Abstract
Until recently it was generally accepted that the only neurotransmitter to be released at central synapses of somatic motoneurons was acetylcholine. However, studies on young mice (P0-10) have provided pharmacological evidence indicating that glutamate may act as a co-transmitter with acetylcholine at synapses between motoneurons and Renshaw cells. We performed a series of anatomical experiments on axon collaterals obtained from intracellularly labelled motoneurons from an adult cat and labelled by retrograde transport in adult rats to determine if glutamate is co-localised with acetylcholine by these terminals. We could find no evidence for the presence of vesicular glutamate transporters in motoneuron axon terminals of either species. In addition, we were unable to establish any obvious relationship between motoneuron terminals and the GluR2 subunit of the AMPA receptor. However we did observe a population of cholinergic terminals in lamina VII which did not originate from motoneurons but were immunoreactive for the vesicular glutamate transporter 2 and formed appositions to GluR2 subunits. These were smaller than motoneuron terminals and, unlike them, formed no relationship with Renshaw cells. The evidence suggests that glutamate does not act as a co-transmitter with acetylcholine at central synapses of motoneurons in the adult cat and rat. However, glutamate is present in a population of cholinergic terminals which probably originate from interneurons where its action is via an AMPA receptor.
Keywords: spinal cord, motor control, Renshaw cell, Acetylcholine, immunocytochemistry
Introduction
The assertion that acetylcholine (ACh) is the only neurotransmitter released at both central and peripheral synapses of somatic motoneurons (e.g. Eccles et al., 1954; Curtis and Ryall, 1964; Windhorst, 1996) has been challenged recently (Herzog et al., 2004; Mentis et al., 2005; Nishimaru et al., 2005). It has been known for many years that the activation of Renshaw cells evoked by stimulation of motoneuron axon collaterals could not be totally blocked by cholinergic antagonists (Eccles et al., 1954; Noga et al., 1987; Schneider et al., 1992; Dourado et al., 2002) and pharmacological studies, using in vitro preparations of young (P0-4) mouse spinal cord, show that this activation can be abolished by application of both cholinergic and glutamatergic antagonists (Mentis et al., 2005; Nishimaru et al., 2005). Recently, Lamotte d’Incamps and Ascher (2008) confirmed that the glutamatergic component of this response is mediated by AMPA and NMDA receptors in P5-10 mice. However anatomical evidence supporting a role for glutamate as a co-transmitter with ACh is inconsistent in adult and young animals. Herzog et al. (2004) provided evidence that in adult rats, motoneurons express mRNA for both vesicular glutamate transporters 1 and 2 (VGLUT1; VGLUT2), a finding that is in conflict with previous reports of absence of mRNA for any of the three known vesicular glutamate transporters in motoneurons (Kullander et al., 2003; Oliveira, et al., 2003). Immunocytochemical studies have also produced conflicting evidence. Nishimaru et al. (2005), were unable to detect the presence of VGLUT1 or VGLUT3 in motoneuron axon collaterals but found some evidence for VGLUT2 co-localisation, but curiously only three cholinergic terminals (labelled with the vesicular cholinergic transporter, VAChT) in this region out of a sample of more than a thousand were positively labelled for VGLUT2. To some extent this is consistent with findings reported by Herzog et al. (2004) who proposed that VAChT and VGLUT2 were not co-localised in the same terminals but that individual collateral branches of motoneurons contained either one or the other. Conversely, Mentis et al. (2005) reported the absence of immunoreactity for any of the vesicular glutamate transporters in motoneuron axon collaterals but nevertheless concluded that some terminals may be enriched with glutamate. In summary, it seems that there is good pharmacological evidence to suggest that glutamate may be co-released along with acetylcholine at synapses formed between motoneurons and Renshaw cells in very young mice but anatomical evidence supporting this is inconsistent. We performed a series of anatomical studies in the adult cat and rat to determine if there was evidence to support the hypothesis that glutamate is co-localised in axon collaterals of mature animals and operates via an AMPA receptor. There were two principal aims of the study: 1) to determine if axon collaterals of adult motoneurons contain vesicular glutamate transporters; 2) to determine if axon collaterals of adult motoneurons are apposed to AMPA receptors. During the course of this study we identified a group of cholinergic axon terminals in the ventral horn that did not originate from motoneurons but contained VGLUT2 and apposed AMPA receptors.
Materials and Methods
Experiments were performed on three adult rats (250–350 g; 10–14 weeks old Harlan, Bicester UK) and one adult cat (3.6 kg; 6 months old) which was bred at the University of Göteborg. Rat experiments were conducted according to British Home Office legislation and were approved by the University of Glasgow Ethics Committee. The cat experiment was conducted according to NIH guidelines and was approved by the Göteborg University Ethics Committee.
Cat experiment
The cat was deeply anaesthetised with sodium pentobarbital (40–44 mg/kg, i.p.) and supplemented with intermittent doses of a-chloralose as required to maintain full anaesthesia (Rhône-Poulenc Santé, France; doses of 5 mg/kg administered every 1–2 hours, up to 55 mg/kg, i.v.). A laminectomy was performed to expose lumbar (L4-L7) segments of the spinal cord. Motoneurons were searched for in L6-L7 segments and identified on the basis of antidromic activation following stimulation of gastrocnemius and soleus nerves. Following identification, motoneurons were labelled intracellularly with a mixture of equal parts of 2% tetramethylrhodamine-dextran (Molecular Probes, Inc, Eugene, Oregon, USA) and 2% Neurobiotin (Vector, UK) in saline (pH 6.5). The marker was injected by passing depolarizing constant current 100–140 nA for 5–8 minutes. New injection sites were a minimum of 2 mm away from previous sites. At the conclusion of the experiment, the animal was given a lethal dose of pentobarbital and perfused, initially with physiological saline, and subsequently with paraformaldehyde (4%) in 0.1 M phosphate buffer (pH 7.4). Lumbar segments of the spinal cord containing labelled cells were removed and placed in the same fixative for 8 hours at 4 °C. Axon collaterals from an intracellularly labelled excitatory interneuron (e.g. see Bannatyne et al., 2006) were obtained from the same animal for control material.
Rat experiments
Three Sprague-Dawley rats were deeply anaesthetised with halothane and the left sciatic nerve was exposed under strict aseptic conditions. A micropipette containing a 1% solution of the B-subunit of cholera toxin (CTb; Sigma-Aldrich Co., Poole, UK) in sterile distilled water was inserted into the perineurium and 3–4 μl of this solution were pressure injected into the nerve. Following 3–5 days survival, the rats were anesthetized with pentobarbitone (l ml i.p.) and perfused through the left ventricle with saline followed by a fixative containing 4% formaldehyde in 0.1 M phosphate buffer pH 7.4. L3 to L5 spinal cord segments were removed from each animal and post-fixed in the same fixative for 2–8 hours at 4 °C. Segments were then cut into two blocks.
The L7 segment of the cat and L3-L5 segments of rats were rinsed several times in 0.1 M phosphate buffer (PB). All the segments were cut into 50 μm thick transverse sections with a Vibratome (Oxford instruments, Technical products international Inc. USA) and sections of cat segments were collected in strict serial order to enable reconstruction of labelled cells. All sections were treated with an aqueous solution of 50% ethanol for 30 minutes to enhance antibody penetration. Following this treatment, cat sections were mounted in serial order on glass slides with Vectashield (Vector Laboratories, Peterborough, UK) and examined with a fluorescence microscope. Sections containing labelled motoneurons were reacted firstly with avidin-rhodamine (1:1000; Jackson Immunoresearch, Luton, UK) and photographed with a digital camera attached to a fluorescent microscope. Sections containing labelled motoneuron axon terminals were then selected for further analysis.
Aim 1: Do adult motoneuron axon collateral terminals contain vesicular glutamate transporters?
Sections were incubated in the combinations of primary antibodies listed in Table 1. All antibodies were diluted in phosphate-buffered saline containing 0.3% Triton X-100 (PBST) and incubated for 48 hours. They were rinsed in phosphate-buffered saline (PBS) and incubated for 3 hours in solutions of secondary antibodies coupled to fluorophores before mounting with anti-fade medium (see Table 1 for details).
Table 1.
Summary of primary and secondary antibody combinations and concentrations used in the current study. All secondary antibodies were raised in donkey and conjugated to Rhodamine Red (Rh Red; 1:100), Cyanine 5.18 (Cy-5;1:100; both supplied by Jackson Immunoresearch, Luton, UK) and Alexa-fluor 488 (Alexa488; 1:500; Molecular Probes, Eugene, Oregon, USA). CB; calbindin, gp, guinea pig; gt, goat; mo, mouse; rbt, rabbit; shp, sheep.
| Primary Antibody combination | Primary Antibody concentration | Supplier | Secondary Antibodies | Sequential Immuno-reaction | Secondary Antibodies | |
|---|---|---|---|---|---|---|
| CAT | gp VGLUT1 | 1:5000 | Chemicon, Harlow, UK | Cy-5 | ||
| rbt VGLUT2 | 1:5000 | Chemicon, Harlow, UK | Alexa488 | |||
|
| ||||||
| RAT | mo CTb | 1:250 | A. Wikström, University of Gothenburg | Rh Red | ||
| A | gt VAChT | 1:5000 | Millipore, USA | Alexa488 | ||
| gp VGLUT1 | 1:5000 | Cy-5 | ||||
|
| ||||||
| B | mo CTb | 1:250 | Rh Red | |||
| gt VAChT | 1:5000 | Alexa488 | ||||
| gp VGLUT2 | 1:5000 | Chemicon, Harlow, UK | Cy-5 | |||
|
| ||||||
| C | mo CTb | 1:250 | Rh Red | |||
| gt VAChT | 1:5000 | Alexa488 | ||||
| gp VGLUT3 | 1:5000 | Chemicon, Harlow, UK | Cy-5 | |||
|
| ||||||
| D | shp NOS | 1:2000 | P. Emson, University of Cambridge | Rh Red | ||
| gp VAChT | 1:5000 | Millipore, USA | Alexa488 | |||
| rbt VGLUT2 | 1:5000 | Chemicon, Harlow, UK | Cy-5 | |||
|
| ||||||
| E | mo CTb | 1:250 | Rh Red | |||
| gp VGLUT1 | 1:5000 | Alexa488 | ||||
| rbt CB | 1:2000 | Swant, Bellizona, Switzerland | Cy-5 | |||
|
| ||||||
| F | mo CTb | 1:250 | Rh Red | |||
| gp VGLUT2 | 1:5000 | Alexa488 | gt VAChT | Cy-5 | ||
| rbt CB | 1:2000 | Cy-5 | ||||
|
| ||||||
| G | mo CTb | 1:250 | Rh Red | |||
| gt VAChT | 1:5000 | Alexa488 | gp VGLUT1 | Cy-5 | ||
| rbt CB | 1:2000 | Cy-5 | ||||
Sections containing axon terminals from the three labelled cat motoneurons were reacted with guinea pig anti-VGLUT1 and rabbit anti-VGLUT2. Motoneuron terminals obtained from rat experiments were identified by the presence of transported CTb from sciatic nerve injections (see above) which was co-localised with immunoreactivity for vesicular acetylcholine transporter (VAChT). Triple immunofluorescence was performed initially with VGLUT1, 2 and 3 antibodies (Groups A, B and C in Table 1). Also, as some cholinergic interneurons co-contain nitric oxide synthase (NOS: Miles et al., 2007), we attempted to determine if any cholinergic axons that were positive for VGLUT2 (see below) originate from this source by reacting tissue with a combination of anti-NOS, anti-VAChT and anti-VGLUT2 antibodies (Group D in Table 1).
As motoneuron axon collaterals are known to form synapses with Renshaw cells and calbindin is thought to be a reliable marker for Renshaw cells (Carr et al., 1998; Alvarez et al., 1999), rat sections containing labelled collaterals were reacted with CTb and calbindin antisera along with one of the following: antibodies: VGLUT1 (Group E), VGLUT2 (Group F), or VAChT (Group G). We performed sequential immunocytochemistry on sections from groups F and G. When analysis of sections with these initial combinations was complete, sections were re-incubated with a fourth antibody: goat anti-VAChT antibody (for group F) or guinea pig anti-VGLUT1 (for group G). The sections were remounted and the same field that had been scanned previously was identified and scanned again. By comparing labelling before and after the re-incubation in the VGLUT1 or VAChT antiserum, we could detect the additional staining, which represents immunoreactivity for VAChT and VGLUT1 in groups F and G, respectively.
Aim 2: Are axon collaterals of adult motoneurons apposed to AMPA receptors?
An antigen unmasking method was used to reveal whether AMPA receptors subunits are associated with labelled motoneuron terminals. Sections containing axon collaterals from three motoneurons and one interneuron were processed. Pepsin treatment was performed by incubating sections at 37°C for 30 min in PBST, followed by 10 min in 0.2M HCl containing 1 mg/ml pepsin (Dako, Glostrup, Denmark) with continuous agitation. After rinsing, sections were incubated in a mouse anti-GluR2 antibody (1:300; Chemicon, Harlow, UK) for 72 hours at 4°C. Sections were then incubated in a species-specific donkey secondary antibody conjugated to Alexa488 (1:500; Molecular Probes, Eugene, Oregon, USA) for 24 hours. Cholinergic terminals in rat tissue were examined to determine if they form associations with the GluR2 subunit of AMPA receptors. As GluR2 subunits are present in 98% AMPA-containing synapses in the rat spinal cord (Nagy et al., 2004), this subunit can be used as a general marker for this class of receptor. Before applying the antigen unmasking method to expose GluR2 subunits as described above, sections from three rats were incubated in goat anti-VAChT antibody (1:50000) for 72 hours and then subjected to a tyramide signal amplification (TSA) reaction in order to preserve labelling for VAChT following pepsin treatment. Sections were initially incubated for 4 hours in anti-goat horseradish peroxidase (HRP; 1:500; Jackson Immunoresearch, West Grove, PA, USA). The sections were then rinsed and processed with a tetramethylrhodamine fluorophore (diluted 1:50 in amplification diluent; PerkinElmer Life Sciences, Boston, MA) for 7 min (see Nagy et al., 2004 for details). Sections were rinsed, treated with pepsin, and incubated for 72 hours in mouse anti-GluR2 (1:300), followed by 24 hours in a species-specific donkey secondary antibody conjugated to Alexa 488 (1:500). We also performed a control experiment to determine the association between glutamatergic boutons and GluR2 subunits. Sections from three rats were incubated for 72 hours in a combination of guinea pig anti-VGLUT1 (1:50000) and guinea pig anti-VGLUT2 (1:50000) antibodies. The sections were rinsed and placed in anti-guinea pig HRP (1:500; Jackson Immunoresearch, West Grove, PA, USA) for 4 hours. They were processed according to the TSA and pepsin treatment described above and incubated in mouse anti-GluR2 (1:300).
Confocal microscopy and analysis
All sections were mounted in a glycerol-based antifade medium (Vectashield, Vector Laboratories, Peterborough, UK) and examined with a BioRad MRC 1024 confocal laser scanning microscope (BioRad, Hemel Hempstead, UK).
Cat sections with VGLUT markers were scanned by using a ×60 oil-immersion lens at a zoom factor of 3.5 and at 0.5 μm steps in the z axis, while sections with GluR2 staining were examined with a ×60 oil-immersion lens at a zoom factor of 2 and at 0.2 μm steps in the z axis. Image stacks were analyzed with Neurolucida for Conofocal software (MBF Bioscience, Colchester, VT, USA). To avoid bias, all labelled motoneuron axon terminals and VGLUT markers (or GluR2 immunoreactivity) were viewed in individual channels for every single optical section initially and then examined in merged image stacks showing all three (or two) channels by switching between different channels. When scanning of immunoreactive boutons was complete, the sections were retrieved from the slides and rinsed in PBS. These sections together with the other sections containing soma and axons of labelled motoneurons were reacted with avidin-biotin-peroxidase complex technique (ABC technique). This involved an overnight incubation in a fresh solution of avidin-biotin-complex (Vector Laboratories Ltd, England) followed by a further rinse with PBS then PB. The sections were then placed in a solution containing hydrogen peroxide plus 3, 3′-diaminobenzidine (DAB; Sigma, Dorset, England) diluted in phosphate buffer for a period of approximately 4 minutes. During this time, the reaction was monitored constantly. Following the DAB reaction, sections were dehydrated in a series of ethanol solutions and mounted in serial order on gelatinised slides for cell reconstruction. This method provides a permanent record of the cells from which detailed reconstructions were made using a drawing tube.
Rat sections from the lumbar segments (L3-L5) were examined within the ventral region of lamina VII, between medial and lateral motoneuron pools, which contains most Renshaw cells (Carr et al., 1998). Sections reacted with antibodies against CTb, VAChT and VGLUT (i.e. group A, B, and C) were scanned by using a ×60 oil-immersion lens with a zoom factor of 2 at 0.5 μm intervals. The stacks of images were analyzed with Neurolucida for Confocal software. Two hundred CTb labelled terminals that were also immunoreactive for VAChT were randomly selected from each rat. To avoid bias, a 100×100 μm grid was placed on the image and the terminal closest to bottom right corner of each grid square was selected. Selected terminals were then examined in the blue channel (representing the VGLUTs) to determine whether they were positive for VGLUT immunoreactivity. Sections reacted with antibodies for CTb, calbindin and VGLUT (i.e. group E and F) or CTb, calbindin and VAChT (i.e. group G) were scanned with a ×40 oil-immersion lens and zoom factor of 2 at 0.5 μm intervals. Image stacks were analyzed with Neurolucida to investigate the proportion of CTb labelled terminals that made contact with Renshaw cells and were also immunolabelled for VGLUT1, VGLUT2 or VAChT in each group.
Sections reacted for VAChT and GluR2 were mounted in Vectashield anti-fade medium and scanned with the confocal microscope. Lamina VII of sections from each of the three rats was scanned by using a ×60 oil-immersion lens with a zoom factor of 2 at 0.2 μm steps in the z axis. In each section, two scanning fields were obtained from both sides with a 100×100 μm scanning area. By using Neurolucida software, image stacks were initially viewed so that only VAChT immunoreactivity was visible. All VAChT immunoreactive boutons within the scanning box from each animal were selected for analysis. GluR2 staining was then examined, and it was determined whether VAChT terminals were apposed to GluR2 puncta. Control material reacted with a combination of VGLUT1 and 2 and GluR2 was analysed in exactly the same way.
In order to determine if axon terminals with different immunochemical characteristics belonged to different populations, we made a comparison between five groups of axon terminals. The equivalent diameter of axonal swellings that were double labelled for: (1) VAChT and GluR2; (2) VAChT and VGLUT2; (3) VAChT and no GluR2; (4) CTb and VAChT and (5) CTb and VGLUT1 were measured from projected confocal images obtained from all 3 rats by using Image J software (National Institutes of Health, USA, http://rsb.info.nih.gov/ij/). A one-way ANOVA was used to determine if there were statistically significant differences in the diameters of these groups (p<0.05). This was followed by a Tukey’s post hoc pairwise comparison to determine which groups were significantly different from each other.
Confocal microscope images for publication were prepared by using Adobe Photoshop to adjust brightness and contrast.
Results
Do adult motoneuron axon collateral terminals contain vesicular glutamate transporters?
In total, 63 axon terminals obtained from 3 intracellularly labelled motoneurons from the cat experiment were analysed. Terminals that could not be traced back to the parent axon were excluded from the sample. Sections, which contained 11 motoneuron axon terminals, were reacted with antibodies against VGLUT1 and VGLUT2. Confocal microscope analysis showed that none of the 11 terminals were positive for either VGLUT1 or VGLUT2 immunoreactivity (Figure 1). The remaining 55 axon terminals were reacted for the presence of GluR2 (see below).
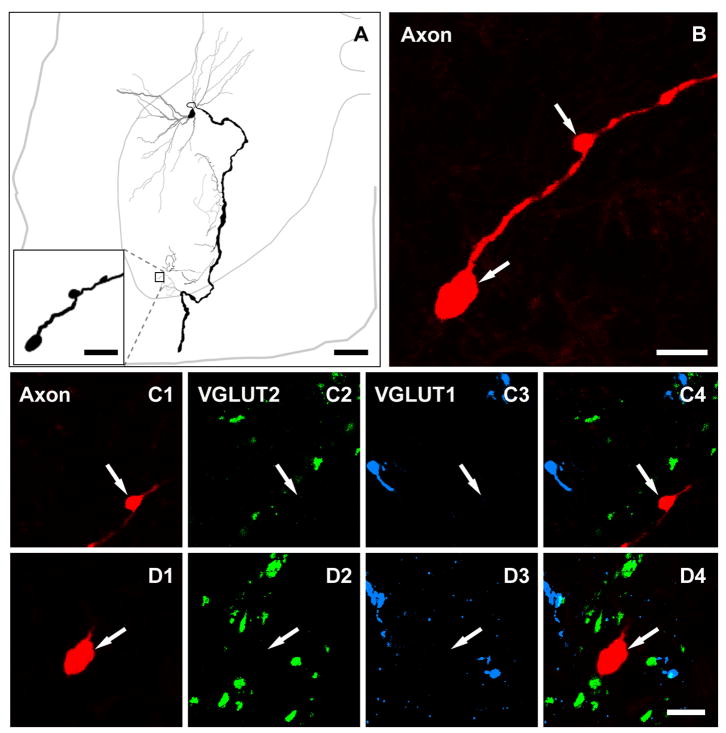
Figure 1.
A reconstruction of a cat motoneuron and its axon collaterals that was intracellularly labelled with Neurobiotin (A) and a series of confocal microscope images illustrating the absence of VGLUT1 and 2 immunoreactivity within the motoneuron axon terminals (B–D). A, the soma and axonal arborisation are shown in black and dendrites in grey. The thinner grey line represents the outline of the grey matter and the central canal; the thicker grey line represents the border of the spinal cord. The axon terminals illustrated in figure B, C, and D are taken from the area outlined by the box in A. B shows a projected image through a series of terminals. C1-C4 are single optical sections and D1-D4 are projected images of two individual terminals (arrows). C4 and D4 are merged images confirming the absence of overlap of immunoreactivity for VGLUT1 and VGLUT2 and labelled motoneuron terminals. Scale bars: 200μm (large panel in A); 10 μm (small panel in A and B); 5 μm (C1-C4 and D1-D4).
Figure 1. A seires of confocal microscope images illustrating the absence of VGLUT1 and 2 immunoreactivity within motoneuron axon terminals in the cat. The large panel on the left shows a projected image through a series of terminals originating from a motoneuron that was intracellularly labelled with Neurobiotin. Small images show single optical sections (top row) and projected images (the bottom row) of two individual terminals (arrows). The right panel of each series is a merged image confirming the absence of overlap of immunoreactivity for VGLUT1 and VGLUT2 and labelled motoneuron terminals and. Scale bars: 10 μm (for the large panel); 5 μm (for the small images).
In rat experiments, both motoneuron axon terminals and primary afferent axons were labelled by injection of the tracer CTb. Retrogradely labelled motoneurons were found in lamina IX ipsilateral to the injection site of the L3 to L5 segments of the spinal cord. Initially, we performed a series of three triple labelling experiments on each animal to determine if VAChT and glutamate vesicular transporters were co-localised in motoneuron collateral terminals. Three groups of sections were reacted: A) CTb +VAChT +VGLUT1; B) CTb +VAChT +VGLUT2; and C) CTb +VAChT +VGLUT3. In each group, a total of six hundred terminals, doubled-labelled for CTb and VAChT, were randomly selected from the three rats (two hundred from each rat). Confocal microscope images revealed that none of the selected terminals was positive for VGLUT1, VGLUT2 and VGLUT3 immunoreactivity (Figure 2). In addition, we also measured the equivalent diameter of 150 axon terminals which were labelled by both CTb and VAChT but not VGLUT1 (50 from each rat), and the equivalent diameter of 150 presumed primary afferent terminals which were labelled by both CTb and VGLUT1 but not VAChT (50 from each rat). As predicted, there was a significant difference between these two types of terminal; the average (±SD) equivalent diameters of the motoneuron terminals and the primary afferent terminals were 1.99±0.51μm and 3.58±0.78μm, respectively (See below). In CTb +VAChT +VGLUT2 (group B) experiments, we not only found that there was no overlap of immunoreactivity for CTb and VGLUT2, but we also identified a small population of VGLUT2-labelled axons with VAChT staining (Figure 2B). The equivalent diameter of VAChT/VGLUT2 terminals was 0.89±0.23μm (50 terminals from each rat). These were significantly smaller than the CTb/VAChT and the CTb/VGLUT1 terminals (see below). We further investigated the relationship between VAChT/VGLUT2 terminals and NOS (Group D) to determine if they originated from the NOS-containing subgroup of cholinergic interneurons (see Miles et al., 2007). A total of fifty VAChT/VGLUT2 terminals were randomly selected but none of them displayed NOS immunoreactivity (Figure 2D).
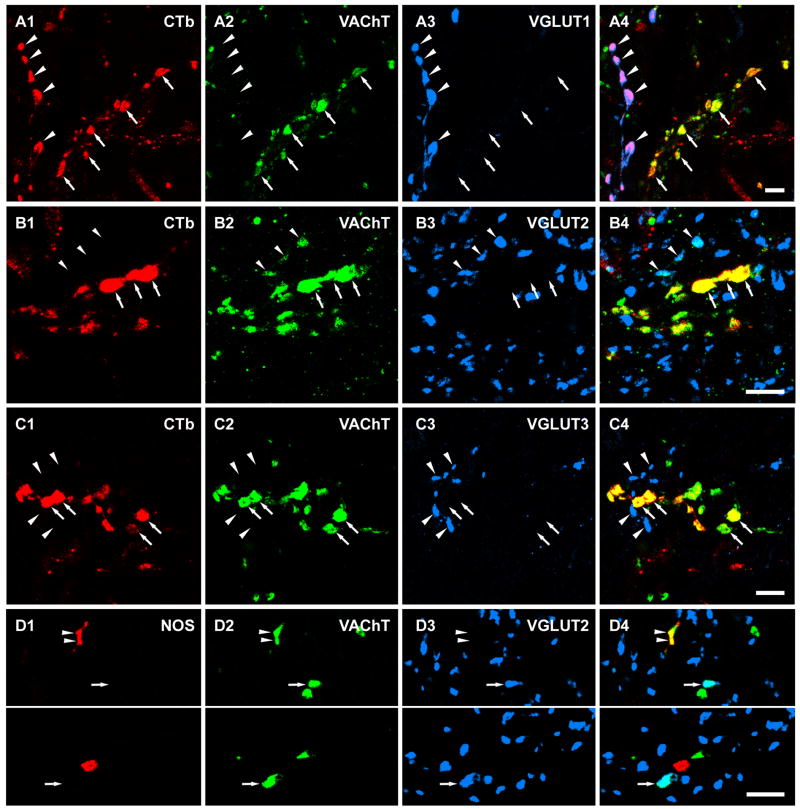
Figure 2.
Three colour confocal images showing CTb labelled motoneuron terminals that are positive for VAChT-immunoreactivity but are negatively labelled for all three vesicular glutamate transporters (A–C) and the relationship of VAChT/VGLUT2 double labelled terminals with NOS immunoreactivity (D). A1-A4 and B1-B4 are projected images of three single optical sections. C1-C4 and D1-D4 are single optical sections. Arrows in A–C indicate CTb labelled motoneuron terminals that were VAChT immunoreactive. Arrows in D indicate terminals that were immunopositive for both VAChT and VGLUT2. Arrowheads in A indicate VGLUT1 immunoreactive terminals also labelled by CTb that are likely to originate from proprioceptive primary afferent fibres. Arrowheads in B indicate VGLUT2 immunoreactive terminals that also contain VAChT staining but are not labelled with CTb. Arrowheads in C indicate VGLUT3 terminals. Arrowheads in D indicate NOS immunopositive terminals that were also labelled by VAChT. A4, B4, C4 and D4 are merged images. Scale bar: 5 μm.
Terminals which formed contacts with Renshaw cells identified by calbindin labelling (Carr et al., 1998; Alvarez et al., 1999) were investigated by performing immunoreactions with two antibody combinations: Group E) CTb +VGLUT1 + calbindin; and Group F) CTb +VGLUT2 + calbindin. For each combination, a sample of 15 calbindin labelled Renshaw cells (5 from each of the three animals) located within ventral lamina VII between motor nuclei was analysed. Each of the scanning areas was 151μm×151μm and contained at least one cell body of a Renshaw cell and its proximal dendrites. In group E, a total of 285 CTb labelled terminals which contacted Renshaw cells was recorded (average ±SD, 19±5.6 contacts per cell). Of these terminals, 99±3% (281 out of 285) was found to be negative for VGLUT1 immunoreactivity (Figure 3A). We then measured the size of the remaining 1% (4 out of 285) terminals which were immunoreactive for VGLUT1. Their equivalent diameters ranged from 3.65μm to 6.15 μm (average ±SD, 4.85±1.25μm). In group F, a total of 339 CTb labelled terminals (average ±SD, 23±3.5 per cell) which were apposed Renshaw cells was analyzed. None of the terminals that contacted Renshaw cells were labelled with VGLUT2 immunoreactivity (Figure 3B). Sequential immunocytochemistry with a fourth antibody against VAChT enabled us to confirm that the CTb-positive VGLUT2-negative terminals originated from motoneurons as all VGLUT2-negative terminals were positively labelled for VAChT immunoreactivity (Figure 3C). In sequential experiments, we also noted that some VGLUT2 positive/CTb negative terminals were positive for VAChT immunoreactivity but that they did not form contacts with Renshaw cells (not shown). These results suggest that some cholinergic terminals located in the deep ventral horn which do not originate from motoneurons contain glutamate.
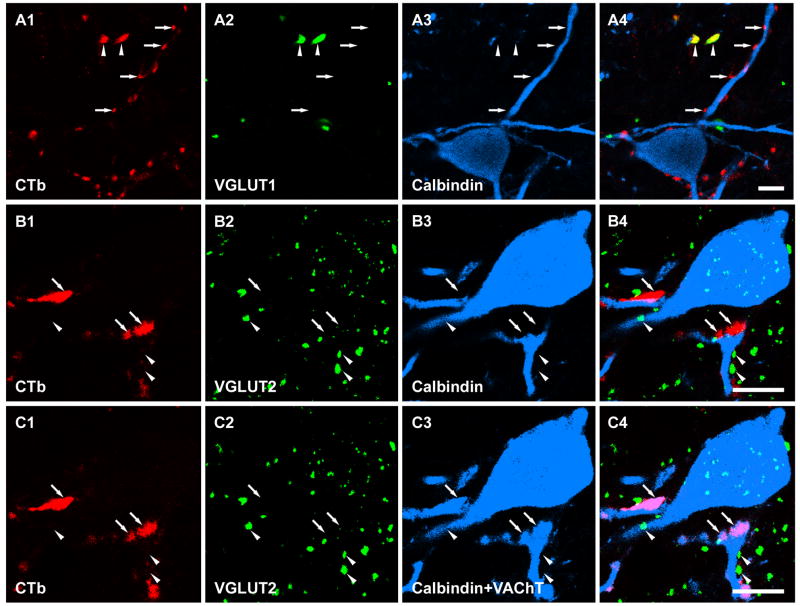
Figure 3.
Single optical sections illustrating CTb labelled motoneuron terminals in contact with Renshaw cells. A1-A4 and B1-B4 show that all of the CTb labelled motoneuron terminals making contact with two calbindin labelled cells were negative for VGLUT1- and VGLUT2-immunoreactivity, respectively. Arrows indicate selected motoneuron terminals which contact the calbindin labelled cells. Arrowheads indicate VGLUT1 and VGLUT2 terminals in A and B, respectively. C1-C4 are single optical sections of the same area shown in B1-B4 that have been rescanned after sequential incubation with a fourth antibody for VAChT. The extra labelling present in C3 (indicated by arrows), which was absent in B3, corresponds to the additional VAChT-immunostaining. Note that all CTb terminals that form contacts with the Renshaw cell are immunoreactive for VAChT (profiles indicated by arrows in C4, merged image on the right) and hence can be confirmed to be motoneuron axon terminals. A4, B4 and C4 are merged images Scale bar: 1μm.
To confirm the above findings, we used a further combination of CTb, VAChT and calbindin (Group G). Another sample of 15 calbindin labelled Renshaw cells was examined (5 from each of the three animals). In 151μm×151μm scanning fields, a total of 364 CTb labelled terminals which contacted Renshaw cells was analysed (average ±SD, 24±5.6 per cell). Of these terminals, 97±4% (352 out of 364) was found to be positive for VAChT (Figure 4A and C). Sequential immunocytochemistry with a fourth antibody against VGLUT1 enabled us to show that the 12 VAChT-negative terminals were immunoreactive for VGLUT1. None of the 352 (out of 364) CTb/VAChT terminals was labelled for VGLUT1 (Figure 6B and D).
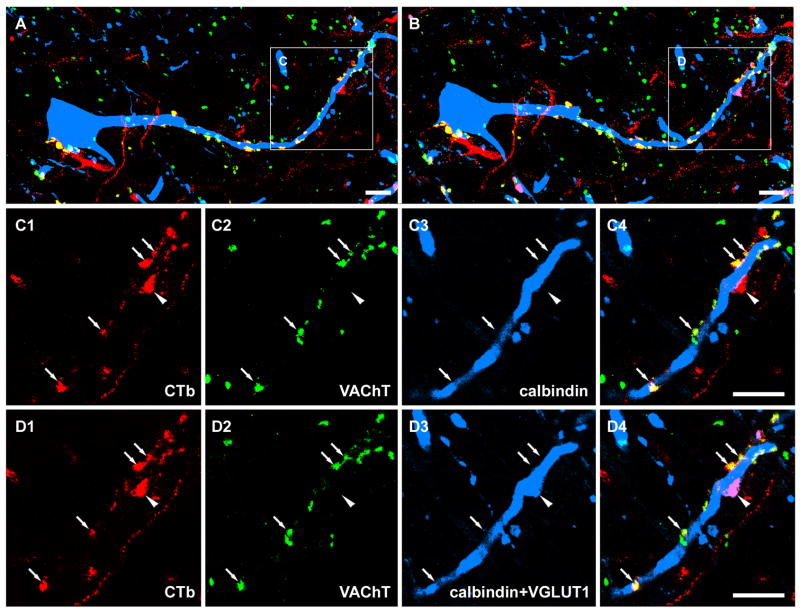
Figure 4.
Sequential immunocytochemistry for CTb labelled terminals in contact with a Renshaw cell. A and B show a general overview of CTb labelled terminals in contact with a calbindin labelled cell before and after a reaction with a fourth antibody against VGLUT1. Details of the areas demarcated by the boxes are shown in C1-C4 and D1-D4. C1-C4 are single optical sections illustrating that most of the CTb labelled terminals in contact with the calbindin cell were positive for VAChT. Arrows indicate double labelled CTb axon terminals with VAChT staining. The arrowhead indicates a single CTb labelled terminal on the Renshaw cell that was negative for VAChT. D1-D4 are single optical sections of the same terminals that were rescanned after sequential incubation with a fourth antibody against VGLUT1. The extra labelling present in D3 (indicated by arrowhead), which was absent in C3, corresponds to additional VGLUT1-immunostaining (see D4). Note that this VGLUT1 positive terminal, which forms an apposition with the Renshaw cell, is bigger than CTb labelled motoneuron terminals, and is likely to be a primary afferent terminal. A, B, C4 and D4 are merged images. Scale bar: 1μm.
Figure 6.
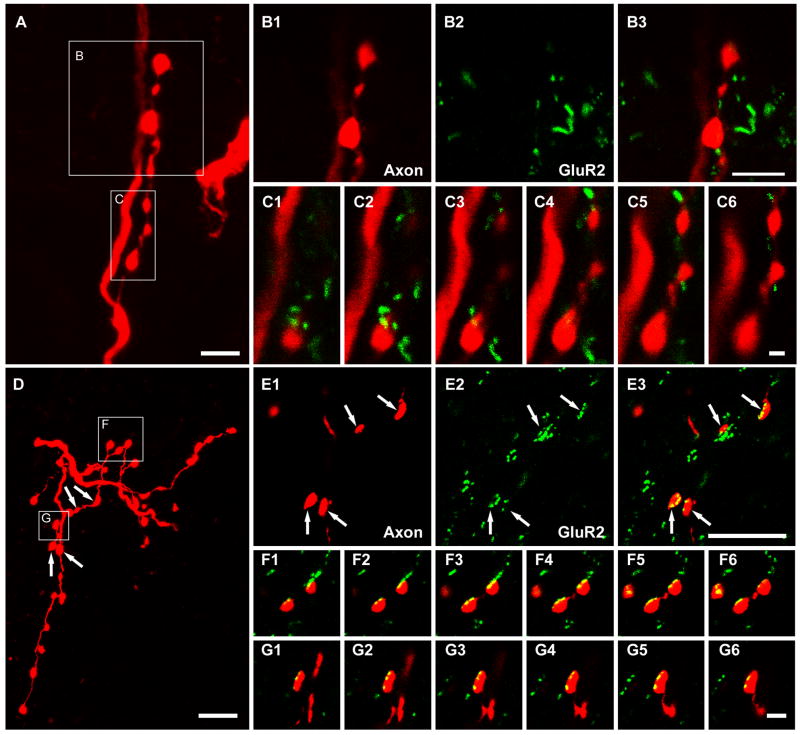
Relationships between intracellularly labelled axon terminals and immunoreactivity for the GluR2 subunit of the AMPA receptor following antigen unmasking with pepsin. A, B and C show that there is no obvious association between motoneuron axon terminals and GluR2 immunoreactivity. D, E and F show associations between interneuronal axon terminals and immunoreactivity for GluR2. The large panels on the top left (A) and on the bottom left (D) show projected images of a series of terminals originating from a motoneuron and an excitatory interneuron, respectively. Details of the areas demarcated by the boxes are shown in B1-B3, C1-C6, E1-E3, F1-F6, and G1-G6. B1-B3 show motoneuron axon terminals (B1), GluR2 (B2), and a merged image (B3) of the same single optical section. C1-C6 show a series of merged single optical sections through the motoneuron terminals taken at intervals of 0.3 μm. E1-E3 show interneuron axon terminals (E1), GluR2 (E2), and a merged image (E3) of the same single optical section. F1-F6 and G1-G6 show series of merged single optical sections through the interneuron terminals taken at intervals of 0.2μm. Scale bar, 5μm (A, B1-B3, D and E1-E3); 1μm (C1-C6); 2μm (F1-F6, and G1-G6).
Are axon collaterals of adult motoneurons apposed to AMPA receptors?
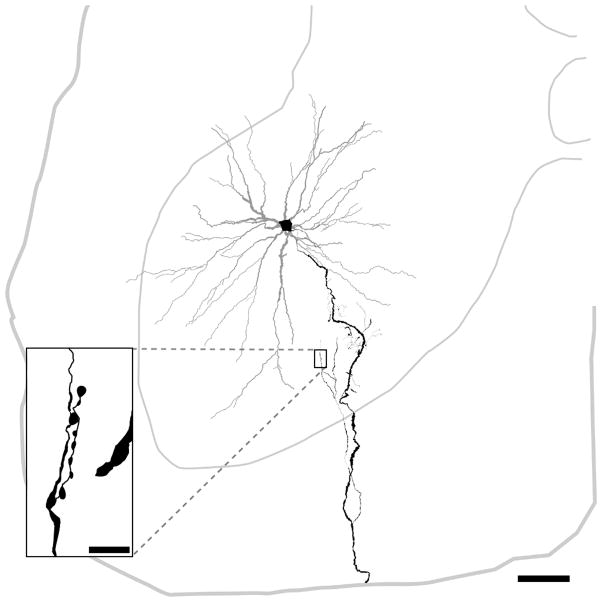
The remaining 52 collateral terminals from the 3 cat motoneurons were used to investigate the relationship between labelled axon collateral swellings and GluR2 subunits in postsynaptic membrane receptors. Figure 5 shows an example of a reconstructed motoneuron and the organisation of its axon. Collateral axons from this cell were used in the analysis (Figure 6A–C). We could find no evidence of any obvious relationship between GluR2-immunoreactive puncta and motoneuron axon collaterals. However, all axon terminals from an interneuron which was labelled in the same animal as the motoneurons were apposed to GLUR2-immunoreactive puncta (Figure 6D–G).
Figure 5.
Reconstruction of a motoneuron and its axon collaterals that was intracellularly labelled with Neurobiotin. The soma and axonal arborisation are shown in black and dendrites in grey. The thinner grey line represents the outline of the grey matter and the central canal; the thicker grey line represents the border of the spinal cord. The axon terminals illustrated in figure 6 are taken from the area outlined by the box. Scale bar, 200μm (in big panel), 10μm (in small panel).
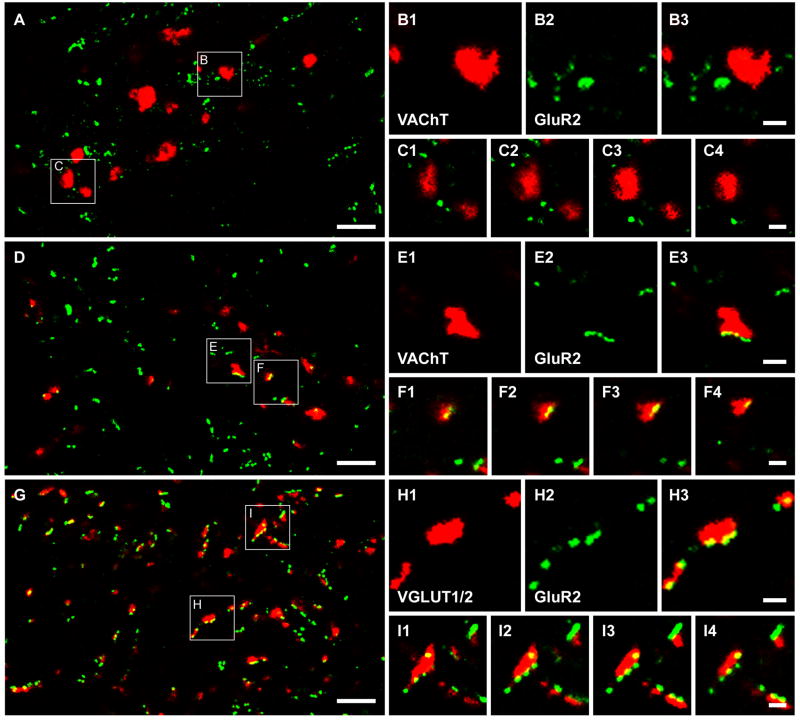
Associations between VAChT-labelled terminals and GluR2 immunoreactive puncta were also investigated in rat tissue. Confocal microscope images showed that although most VAChT immunoreactive boutons were not apposed to GluR2 puncta (Figure 7A–C), a small number of VAChT boutons were associated with GluR2 staining (Figure 7D–F). The average (±SD) equivalent diameters of the former group and the latter group were 1.95±0.53μm and 0.87±0.23μm, respectively. For comparison, we examined the relationship between glutamatergic terminals (labelled with a combination of VGLUT1 and 2 antibodies) and GluR2 immunoreactive puncta in deep lamina VII. Despite an intensive search, we only detected an occasional VGLUT immunoreactive axon terminal which was not apposed to GluR2-immunoreactive puncta (Figure 7G–I).
Figure 7.
Relationships between VAChT labelled terminals and VGLUT labelled terminals and immunoreactivity for the GluR2 subunit of the AMPA receptor following antigen unmasking with pepsin. A and D show general overviews of groups of VAChT immunostained terminals (red) and their relationships with GluR2-immunoreactive puncta (green). G shows a general overview of a group of VGLUT1 and VGLUT2 immunoreactive terminals in lamina VII and their relationship with GluR2-immunoreactivie puncta. Details of the areas demarcated by the boxes are shown in B1-B3, C1-C4, E1-E3, F1-F4, H1-H3, and I1-I4. B1-B3, E1-E3 and H1-H3 show VAChT terminals (B1 and E1), VGLUT1 and VGLUT2 terminals (H1), GluR2 puncta (B2, E2 and H2), and merged images (B3, E3 and H3) of the same single optical section. C1-C4, F1-F4 and I1-I4 show series of merged single optical sections through the selected terminals taken at intervals of 0.2 μm. Note that the VAChT terminals which lack any association with GluR2 puncta (shown in B and C), are bigger than the terminals which form appositions with GluR2 puncta (shown in E and F). Scale bar, 5μm A, D and G; 1μm B, C, E, F, H and I.
Comparison of equivalent diameters of populations of cholinergic and glutamatergic terminals in the rat
We made a comparison of the equivalent diameters of five groups of terminals investigated in this study: A) those that were labelled with VAChT and were associated with GluR2; B) those that were labelled with VAChT and VGLUT2; C) those that were labelled with VAChT but were not associated with GluR2; D) those that were labelled with VAChT and CTb; and E) those that were labelled with CTb and VGLUT1 (see Figure 8). Each group consisted of 50 randomly selected terminals obtained from each of the three rats. By using ANOVA analysis, we found that there was a highly significant difference between these groups (P<0.0005). A post hoc Tukey’s pairwise comparison showed that there was no statistical difference between groups A and B or between groups C and D but that all other comparisons were significantly different.
Figure 8.
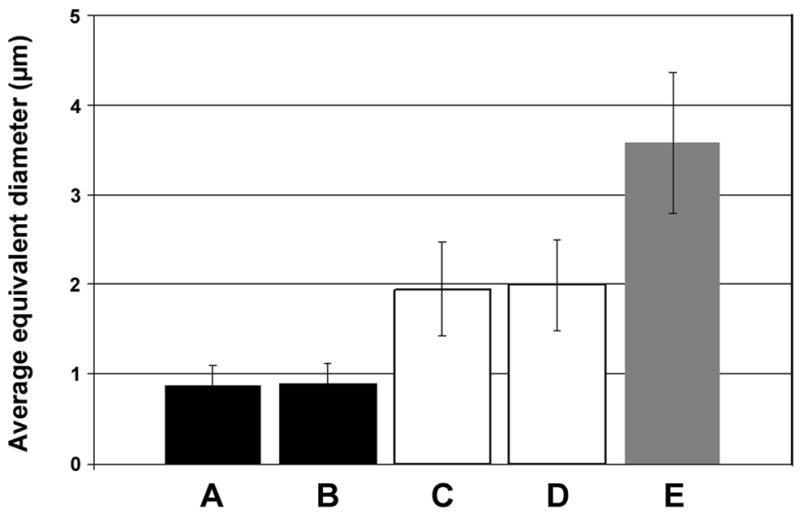
Comparison of the average equivalent diameters of five groups of terminals. Columns A and B show the average equivalent diameters of VAChT terminals with GluR2 association and with VGLUT2 labelling, respectively. Columns C and D show the average equivalent diameters of VAChT terminals without association with GluR2 and with CTb labelling, respectively. Column E represents the average equivalent diameters of CTb terminals with VGLUT1 immmunoreactivity. One way ANOVA showed that there was a significant difference between these groups and a post hoc comparison showed no difference between groups A and B and between groups C and D. All other comparisons were significantly different. Bars show standard deviations.
Discussion
In the series of experiments reported here we have shown: 1) that axon collateral swellings of adult cat motoneurons do not contain the vesicular glutamate transporters VGLUT1 or VGLUT2 and have no obvious association with the GluR2 subunit of the AMPA receptor; 2) that axon collateral swellings of adult rat motoneurons also do not contain vesicular glutamate transporters VGLUT1, VGLUT2 or VGLUT3 and have no obvious association with the GluR2 subunit of the AMPA receptor; 3) that a group of cholinergic axon terminals within lamina VII of the rat spinal cord, contain VGLUT2 and form associations with the GluR2 subunit of the AMPA receptor.
In the cat experiment, our analysis was confined to terminals which could be traced back to the parent axon and hence could be identified unequivocally as motoneuron collaterals. In rat experiments, terminals were classified into three distinct populations according to their immunocytochemical characteristics and equivalent diameters: 1) CTb labelled cholinergic terminals were identified as motoneuron axon collateral terminals. This group of axons had intermediate-sized terminals (approximately 2μm in diameter) and made numerous contacts with Renshaw cells which were labelled with calbindin (Carr et al., 1998; Alvarez et al., 1999). Alvarez et al., (1999) also measured size of VAChT terminals contacting Renshaw cells and the mean diameter (2.26±0.94μm) was similar to that described here. 2) CTb labelled non-cholinergic terminals that were immunopositive for VGLUT1 which were classified as primary afferent terminals because VGLUT1 is generally present in terminals of myelinated primary afferent axons but not in spinal interneurons (Varoqui, et al., 2002; Todd et al., 2003). These terminals were the largest of the three groups and had an average diameter of approximately 3.52μm. This is consistent with previous reports of the sizes of Group Ia afferent terminals (Watson and Bazzaz, 2001) which are likely to constitute the majority of primary afferents terminating in lamina VII (Brown, 1981). These terminals made occasional contacts with Renshaw cells. 3) The third group of terminals was the most intriguing of all. This group comprised of small cholinergic terminals (approximately 0.92μm in diameter), that were never labelled with CTb but were immunoreactive for VGLUT2 immunoreactivity. These axons apparently made no contacts with Renshaw cells.
Our observation that adult cat and rat motoneuron axon collaterals do not contain VGLUT 2 is at variance with findings from previous studies where this vesicular transporter was reported to be present in axon collaterals of adult rat (Herzog et al., 2004) and neonatal mouse (Nishimaru, et al., 2005). However they are consistent with the findings of Mentis et al. (2005) who also noted absence of immunoreactivity for all three VGLUTs in collaterals of young mice. In addition, it is also consistent with reports of absence of mRNAs for all three VGLUTs in motoneurons (Kullander et al., 2003; Oliveira, et al., 2003; but cf Herzog et al., 2004). Herzog et al. (2004) suggested that motoneuron collaterals gave rise to axon branches which contained acetylcholine but not glutamate and vice versa. We could find no evidence for this arrangement and, in fact, any CTb labelled terminals that were not immunoreactive for VAChT were invariably immunoreactive for VGLUT1 and were most probably terminals of primary afferent proprioceptors (see above). We also could detect no obvious relationship between cat or rat motoneuron terminals and immunoreactivity for the GluR2 subunit of the AMPA receptor. According to Nagy et al. (2004), 98% of AMPA receptors in the rat spinal grey matter contain this subunit and therefore it can be considered to be a marker for almost all AMPA receptors. In theory, there is a small probability that synapses formed by motoneuron collaterals appose AMPA receptors that do not possess this subunit. However, this seems an improbable explanation for our negative findings. Firstly, our control experiments showed that GluR2 subunits were abundant in deep lamina VII in rat and cat tissue and that, in the rat, VGLUT immunoreactive terminals were invariably associated with them. Secondly,Lamotte d’Incamps and Ascher (2008) provided evidence that the AMPA receptor mediated current recorded between motoneurons and Renshaw cells in young mice has a low permeability to calcium ions and therefore it would be predicted that the receptors present at this synapse possess GluR2 subunits. In the cat experiment, all axon terminals could be traced to the parent motoneuron axon but in the rat we had to confirm by statistical analysis that the population of VAChT terminals which did not appose GluR2 puncta belonged to the same population that were labelled for VAChT and CTb (Group 1 discussed above) because the antigen retrieval method combined with TSA only enables two antibodies to be used on the same tissue and, for this reason, we were unable to label these terminals for CTb also. Taken together, the two sets of observations presented above suggest that glutamate is not co-localised with acetylcholine at central synapses of adult motoneurons and does not act through an AMPA receptor.
Mentis et al. (2005), Nishimaru et al. (2005) and Lamotte d’Incamps and Ascher (2008) produced pharmacological evidence that glutamate is co-released along with acetylcholine at motoneuron/Renshaw cell synapses in young mice (P0-10) and therefore a possible reason for differences in the findings of these studies and our anatomical observations may be related to the maturity of the experimental animals they used. The original observations of Eccles et al. (1954) on adult cats indicated that cholinergic antagonists do not completely block transmission at motoneuron/Renshaw cell synapses and therefore it remains possible that another transmitter is co-released along with acetylcholine. Our data suggest that if glutamate is this transmitter, then it is not stored conventionally in vesicles and it does not act via AMPA receptors. Mentis et al. (2005) reported that motoneuron central terminals did not contain vesicular glutamate transporters but that glutamate was enriched in them and argued that this was tentative evidence for a transmitter pool of glutamate. However, glutamate may be enriched in structures for a variety of reasons which are unrelated to transmitter function. It is also possible that glutamate acts via kainate or activated NMDA receptors in the adult. However, the antagonist, CNQX, used by Mentis et al. (2005) and Nishimaru et al. (2005) and the NBQX, used by Lamotte d’Incamps and Ascher (2008) demonstrated a specific AMPA component of the current. The most likely explanation of this discrepancy with our findings is that the expression of AMPA receptors decreases during development to adulthood but further studies on young animals will be required to establish this.
Two further observations arose from this study. Firstly, we found evidence that a small number of primary afferent axons form contacts with Renshaw cells in adult rats. The classical view of Renshaw cells is that they are not monosynaptically activated by primary afferent axons (Renshaw 1946; Eccles et al., 1954). However according to a recent report by Mentis et al. (2006), the density of primary afferent contacts on Renshaw cells decreases during development but the overall numbers of contacts remains approximately the same as a consequence of the enlargement of the cell. They suggest that there is a ‘functional deselection’ of such contacts during development and although such synapses are still present, they become less effective and have a limited influence on Renshaw cells in adulthood. Although, we did not attempt to quantify the numbers of CTb/VGLUT1 contacts on Renshaw cells, our impression was that that they were sparse in comparison to cholinergic contacts which would be consistent with their limited influence in adulthood.
The second observation was perhaps the most novel of all. We found a population of cholinergic axons in lamina VII which were immunoreactive for VGLUT2 and also formed associations with GluR2 subunit immunoreactivity. These axon terminals were considerably smaller than those originating from motoneurons and were not seen to make contacts onto Renshaw cells. At present we are unable to determine the origin of these axons but it is very unlikely that they originate from descending systems or primary afferents as neither of these groups of neuron uses acetylcholine as a neurotransmitter (e.g. Rustioni and Weinberg, 1989). However, it is highly likely that they originate from local interneurons (Barber et al., 1984) or propriospinal neurons (Sheriff and Henderson, 1994) which are known to be cholinergic. In the intermediate grey matter of the lumbar spinal cord there is a concentration of cholinergic neurons in lamina X and lateral to the central canal in lamina VII (Barber et al., 1984). There are two principal types of cholinergic cell in this region which are characterised by the presence or absence of NOS in their cell bodies (Miles et al., 2007). Our findings indicate that VAChT/VGLUT2 terminals do not originate from the NOS-containing group and so, at present, their precise origin remains uncertain. Some cholinerergic interneurons which lack NOS are located principally in lamina VII lateral to the central canal and are likely to be the origin of the large ‘C-terminals’ on motoneurons. These cells therefore are also unlikely to be the source of the small terminals we observed.
Conclusion
We have not found any evidence to support the proposition that glutamate is co-localised in central synapses of adult motoneurons and acts via an AMPA receptor. However, some cholinergic terminals which probably originate from interneurons are likely to use glutamate as a co-transmitter and act via AMPA receptors.
Acknowledgments
The study was supported by a grant from NINDS/NIH (R01 NS040863). Ting Ting Liu is a University of Glasgow FBLS Scholar.
Abbreviations
- ACh
Acetylcholine
- CTb
b subunit of cholera toxin
- GluR2
R2 subunit of the AMPA receptor
- NOS
nitric oxide synthase
- PB
phosphate buffer
- PBS
phosphate buffered saline
- PBST
phosphate buffered saline Triton
- VAChT
vesicular acetylcholine transporter
- VGLUT
vesicular glutamate transporter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez FJ, Dewey DE, McMillin P, Fyffe REW. Distribution of cholinergic contacts on Renshaw cells in the rat spinal cord: a light microscopic study. J Physiol (Lond) 1999;515:787–797. doi: 10.1111/j.1469-7793.1999.787ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannatyne BA, Edgley SA, Hammar I, Jankowska E, Maxwell DJ. Differential projections of excitatory and inhibitory dorsal horn interneurons relaying information from group II muscle afferents in the cat spinal cord. J Neurosci. 2006;26:2871–2880. doi: 10.1523/JNEUROSCI.5172-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber RP, Phelps PE, Houser CR, Crawford GD, Salvaterra PM, Vaughn JE. The morphology and distribution of neurons containing choline acetyltransferase in the adult rat spinal cord: an immunocytochemical study. J Comp Neurol. 1984;229:329–346. doi: 10.1002/cne.902290305. [DOI] [PubMed] [Google Scholar]
- Brown AG. Organization in the Spinal Cord. Berlin. Heidelberg; New York: Springer Verlag; 1981. [Google Scholar]
- Carr PA, Alvarez FJ, Leman EA, Fyffe REW. Calbindin D28k expression in immunohistochemically identified Renshaw cells. Neuroreport. 1998;9:2657–2661. doi: 10.1097/00001756-199808030-00043. [DOI] [PubMed] [Google Scholar]
- Curtis DR, Ryall RW. Nicotinic and Muscarinic Receptors of Renshaw Cells. Nature. 1964;203:652–653. doi: 10.1038/203652a0. [DOI] [PubMed] [Google Scholar]
- Dourado M, Sargent PB. Properties of nicotinic receptors underlying Renshaw cell excitation by a-motor neurons in neonatal rat spinal cord. J Neurophysiol. 2002;87:3117–3125. doi: 10.1152/jn.2002.87.6.3117. [DOI] [PubMed] [Google Scholar]
- Eccles JC, Fatt P, Koketsu K. Cholinergic and inhibitory synapses in a pathway from motor axon collaterals to motoneurones. J Physiol (Lond) 1954;126:524–562. doi: 10.1113/jphysiol.1954.sp005226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog E, Landry M, Buhler E, Bouali-Benazzouz R, Legay C, Henderson CE, Nagy F, Dreyfus P, Giros B, El Mestikawy S. Expression of vesicular glutamate transporters, VGLUT1 and VGLUT2, in cholinergic spinal motoneurons. Eur J Neurosci. 2004;20:1752–1760. doi: 10.1111/j.1460-9568.2004.03628.x. [DOI] [PubMed] [Google Scholar]
- Kullander K, Butt SJ, Lebret JM, Lundfald L, Restrepo CE, Rydström A, Klein R, Kiehn O. Role of EphA4 and EphrinB3 in local neuronal circuits that control walking. Science. 2003;299:1889–1892. doi: 10.1126/science.1079641. [DOI] [PubMed] [Google Scholar]
- Lamotte d’Incamps B, Ascher P. Four excitatory postsynaptic ionotropic receptors coactivated at the motoneuron-Renshaw cell synapse. J Neurosci. 2008;28:14121–14131. doi: 10.1523/JNEUROSCI.3311-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentis GZ, Alvarez FJ, Bonnot A, Richards DS, Gonzalez-Forero D, Zerda R, O’Donovan MJ. Noncholinergic excitatory actions of motoneurons in the neonatal mammalian spinal cord. Proc Natl Acad Sci USA. 2005;102:7344–7349. doi: 10.1073/pnas.0502788102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentis GZ, Siembab VC, Zerda R, O’Donovan MJ, Alvarez FJ. Primary afferent synapses on developing and adult Renshaw cells. J Neurosci. 2006;26:13297–13310. doi: 10.1523/JNEUROSCI.2945-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles GB, Hartley R, Todd AJ, Brownstone RM. Spinal cholinergic interneurons regulate the excitability of motoneurons during locomotion. Proc Natl Acad Sci USA. 2007;104:2448–2453. doi: 10.1073/pnas.0611134104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy GG, Al Ayyan M, Andrew D, Fukaya M, Watanabe M, Todd AJ. Widespread expression of the AMPA receptor GluR2 subunit at glutamatergic synapses in the rat spinal cord and phosphorylation of GluR1 in response to noxious stimulation revealed with an antigen-unmasking method. J Neurosci. 2004;24:5766–5777. doi: 10.1523/JNEUROSCI.1237-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimaru H, Restrepo CE, Ryge J, Yanagawa Y, Kiehn O. Mammalian motor neurons corelease glutamate and acetylcholine at central synapses. Proc Natl Acad Sci USA. 2005;102:5245–5249. doi: 10.1073/pnas.0501331102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noga BR, Shefchyk SJ, Jamal J, Jordan LM. The role of Renshaw cells in locomotion: antagonism of their excitation from motor axon collaterals with intravenous mecamylamine. Exp Brain Res. 1987;66:99–105. doi: 10.1007/BF00236206. [DOI] [PubMed] [Google Scholar]
- Oliveira AL, Hydling F, Olsson E, Shi T, Edwards RH, Fujiyama F, Kaneko T, Hokfelt T, Cullheim S, Meister B. Cellular localization of three vesicular glutamate transporter mRNAs and proteins in rat spinal cord and dorsal root ganglia. Synapse. 2003;50:117–129. doi: 10.1002/syn.10249. [DOI] [PubMed] [Google Scholar]
- Renshaw B. Central effects of centripetal impulses in axons of spinal central roots. J Neurophysiol. 1946;9:191–204. doi: 10.1152/jn.1946.9.3.191. [DOI] [PubMed] [Google Scholar]
- Rustioni A, Weinberg RJ. The somatosensory system. In: Björklund A, Hökfelt T, Swanson LW, editors. Handbook of Chemical Neuroanatomy. Amsterdam: Elsevier; 1989. pp. 219–320. [Google Scholar]
- Schneider SP, Fyffe REW. Involvement of GABA and glycine in recurrent inhibition of spinal motoneurones. J Neurophysiol. 1992;68:397–406. doi: 10.1152/jn.1992.68.2.397. [DOI] [PubMed] [Google Scholar]
- Sherriff FE, Henderson Z. A cholinergic propriospinal innervation of the rat spinal cord. Brain Res. 1994;634:150–154. doi: 10.1016/0006-8993(94)90268-2. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Hughes DI, Polgár E, Nagy GG, Mackie M, Ottersen OP, Maxwell DJ. The expression of vesicular glutamate transporters VGLUT1 and VGLUT2 in neurochemically defined axonal populations in the rat spinal cord with emphasis on the dorsal horn. Eur J Neurosci. 2003;17:13–27. doi: 10.1046/j.1460-9568.2003.02406.x. [DOI] [PubMed] [Google Scholar]
- Varoqui H, Schafer MK, Zhu H, Weihe E, Erickson JD. Identification of the differentiation-associated Na+/PI transporter as a novel vesicular glutamate transporter expressed in a distinct set of glutamatergic synapses. J Neurosci. 2002;22:142–155. doi: 10.1523/JNEUROSCI.22-01-00142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson AHD, Bazzaz AA. GABA and glycine-like immunoreactivity at axoaxonic synapses on 1a muscle afferent terminals in the spinal cord of the rat. J Comp Neurol. 2001;433:335–348. doi: 10.1002/cne.1143. [DOI] [PubMed] [Google Scholar]
- Windhorst U. On the role of recurrent inhibitory feedback in motor control. Prog Neurobiol. 1996;49:517–587. doi: 10.1016/0301-0082(96)00023-8. [DOI] [PubMed] [Google Scholar]