Abstract
Our long-term goal is to understand the mechanisms by which relaxin and estrogen potentially contribute to joint diseases particularly those afflicting the fibrocartilaginous temporomandibular joint (TMJ). Previously, we showed that relaxin produces a dose-dependent induction of tissue degrading enzymes of the matrix metalloproteinase (MMP) family, specifically MMP-1 (collagenase-1), −3 (stromelysin-1), −9 (92-kDa-gelatinase) and −13 (collagenase-3) in cell isolates and tissue explants from TMJ fibrocartilage. The induction of these MMPs is accompanied by loss of collagen and glycosaminoglycans (GAGs), which was blocked by a pan-MMP inhibitor. We also found the targeted in vivo loss of collagen and GAGs in TMJ discs of ovariectomized rabbits treated with β-estradiol or relaxin or both hormones together. Progesterone attenuated the induction of MMPs and matrix loss by relaxin and estrogen. The modulation of matrix composition in TMJ fibrocartilage by these hormones was similar to that observed in the pubic symphysis, and differed from that of the knee meniscus. The two target tissues showing the greatest modulation of MMPs and matrix loss, namely the TMJ disc and pubic symphysis, had similar expression profiles of the estrogen receptors (ER)-α and –β, relaxin-1 receptor (RXFP1, LGR7), and INSL3 receptor (RXFP2, LGR8) that differed from those in cells from the knee meniscus. These findings suggest a novel model for targeted tissue turnover of cartilages of specific joints through hormone-mediated induction of select MMPs.
Keywords: Degenerative joint disease, fibrocartilage, relaxin, estrogen, progesterone, matrix metalloproteinases, extracellular matrix
Introduction
Degenerative joint diseases (DJDs) of most systemic joints are more prevalent in women than in men, and occur primarily in postmenopausal years.1 However, diseases of the fibrocartilaginous TMJ, which also show a high female to male preponderance,2 primarily afflict women between 18 and 45 years of age.3 Symptoms of TMJ related disorders occur in approximately 6 to 12% of the adult population or approximately 10 million individuals in the United States.2,4 The reasons for the marked sexual dimorphism and age distribution of TMJ disorders remain unclear. However, a potential role of female reproductive hormones, particularly estrogen in the etiology of these diseases has been proposed. Both estrogen and progesterone receptors have been localized in the TMJ of humans and other mammals,5–8 with some findings suggesting a sexual dimorphism in the presence of estrogen receptors.5,8 Despite these studies, until recently no direct evidence existed linking female reproductive hormones to TMJ disease or defining the mechanisms by which these hormones may cause TMJ disease. Additionally, the potential role of other female hormones such as relaxin in these diseases has only been examined recently. These findings show that relaxin H2, which is found systemically in cycling and pregnant women but not in men, causes the targeted induction of tissue degrading enzymes of the MMP family in the fibrocartilaginous tissues of the TMJ potentially predisposing to TMJ diseases.9–11
MMPs are a family of up to 25 enzymes that are characterized by their extracellular matrix (ECM) substrate specificity, zinc-dependent activity, inhibition by tissue inhibitors of metalloproteinases (TIMPs), secretion as a zymogen and sequence similarities. Between them, MMPs can degrade the major matrix macromolecules of cartilage, namely collagen and proteoglycans as well as most of the minor proteins in this tissue. Matrix degradation by MMPs is considered to be a primary event in the initiation and progression of joint diseases. The modulation of MMPs by hormones such as relaxin and estrogen in cartilage that may initiate or predispose to a subset of non-inflammatory joint diseases has only recently come under scrutiny.9,11,12
Relaxin, β-estradiol and progesterone modulate MMP expression and matrix content in various tissues
Relaxin H2, a 6-kDa polypeptide, is the major stored and circulating form of relaxin in humans13, and appears to have long term effects on connective tissues by altering the turnover of collagen and proteoglycans. In most reproductive tissues including the cervix, uterus, ovary, breast and deciduas, relaxin mediates changes in matrix composition and organization by modulating the synthesis of matrix macromolecules,14 or altering the expression of matrix-degrading enzymes15 or both.14 In fibocartilaginous tissues of the pubic symphysis and TMJ, alterations in matrix composition appear to result due to increased degradative responses rather than due to changes in matrix synthesis.11,16,17 Relaxin appears to contribute to tissue degradation by primarily inducing several MMPs including MMP-1, -3, -9 and -13 in cells of reproductive and non-reproductive tissues including skin and lung alvelolar fibroblasts,15,18–20 and, as discussed later, of TMJ fibrocartilage9,11.
Estrogens are also capable of modulating degradation of the ECM by regulating the expression of various MMPs in several cell types.21,22 Additionally, β-estradiol is known to potentiate MMP induction and matrix loss by relaxin in cells from reproductive and non-reproductive tissues including the TMJ fibrocartilage9,15,17 (Figs. 1 and 2). In contrast to the effects of estrogen and relaxin in enhancing MMP expression, progesterone downregulates several MMPs including MMP-1, -2, -3 and -9 in cells from several reproductive tissues,23–26 and, as discussed below, of MMP-3, -9 and -13 in mouse TMJ disc cells.
Figure 1.
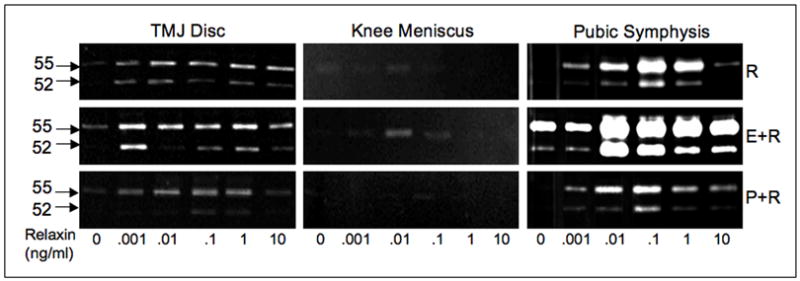
Dose-dependent modulation of 52/55-kDa gelatinolytic proteinase by relaxin (R) in the absence or presence of β-estradiol (E) or progesterone (P) in mice TMJ disc, knee meniscus and pubic symphysis fibrochondrocytes. Early passage cells plated at 105/cm2 in serum-free medium were exposed to increasing concentrations of relaxin (0 to 10 ng/ml) in the absence or presence of β-estradiol (0.1 ng/ml) or progesterone (10 ng/ml) for 48 hours. The cell-conditioned medium, standardized by total protein, was analyzed by gelatin substrate zymography. Relaxin produced a bimodal dose-dependent increase in expression of the 52/55-kDa-gelatinolytic proteinase (presumably MMP-13) in the TMJ disc and pubic symphysis but very minimally in the knee meniscus. The activity of this proteinase band was inhibited in the presence of the MMP inhibitor 1,10 phenanthroline (data not shown) characterizing this enzyme as a metalloproteinase. In TMJ disc and pubic symphysis cells the induction of this proteinase by relaxin was potentiated by β-estradiol and attenuated by progesterone.
Figure 2.
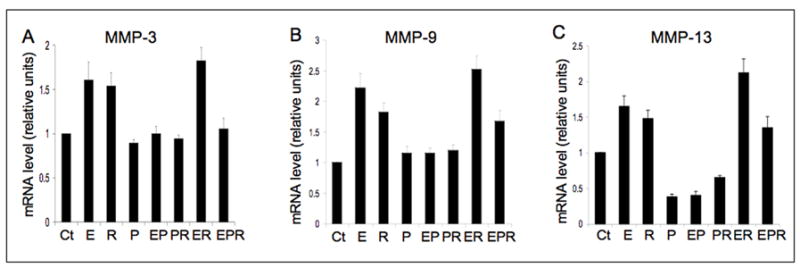
β-estradiol (E), relaxin (R) and progesterone (P) modulate MMP-3 (A), -9 (B) and -13 (C) gene expression in mouse TMJ disc fibrochondrocytes. Early passage fibrochondrocytes were treated as described in figure 1 except that only a single concentration of relaxin (0.1 ng/ml) was used. Total RNA was extracted and subjected to qRT-PCR for the MMPs following standardization by total RNA concentration. 18s RNA was used as an internal control. All three MMPs were significantly up-regulated by E or R or E+R treatments. E increased the induction of MMP-9 and -13 by relaxin, while P attenuated the induction of MMP-3, -9, and -13 by relaxin or β-estradiol. Data represents means ± SD fold increase relative to controls from 3 different experiments. (Ct=control).
Female hormones cause a targeted modulation of MMPs in TMJ and pubic symphysis, but not in knee meniscus fibrochondrocytes
Previously we demonstrated that rabbit TMJ disc fibrocartilaginous cells show a dose-dependent induction of MMP-1 and -3, which is potentiated by exposure of the cells to β-estradiol.9 More recently we performed studies to assess the MMP responses of TMJ disc cells from young adult cycling female mice to increasing concentrations of relaxin alone or in combination with β-estradiol or progesterone. We also examined if the responses of pubic symphysis and knee meniscus fibrochondrocytes to these hormones are similar to those of TMJ fibrochondrocytes. We found that relaxin produces an induction of 52/55-kDa gelatinolytic enzyme (presumably MMP-13) in TMJ disc and pubic symphysis cells, but minimally in the knee meniscus cells (Fig. 1). Relaxin’s induction of this proteinase in TMJ disc and pubic symphysis cells was potentiated by exposure to β-estradiol and attenuated by progesterone. Whether used alone or with β-estradiol or progesterone, the peak induction of the 52/55-kDa proteinase by relaxin occurred at relaxin concentrations of 0.01 to 1 ng/ml, which falls within the physiologic range in cycling and pregnant women.
Next, we assessed the effects of β-estradiol alone or progesterone alone or in various combinations with relaxin on the modulation of MMP-3, -9 and -13 mRNA in mouse TMJ disc cells. β-estradiol or relaxin alone or together produced a significant induction of all three MMPs relative to baseline controls (Fig. 2). Progesterone alone or in combination with β-estradiol or relaxin caused a significant repression of MMP-13 relative to baseline controls, and maintained that of MMP-3 and -9 mRNAs at control levels. More importantly, progesterone caused a significant attenuation of the induction MMP-3, -9 and -13 by β-estradiol and/or relaxin. Together, these findings show the targeted induction by relaxin and β-estradiol of MMP-3, -9 and -13 in the TMJ disc and pubic symphysis fibrocartilages but not in the knee meniscus, and the attenuation of this response by progesterone. The divergent regulation of MMPs by estrogen and relaxin on one hand and progesterone on the other, suggests that the hormonal cross-talk may be an important determinant of the matrix composition and organization of target cartilaginous tissues.
Relaxin, β-estradiol and progesterone’s modulation of MMPs is paralleled by changes in matrix content of TMJ disc and pubic symphysis fibrocartilages
Although the induction of MMPs by relaxin and β-estradiol in TMJ disc cells may be an indicator of enhanced matrix degradative activity, it does not necessarily follow that this results in a net loss of matrix macromolecules. Therefore, we determined whether fibrocartilaginous cells in their native matrix environment show similar responses to relaxin as isolated cells, and whether the induction of MMPs is associated with the loss of collagen and GAGs in fibrocartilaginous explants.11 We found that relaxin’s induction of MMP-1 and MMP-3 in rabbit TMJ disc explants is accompanied by loss of disc collagen and GAGs. The loss of these matrix molecules was prevented when a pan-MMP inhibitor was added to the cultures demonstrating the contribution of relaxin-induced MMPs to matrix loss.
We have also addressed the question of whether the loss of collagen and GAGs by relaxin with or withoutβ-estradiol observed in TMJ explants occurs in vivo and determined the specificity of these effects on TMJ fibrocartilage versus that on the knee meniscus.12 Additionally, we assessed whether progesterone attenuates the effects of relaxin and β-estradiol in causing the loss of tissue matrices. We found that β-estradiol or relaxin alone or together caused a significant loss of GAGs and collagen from the pubic symphysis and TMJ disc, but not from the knee meniscus. Progesterone prevented the relaxin- or β-estradiol-mediated loss of these molecules. The findings suggest that, by differentially modulating matrix loss from these tissues, female hormones may selectively contribute to diseases of the TMJ. Pertinent to our studies on the fibrocartilaginous TMJ disc, a relationship between peak levels of relaxin during pregnancy and a decrease in total collagen has been demonstrated in the rat pubic symphysis fibrocartilage.16 Additional evidence for the importance of relaxin to normal tissue turnover is provided by studies on relaxin null mice that show an increase in collagen density and failure in relaxation of the pubic symphysis during parturition.27 The reasons for why relaxin, β-estradiol and progesterone have similar and targeted effects on matrix remodeling activites within the TMJ disc and pubic symphysis fibrocartilages are not known, but may be associated with the quantity and types of hormone receptors present in these tissues.
The contributions of the known estrogen and relaxin receptors to MMP induction and matrix loss remain to be determined
Although relaxin and estrogen each have two known receptors each- relaxin family peptide 1 receptor (RXFP1 or LGR7) and RXFP2 (or LGR8) for relaxin, and estrogen receptor (ER)-α and ER-β for estrogen, little is currently known about the contributions that each of these receptors make to the induction of MMPs and to matrix turnover. Relaxin binds with high affinity to RXFP1 receptor and with low affinity to RXFP2 receptor.28,29 Indirect evidence for the role of RXFP1 in in vivo matrix remodeling activity is provided by the phenotypic characteristics of the female RXFP1 null mice that have abnormal matrices similar to those found in relaxin-deficient mice.27,30 These mice show increased density of collagen of the nipple and of the pubic symphysis, suggesting that relaxin acts on its target tissues via RXFP1 to regulate collagen breakdown and reorganization. In light of the fact that the changes in matrix composition by relaxin parallel its modulation of MMPs in target tissues, it is likely that these changes in matrices result from relaxin’s induction of MMPs. However, no evidence is currently available on the involvement of RXFP1 or RXFP2 in relaxin’s induction of specific MMPs in any tissues including those in synovial joints.
While the biological effects of estrogens can be mediated via one of four ER signaling pathways, evidence indicates that modulation of MMPs by these hormones results from the classical ligand-dependent pathway,31–33 in which estrogen-ER complexes regulate gene transcription. Although, little is known about the precise contributions of ER-α and ER-β to MMP regulation, the findings that the exposure of endometrial cancer (Ishikawa) cells transfected with ER-α expression vector to β-estradiol results in their increased invasiveness concomitant with an enhanced expression of MMPs, provides indirect evidence for the role of ER-α in the induction of MMPs.34 It is plausible that a similar mechanism may be relevant to estrogen’s induction of MMPs in TMJ fibrocartilage leading to its enhanced degradation. Evidence that estrogen and relaxin likely contribute to the activities of TMJ disc cells is provided by our studies showing that these cells express ER-α, ER-β, RXFP1 and RXFP2.8 We also found that the two target tissues, namely the TMJ disc and pubic symphysis, showing the greatest induction of MMPs and matrix loss in response to relaxin and β-estradiol have similar expression profiles of their receptors that differ from those in cells from the minimally responsive knee meniscus. Although these observations provide important clues for the basis of differential modulation of MMPs by their respective hormones in different tissues, further studies are required to determine the contribution of each receptor type to these responses.
Conclusion
The initiation of matrix loss is a critical first step in DJDs and leads to adverse changes in mechanical properties, inability to sustain function and eventually to disease. Therefore, understanding the key initial events that lead to DJD, possibly including those initiated by relaxin and β-estradiol, may be critical to preventing or reversing these disorders. The findings presented in this manuscript offer insights into modulation of joint cartilage turnover by female hormones. However, much work remains to be done to establish a definitive link between the hormone-hormone receptor-MMP axis and joint degeneration.
Acknowledgments
Our studies were supported by NIH R29 DE11993, KO2 DE00458 and RO1 DE018455 to SK. Wethank Bas Medical Corporation for providing us with reagents used in our studies.
Literature Cited
- 1.Felson DT, Nevitt MC. The effects of estrogen on osteoarthritis. Curr Opin Rheumatol. 1998;10:269–72. doi: 10.1097/00002281-199805000-00019. [DOI] [PubMed] [Google Scholar]
- 2.Von Korff M, et al. An epidemiologic comparison of pain complaints. Pain. 1988;32:173–83. doi: 10.1016/0304-3959(88)90066-8. [DOI] [PubMed] [Google Scholar]
- 3.Warren MP, Fried JL. Temporomandibular disorders and hormones in women. Cells Tissues Organs. 2001;169:187–92. doi: 10.1159/000047881. [DOI] [PubMed] [Google Scholar]
- 4.Lipton JA, Ship JA, Larach-Robinson D. Estimated prevalence and distribution of reported orofacial pain in the United States. J Am Dent Assoc. 1993;124:115–21. doi: 10.14219/jada.archive.1993.0200. [DOI] [PubMed] [Google Scholar]
- 5.Milam SB, et al. Sexual dimorphism in the distribution of estrogen receptors in the temporomandibular joint complex of the baboon. Oral Surg Oral Med Oral Pathol. 1987;64:527–32. doi: 10.1016/0030-4220(87)90025-9. [DOI] [PubMed] [Google Scholar]
- 6.Abubaker AO, Raslan WF, Sotereanos GC. Estrogen and progesterone receptors in temporomandibular joint discs of symptomatic and asymptomatic persons: a preliminary study. J Oral Maxillofac Surg. 1993;51:1096–100. doi: 10.1016/s0278-2391(10)80448-3. [DOI] [PubMed] [Google Scholar]
- 7.Yamada K, et al. Expression of estrogen receptor alpha (ER alpha) in the rat temporomandibular joint. Anat Rec A Discov Mol Cell Evol Biol. 2003;274:934–41. doi: 10.1002/ar.a.10107. [DOI] [PubMed] [Google Scholar]
- 8.Wang W, Hayami T, Kapila S. Female hormone receptors are differentially expressed in mouse fibrocartilages. Osteoarthritis Cartilage. 2008 doi: 10.1016/j.joca.2008.09.015. (Submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kapila S, Xie Y. Targeted induction of collagenase and stromelysin by relaxin in unprimed and beta-estradiol-primed diarthrodial joint fibrocartilaginous cells but not in synoviocytes. Lab Invest. 1998;78:925–38. [PubMed] [Google Scholar]
- 10.Kapila S. Does the relaxin, estrogen and matrix metalloproteinase axis contribute to degradation of TMJ fibrocartilage? J Musculoskelet Neuronal Interact. 2003;3:401–5. discussion 406–7. [PubMed] [Google Scholar]
- 11.Naqvi T, et al. Relaxin’s induction of metalloproteinases is associated with the loss of collagen and glycosaminoglycans in synovial joint fibrocartilaginous explants. Arthritis Res Ther. 2005;7:R1–11. doi: 10.1186/ar1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hashem G, et al. Relaxin and beta-estradiol modulate targeted matrix degradation in specific synovial joint fibrocartilages: progesterone prevents matrix loss. Arthritis Res Ther. 2006;8:R98. doi: 10.1186/ar1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samuel CS, Parry LJ, Summers RJ. Physiological or pathological - a role for relaxin in the cardiovascular system? Curr Opin Pharmacol. 2003;3:152–158. doi: 10.1016/s1471-4892(03)00011-0. [DOI] [PubMed] [Google Scholar]
- 14.Hwang JJ, Macinga D, Rorke EA. Relaxin modulates human cervical stromal cell activity. J Clin Endocrinol Metab. 1996;81:3379–84. doi: 10.1210/jcem.81.9.8784100. [DOI] [PubMed] [Google Scholar]
- 15.Mushayandebvu TI, Rajabi MR. Relaxin stimulates interstitial collagenase activity in cultured uterine cervical cells from nonpregnant and pregnant but not immature guinea pigs; estradiol-17 beta restores relaxin’s effect in immature cervical cells. Biol Reprod. 1995;53:1030–7. doi: 10.1095/biolreprod53.5.1030. [DOI] [PubMed] [Google Scholar]
- 16.Samuel CS, et al. The effect of relaxin on collagen metabolism in the nonpregnant rat pubic symphysis: the influence of estrogen and progesterone in regulating relaxin activity. Endocrinology. 1996;137:3884–90. doi: 10.1210/endo.137.9.8756561. [DOI] [PubMed] [Google Scholar]
- 17.Samuel CS, Coghlan JP, Bateman JF. Effects of relaxin, pregnancy and parturition on collagen metabolism in the rat pubic symphysis. J Endocrinol. 1998;159:117–25. doi: 10.1677/joe.0.1590117. [DOI] [PubMed] [Google Scholar]
- 18.Palejwala S, et al. Relaxin positively regulates matrix metalloproteinase expression in human lower uterine segment fibroblasts using a tyrosine kinase signaling pathway. Endocrinology. 2001;142:3405–13. doi: 10.1210/endo.142.8.8295. [DOI] [PubMed] [Google Scholar]
- 19.Unemori EN, Amento EP. Relaxin modulates synthesis and secretion of procollagenase and collagen by human dermal fibroblasts. J Biol Chem. 1990;265:10681–5. [PubMed] [Google Scholar]
- 20.Unemori EN, et al. Relaxin induces an extracellular matrix-degrading phenotype in human lung fibroblasts in vitro and inhibits lung fibrosis in a murine model in vivo. J Clin Invest. 1996;98:2739–45. doi: 10.1172/JCI119099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Potier M, et al. Expression and regulation of estrogen receptors in mesangial cells: influence on matrix metalloproteinase-9. J Am Soc Nephrol. 2001;12:241–51. doi: 10.1681/ASN.V122241. [DOI] [PubMed] [Google Scholar]
- 22.Marin-Castano ME, et al. Regulation of estrogen receptors and MMP-2 expression by estrogens in human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2003;44:50–9. doi: 10.1167/iovs.01-1276. [DOI] [PubMed] [Google Scholar]
- 23.Di Nezza LA, Jobling T, Salamonsen LA. Progestin suppresses matrix metalloproteinase production in endometrial cancer. Gynecol Oncol. 2003;89:325–33. doi: 10.1016/s0090-8258(03)00089-1. [DOI] [PubMed] [Google Scholar]
- 24.Shimonovitz S, et al. Expression of gelatinase B by trophoblast cells: down-regulation by progesterone. Am J Obstet Gynecol. 1998;178:457–61. doi: 10.1016/s0002-9378(98)70420-x. [DOI] [PubMed] [Google Scholar]
- 25.Keller NR, et al. Progesterone exposure prevents matrix metalloproteinase-3 (MMP-3) stimulation by interleukin-1alpha in human endometrial stromal cells. J Clin Endocrinol Metab. 2000;85:1611–9. doi: 10.1210/jcem.85.4.6502. [DOI] [PubMed] [Google Scholar]
- 26.Salamonsen LA, Woolley DE. Menstruation: induction by matrix metalloproteinases and inflammatory cells. J Reprod Immunol. 1999;44:1–27. doi: 10.1016/s0165-0378(99)00002-9. [DOI] [PubMed] [Google Scholar]
- 27.Zhao L, et al. Collagen studies in late pregnant relaxin null mice. Biol Reprod. 2000;63:697–703. doi: 10.1095/biolreprod63.3.697. [DOI] [PubMed] [Google Scholar]
- 28.Hsu SY, et al. Activation of orphan receptors by the hormone relaxin. Science. 2002;295:671–4. doi: 10.1126/science.1065654. [DOI] [PubMed] [Google Scholar]
- 29.Kumagai J, et al. INSL3/Leydig insulin-like peptide activates the LGR8 receptor important in testis descent. J Biol Chem. 2002;277:31283–6. doi: 10.1074/jbc.C200398200. [DOI] [PubMed] [Google Scholar]
- 30.Krajnc-Franken MA, et al. Impaired nipple development and parturition in LGR7 knockout mice. Mol Cell Biol. 2004;24:687–96. doi: 10.1128/MCB.24.2.687-696.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grant-Tschudy KS, Wira CR. Effect of estradiol on mouse uterine epithelial cell transepithelial resistance (TER) Am J Reprod Immunol. 2004;52:252–62. doi: 10.1111/j.1600-0897.2004.00218.x. [DOI] [PubMed] [Google Scholar]
- 32.Lu T, et al. Estrogen receptor alpha regulates matrix metalloproteinase-13 promoter activity primarily through the AP-1 transcriptional regulatory site. Biochim Biophys Acta. 2006;1762:719–31. doi: 10.1016/j.bbadis.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 33.Stevens TA, Meech R. BARX2 and estrogen receptor-alpha (ESR1) coordinately regulate the production of alternatively spliced ESR1 isoforms and control breast cancer cell growth and invasion. Oncogene. 2006;25:5426–35. doi: 10.1038/sj.onc.1209529. [DOI] [PubMed] [Google Scholar]
- 34.Mizumoto H, et al. Acceleration of invasive activity via matrix metalloproteinases by transfection of the estrogen receptor-alpha gene in endometrial carcinoma cells. Int J Cancer. 2002;100:401–6. doi: 10.1002/ijc.10504. [DOI] [PubMed] [Google Scholar]


