Abstract
Apolipoprotein E (apoE) is a protein mainly synthesized in the liver and brain. To further understand the role of brain apoE in the management of daily food intake, we have examined the circadian pattern of hypothalamic apoE gene and protein expression in freely-fed (FF) and food-restricted (RF, food provided 4 h daily between 1000 h and 1400 h) rats sacrificed at 3-h intervals throughout the light-dark cycle. In FF rats, hypothalamic apoE mRNA and protein levels fluctuated with peaks occuring during the dark phase and the nadirs occuring during the light phase. This pattern was altered in RF rats, which had a marked increase in hypothalamic apoE mRNA and protein levels during the 4-h feeding period in the light phase. Although corticosterone (CORT) levels temporally coincided with the increasing phase of apoE in the hypothalamus in both FF and RF rats, depletion of CORT by adrenalectomy (ADX) did not significantly influence the hypothalamic apoE levels during either period, implying that the circadian pattern of hypothalamic apoE is regulated by factors other than circulating CORT. The finding that hypothalamic apoE and food intake are positively associated during the normal circadian cycle as well as in the period of restricted feeding suggests that hypothalamic apoE is food-entrained and likely involved in the physiological regulation of daily food intake.
Keywords: circadian rhythm, apolipoprotein, food restriction, hypothalamus
1. Introduction
Apolipoprotein E (apoE) is an important protein required for the homeostasis of cholesterol and other lipids in the circulation [Schaefer et al., 1986;Plump et al., 1992]. It facilitates transport of high-density lipoprotein (HDL) and very low density lipoprotein (VLDL) particles, and it initiates transport of cholesterol and phospholipids into cells [Mahley, 1988]. Although apoE is synthesized in several areas of the body, the liver accounts for the major portion of circulating apoE. ApoE is a ligand for the low density lipoprotein (LDL) receptor family [Herz and Bock, 2002;Kim et al., 1997;Kim et al., 1996]. Previous studies have demonstrated that mutations to the LDL receptor are associated with high plasma cholesterol levels and lead to premature atherosclerosis in humans and experimental animals [Rall, Jr. et al., 1989;Wardell et al., 1987].
The brain is also a major site of apoE expression in humans and rodents [Boyles et al., 1985], and it is possible that it also plays an important role in lipid homeostatic mechanisms in the brain. Recently, apoE has been proposed as a physiological controller of food intake, and the inhibition of food intake by apoE is mediated centrally. Intracerebroventricular administration of apoE dose-dependently reduces food intake without eliciting signs of toxicity. Blocking the action of endogenous apoE by central administration of its antibody increases meal size, implying that endogenous brain apoE exerts an inhibitory tone on feeding [Shen et al., 2008]. ApoE has been demonstrated to be present in the hypothalamus, and hypothalamic apoE gene expression is reduced by food deprivation and restored by chow re-feeding [Shen et al., 2008]. In view of these effects on feeding elicited by apoE, we hypothesized that under physiological conditions, feeding behavior and levels of apoE in the hypothalamus will be closely associated. We specifically hypothesized that hypothalamic apoE expression will be increased during times of active feeding because a higher level of apoE, being a satiation factor, would circumvent overeating [Woods, 1991]. To test this hypothesis, gene and protein expression of hypothalamic apoE were assessed in freely feeding (FF) rats at selected times across the 24-h period. The existence of a circadian pattern of hypothalamic apoE would be consistent with the possibility that its circadian periodicity plays a role in the regulation of feeding. To determine if there is a causal relationship between feeding and hypothalamic apoE, a restricted feeding (RF) paradigm, which constrains feeding to a specific period during the light phase while leaving the 24-h light-dark cues unchanged [Krieger and Hauser, 1978], was used in one group of animals.
A role of adrenal glucocorticoids in the regulation of feeding and the development of obesity has been well demonstrated. The peak and nadir of glucocorticoid circadian secretion over 24 h coincide with the initiation and termination, respectively, of the active feeding period and associated locomotor activities [Akana et al., 1994;Akana et al., 1999]. Central administration of corticosterone (CORT) or its analogs stimulates food intake and promotes obesity [Tempel and Leibowitz, 1994;Therrien and Drouin, 1993]. Most obese rodent models are hypercorticosteronemic [Green et al., 1992;Zakrzewska et al., 1999]. However, no studies have examined the effects of CORT on apoE gene and protein expression in the hypothalamus. Thus, a second aim of the present study was to examine the circadian profiles of hypothalamic apoE expression and their relation to circulating levels of CORT.
2. Results
2.1. Circadian pattern of food intake
Food intake was monitored every 3 h throughout the 24-h light/dark cycle. In the free feeding condition, food intake in the light phase was low, with a dramatic increase occurring just after the onset of dark (1800 h), and active food intake was maintained from 1800-0300 h. Subsequently, food intake declined until lights-on at 0600 h. Food intake during the light period was 4.1 ± 0.5 g and during the dark period was 24.3 ± 1.2 g (P < 0.01). Thus, as observed in many experiments, food intake of FF rats had a strong nocturnal feeding pattern.
In the RF rats, all of the food intake occurred in the 4 h that food was available from 1000 to 1400 h. During the first 3 days of the restricted feeding paradigm (food available only from 1000 to 1400 h), animals lost body weight. By Day 9, the rats had regained their initial body weight and then gained weight at a steady but slower rate than the FF rats. After 4 weeks, the body weight of RF rats was 12% less than that of FF rats (P < 0.05) and reflected lower average food consumption. For example, on Day 26, the RF rats consumed 20.8 ± 0.80 g compared with 28.4 ± 0.75 g for FF rats (P < 0.05).
In the following results, apoE mRNA and protein, and plasma CORT levels were determined at 3 h intervals across a 24-h period in two groups (FF and FR) of rats.
2.2. Circadian pattern of hypothalamic apoE mRNA and protein in FF rats
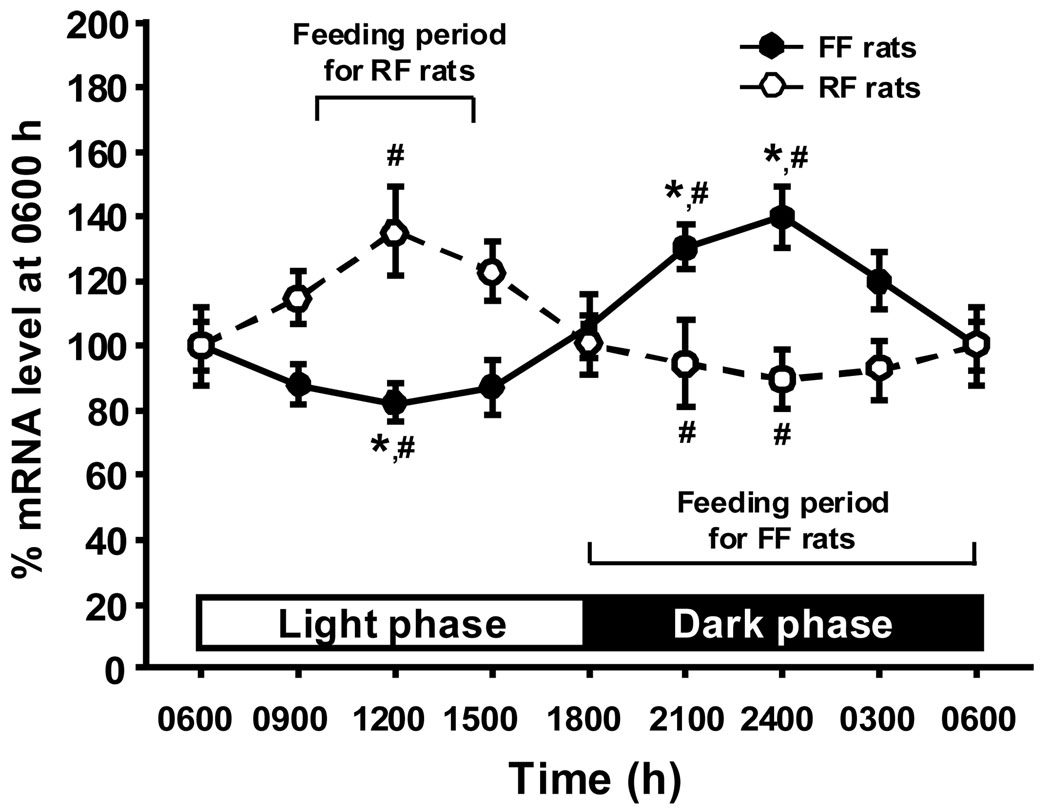
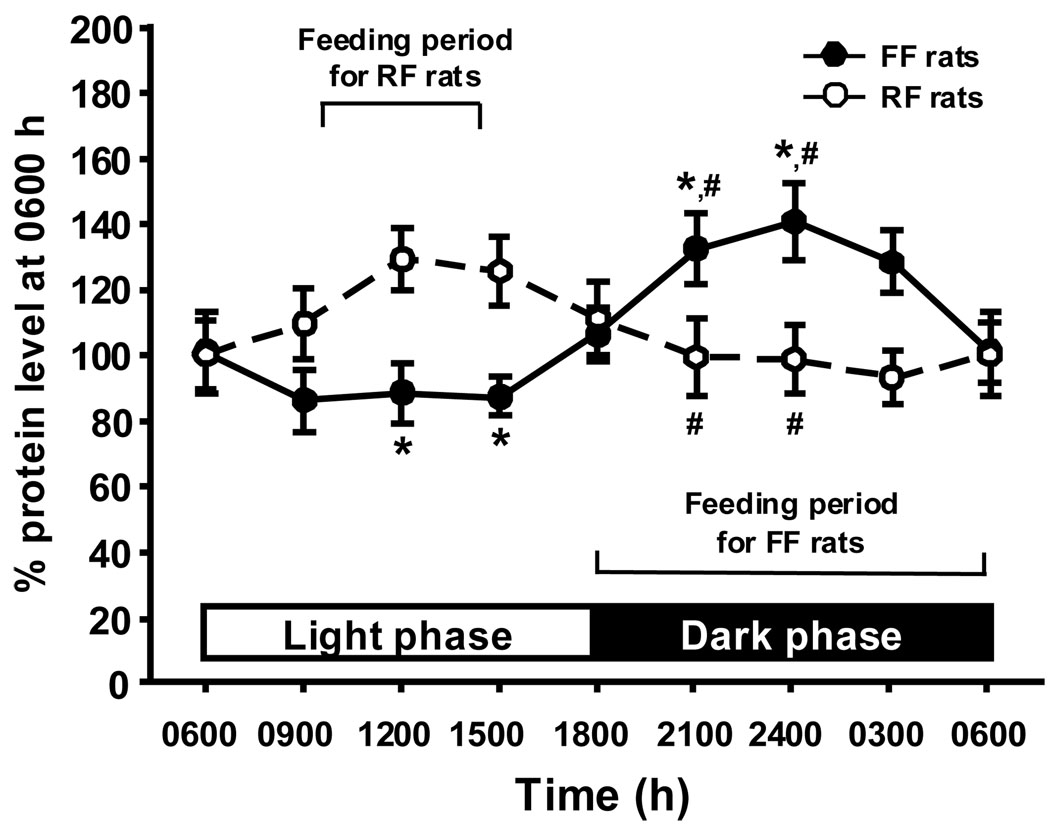
In FF rats, hypothalamic apoE mRNA levels and food intake were positively associated throughout the day. As depicted in Fig. 1, apoE mRNA level decreased starting around 0600 h and remained low until 1500 h. ApoE mRNA then increased until 1800 h (during the dark), with peak levels at 2400 h. The relatively high level was sustained throughout the dark phase (Fig. 1). Mean hypothalamic apoE mRNA in the light (from 0600–1800 h) was significantly lower than in the dark (1800-0600 h, P < 0.01). Statistical analysis indicated that the levels of apoE mRNA at 0900, 1200 and 1500 h in the light cycle differed significantly from those at 2100 and 2400 h in the dark cycle (F(7, 29) = 7.242, P < 0.05 for each comparison, Fig. 1). Protein analysis measured by Western blot confirmed that the circadian fluctuation of hypothalamic apoE protein (Fig. 3) was highly parallel with apoE mRNA.
Fig 1.
Comparison of circadian rhythms in hypothalamic apoE mRNA levels between FF and RF rats. The apoE mRNA values are depicted as a percentage of apoE mRNA levels at 0600 h set at 100%. Values are expressed as means ± SEM, n = 4 – 6. * P < 0.05, significant differences at the tested time points between light and dark phases in FF rats. #P < 0.05, significant differences at the same time points between the FF rats and RF rats.
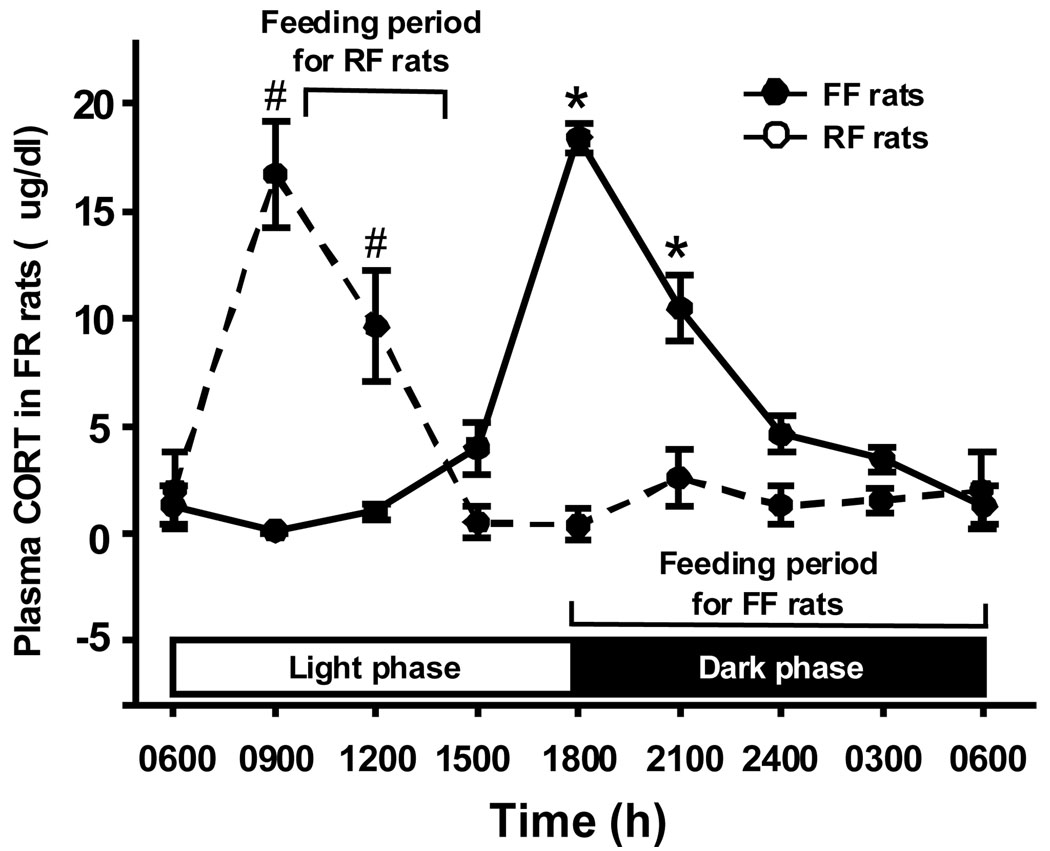
Fig 3.
Circadian changes in plasma CORT levels in rats. Values are expressed as means ± SEM, n = 5–6 (for FF rats), and n = 4–6 (for RF rats). *P < 0.05, significantly different, compared with all other time points in FF rats. # P < 0.05, significantly different, compared with all other time points in RF rats.
2.3. Circadian pattern of hypothalamic apoE mRNA and protein in RF rats
As depicted in Figure 1 and Figure 2, RF rats had a marked increase in hypothalamic apoE mRNA and protein levels during the 4-h feeding period in the middle of the light phase. In contrast to the decrease observed during the light in FF rats, the apoE levels started to increase in the RF rats when the animals started to eat, and the difference between the two groups at 1200 h (midpoint of the 4 h of feeding) was statistically signficant (F(7, 53) = 7.765, P < 0.05). After the 4 h of active feeding, hypothalamic apoE levels in RF rats started to decrease, and low levels of apo E mRNA and protein were sustained throughout the dark phase (Fig. 1 and Fig 2). The difference in hypothalamic apoE mRNA between the two groups was statistically signficant at 2100 and 2400 h (P < 0.05).
Fig 2.
Comparison of rhythmic changes in hypothalamic apoE protein levels between FF and RF rats. The apoE protein values are depicted as a percentage of apoE protein levels at 0600 h set at 100%. Values are expressed as means ± SEM, n = 4 – 6. *P < 0.05, significant differences at the tested time points between light and dark phases in FF rats.#P < 0.05, significant differences at the same time points between the FF rats and RF rats.
2.4. Daily fluctuations of plasma CORT levels in FF and RF rats
Consistent with previous reports [Wilkinson et al., 1979], circulating CORT had a well-defined circadian pattern in FF rats (Fig. 3). Plasma CORT levels were low in the light (from 0600–1500 h), ranging from 0.1–1.3 µg/dl and started to increase late in the light phase, with a peak at the time of the light/dark transition (1800 h; 18.5 ± 0.67 µg/dl). Subsequently, plasma CORT declined to 10.5 ± 1.57 µg/dl at 2100 h, and to 3.4 ± 0.58 µg/dl 3 h before returning to the nadir at 0600 h. Statistical analysis indicated that the levels of CORT at 1800 and 2100 h differed significantly from all other tested time points in FF rats (F(7, 29) = 47.708, P < 0.01, Fig. 3).
Food availability restricted to 1000–1400 h shifted the pattern of plasma CORT. CORT levels rose at 0900 h, preceding the period of food availability, and returned to the basal range at 1500 h, one hour after feeding (Fig. 3). An important point is that a sharp rise in CORT preceded the increasing phase of apoE expression in the hypothalamus of both FF and RF rats. Specifically, the peak levels of apoE expression lagged 3–6 h behind the peak plasma CORT (Fig. 3). Statistical analysis indicated that the levels of CORT at 0900 and 1200 h differed significantly from all other tested time points in FF rats (F(7, 35) = 13.678, P < 0.01, Fig. 3).
2.5. Effect of ADX on apoE protein expression in the hypothalamus
In SHAM-operated rats, plasma CORT had a similar pattern as observed in intact FF rats, for example, the peak level of CORT at 2100 h was 9.4 ± 2.48 µg/dl in SHAM rats, compared with 10.5 ± 1.57 µg/dl in FF rats. CORT was undetectable in ADX rats. ADX caused a significant reduction in body weight (−11.5 ± 2.68 g from the presurgical body weight), whereas SHAM controls gained 17.3 ± 3.03 g from their presurgical body weight over the same 7 d. Daily food intake was reduced in ADX rats over the 7 d after surgery, but the general feeding pattern was not altered by ADX (data not shown).
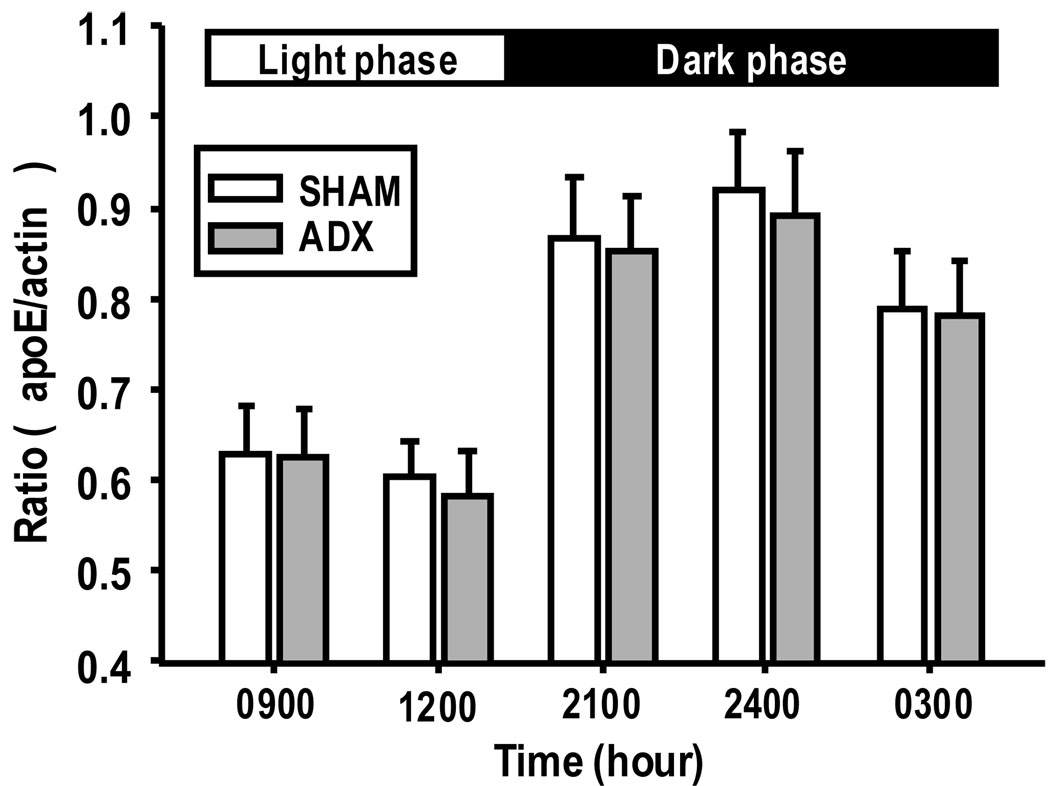
To address the question as to whether CORT regulates hypothalamic apoE, the levels of apoE protein in the hypothalamus were compared in SHAM and ADX rats sacrificed at 0900 and 1200 h, two time points with a relatively low level of apoE in FF rats, as well at 2100, 2400 and 0300 h, three time points with a relatively high level of apoE in FF rats. These time points were selected because they represented significant changes in both plasma CORT and hypothalamic apoE levels across a 24-h period. SHAM controls had similar levels of apoE protein in the hypothalamus (Fig. 4) as observed in the unoperated FF rats at all time points evaluated (Fig. 3), and quantitative analyses indicated no significant difference in hypothalamic apoE protein levels between ADX rats and SHAM-operated controls (Fig. 4).
Fig 4.
Effect of ADX on the expression of apoE protein levels in the hypothalamus. No significant difference was found in apoE protein levels between SHAM and ADX rats at any of the tested time points. Values are expressed as means ± SEM, n = 5–6.
3. Discussion
The present studies demonstrate for the first time that apoE mRNA and protein expression in the hypothalamus of FF rats varies with the light cycle. The circadian pattern of hypothalamic apoE mRNA and protein levels were positively associated with the pattern of food intake; i.e., all of these parameters were significantly higher during the dark than during the light, suggesting that the presence and consumption of food during the dark period may increase the nocturnal gene and protein expression of apoE in the hypothalamus. Because these parameters are only correlated, it was important to determine whether the changes in hypothalamic apoE levels were due to the light cycle per se or to the increased food consumption in these FF rats that normally occurs in the dark.
To differentiate these two possibilities, we performed an additional experiment on restricted fed rats. In that experiment, the period of food availability was restricted to a 4-h period in the light phase (1000 h to 1400 h). The RF animals initially lost weight, but soon began to regain the lost body weight and gain weight at a significantly reduced rate. Despite an unchanged light-dark cycle, the circadian pattern of hypothalamic apoE expression was modified significantly in the RF rats as compared to the FF rats (Fig. 2). Instead of increasing during the dark as occurred in FF rats, the peak of hypothalamic apoE mRNA was shifted to the light phase, coinciding with the actual period of feeding. Thus, it would appear that the period of food availability and/or active feeding caused the hypothalamic apoE gene expression to shift and increase at a different time. The fact that hypothalamic apoE expression started increasing prior to actual food availability indicates that it is an example of a learned meal-related anticipatory response [Woods, 1991;Strubbe and Woods, 2004]. Future studies should address the specific localization of cephalic apoE within the hypothalamus by in-situ hybridization and/or immunohistochemistry, because it is possible that it is localized to specific sites in the hypothalamus.
The pattern of hypothalamic apoE in both FF and RF rats is consistent with our current understanding of the role of brain apoE in the regulation of food intake. Central administration of apoE dose-dependently reduces food intake without causing malaise [Shen et al., 2008], and blocking the action of endogenous apoE with a specific antibody increases food intake during the light when there is normally little or no food intake [Shen et al., 2008]. This implies that endogenous apoE, acting within the brain, normally acts to limit food intake. The implication is that hypothalamic apoE acts as a satiation factor limiting meal size in the light.
Fasted rats have decreased hypothalamic apoE gene expression, and following 4 h of refeeding there is a significant increase of hypothalamic apoE mRNA [Shen et al., 2008]. If brain apoE were providing a physiological brake on meal size, hypothalamic apoE levels would be predicted to be low in fasted animals but to increase at times when animals normally eat [Woods, 1991;Strubbe and Woods, 2004], and this is exactly what we observed. The increased apoE at meal time may normally therefore prevent animals from consuming too many calories in one meal. Our current observations therefore lend support to the concept that apoE’s physiological role may be an important modulator of daily food intake.
While the mechanism by which hypothalamic apoE mRNA and protein expression fluctuates diurnally is not clear, analysis of circadian events that precede the phases of increased apoE expression may help identify the key regulatory factors. Wilkinson et al. reported that peak CORT levels occur just prior to feeding in free-feeding rats [Wilkinson et al., 1979], making CORT a potential candidate to regulate hypothalamic apoE. We therefore determined circulating CORT levels in the animals in our FF and RF rats. Peak CORToccurred just before the active feeding period in FF rats (i.e., dark onset), and this peak in CORT was shifted to the time of food availability in RF rats. Interestingly, plasma CORT levels in RF rats rose at the onset of feeding and decreased to the basal range by the end of 4 h of feeding, demonstrating a tight relationship of CORT with the onset of feeding behavior [Dallman et al., 1993]. Analysis of the temporal relationship between CORT and apoE protein revealed a strong correspondence between peak circulating CORT and the increased hypothalamic apoE protein in both FF and RF rats (Fig. 3). The rise in plasma CORT preceded the increase in apoE, suggesting that CORT may act as a signal for increased apoE expression. This possibility, however, was not supported by the observation that ADX did not significantly influence hypothalamic apoE protein, implying that CORT is not necessary for the feeding-related rise in apoE expression, nor permissive for baseline apoE expression. It is more likely that the presence and consumption of food act as a causative factor of the rhythmic fluctuation of apoE. Further experiments will be needed to determine the mechanism(s) as to how the food intake affects hypothalamic apoE gene and protein expression.
In summary, the present results demonstrate that there is a circadian rhythm in hypothalamic apoE mRNA and protein levels in FF rats, and that this pattern of apoE is altered by food restriction. The present results also provide strong evidence that the circadian pattern of apoE protein expression, while normally correlated with, is actually independent of circulating CORT. Other factor(s) which may affect hypothalamic apoE gene and protein expression and the precise mechanism as to how the hypothalamic apoE regulates feeding behavior under physiological conditions remain to be determined.
4. Experimental procedures
4.1. Animals
Adult male Sprague-Dawley rats (Harlan, Indianapolis, IN) were housed individually in a light-and temperature-controlled room (lights on 0600–1800, 21°C). Unless stated otherwise, food (pelleted chow, Teklad Rodent Chow, Harlan) and water were available ad libitum. Animals were allowed to acclimate to these housing conditions for one week before experiments were started. All procedures were performed in accordance with institutional guidelines of the Institutional Animal Care and Use Committee at the University of Cincinnati.
There were 2 experiments. In the first experiment, the rats were allowed to feed freely. In the second experiment, the rats were food restricted with food available only from 1000 h to 1400 h for 4 weeks. Water was available ad libitum to all rats. Body weight, 24-h food intake for FF rats, and 4 h-food intake for RF rats were recorded daily. The animals were sacrificed by decapitation at 3-h intervals (5–6 FF rats/time point and 4–6 RF rats/time point) throughout a 24-h period. Utmost care was taken to cause minimal stress to the rats before decapitation, which was completed within 1 min of disturbing a cage. The brain was rapidly removed from each rat, and the entire hypothalamus was dissected according to the atlas of Paxinos and Watson [Paxinos and Watson, 1982] and immediately frozen in liquid nitrogen. The hypothalamus was stored at −80°C until total RNA and protein extraction. Trunk blood was collected and centrifuged, and plasma was stored at −80°C. Separate aliquots of plasma were taken for assay of CORT levels by radioimmunoassay.
An additional two groups of rats were used for testing the effect of CORT on the circadian variation in hypothalamic apoE protein expression. One group was sham-operated (SHAM), and the other was adrenalectomized (ADX) by a dorsolateral approach to remove the adrenal glands bilaterally [Chao et al., 1998]. Drinking water for all ADX rats was replaced with 0.9% saline. Body weight and food intake were recorded daily after surgery. All of the animals were killed by decapitation at 0900, 1200, 2100, 2400 and 0300 h (5–6 rats per time point), 8 days after surgery. Trunk blood was collected, and the hypothalamus was quickly removed as described above. Only ADX rats with plasma CORT levels less than 1 µg/dl at the time of sacrifice were regarded as indicative of the completeness of ADX and were included in the results.
4.2. Quantitative real time PCR for apoE mRNA measurement
Total RNA (100 ng), extracted from hypothalamus with Tri Reagent (Molecular Research Center, Inc., Cincinnati, OH) was reverse-transcribed to first-strand complementary cDNA (GE Healthcare Bio-Sciences Corp., Piscataway, NJ). Quantitative real time PCR (qPCR) was performed in a 25 µl final reaction volume with an iCycler iQ Detection System using iQ™ SYBR Green Supermix (Bio-Rad, Laboratories Inc., Hercules, CA). qPCR conditions were as follows: 95° C for 3 min for one cycle, followed by 38 cycles of 95° C for 30 sec and 58° C for 30 sec. Melt curve analysis revealed one peak per sample. Threshold cycle (Ct) readings for unknown samples were calculated, and results were analyzed using the delta delta CT method (Perkin-Elmer Applied Biosystems) [Liu et al., 2004]. Cyclophilin was used as the internal control. Primer sequences, listed in Table 1, for rat apoE and cyclophilin were determined using primer design Software (Integrated DNA Technologies, Coralville, IA).
Table 1.
Primer sequences for real-time RT-PCR
4.3. Western blot for apoE protein
Hypothalamic samples were homogenized and centrifuged at 15,000 rpm for 20 min at 4°C. The supernatant (containing 20 µg protein per sample) was separated by 12% polyacrylamide gel electrophoresis, transferred to nitrocellulose sheets, and blotted with a goat polyclonal antibody against apoE (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA). Immuno-complexes were quantified using an enhanced chemiluminescence detection system (Amersham Pharmacia Biotech, Piscataway, NJ). Blots were stripped and re-incubated with a monoclonal antibody against actin (1:10,000, Chemicon International, Inc. Temecula, CA). Membranes were exposed to X-ray film (Kodak Scientific imaging film, Rochester, NY), and image density, measured as transmittance, was expressed as volume-adjusted optical density. The amount of apoE protein was normalized to the respective individual density values reflecting actin protein level and is expressed as a ratio.
4.4. Measurement of plasma CORT
Total plasma CORT was measured by radioimmuniassay (RIA) using rabbit antiserum raised against CORT (B3-163) obtained from Endocrine Sciences (Calabasas Hills, CA). Briefly, 20 µl duplicate samples of plasma were heated at 60°C for 2 h to denature binding protein and were incubated overnight with CORT antibody. [3H]corticosterone (NEN Life Sciences, Inc. Boston, MA) was used as a radioactive tracer. Free and bound CORT were separated by incubating with charcoal. CORT concentrations were calculated using an equation derived from a standard curve.
4.5. Statistical analysis
Daily body weight is presented as percent of initial body weight. Data were analyzed by one-way (the time-course data of apoE mRNA and protein levels) or two-way (the time-course data of apoE mRNA levels between FF and RF groups) analysis of variance (ANOVA), followed by Tukey’s multiple comparison. Results are expressed as the mean ± SEM, and P < 0.05 is considered statistically significant.
Acknowledgements
This work was supported by research grants from National Institutes of Health DK70992, DK63907, and DK067550.
Abbreviations
- apoE
apolipoprotein E
- FF
free feeding
- RF
restricted feeding
- CORT
corticosterone
- ADX
adrenalectomy
- qPCR
quantitative real time PCR
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akana SF, Strack AM, Hanson ES, Dallman MF. Regulation of activity in the hypothalamo-pituitary-adrenal axis is integral to a larger hypothalamic system that determines caloric flow. Endocrinology. 1994;135:1125–1134. doi: 10.1210/endo.135.3.8070356. [DOI] [PubMed] [Google Scholar]
- Akana SF, Strack AM, Hanson ES, Horsley CJ, Milligan ED, Bhatnagar S, Dallman MF. Interactions among chronic cold, corticosterone and puberty on energy intake and deposition. Stress. 1999;3:131–146. doi: 10.3109/10253899909001118. [DOI] [PubMed] [Google Scholar]
- Boyles JK, Pitas RE, Wilson E, Mahley RW, Taylor JM. Apolipoprotein E associated with astrocytic glia of the central nervous system and with nonmyelinating glia of the peripheral nervous system. J Clin Invest. 1985;76:1501–1513. doi: 10.1172/JCI112130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao HM, Sakai RR, Ma LY, McEwen BS. Adrenal steroid regulation of neurotrophic factor expression in the rat hippocampus. Endocrinology. 1998;139:3112–3118. doi: 10.1210/endo.139.7.6114. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Strack AM, Akana SF, Bradbury MJ, Hanson ES, Scribner KA, Smith M. Feast and famine: critical role of glucocorticoids with insulin in daily energy flow. Front Neuroendocrinol. 1993;14:303–347. doi: 10.1006/frne.1993.1010. [DOI] [PubMed] [Google Scholar]
- Green PK, Wilkinson CW, Woods SC. Intraventricular corticosterone increases the rate of body weight gain in underweight adrenalectomized rats. Endocrinology. 1992;130:269–275. doi: 10.1210/endo.130.1.1727703. [DOI] [PubMed] [Google Scholar]
- Herz J, Bock HH. Lipoprotein receptors in the nervous system. Annu Rev Biochem. 2002;71:405–434. doi: 10.1146/annurev.biochem.71.110601.135342. [DOI] [PubMed] [Google Scholar]
- Kim DH, Iijima H, Goto K, Sakai J, Ishii H, Kim HJ, Suzuki H, Kondo H, Saeki S, Yamamoto T. Human apolipoprotein E receptor 2. A novel lipoprotein receptor of the low density lipoprotein receptor family predominantly expressed in brain. J Biol Chem. 1996;271:8373–8380. doi: 10.1074/jbc.271.14.8373. [DOI] [PubMed] [Google Scholar]
- Kim DH, Magoori K, Inoue TR, Mao CC, Kim HJ, Suzuki H, Fujita T, Endo Y, Saeki S, Yamamoto TT. Exon/intron organization, chromosome localization, alternative splicing, and transcription units of the human apolipoprotein E receptor 2 gene. J Biol Chem. 1997;272:8498–8504. doi: 10.1074/jbc.272.13.8498. [DOI] [PubMed] [Google Scholar]
- Krieger DT, Hauser H. Comparison of synchronization of circadian corticosteroid rhythms by photoperiod and food. Proc Natl Acad Sci U S A. 1978;75:1577–1581. doi: 10.1073/pnas.75.3.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Shen L, Liu Y, Tajima D, Sakai R, Woods SC, Tso P. Diurnal Rhythm of Apolipoprotein A-IV in Rat Hypothalamus and Its Relation to Food Intake and Corticosterone. Endocrinology. 2004;145:3232–3238. doi: 10.1210/en.2003-1554. [DOI] [PubMed] [Google Scholar]
- Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates (the 4th version) New York: Academic Press, Harcourt Brace & Company; 1982. [Google Scholar]
- Plump AS, Smith JD, Hayek T, Aalto-Setala K, Walsh A, Verstuyft JG, Rubin EM, Breslow JL. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71:343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- Rall SC, Jr, Newhouse YM, Clarke HR, Weisgraber KH, McCarthy BJ, Mahley RW, Bersot TP. Type III hyperlipoproteinemia associated with apolipoprotein E phenotype E3/3. Structure and genetics of an apolipoprotein E3 variant. J Clin Invest. 1989;83:1095–1101. doi: 10.1172/JCI113988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer EJ, Gregg RE, Ghiselli G, Forte TM, Ordovas JM, Zech LA, Brewer HB., Jr Familial apolipoprotein E deficiency. J Clin Invest. 1986;78:1206–1219. doi: 10.1172/JCI112704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Tso P, Woods SC, Clegg DJ, Barber KL, Carey K, Liu M. Brain apolipoprotein E: an important regulator of food intake in rats. Diabetes. 2008;57:2092–2098. doi: 10.2337/db08-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strubbe JH, Woods SC. The timing of meals. Psychol Rev. 2004;111:128–141. doi: 10.1037/0033-295X.111.1.128. [DOI] [PubMed] [Google Scholar]
- Tempel DL, Leibowitz SF. Adrenal steroid receptors: interactions with brain neuropeptide systems in relation to nutrient intake and metabolism. J Neuroendocrinol. 1994;6:479–501. doi: 10.1111/j.1365-2826.1994.tb00611.x. [DOI] [PubMed] [Google Scholar]
- Therrien M, Drouin J. Molecular determinants for cell specificity and glucocorticoid repression of the proopiomelanocortin gene. Ann N Y Acad Sci. 1993;680:663–671. doi: 10.1111/j.1749-6632.1993.tb19768.x. [DOI] [PubMed] [Google Scholar]
- Wardell MR, Brennan SO, Janus ED, Fraser R, Carrell RW. Apolipoprotein E2-Christchurch (136 Arg----Ser). New variant of human apolipoprotein E in a patient with type III hyperlipoproteinemia. J Clin Invest. 1987;80:483–490. doi: 10.1172/JCI113096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson CW, Shinsako J, Dallman MF. Daily rhythms in adrenal responsiveness to adrenocorticotropin are determined primarily by the time of feeding in the rat. Endocrinology. 1979;104:350–359. doi: 10.1210/endo-104-2-350. [DOI] [PubMed] [Google Scholar]
- Woods SC. The eating paradox: how we tolerate food. Psychol Rev. 1991;98:488–505. doi: 10.1037/0033-295x.98.4.488. [DOI] [PubMed] [Google Scholar]
- Zakrzewska KE, Cusin I, Stricker-Krongrad A, Boss O, Ricquier D, Jeanrenaud B, Rohner-Jeanrenaud F. Induction of obesity and hyperleptinemia by central glucocorticoid infusion in the rat. Diabetes. 1999;48:365–370. doi: 10.2337/diabetes.48.2.365. [DOI] [PubMed] [Google Scholar]