke I, Mirza N, Dupont J, et al. Vascular endothelial growth factor-trap overcomes defects in dendritic cell differentiation but does not improve antigen-specific immune responses. Clin Cancer Res. 2007;13:4840–8. doi: 10.1158/1078-0432.CCR-07-0409. [DOI] [PubMed] [Google Scholar]
- 20.Markovic SN, Nevala WK, Uhl CB, Celis E, McKean DJ. A reproducible method for the enumeration of functional (cytokine producing) versus non-functional peptide-specific cytotoxic T lymphocytes in human peripheral blood. Clin Exp Immunol. 2006;145:438–47. doi: 10.1111/j.1365-2249.2006.03157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–93. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- 22.Pellegrini P, Berghella AM, Del Beato T, Cicia S, Adorno D, Casciani CU. Disregulation in TH1 and TH2 subsets of CD4+ T cells in peripheral blood of colorectal cancer patients and involvement in cancer establishment and progression. Cancer Immunol Immunother. 1996;42:1–8. doi: 10.1007/s002620050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi M, Kobayashi H, Pollard RB, Suzuki F. A pathogenic role of Th2 cells and their cytokine products on the pulmonary metastasis of murine B16 melanoma. J Immunol. 1998;160:5869–73. [PubMed] [Google Scholar]
- 24.Sester U, Presser D, Dirks J, Gartner BC, Kohler H, Sester M. PD-1 Expression and IL-2 Loss of Cytomegalovirus- Specific T Cells Correlates with Viremia and Reversible Functional Anergy. Am J Transplant. 2008 doi: 10.1111/j.1600-6143.2008.02279.x. [DOI] [PubMed] [Google Scholar]
- 25.Wong RM, Scotland RR, Lau RL, et al. Programmed death-1 blockade enhances expansion and functional capacity of human melanoma antigen-specific CTLs. Int Immunol. 2007;19:1223–34. doi: 10.1093/intimm/dxm091. [DOI] [PubMed] [Google Scholar]
- 26.Tas F, Duranyildiz D, Oguz H, Camlica H, Yasasever V, Topuz E. Circulating serum levels of angiogenic factors and vascular endothelial growth factor receptors 1 and 2 in melanoma patients. Melanoma Res. 2006;16:405–11. doi: 10.1097/01.cmr.0000222598.27438.82. [DOI] [PubMed] [Google Scholar]
- 27.Gabrilovich DI, Chen HL, Girgis KR, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2:1096–103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 28.Lee CG, Link H, Baluk P, et al. Vascular endothelial growth factor (VEGF) induces remodeling and enhances TH2-mediated sensitization and inflammation in the lung. Nat Med. 2004;10:1095–103. doi: 10.1038/nm1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahmoud FA, Rivera NI. The role of C-reactive protein as a prognostic indicator in advanced cancer. Current oncology reports. 2002;4:250–5. doi: 10.1007/s11912-002-0023-1. [DOI] [PubMed] [Google Scholar]
- 30.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 31.Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323:1228–33. doi: 10.1056/NEJM199011013231802. [DOI] [PubMed] [Google Scholar]
- 32.Medrano EE, Farooqui JZ, Boissy RE, Boissy YL, Akadiri B, Nordlund JJ. Chronic growth stimulation of human adult melanocytes by inflammatory mediators in vitro: implications for nevus formation and initial steps in melanocyte oncogenesis. Proc Natl Acad Sci U S A. 1993;90:1790–4. doi: 10.1073/pnas.90.5.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–7. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 34.Chen W. Dendritic cells and (CD4+)CD25+ T regulatory cells: crosstalk between two professionals in immunity versus tolerance. Front Biosci. 2006;11:1360–70. doi: 10.2741/1889. [DOI] [PubMed] [Google Scholar]
- 35.de Visser KE, Coussens LM. The inflammatory tumor microenvironment and its impact on cancer development. Contrib Microbiol. 2006;13:118–37. doi: 10.1159/000092969. [DOI] [PubMed] [Google Scholar]
- 36.Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: The role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2007 doi: 10.1016/j.critrevonc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Riccobon A, Gunelli R, Ridolfi R, et al. Immunosuppression in renal cancer: differential expression of signal transduction molecules in tumor-infiltrating, near-tumor tissue, and peripheral blood lymphocytes. Cancer Invest. 2004;22:871–7. doi: 10.1081/cnv-200039653. [DOI] [PubMed] [Google Scholar]
- 38.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nelson BH. The impact of T-cell immunity on ovarian cancer outcomes. Immunol Rev. 2008;222:101–16. doi: 10.1111/j.1600-065X.2008.00614.x. [DOI] [PubMed] [Google Scholar]
- 40.Mittal S, Marshall NA, Duncan L, Culligan DJ, Barker RN, Vickers MA. Local and systemic induction of CD4+CD25+ regulatory T-cell population by non-Hodgkin lymphoma. Blood. 2008;111:5359–70. doi: 10.1182/blood-2007-08-105395. [DOI] [PubMed] [Google Scholar]
- 41.Kaporis HG, Guttman-Yassky E, Lowes MA, et al. Human basal cell carcinoma is associated with Foxp3+ T cells in a Th2 dominant microenvironment. J Invest Dermatol. 2007;127:2391–8. doi: 10.1038/sj.jid.5700884. [DOI] [PubMed] [Google Scholar]
- 42.Strauss L, Bergmann C, Whiteside TL. Functional and phenotypic characteristics of CD4+CD25highFoxp3+ Treg clones obtained from peripheral blood of patients with cancer. Int J Cancer. 2007;121:2473–83. doi: 10.1002/ijc.23001. [DOI] [PubMed] [Google Scholar]
- 43.Sarih M, Bouchrit N, Benslimane A. Different cytokine profiles of peripheral blood mononuclear cells from patients with persistent and self-limited hepatitis C virus infection. Immunology letters. 2000;74:117–20. doi: 10.1016/s0165-2478(00)00210-8. [DOI] [PubMed] [Google Scholar]
- 44.Sindhu S, Toma E, Cordeiro P, Ahmad R, Morisset R, Menezes J. Relationship of in vivo and ex vivo levels of TH1 and TH2 cytokines with viremia in HAART patients with and without opportunistic infections. Journal of medical virology. 2006;78:431–9. doi: 10.1002/jmv.20558. [DOI] [PubMed] [Google Scholar]
- 45.Berin MC, Dwinell MB, Eckmann L, Kagnoff MF. Production of MDC/CCL22 by human intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1217–26. doi: 10.1152/ajpgi.2001.280.6.G1217. [DOI] [PubMed] [Google Scholar]
- 46.Mellor AL, Munn DH. Immunology at the maternal-fetal interface: lessons for T cell tolerance and suppression. Annu Rev Immunol. 2000;18:367–91. doi: 10.1146/annurev.immunol.18.1.367. [DOI] [PubMed] [Google Scholar]
- 47.Lin YL, Liang YC, Chiang BL. Placental growth factor down-regulates type 1 T helper immune response by modulating the function of dendritic cells. J Leukoc Biol. 2007;82:1473–80. doi: 10.1189/jlb.0307164. [DOI] [PubMed] [Google Scholar]
- 48.Brychtova S, Bezdekova M, Brychta T, Tichy M. The role of vascular endothelial growth factors and their receptors in malignant melanomas. Neoplasma. 2008;55:273–9. [PubMed] [Google Scholar]
- 49.Powell DJ, Jr, Dudley ME, Hogan KA, Wunderlich JR, Rosenberg SA. Adoptive transfer of vaccine-induced peripheral blood mononuclear cells to patients with metastatic melanoma following lymphodepletion. J Immunol. 2006;177:6527–39. doi: 10.4049/jimmunol.177.9.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yee C, Thompson JA, Byrd D, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U S A. 2002;99:16168–73. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.