Abstract
Background
Current World Health Organization (WHO) guidelines for treatment of HIV in resource-limited settings call for two antiretroviral regimens. The effectiveness and cost-effectiveness of increasing the number of antiretroviral regimens is unknown.
Methods
Using a simulation model, we compared the survival and costs of current WHO regimens with two 3-regimen strategies: an initial regimen of three nucleoside reverse transcriptase inhibitors followed by the WHO regimens; and the WHO regimens followed by a regimen with a second-generation boosted protease inhibitor (2bPI). We evaluated monitoring with CD4 counts only and with both CD4 counts and viral load. We used cost and effectiveness data from Cape Town, and tested all assumptions in sensitivity analyses.
Results
Over the lifetime of the cohort, 25.6% of individuals failed both WHO regimens by virologic criteria. However, when patients were monitored using CD4 counts alone, only 6.5% were prescribed additional HAART, due to missed and delayed detection of failure. The life expectancy gain for individuals who took a 2bPI was 6.7–8.9 months, depending on the monitoring strategy. When CD4 alone was available, adding a regimen with a 2bPI was associated with an incremental cost-effectiveness ratio of $2,581 per year-of-life gained, and when viral load was available, the ratio was $6,519 per year-of-life gained. Strategies with triple-NRTI regimens in initial therapy were dominated. Results were sensitive to the price of 2bPIs.
Conclusions
About 1 in 4 individuals who start HAART in sub-Saharan Africa will fail currently recommended regimens. At current prices, adding a regimen with a 2bPI is cost-effective for South Africa and other middle-income countries by WHO standards.
Introduction
Africa is home to more than twenty million HIV-infected individuals, two-thirds of all new infections, and three-quarters of all HIV-related deaths.1 By the end of 2007, more than 2 million people have initiated Highly Active Antiretroviral Therapy (HAART) in sub-Saharan Africa, and access to treatment continues to expand.2 However, HIV treatment in publicly funded programs in sub-Saharan Africa is restricted to two regimens.3, 4 The World Health Organization (WHO) provides no guidelines beyond second-line therapy, and individuals who fail or cannot tolerate first- and second-line regimens do not have access to further antiretroviral treatment options.4 In contrast, six different classes of antiretrovirals are available in developed countries yielding multiple possible regimens for patients who fail initial therapy.5–7 The growing experience with treatment of drug-resistant infections may make new regimens in resource-limited settings feasible.8
The first-line HAART regimen recommended for low and middle-income countries includes a backbone of two nucleotide/nucleoside reverse transcriptase inhibitors (NRTIs) with a non-nucleoside reverse transcriptase inhibitor (NNRTI), either nevirapine or efavirenz. The second-line regimen includes 2 similar but not identical NRTIs with a boosted protease inhibitor (bPI) such as lopinavir/ritonavir.4 Previous studies evaluated the effectiveness and cost-effectiveness of 2-regimen strategies for Africa,9, 10 but to date no evaluation of strategies employing additional HAART regimens has been done. We evaluated strategies where the current treatment guidelines are expanded to three lines of treatment. In addition, we examine the relationship of treatment monitoring to antiretroviral strategies. Recent studied examined the role of monitoring for the current 2-regimen strategies,11, 12 but the importance of monitoring when using an expanded range of HAART regimens has not been studied. As experience with HAART continues to expand in Africa, additional treatment options are needed. This work will help in planning for the next phase of antiretroviral therapy options in sub-Saharan Africa.
Methods
Overview
We developed a mathematical simulation model of the clinical course of HIV infected individuals who present to care in South Africa (see Supplementary Appendix for more details). We compared the effectiveness and costs of three alternative HAART strategies: 1) the World Health Organization’s (WHO) 2-regimen strategy 2) a triple NRTI regimen followed by the WHO regimens, and 3) the WHO regimens followed by a second generation bPI-based regimen. We only examined regimens that are as easy to administer as the currently available regimens used in sub-Saharan Africa: oral regimens, simple dosing, and little or no requirement for refrigeration (Table 1). We followed each person’s health in one-month increments, though clinical and laboratory data was only available to the patient’s clinician during routinely scheduled clinic visits, or sooner for acute events.
Table 1.
Antiretroviral Regimen Strategie
| Regimen | Suppressed at 1yr |
Range | Sources |
|---|---|---|---|
| Strategy A: Current WHO Guidelines | |||
| Regimen 1: 2 NRTIs + NNRTI (based on AZT + 3TC + NVP or EFV) |
85 | 60–90 | Boulle,39 Orrell,25 Calmy40 |
| Regimen 2: 2 NRTIs + PI/r (based on TDF + 3TC + LPV/r) |
70 | 50–80 | De mendoza41, Kaufman42, Robbins43 |
| Strategy B: Initial Triple NRTI Regimen | |||
| Regimen 1: 3 NRTIs (based on AZT + 3TC + ABC) |
60 | 40–70 | Staszewski44, DART14, Srikantiah45 |
| Regimen 2: 2 NRTIs + NNRTI (based on TDF + 3TC + NVP or EFV) |
80 | 50–90 | Gullick46 |
| Regimen 3: 2 NRTIs + PI/r (based on TDF + 3TC + LPV/r) |
70 | 50–80 | De mendoza41, Kaufman42, Robbins43 |
| Strategy C: 3rd Regimen with 2nd Generation PI | |||
| Regimen 1: 2 NRTIs + NNRTI (based on AZT + 3TC + NVP or EFV) |
85 | 60–90 | Boulle,39 Orrell,25 Calmy40 |
| Regimen 2: 2 NRTIs + PI/r (based on TDF + 3TC + LPV/r) |
70 | 50–80 | De mendoza41, Kaufman42, Robbins43 |
| Regimen 3: 2 NRTIs + 2nd gen PI/r (based on TDF + 3TC + DNV/r) |
60 | 30–75 | Madruga16 |
3TC - Lamivudine NVP – Nevirapine EFV – Efavirenz LPV/r – Ritonavir-boosted lopinavir AZT – Zidovudine TDF – Tenofovir ABC – Abacavir DNV/r – Ritonavir-boosted darunavir
Treatment and Monitoring Strategies
We evaluated three HAART strategies, one with two lines of treatment and two with three lines (Table 1). The 2-line WHO strategy (A) included a regimen with 2 NRTIs plus an NNRTI followed by a regimen with a different combination of NRTIs and a bPI.4 The second strategy (B) started with a triple NRTI regimen followed by two regimens similar to the WHO strategy, and the third strategy (C) consisted of the WHO sequence followed by a regimen containing a second-generation bPI.
Strategies B and C are realistic in resource-limited settings for clinical and practical reasons. Triple NRTI regimen hold several advantages as initial therapy in resource-limited regions: they are inexpensive, have relatively low pill burden, avoid important drug interactions, and spare both NNRTIs and PIs, thus maintaining the effectiveness of both drug classes for subsequent regimens. While initial therapy with triple NRTI regimens have higher failure rates than initial therapy with NNRTI or bPI, triple NRTI regimens have been studied in both clinical and cohort trials in Africa.13–15 A third-line regimen with a second-generation bPI is also reasonable, especially after failure of a regimen with first-generation bPI. Easy dosing, side-effect profile comparable to first-generation bPIs, increasing clinical experience, and decreasing costs make this regimen a real possibility for sub-Saharan Africa.16, 17
The data on rates of failure were taken from cohort and clinical trials (Table 1). Previous estimates of failure rates vary widely based on the clinical setting, definition of failure, existing or new drug resistance mutations, and multiple determinants of adherence, including pill burden, funding source for antiretrovirals, and adherence counseling.18–22 Most of the available data on treatment failure come from trials which enrolled individuals with HIV subtype B, a rare clade in southern Africa. However, there is no evidence that antiretroviral activity is significantly altered in non-subtype-B viruses that predominate in southern Africa.23 For the base case estimates, we used data from South Africa, followed by data from other sub-Saharan cohort studies, and non-African trials where no other data was available (Table 2). Due to the uncertainty in the estimates, we varied the rates of virologic failure broadly to examine how uncertainty changes the effectiveness and cost-effectiveness of the strategies.
Table 2.
Estimates for Model Variable
| Variable | Base Case | Range | Source | ||
|---|---|---|---|---|---|
| Demographic variables | |||||
| Age at presentation (mean ± SD) | 33±9 | Holmes29, Badri47 | |||
| Male | 60% | 50–70% | Holmes29, Badri28, Orrell21 | ||
| CD4 at presentation, cells/µl (mean ± SD) |
307±227 | Holmes29, Badri47 | |||
| Viral load at presentation, log copies/ml (mean ± SD) |
5.0± 0.8 | Badri48 | |||
| Disease Progression Variables | |||||
| Decline in CD4 (cells/µl/mo), viral load >105 |
Holmes29, Mellors49, Rodriguez50, PLATO51 |
||||
| Baseline CD4 >500 cells/µl | 5.9 | ± 50% | |||
| Baseline CD4 351–500 cells/µl | 3.8 | ± 50% | |||
| Baseline CD4 201–350 cells/µl | 2.6 | ± 50% | |||
| Baseline CD4 <200 cells/µl | 2.0 | ± 50% | |||
| Decline in CD4 (cells/µl/mo), viral load 103–105 |
Holmes29, Mellors49, Rodriguez50, PLATO51 |
||||
| Baseline CD4 >500 cells/µl | 3.9 | ± 50% | |||
| Baseline CD4 351–500 cells/µl | 2.6 | ± 50% | |||
| Baseline CD4 201–350 cells/µl | 1.7 | ± 50% | |||
| Baseline CD4 <200 cells/µl | 1.3 | ± 50% | |||
| Monthly probability of developing severe opportunistic diseases (%), by CD4 |
<50 cells/µl |
51–200 cells/µl |
201–350 cells/µl |
>350 cells/µl |
Holmes29 |
| Oral candidiasis | 3.50% | 2.04% | 1.26% | 0.57% | |
| Chronic diarrhea | 2.00% | 0.49% | 0.18% | 0.00% | |
| Esophageal candidiasis | 1.46% | 0.34% | 0.09% | 0.06% | |
| Wasting syndrome | 1.29% | 0.23% | 0.02% | 0.00% | |
| Severe bacterial | 1.15% | 0.04% | 0.03% | 0.00% | |
| Pulmonary TB | 1.15% | 0.71% | 0.47% | 0.11% | |
| Extrapulmonary TB | 0.98% | 0.47% | 0.18% | 0.05% | |
| PCP | 0.67% | 0.05% | 0.02% | 0.00% | |
| CMV | 0.52% | 0.07% | 0.02% | 0.00% | |
| Cryptococcal meningitis | 0.52% | 0.05% | 0.00% | 0.00% | |
| Risk of death | Badri28 | ||||
| CD4 <50 cells/µl | 2.1%/mo | ||||
| CD4 51–200 cells/µl | 1.7%/mo | ||||
| CD4 201–350 cells/µl | 1.1%/mo | ||||
| CD4 >350 cells/µl | 0.8%/mo | ||||
| Additional risk of death from severe opportunistic disease |
Goldie9 | ||||
| CD4 <50 cells/µl | 7.69%/mo | ||||
| CD4 51–200 cells/µl | 4.48%/mo | ||||
| CD4 201–350 cells/µl | 0.66%/mo | ||||
| Risk of virologic failure | See Table 1 | ||||
| Harm from discontinuation of a non- suppressive regimen |
Deeks52 | ||||
| Drop in CD4, cells/µl | 128 | ||||
| Rise in viral load set point, log copies/ml |
0.8 | ||||
| Risk of regimen change or discontinuation due to toxicity, %/mo |
|||||
| AZT + 3TC + ABC | 0.3 | 0.1–0.5 | DART14, Munderi53 | ||
| AZT + 3TC + NVP or EFV | 0.5 | 0.3–0.7 | Orrell25, Boulle39 | ||
| TDF + 3TC + LPV/r | 0.3 | 0.1–0.5 | Amoroso54, Calmy40 | ||
| TDF + 3TC + DNV/r | 0.5 | 0.3–0.7 | Madruga16 Clotet17 | ||
| Utilization and Cost Variables | |||||
| Annual inpatient cost (2007USD) | Badri30, Govender55, Cleary56, Thomas57 |
||||
| No AIDS on HAART | 255 | 230–282 | |||
| AIDS on HAART | 483 | 386–596 | |||
| No AIDS off HAART | 887 | 839–939 | |||
| AIDS off HAART | 3,632 | 3,304– 3,985 |
|||
| Annual Outpatient cost | Badri30, Govender55, Cleary56 |
||||
| No AIDS on HAART | 316 | 305–328 | |||
| AIDS on HAART | 276 | 248–308 | |||
| No AIDS off HAART | 158 | 149–203 | |||
| AIDS off HAART | 240 | 207–277 | |||
| Annual cost of HAART Regimens (2007USD) |
MSF58, Aidsmap31, Badri30 | ||||
| 3 NRTIs (based on AZT + 3TC + ABC) |
548 | ||||
| 2 NRTIs + NNRTI (based on AZT + 3TC + NVP or EFV) |
199 | ||||
| 2 NRTIs + PI/r (based on TDF + 3TC + LPV/r) |
737 | ||||
| 2 NRTIs + 2nd gen PI/r (based on TDF + 3TC + DNV/r) |
1,332 | ||||
| Cost of CD4 test (2007 USD) | 15 | 10–25 | Badri24, Zijenah59 | ||
| Cost of viral load test (2007 USD) | 45 | 15–75 | Rouet60, Calmy61 | ||
We tested strategies where CD4 counts alone or in combination with viral load were available for patient monitoring. Viral load monitoring is relatively expensive and rarely available in Africa, although there is interest in increasing viral load capacity with the scale up on HAART as it is the most direct virologic measure of treatment failure.24 Failure was defined as two successive measurements above 1,000 copies/µl.25 When CD4 counts only were available, a decline to half the highest measured CD4 count after an initial response was considered failure, and prompted a regimen change.4 Although the WHO currently recommends treatment initiation at 200 cells/µl, recent evidence and ongoing trials are favoring earlier treatment initiation, thus in our model treatment was initiated at a CD4 threshold of 200–350 cells/µl.26, 27
Disease Progression
The disease model is described in the Supplementary Appendix and elsewhere.11 The principal determinants of short term mortality were the CD4 counts and development of severe opportunistic diseases.28 The CD4 counts changed according to HAART regimen, duration of treatment, CD4 at the start of treatment, age, viral load, and opportunistic diseases (Table 2). We modeled the incidence of AIDS-defining opportunistic diseases based on clinical experience in Cape Town, and estimated their contribution to mortality, utilization of resources, and costs separately.29, 30
Costs and Benefits
We included all direct costs of HIV care: inpatient, outpatient, HAART, and monitoring costs. Inpatient and outpatient costs were derived from costing studies in Cape Town, and costs of HAART were taken from published sources and estimated at the lowest available price in 2007 USD. Second generation bPI are rarely available in South Africa, and we used expected prices for low- and middle-income countries.31 We measured the cost-effectiveness as the ratio of the incremental costs to the incremental benefits of each strategy compared with the next least cost-effective strategy in 2007 USD per year-of-life gained. We adopted a societal perspective, although some indirect costs were excluded as we assumed they would be equivalent between strategies. We discounted all costs and benefits at 3% annually.
Sensitivity Analysis
In sensitivity analysis, we varied the rates of virologic failure to reflect uncertainty in published estimates. We also varied several important cost parameters that are expected to change. Specifically, bPIs are likely to become less expensive due to entry of additional drugs and continued price negotiations; and viral load monitoring is increasingly affordable due to cheaper technologies, durable measurement devices, and improving infrastructure.32 We performed a probabilistic sensitivity analysis, where we specified distributions for model parameters and employed a Monte Carlo simulation to sample from these distributions. We used the results to calculate confidence intervals around our incremental cost-effectiveness ratio estimates.33 Additional details on the distributions used is in the Supplementary Appendix.
Results
We estimate that over the lifetime of the cohort 25.6% of individuals would experience virologic failure of WHO’s 1st and 2nd line regimens. All individuals with virologic failure who remained in care were detected where viral load monitoring was available. However, where CD4 monitoring alone was available, treatment failure based on immunologic criteria was detected in only 6.5% of the cohort. Monitoring individuals with CD4 counts alone led to lower rates of detecting treatment failure primarily due to the insensitivity of immunologic criteria for detecting virologic failure.34 In addition, monitoring CD4 counts only was associated with a delay of 16.1 months on average in detecting failure. This, in turn, was associated with higher rates of mortality (Table 3), and decreasing the opportunities for detecting treatment failure.
Table 3.
Lifetime Costs and Outcomes of Alternative Strategie
| Strategy* | Discounted Life Expectancy (mo) |
Discounted Lifetime Cost ($) |
Percent Utilizing 3rd Line HAART |
Rate of ODs (per 100 PY) ** |
Incremental Cost-Effectiveness Ratio ($/yr of life gained) |
|---|---|---|---|---|---|
| CD4 counts only available | |||||
| A | 78.7 | 6,299 | NA | 11.7 | – |
| B | 76.1 | 6,892 | 18.1 | 12.8 | Dominated† |
| C | 79.3 | 6,423 | 6.5 | 10.9 | 2,581 |
| Viral loads available | |||||
| A | 81.0 | 7,645 | NA | 10.9 | - |
| B | 81.1 | 8,006 | 30.3 | 11.3 | Dominated by extended dominance‡ |
| C | 82.6 | 8,526 | 25.6 | 9.7 | 6,519 |
The letter refers to the strategies listed in Table 1 (A, 2 regimens; B, 3 regimens with triple NRTI as initial therapy; and C, 3 regimens with second generation PI).
ODs – opportunistic diseases.
Dominated strategies had at least one other strategy that was more effective and less costly than the dominated strategy. Extended dominance means that some blend of two strategies was more effective and less costly than the dominated strategy.
HAART Strategies with CD4 Monitoring
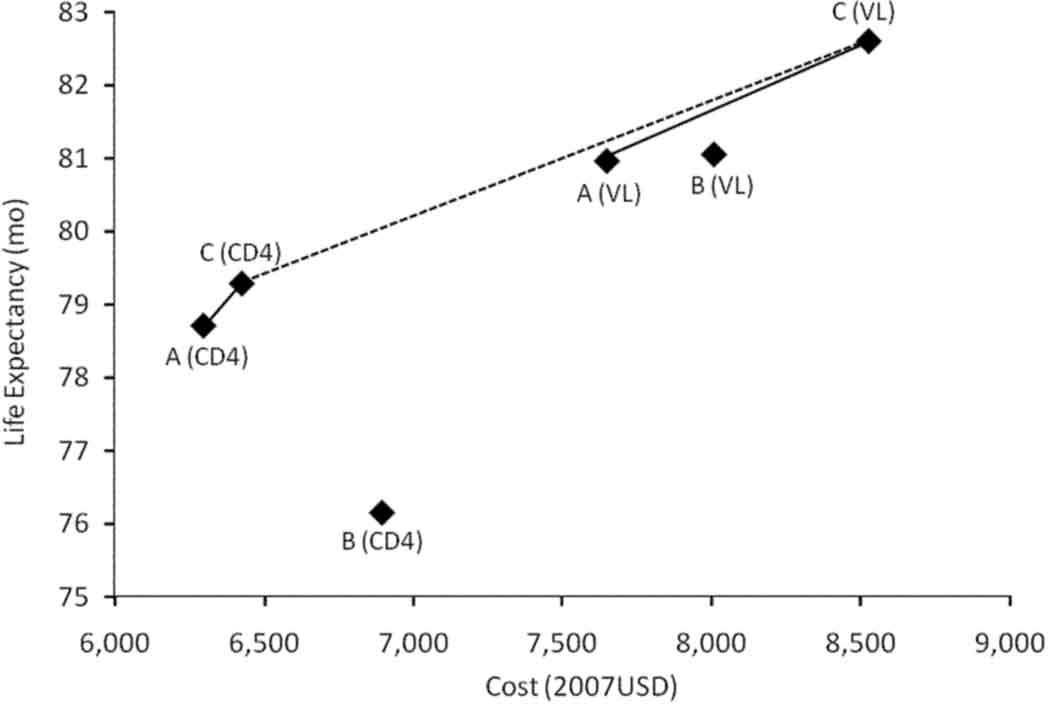
Where CD4 alone was used to monitor treatment success, the base strategy (A) was associated with a discounted life expectancy of 78.7 months, and lifetime costs of $6,299 from the time of presentation to care. In comparison, the strategy with a triple NRTI as initial therapy (B) was dominated; that is, it decreased life expectancy and increased costs (Figure 1). The strategy where a second-generation bPI was used as a third regimen (C) was associated with an increase in life expectancy of 18 discounted days and an increase in lifetime costs of $124, an incremental cost effectiveness ratio of $2,581/year-of-life gained (95%CI 2,044–3,006, using probabilistic sensitivity analysis). Only 6.5% of the population were placed on third line HAART in strategy C, but the life expectancy of individuals who utilized additional HAART in that strategy was, on average, 8.9 discounted (14.9 undiscounted) months longer than the equivalent population in the WHO strategy (A). The gains in life expectancy were associated with an overall reduction in the incidence of severe opportunistic diseases (Table 3).
Figure 1. Health and Cost Outcomes of Alternative HAART Regimens.
Life expectancy and lifetime costs of alternative HAART strategies. Letters correspond to strategies in Table 1 (A, 2 regimens; B, 3 regimens with triple NRTI as initial therapy; C, 3 regimens with second generation bPI). Monitoring strategy is represented as either CD4 counts only (CD4) or CD4 and viral loads (VL). Strategy B is strictly dominated when only CD4 counts are available (it is more costly and less effective than other strategies), and dominated by extended dominance when viral loads are available (it is more costly and less effective than some combination of A and C). Solid lines connect the cost-effective strategies within a given monitoring strategy, and the dotted line connect the cost-effective strategies regardless of monitoring strategy.
HAART strategies with Viral Load Monitoring
When viral load monitoring was available, the WHO strategy was associated with a discounted life expectancy of 81.0 months and a lifetime cost of $7,645. Compared with the WHO strategy, adding a regimen with a second generation bPI (C) was associated with a gain in life expectancy of 1.6 months and additional $881 in lifetime costs, an incremental cost-effectiveness ratio of $6,519 per year-of-life gained (95% CI 5,673 to 8,129). The cost of HAART alone was $873 higher in strategy C compared with the WHO strategy. Strategy C was associated with a lower incidence of severe opportunistic diseases, 9.7 per 100 patient-years (PYs), compared with 10.9 per 100PYs in the WHO strategy. With viral load monitoring, 25.6% of individuals were eligible for third-line treatment under strategy C. The life expectancy of those who were placed on a second-generation bPI was 10.4 undiscounted months longer than the equivalent population in the WHO strategy. Strategy B was dominated by extended dominance. That is, it was more costly and less effective than a blend of strategies A and C, but not compared with either strategy alone.
Sensitivity Analyses
Rates of Failure
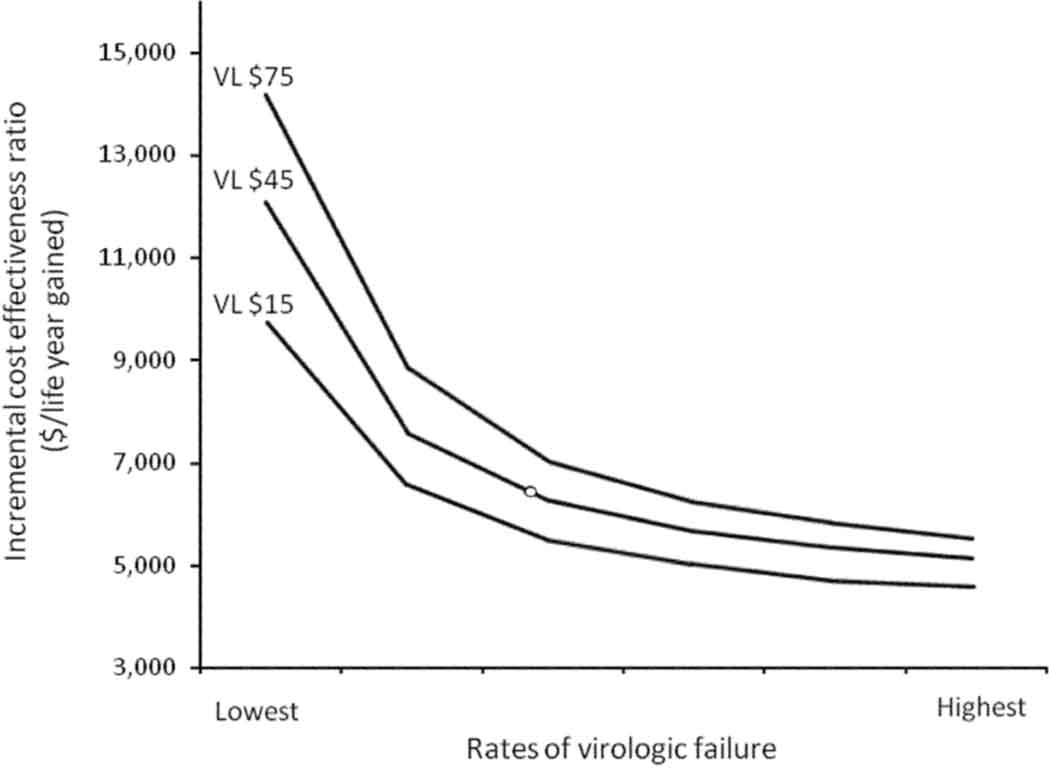
Rates of failure vary widely based on the HAART regimen, geography, clinical setting (e.g. trial or cohort), and individual treatment history. For that reason, we varied the rates of virologic suppression to reflect a broad range of uncertainty. Decreasing the rates of virologic failure from highest to lowest was associated with an average increase in life expectancy of 5.6 months across all strategies (range 4.9–6.1 months). Decreasing rates of virologic failure were also associated with an less attractive incremental cost-effectiveness ratio of additional HAART regimens. With viral load monitoring, the incremental cost effectiveness ratio of strategy C compared with the WHO strategy varied from $12,098 to $5,178 per life-year gained as the rate of virologic failure varied from lowest to highest rate. Thus, the value adding an effective 3rd line HAART regimen is greatest where rates of failure are relatively high (Figure 2).
Figure 2. Effect of Failure Rates and Viral Load Cost on Cost-Effectiveness of a Third HAART Regimen.
The incremental cost-effectiveness ratio of strategy C compared with the WHO strategy with viral load monitoring as a function of rates of failure and price of viral load monitoring. With lower rates of failure, fewer people require advanced HAART regimens, and the incremental benefits of those regimens diminishes, making the incremental cost-effectiveness ratio higher. The incremental cost-effectiveness ratio depends on the per-test cost of viral load monitoring.
Cost Implications
We varied several cost parameters to examine the implications as drug and diagnostic technologies are increasingly affordable. Specifically, we varied the costs of second-generation bPIs, and the cost of viral load testing. When the annual cost of a second-generation bPI dropped below $540, strategy C with CD4 count monitoring dominated the WHO strategy. That is, it saved costs and improved outcomes relative to the WHO strategy. Reducing the cost of viral load monitoring from $75 to $15 per test decreased the incremental cost-effectiveness ratio of adding a second-generation bPI to $5,427 per life-year gained. Figure 2 shows the effect of reducing the cost of viral load monitoring on the incremental cost-effectiveness ratio of strategy C compared with the WHO strategy.
Probabilistic Sensitivity Analysis
In probabilistic sensitivity analysis we examined the joint effect of parameter uncertainty. The analysis was repeated 1,000 times, and we used the results to obtain confidence intervals for our estimates. Our 95% confidence bounds for the portion of the population who had virologic failure to WHO’s 1st and 2nd line were 23.4%–28.8%. In our analysis, the strategy where a triple NRTI was used in initial regimen with CD4 count monitoring was dominated in 88% of the scenarios we simulated, and it never dominated the WHO strategy.
Discussion
We analyzed the benefits, costs, and cost-effectiveness of adding HAART regimens for resource-limited settings using data from South Africa. We show that adding an effective third antiretroviral regimen could provide substantial benefits for those who fail 1st and 2nd line therapy. Our estimates suggest that individuals who fail both existing regimens may gain between 6.7 and 8.9 months of life with third line HAART. Although at most a quarter of the infected population could derive benefit from an effective third line, we estimate that that is sufficient to improve the average life expectancy of the entire infected population by 0.6–1.6 months, depending on the monitoring technology. Our estimates of the need for additional regimens, which suggest that the current recommended regimens will provide adequate lifelong benefits for about three-quarters of the infected population on HAART, are consistent with recent evidence showing low rates of failure in low-income countries.35
The WHO and World Bank suggest that interventions with an incremental cost-effectiveness ratio less than three times the GDP per capita represent good value.36, 37 By that criteria, adding a third-line regimen based on a second-generation bPI to the existing WHO regimens should be acceptable in South Africa. While the incremental cost-effectiveness ratio is higher with viral load monitoring (due to higher HAART and monitoring costs), adding a third regimen may be acceptable in countries with an annual per capita GDP above $2000. Further reductions in the price of second-generation bPIs will improve the cost-effectiveness of adding a third regimen, and may be cost-saving below $540 per year.
Our analysis, however, suggests that adding a less efficacious first-line regimen may worsen outcomes where viral load monitoring is not available. When methods for timing of regimen change are associated with a significant delay, such as when using CD4 counts, adding an initial regimen with rates of failure higher than subsequent regimens may lead to worse outcomes. While preserving drug classes has intuitive appeal, the delay in diagnosis of treatment failure without viral load monitoring led to additional opportunistic diseases and higher mortality in our study. Even when viral load monitoring is available, we estimate that the cost-effectiveness of adding an initial triple NRTI regimen is not as cost-effective as adding a second-generation bPI as a third regimen.
We also show the importance of preventing virologic failure in improving patient outcomes. The variability in the rates of failure reported in the literature may be attributed to clinical practices and behavioral factors. Taking HAART regularly is directly related to maintaining virologic suppression, and our study suggests that HAART outcomes improve substantially with lower rates of failure. We find that the relative value of additional HAART regimens is highest where the rates of failure are also high.
Use of CD4 counts to determine when to initiate HAART in resource-limited settings improves life expectancy substantially and may reduce costs.11 Here we highlight several important roles which viral load monitoring plays. It is the preferred method for timing regimen change: it is more accurate than using CD4 counts for determining treatment failure, and leads to a significantly shorter lag in diagnosis and fewer opportunistic diseases. The findings in our analysis dovetail with increasing evidence about the inaccuracy of using CD4 count monitoring alone for determining treatment failure.34, 38 In addition, the benefits of viral load monitoring are greater with more complex treatment options. However, substantial expenditures, lack of infrastructure, and shortage of skilled labor needed for viral load monitoring may continue to be a barrier in many places.
Our model has several important limitations. We estimate rates of virologic failure and medication toxicities from clinical trials and cohort trials. Most of those were done in sub-Saharan Africa on HIV subtype non-B, but where no estimates were available from our region of interest, we used data from developed countries. In addition, available data on the effectiveness of sequential regimens is sparse, and our estimates are partly based on conditional predictions. We also do not account for rates of loss to follow-up, which some suggest may be lower where diagnostic monitoring and medical care is more extensive. Finally, it is also possible that the pathways to resistance of HIV subtype non-B may differ from reported experience. For all these reasons, we vary the rates of failure across a wide range, and indicate the limitations of our estimates.
As access to HIV treatment continues to expand across sub-Saharan Africa, where over 20 million are infected and thousands are started on treatment weekly, the number of people who will fail the existing regimens will continue to increase. We suggest that offering additional effective regimens provide substantial benefits to individuals who fail existing therapies, is cost-effective in many parts of southern Africa where CD4 count monitoring is available, and may be cost-saving with substantial price reductions of second-generation bPIs. Our analysis also shows that reducing treatment failure is an effective way to minimize the need for additional regimens and maximize the benefits of the regimens that are currently available.
Supplementary Material
Acknowledgments
This research is supported in part by the National Institute on Drug Abuse (R01 DA15612-01), the Department of Veterans Affairs, and the Agency for Healthcare Research and Quality (T32-HS000028). The funding organizations had no part in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Dr. Bendavid had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.UNAIDS. Report on the Global AIDS Epidemic. Geneva: Joint United Nations Programme on HIV/AIDS; 2008. [Google Scholar]
- 2.UNAIDS. Towards universal access: scaling up priority HIV/AIDS interventions in the health sector. Progress Report. Geneva: World Health Organization, UNAIDS; 2007. [Google Scholar]
- 3.Hammer S, Havlir D, Klement E, et al. Scaling up Antiretroviral Therapy in Resource-Limited Settings: Treatment Guidelines for a Public health Approach. Geneva: World Health Organization; 2003. [Google Scholar]
- 4.Antiretroviral Therapy For HIV Infection in Adults And Adolescents: Recommendations for a public health approach. Geneva: World Health Organization; 2006. [PubMed] [Google Scholar]
- 5.Hardy D, Reynes J, Konourina I, et al. Efficacy and Safety of Maraviroc plus Optimized Background Therapy in Treatment-experienced Patients Infected with CCR5-Tropic HIV-1: 48-Week Combined Analysis of the MOTIVATE Studies. Boston: CROI; 2008. [Google Scholar]
- 6.Steigbigel R, Kumar P, Eron J, et al. 48-Week Results from BENCHMRK-2, a Phase III Study of Raltegravir (RAL) in Patients Failing Antiretroviral Therapy (ART) with Triple-Class Resistant HIV-1. Boston: CROI; 2008. [Google Scholar]
- 7.Egger M. Outcome of ART in Resource-Limited and Industrialized Countries. CROI. LA Conference Center; 2007. [Google Scholar]
- 8.Mills EJ, Nachega JB. A wake-up call for global access to salvage HIV drug regimens. Lancet. 2007;370(9603):1885–1887. doi: 10.1016/S0140-6736(07)61790-5. [DOI] [PubMed] [Google Scholar]
- 9.Goldie SJ, Yazdanpanah Y, Losina E, et al. Cost-effectiveness of HIV treatment in resource-poor settings--the case of Cote d'Ivoire. N Engl J Med. 2006;355(11):1141–1153. doi: 10.1056/NEJMsa060247. [DOI] [PubMed] [Google Scholar]
- 10.Bishai D, Colchero A, Durack DT. The cost effectiveness of antiretroviral treatment strategies in resource-limited settings. AIDS. 2007;21(10):1333–1340. doi: 10.1097/QAD.0b013e328137709e. [DOI] [PubMed] [Google Scholar]
- 11.Bendavid E, Young SD, Katzenstein DA, Bayoumi AM, Sanders GM, Owens DK. Cost-Effectiveness of HIV Monitoring Strategies in Resource-Limited Settings – A Southern African Analysis. Archives of Internal Medicine. 2008;168(17):1910–1918. doi: 10.1001/archinternmed.2008.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phillips A, Pillay D, Miners AH, Bennett DE, Gilks C, Lundgren JD. Outcomes from monitoring of patients on antiretroviral therapy in resource-limited settings with viral load, CD4 cell count, or clinical observation alone: a computer simulation model. Lancet. 2008;371(9622):1443–1451. doi: 10.1016/S0140-6736(08)60624-8. [DOI] [PubMed] [Google Scholar]
- 13.Gulick RM, Ribaudo HJ, Shikuma CM, et al. Triple-nucleoside regimens versus efavirenz-containing regimens for the initial treatment of HIV-1 infection. N Engl J Med. 2004;350(18):1850–1861. doi: 10.1056/NEJMoa031772. [DOI] [PubMed] [Google Scholar]
- 14.DART Virology Group and Trial Team. Virological response to a triple nucleoside/nucleotide analogue regimen over 48 weeks in HIV-1-infected adults in Africa. AIDS. 2006;20(10):1391–1399. doi: 10.1097/01.aids.0000233572.59522.45. [DOI] [PubMed] [Google Scholar]
- 15.Walker S, Kityo C, Kaleebu P, et al. Superior Virological Suppression with Nevirapine, Zidovudine, and Lamivudine vs Abacavir, Zidovudine, and Lamivudine without Evidence of Clinical Benefit to 48 Weeks: A Randomized Comparison in Patients with Low CD4 Counts in Africa. Los Angeles: CROI; 2007. [Google Scholar]
- 16.Madruga JV, Berger D, McMurchie M, et al. Efficacy and safety of darunavir-ritonavir compared with that of lopinavir-ritonavir at 48 weeks in treatment-experienced, HIV-infected patients in TITAN: a randomised controlled phase III trial. Lancet. 2007;370(9581):49–58. doi: 10.1016/S0140-6736(07)61049-6. [DOI] [PubMed] [Google Scholar]
- 17.Clotet B, Bellos N, Molina JM, et al. Efficacy and safety of darunavir-ritonavir at week 48 in treatment-experienced patients with HIV-1 infection in POWER 1 and 2: a pooled subgroup analysis of data from two randomised trials. Lancet. 2007;369(9568):1169–1178. doi: 10.1016/S0140-6736(07)60497-8. [DOI] [PubMed] [Google Scholar]
- 18.Taylor BS, Sobieszczyk ME, McCutchan FE, Hammer SM. The challenge of HIV-1 subtype diversity. New England Journal of Medicine. 2008;358(15):1590–1602. doi: 10.1056/NEJMra0706737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conway B. The role of adherence to antiretroviral therapy in the management of HIV infection. Journal of acquired immune deficiency syndromes. 2007;45 Suppl 1:S14–S18. doi: 10.1097/QAI.0b013e3180600766. [DOI] [PubMed] [Google Scholar]
- 20.Nettles RE, Kieffer TL, Kwon P, et al. Intermittent HIV-1 viremia (Blips) and drug resistance in patients receiving HAART. JAMA. 2005;293(7):817–829. doi: 10.1001/jama.293.7.817. [DOI] [PubMed] [Google Scholar]
- 21.Orrell C, Bangsberg DR, Badri M, Wood R. Adherence is not a barrier to successful antiretroviral therapy in South Africa. AIDS. 2003;17(9):1369–1375. doi: 10.1097/00002030-200306130-00011. [DOI] [PubMed] [Google Scholar]
- 22.Ramadhani HO, Thielman NM, Landman KZ, et al. Predictors of incomplete adherence, virologic failure, and antiviral drug resistance among HIV-infected adults receiving antiretroviral therapy in Tanzania. Clin Infect Dis. 2007;45(11):1492–1498. doi: 10.1086/522991. [DOI] [PubMed] [Google Scholar]
- 23.Kantor R, Katzenstein DA, Efron B, et al. Impact of HIV-1 subtype and antiretroviral therapy on protease and reverse transcriptase genotype: results of a global collaboration. PLoS Med. 2005;2(4):e112. doi: 10.1371/journal.pmed.0020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rewari B, Rajasekaran S, Deshpande A, Chan P, Bachani D, Srikantiah P. Evaluating Patients for Second-line ART in India: Confirmation of Virologic Failure Prevents Unnecessary Treatment Switches. Montreal, Canada: CROI; 2009. [Google Scholar]
- 25.Orrell C, Harling G, Lawn SD, et al. Conservation of first-line antiretroviral treatment regimen where therapeutic options are limited. Antiviral therapy. 2007;12(1):83–88. [PubMed] [Google Scholar]
- 26.Kitahata M, SJ G, RD M, Kuritzkes D. Initiating Rather than Deferring Haart at a CD4+ Count Between 351–500 Cells/mm3 is Associated with Improved Survival 2008. Washington, DC: ICAAC/IDSA; 2008. [Google Scholar]
- 27.Walensky RP, Wolf L, Wood R, et al. When to Start ART—A Policy Evaluation While Awaiting Trial Results: South Africa. Montreal, Canada: CROI; 2009. [Google Scholar]
- 28.Badri M, Lawn SD, Wood R. Short-term risk of AIDS or death in people infected with HIV-1 before antiretroviral therapy in South Africa: a longitudinal study. Lancet. 2006;368(9543):1254–1259. doi: 10.1016/S0140-6736(06)69117-4. [DOI] [PubMed] [Google Scholar]
- 29.Holmes CB, Wood R, Badri M, et al. CD4 Decline and Incidence of Opportunistic Infections in Cape Town, South Africa: Implications for Prophylaxis and Treatment. J Acquir Immune Defic Syndr. 2006;42(4):464–469. doi: 10.1097/01.qai.0000225729.79610.b7. [DOI] [PubMed] [Google Scholar]
- 30.Badri M, Maartens G, Mandalia S, et al. Cost-effectiveness of highly active antiretroviral therapy in South Africa. PLoS Med. 2006;3(1):e4. doi: 10.1371/journal.pmed.0030004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. [Accessed April 2, 2008];Drug Access - Aspen Pharmacare, Tibotec Enter Agreement To Distribute Protease Inhibitor Prezista in Sub-Saharan Africa. 2007 at http://www.theglobalfund.org/programs/news_summary.aspx?newsid=19&countryid=NMB&lang=EN.
- 32.Stevens G, Rekhviashvili N, Scott LE, Gonin R, Stevens W. Evaluation of two commercially available, inexpensive alternative assays used for assessing viral load in a cohort of human immunodeficiency virus type 1 subtype C-infected patients from South Africa. Journal of clinical microbiology. 2005;43(2):857–861. doi: 10.1128/JCM.43.2.857-861.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Briggs A, Fenn P. Confidence intervals or surfaces? Uncertainty on the cost-effectiveness plane. Health Econ. 1998;7(8):723–740. doi: 10.1002/(sici)1099-1050(199812)7:8<723::aid-hec392>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 34.Badri M, Lawn SD, Wood R. Utility of CD4 cell counts for early prediction of virological failure during antiretroviral therapy in a resource-limited setting. BMC Infect Dis. 2008;8:89. doi: 10.1186/1471-2334-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keiser O. IeDEA, ART-LINC. Switching to Second-line ART, and Mortality in Resource-limited Settings: Collaborative Analysis of Treatment Programs in Africa, Asia, and Latin America. Montreal, Canada: CROI; 2009. [Google Scholar]
- 36.World Health Report. Reducing Risk, Promoting Healthy Life. Geneva: World Health Organization; 2002. [Google Scholar]
- 37.Macroeconomics and health. investing in health for economic development. Report of the Commission on Macroeconomics and Health. Geneva: World Health Organization; 2001. [Google Scholar]
- 38.Etiebet M, Gebi U, Shepherd J, et al. Performance of WHO Immunological Criteria for Determining Virological Treatment Failure in Low-resource Settings. Montreal, Canada: CROI; 2009. [Google Scholar]
- 39.Boulle A, Orrel C, Kaplan R, et al. Substitutions due to antiretroviral toxicity or contraindication in the first 3 years of antiretroviral therapy in a large South African cohort. Antiviral therapy. 2007;12(5):753–760. doi: 10.1177/135965350701200508. [DOI] [PubMed] [Google Scholar]
- 40.Calmy A. Outcomes of Adults Receiving Second-line ART in Médecins Sans Frontiéres-supported Projects in Resources-Limited Countries. CROI. Los Angeles Conference Center; 2007. [Google Scholar]
- 41.de Mendoza C, Valer L, Ribera E, et al. Performance of six different ritonavir-boosted protease inhibitor-based regimens in heavily antiretroviral-experienced HIV-infected patients. HIV clinical trials. 2006;7(4):163–171. doi: 10.1310/hct0704-163. [DOI] [PubMed] [Google Scholar]
- 42.Kaufmann GR, Khanna N, Weber R, et al. Long-term virological response to multiple sequential regimens of highly active antiretroviral therapy for HIV infection. Antiviral therapy. 2004;9(2):263–274. [PubMed] [Google Scholar]
- 43.Robbins GK, De Gruttola V, Shafer RW, et al. Comparison of sequential three-drug regimens as initial therapy for HIV-1 infection. N Engl J Med. 2003;349(24):2293–2303. doi: 10.1056/NEJMoa030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Staszewski S, Keiser P, Montaner J, et al. Abacavir-lamivudine-zidovudine vs indinavir-lamivudine-zidovudine in antiretroviral-naive HIV-infected adults: A randomized equivalence trial. JAMA. 2001;285(9):1155–1163. doi: 10.1001/jama.285.9.1155. [DOI] [PubMed] [Google Scholar]
- 45.Srikantiah P, Walusimbi MN, Kayanja HK, et al. Early virological response of zidovudine/lamivudine/abacavir for patients co-infected with HIV and tuberculosis in Uganda. AIDS. 2007;21(14):1972–1974. doi: 10.1097/QAD.0b013e32823ecf6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gulick RM, Lalama CM, Ribaudo HJ, et al. Intensification of a triple-nucleoside regimen with tenofovir or efavirenz in HIV-1-infected patients with virological suppression. AIDS. 2007;21(7):813–823. doi: 10.1097/QAD.0b013e32805e8753. [DOI] [PubMed] [Google Scholar]
- 47.Badri M, Wilson D, Wood R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet. 2002;359(9323):2059–2064. doi: 10.1016/S0140-6736(02)08904-3. [DOI] [PubMed] [Google Scholar]
- 48.Badri M, Cleary S, Maartens G, et al. When to initiate highly active antiretroviral therapy in sub-Saharan Africa? A South African cost-effectiveness study. Antivir Ther. 2006;11(1):63–72. [PubMed] [Google Scholar]
- 49.Mellors JW, Muñoz A, Giorgi JV, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Annals of internal medicine. 1997;126(12):946–954. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 50.Rodríguez B, Sethi AK, Cheruvu VK, et al. Predictive value of plasma HIV RNA level on rate of CD4 T-cell decline in untreated HIV infection. JAMA. 2006;296(12):1498–1506. doi: 10.1001/jama.296.12.1498. [DOI] [PubMed] [Google Scholar]
- 51.Ledergerber B, Lundgren JD, Walker AS, et al. Predictors of trend in CD4-positive T-cell count and mortality among HIV-1-infected individuals with virological failure to all three antiretroviral-drug classes. Lancet. 2004;364(9428):51–62. doi: 10.1016/S0140-6736(04)16589-6. [DOI] [PubMed] [Google Scholar]
- 52.Deeks SG, Wrin T, Liegler T, et al. Virologic and immunologic consequences of discontinuing combination antiretroviral-drug therapy in HIV-infected patients with detectable viremia. The New England journal of medicine. 2001;344(7):472–480. doi: 10.1056/NEJM200102153440702. [DOI] [PubMed] [Google Scholar]
- 53.Munderi P. DART. Safety of Nevirapine Compared to Abacavir on a Background of Zidovudine/Lamivudine as First-line Antiretroviral Therapy: A Randomized Double-blind Trial. Denver, CO: CROI; 2006. [Google Scholar]
- 54.Amoroso A. ART-Associated Toxicities Leading to a Switch in Medication: Experience in Uganda, Kenya, and Zambia. Los Angeles: CROI; 2007. [Google Scholar]
- 55.Govender V, McIntyre D, Grimwood A, Maartens G. The Costs and Perceived Quality of Care for People Living with HIV/AIDS in the Western Cape Province in South Africa. Bethesda, MD: Partnerships for Health Reform; 2000. [Google Scholar]
- 56.Cleary SM, McIntyre D, Boulle AM. The cost-effectiveness of Antiretroviral Treatment in Khayelitsha, South Africa - a primary data analysis. Cost effectiveness and resource allocation. 2006;4:1–14. doi: 10.1186/1478-7547-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thomas LS, Manning A, Holmes CB, et al. Comparative Costs of Inpatient Care for HIV-Infected and Uninfected Children and Adults in Soweto, South Africa. J Acquir Immune Defic Syndr. 2007 doi: 10.1097/QAI.0b013e318156ec90. [DOI] [PubMed] [Google Scholar]
- 58.Medicines Sans Frontiers, Untangling the web of price reductions: a pricing guide for the purchase of ARVs for developing countries. 2007 (Accessed at http://www.accessmed-msf.org/.)
- 59.Zijenah LS, Kadzirange G, Madzime S, et al. Affordable flow cytometry for enumeration of absolute CD4+ T-lymphocytes to identify subtype C HIV-1 infected adults requiring antiretroviral therapy (ART) and monitoring response to ART in a resource-limited setting. Journal of translational medicine. 2006;4:33–39. doi: 10.1186/1479-5876-4-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rouet F, Rouzioux C. HIV-1 viral load testing cost in developing countries: what's new? Expert Rev Mol Diagn. 2007;7(6):703–707. doi: 10.1586/14737159.7.6.703. [DOI] [PubMed] [Google Scholar]
- 61.Calmy AA, Ford NN, Hirschel BB, et al. HIV viral load monitoring in resource-limited regions: optional or necessary? Clinical infectious diseases. 2007;44(1):128–134. doi: 10.1086/510073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.