Abstract
Background
Growth hormone (GH) secretion and muscle mass decline from mid-puberty throughout life culminating in sarcopenia, frailty, decreased function and loss of independence.
Objective
Determine if an oral ghrelin mimetic (MK-677) would enhance GH secretion into the young adult range without serious adverse effects, prevent the decline of fat-free mass (FFM), and decrease abdominal visceral fat (AVF) in healthy older adults.
Design
Two-year, double-blind, randomized, placebo-controlled, modified-crossover clinical trial.
Setting
General Clinical Research Center study performed at a University Hospital.
Participants
Sixty-five healthy men and women (on or off hormone replacement therapy) ages 60-81.
Intervention
Oral administration of MK-677 (25 mg) or placebo once daily.
Measurements
Growth hormone and insulin-like growth factor-I (IGF-I); FFM and AVF were the primary endpoints after one year of treatment. Other endpoints: weight, fat mass, insulin sensitivity, lipid and cortisol levels, bone mineral density, limb lean and fat mass, isokinetic strength, function and quality of life; all endpoints were assessed at baseline and every 6 months.
Limitations
Study design (duration and subject number) not sufficient to evaluate functional endpoints in healthy elderly
Results
Daily MK-677 significantly increased GH and IGF-I levels to those of healthy young adults without serious adverse effects. With placebo, mean (95% Cl) FFM decreased -0.5 (-1.1 to 0.2) kg, however, FFM increased 1.1 (0.7 to 1.5) kg with MK-677 (P<0.001, MK-677 vs. placebo); body cell mass as reflected by intracellular water decreased -1.0 (-2.1 to 0.2) kg with placebo, but increased 0.8 (-0.1 to 1.6) kg with MK-677 (P=0.021). There were no significant differences in AVF or total fat mass. However, the average increase in limb fat in the MK-677 group (1.1 kg) was greater than with placebo (0.24 kg); P=0.001. Body weight increased 0.8 (-0.3 to 1.8) kg with placebo and 2.7 (2.0 to 3.5) kg with MK-677 (P=0.003). Fasting blood glucose increased an average of 0.3 mmol/L (5 mg/dL) with MK-677 (P=0.015) and insulin sensitivity declined. The most frequent side effects were an increase in appetite that subsided within a few months and transient, mild lower extremity edema and muscle pain. Low density lipoprotein cholesterol decreased -0.14 (-0.27 to -0.01) mmol/L [-5.4 (-10.4 to -0.4) mg/dL] with MK-677 (P=0.026); there were no differences in total or high density lipoprotein cholesterol. Cortisol increased 47 (28 to 71) nmol/L [1.7 (1.0 to 2.6 µg/dL)] with MK-677 (P=0.020). Changes in bone mineral density consistent with increased bone remodeling occurred in MK-677-treated subjects. Increased FFM did not result in changes in strength or function. Two-year exploratory analyses confirmed the 1-year results.
Conclusions
The ghrelin mimetic MK-677 enhanced pulsatile GH secretion and significantly increased FFM over 12 months and was generally well tolerated. Long-term functional, and ultimately pharmaco-economic, studies in elderly adults are indicated.
Keywords: Ghrelin, ghrelin mimetic, body composition, aging, sarcopenia, frailty, healthspan, growth hormone, growth hormone secretagogue
INTRODUCTION
Aging is an inevitable process across all species. In humans, muscle mass declines following its peak in the third decade of life. Muscle mass is important for physical fitness and metabolic regulation; development of sarcopenia is a major risk factor for developing frailty, loss of independence and physical disability in the elderly (1) and is associated with shortened survival in critically-ill patients (2). With increased lifespan, increasing numbers of adults are becoming frail and dependent upon others, creating challenges for them, their families and society.
The decline in fat-free mass (FFM) correlates with the aging-associated decline of growth hormone (GH) secretion (3, 4). Rudman et al. noted that aging adults show similar declines in FFM and GH secretion as seen in GH-deficient young adults (5). By the 8th decade, men and women have lost about 7 and 3.8 kg of muscle mass, respectively (3), with an increase in intra-abdominal fat (6, 7).
Previous trials using GH in the elderly were small, poorly controlled and/or too short (8); in addition, GH replacement does not restore pulsatile GH secretion. MK-677, the first orally-active ghrelin mimetic (GH secretagogue; GH secretagogue-receptor agonist), increases pulsatile GH secretion in older adults to that observed in young adults (9, 10). The primary objectives were to determine if oral MK-677 (25 mg daily) in healthy older adults would increase GH and IGF-I levels, prevent the decline of FFM and decrease abdominal visceral fat (AVF) with acceptable tolerability.
METHODS
Study Design
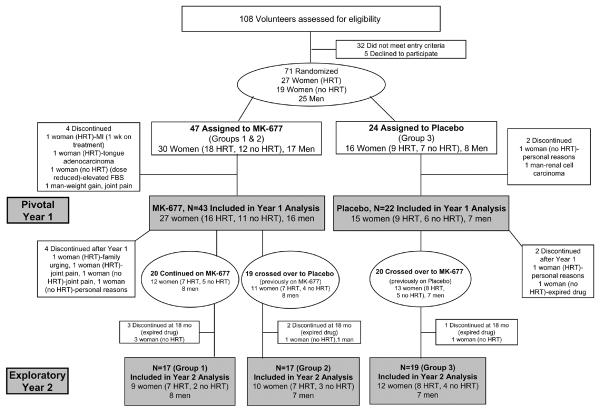
This study was approved by the General Clinical Research Center (GCRC) and the University of Virginia Institutional Review Boards under IND # 54,041. All subjects gave written informed consent. A two-year, randomized, double-blind, modified-crossover trial of once-daily oral administration of 25 mg MK-677 or placebo (2:1 ratio) to healthy older adults (men, women on and women off hormone replacement therapy) was performed. After 1 year, MK-677-treated subjects were randomized to continue on MK-677 (Group 1) or change to placebo (Group 2); the placebo-treated subjects were given MK-677 during year 2 (Group 3). A schematic of the study design is provided in APPENDIX FIGURE 1.
Setting and Participants
Healthy older volunteers ≥ 60 years were recruited from the general population by advertisement and were screened by medical history, physical examination and laboratory testing to rule out underlying disease. Exclusion criteria included body mass index ≥ 35 kg/m2, strenuous exercise > 60 minutes per day, smoking, diabetes, history of malignancy (other than some skin cancers), untreated hypertension, thyroid disease or medications known to affect GH secretion. Participants were asked to maintain their typical diet and exercise throughout the study and to report any illnesses, medical procedures or adverse effects. All subjects were Caucasian, with the exception of 1 Hispanic and 1 African-American man.
At baseline and every 6 months for 2 years, subjects were admitted to the GCRC for body composition, body water, lipid and bone mineral density measurements, frequent blood sampling and completion of quality of life questionnaires. Tests of strength and function also were performed. During GCRC admissions, meals were standardized for caloric and nutrient content. Blood samples for GH were drawn from an indwelling venous cannula every 10 minutes for 24 hours; subjects were allowed to sleep after 21:00.
Randomization and Intervention
Blinded supplies of MK-677 and placebo tablets were provided by Merck Research Laboratories, Inc., stored by a research pharmacist, and were dispensed in a blinded manner according to a randomization table with stratification for gender and hormone replacement therapy. Ten mg tablets were provided for blind back-titration. Placebo or MK-677 (25 mg) tablets were taken once daily between 7 and 9:00 AM (at 9:00 AM during admissions). All research staff and the volunteers remained blinded throughout the study and during data verification. Compliance was monitored by pill counts.
Outcome Measures
Growth Hormone and IGF-I
Serum GH and IGF-I levels were measured in duplicate in the GCRC Core Laboratory. 24-h mean GH and endogenous GH secretory dynamics were assessed by Cluster (11) and an automated (12) multiple-parameter deconvolution method (9). Details of all assay methods are provided in APPENDIX 1.
Body Composition and Bone Mineral Density
FFM and total body fat were evaluated by a 4-compartment (4-C) model (13) and by dual x-ray absorptiometry (DXA) on a Hologic QDR-2000 (Hologic Inc., Bedford MA) in pencil beam mode (14). DXA measurements included: i. appendicular lean soft tissue of the arms and legs as an estimate of total appendicular skeletal muscle mass (TASM) (15); ii. appendicular fat; iii. bone mineral density of the femoral neck, spine (L2-4) and total hip.
T-score for TASM/ht2
The DXA TASM estimates were divided by height squared in meters [TASM (kg)/ht2] (15). This index of relative limb muscle mass was used to compute a t-score for each individual, relating the TASM/ht2 to those of gender-concordant young adults (16). Sarcopenia was defined as ≤ 2 SD below young, gender-specific reference populations (17, 18).
Computed Tomography
Cross-sectional computed tomography images were used to measure the areas (cm2) of abdominal visceral (AVF) and subcutaneous fat, and mid-thigh skeletal muscle at pre-defined anatomic locations (19); data were not included when the subsequent scan location differed or there were technical difficulties [N=4 placebo, N=3 MK-677].
DXA and CT scans were analyzed by a single blinded observer (J.L.C.)
Body Water
Total body water, using the deuterium oxide (D2O) dilution technique (20), and extracellular water by bromide dilution (21) were measured. Intracellular water was assessed as the difference between total body water and extracellular water. To determine the relative relationships of total-, extra- and intra-cellular water, each component (in kg) was expressed per kg of FFM at each time point. The scale of measure used in the analysis was chosen a priori; the raw data also were analyzed and are reported in typical units for comparison.
Isokinetic Muscle Strength
Concentric force during flexion and extension of the knee and shoulder were determined every 6 months using an isokinetic dynamometer (Cybex II, CSM, Inc., Boston, MA). Six repetitions of maximal effort over 90 degrees at 60 degrees/second were performed with the mean of the last 5 repetitions computed by proprietary software (22). Total work (Newton.metres) was calculated by multiplying the mean per repetition by 5.
Function
Function tests performed every 6 months included walking 30 meters as quickly as possible (best of 2 trials), walking as far as possible in 6 minutes on an indoor track, descending and ascending 4 flights of stairs, and rising and sitting 5 times from an armless chair with an 18” seat height.
Correction for Height and Gender
To compensate for differences in muscle mass between men and women, all strength and function measurements were analyzed per kg of baseline appendicular skeletal muscle (lean) from DXA. Arm lean and leg lean were used for shoulder and knee strength, respectively; baseline TASM (sum of arms and legs) was used for the function tests. The scale of measure used in the analysis was chosen a priori; the raw data also are reported.
Quality of Life Assessments
Subjects completed 4 questionnaires every 6 months to assess quality of life and general wellbeing: the 20-item Short Form Health Survey; Beck Depression Inventory; Pittsburgh Sleep Quality Index; and the Body Cathexis Scale.
Additional details of quality of life, muscle strength and function assessments are provided in APPENDIX 1.
Clinical Outcomes
Every 6 months, cholesterol, cortisol and insulin sensitivity, estimated by fasting insulin and glucose using the Quicki Index method of Katz et al. (23), were measured.
Exploratory 2-year Outcomes
To determine if the effects of MK-677 treatment were sustained over 2 years, or reversed when changed to placebo, several endpoints were analyzed in a subgroup of subjects who completed 24 months in each of the 3 treatment groups; FIGURE 1.
FIGURE 1.
Participant Flow Through the Study
Monitoring for Adverse Effects
Each year volunteers were seen monthly for the first 3 months and every 3 months thereafter for a physical examination, documentation of medications and vital signs, and questioning for side effects and overall well being. A complete blood count, chemistry panel, hemoglobin A1c (HbA1c), fasting blood glucose, and prostate-specific antigen and testosterone levels in men were monitored. Women had annual pap smears and mammograms.
Statistical Analysis
The two primary endpoints were FFM and AVF. The study was powered for the pivotal first 12 months; the power analysis is described in detail in APPENDIX 1. It was unknown whether there would be differences in responses among men, women on and women off hormone replacement therapy. Consequently, the randomization was conducted a priori so that equal numbers of these 3 populations would be randomly assigned to MK-677 and to placebo. Therefore, the power analyses were focused on the pivotal 12-month change comparison between the 2 treatment groups as a whole and did not focus on specific subgroup comparisons (gender or hormone replacement therapy).
All statistical analyses were conducted under the guidelines of the intention-to-treat principle and missing data points were not imputed. The analyses were performed on the baseline and the 6- and 12-month primary and secondary outcomes and the data analysis plan was decided a priori. The primary outcome data for the 6- and 12-month changes in FFM and AVF as well as for IGF-I and GH were analyzed via repeated measures analysis of covariance (ANCOVA). For each ANCOVA, treatment (MK-677 or placebo) and time (6- or 12-months) were considered potential sources of variability, as was treatment by time interaction. The subjects' baseline measurements were treated as the ANCOVA covariate. The FFM and AVF data were analyzed on the same scale on which they were measured and are reported as a difference between arithmetic means. The GH and IGF-I data were transformed to the natural logarithmic scale before conducting the statistical analyses so that the variance and normality assumptions of the linear model were not violated; results are reported as a ratio of geometric means (fold change).
To estimate the mean within-subject change in the response at 6 and at 12 months, linear contrasts were constructed. Similarly, linear contrasts were constructed to estimate the baseline-adjusted difference in changes in the response at 6 and 12 months between the MK-677 and placebo groups. For the pivotal 12-month comparison (MK-677 versus placebo), the null hypothesis of equality of means was rejected if the p-value of the F-statistic was ≤ 0.05. For the 6-month between-group comparison, the null hypothesis was rejected if the p-value of the F-statistic was ≤ 0.05 after implementing the Bonferonni post-hoc test correction. For the 12-month comparison, the 95% confidence interval was constructed based on the Students' t-distribution quantile values at the 2.5 and 97.5 percentiles, while the 6-month comparison was based on the 1.25 and 98.75 percentiles of the distribution. With the exception of the quality of life data, all secondary outcome data were analyzed via repeated measure ANCOVA and are reported exactly as the primary outcome data. For the quality of life data, a Factor Analysis (24) of the different scales of the questionnaires was performed to create an overall wellbeing factor; this is described in detail in APPENDIX 1.
The effects of year-2 treatment were designed as exploratory. To determine whether the effects were maintained or reversed, a separate post hoc analysis of endpoints based on responses in year 1, was performed in subjects who had complete data at baseline, 12 and 24 months. Only the change from baseline to 24 months was analyzed, in exactly the same way as the year 1 data; a post hoc correction for the 3 treatment groups was implemented. The software of SAS version 9.1 (SAS Institute Inc, Cary NC) was used to conduct the statistical analyses.
Role of Funding Source
This was an investigator-initiated GCRC study funded by the NIH; it was conducted and analyzed at the University of Virginia.
RESULTS
Characteristics of the participants
FIGURE 1 shows the flow of study participants and baseline demographics are shown in TABLE 1.
TABLE 1.
DEMOGRAPHICS
| Placebo | MK-677 | |
|---|---|---|
| Men | N=7 | N=16 |
| Women [HRT] | N=9 | N=16 |
| Women [no HRT] | N=6 | N=11 |
| N=22 | N=43 | |
| Mean (SD), minimum-maximum | Mean (SD), minimum-maximum | |
| Age, y | 66.5 (5.5), 60.7-80.7 | 67.9 (5.4), 60.2-80.4 |
| BMI, kg/m2 | 26.2 (3.9), 19.0-34.5 | 26.1 (3), 20.8-33.8 |
All data presented are the arithmetic mean and standard deviation (SD) and the minimum and maximum values. There were no significant differences between the placebo and MK-677 treatment groups at baseline.
Abbreviations: HRT = hormone replacement therapy; BMI = body mass index
Treatment occurred between September 1998 and November 2003. Because the study drug expired in November 2003, 6 subjects were treated for only 18 months (2 on placebo, 4 on MK-677) and 1 for only 12 months (on placebo). Seventy-one volunteers were randomized and 65 completed year 1 (dropout rate, 8.5%); 23 men and 42 women (25 on and 17 not on hormone replacement therapy). The treatment groups were well-matched at baseline, with no significant differences between groups. Fifty-nine subjects completed 18 months and 53 completed 24 months (FIGURE 1).
Back titration
In 4 subjects, the dose of study drug was blindly back titrated to 10 mg daily because of: increased fasting glucose after crossover from placebo to MK-677 in year 2 (81-year-old man, completed 2 years); increased fasting glucose in 1 woman on MK-677 (withdrawn after 3 months); and increased joint pain in 2 women on MK-677 (withdrew after 12 months).
Additional methods, baseline data and all results are presented in supplementary appendix materials. Data presented are mean (95% confidence limits). All statistical comparisons at 6 and 12 months are between MK-677 and placebo.
Growth Hormone and IGF-I Levels
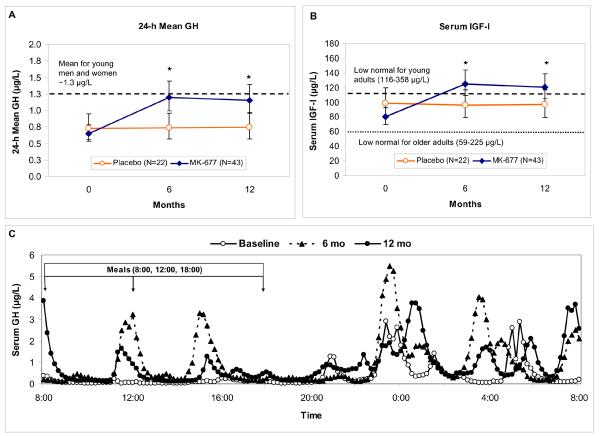
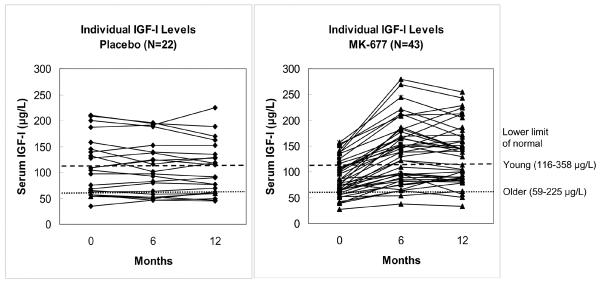
The effects of treatment in the pivotal year 1 are shown in FIGURE 2. After 12 months of MK-677 treatment, there was a 1.8 (1.56 to 2.0) -fold increase from baseline in 24-h mean GH (P<0.001). This was accounted for by enhanced pulsatile GH secretion (APPENDIX TABLE 1), as shown in a representative subject, FIGURE 2C. Serum IGF-I levels also were increased by 1.5 (1.4 to 1.6) -fold, P<0.001. In 22/43 subjects, IGF-I levels were sustained in the normal range for young adults; individual IGF-I results are shown in APPENDIX FIGURE 2.
FIGURE 2. Serum 24-h mean GH and IGF-I results in pivotal year 1.
GH and IGF-I data were not normally distributed and were analyzed on the natural logarithmic scale; line graphs show geometric means and 95% confidence intervals. Results of GH deconvolution analysis are included in APPENDIX TABLE 1.
Panels A and B: 24-h mean GH and serum IGF-I levels at baseline, 6 and 12 months. An asterisk indicates P<0.001, MK-677 vs. placebo. In Panel A, the 24-h mean GH for young men and women combined (~1.3 μg/L) is indicated by a dashed line. In Panel B the lower limit of the IGF-I normal range is indicated: older adults (59-225 μg/L, lower dotted line); young adults aged 21-25 (116-358 μg/L, upper dashed line). GH=growth hormone, IGF-I=insulin-like growth factor-I.
Panel C: Representative 24-h GH profiles in one 70-year-old man, BMI, 23.2 kg/m2, treated for one year with MK-677. 24-h mean GH levels were 0.37, 1.0 and 0.86 μg/L at baseline (open circles), 6 months (triangles) and 12 months (closed circles), respectively. Note that the pulsatile pattern of GH secretion at baseline is maintained and enhanced at 6 and 12 months, primarily as a result of increased secretion per peak rather than peak frequency.
APPENDIX TABLE 1.
EFFECTS OF TREATMENT ON 24-H MEAN GH AND IGF-I AND GH SECRETORY DYNAMICS
| BASELINE | 6 MONTHS | 12 MONTHS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arithmetic Mean (SD) * | minimum-maximum | Arithmetic Mean Change * | Ratio of Geometric Means [Fold change] † | 95% Cl | P Value MK-677 vs. Placebo ‡ | Arithmetic Mean Change * | Ratio of Geometric Means [Fold change] † | 95% Cl | P Value MK-677 vs. Placebo ‡ | |||
| GH and IGF-I | ||||||||||||
| 24-h Mean GH, μg/L | Placebo | 0.89 (0.58) | 0.16-2.28 | N=22 | -0.03 | 1.0 | 0.8-1.2 | -0.01 | 1.0 | 0.9-1.2 | ||
| MK-677 | 0.81 (0.53) | 0.09-2.33 | N=43 | 0.57 | 1.9 | 1.6-2.1 | <0.001 | 0.55 | 1.8 | 1.6-2.0 | <0.001 | |
| IGF-I, μg/L | Placebo | 112 (55) | 35-210 | N=22 | -3 | 1.0 | 0.9-1.1 | -4 | 1.0 | 0.9-1.1 | ||
| MK-677 | 87 (34) | 27-158 | N=43 | 50 | 1.6 | 1.4-1.7 | <0.001 | 45 | 1.5 | 1.4-1.6 | <0.001 | |
| GH Secretory Dynamics § | ||||||||||||
| # Secretion Events, pulses/24 h | Placebo | 18.5 (3.1) | 11-23 | N=22 | -0.4 | 1.0 | 0.9-1.1 | -1.2 | 0.9 | 0.9-1.0 | ||
| MK-677 | 18.5 (3.2) | 12-27 | N=43 | 0.9 | 1.1 | 1.0-1.1 | 0.189 | 1.3 | 1.1 | 1.0-1.1 | 0.003 ‖ | |
| Total 24-h Production Rate, μg/L/min | Placebo | 57.9 (35.4) | 5.7-122.7 | N=22 | 3.1 | 1.1 | 0.8-1.3 | -1.2 | 1.0 | 0.8-1.2 | ||
| MK-677 | 52.8 (33.5) | 6.9-137.2 | N=43 | 31.9 | 1.8 | 1.6-2.2 | <0.001 | 29.9 | 1.7 | 1.5-2.0 | <0.001 | |
| Total Pulsatile Production Rate, μg/L/min | Placebo | 51.8 (33.7) | 4.5-116.8 | N=22 | 2.9 | 1.1 | 0.8-1.4 | -1.4 | 1.1 | 0.8-1.4 | ||
| MK-677 | 47.3 (31.6) | 5.7-131.5 | N=43 | 27.9 | 1.8 | 1.5-2.2 | <0.001 | 26.7 | 1.7 | 1.5-2.0 | <0.001 | |
Baseline data presented are the arithmetic mean (SD) and the minimum and maximum values; mean changes (arithmetic differences) at 6 and 12 months are provided for reference.
GH data were transformed to the natural logarithmic scale before conducting the statistical analyses; results are reported as the ratio of geometric means (fold-changes from baseline) with upper and lower 95% confidence limits.
P values are for the pivotal comparison of the fold changes from baseline between the two groups at 6 and at 12 months.
Endogenous GH secretory dynamics were assessed using an automated multiple-parameter deconvolution method.
The increase in peak number was statistically significant with MK-677, however, the mean change from 18.5 to 19.7 (vs. 18.5 to 17.3 with placebo) is not considered biologically significant.
Abbreviations: GH = growth hormone; IGF-I = insulin-like growth factor I
Body Composition
Fat-Free Mass, Total Appendicular Skeletal Muscle Mass (TASM) (FIGURE 3A) and Thigh Muscle Area (APPENDIX TABLE 2)
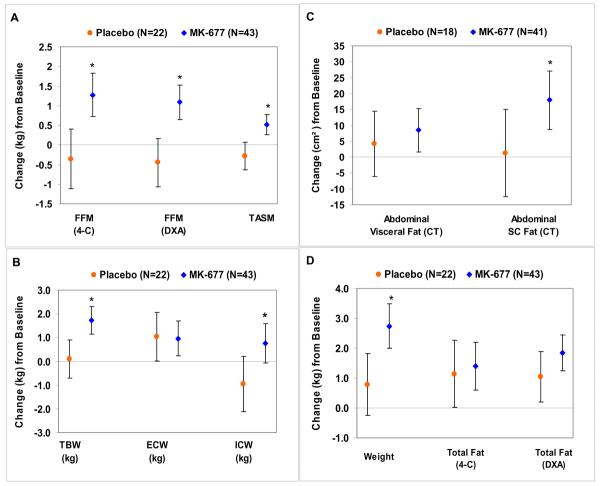
FIGURE 3. Changes in body composition at 12 months.
Graphs show arithmetic differences (95% CI). An asterisk indicates a significant difference (MK-677 vs. placebo) at 12 months. Panel A: Changes in fat-free mass (FFM, by 4-C model and by DXA) and TASM (total appendicular skeletal muscle mass by DXA). Panel B: Changes in TBW (total body water), ECW (extracellular water) and ICW (intracellular water). The increase in TBW and increase in ICW with active drug are consistent with the anabolic effects of MK-677. Panel C: Changes in abdominal visceral and abdominal subcutaneous fat cross-sectional areas measured by CT. Panel D: Changes in body weight and total fat mass (by 4-C model and DXA). CT = computed tomography; DXA = dual energy x-ray absorptiometry
APPENDIX TABLE 2.
EFFECTS OF TREATMENT ON BODY COMPOSITION
| BASELINE | 6 MONTHS | 12 MONTHS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BODY COMPOSITION | Mean (SD) | minimum-maximum | Mean Change * | 95% CI | P Value MK-677 vs. Placebo † | Mean Change * | 95% CI | P Value MK-677 vs. Placebo † | ||||
| Primary Endpoints | ||||||||||||
| FFM, kg [4-C] | Placebo | 47.9 (11.9) | 33.5-77.5 | -0.2 | -1.0 - 0.7 | -0.4 | -1.1 - 0.4 | |||||
| MK-677 | N=42 | 47.6 (9.9) | 33.6-69.3 | N=42 | 1.1 | 0.5 - 1.8 | 0.014 | N=42 | 1.3 | 0.7 - 1.8 | 0.001 | |
| FFM, kg [DXA] | Placebo | 48.2 (12.4) | 34.3-76.5 | -0.2 | -0.9 - 0.5 | -0.5 | -1.1 - 0.2 | |||||
| MK-677 | 48.3 (11.3) | 30.8-71.2 | 1.0 | 0.5 - 1.5 | 0.006 | 1.1 | 0.7 - 1.5 | <0.001 | ||||
| AVF, cm2 [CT] | Placebo | N=18 | 104.8 (46.7) | 35.6-214.0 | N=18 | 6.0 | -5.9 - 17.8 | N=18 | 4.2 | -6.2 - 14.5 | ||
| MK-677 | N=41 | 128.8 (58.0) | 36.7-313.9 | N=41 | 2.5 | -5.4 - 10.3 | 0.83 | N=41 | 8.4 | 1.6 - 15.3 | 0.68 | |
| Other Endpoints | ||||||||||||
| Weight, kg | Placebo | 74.5 (13.7) | 51.5-97.9 | 0.7 | -0.5-1.9 | 0.8 | -0.3 - 1.8 | |||||
| MK-677 | 75.2 (11.1) | 53.5-107.5 | 3.1 | 2.2 - 3.9 | 0.001 | 2.7 | 2.0 - 3.5 | 0.003 | ||||
| TBF, kg [4-C] | Placebo | 26.6 (8.9) | 12.0-49.2 | 0.9 | -0.4 - 2.2 | 1.1 | 0.0 - 2.3 | |||||
| MK-677 | N=42 | 27.7 (7.0) | 10.9-45.7 | N=42 | 1.8 | 0.9 - 2.8 | 0.37 | N=42 | 1.4 | 0.6 - 2.2 | 0.76 | |
| TBF, kg [DXA] | Placebo | 26.1 (9.2) | 11.6-49.7 | 0.9 | 0.0 - 1.9 | 1.1 | 0.2 - 1.9 | |||||
| MK-677 | 26.5 (7.6) | 7.5-44.9 | 2.1 | 1.4 - 2.8 | 0.040 | 1.8 | 1.2 - 2.5 | 0.130 | ||||
| Fat, % [4-C] | Placebo | 35.7 (9.5) | 19.0-53.2 | 0.8 | -0.4 - 2.1 | 1.3 | 0.2 - 2.4 | |||||
| MK-677 | N=42 | 36.9 (7.9) | 14.7-50.8 | N=42 | 0.9 | 0.0 - 1.8 | >.99 | N=42 | 0.4 | -0.4 - 1.2 | 0.196 | |
| Fat, % [DXA] | Placebo | 35.2 (10.3) | 19.4-53.8 | 0.9 | 0.1 - 1.8 | 1.2 | 0.4 - 0.9 | |||||
| MK-677 | 35.7 (9.5) | 9.8-49.9 | 1.1 | 0.5 - 1.7 | >.99 | 0.8 | 0.3 - 1.3 | 0.39 | ||||
| Other Endpoints | ||||||||||||
| ASCF, cm2 [CT] | Placebo | N=18 | 281.2 - 124.2) | 110.6-620.7 | N=18 | 8.3 | -7.5 - 24.1 | N=18 | 1.3 | -12.5 - 15.0 | ||
| MK-677 | N=40 | 292.8 (97.0) | 53-506.8 | N=40 | 22.0 | 11.4 - 32.6 | 0.23 | N=40 | 18.0 | 8.7 - 27.2 | 0.054 | |
| Limb Fat, kg [DXA] | Placebo | 12.5 (4.6) | 6.2-23.3 | 0.24 | -0.2 - 0.7 | 0.29 | -0.1 - 0.7 | |||||
| MK-677 | 12.53 (4.1) | 3.3-20.7 | 1.1 | 0.7 - 1.4 | 0.001 | 0.9 | 0.6 - 1.2 | 0.011 | ||||
| Limb Lean, kg [DXA] | Placebo | 18.2 (6.0) | 11.8-32.6 | -0.17 | -0.6 - 0.2 | -0.3 | -0.6 - 0.1 | |||||
| MK-677 | 18.2 (5.4) | 10.6-30.1 | 0.5 | 0.2 - 0.8 | 0.008 | 0.5 | 0.3 - 0.8 | <0.001 | ||||
| T-Score for TASM/ht2 | Placebo | -0.1 (1.4) | -2.5 - 2.4 | -0.11 | -0.32 - 0.11 | -0.13 | -0.32 - 0.06 | |||||
| MK-677 | -0.2 (1.1) | -2.4 - 2.5 | 0.26 | 0.10 - 0.41 | 0.005 | 0.29 | 0.16 - 0.42 | <0.001 | ||||
| Thigh Muscle Area, cm2 [CT] | Placebo | 112.9 (33.3) | 75.3-175.4 | N=21 | 1.5 | -1.7 - 4.7 | -0.5 | -3.2 - 2.2 | ||||
| MK-677 | N=41 | 111.2 (26.9) | 78.3-185.4 | N=41 | 2.6 | 0.3 - 4.9 | >.99 | N=41 | 1.5 | -0.5 - 3.5 | 0.20 | |
| Leg Lean, kg [DXA] | Placebo | 13.8 (4.1) | 9.3-23.7 | -0.11 | -0.4 - 0.2 | -0.25 | -0.5 - 0.0 | |||||
| MK-677 | 13.7 (3.7) | 9.3-21.0 | 0.4 | 0.2 - 0.6 | 0.011 | 0.4 | 0.2 - 0.6 | 0.001 | ||||
| Arm Lean, kg [DXA] | Placebo | 4.5 (2.0) | 2.4-8.8 | -0.1 | -0.2 - 0.1 | 0.0 | -0.2 - 0.1 | |||||
| MK-677 | 4.5 (1.8) | 2.3-9.1 | 0.09 | 0.0 - 0.2 | 0.104 | 0.16 | 0.1 - 0.3 | 0.019 | ||||
Baseline data presented are arithmetic mean (SD) and the minimum and maximum values; Placebo (N=22) and MK-677 (N=43) unless otherwise indicated.
Mean change (arithmetic difference) from baseline with 95% confidence limits
P values are for the pivotal comparisons between the two groups at 6 months and at 12 months.
FFM (fat-free mass) and TBF (total body fat) were measured by a 4-compartment (4-C) model and by DXA (dual energy x-ray absorptiometry)
AVF (abdominal visceral fat) and ASCF (abdominal subcutaneous fat) measured from a single-slice CT (computed tomography) scan
Limb fat (appendicular fat mass) from DXA, measured as the sum of the fat for the arms and the legs
Limb lean (total appendicular skeletal muscle mass; TASM) from DXA, measured as the sum of the lean soft tissue for the arms (arm lean) and the legs (leg lean)
Thigh muscle area (average of R and L thighs) from a single-slice CT scan
A t-score was computed for each individual, relating the TASM/ht2 to those of gender-concordant young adults.
FFM measured by DXA decreased -0.5 (-1.10 to 0.2) kg with placebo and increased 1.1 (0.7 to 1.5) kg with MK-677 (P<0.001). This increase was observed with DXA and the 4-compartment model (r2=0.98). TASM (limb lean) decreased -0.3 (-0.6 to 0.1) kg with placebo and increased 0.5 (0.3 to 0.8) kg with MK-677 (P<0.001).
Thigh muscle area by computed tomography was -0.5 (-3.2 to 2.2) cm2 with placebo and 1.5 (-0.5 to 3.5) cm2 with MK-677(P=0.20).
Total Body Water and Cell Mass
When each body water compartment was expressed per FFM at each timepoint there were no significant differences between treatment groups (APPENDIX TABLE 3).
APPENDIX TABLE 3.
EFFECTS OF TREATMENT ON BODY WATER
| BASELINE | 6 MONTHS | 12 MONTHS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | minimum-maximum | Mean Change * | 95% CI | P Value MK-677 vs. Placebo † | Mean Change * | 95% CI | P Value MK-677 vs. Placebo † | |||
| BODY WATER | ||||||||||
| TBW, kg | Placebo | N=23 | 35.3 (8.4) | 24.4-56.5 | 0.5 | -0.4 - 1.4 | 0.1 | -0.7 - 0.9 | ||
| MK-677 | N=43 | 35.1 (7.2) | 23.9-49.5 | 1.3 | 0.6 - 1.9 | 0.22 | 1.7 | 1.2 - 2.3 | 0.001 | |
| TBW/FFM, kg/kg ‡ | Placebo | N=23 | 73.7 (3.6) | 68.1-82.7 | 1.3 | -0.6 - 3.1 | 0.7 | -0.9 - 2.3 | ||
| MK-677 | N=43 | 73.3 (4.3) | 64.6-87.3 | 0.9 | -0.4 - 2.2 | 0.95 | 1.7 | 0.6 - 2.9 | 0.32 | |
| ECW | Placebo | N=23 | 16.5 (2.3) | 12.1-22.0 | 0.7 | -0.4 - 1.9 | 1.0 | 0.02 - 2.1 | ||
| MK-677 | N=43 | 17.0 (2.8) | 12.0-25.1 | 0.7 | -0.1 - 1.6 | >.99 | 1.0 | 0.2 - 1.7 | 0.96 | |
| ECW/FFM, kg/kg ‡ | Placebo | N=23 | 35.6 (6.7) | 24.8-45.9 | 1.9 | -0.2 - 3.9 | 2.3 | 0.3 - 4.3 | ||
| MK-677 | N=43 | 36.3 (6.2) | 23.0-48.3 | 0.5 | -0.9 - 2.0 | 0.66 | 1.1 | -0.3 - 2.6 | 0.39 | |
| ICW, kg | Placebo | N=23 | 18.8 (7.3) | 10.0-34.5 | -0.2 | -1.6 - 1.1 | -1.0 | -2.1 - 0.2 | ||
| MK-677 | N=43 | 18.1 (5.8) | 10.2-32.3 | 0.5 | -0.5 - 1.5 | 0.59 | 0.8 | -0.1 - 1.6 | 0.021 | |
| ICW/FFM, kg/kg ‡ | Placebo | N=23 | 38.1 (6.3) | 27.5-49.2 | -0.6 | -3.2 - 2.1 | -1.7 | -3.9 - 0.7 | ||
| MK-677 | N=43 | 37.1 (5.2) | 25.8-49.6 | 0.4 | -1.5 - 2.3 | >.99 | 0.6 | -1.1 - 2.2 | 0.170 | |
Baseline data presented are arithmetic mean (SD) and the minimum and maximum values
Mean change (arithmetic difference) from baseline with 95% confidence limits
P values are for the pivotal comparisons between the two groups at 6 months and at 12 months.
For analysis, each body water component (in kg) was expressed per kg FFM at each time point; the raw data were also analyzed without correction for FFM.
TBW = total body water; ECW = extracellular water; ICW= intracellular water; FFM = fat-free mass by DXA (dual energy x-ray absorptiometry)
Total body water increased 0.1 (-0.7 to 0.9) kg with placebo and 1.7 (1.2 to 2.3) kg with MK-677 (P=0.001). Both treatment groups had similar increases in extracellular water; 1.0 (0.02 to 2.1) kg with placebo and 1.0 (0.2 to 1.7) kg with MK-677. Intracellular water decreased -1.0 (-2.1 to 0.2) kg with placebo, but increased 0.8 (-0.1 to 1.6) kg with MK-677 (P=0.021) (FIGURE 3B). The loss of intracellular water or cell mass with placebo is reflected in a loss of total FFM (FIGURE 3A), specifically TASM. Conversely, the increase in cell mass with MK-677 is reflected in the significant increase in total FFM and TASM (FIGURE 3A).
Abdominal Visceral and Abdominal Subcutaneous Fat
Abdominal visceral fat increased 4.2 (-6.2 to 14.5) cm2 with placebo and 8.4 (1.6 to 15.3) cm2 with MK-677 (P=0.68) (FIGURE 3C). Abdominal subcutaneous fat increased 1.3 (-12.5 to 15.0) cm2 with placebo and 18.0 (8.7 to 27.2) cm2 with MK-677 (P=0.054).
Body Weight and Total Body Fat
Body weight increased 0.8 (-0.3 to 1.8) kg with placebo and 2.7 (2.0 to 3.5) kg with MK-677 (P=0.003) (FIGURE 3D). Total body fat by DXA increased 1.1 (0.2 to 1.9) kg with placebo and 1.8 (1.2 to 2.5) kg with MK-677 (P=0.130).
Limb Lean (TASM) and Limb Fat
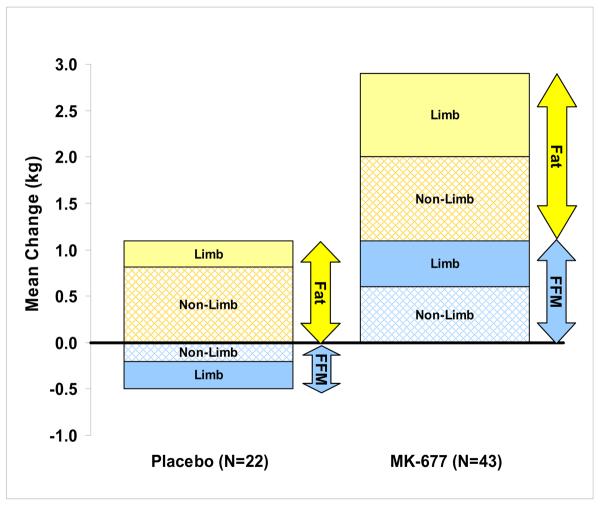
FIGURE 4 compares the mean changes in limb lean and limb fat as they relate to the mean changes in FFM and total fat mass in both treatment groups. There was a loss of limb lean with placebo and ~50% of the increase in FFM with MK-677 was in the limbs. The average increase in limb fat in the MK-677 group (1.1 kg) was greater than with placebo (0.24 kg); P=0.001.
FIGURE 4. Summary of mean changes in fat and FFM at 12 months.
Fat mass (yellow) and fat-free mass (blue) from DXA; limb (solid bars) = appendicular lean soft tissue and appendicular fat; non-limb (hatched bars) = total minus limb.
DXA = dual energy x-ray absorptiometry
T-score for TASM/ht2
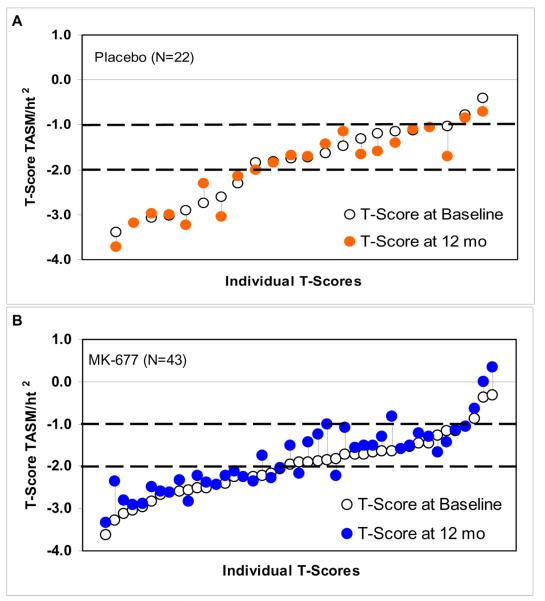
Individual t-scores for TASM/ht2 at baseline and 12 months are shown in APPENDIX FIGURE 3. After one year of MK-677 treatment, the t-score improved in 27/43 (63%) subjects [mean 0.29 (0.16 to 0.42)], while in 18/22 (82%) subjects receiving placebo there was no change or a decrease in t-score [mean -0.13 (-0.32 to 0.06)]; P<0.001.
Bone Mineral Density
After 12 months, femoral neck bone mineral density increased 0.012 (0.002 to 0.022) g/cm2 with placebo and decreased -0.005 (-0.012 to 0.002) g/cm2 with MK-677. When MK-677 was compared with placebo, there was a small, but significant difference (-0.018 g/cm2; P=0.004). There were no significant differences in spine or total hip bone mineral density. When 5 subjects on alendronate were excluded (3 on MK-677, 2 on placebo), the same pattern was observed.
The detailed effects on other body composition endpoints and bone mineral density are provided in APPENDIX TABLES 2 and 4, respectively.
APPENDIX TABLE 4.
EFFECTS OF TREATMENT ON BONE MINERAL DENSITY
| BASELINE | 6 MONTHS | 12 MONTHS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | minimum-maximum | Mean Change * | 95% CI | P Value MK-677 vs. Placebo † | Mean Change * | 95% CI | P Value MK-677 vs. Placebo † | |||
| BONE MINERAL DENSITY | ||||||||||
| Femoral Neck, g/cm2 | Placebo | N=22 | 0.752 (0.137) | 0.584-1.101 | 0.010 | -0.001-0.021 | 0.012 | 0.002-0.022 | ||
| MK-677 | N=43 | 0.748 (0.133) | 0.522-1.066 | -0.003 | -0.011-0.005 | 0.079 | -0.005 | -0.01-0.002 | 0.004 | |
| Spine [L2-L4], g/cm2 | Placebo | N=22 | 1.046 (0.164) | 0.770-1.441 | 0.004 | -0.013-0.022 | 0.005 | -0.010-0.020 | ||
| MK-677 | N=43 | 1.085 (0.196) | 0.761-1.630 | 0.001 | -0.012-0.013 | >.99 | 0.008 | -0-0.019 | 0.69 | |
| Total Hip, g/cm2 | Placebo | N=22 | 0.910 (0.160) | 0.688-1.315 | 0.005 | -0.003-0.014 | 0.005 | -0.003-0.013 | ||
| MK-677 | N=43 | 0.896 (0.138) | 0.686-1.183 | -0.002 | -0.008-0.004 | 0.22 | -0.004 | -0.01-0.002 | 0.063 | |
Baseline data presented are the arithmetic mean (SD) and the minimum and maximum values
Mean change (arithmetic difference) from baseline with 95% confidence limits at 6 months and at 12 months
P values are for the pivotal comparisons between the two groups at 6 months and at 12 months.
Bone mineral density was determined by DXA.
Muscle Strength
Shoulder flexion total work decreased with placebo, but there was no decline with MK-677. When corrected for baseline arm ASM, shoulder flexion total work decreased -5.7 (-10.1 to -1.4) N.m/kg with placebo, with a smaller decline of -1.0 (-4.1 to 2.1) N.m/kg with MK-677 (P=0.073). There were no significant changes in total work in knee extension or flexion or in shoulder extension between groups. (APPENDIX TABLE 5)
APPENDIX TABLE 5.
EFFECTS OF TREATMENT ON MUSCLE STRENGTH
| BASELINE | 6 MONTHS | 12 MONTHS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | minimum-maximum | Mean Change * | 95% CI | P Value MK-677 vs. Placebo † | Mean Change * | 95% CI | P Value MK-677 vs. Placebo † | |||||
| MUSCLE STRENGTH | ||||||||||||
| KNEE | ||||||||||||
| Extension | Placebo | N=20 | 518 (225) | 149-1044 | N=20 | -10 | N=20 | -3 | ||||
| MK-677 | N=41 | 501 (173) | 193-1014 | N=39 | -14 | N=41 | -8 | |||||
| Extension/Leg Lean, N.m/kg ‡ | Placebo | N=20 | 72.1 (20.1) | 26.9-113.9 | N=20 | -0.8 | -5.1 - 3.6 | N=20 | -0.4 | -4.2 - 3.4 | ||
| MK-677 | N=41 | 72.6 (14.4) | 35.8-96.6 | N=39 | -2.1 | -5.2 - 1.0 | >.99 | N=41 | -1.3 | -3.9 - 1.4 | 0.73 | |
| Flexion | Placebo | N=20 | 336 (149) | 142-702 | N=20 | 9 | N=20 | 7 | ||||
| MK-677 | N=41 | 341 (120) | 166-624 | N=39 | 6 | N=41 | 15 | |||||
| Flexion/Leg Lean, N.m/kg ‡ | Placebo | N=20 | 51.1 (13.0) | 25.6-76.6 | N=20 | 1.9 | -1.0 - 4.8 | N=20 | 0.8 | -1.7 - 3.4 | ||
| MK-677 | N=41 | 49.3 (9.9) | 30.8-71.8 | N=39 | 0.8 | -1.3 - 2.9 | 0.91 | N=41 | 2.1 | 0.4 - 3.9 | 0.46 | |
| SHOULDER | ||||||||||||
| Extension | Placebo | N=20 | 389 (162) | 163-705 | N=20 | -3 | N=20 | 6 | ||||
| MK-677 | N=40 | 375 (134) | 210-746 | N=38 | 14 | N=40 | 20 | |||||
| Extension/Arm Lean, N.m/kg ‡ | Placebo | N=20 | 171.5 (36.0) | 98.9-232 | N=20 | -2.1 | -13.7-9.6 | N=20 | 0.9 | -9.3 - 11.0 | ||
| MK-677 | N=40 | 170.8 (41.6) | 87.1-306.1 | N=38 | 5.3 | -2.8 - 14.0 | 0.42 | N=40 | 6.4 | -0.8 - 13.6 | 0.36 | |
| Flexion | Placebo | N=20 | 203 (114) | 95-454 | N=20 | -12 | N=20 | -13 | ||||
| MK-677 | N=40 | 194 (75) | 81-427 | N=38 | -3 | N=40 | 0 | |||||
| Flexion/Arm Lean, N.m/kg ‡ | Placebo | N=20 | 86 (20.8) | 45.5-127.4 | N=20 | -5.1 | -10.1 to -0.1 | N=20 | -5.7 | -10.1 to -1.4 | ||
| MK-677 | N=40 | 87.4 (20.9) | 46.8-145.8 | N=38 | -1.0 | -4.7 - 2.6 | 0.24 | N=40 | -1.0 | -4.1 - 2.1 | 0.073 | |
Baseline data presented are the arithmetic mean (SD) and the minimum and maximum values; N for each test is indicated.
Mean change (arithmetic difference) from baseline with 95% confidence limits at 6 months and at 12 months
P values are for the pivotal comparisons between the two groups at 6 months and at 12 months.
Strength variables were analyzed per kg leg lean (for knee) and arm lean (for shoulder) at baseline.
Muscle strength was determined using an isokinetic dynamometer; Cybex II.
Empty cells = no statistical analyses were performed; raw data are reported in typical units for comparison
Leg Lean and Arm Lean = sum of appendicular skeletal muscle mass of legs and of arms (from DXA)
Function and Quality of Life
There were no significant changes in any measurements of function (APPENDIX TABLE 6) or quality of life (APPENDIX 1) in this healthy older population.
APPENDIX TABLE 6.
EFFECTS OF TREATMENT ON FUNCTION
| BASELINE | 6 MONTHS | 12 MONTHS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | minimum-maximum | Mean Change * | 95% CI | P Value MK-677 vs. Placebo † | Mean Change * | 95% CI | P Value MK-677 vs. Placebo † | |||||
| FUNCTION TESTS | ||||||||||||
| 30 m walk, sec | Placebo | N=21 | 14.5 (2.8) | 9.5-21.2 | N=21 | -0.1 | N=21 | 0.2 | ||||
| MK-677 | N=40 | 14.3 (2.9) | 9.0-20.5 | N=40 | -0.3 | N=40 | -0.2 | |||||
| 30 m walk, m/sec | Placebo | N=21 | 2.14 (0.40) | 1.42-3.17 | N=21 | 0.04 | N=21 | -0.01 | ||||
| MK-677 | N=40 | 2.19 (0.47) | 1.46-3.32 | N=40 | 0.05 | N=40 | 0.06 | |||||
| 30 m walk/TASM, sec/kg ‡ | Placebo | N=21 | 0.89 (0.39) | 0.36-1.64 | N=21 | -0.01 | -0.04 - 0.03 | N=21 | 0.01 | -0.02 - 0.04 | ||
| MK-677 | N=40 | 0.88 (0.33) | 0.32-1.42 | N=40 | -0.01 | -0.04 - 0.01 | >.99 | N=40 | -0.01 | -0.03 - 0.01 | 0.30 | |
| 6 min walk, m | Placebo | N=20 | 422 (58) | 267-534 | N=19 | 8 | N=20 | 5 | ||||
| MK-677 | N=40 | 426 (58) | 334-567 | N=40 | 9 | N=40 | 11 | |||||
| 6 min walk, # laps | Placebo | N=20 | 4.7 (0.7) | 3.0-6.0 | N=19 | 0.1 | N=20 | 0.1 | ||||
| MK-677 | N=40 | 4.8 (0.7) | 3.8-6.4 | N=40 | 0.1 | N=40 | 0.1 | |||||
| 6 min walk, # laps/TASM ‡ | Placebo | N=20 | 0.28 (0.08) | 0.15-0.41 | N=19 | 0.004 | -0.004-0.013 | N=20 | 0.002 | -0.005-0.009 | ||
| MK-677 | N=40 | 0.28 (0.06) | 0.16-0.45 | N=40 | 0.005 | -0.001-0.011 | >.99 | N=40 | 0.006 | 0.001-0.011 | 0.44 | |
| Sit-Stand x 5, sec | Placebo | N=17 | 11.0 (2.8) | 7.4-18.2 | N=17 | -0.6 | N=17 | -0.1 | ||||
| MK-677 | N=30 | 11.2 (2.2) | 7.3-16.6 | N=30 | -0.6 | N=30 | -0.3 | |||||
| Sit-Stand x 5/TASM, sec/kg ‡ | Placebo | N=17 | 0.70 (0.31) | 0.27-1.3 | N=17 | -0.04 | -0.10 - 0.02 | N=17 | 0.00 | -0.06 - 0.05 | ||
| MK-677 | N=30 | 0.68 (0.23) | 0.26-1.04 | N=30 | -0.04 | -0.09 - 0.01 | >.99 | N=30 | -0.03 | -0.07 - 0.01 | 0.37 | |
| FUNCTION TESTS | ||||||||||||
| Stairs - DOWN, sec | Placebo | N=19 | 16.6 (3.7) | 12.5-27.5 | N=19 | 0.1 | N=19 | 0.4 | ||||
| MK-677 | N=38 | 17.7 (4.8) | 10.7-32.2 | N=38 | -0.3 | N=38 | -0.3 | |||||
| Stairs -DOWN/TASM, sec/kg ‡ | Placebo | N=19 | 1.0 (0.5) | 0.4-1.9 | N=19 | 0.01 | -0.05 - 0.07 | N=19 | 0.03 | -0.03 - 0.08 | ||
| MK-677 | N=38 | 1.1 (0.5) | 0.4-2.0 | N=38 | -0.02 | -0.07 - 0.02 | 0.91 | N=38 | -0.03 | -0.06 - 0.01 | 0.180 | |
| Stairs - UP, sec | Placebo | N=20 | 17.9 (4.1) | 12.7-29.6 | N=20 | 0.0 | N=20 | 0.2 | ||||
| MK-677 | N=39 | 19.4 (4.9) | 10.8-31.9 | N=39 | 0.1 | N=39 | 0.3 | |||||
| Stairs - UP/TASM, sec/kg ‡ | Placebo | N=20 | 1.09 (0.49) | 0.44-2.06 | N=20 | -0.01 | -0.06 - 0.05 | N=20 | 0.02 | -0.03 - 0.07 | ||
| MK-677 | N=39 | 1.20 (0.51) | 0.36-2.09 | N=39 | 0.01 | -0.03 - 0.05 | >.99 | N=39 | 0.02 | -0.02 - 0.05 | 0.97 | |
Baseline data presented are the arithmetic mean (SD) and the minimum and maximum values; N for each test is indicated.
Mean change (arithmetic difference) from baseline with 95% confidence limits at 6 months and at 12 months
P values are for the pivotal comparisons between the two groups at 6 months and at 12 months.
Function variables were analyzed per kg TASM at baseline.
Empty cells = no statistical analyses were performed; raw data are reported in typical units for comparison
TASM = total appendicular skeletal muscle mass from DXA
Clinical Outcomes
Lipids
At 12 months, low density lipoprotein cholesterol increased 0.12 (-0.07 to 0.3) mmol/L [4.6 (-2.7 to 11.6) mg/dL] with placebo, but decreased -0.14 (-0.27 to -0.01) mmol/L [-5.4 (-10.4 to -0.4) mg/dL] with MK-677 (P=0.026). There was no difference in total or high density lipoprotein cholesterol between groups. (APPENDIX TABLE 7).
APPENDIX TABLE 7.
EFFECTS OF TREATMENT ON CHOLESTEROL, CORTISOL, TESTOSTERONE AND PSA
| BASELINE | 6 MONTHS | 12 MONTHS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | minimum-maximum | Mean Change * | 95% CI | P Value MK-677 vs. Placebo † | Mean Change * | 95% CI | P Value MK-677 vs. Placebo † | |||||
| CHOLESTEROL | ||||||||||||
| Total Cholesterol, mmol/L | Placebo | N=21 | 4.65 (0.79) | 3.01-5.76 | N=21 | 0.05 | -0.19 - 0.28 | N=21 | 0.19 | -0.01 - 0.40 | ||
| MK-677 | N=43 | 4.93 (0.84) | 3.22-6.91 | N=43 | 0.09 | -0.08 - 0.25 | >.99 | N=43 | -0.01 | -0.16 - 0.13 | 0.087 | |
| Total Cholesterol, mg/dL | Placebo | N=21 | 179.5 (30.5) | 116-222 | N=21 | 1.9 | -7.3 - 10.8 | N=21 | 7.3 | -0.4 - 15.4 | ||
| MK-677 | N=43 | 190.3 (32.4) | 124-267 | N=43 | 3.5 | -3.1 - 9.7 | >.99 | N=43 | -0.4 | -6.2 - 5.0 | 0.087 | |
| LDL Cholesterol, mmol/L | Placebo | N=21 | 2.91 (0.71) | 1.45-4.11 | N=21 | -0.04 | -0.25 - 0.17 | N=21 | 0.12 | -0.07 - 0.30 | ||
| MK-677 | N=43 | 3.16 (0.71) | 1.82-4.84 | N=43 | -0.04 | -0.19 - 0.11 | >.99 | N=43 | -0.14 | -0.27 - -0.01 | 0.026 | |
| LDL Cholesterol, mg/dL | Placebo | N=21 | 112.4 (27.4) | 56-159 | N=21 | -1.5 | -9.7 - 6.6 | N=21 | 4.6 | -2.7 - 11.6 | ||
| MK-677 | N=43 | 122.0 (27.4) | 70-187 | N=43 | -1.5 | -7.3 - 4.2 | >.99 | N=43 | -5.4 | -10.4 - -0.4 | 0.026 | |
| HDL Cholesterol, mmol/L | Placebo | N=21 | 1.30 (0.39) | 0.69-2.12 | N=21 | -0.06 | -0.15 - 0.04 | N=21 | 0.05 | -0.03 - 0.13 | ||
| MK-677 | N=43 | 1.38 (0.43) | 0.62-2.51 | N=43 | 0.09 | 0.02 - 0.16 | 0.014 | N=43 | 0.07 | 0.02 - 0.13 | 0.73 | |
| HDL Cholesterol, mg/dL | Placebo | N=21 | 50.2 (15.1) | 27-82 | N=21 | -2.3 | -5.8 - 1.5 | N=21 | 1.9 | -1.2 - 5.0 | ||
| MK-677 | N=43 | 53.3 (16.6) | 24-97 | N=43 | 3.5 | 0.8 - 6.2 | 0.014 | N=43 | 2.7 | 0.8 - 5.0 | 0.73 | |
| CORTISOL, TESTOSTERONE AND PSA | ||||||||||||
| Cortisol, nmol/L | Placebo | 367 (77) | 251-519 | N=22 | -18 | -55 - 19 | N=22 | -2 | -36 - 30 | |||
| MK-677 | N=42 | 353 (74) | 218-530 | N=42 | 28 | 0 - 54 | 0.071 | N=42 | 47 | 28 - 71 | 0.020 | |
| Cortisol, μg/dL | Placebo | 13.3 (2.8) | 9.1-18.8 | N=22 | -0.7 | -2.0 - 0.7 | N=22 | -0.1 | -1.3 - 1.1 | |||
| MK-677 | N=42 | 12.8 (2.7) | 7.9-19.2 | N=42 | 1.0 | 0.0 - 1.9 | 0.071 | N=42 | 1.7 | 1.0 - 2.6 | 0.020 | |
| Total Testosterone, nmol/L | Placebo | N=7 | 16.6 (4.3) | 12.3-22.7 | N=7 | 1.1 | -1.8 - 4.1 | N=7 | -0.5 | -3.0 - 2.1 | ||
| MK-677 | N=16 | 18.7 (5.2) | 12.1-29.8 | N=16 | -0.6 | -2.6 - 1.3 | 0.50 | N=16 | -0.6 | -2.3 - 1.1 | <.99 | |
| Total Testosterone, ng/dL | Placebo | N=7 | 479 (123) | 354-655 | N=7 | 33 | -51 - 118 | N=7 | -13 | -86 - 61 | ||
| MK-677 | N=16 | 538 (149) | 350-858 | N=16 | -18 | -74 - 37 | 0.50 | N=16 | -16 | -65 - 32 | <.99 | |
| PSA, μg/L | Placebo | N=7 | 1.8 (1.6) | 0.6-4.9 | N=7 | 0.2 | -0.4 - 0.8 | N=7 | 0.1 | -0.4 - 0.5 | ||
| MK-677 | N=16 | 2 (1.6) | 0.4-5.1 | N=16 | -0.4 | -0.8 - 0.0 | 0.073 | N=16 | -0.3 | -0.6 - 0.1 | 0.30 | |
Baseline data presented are the arithmetic mean (SD) and the minimum and maximum values; N for each test is indicated.
Mean change (arithmetic difference) from baseline with 95% confidence limits at 6 months and at 12 months
P values are for the pivotal comparisons between the two groups at 6 months and at 12 months.
Total testosterone normal range for men >50 y is 6.3 - 26.8 nmol/L (181 - 772 ng/dL)
PSA normal range for men 66-70 y is <4.5 μg/L; for >70 y it is <6.5 μg/L
Abbreviations: LDL = low-density lipoprotein; HDL = high-density lipoprotein; PSA = prostate-specific antigen
Cortisol
24-hour mean cortisol concentrations decreased -18 (-55 to 19) nmol/L [-0.1 (-1.4 to 1.2) μg/dL] with placebo and increased 47 (28 to 71) nmol/L [2.0 (1.0 to 2.9) μg/dL] with MK-677 (P=0.020). (APPENDIX TABLE 7)
Safety Data
Total testosterone levels in men did not change (APPENDIX TABLE 7). Prostatic-specific antigen levels remained in the normal range or declined with MK-677 treatment, except for a transient increase in one man. There were no changes in mammograms, pap smears or routine laboratory tests during the 2-year study.
Insulin Sensitivity
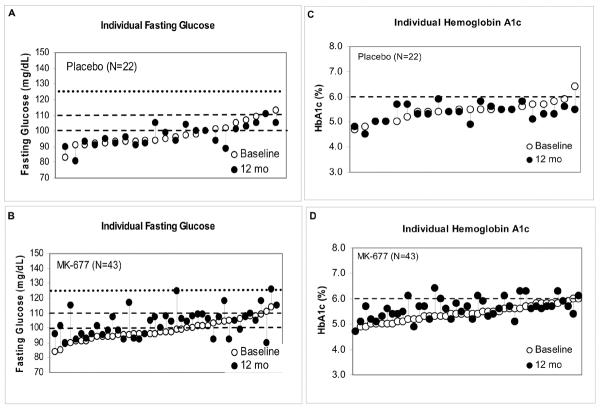
Individual glucose and HbA1c responses are shown in APPENDIX FIGURE 4. Mean fasting blood glucose did not change 0.0 (-0.3 to 0.2) mmol/L [1.0 (-5.0 to 3.0) mg/dL] with placebo and increased 0.3 (0.1 to 0.4) mmol/L [5.0 (2.0 to 7.0) mg/dL] with MK-677 (P=0.015). In 2 subjects on placebo and 16 on MK-677, fasting glucose increased from <5.6 to between 5.6 and 6.1 mmol/L [101 to 110 mg/dL]. In 3 subjects on MK-677 with baseline fasting glucose concentrations <5.6 and 3 subjects with >5.6 mmol/L [100 mg/dL], glucose increased to between 6.1 and 6.7 mmol/L [110 to 120 mg/dL] in 4 subjects and to 6.9 mmol/L [125 and 126 mg/dL] in 2 others. Mean HbA1c decreased -0.1 (-0.2 to 1.0) % with placebo and increased 0.2 (0.1 to 0.3) % with MK-677 (P=0.002); at 12 months, 8 subjects had a HbA1c >6% (6.1% in 4, 6.3% in 3 and 6.4% in 1). Insulin sensitivity estimated by Quicki Index also was reduced after 12 months of treatment (P<0.001) (APPENDIX TABLE 8). One 81 year-old man had an increase in fasting blood glucose and HbA1c after crossover from placebo to MK-677; after dose reduction and institution of a low carbohydrate diet, the values returned to normal.
APPENDIX TABLE 8.
EFFECTS OF TREATMENT ON FASTING BLOOD GLUCOSE, HbA1c AND QUICK INDEX
| BASELINE | 6 MONTHS | 12 MONTHS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | minimum-maximum | Mean Change * | 95% CI | P Value MK-677 vs. Placebo † | Mean Change * | 95% CI | P Value MK-677 vs. Placebo † | |||||
| FBG, HbA1c, Quicki Index | ||||||||||||
| FBG, mmol/L | Placebo | 5.4 (0.4) | 4.6-6.3 | 0.1 | -0.2 - 0.3 | 0.0 | -0.3 - 0.2 | |||||
| MK-677 | 5.5 (0.4) | 4.7-6.4 | 0.2 | 0.1 - 0.4 | 0.27 | 0.3 | 0.1 - 0.4 | 0.015 | ||||
| FBG, mg/dL | Placebo | 98 (8) | 83-113 | 1 | -3 - 6 | -1 | -5 - 3 | |||||
| MK-677 | 99 (7) | 84-115 | 4 | 1 - 8 | 0.27 | 5 | 2 - 7 | 0.015 | ||||
| HbA1c, % | Placebo | 5.4 (0.4) | 4.7-6.4 | -0.2 | -0.3 - 0.0 | -0.1 | -0.2 - 0.1 | |||||
| MK-677 | 5.4 (0.3) | 4.7-6.0 | 0.2 | 0.0 - 0.3 | 0.001 | 0.2 | 0.1 - 0.3 | 0.002 | ||||
| Quicki Index | Placebo | N=21 | 0.36 (0.03) | 0.33-0.41 | N=21 | 0.004 | -0.004-0.012 | N=21 | 0.005 | -0.003-0.013 | ||
| MK-677 | N=41 | 0.36 (0.03) | 0.27-0.43 | N=41 | -0.012 | -0.018 to -0.006 | <0.001 | N=41 | -0.013 | -0.019 to -0.008 | <0.001 | |
Baseline data presented are the arithmetic mean (SD) and the minimum and maximum values; Placebo (N=22) and MK-677 (N=43) unless otherwise indicated.
Mean change (arithmetic difference) from baseline with 95% confidence limits at 6 months and at 12 months
P values are for the pivotal comparisons between the two groups at 6 months and at 12 months.
Quicki Index - estimate of insulin sensitivity from fasting glucose and insulin
Abbreviations: FBG = fasting blood glucose; HbA1C = hemoglobin A1c
Adverse Effects
An increase in appetite was reported in 8/22 (36%) subjects on placebo vs. 29/43 (67%) on MK-677 (P=0.02); in 50% of subjects on MK-677, appetite returned to normal within 3 months and more gradually in others. Mild, transient edema [6/22 (27%) on placebo vs. 19/43 (44%) on MK-677, P=0.3] and transient muscle pain [2/22 (9%) on placebo vs. 14/43 (33%) on MK-677, P=0.1] were recorded. Joint pain was reported in both groups [17/22 (77%) on placebo vs. 25/43 (58%) on MK-677, P=0.2].
In year 1, an 82 year-old woman on MK-677 developed adenocarcinoma of the tongue, diagnosed at 12 months, and a 68 year-old woman had a myocardial infarction 7 days after starting MK-677. One man on placebo was diagnosed with renal cell carcinoma at 6 months. All were withdrawn from the study. At the end of year 2, an 83 year-old woman was diagnosed with colon cancer (on MK-677 in year 1 and placebo in year 2).
Two-year Exploratory Results
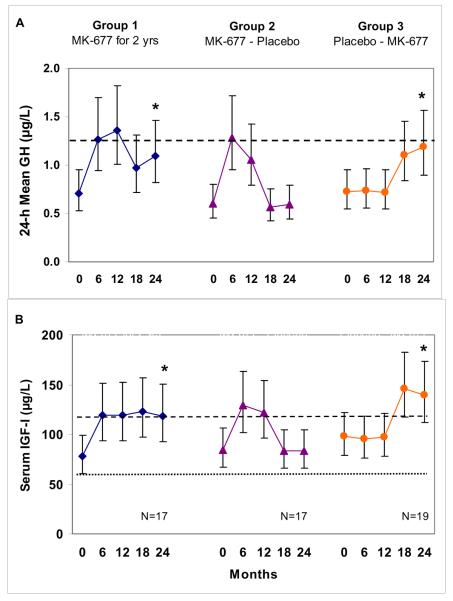
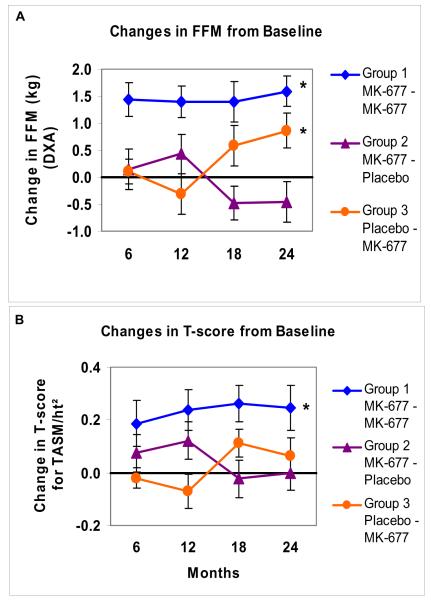
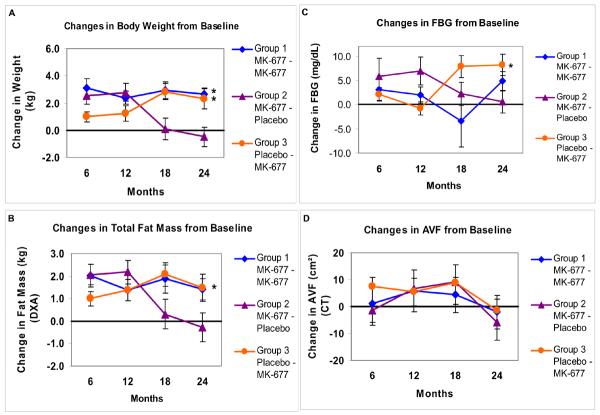
Two-year exploratory analyses of changes from baseline to 24 months in a subgroup of 53 subjects (APPENDIX TABLE 9) confirmed that the significant changes observed in the pivotal first year were sustained: GH and IGF-I (APPENDIX FIGURE 5) and FFM (by DXA only) and t-score for TASM/ht2 (APPENDIX FIGURE 6). Changes in weight, total fat mass, fasting blood glucose and AVF are shown in APPENDIX FIGURE 7. In the group treated for 2 years with MK-677, fasting blood glucose was not significantly increased (P=0.093; vs. baseline).
APPENDIX TABLE 9.
TWO-YEAR EXPLORATORY DATA
| Group 1 | MK-677 Yr 1 MK-677 Yr 2 |
Group 2 | MK-677 Yr 1 Placebo Yr 2 |
Group 3 | Placebo Yr 1 MK-677 Yr 2 |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean Change * |
95% CI | P vs. Baseline † | Mean (SD) | Mean Change * |
95% CI | P vs. Baseline † | Mean (SD) | Mean Change * |
95% CI | P vs. Baseline † | |||||
| 24-h Mean GH, μg/L |
Baseline | N=17 | 0.91 (0.65) | N=17 | 0.73 (0.41) | N=19 | 0.90 (0.61) | |||||||||
| 24 mo | N=17 | 1.32 (0.91) | 1.54 ‡ | 1.11 to 2.16 | 0.001 | N=17 | 0.70 (0.38) | 0.98 ‡ | 0.70 to 1.37 | >.99 | N=19 | 1.35 (0.77) | 1.64 ‡ | 1.20 to 2.25 | <0.001 | |
| IGF-I, μg/L |
Baseline | N=16 | 86 (41) | N=17 | 91 (35) | N=19 | 113 (59) | |||||||||
| 24 mo | N=16 | 133 (63) | 1.53 ‡ | 1.26 to 1.84 | <0.001 | N=17 | 90 (36) | 0.98 ‡ | 0.82 to 1.18 | >.99 | N=19 | 153 (66) | 1.42 ‡ | 1.20 to 1.69 | <0.001 | |
| FFM, kg [4-C] |
Baseline | N=17 | 50.7 (11.0) | N=17 | 48.0 (8.9) | N=19 | 49.0 (12.1) | |||||||||
| 24 mo | N=17 | 51.5 (11.5) | 0.8 | -0.7 to 2.2 | 0.30 | N=17 | 48.6 (9.1) | 0.6 | -0.9 to 2.0 | 0.74 | N=19 | 49.7 (12.3) | 0.7 | -0.7 to 2.1 | 0.38 | |
| FFM, kg [DXA] |
Baseline | N=17 | 50.9 (12.7) | N=17 | 49.9 (10.6) | N=19 | 49.2 (12.9) | |||||||||
| 24 mo | N=17 | 52.5 (12.6) | 1.6 | 0.6 to 2.6 | <0.001 | N=17 | 49.4 (10.3) | -0.5 | -1.5 to 0.6 | 0.54 | N=19 | 50.0 (12.8) | 0.9 | -0.1 to 1.8 | 0.026 | |
| T-Score for Limb Lean (TASM)/ht2 |
Baseline | N=17 | -1.9 (0.9) | N=17 | -1.9 (0.7) | N=19 | -1.8 (0.9) | |||||||||
| 24 mo | N=17 | -1.7 (0.8) | 0.25 | 0.02 to 0.48 | 0.006 | N=17 | -1.9 (0.7) | -0.001 | -0.231 to 0.229 | >.99 | N=19 | -1.8 (0.8) | 0.07 | -0.153 to 0.283 | >.99 | |
| AVF, cm2 [CT] |
Baseline | N=14 | 127.5 (59.5) | N=16 | 135.9 (63.2) | N=16 | 109.7 (47.4) | |||||||||
| 24 mo | N=14 | 125.5 (52.4) | -2.0 | -20.8 to 16.7 | >.99 | N=16 | 130.0 (59.0) | -6.0 | -23.5 to 11.6 | 0.89 | N=16 | 108.2 (45.2) | -1.4 | -18.9 to 16.1 | >.99 | |
| ASCF, cm2 [CT] |
Baseline | N=13 | 252.4 (95.9) | N=16 | 308.3 (106.1) | N=16 | 275.0 (128.6) | |||||||||
| 24 mo | N=13 | 269.2 (98.2) | 16.8 | -7.7 to 41.2 | 0.116 | N=16 | 301.1 (98.6) | -7.2 | -29.3 to 14.8 | 0.94 | N=16 | 280.0 (133.1) | 5.0 | -17.0 to 27.1 | >.99 | |
| Weight, |
Baseline | N=17 | 76.5 (12.1) | N=17 | 77.5 (10.9) | N=19 | 74.5 (13.4) | |||||||||
| 24 mo | N=17 | 79.2 (12.8) | 2.7 | 0.6 to 4.7 | 0.001 | N=17 | 77.0 (11.6) | -0.5 | -2.5 to 1.5 | >.99 | N=19 | 76.8 (14.0) | 2.3 | 0.4 to 4.2 | 0.002 | |
| Fat, kg [4-C] |
Baseline | N=17 | 25.8 (8.1) | N=17 | 29.5 (6.3) | N=19 | 25.5 (8.5) | |||||||||
| 24 mo | N=17 | 27.7 (8.1) | 1.9 | 0.3 to 3.5 | 0.016 | N=17 | 28.5 (7.1) | -1.0 | -2.6 to 0.6 | 0.34 | N=19 | 27.1 (8.9) | 1.6 | 0.1 to 3.1 | 0.033 | |
| Fat, kg [DXA] |
Baseline | N=17 | 25.1 (8.7) | N=17 | 27.6 (7.3) | N=19 | 25.0 (8.9) | |||||||||
| 24 mo | N=17 | 26.5 (9.0) | 1.4 | -0.1 to 2.9 | 0.062 | N=17 | 27.3 (7.7) | -0.3 | -1.8 to 1.2 | >.99 | N=19 | 26.5 (9.3) | 1.5 | 0.1 to 2.9 | 0.030 | |
| FBG, mmol/L [mg/dL] |
Baseline | N=17 | 5.7 (0.3) [102 (6)] | N=17 | 5.3 (0.4) [95 (7)] | N=19 | 5.4 (0.4) [98 (8)] | |||||||||
| 24 mo | N=17 | 5.9 (0.6) [107 (10)] | 0.3 [5] | -0.06 to 0.6 [-1 to 10] | 0.093 | N=17 | 5.3 (0.3) [95 (5)] | 0.06 [1] | -0.3 - 0.6 [-5-6)] | >0.99 | N=19 | 5.9 (0.5) [106 (9)] | 0.4 [8] | 0.2 to 0.7 [3 to13)] | 0.001 | |
| Quicki Index |
Baseline | N=17 | 0.35 (0.03) | N=17 | 0.36 (0.03) | N=19 | 0.36 (0.03) | |||||||||
| 24 mo | N=17 | 0.34 (0.04) | -0.014 | -0.030 to 0.002 | 0.028 | N=17 | 0.37 (0.04) | 0.003 | -0.012 to 0.019 | >.99 | N=19 | 0.35 (0.03) | -0.010 | -0.025 to 0.005 | 0.120 | |
| Total Cholesterol, mmol/L [mg/dL] |
Baseline | N=17 | 5.04 (0.86) [195 (33)] | N=17 | 4.61 (0.88) [178 (34)] | N=18 | 4.66 (0.85) [180 (33)] | |||||||||
| 24 mo | N=17 | 4.88 (0.78) [188 (30)] | -0.17 [-7] | -0.54 to 0.21 [-21 to 8] | 0.83 | N=17 | 4.59 (0.66) [177 (25)] | -0.02 [-0.8] | -0.39 to 0.36 [-15 to 14] | >.99 | N=18 | 4.79 (0.82) [185 (32)] | 0.13 [5] | -0.23 to 0.49 [-9 to19] | >.99 | |
| LDL Cholesterol, mmol/L [mg/dL] |
Baseline | N=17 | 3.31 (0.72) [128 (28)] | N=17 | 2.90 (0.75) [112 (29)] | N=18 | 2.89 (0.75) [112 (29)] | |||||||||
| 24 mo | N=17 | 2.96 (0.51) [114 (20)] | -0.35 [-14] | -0.69 to -0.01 [-27 to -0.4] | 0.042 | N=17 | 2.89 (0.56) [112 (22)] | -0.01 [-0.4] | -0.35 to 0.33 [-14 to 13] | >.99 | N=18 | 2.86 (0.69) [110 (27)] | -0.03 [-1] | -0.36 to 0.3 [-14 to12] | >.99 | |
| HDL Cholesterol, mmol/L [mg/dL] |
Baseline | N=17 | 1.32 (0.43) [51 (17)] | N=17 | 1.25 (0.38) [48 (15)] | N=18 | 1.32 (0.39) [51 (15)] | |||||||||
| 24 mo | N=17 | 1.42 (0.53) [55 (21)] | 0.11 [4] | -0.20 to 0.23 [-1 to 9] | 0.120 | N=17 | 1.34 (0.35) [52 (14)] | 0.09 (-0.03) [4 (-1)] | -0.03 to 0.21 [-1 to 8] | 0.23 | N=18 | 1.50 (0.53) [58 (21)] | 0.18 [7] | 0.06 to 0.3 [2 to 12] | 0.001 | |
Data presented are the arithmetic mean (SD) at baseline and 24 months.
Changes from baseline are the arithmetic difference with 95% confidence intervals, unless otherwise indicated.
Bonferroni-adjusted P values are for the difference from baseline to 24 months within the group.
GH and IGF-I data were transformed to the natural logarithmic scale before conducting the statistical analyses; results are reported as the ratio of geometric means (fold-changes from baseline) with upper and lower 95% confidence intervals.
FFM measured by 4-compartment (4-C) model and by DXA
AVF and ASCF areas from single-slice abdominal CT scan
TFM measured by 4-compartment (4-C) model and by DXA
Quicki Index is insulin sensitivity estimated from fasting glucose and insulin.
Abbreviations: GH = growth hormone; IGF-I = insulin-like growth factor-I; FFM = fat-free mass; TFM = total fat mass; AVF = abdominal visceral fat; ASCF = abdominal subcutaneous fat; FBG = fasting blood glucose; LDL = low density lipoprotein; HDL = high density lipoprotein
Similar changes were observed in subjects who received MK-677 in year 2 after placebo in year 1 (Group 3), while the changes were reversed in those treated with MK-677 in year 1 and placebo in year 2 (Group 2). One month after crossover, and at 3 and 6 months after study completion, IGF-I concentrations returned to pretreatment levels.
DISCUSSION
Healthy elderly individuals who took the ghrelin mimetic MK-677 experienced a sustained increase in the amplitude of pulsatile GH secretion and IGF-I to levels seen in young adults. The likely mechanism was activation of the ghrelin receptor by MK-677, with feedback by IGF-I preventing excess GH production. MK-677 increased fat-free mass (FFM) by 1.6 kg relative to placebo. To provide perspective, an adult's average lifetime loss of FFM is ~ 5.5 kg (3). The concomitant increase in intracellular water, which reflects body cell mass (25), is the likely mechanism for the increase in FFM.
Ghrelin stimulates GH secretion, but it also has effects that are not attributable to increased GH. In this study, a ghrelin mimetic transiently increased appetite, a novel effect that might counteract physiological anorexia, which is one cause of weight loss in the elderly (26, 27), Ghrelin increases fat stores, unlike GH which is lipolytic. We found that body weight increased more after MK-677 than placebo. Although total fat mass increased in both groups, limb fat and limb lean mass increased more with MK-677 than placebo. Surprisingly, thigh muscle cross-sectional area did not increase, although the study is not powered to detect small but potentially important differences with the imprecise single slice CT method that we used. GH reduces abdominal visceral fat (AVF) in GH-deficient adults (28) and abdominally obese, postmenopausal women (29), but not normal elderly subjects (30). Despite increasing GH levels, MK-677 did not affect AVF, perhaps because its combined orexigenic and adipogenic effects counteracted the lipolytic effects of enhanced GH. Finally, although MK-677 did not reduce AVF, it did reduce LDL levels at 12 months, an effect not seen with GH in normal elderly subjects (8).
Strength, function and quality of life did not improve after administration of MK-677 in this study of a small, healthy cohort. Perhaps, we should have expected this result. Although strength improved in elderly hypopituitary patients after daily injections of GH for two to three years (31), GH alone does not increase strength in the healthy elderly (8, 32, 33). Indeed, strength improved in healthy older men taking GH only in combination with testosterone for 26 weeks (33). Finally, the relationship between strength and physical performance is non-linear (34); we speculate that increased physical capacity might substantially improve performance in frail adults but not healthy adults.
Sarcopenia is a hallmark of frailty (35, 36), and is associated with increased mortality in the elderly (37-40). In our study of healthy older adults, MK-677 counteracted three important factors that contribute to the development of sarcopenia: reduced secretion of GH, loss of FFM and inadequate food intake. We did not study patients with sarcopenia, and their response to a ghrelin mimetic is not known.
Importantly, our subjects tolerated daily administration of MK-677 for the two-year period of this study. The most frequent side effects were mild, transient, lower extremity edema, transient muscle pain, and increased appetite which subsided within a few months. These effects of physiologically stimulated GH secretion contrast with those of GH administered by injection: edema; arthralgia; carpal tunnel syndrome; gynecomastia; and new onset of impaired fasting glucose and diabetes mellitus in some subjects (8).
Both GH and MK-677 increase insulin resistance and blood glucose in the elderly (22, 33, 41, 42). We found statistically significant but small increases in fasting blood glucose and HbA1c at 12 months. Based on the results of short-term studies with MK-677, which found no statistically significant increase in serum cortisol (9, 43), we do not think that the small increase in serum cortisol found in our study is likely to underlie the increase in fasting glucose. In patients treated with GH, bone mineral density (BMD) initially falls (44); treatment for at least 18 months is needed to demonstrate an increase in BMD (45). Femoral neck BMD declined at 12 months with MK-677, which is consistent with increased bone remodeling, as occurs with GH (44). Fracture risk would be the best measure of MK-677 effects on bone, but this outcome would require studies of large numbers of subjects over many years.
This study has limitations. Its duration was relative short. The number of participants was small. Combining the results in men and women may have missed important gender effects. As a small randomized study in healthy older adults, ours was a “proof-of concept” study. It showed, apparently for the first time, that a drug can maintain IGF-I and a physiological pattern of GH secretion seen in young adults over at least one year, and partially reverse age-related body composition changes.
Frailty is one of the scourges of the oldest old, and as researchers are beginning to learn about its causes, they are asking if growth hormone deficiency is one cause. A systematic review has concluded that the risks of exogenous GH outweigh the benefits and that it is not the long-sought solution to frailty (8). The promise of MK-677 is that it appears to restore endogenous GH levels in a physiological secretory pattern, unlike the single high-amplitude pulse observed after exogenous GH administration. We believe that our study sets the stage for an adequately powered clinical trial of sufficient duration in a population vulnerable to frailty.
Acknowledgements
This study was supported by NIH grants DK-32632 (to MOT) and RR-00847 (to GCRC); RN was supported in part by a grant from the Deutsche Forschungsgemeinschaft (Na 317/1-1, Na 317/1-2). MK-677 and placebo were kindly provided by Merck & Co., Inc.
We acknowledge the contributions of Mark L. Hartman, M.D. (Eli Lilly and Company, Indianapolis, IN) in the conception and design of this study when he was on the faculty at UVA, as well as those of Arthur Weltman, Ph.D. We also are grateful for the expert advice offered by Bette Ann Harris, D.P.T., M.S. (MGH Institute of Health Professions, Boston MA) and Stephanie Studenski, M.D., M.P.H. (Department of Medicine, Geriatrics, University of Pittsburgh) who reviewed the strength and function data and to Sue Brown, M.D. (Department of Medicine, Endocrinology, UVA) who reviewed the bone data.
The deconvolution program was provided by Michael Johnson, Ph.D. (Department of Pharmacology, UVA) and the analyses were performed by Paula Veldhuis.
Total body water (TBW) was measured by Isao Eto, Ph.D. and Alexandra Vyazorkina using the deuterium oxide (D2O) dilution technique in the Clinical Nutrition Research Unit (NIH P30-DK56336) of Drs. Barbara Gower and Timothy Nagy at the University of Alabama at Birmingham. Extracellular water (ECW) by bromide dilution was measured by Jack Wang in the Body Composition Unit (NIH PO1-DK42618) at the Columbia University College of Physicians and Surgeons at St. Luke's-Roosevelt Hospital, New York, NY. Lipids were measured by Jean Bergeron, M.D. at Centre Hospitalier Universitaire de Quebec, Laval University, Quebec, Canada. The laboratories mentioned above were paid from NIH grants (to MOT or the GCRC) for performing the assays.
The factor analysis of the quality of life and overall wellbeing questionnaires was performed by Shigehiro Oishi, Ph.D. (Department of Psychology at UVA); all other statistical analyses were performed by James T. Patrie, M.S.
We are grateful for the care of our wonderful volunteers by the GCRC staff over the course of this study. We thank the nurses, the Core Laboratory and Metabolic Kitchen personnel, and the dedicated staff of the Exercise Physiology Laboratory, under the direction of Arthur Weltman, Ph.D., for performing the body composition, strength and function testing.
All individuals acknowledged in this manuscript have provided their written permission.
Non-standard acronyms
- FFM
Fat-free mass, measured by DXA and by a 4-compartment model
- TASM
Total appendicular skeletal muscle mass (limb lean) from DXA; sum of lean soft tissue for arms and legs
- T-score for TASM/ht2
TASM divided by height (meters) squared; an index of relative limb muscle mass was used to compute a t-score by relating it to gender-concordant young adults
- ASM
Appendicular skeletal muscle mass of the limbs, arms and legs separately, used to correct strength measurements; estimated from DXA
- AVF
Abdominal visceral fat area; measured from a single-slice CT scan 33
APPENDIX
METHODS
Assays
Growth Hormone
GH was measured in duplicate in a sensitive chemiluminescence assay, Nichols (San Juan, Capistrano, CA), as previously reported (46).
Other Assays
Plasma glucose by glucose oxidase method was measured on a Beckman analyzer. Total IGF-I, insulin, cortisol (mean of samples drawn every 4 hours over 24 hours) and total testosterone (mean of samples drawn every 6 hours over 24 hours) were measured on an Immulite 2000 (Diagnostic Products Corporation, Los Angeles, CA); other hormones to verify normal pituitary function were measured on the Chiron Diagnostics ACS:180 (Norwood, MA); data not shown. Samples for plasma lipids were obtained after an overnight fast of at least 12 hours and fresh samples were analyzed within 10 days (47, 48). Additional safety laboratory tests were performed by standard methods in the UVA Clinical Laboratories.
Isokinetic Muscle Strength
Limbs were properly aligned according to the manufacturer; settings at baseline were reproduced for subsequent tests. Scripted instructions and encouragement were given during testing. After moving through the range of motion 2-3 times with minimal effort, both knees and both shoulders were tested at maximal effort and the average total work for each joint was used; order of testing was determined by hand dominance. Testing of joints after recent injury or surgery and those determined to be at risk of injury was omitted. Data from a single limb (2 knees, 8 shoulders) were used when the average of both limbs was not available due to testing error, pain or injury.
Function
All tests were repeated in the same order each time using scripted verbal instructions; no encouragement was given during testing. Testing was not done after recent injury or surgery and in those determined to be at risk of injury. The number of subjects for each test is indicated in the tables. Due to inconsistencies during the testing procedures, as well as injury or joint replacement in 3 volunteers, not all data were available. Flexibility was measured using a movable block while the subject was seated in a box on the floor (data not shown).
Quality of Life Assessments
A Factor Analysis (24) of the different scales of the 4 questionnaires was performed to create an overall wellbeing factor. Initially, study subjects were used as their own internal controls and assessed if there was change over time in overall wellbeing. Using the hierarchical linear modeling (HLM), a growth curve analysis was conducted, testing within-person linear and curvilinear change in overall wellbeing over time.
Power Calculations
The two primary endpoints were FFM and AVF. The study was powered for the pivotal first 12 months, based on a sample size of 60 subjects (40 randomized to MK-677 and 20 to placebo in year 1). This cross-over study was designed to have at least 90% power to detect a 4.1% or greater between-group difference in the change in percent FFM, with the same power to detect a 12.6% or greater between-group difference in the change in AVF (cm2) after the initial 12 months of treatment. The sample size formula for two-sample Student's t-test was used to conduct the power analysis. The standard deviations used in the power calculations were 4.4% for FFM and 14.1% for AVF. Both power calculations reflect the power of a two-sided test with a type I error rate of 0.05.
RESULTS
Quality of Life and Overall Wellbeing
There were no differences between the MK-677 and placebo groups in a linear change in overall wellbeing; t (61) = -1.47, P=0.1. No difference in linear trend of pain between the MK-677 and placebo groups was observed; t (61) = 1.80, P=0.07. Subjects in the MK-677 group reported more depression over time (not significant), whereas subjects in the placebo group reported less depression over time; t (61) = 1.66, P=0.1. However, it should be noted that subjects in the placebo group had a higher level of depression than subjects in the MK-677 group at baseline; t (61) = -2.28, P=0.02.
APPENDIX FIGURE 1. Study Design.
Year 1: In each of 3 cohorts [23 men, 25 women on HRT and 17 women off HRT], 2/3 received oral MK-677 (25 mg daily) and 1/3 received placebo. At the end of year 1, MK-677-treated subjects were randomized to continue on MK-677 (Group 1) or change to placebo (Group 2); the placebo-treated subjects were given MK-677 treatment in year 2 (Group 3).
APPENDIX FIGURE 2. Individual IGF-I Results.
Serum IGF-I levels at baseline, 6 and 12 months in each subject. The lower limit of the IGF-I normal range is indicated: older adults (59-225 μg/L, lower dotted line); young adults aged 21-25 (116-358 μg/L, upper dashed line).IGF-I = insulin-like growth factor-I
APPENDIX FIGURE 3. Individual t-scores for TASM/ht2.
Sarcopenia was defined as a t-score ≤ 2 SD below the young, gender-concordant population (17, 18). Dashed lines indicate -1 and -2 SD for t-score. TASM = total appendicular skeletal muscle mass by dual energy x-ray absorptiometry (DXA). TASM divided by height (meters) squared = an index of relative limb muscle mass used to compute a t-score by relating it to gender-concordant young adults
APPENDIX FIGURE 4. Individual FBG (Panels A and B) and HbA1c levels (Panels C and D).
Fasting blood glucose (FBG) and hemoglobin A1c (HBA1c) after 12 months of placebo (upper panels) and MK-677 (lower panels). FBG (lower dashed line=upper limit normal range, middle dashed line=prediabetic range, upper dotted line=diabetes mellitus); HbA1c (dashed line=upper limit of normal range).
APPENDIX FIGURE 5. Serum 24-h mean GH and IGF-I levels every 6 months over 2 years.
GH (Panel A) and IGF-I (Panel B) data were not normally distributed and were analyzed on the natural logarithmic scale; line graphs show geometric means and 95% confidence intervals. An asterisk indicates that the fold change from baseline to 24 months was significant for that group (Bonferroni-adjusted P<0.001). In Panel A, the 24-h mean GH for young men and women combined (~1.3 μg/L) is indicated by a dashed line. In Panel B the lower limit of the IGF-I normal range is indicated: older adults (59-225 μg/L, lower dotted line); young adults aged 21-25 (116-358 μg/L, upper dashed line). GH=growth hormone, IGF-I=insulin-like growth factor-I
APPENDIX FIGURE 6. Changes from baseline in FFM and t-scores for TASM/ht2 every 6 months for 2 years.
Data presented are arithmetic mean (SE) changes from baseline at 6, 12, 18 and 24 months; FFM (Panel A), t-scores for TASM/ht2 (Panel B). An asterisk indicates a significant difference in the change from baseline to 24 months within the group (Bonferroni-adjusted P values). In subjects treated with placebo followed by MK-677 in year 2 (Group 3), the change from baseline in FFM was significant (P=0.026), however, the t-score for TASM/ht2 was not significant compared with baseline (t-scores did increase in response to MK-677 in year 2, but had declined in year 1 during placebo). FFM = fat-free mass by DXA, TASM=total appendicular skeletal muscle mass from DXA, TASM divided by height (meters) squared = an index of relative limb muscle mass used to compute a t-score by relating it to gender-concordant young adults
APPENDIX FIGURE 7. Changes from baseline in body weight, total body fat, FBG and AVF every 6 months for 2 years.
Data presented are arithmetic mean (SE) changes from baseline at 6, 12, 18 and 24 months; body weight (Panel A), total body fat mass by DXA (Panel B), FBG (Panel C) and AVF (Panel D). An asterisk indicates a significant difference in the change from baseline to 24 months within the group (Bonferroni-adjusted P values). In subjects treated with placebo followed by MK-677 in year 2 (Group 3), the change from baseline in body weight and FBG were significant (P=0.002 and P=0.001, respectively). In subjects treated with MK-677 for 2 years (Group 1), body weight was significantly increased (P=0.001), but fasting blood glucose was not (P=0.093). Total body fat mass by 4-C model was significantly increased (P=0.016) in Group 1, but body fat by DXA was not (P=0.062). There were no significant changes in AVF from baseline to 24 months in any group. FBG=fasting blood glucose), AVF=abdominal visceral fat from computed tomography (CT), DXA = dual energy x-ray absorptiometry
Footnotes
Publisher's Disclaimer: “This is the prepublication, author-produced version of a manuscript accepted for publication in Annals of Internal Medicine. This version does not include post-acceptance editing and formatting. The American College of Physicians, the publisher of Annals of Internal Medicine, is not responsible for the content or presentation of the author-produced accepted version of the manuscript or any version that a third party derives from it. Readers who wish to access the definitive published version of this manuscript and any ancillary material related to this manuscript (e.g., correspondence, corrections, editorials, linked articles) should go to www.annals.org or to the print issue in which the article appears. Those who cite this manuscript should cite the published version, as it is the official version of record.”
Protocol: available to interested readers by contacting Dr. Thorner at MOT@virginia.edu
Statistical Code: available to interested readers by contacting Dr. Thorner at MOT@virginia.edu
REFERENCES
- (1).Fantin F, Francesco VD, Fontana G, Zivelonghi A, Bissoli L, Zoico E, et al. Longitudinal body composition changes in old men and women: interrelationships with worsening disability. J Gerontol A: Biol Sci Med Sci. 2007;62:1375–81. doi: 10.1093/gerona/62.12.1375. [DOI] [PubMed] [Google Scholar]
- (2).Kotler DP, Tierney AR, Wang J, Pierson RN., Jr Magnitude of body-cell-mass depletion and the timing of death from wasting in AIDS. Am J Clin Nutr. 1989;50:444–47. doi: 10.1093/ajcn/50.3.444. [DOI] [PubMed] [Google Scholar]
- (3).Janssen I, Heymsfield SB, Wang Z, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol. 2000;89:81–88. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- (4).Zadik Z, Chalew SA, McCarter RJ, Jr., Meistas M, Kowarski AA. The influence of age on the 24-hour integrated concentration of growth hormone in normal individuals. J Clin Endocrinol Metab. 1985;60:513–16. doi: 10.1210/jcem-60-3-513. [DOI] [PubMed] [Google Scholar]
- (5).Rudman D, Kutner MH, Rogers CM, Lubin MF, Fleming GA, Bain RP. Impaired growth hormone secretion in the adult population: relation to age and adiposity. J Clin Invest. 1981;67:1361–69. doi: 10.1172/JCI110164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Haarbo J, Marslew U, Gotfredsen A, Christiansen C. Postmenopausal hormone replacement therapy prevents central distribution of body fat after menopause. Metabolism. 1991;40:1323–26. doi: 10.1016/0026-0495(91)90037-w. [DOI] [PubMed] [Google Scholar]
- (7).DeNino WF, Tchernof A, Dionne IJ, Toth MJ, Ades PA, Sites CK, et al. Contribution of abdominal adiposity to age-related differences in insulin sensitivity and plasma lipids in healthy nonobese women. Diabetes Care. 2001;24:925–32. doi: 10.2337/diacare.24.5.925. [DOI] [PubMed] [Google Scholar]
- (8).Liu H, Bravata DM, Olkin I, Nayak S, Roberts B, Garber AM, et al. Systematic review: the safety and efficacy of growth hormone in the healthy elderly. Ann Intern Med. 2007;146:104–15. doi: 10.7326/0003-4819-146-2-200701160-00005. [DOI] [PubMed] [Google Scholar]
- (9).Chapman IM, Bach MA, Van Cauter E, Farmer M, Krupa D, Taylor AM, et al. Stimulation of the growth hormone (GH)-insulin-like growth factor I axis by daily oral administration of a GH secretagogue (MK-677) in healthy elderly subjects. J Clin Endocrinol Metab. 1996;81:4249–57. doi: 10.1210/jcem.81.12.8954023. [DOI] [PubMed] [Google Scholar]
- (10).Smith RG. Development of growth hormone secretagogues. Endocr Rev. 2005;26:346–60. doi: 10.1210/er.2004-0019. [DOI] [PubMed] [Google Scholar]
- (11).Veldhuis JD, Johnson ML. Cluster analysis: a simple, versatile, and robust algorithm for endocrine pulse detection. Am J Physiol. 1986;250:E486–E493. doi: 10.1152/ajpendo.1986.250.4.E486. [DOI] [PubMed] [Google Scholar]
- (12).Johnson ML, Veldhuis PP, Farhi LS, Evans WS. Performance properties of an automatic multi-parameter deconvolution method (AutoDecon). Proc 88th Meeting of the Endocrine Soc P3.2006. [Google Scholar]
- (13).Heymsfield SB, Lichtman S, Baumgartner RN, Wang J, Kamen Y, Aliprantis A, et al. Body composition of humans: comparison of two improved four-compartment models that differ in expense, technical complexity, and radiation exposure. Am J Clin Nutr. 1990;52:52–58. doi: 10.1093/ajcn/52.1.52. [DOI] [PubMed] [Google Scholar]
- (14).Clasey JL, Kanaley JA, Wideman L, Heymsfield SB, Teates CD, Gutgesell ME, et al. Validity of methods of body composition assessment in young and older men and women. J Appl Physiol. 1999;86:1728–38. doi: 10.1152/jappl.1999.86.5.1728. [DOI] [PubMed] [Google Scholar]
- (15).Heymsfield SB, Smith R, Aulet M, Bensen B, Lichtman S, Wang J, et al. Appendicular skeletal muscle mass: measurement by dual-photon absorptiometry. Am J Clin Nutr. 1990;52:214–18. doi: 10.1093/ajcn/52.2.214. [DOI] [PubMed] [Google Scholar]
- (16).Gallagher D, Visser M, De Meersman RE, Sepulveda D, Baumgartner RN, Pierson RN, et al. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J Appl Physiol. 1997;83:229–39. doi: 10.1152/jappl.1997.83.1.229. [DOI] [PubMed] [Google Scholar]
- (17).Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–63. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- (18).Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. RE: “Epidemiology of sarcopenia among the elderly in New Mexico”. Am J Epidemiol. 1999;149:1160. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- (19).Clasey JL, Bouchard C, Wideman L, Kanaley J, Teates CD, Thorner MO, et al. The influence of anatomical boundaries, age and sex on the assessment of abdominal visceral fat. Obesity Res. 1997;5:395–401. doi: 10.1002/j.1550-8528.1997.tb00661.x. [DOI] [PubMed] [Google Scholar]
- (20).Silva AM, Wang J, Pierson RN, Jr., Wang Z, Heymsfield SB, Sardinha LB, et al. Extracellular water: greater expansion with age in African Americans. J Appl Physiol. 2005;99:261–67. doi: 10.1152/japplphysiol.01317.2004. [DOI] [PubMed] [Google Scholar]
- (21).Schoeller DA, van Santen E, Peterson DW, Dietz W, Jaspan J, Klein PD. Hydrometry. In: Roche AF, Heymsfield SB, Lohman TG, editors. Human Body Composition. Human Kinetics; Champagne,IL: 1996. pp. 25–49. [Google Scholar]
- (22).Plotkin D, Ng J, Farmer M, Gelato M, Kaiser F, Kiel D, Korenman S, McKeever C, Munoz, Schwartz R, Krupa D, Gormley G, Bach MA. Use of MK-677, an oral GH secretagogue in frail elderly subjects. Endocrinol Metab. 1997;4(SupplA):35–36. [Google Scholar]
- (23).Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–10. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- (24).Tabachnick BG, Fidell LS. Using multivariate statistics. 5th ed. Pearson Education, Inc.; 2007. [Google Scholar]
- (25).Wang Z, Onge MP, Lecumberri B, Pi-Sunyer FX, Heshka S, Wang J, et al. Body cell mass: model development and validation at the cellular level of body composition. Am J Physiol Endocrinol Metab. 2004;286:E123–E128. doi: 10.1152/ajpendo.00227.2003. [DOI] [PubMed] [Google Scholar]
- (26).Morley JE. Weight loss in older persons: new therapeutic approaches. Curr Pharm Des. 2007;13:3637–47. doi: 10.2174/138161207782794149. [DOI] [PubMed] [Google Scholar]
- (27).Morley JE. Anorexia and weight loss in older persons. J Gerontol A: Biol Sci Med Sci. 2003;58:M131–M137. doi: 10.1093/gerona/58.2.m131. [DOI] [PubMed] [Google Scholar]
- (28).Bengtsson BA, Eden S, Lonn L, Kvist H, Stokland A, Lindstedt G, et al. Treatment of adults with growth hormone (GH) deficiency with recombinant human GH. J Clin Endocrinol Metab. 1993;76:309–17. doi: 10.1210/jcem.76.2.8432773. [DOI] [PubMed] [Google Scholar]
- (29).Franco C, Brandberg J, Lonn L, Andersson B, Bengtsson BA, Johannsson G. Growth hormone treatment reduces abdominal visceral fat in postmenopausal women with abdominal obesity: a 12-month placebo-controlled trial. J Clin Endocrinol Metab. 2005;90:1466–74. doi: 10.1210/jc.2004-1657. [DOI] [PubMed] [Google Scholar]
- (30).Münzer T, Harman SM, Hees P, Shapiro E, Christmas C, Bellantoni MF, et al. Effects of GH and/or sex steroid administration on abdominal subcutaneous and visceral fat in healthy aged women and men. J Clin Endocrinol Metab. 2001;86:3604–10. doi: 10.1210/jcem.86.8.7773. [DOI] [PubMed] [Google Scholar]
- (31).Götherström G, Bengtssom B, Sunnerhagen KS, Johannsson G, Svensson J. The effects of five-year growth hormone replacement therapy on muscle strength in elderly hypopituitary patients. Clin Endocrin. 2005;62:105–13. doi: 10.1111/j.1365-2265.2004.02181.x. [DOI] [PubMed] [Google Scholar]
- (32).Papadakis MA, Grady D, Black D, Tierney MJ, Gooding GA, Schambelan M, et al. Growth hormone replacement in healthy older men improves body composition but not functional ability. Ann Intern Med. 1996;124:708–16. doi: 10.7326/0003-4819-124-8-199604150-00002. [DOI] [PubMed] [Google Scholar]
- (33).Blackman MR, Sorkin JD, Munzer T, Bellantoni MF, Busby-Whitehead J, Stevens TE, et al. Growth hormone and sex steroid administration in healthy aged women and men: a randomized controlled trial. JAMA. 2002;288:2282–92. doi: 10.1001/jama.288.18.2282. [DOI] [PubMed] [Google Scholar]
- (34).Buchner DM, Larson EB, Wagner EH, Koepsell TD, De Lateur BJ. Evidence for a non-linear relationship between leg strength and gait speed. Age Ageing. 1996;25:386–91. doi: 10.1093/ageing/25.5.386. [DOI] [PubMed] [Google Scholar]
- (35).Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M157. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- (36).Walston J, Hadley EC, Ferrucci L, Guralnik JM, Newman AB, Studenski SA, et al. Research agenda for frailty in older adults: Toward a better understanding of physiology and etiology: Summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54:991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- (37).Thomas DR. Loss of skeletal muscle mass in aging: examining the relationship of starvation, sarcopenia and cachexia. Clin Nutr. 2007;26:389–99. doi: 10.1016/j.clnu.2007.03.008. [DOI] [PubMed] [Google Scholar]
- (38).Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW. Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341:1097–105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- (39).Murden R, Ainslie N. Recent weight loss is related to short-term mortality in nursing homes. J Gen Intern Med. 1994;9:648–50. doi: 10.1007/BF02600311. [DOI] [PubMed] [Google Scholar]
- (40).Ryan C, Bryant E, Eleazer P, Rhodes A, Guest K. Unintentional weight loss in long-term care: predictor of mortality in the elderly. South Med J. 2008;88:721–24. doi: 10.1097/00007611-199507000-00005. [DOI] [PubMed] [Google Scholar]
- (41).del Rincon JP, Iida K, Gaylinn BD, McCurdy CE, Leitner JW, Barbour LA, et al. Growth hormone regulation of p85 alpha expression and phosphoinositide 3-kinase activity in adipose tissue: mechanism for growth hormone-mediated insulin resistance. Diabetes. 2007;56:1638–46. doi: 10.2337/db06-0299. [DOI] [PubMed] [Google Scholar]
- (42).Murphy MG, Weiss S, McClung M, Schnitzer T, Cerchio K, Connor J, et al. Effect of alendronate and MK-677 (a growth hormone secretagogue), individually and in combination, on markers of bone turnover and bone mineral density in postmenopausal osteoporotic women. J Clin Endocrinol Metab. 2001;86:1116–25. doi: 10.1210/jcem.86.3.7294. [DOI] [PubMed] [Google Scholar]
- (43).Svensson J, Lonn L, Jansson JO, Murphy G, Wyss D, Krupa D, et al. Two-month treatment of obese subjects with the oral growth hormone (GH) secretagogue MK-677 increases GH secretion, fat-free mass, and energy expenditure. J Clin Endocrinol Metab. 1998;83:362–69. doi: 10.1210/jcem.83.2.4539. [DOI] [PubMed] [Google Scholar]
- (44).Gotherstrom G, Bengtsson BA, Bosaeus I, Johannsson G, Svensson J. Ten-year GH replacement increases bone mineral density in hypopituitary patients with adult onset GH deficiency. Eur J Endocrinol. 2007;156:55–64. doi: 10.1530/eje.1.02317. [DOI] [PubMed] [Google Scholar]
- (45).Landin-Wilhelmsen K, Nilsson A, Bosaeus I, Bengtsson BA. Growth hormone increases bone mineral content in postmenopausal osteoporosis: a randomized placebo-controlled trial. J Bone Mineral Res. 2003;18:393–405. doi: 10.1359/jbmr.2003.18.3.393. [DOI] [PubMed] [Google Scholar]
- (46).Chapman IM, Hartman ML, Straume M, Johnson ML, Veldhuis JD, Thorner MO. Enhanced sensitivity growth hormone (GH) chemiluminescence assay reveals lower postglucose nadir GH concentrations in men than women. J Clin Endocrinol Metab. 1994;78:1312–19. doi: 10.1210/jcem.78.6.8200931. [DOI] [PubMed] [Google Scholar]
- (47).Weltman A, Clasey JL, Weltman JY, Wideman L, Kanaley J, Patrie J, et al. Impact of abdominal visceral fat (AVF), growth hormone (GH), fitness and insulin on serum lipids in older adults. Metabolism: Clinical & Experimental. 2003;52:73–80. doi: 10.1053/meta.2003.50007. [DOI] [PubMed] [Google Scholar]
- (48).Despres JP, Gagnon J, Bergeron J, Couillard C, Leon AS, Rao DC, et al. Plasma post-heparin lipase activities in the HERITAGE family study: the reproducibility, gender differences, and associations with lipoprotein levels. Clin Biochem. 1999;32:157–65. doi: 10.1016/s0009-9120(98)00106-4. [DOI] [PubMed] [Google Scholar]