Abstract
The inability to name objects (anomia) is one of the most common findings in the neurologic examination of primary progressive aphasia (PPA). In the semantic variant of PPA, the anomia is profound and reflects a combination of object naming and word comprehension deficits. In contrast, nonsemantic variants of PPA display a more selective impairment of object naming, without corresponding impairments of word comprehension. The aim of the present study was to explore the nature of the anomia in nonsemantic variants of PPA with a sensitive chronometric test of covert word/picture association. We tested priming effects in 12 patients with nonsemantic variant of PPA and 18 controls. Stimuli consisted of written words and line pictures of concrete objects. Within-format (word-word and picture-picture) and cross-format (word-picture and picture-word) priming effects were assessed by measuring the shortening of response times to the second versus initial presentation of corresponding stimulus pairs. In addition to the expected impairment of picture-to-word priming, a condition simulating object naming, the nonsemantic PPA patients also showed unexpected impairments of word-to-picture and word-to-word priming. Picture-to-picture priming was preserved, demonstrating the selectivity of the deficit for lexical processing. These findings show that the information processing bottleneck in patients with nonsemantic variants of PPA is not confined to the stage of lexical access but that it also extends into the prior levels of lexical semantics. The boundaries between the semantic and nonsemantic variants are therefore far from rigid.
Keywords: dementia, frontotemporal dementia, priming, naming, anomia
Primary progressive aphasia (PPA) is a neurodegenerative syndrome that is being diagnosed with increasing frequency. It is characterized by a selective disruption of word usage and comprehension in the face of relative preservation of other cognitive abilities during the early stages of the disease.1 Initially, the left-hemisphere language network of the brain is the preferential focus of atrophy and functional disruption.2-4 On the basis of the nature of the language impairment, patients with PPA can be subdivided into agrammatic/dysfluent, logopenic, and semantic subtypes.1,5,6 According to this classification system, the semantic variant is characterized by poor single word comprehension but relatively preserved fluency and syntax; the agrammatic variant by poor syntax and fluency but relatively preserved word comprehension; and the logopenic subtype by preserved syntax and comprehension but variable fluency. In the literature, the logopenic and agrammatic subtypes also fulfill the criteria for progressive nonfluent aphasia and the semantic subtype partially overlaps with the syndrome of semantic dementia.7,8 The single most prominent deficit commonly encountered in almost all PPA patients is an impairment of word-finding.9 Such impairments can lead to effortful groping for the right word during spontaneous speech or to anomia during object naming to confrontation.
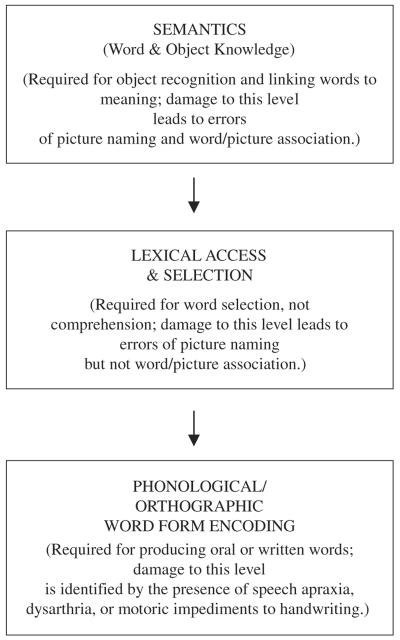
The naming of objects is a uniquely human faculty of immense complexity. Several models have been proposed to describe the processes that link the percept of an object to its name. A model proposed by Hillis and colleagues is based on 3 stages: semantics, lexical access, and word form encoding (Fig. 1).10,11 The semantic level corresponds to the distributed associations required for object recognition and for linking words to meaning. A disruption at this level leads to multimodal impairments of confrontation naming and in linking a word to its corresponding picture. There is considerable discussion about the internal organization of this level, particularly whether it contains a single modal representation of words and objects or 2 separate but converging routes, 1 for object knowledge and the other for word meaning.7,12 The intermediate level of lexical access in Figure 1 is required for word selection but not for word comprehension. Disruption at this level leads to impairment in picture naming abnormalities but not in establishing word/picture associations.10 The third level incorporates the transformation of lexical representations into spoken and written words. Speech apraxia, a motor speech disorder characterized by impaired ability to coordinate the sequential, articulatory movements that generate speech sounds to form words, is an example of a disruption at this level of the processing hierarchy. The processing stages in Figure 1 are consistent with the more extensively characterized models of Levelt et al,13 Ellis and Young,14 and Caramazza.15
FIGURE 1.
A simplified 3-stage model of object naming adapted from DeLeon et al10 and Hillis11 2007.
Naming in PPA has been investigated most intensely in the semantic subtypes, leading to the conclusion that the impairments in these patients involve a dysfunction of the semantic level in Figure 1.7,16,17 Such patients cannot name pictures of objects and cannot point to the correct object when the name is presented by the examiner. In the nonsemantic subtypes of PPA, confrontation naming is impaired, whereas the ability to point to the correct picture on hearing the word is intact, indicating that the name of the object is understood, at least when it is tested by pointing to the corresponding object. In many instances cueing assists a patient with nonsemantic PPA in finding the correct word, and their paraphasias are phonemic rather than semantic.9 Furthermore, the patients frequently state that they “know what the word is but cannot say it.” These features of the anomia in the nonsemantic variants of PPA suggest that the impairment is at the lexical access and/or phonologic encoding stages but that it does not involve more upstream semantic levels of the naming process.
To test this formulation and explore the nature of anomia in nonsemantic PPA, we designed a novel task, based on the principles of repetition priming. The term “priming” refers to the idea that exposure to a stimulus (prime) automatically triggers neural activity that influences the processing of an identical or related stimulus (target) encountered at a later time. The influence of the stimulus on the speed or accuracy of responding to the target is defined as priming.18,19
We used pictures of objects and written words to remain within a single sensory modality, namely, visual. This choice is unlikely to undermine the generality of our findings because most of the anomias interfere with the written and oral production of the object name.10 Our task did not require the patient to explicitly name objects but chronometrically probed levels of information processing that link words to corresponding objects. It is chronometric in that, differences in response times are used as a measure of cognitive processing, specifically, the integrity of lexical processing in PPA.20
The subjects, individuals with impaired naming, were asked to make a “natural or manmade” semantic judgment for sequentially presented written names or pictures of objects. Responses were made by pressing a button on a computer keypad and so, no overt naming was required. Each word and picture appeared in only 1 priming condition. There were 4 possible priming conditions: word followed by identical word; picture followed by identical picture; word followed by the picture it named; picture followed by the word that represented its name. We compared the speed of responding to the first versus second member of a given pair to see if exposure to the first member of a pair automatically triggered neural associations that facilitated the response to the second. The difference in response speeds was used to measure the magnitude of priming.
We predicted that the picture-word (PW) priming would be weaker in patients than controls because it simulated confrontation naming that was impaired in all of the patients. We were also particularly interested in the reciprocal word-picture (WP) priming. This condition simulates the ability to point to an object upon hearing its name, a process that was preserved in our subjects and that is more closely related to the semantic stage in Figure 1. These hypotheses were tested in a group of 12 nonsemantic PPA patients and 18 neurologically intact controls.
METHODS
Participants
Twelve PPA patients with poor confrontation naming as determined by the Boston Naming Test (BNT), but preserved single word comprehension, participated in the study (Table 1). The patients would thus fall into the agrammatic and logopenic subtypes of PPA, as defined by Gorno-Tempini and colleagues.5 No attempt was made to further subdivide these patients because the criteria for distinguishing the logopenic and agrammatic variants are still being refined.21 All patients were required to have a study partner (typically a caregiver) to provide historical information to aid in the diagnosis.
TABLE 1.
Subject Demographics
| PPA | NC | |
|---|---|---|
| n = 12 Mean (SD) | n = 18 Mean (SD) | |
| Sex: M/F | 7/5 | 6/12 |
| Age | 72.2 (65) | 72.3 (60) |
| Education | 15.1 (22) | 15.8 (20) |
| Symptom duration: years | 4.5 (18) | NA |
No significant demographic differences between groups.
NC indicates normal control; PPA, primary progressive aphasia.
Eighteen age-matched and education-matched cognitively intact normal control subjects (NC) were recruited from the Clinical Core registry of the Northwestern Alzheimer's Disease Center (NADC) (Table 1). Written informed consent was obtained from all subjects and study partners and the study was approved by the Institutional Review Board at Northwestern University.
All participants received a neuropsychologic test battery. To participate in the study, control subjects were required to score no lower than 2 standard deviations below age-adjusted and education-adjusted normative values on all neuropsychologic tests. Additional exclusion criteria were the presence of neurologic, medical, or psychiatric history, and long-term use of psychoactive medication. All subjects were required to have a minimum visual acuity of 20/40 (correction permitted) to view the stimuli.
The diagnosis of PPA was based on consensus of a speech pathologist, a neurologist, and a neuropsychologist at the NADC using the criteria outlined by Mesulam1 and adopted by the Uniform Data Set of the Alzheimer's Disease Center programs of the National Institute on Aging (http://www.alz.washington.edu/).22 Clinical neuroimaging (eg, magnetic resonance imaging, positron emission tomography) had been obtained on all patients (Table 2).
TABLE 2.
Clinical Characterization
| PPA Subject No. |
Age/Sex* | Symptom Duration: Years† |
MMSE | CDR | ADLQ | Imaging | BNT (60) | Category Fluency: Animals |
WAB Yes/ No (60) |
WAB Aphasia Quotient |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 73/F | 3 | 13 | 1 | 19% | PET: left temporoparietal hypometabolism | 1 | 2 | 60 | 73.6 |
| 2 | 71/F | 8.5 | NVL | 1 | 10% | PET: bilateral parietal hypometabolism left > right | 4 | 1 | 60 | 48.9 |
| 3 | 79/F | 4.5 | 10 | 0.5 | 26% | MRI: nonspecific hyperintensities | 19 | 2 | 57 | 92.8 |
| 4 | 62/F | 3.5 | 27 | 0.5 | 13% | SPECT: posterior hypoperfusion, left posterior cerebral hemisphere; EEG: minimal left slowing | 22 | 4 | 57 | 88.7 |
| 5 | 77/M | 5 | 20 | 0.5 | 24% | PET: left temporoparietal hypometabolism | 32 | 8 | 60 | 85.2 |
| 6 | 76/F | 7 | 15 | 1 | 12% | SPECT: temporoparietal hypoperfusion greater on the left | 35 | 10 | 60 | 85.2 |
| 7 | 72/M | 4 | 21 | 0.5 | 27% | MRI: mild to moderate generalized atrophy | 36 | 3 | 60 | 89 |
| 8 | 58/M | 2.5 | 20 | 1 | 22% | MRI: atrophy and widening of the left perisylvian fissure | 39 | 8 | 60 | 73.9 |
| 9 | 76/M | 3 | 26 | 0.5 | 11% | MRI: mild bilateral atrophy | 41 | 13 | 60 | 71.6 |
| 10 | 70/M | 4.5 | 22 | 1 | 18% | MRI: nonspecific white matter intensities, age specific atrophy | 44 | 14 | 57 | 88.9 |
| 11 | 72/M | 3 | 24 | 0.5 | ND | MRI: slightly asymmetrical widening of the left perisylvian and anterior temporal regions; SPECT: abnormal, decreased perfusion, left temporal, parietal areas | 47 | 10 | 60 | 74.8 |
| 12 | 80/M | 5 | 25 | 1 | 8% | MRI: mild cerebral atrophy, periventricular white matter changes | 47 | 5 | 60 | 61.8 |
| Control subject average: no. 13-30 | 72.3 | NA | 29.6 | 0 | NA | NA | 58.8 | 24.5 | NA | NA |
Numbers in parenthesis denote the maximum score.
Age at time of testing.
Reported length of symptom duration at the time of testing.
ADLQ indicates activities of daily living questionnaire; BNT, Boston Naming Test; CDR, Clinical Dementia Rating Scale (0 = none, 0.5 = Questionable/Very Early, 1 = Early, 2 = Moderate, 3 = Severe Dementia); EEG, electroencephalography; MMSE, Mini Mental State Examination; MRI, magnetic resonance imaging; NA, variable not relevant for control participants; ND, not done; NVL, not valid due to language deficits; PET, positron emission tomography; PPA, primary progressive aphasia; SPECT, single photon emission computed tomography; WAB, Western Aphasia Battery Yes No Questions comprehension subtest.
The ADLQ score on this questionnaire is a composite of informants' ratings of activities related to self-care, household care, recreation travel, employment, and communication. The score is a percentage reflecting the degree to which ADL's are comprised (O% to 33% mild, 34 to 66% moderate, 67% to l00% severe).
Language comprehension is complex and can vary depending on the nature of the test used. For this study, the level of language comprehension was characterized quantitatively by the score on the “yes/no questions” auditory comprehension subtest of the Western Aphasia Battery (WAB)23 and qualitatively, on the basis of reports from comprehensive neurologic, neuropsychologic, and speech language pathology examinations. Patients with moderate-to-severe language comprehension deficits were excluded from this study (WAB yes/no questions subtest scores ≤54).
The Mini Mental State Examination (MMSE) score was used as a measure of dementia severity.24 Because aphasia could influence performance on the MMSE,25 the Activities of Daily Living Questionnaire (ADL-Q), completed by caregivers, was obtained as an independent measure of functional level in daily activities.26,27
Word-finding abilities were measured in 3 ways: (1) visual confrontation naming using the BNT28; (2) Semantic Fluency Test (animals)29; and (3) The Fluency subscore in the Spontaneous Speech section of the WAB.23 Only poor namers, that is, subjects whose scores were ≤47/60 on the BNT (abnormal for age and education), were studied. Naming deficits were not because of dysarthria or other motor speech deficits in this sample.
Individual test scores and clinical neuroimaging results are presented in Table 2, which also contains relevant means and standard deviations for the entire NC group.
Design and Materials
Repetition Priming Task
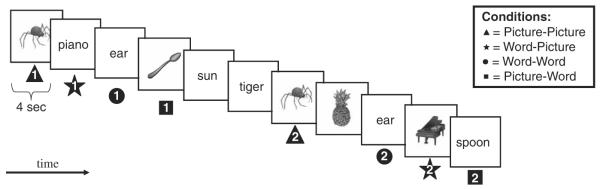
Four hundred stimuli were presented to each subject, 200 primes and 200 targets. Prime-target pairs were equally divided into 4 possible priming conditions: word-word (WW) in which the target word was primed by an identical word; picture-picture (PP) in which the target picture of an object was primed by an identical picture; PW in which the name of an object was primed by the picture of its corresponding object; and WP where the target picture of an object was primed by the word that represented its name. None of the 400 stimuli appeared in more than 1 prime-target pair.
Items were presented in blocks of 80 stimuli. Within each block of 80 trials, there were 20 prime-target pairs from each of the 4 conditions (WP, PW, PP, and WW). Each stimulus was presented for 4 seconds on a computer screen using SuperLab Pro version 2.0.3, with a 50 ms interstimulus interval. The different conditions were randomly distributed throughout the run, and stimulus order was semirandom with the repeat (target) stimulus presented 5 to 10 stimuli (20 to 40 s) after presentation of its prime (Fig. 2). All subjects received the stimuli in the same order.
FIGURE 2.
Example stimuli and conditions for the task. Stimuli are presented one at a time for a duration of 4 seconds. Subjects make a natural/manmade judgment for each item by pressing the appropriate button on the keyboard. Each stimulus is repeated once either in the same format (WW and PP) or different format (WP and PW). Exemplars from each of the four conditions (PP, WW, WP, and PW) are given above. Repetitions are separated by 5 to 10 items (20 to 40 s). 1 = Initial presentation of the stimulus. 2 = Second presentation of the stimulus. PP indicates picture-picture; PW, picture-word; WP, word-picture; WW, word-word.
Words were presented in their canonical orientation on a computer in bold lowercase 150 point Times New Roman font. Pictures consisted of gray-scale versions of the Snodgrass and Vanderwart stimuli from the following website: http://www.cog.brown.edu/~tarr/stimuli.html#sv. The images had been validated by Rossion and Pourtois30 and their familiarity ratings were used to match the stimulus groups. The mean length of the object names and words was 5.87 ± 2.34 letters (range, 2 to 12 letters). The average familiarity rating was 3.58 ± 0.88 (range, 1.76 to 5.00), based on a scale from 1 to 5 where 1 represented “very unfamiliar” and 5 “very familiar.” 30 Separate t tests or 1-way analysis of variances (ANOVAs) were completed to ensure that there were no statistically significant differences in familiarity ratings or word length by (1) category (natural/manmade), (2) condition (WP, PW, PP, and WW), or (3) block of trials.1-5
Procedure
A 10-item practice run immediately preceded the experiment to ensure task comprehension. Subjects were asked to view each stimulus, and to prompt deeper semantic encoding,31,32 to decide whether the item represented something “natural” or “manmade” by pressing the appropriately labeled button on the computer keypad (Fig. 2). Auditory instructions were accompanied by written instructions on the computer screen to promote optimal comprehension of the task. Subjects were told that the stimuli would stay on the screen for 4 seconds regardless of their speed of response but were encouraged to make their decision and respond as quickly and as accurately as possible.
Data Analysis
A t test revealed a significant difference in the overall mean reaction times (RTs) by group (mean ± standard deviation: PPA: 1485.03 ± 106.6; NC: 1048.0 ± 125.9; t = 4.17, df = 28; P < 0.001). Given this difference, all remaining analyses were completed using a priming index proportion score for each condition (see example of computation below). This method, proposed by Paller and colleagues,33 takes into account the expectation that larger difference scores are associated with slower responses in situations such as the present study where response latencies vary among individuals, diagnostic groups, and format (word/picture). For example, the results could be skewed by a scaling effect, whereby a normal priming effect for a 2-second RT might be larger than a normal priming effect for a 1-second RT because there is more room for improvement for an initial RT of 2 seconds than of 1 second. The proportion score is one way to account for this potential confound.
Four priming indices, 1 for each condition (WP, PW, PP, and WW), were computed for each subject using the formula:
To avoid bias in the priming scores by format (word/picture), primes of the same format (word or picture) were used as both the minuend and divisor for each condition. For example, in the PW condition the mean RTs for all target words in the PW condition was subtracted from the mean RTs of all prime words and this difference was then divided by the mean RT of all prime words to yield the priming index (Appendix). As a result, each subject had 4 priming indices, 1 for each condition that was used for the remainder of the analyses.
Individual 1-sample t tests were used to determine if the priming effects were greater than zero for each diagnostic group by condition. To examine the priming index and the percent of correct responses, a repeated measures ANOVA was used with 1 between-subjects factor: (diagnostic group, 2 levels: PPA and NC) and 1 within-subjects factor (condition, 4 levels: PW, WP, WW, and PP). Post-hoc comparisons were made using t tests to identify differences between the groups by condition. For the 1-sample t tests and for any pairwise comparisons between the 2 groups by condition, a Bonferroni correction was used with a criterion for significance of P < 0.0125 (0.05/4).
Items misidentified as being natural or manmade and/or response latencies less than 485 ms (2.5 standard deviations below the uncorrected mean) and/or greater than 4 seconds were counted as errors and excluded from analysis.
RESULTS
The common feature shared by all 12 PPA patients was the combination of abnormal BNT, decreased category fluency, and preserved word comprehension. These characteristics are consistent with a classification of all patients in the nonsemantic PPA group.
On average, the PPA group correctly discriminated natural items from manmade items with 90% accuracy (range, 76% to 97%) and the controls averaged 98% accuracy (range, 88.0% to 100%). The ANOVA demonstrated that the percentage of correct responses for the PPA group was significantly lower than the NC group (P < 0.004). There were no significant differences in accuracy by condition (PW, WP, WW, and PP) within the PPA group (P = 0.725).
Priming Effects Within Each Group by Condition
Results from the individual t tests revealed a priming effect for each of the 4 conditions (PW, WP, WW, and PP; all P < 0.001) in the NC's, whereas the PPA group only showed significant priming effects for the PP condition (P < 0.001; Fig. 3).
FIGURE 3.
Priming effects within each group by condition. *Indicates priming effects that are significantly greater than zero (P<0.05). Values are reported as means and (standard errors).
The ANOVA demonstrated significant differences by condition within the PPA group (P < 0.001). Post-hoc comparisons showed that the priming effects for the PP condition were significantly larger than for each of the other conditions (PW, WP, and WW; P ≤ 0.0125). The PW condition showed the smallest average priming effect (mean = -0.002); however, this mean was not statistically different from the WP or WW conditions after a Bonferroni correction.
Between Group Comparisons by Condition
The repeated measures ANOVA revealed significant main effects: (1) diagnosis, [F(1, 28) = 14.8, P = 0.001] and (2) condition [F(3, 28) = 15.4, P≤0.001] and a significant interaction effect of diagnosis × condition [F(3, 28) = 3.1, P = 0.03]. Post-hoc comparisons showed that the PPA patients had significantly reduced priming effects for 3 of the 4 conditions (PW, WP, and WW) compared with the controls (P < 0.009). Performance on the PP condition did not differ between groups (P = 0.78).
To illustrate individual patterns, priming magnitudes for each subject were graded on a scale from 0 to 3 (Table 3). The score of 0 indicated no priming and 1 to 3 reflected the 3 levels obtained by dividing the maximum Priming Index Score for all subjects into 3 equal ranges. The table shows the considerable heterogeneity of priming patterns in patients and controls. However, there were several general findings that reflected the group results. The PP condition was the only one where all patients showed priming effects and the majority of PPA patients (67%) had priming magnitudes above 1. In the other 3 conditions, only 8 of the 36 (22%) priming scores in the PPA group had a magnitude above 1. In comparison, 31 of 54 (57%) of priming scores in the PW, WP, and WW conditions had a magnitude greater than 1 in the control group. The PW condition seemed to be the least likely to demonstrate a priming effect, as only 5/18 (28%) of controls reached a magnitude above 1. In the PPA group, a PW priming effect above a magnitude of 1 was reached in only 1 of the 12 patients (8% of the group). There was no obvious relation of PW priming to BNT or category fluency scores although subject no. 10 who had the best PW priming score also had one of the 3 highest BNT scores. All subjects obtained nearly perfect scores in the WAB comprehension subtest so that the scores of this test could not be correlated with priming scores in the WP and WW conditions that might have been expected to reflect processes related to word comprehension.
TABLE 3.
Magnitude of Priming by Condition for Individual Subjects
| Subject No. | PW | WP | WW | PP |
|---|---|---|---|---|
| NC Magnitude of Priming by Condition | ||||
| 1 | 1 | 3 | 3 | 2 |
| 2 | 1 | 1 | 2 | 2 |
| 3 | 1 | 2 | 2 | 1 |
| 4 | 1 | 1 | 2 | 2 |
| 5 | 1 | 2 | 2 | 3 |
| 6 | 2 | 1 | 1 | 2 |
| 7 | 1 | 3 | 2 | 3 |
| 8 | 2 | 2 | 2 | 3 |
| 9 | 1 | 2 | 3 | 2 |
| 10 | 2 | 2 | 3 | 2 |
| 11 | 1 | 2 | 1 | 3 |
| 12 | 2 | 1 | 2 | 0 |
| 13 | 0 | 2 | 1 | 0 |
| 14 | 1 | 2 | 2 | 2 |
| 15 | 1 | 1 | 1 | 2 |
| 16 | 1 | 2 | 2 | 3 |
| 17 | 1 | 1 | 2 | 2 |
| 18 | 2 | 2 | 3 | 3 |
| PPA Magnitude od Priming by Condition | ||||
| 1 | 1 | 0 | 2 | 3 |
| 2 | 1 | 0 | 1 | 1 |
| 3 | 0 | 0 | 0 | 1 |
| 4 | 1 | 2 | 1 | 3 |
| 5 | 0 | 1 | 1 | 2 |
| 6 | 0 | 2 | 1 | 2 |
| 7 | 0 | 0 | 2 | 2 |
| 8 | 0 | 0 | 2 | 1 |
| 9 | 1 | 2 | 0 | 3 |
| 10 | 2 | 0 | 0 | 1 |
| 11 | 1 | 2 | 0 | 2 |
| 12 | 1 | 0 | 1 | 2 |
| Key Code | Priming Index Cut-off | % Priming | ||
|---|---|---|---|---|
| 0 | <0 | No priming | ||
| 1 | 0-0.063 | 0%-33% | ||
| 2 | 0.064-0.0126 | 34%-66% | ||
| 3 | > 0.126 | > 66% |
Priming magnitudes were graded on a scale from 0 to 3 for each subject. The score of 0 indicated no priming and 1-3 reflected the 3 levels obtained by dividing the maximum Priming Index Score for all subjects into 3 equal ranges.
NC indicates normal control; PP, picture-picture; PW, picture-word; WP, word-picture; WW, word-word.
DISCUSSION
The anomia in nonsemantic variants of PPA has been investigated by contrasting overt picture naming according to word-class (verbs vs. nouns), semantic category (eg, living vs. nonliving items), and error type (semantic vs. phonemic).34-39 Our experiment did not involve overt naming but probed covert aspects of word and picture processing.
We designed a novel task, based on the general principles of priming, for the chronometric assessment of automatic neural processes that link names and pictures of objects to each other. The task did not involve overt naming and required the subject to process a written word or pictorial stimulus by determining whether it was natural or manmade. Priming was measured by comparing the RT at first presentation of a word or picture to the RT upon seeing a semantically identical stimulus of the same format (WW and PP) or of the corresponding format (WP and PW).
In the group of control subjects, exposure to the name or picture of a concrete object triggered a set of neural responses that automatically speeded the processing of the corresponding word or picture in all 4 priming conditions described above. The presence of these priming effects in the control group establishes the face validity of the task we used. Even in controls, patterns of priming magnitudes displayed individual variations although none of the control subjects had any impairment in the BNT or category fluency. It seems that this chronometric priming task probes covert aspects of lexical processing that may have no one-to-one relationship to traditional neuropsychologic tasks such as the BNT and category fluency.
The PPA patients showed less efficient priming than controls in the PW condition. Although there was no obvious concordance of PW priming magnitudes with BNT scores at the level of individual patients, it is reasonable to assume that this type of priming probes the associative linkage of objects to corresponding lexical labels, a process that was defective in the patient group we tested. The patients had no impairment of word comprehension when tested by standardized tests and could readily point to the appropriate object upon hearing or seeing its name during routine clinical examination. We therefore expected that the corresponding WP priming condition might be intact. The somewhat unexpected weakness of WP priming in the patient group suggests that the information processing deficit in nonsemantic PPA may not be confined to the stage of lexical access but that it may also extend, at least in part, into the more upstream semantic stage. Conceivably, more challenging tasks of word comprehension might have elicited subtle impairments to match the weakness of WP priming in at least some of our patients, indicating that comprehension is relatively preserved but not necessarily intact even in nonsemantic PPA variants. However, it is also important to point out that the correspondence between WP priming and overt word comprehension, although plausible, has not yet been established.
Our results are consonant with 2 previous experiments from our laboratory. In 1 investigation on mostly nonsemantic PPA patients, it was shown that exposure to a word unexpectedly slowed down the naming of a semantically related object.40 This finding suggested that the priming word had activated the appropriate semantic field but that it paradoxically slowed the naming of a related object because of a deficit in the ability to discriminate among members of the same semantic group. This is somewhat similar to the situation in spatial attention where target detection becomes progressively slower as the target is made more similar to the distracters.41 In another experiment, it was shown that nonsemantic PPA patients were not as good as controls in verbal memory tasks because of false-positive recognition of semantically related items.42 These results suggest that nonsemantic PPA patients may have impairments that are not limited to lexical access and that may extend to the level of semantic mapping. Such a possibility would support the view that PPA is a syndrome of the language network as a whole, such that, individual patients may differ in the type of language function that is most severely impaired but no function of the language network remains completely spared.
The within-format priming of identical stimuli in the WW and PW conditions could reflect shallow perceptual processing and also the deeper semantic encoding elicited by the requirement to decide if the stimulus represented a natural or manmade object. We did not expect to see an impairment of WW priming in our patients, because they had no difficulty in matching identical words or understanding words in traditional clinical tests. The chronometric assessment of priming in this task seems to have provided a more sensitive measure of the neural processes related to the overall integrity of the language network. Presumably words automatically elicited semantic associations that mediated at least some of the priming effects of the WW condition, a process that would be vulnerable to the degeneration of the language network in PPA. By the same token, the preservation of the PW priming indicates that perceptual encoding and perhaps also nonverbal semantics were preserved in these PPA patients. It will be interesting to see if this sort of priming is impaired in semantic PPA.
In conclusion, the validity of a novel task for assessing relatively automatic neural processes that interlink names and pictures of objects was demonstrated through significant priming effects obtained in neurologically intact control subjects. Nonsemantic variants of PPA, identified by the presence of confrontation naming deficits in the BNT but preserved word comprehension in standard tests, showed weaker priming effects not only in the PW but also in the reciprocal WP condition, indicating that the impairment in linking the picture of an object to its name in these patients may have a reciprocal component even when the clinical testing seems to show a deficit confined to confrontation naming.
Acknowledgments
Supported by the Ruth L. Kirschstein National Research Service Award no. F31 NS055557; National Institute of Neurological Disorders and Stroke (NINDS); Alzheimer's Disease Center Grant AG13854 NIA; Cellular and Behavioral Aspects of Aging and Dementia Training Grant AG20506; and the National Institute on Deafness and Other Communication Disorders DC008552.
APPENDIX
Priming Index Computations by Condition
REFERENCES
- 1.Mesulam MM. Primary progressive aphasia. Ann Neurol. 2001;49:425–432. [PubMed] [Google Scholar]
- 2.Turner RS, Kenyon LC, Trojanowski JQ, et al. Clinical, neuroimaging, and pathologic features of progressive nonfluent aphasia. Ann Neurol. 1996;39:166–173. doi: 10.1002/ana.410390205. [DOI] [PubMed] [Google Scholar]
- 3.Chawluk JB, Mesulam MM, Hurtig H, et al. Slowly progressive aphasia without generalized dementia: studies with positron emission tomography. Ann Neurol. 1986;19:68–74. doi: 10.1002/ana.410190112. [DOI] [PubMed] [Google Scholar]
- 4.Mesulam MM, Weintraub S. Primary progressive aphasia: Sharpening the focus on a clinical syndrome. In: Boller F, Forette F, Khachaturian Z, et al., editors. Heterogeneity of Alzheimer's Disease. Springer Verlag; Berlin, Germany: 1992. pp. 43–66. [Google Scholar]
- 5.Gorno-Tempini ML, Dronkers NF, Rankin KP, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55:335–346. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rogalski E, Mesulam M. An update on primary progressive aphasia. Curr Neurol Neurosci Rep. 2007;7:388–392. doi: 10.1007/s11910-007-0060-0. [DOI] [PubMed] [Google Scholar]
- 7.Adlam AL, Patterson K, Rogers TT, et al. Semantic dementia and fluent primary progressive aphasia: two sides of the same coin? Brain. 2006;129:3066–3080. doi: 10.1093/brain/awl285. [DOI] [PubMed] [Google Scholar]
- 8.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration. A consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 9.Weintraub S, Rubin NP, Mesulam MM. Primary progressive aphasia. Longitudinal course, neuropsychological profile, and language features. Arch Neurol. 1990;47:1329–1335. doi: 10.1001/archneur.1990.00530120075013. [DOI] [PubMed] [Google Scholar]
- 10.DeLeon J, Gottesman RF, Kleinman JT, et al. Neural regions essential for distinct cognitive processes underlying picture naming. Brain. 2007;130:1408–1422. doi: 10.1093/brain/awm011. [DOI] [PubMed] [Google Scholar]
- 11.Hillis AE. Aphasia: progress in the last quarter of a century. Neurology. 2007;69:200–213. doi: 10.1212/01.wnl.0000265600.69385.6f. [DOI] [PubMed] [Google Scholar]
- 12.Mesulam MM. From sensation to cognition. Brain. 1998;121:1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- 13.Levelt WJ, Roelofs A, Meyer AS. A theory of lexical access in speech production. Behav Brain Sci. 1999;22:1–38. doi: 10.1017/s0140525x99001776. discussion 38-75. [DOI] [PubMed] [Google Scholar]
- 14.Ellis AW, Young AW. Human Cognitive Neuropsychology. Lawrence Erlbaum Associates; London: 1988. [Google Scholar]
- 15.Caramazza A. How many levels of processing are there in lexical access? Cogn Neuropsychol. 1997;14:177–208. [Google Scholar]
- 16.Jefferies E, Lambon Ralph MA. Semantic impairment in stroke aphasia versus semantic dementia: a case-series comparison. Brain. 2006;129:2132–2147. doi: 10.1093/brain/awl153. [DOI] [PubMed] [Google Scholar]
- 17.Lambon Ralph MA, Graham KS, Ellis AW, et al. Naming in semantic dementia-what matters? Neuropsychologia. 1998;36:775–784. doi: 10.1016/s0028-3932(97)00169-3. [DOI] [PubMed] [Google Scholar]
- 18.Tulving E, Schacter DL. Priming and human memory systems. Science. 1990;247:301–306. doi: 10.1126/science.2296719. [DOI] [PubMed] [Google Scholar]
- 19.Thompson-Schill SL, Kan IP. Perceptual and conceptual sources of priming on a word generation task. Mem Cognit. 2001;29:698–706. doi: 10.3758/bf03200472. [DOI] [PubMed] [Google Scholar]
- 20.Donders FC. On the speed of mental processes. In: Koster WG, editor. Attention and Performance II. Acta Psychologica. North Holland Publishing; Netherlands: 1969. pp. 412–431. [DOI] [PubMed] [Google Scholar]
- 21.Gorno-Tempini M, Hillis A, Grossman M, PPA Subtyping Task Group 5th International Conference on Frontotemporal Dementia; San Francisco, CA: 2006. [Google Scholar]
- 22.Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20:210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 23.Kertesz A. Western Aphasia Battery. The Psychological Corporation; San Antonio, TX: 1982. [Google Scholar]
- 24.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 25.Osher JE, Wicklund AH, Rademaker A, et al. The mini-mental state examination in behavioral variant frontotemporal dementia and primary progressive aphasia. Am J Alzheimers Dis Other Demen. 2007;22:468–473. doi: 10.1177/1533317507307173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wicklund AH, Johnson N, Rademaker A, et al. Profiles of decline in activities of daily living in non-Alzheimer dementia. Alzheimer Dis Assoc Disord. 2007;21:8–13. doi: 10.1097/WAD.0b013e3180324549. [DOI] [PubMed] [Google Scholar]
- 27.Johnson N, Barion A, Rademaker A, et al. The Activities of Daily Living Questionnaire: a validation study in patients with dementia. Alzheimer Dis Assoc Disord. 2004;18:223–230. [PubMed] [Google Scholar]
- 28.Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Lea and Febiger; Philadelphia: 1983. [Google Scholar]
- 29.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 30.Rossion B, Pourtois G. Revisiting Snodgrass and Vanderwart's object pictorial set: the role of surface detail in basic-level object recognition. Perception. 2004;33:217–236. doi: 10.1068/p5117. [DOI] [PubMed] [Google Scholar]
- 31.Craik FIM, Lockhart RS. Levels of processing: a framework for memory research. Journal of Verbal Learning and Verbal Behavior. 1972;11:671–684. [Google Scholar]
- 32.Craik FIM, Tulving E. Depth of processing and the retention of words in episodic memory. J Exp Psychol. 1975;104:268–294. [Google Scholar]
- 33.Paller KA, Mayes AR, Thompson KM, et al. Priming of face matching in amnesia. Brain Cogn. 1992;18:46–59. doi: 10.1016/0278-2626(92)90110-8. [DOI] [PubMed] [Google Scholar]
- 34.Thompson CK, Ballard K, Tait M, et al. Patterns of language decline in non-fluent primary progressive aphasia. Aphasiology. 1997;11:297–321. [Google Scholar]
- 35.Hillis AE, Oh S, Ken L. Deterioration of naming nouns versus verbs in primary progressive aphasia. Ann Neurol. 2004;55:268–275. doi: 10.1002/ana.10812. [DOI] [PubMed] [Google Scholar]
- 36.Clark DG, Charuvastra A, Miller BL, et al. Fluent versus nonfluent primary progressive aphasia: a comparison of clinical and functional neuroimaging features. Brain Lang. 2005;94:54–60. doi: 10.1016/j.bandl.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 37.Cotelli M, Borroni B, Manenti R, et al. Action and object naming in frontotemporal dementia, progressive supranuclear palsy, and corticobasal degeneration. Neuropsychology. 2006;20:558–565. doi: 10.1037/0894-4105.20.5.558. [DOI] [PubMed] [Google Scholar]
- 38.Tyler LK, Stamatakis EA, Jones RW, et al. Deficits for semantics and the irregular past tense: a causal relationship? J Cogn Neurosci. 2004;16:1159–1172. doi: 10.1162/0898929041920559. [DOI] [PubMed] [Google Scholar]
- 39.McMillan C, Gee J, Moore P, et al. Confrontation naming and morphometric analyses of structural MRI in frontotemporal dementia. Dement Geriatr Cogn Disord. 2004;17:320–323. doi: 10.1159/000077163. [DOI] [PubMed] [Google Scholar]
- 40.Vandenberghe RR, Vandenbulcke M, Weintraub S, et al. Paradoxical features of word finding difficulty in primary progressive aphasia. Ann Neurol. 2005;57:204–209. doi: 10.1002/ana.20362. [DOI] [PubMed] [Google Scholar]
- 41.Verghese P. Visual search and attention: a signal detection theory approach. Neuron. 2001;31:523–535. doi: 10.1016/s0896-6273(01)00392-0. [DOI] [PubMed] [Google Scholar]
- 42.Rogalski E, Blum D, Rademaker A, et al. False recognition of incidentally learned pictures and words in primary progressive aphasia. Neuropsychologia. 2007;45:368–377. doi: 10.1016/j.neuropsychologia.2006.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]