Summary
Stem cell antigen-1 (Sca1; Ly6A/E) is a well-established marker of murine hematopoietic stem cells, and also is expressed on memory T cells. It has been suggested that the functional maintenance of T cell memory requires the expression of Sca1 on a specialized population of memory T cells termed “memory stem cells”. Here, we evaluate the requirement for Sca1 in the primary T cell response to virus infection, and in the establishment and maintenance of T cell memory. We find that Sca1 expression increases on almost all CD4+ and CD8+ T cells during virus infection, and remains high on virus-specific memory cells. However, Sca1-deficient (Sca1KO) mice generate normal primary T cell responses to infection; the kinetics, the immunodominance hierarchy, and the absolute numbers of CD4+ and CD8+ T cells are essentially indistinguishable from those observed in wildtype mice. Furthermore, by several criteria, primary and memory T cells in Sca1KO mice are phenotypically and functionally normal. These data indicate that Sca1, although perhaps a useful marker of virus-specific memory T cells, is not required for the regulation of T cell quantity or quality, or for the development of a competent pool of memory cells.
Keywords: T cells, memory cells, cellular activation
Introduction
Stem cell antigen-1 (Sca1) is expressed on murine hematopoietic stem cells, and it is a commonly-used marker of these cells [1]. The Sca1 protein was first identified more than 3 decades ago [2] and is a member of the Ly-6 gene superfamily, so-named because several of the gene products are markers of lymphocyte activation and differentiation [3, 4]. The Ly-6 superfamily comprises at least 18 highly homologous genes, the expression of two of which are quite commonly measured by immunologists: Ly-6C, often considered a marker of memory cells [5], and Sca1, also known by its alternative name, Ly-6A/E [6–8]. Sca1/Ly-6A is encoded by the Ly6a gene, the prototypical member of the Ly-6 superfamily. The Ly6a gene has two common alleles whose coding sequences differ by 2 amino-acids; C57BL/6 mice encode the Ly-6A protein, and BALB/c mice express the Ly-6E allele [6, 8, 9]. Cross-reactive antibodies have been widely used to evaluate the expression of these proteins on murine T cells, and so these allelic variants often are referred to in combination, as Ly-6A/E. Hereinafter, we shall refer to the allelic proteins as Sca1.
Sca1 expression fluctuates during lymphocyte development. As noted above, it is expressed on their earliest precursors (hematopoietic stem cells), and expression is maintained on immature thymocytes and double-negative T cells, lost at the double-positive stage of development, and regained by naïve single-positive CD4+ T cells and CD8+ T cells [10–12]. Thus, all naïve T cells are thought to express low levels of Sca1. Expression increases following T cell activation, e.g. when CD4+ T cells differentiate from uncommitted precursor cells to Th1 or Th2 cells, and it has been suggested that CD4+ memory cells may contain both Sca1lo and Sca1hi populations [13]. Both primary and memory CD8+ T cells show increased levels of cell-surface Sca1 in a graft-versus-host model [14]; and in a model of virus infection, Sca1 was one of two molecules that showed the greatest increase between naïve T cell receptor (TcR)-transgenic CD8+ T cells and their memory counterparts [15]. Indeed, a role for Sca1 in memory T cell development has been proposed. Memory T cells are a self-renewing population of cells that, when appropriately stimulated, can rapidly divide and can show concurrent phenotypic changes such as altered effector function. These attributes of memory cells (self renewal and progeny differentiation), together with their increased expression of Sca1, a marker of stem cells in mice, has led some workers to propose the existence of a specialized “memory-precursor” population of T cells termed “memory stem cells” [14, 16]. By this hypothesis, Sca1 is not only a marker of those cells, but also is a key regulator of memory T cell establishment and function.
Consistent with the above hypothesis, there is evidence that Sca1 might be involved in the regulation of T cell responses. Both positive and negative effects have been reported. Supporting a positive effect, agonistic antibodies against Sca1 induce a Ca2+ flux and increase IL-2 production [17], and antigen-stimulated CD8+ T cells are capable of producing IL-2 and killing target cells when co-stimulated with immobilized antibody against Sca1 [18]; but, conversely, anti-CD3-mediated IL-2 production is inhibited by anti-Sca1 antibodies [19]. Thus, the effect of Sca1 signaling on cytokine production varies in different in vitro models. Variable effects also have been reported with regard to T cell proliferation; monoclonal antibodies to Sca1 augment T cell proliferation [20], but anti-TcR-induced proliferation is inhibited by antibody against Sca1 [18], and T cells from mice that over-express Sca1 proliferate poorly in vitro [21]. Sca1-deficient mice have been described by two research groups [22, 23]. These animals show no marked developmental abnormalities; and in both genotypes, hematopoiesis is essentially normal in homozygous mutant (Sca null) mice [22, 23], with only very minor lineage skewing [24]. Perhaps most surprisingly, given the complex pattern of Sca1 expression in thymocytes, the naïve T cell compartment appears to be normal in these mice. However, some in vitro data suggest that T cells from Sca1KO mice may be hyper-responsive. The cells proliferate to a greater extent in vitro than wildtype T cells when stimulated by concanavalin-A or anti-CD3, or by a mixed lymphocyte reaction [22]. Furthermore, when Sca1KO mice received three injections of keyhole limpet hemocyanin, T cells from the mice were more responsive to the antigen in vitro [22]. The in vitro hyper-responsiveness of Sca1KO T cells may require TcR engagement, because the effect is not seen in cultures stimulated with PMA or ionomycin, signals that bypass TcR complex signaling [22]. Taken together, the foregoing work suggests that (i) Sca1 is not required for the development of naïve T cells; (ii) therefore, if Sca1 plays a key regulatory role in T cell biology, it most likely does so at a subsequent stage – for example, during an immune response, when naïve cells differentiate into effector & memory progeny; and (iii) the in vivo regulatory effects of the Sca1 protein, if any, are most likely to be negative.
T cells are, of course, key components of protective immunity against many microbial infections but, surprisingly, the importance of Sca1 for antigen-specific T cell responses has not previously been evaluated in this regard. We report herein the results of such a study, using the lymphocytic choriomeningitis virus (LCMV) model. We describe the changes in Sca1 expression that occur on virus-specific and non-virus-specific CD4+ and CD8+ T cells during and after virus infection; and we demonstrate that Sca1-deficient mice develop antiviral primary and memory CD4+ and CD8+ T cell responses that, by several criteria, are quantitatively and qualitatively normal.
Results
During acute infection most T cells become Sca1hi, and this does not require IFN signaling
We considered it important first to confirm and expand the work of other labs, by evaluating Sca1 expression on normal (endogenous, non-TcR-transgenic) CD4+ and CD8+ T cells before, and during, a virus infection. To this end, wild-type C57BL/6 mice were infected with LCMV; Sca1KO mice also were infected, and were used here as negative controls for evaluating Sca1 expression levels. In uninfected mice (day 0), most wt CD8+ T cells were Sca1lo; but, contrary to our expectations, the majority of naïve CD4+ T cells were Sca1hi (Figure 1A). By day 7 after infection, almost all CD4+ and CD8+ T cells expressed high levels of Sca1. Early work indicated that type I and type II interferons (IFN) could up-regulate Sca1 expression on T cells both in vitro and in vivo [7, 25–27]. To evaluate the requirements for IFNs in Sca1 up-regulation in vivo, single knockout mice, lacking the receptor for one or other type of IFN, were infected with LCMV (Figure 1B). Two interesting observations were made. First, compared to wt mice, Sca1 expression was much reduced on naïve CD4+ and CD8+ T cells in IFNαβRKO mice; in contrast, expression on naïve cells was similar in wt and IFNγRKO animals. Second, in both of the receptor-deficient strains, almost all T cells expressed high levels of Sca1 at 7 days p.i. Taken together, these data indicate that (i) type I IFNs may up-regulate constitutive Sca1 expression on naïve T cells; (ii) during LCMV infection, almost all T cells (CD4+ and CD8+) become Sca1hi; and (iii) the up-regulation of Sca1 has no absolute requirement for either IFNα/β, or for IFNγ. However, since both type I IFNs and IFNγ are reported to upregulate Sca1, it was possible that of one or other receptor would have little effect on Sca1 regulation, because the other IFN pathway would remain intact. Therefore, Sca1 expression also was analyzed in mice deficient in both receptors (DKO mice). As was observed in IFNαβRKO mice, T cells from the DKO mice showed reduced basal expression of Sca1 (Figure 1B), and at 7 days p.i. their T cells expressed greater levels of Sca1. Thus, we conclude that there is no requirement for type I IFN or IFNγ in the upregulation of Sca1 that takes place on most T cells after virus infection.
Figure 1. Sca1 expression on CD4+ and CD8+ T cells changes over the course of infection, and the changes require neither type I nor type II IFNs.
The expression level of Sca1 on T cells was analyzed by flow cytometry before and at various times after acute infection with LCMV-Armstrong. For mice of the five indicated strains, the levels of Sca1 expression on T cells were determined in naïve mice (Day 0) or 7 days after infection with LCMV. Panel A. T cells from Sca1KO mice serve as controls for the analyses, and define the cutoff point for Sca1 detection in wt mice (naïve and day 7 p.i.). The numbers indicate the percentage of all spleen cells in each quadrant. Panel B. Similar analyses were carried out in mice lacking the receptor either for type I IFNs (top row), for IFNγ (middle row) or for both receptors (DKO mice, bottom row). The data are representative of at least 2 independent experiments; and in all cases, there were at least 2 mice in the uninfected groups, and 7–16 mice in the infected groups.
Only virus-specific T cells retain high levels of Sca1 after infection is cleared
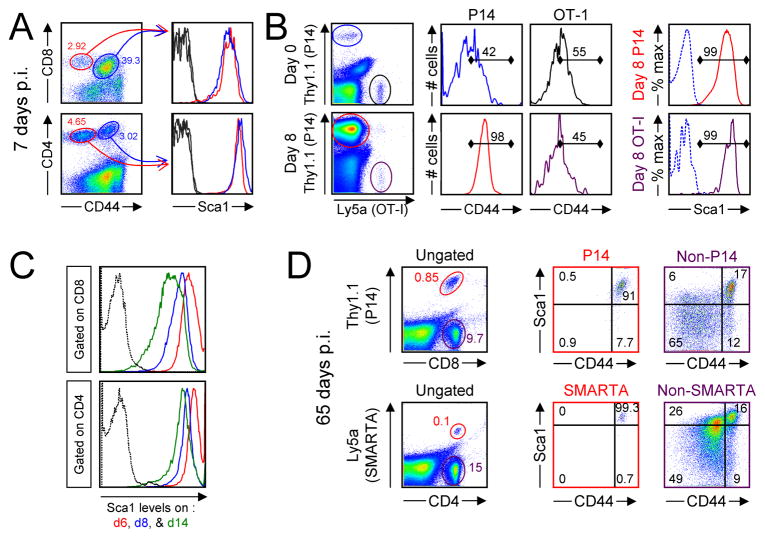
LCMV infection induces a profound expansion of virus-specific T cells, but most of the naïve T cells in a wildtype mouse are not LCMV-specific and, as shown in many prior studies, these cells do not significantly respond to the infection. Thus, the up-regulation of Sca1 on essentially all T cells was surprising to us, and suggested that even non-virus-specific T cells, which are considered non-responders by several other criteria, might up-regulate Sca1 during LCMV infection. To investigate this, we identified antigen-experienced cells by staining for CD44. As shown in Figure 2A, at 7 days after LCMV infection ~93% of CD8+ T cells, and ~40% of CD4+ T cells, are CD44hi. This is consistent with the more dramatic expansion of virus-specific CD8+ T cells compared to their CD4+ counterparts. For both CD8+ and CD4+ T cells, we gated on the CD44lo and CD44hi populations (red and blue ovals respectively) and determined their Sca1 expression. As shown, Sca1 expression was high on all T cells, regardless of their CD44 status. It might be argued that CD44 expression is not a definitive criterion of virus specificity so, to address this, we next evaluated Sca1 expression on two different populations of TcR-transgenic CD8+ T cells, only one of which is LCMV-specific. P14 mice contain CD8+ TcR-transgenic T cells that are specific for the GP33-41 epitope of LCMV, and OT-1 mice contain CD8+ TcR-transgenic T cells specific for an ovalbumin epitope [28]. Equal numbers of these cells were mixed, and adoptively transferred into wildtype mice. 2 days later, some of the mice were infected with LCMV, and 8 days later the extent of Sca1 expression was determined on each of the cell populations in infected and uninfected mice. As shown in Figure 2B, the input (naïve, day 0) P14 cells (Thy1.1) and OT-I cells (Ly5a) were similar in number (top row dotplot, blue and black ovals respectively) and similar in CD44 levels (~50% CD44hi). By 8 days p.i., (bottom row dotplot) the P14 cells (red oval) were far more abundant than the OT-1 cells (purple oval); this is as expected, because only the P14 cells will have divided in response to virus infection. Consistent with the P14 cells’ being antigen-experienced, ~98% are CD44hi at 8 days p.i., while the proportion of CD44hi OT-I cells has not increased. However, at 8 days p.i., ~99% of both populations of TcR-transgenic CD8+ T cells express high levels of Sca1 (Figure 2B, right column). Thus, Sca1 is upregulated on all CD8+ T cells, regardless of their antigen specificity; and this upregulation requires neither antigen stimulation nor extensive cell division.
Figure 2. During infection, Sca1 is upregulated on all T cells, but only virus-specific cells remain Sca1hi in the memory phase.
Panel A. Wt mice were infected with LCMV and, 7 days later, splenocytes were isolated and stained for expression of CD4, CD8, CD44 and Sca1. For both CD4+ and CD8+ T cells, cells were gated on CD44lo and CD44hi populations (left dotplots, red and blue ovals respectively), and those populations were analyzed for Sca1 expression (right histograms, color-coded as for the ovals). The dotted grey lines show signal from isotype control antibody. The data shown are representative of 3 separate experiments, with a total of 7 mice analyzed. Panel B. Equal numbers of CD8+ P14 cells (Thy1.1) and CD8+ OT-I cells (Ly5a) (5×105 each) were mixed and transferred into C57BL/6 mice. Two days later, some of the recipient mice were infected with LCMV. 8 days later, spleen cells were harvested from both infected and uninfected mice, and were analyzed by flow cytometry. The TcR-transgenic CD8+ T cells are enclosed in color-coded ovals (left column), and their CD44 expression, also color-coded, is shown in the two middle columns. Finally, Sca1 expression of both populations in virus-infected mice is shown (right column); the dotted line indicates isotype control staining. Representative data from one of 3 experiments are shown, and a total of 6 mice were analyzed. Panel C. At days 6, 8, and 14 days after infection (2 mice per time point), spleen cells were harvested and stained for Sca1 expression. The histograms are gated on either CD8+ or CD4+ T cells, and show the level of Sca1 fluorescence at each of the three time points for an individual mouse; in each case, the second mouse showed a similar phenotype. The dashed black line represents the isotype control staining on day 6 cells. Panel D. P14/Thy1.1 spleen cells or SMARTA/Ly5a+ spleen cells were mixed and were adoptively transferred into three C57BL/6 mice (Thy1.2, Ly5a−), and after 2 days, the recipient mice were given LCMV. 65 days later, the spleen cells from the immune recipients were stained to identify the TcR-transgenic T cells and their expression of Sca1. The ovals in the top dotplot show the P14 CD8+ T cells (red oval) and the host CD8+ cells (purple oval). The numbers in the dot plots indicate the percentage of all splenocytes that are within the ovals. After gating on these two populations, the adjacent histograms show the expression of Sca1 and CD44 on the P14 cells and on the host cells. The numbers in the histograms indicate the percentages of gated T cells in each quadrant. The lower dot plots show similar analyses for SMARTA CD4+ memory T cells and the host CD4+ T cells. The data shown are from 1 of 5 experiments, comprising a total of 12 recipient mice.
Next, we evaluated the kinetics of Sca1 expression in more detail. Wt C57BL/6 mice were infected with LCMV, and were sacrificed 6, 8, or 14 days later. As shown in Figure 2C, Sca1 peaked in abundance at day 6 on both CD4+ and CD8+ T cells. Thereafter, expression levels fell somewhat, although not to the low levels observed on naïve cells. Notably, Sca1 expression was monophasic at all time points, consistent with our observation that, during the primary immune response, virus-specific cells cannot readily be distinguished from non-specific cells by their level of Sca1 expression. However, the range of Sca1 expression (as measured by the breadth of signal on the x axis) appeared to be slightly greater at day 14 (green line) than at day 6 or day 8 (red and blue lines respectively). This suggested that, as the antiviral T cell response entered the contraction phase, the extent to which Sca1 expression declined (or was maintained) might vary among different populations of T cells. To evaluate this possibility on both CD8+ and CD4+ T cells, we once again made use of P14 TcR-transgenic cells, this time in combination with cells taken from SMARTA mice, which contain CD4+ T cells that are specific for the LCMV GP61-80 epitope [29]. A mixture of P14 cells (Thy1.1) and SMARTA cells (Ly5a) were adoptively transferred into wt mice (Thy1.2, Ly5b). The mice then were infected with LCMV and, 65 days later, Sca1 expression was measured on TcR-transgenic (P14 and SMARTA, donor) cells and on non-TcR-transgenic (host) T cells. Analyses of CD8+ T cells (Figure 2D, top row) showed that ~98.7% of P14 cells were CD44hi (consistent with their being virus-specific memory cells), and almost all of those cells also were Sca1hi. This indicates that memory T cells retain the high level of Sca1 expression that was present on their predecessors during the acute phase of the immune response. In contrast, at 65 days p.i. the host CD8+ T cells (non-P14, purple oval and dotplot) were much more heterogeneous. This population comprises a mixture of naïve cells (most of which are not virus-specific) and of virus-specific memory cells. Within this endogenous CD8+ T cell population, most of the CD44hi cells (many of which will be LCMV-specific memory cells) were also Sca1hi; and most of the Sca1hi cells were CD44hi. However, the single most abundant population (65% of the endogenous CD8+ T cells) was CD44lo/Sca1lo. Similar observations apply to the CD4+ memory T cells (Figure 2D, bottom row): 100% of SMARTA cells are CD44hi and, of these, almost all are Sca1hi; and again the endogenous (host) cells are more heterogeneous, with the largest single population being CD44lo/Sca1lo.
From the foregoing expression data we conclude that, during a virus infection, (i) Sca1 is up-regulated at early times post-infection on all T cells, regardless of antigen specificity (Figure 1, Figure 2A & B); (ii) Sca1 expression on non-virus-specific T cells declines as the response enters the contraction phase (Figure 2C); but (iii) Sca1 remains high on virus-specific T cells well into the memory phase (Figure 2D). Thus, Sca1 protein may be a marker of T cell activation and/or memory; but, more importantly, does the protein subserve a required function in T cell development?
Sca1 deficiency causes no major change in the magnitude or kinetics of the primary T cell response
First, the magnitude and kinetics of endogenous antiviral CD4+ and CD8+ T cell responses in mice deficient in Sca1 [22] were determined, and were compared to those observed in wildtype mice on the same genetic background (C57BL/6). The results are shown in Figure 3. Epitope-specific responses were measured by intracellular cytokine staining (ICCS) at days 6, 8 and 14 post-infection for 3 dominant epitopes (one for CD4+ T cells, GP61-80, panel A; and two for CD8+ T cells, GP33-41 and NP396-404, panels B and C). In addition, responses were measured at day 8 p.i. (the peak of the response) for two additional, subdominant, CD8+ T cell epitopes (GP276-286 and NP205-212, panels D and E). The responses in Sca1KO mice closely paralleled those found in wt animals, and none of the differences were statistically significant; for example, comparison of the day 6 GP33-specific response (Figure 3B) showed a p value >0.27; and a similar comparison of the CD4+ response at day 6 (Figure 3A) also had a p value of >0.27. Of the 11 pairwise comparisons in Figure 3, 8 of the T cell responses are marginally higher in Sca1KO mice compared to wt mice, including all 5 day 8 comparisons. This raised the possibility that Sca1 deficiency caused a small degree of hyper-responsiveness than was not significant for any one epitope-specific population, but that might be significant when considered at the level of the overall antiviral T cell response. Consequently, the total numbers of antiviral T cells in spleens of wt and Sca1KO mice at d8 post infection were calculated and compared; no significant difference was found (p>0.16, wt n=7, Sca1KO n=8, data not shown). Thus, despite the fact that Sca1 expression on T cells appears to be regulated over the course of the immune response (Figure 1), these data (Figure 3) indicate that deficiency in Sca1 expression does not significantly impact the abundance of antiviral CD4+ or CD8+ T cells over the course of the primary immune response.
Figure 3. Sca1 deficiency causes no significant change in the magnitude or kinetics of the primary CD4+ or CD8+ T cell response.
ICCS assay was used to quantify the number of epitope-specific T cells found in Sca-1+ (wt) and Sca1KO mice (2–6 mice per group at each time point). For the GP61-80 CD4+ T cell epitope (panel A) and for the two dominant CD8+ T cells epitopes (panels B & C), analyses were carried out at 6, 8 and 14 days after infection. For the remaining two CD8+ T cell epitopes, analyses were limited to the day 8 time point (panels D & E). The bar graphs show the total number of IFNγ+ CD8+ T cells or CD4+ T cells found in the spleen. In all cases, the data are shown as the mean +/− SEM. T cell responses in wt and Sca1KO were compared as detailed in the text, and in no case was a statistically-significant difference observed (Student’s t test).
Figure 8. Sca1KO mice contain normal numbers of CD4+ and CD8+ memory cells.
The absolute numbers of virus-specific T cells in the spleens of 4 immune wildtype and 4 Sca1KO mice was determined by ICCS assay 60 days after acute infection with LCMV. The spleen cells were stimulated with the indicated peptides, and the frequency of IFNγ+ T cells was determined by flow cytometry.
Responses to low dose, high dose, or persistent infection, are similar in Sca1-deficient and WT mice
In the experiments presented above the mice were infected with LCMV Armstrong strain, a virus that is rapidly cleared by mice; in this situation the virus-specific T cells are transiently exposed to a relatively high dose of antigen. It was conceivable that Sca1 might have some effect on T cell responses when the antigen load was lower, and/or during chronic antigen stimulation. To evaluate these possibilities, groups of WT and Sca1KO mice were infected with two different doses of LCMV-Armstrong, over a 1000-fold range, or with LCMV-Clone13, a viral variant that establishes persistent infection. T cell responses were compared eight days later by ICCS assay (Figure 4). The frequency of virus-specific CD8+ T cells and CD4+ T cells was very similar between the WT and Sca1KO groups. When given 200 PFU or 2×105 PFU of LCMV-Armstrong, both groups of mice generated robust and overlapping primary T cell responses, indicating that Sca1 is not required, even when the mice are challenged with low amounts of virus. WT mice that were given LCMV-clone13 generated low numbers of functional virus-reactive T cells (Figure 4), as expected [30], and a weakened response was also found in the Sca1KO mice (Figure 4). Moreover, T cell responses continued to be diminished in the LCMV-Clone13 infected mice during the persistent phase, and there were no differences between the two groups of mice in the frequency or number of virus-reactive T cells or in the viral load during that time (data not shown). So, Sca1 does not appear to impact primary T cell responses following low-dose or high-dose infection, nor does it impact the deletion of cells when LCMV persists.
Figure 4. T cell responses to low dose and high dose infection, and to persistent infection, are similar in Sca1-deficient and WT mice.
Groups of 2–3 wt or Sca1KO mice were given 200 PFU LCMV-Armstrong, 2×105 PFU LCMV Armstrong, or 2×106 PFU LCMV clone13. ICCS was used to quantify the epitope-specific T cell response in the spleen 8 days after infection. Panel A. The ovals within the dot plots show CD8+ T cells that made IFNγ in response to the indicated peptides; the numbers indicate the frequency of these cells among all spleen cells. Panel B. The ovals within the dot plots show CD4+ T cells that made IFNγ in response to stimulation with GP61-80 or no peptide, and the numbers indicate the frequency of these cells among all spleen cells.
Responding T cells in Sca1KO mice show normal CD44 phenotype and cytokine function
Next, we analyzed the requirement for Sca1 in regulating selected phenotypic and functional attributes of T cells. As shown in Figure 5A, in naïve (day 0) wt mice, 60–70% of CD8+ and CD4+ T cells were CD44lo. Very similar proportions were observed in uninfected Sca1KO mice, consistent with an earlier report [22]. At 8 days after LCMV infection of wt mice, most CD8+ and CD4+ T cells (~96% and ~62% respectively) expressed high amounts of CD44; similar proportions of CD8+ and CD4+ cells became CD44hi in Sca1KO mice (~96% and ~55% respectively). Thus, the proportion of T cells acquiring high levels of CD44 is unaffected by the absence of Sca1. However, it remained possible that Sca1 deficiency might alter the relative proportions of different epitope-specific populations within the overall virus-specific pool of cells. To investigate this possibility, four distinct epitope-specific CD8+ T cell populations were enumerated by ICCS at day 8 after infection (Figure 5B). The frequencies of all four populations were similar in wildtype and Sca1KO mice, indicating that Sca1 expression does not markedly impact the epitope hierarchy of the response. These findings are based on a functional assay (ICCS), suggesting that at least one CD8+ T cell effector function (in this case, IFNγ production) is unaffected by the absence of Sca1. This conclusion is supported by the fact the amounts of IFNγ produced by the responding cells (as measured by geometric mean fluorescence intensities; gMFI) are similar between wt and Sca1KO CD8+ T cell populations. Finally, similar analyses were applied to CD4+ T cells (Figure 5C). At 8 days p.i., wildtype and Sca1KO mice contained comparable frequencies of IFNγ-producing CD4+ T cells, and also of IL-2-producing cells. Within each of the population pairs (the IFNγ pair, and the IL-2 pair), the gMFI were similar indicating that Sca1 is not required for the production of either of these cytokines. We considered the possibility that virus-specific T cells from Sca1KO mice may be less sensitive to low amounts of cognate peptide – that is, have lower functional avidity [31]. Therefore, the functional avidity of the memory T cells isolated from day 60 infected mice was determined by flow cytometry. The dose response of the wt and Sca1KO T cells was overlapping (data not shown), indicating that Sca1 expression does not regulate the ability of T cells to recognize, and respond to, low quantities of peptide.
Figure 5. Responding T cells in Sca1KO mice show normal CD44 phenotype and cytokine function.
Wildtype and Sca1KO mice were given LCMV-Armstrong, and the splenic T cell responses of these mice were quantified by flow cytometry. Panel A. The dot plots and associated matrices show the proportion of CD8+ T cells or CD4+ T cells that express CD44. The numbers in the matrices indicate the percentage of spleen cells in each quadrant of the adjacent dotplot. Panel B. IFNγ production by epitope-specific CD8+ T cells at 8 days after infection were determined by ICCS. The numbers in the dot plots identify the fraction of CD8+ T cells (as a percentage of all spleen cells) that made IFNγ upon ex vivo stimulation with the indicated peptide. Panel C. GP61-80-specific CD4+ T cells were similarly quantified by ICCS. The numbers within the upper set of dot plots identify the cells that made IFNγ (as a percentage of all spleen cells); and the numbers in the lower set of dot plots show the percentage of cells that made IL-2. The data are representative of 13 mice analyzed, in 5 independent experiments.
Sca1KO mice develop normal in vivo cytotoxic T cell activity, and rapidly control LCMV infection
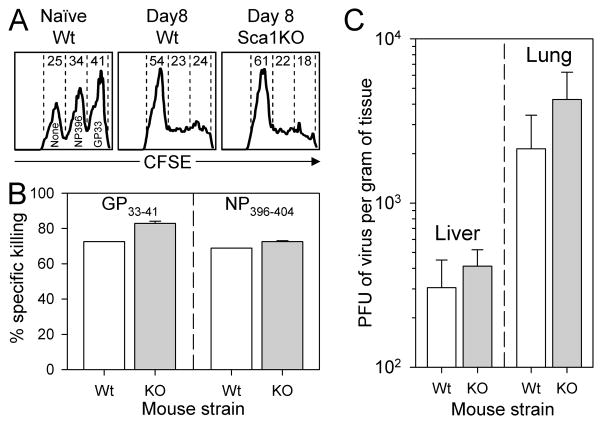
Cytotoxic CD8+ T cells are required to eliminate LCMV infection [32–34], and defects in CTL activity allow the virus to establish a persistent infection, sometimes for the lifetime of the animal. Thus, we next determined the capacity of Sca1KO mice to (i) demonstrate epitope-specific cytotoxicity, using an in vivo cytotoxicity assay; and (ii) to control, and ultimately eradicate, LCMV infection. Three populations of CFSE-labeled potential target cells were prepared, differing in the level of CFSE staining (low, medium, high), and also in their peptide-coating (none, NP396-404 or GP33-41). The potential target cells were mixed, and inoculated into wt mice or Sca1KO mice at 8 days after LCMV infection, a time point at which cytotoxic T cells should be abundant. As a control, the cells also were inoculated into naïve wt mice. 1 hour later, the mice were sacrificed, splenocytes were harvested, and the three populations of target cells were enumerated based on their CFSE fluorescence. In naïve animals, all three populations were abundant (Figure 6A, left histogram). In the infected mice, CFSElo cells, which were not coated with epitope peptide, remained abundant, as expected. In contrast, both the CFSEmed and CFSEhi populations (coated respectively with the NP396-404 or GP33-41 peptides) were largely absent, in the infected wt and Sca1KO mice (Figure 6A, middle and right panels respectively). Calculation of the percentage of specific killing for each of the peptide-coated populations showed that Sca1KO mice developed highly-effective cytotoxic responses against both GP33-41 and NP396-404 epitopes (Figure 6B). Consistent with their generating strong virus-specific cytotoxic responses, virus titers in the liver and lung of Sca1KO mice were similar to those observed in wildtype mice at at 8 days p.i. (Figure 6C), and both strains of mice completely eradicated LCMV at later time points (data not shown). Thus, Sca1KO mice develop strong protective antiviral immune responses.
Figure 6. Sca1KO mice develop normal in vivo cytotoxic T cell activity, and rapidly control LCMV infection.
The ability of infected Sca1KO mice to generate CTL and eliminate virus infection was measured 8 days after infection. Panel A. An in vivo CTL assay was used to determine how efficiently Sca1KO mice eliminate peptide-coated target cells. Target cells were coated with GP33-41 peptide, NP396-404 peptide, or no peptide; and then were labeled with differing concentrations of CFSE so that they would fluoresce at different intensities. The labeled cells were transferred into the wt mice or Sca1KO mice, as indicated above each histogram. 1 hour later, the spleens from these mice were harvested, and the relative loss of each target cell population was determined by flow cytometry. The histograms are gated on CFSE+ spleen cells, and the numbers indicate the percentage of these present in each peak. Panel B. The bar graphs indicate the percent specific killing of the peptide-coated target cells in 1–2 mice per group. Panel C. Plaque assay was used to quantify infectious virus in the liver and lung of 3–4 mice per group; the graphs show plaque forming units (PFU) per gram of tissue. p values (Student’s t test) are >0.28 (liver) and >0.22 (lung).
Memory cell frequencies and absolute numbers are similar in wt and Sca1KO mice
Memory lymphocytes are the cornerstone of adaptive immunity, and the frequency and number of memory T cells are dependent upon their early differentiation, and their cytokine-mediated homeostatic maintenance [35, 36]. Sca1 is highly expressed on memory T cells (Figure 2), and it is plausible that its expression plays some role in memory development or maintenance, as others have proposed [14, 16]. To determine whether the absence of Sca1 impacts T cell memory, the frequencies and absolute numbers of several populations of epitope-specific CD8+ and CD4+ T cells were quantified in wt and Sca1KO mice at 2–3 months after LCMV infection. The frequencies of virus-specific CD8+ T cells that made IFNγ or IL-2 when re-stimulated ex vivo were similar in wt and Sca1KO mice, for 5 different epitope-specific populations (Figure 7A); the same was true for 2 epitope-specific populations CD4+ T cells (Figure 7B). The gMFIs for IFNγ and for IL-2 were similar in all pairs (wt versus Sca1KO) of T cell populations, indicating that Sca1 deficiency has little impact on these memory cell functions. The similar frequencies of memory cells were reflected in the absolute numbers that were present (Figure 8). These data indicate that the expression of Sca1 is not required during early T cell differentiation or during the maintenance phase of CD4+ and CD8+ T cell memory. To determine whether Sca1 expression on memory T cells is needed for biologically-effective recall responses, wt and Sca1KO mice that had been inoculated with LCMV 9 weeks earlier were re-challenged with LCMV-Armstrong (2×106 PFU). Six days later, virus titers had been reduced to undetectable levels in both strains of mice.
Figure 7. Memory cell frequencies are similar in wt and Sca1KO mice.
T cell memory in Sca1KO and wt mice was quantified by ICCS 50–60 days after infection. Panel A. Epitope-specific CD8+ T cells; and panel B, epitope-specific CD4+ T cells. In each panel, the numbers within the left set of dot plots identify the epitope-specific IFNγ+ T cells, and the numbers in the right set of dot plots identify memory IL-2+ T cells. The numbers represent the fraction of cytokine-producing cells as a percentage of all spleen cells. Data are representative of 6 mice per group, analyzed in 3 independent experiments.
Discussion
The data presented herein show that Sca1 is expressed on many naïve T cells, and that the level of constitutive expression may be up-regulated by type I IFNs. Furthermore, Sca1 is strongly induced on all T cells during acute LCMV infection, and its expression is sustained on virus-specific memory T cells long after infection has been cleared. In addition, we demonstrate that Sca1KO mice generate normal numbers of antiviral T cells that are capable of producing IL-2 and IFNγ in response to viral peptide stimulation, and can kill peptide coated targets cells in vivo. The near-equivalence of the T cell responses mounted by wt and Sca1KO mice is retained after both low-dose and high-dose infections, and following inoculation of a variant virus that establishes persistent infection. Finally, memory T cells developed normally in these mice, and the cells were sustained over time. Taken together our data suggest that – in contrast to several published reports, most of which relied on in vitro analyses – Sca1 is dispensable for T cell differentiation and for the homeostatic maintenance of T cell memory. Therefore, our findings and broad conclusions conflict with previous suggestions that Sca1 signaling is a key factor that regulates T cell function. Interestingly, Sca1 is not present in the human genome, which suggests that there was no selection for its maintenance during subsequent speciation events. However, these conclusions must be tempered with caution. For example, we show here only that Sca1 is not required for normal T cell responses to virus infection; it remains possible that the Sca1 protein does play a role in regulating T cells but that, in its absence, other proteins provide compensatory functions that obscure the effects of Sca1 deficiency. Furthermore, we cannot exclude the possibility that Sca1 exerts subtle effects on T cell numbers and/or function; we discuss several possible effects, and other caveats, below.
First, we show here that Sca1KO mice generate normal numbers of T cells by day 8 after infection, implying that Sca1 probably does not profoundly limit cell division. However, we did not directly measure cell division rates, and thus we cannot exclude the possibility that Sca1 exerts two opposing effects in wt mice in vivo; one negative (that limits cell division), and one positive (that, for example, may decrease the rate of cell death). Under such a circumstance, Sca1KO mice may have slightly more T cell proliferation (as predicted by the earlier studies) counterbalanced by increased cell death, resulting in T cell numbers that are similar to those observed in wildtype mice. Second, most of our studies presented herein focused on T cells that were isolated from the spleen. However, Sca1 is expressed on certain non-immune cells, such as intestinal epithelium, where its activation can alter chemokine expression [37]. This raises the possibility that T cell trafficking could be altered in the absence of Sca1 signaling. However, we found that the antiviral CD8+ T cells generated in Sca1KO mice controlled infection in peripheral tissues (liver and lung) as efficiently as did the cells in wt mice (Figure 6C), suggesting that T cell trafficking (at least to these organs) was not dramatically impaired. Third, CD4+ T cells play an important role in controlling many antibody responses, but these have not been evaluated in Sca1KO mice. Possibly relevant to this is the difference that we observed in the basal expression of Sca1 on naïve T cells in wt mice; many naïve CD8+ T cells showed very low expression, whereas most naïve CD4+ T cells showed a higher level of expression (Figure 1). The ligand of Sca1 is unknown; current evidence suggests that the ligand is expressed on B cells, and may be CD22 [38]. Thus, one might speculate that the increased amount of Sca1 on naïve CD4+ T cells could improve their local association with B cells in the lymphoid organs, poising them to respond to exogenous antigens presented by these cells. Interestingly, B cells express Sca1 following some forms of activation [39], and the amount of Sca1 expression on B cells is associated with the severity of disease in lupus prone mice [40]. Moreover, antibody-producing cells tend to be Sca-1+ and those B cells that are not making antibody are Sca1− [39], consistent with Sca1-dependent signals enhancing B cell activation and differentiation. Taken together with the evidence that B cells express a ligand for Sca1, these data suggest that complex intercellular interactions may be mediated by this molecular pair. These interactions could be particularly important for immunity against infections that are prone to persist, or infections where B cells play a key role in defense.
In conclusion: it remains possible that Sca1 does contribute to some aspects of T cell biology, but this study provides definitive evidence that this protein is not required for the development of normal CD4+ and CD8+ T cell responses to acute and chronic virus infections.
Materials and Methods
Mice and virus
C57BL/6 mice were purchased from the breeding colony at The Scripps Research Institute (TSRI). Sca1KO mice [22] that were backcrossed 10 generations to C57BL/6 were kindly given to us by Dr. William Stanford, Division of Comparative Medicine, University of Toronto. SMARTA TcR-transgenic mice [29] specific for the I-Ab LCMV epitope GP61-80 were crossed to C57BL/6.Ly5a mice (B6.SJL-PtprcaPep3b/BoyJ) to generate SMARTA.Ly5a mice [41]. P14 TcR-transgenic mice specific for the LCMV epitope GP33-41 on the H-2b background [42] were crossed to B6.Ly5a mice to generate the P14.Ly5a strain [43]. OT-I mice [28] were obtained from Dr. Charles Surh at TSRI. IFNαβRKO mice [originally described in ref 44, and subsequently backcrossed onto the B6 background] were obtained from Dr. Charles Surh (TSRI). IFNγRKO mice backcrossed onto the B6 background (strain B6.129S7-Ifngrtm1Agt/J) were purchased from Jackson labs (Bar Harbor, ME). Double KO mice were acquired from Dr. Bruce Beutler (TSRI) and were analyzed genotypically (PCR) and phenotypically (flow cytometry) before use. Mice were infected by intraperitoneal administration of the indicated doses of LCMV (Armstrong strain), or intravenous administration of LCMV clone13, a viral variant that establishes persistent infection when inoculated into adult mice. Quantitation of virus in the tissues was done by plaque assay on Vero cell monolayers. All animal experiments were approved by TSRI Animal Care and Use Committee and were carried out in accordance with national regulations.
Flow cytometry
The intracellular staining assay was performed as described previously [45]. In brief: spleen cells were cultured for 5 hours in the presence of Brefeldin-A (1μg/ml) with or without peptide (1μg/ml). The cells were surface stained using antibodies described below, fixed, and permeabilized using the IC Fixation Buffer and IC Permeabilization buffers from eBioscience.com (San Diego, CA). The permeabilized cells were stained using fluorescent antibodies against IFNγ, TNF, or IL-2. Anti-CD4 (clone RN4-5), anti-CD8 (clone 53-6.7), anti-CD44 (clone IM&), anti-Ly5a (Ly5.1, clone A20), and Rat-IgG2a,K isotype control were all purchased from eBioscience. The anti-Sca1 (Sca1, clone D7) was purchased from BD-Biosciences. Cell staining was analyzed by four-color flow cytometry at TSRI core facility using a BD Biosciences FACScalibur and FloJo software (Tree Star).
In vivo cytotoxicity
Naïve C57BL/6 splenocytes were incubated with 4μM, 1μM, or 0.25μM CFSE (5,6-carboxy-fluorescein diacetate succinimidyl ester; Molecular Probes). The CFSEhi cells were coated with the GP33-41 peptide, the CFSEmed were coated with the NP396-404 peptide, and the CFSElo cells were left uncoated. After extensive washing, equal numbers of the CFSEhi, CFSEmed, and CFSElo cells were mixed, and transferred into either naïve recipients or day 8-infected mice. After 1 hour, splenocytes were harvested and the abundances of each of the three populations of CFSE+ cells were identified by flow cytometry; the percent killing was calculated as 100−{[(% peptide coated in infected/% uncoated in infected)/(% peptide coated in uninfected/% uncoated in uninfected)] × 100}.
Acknowledgments
The authors thank Annette Lord for expert administrative assistance. This is manuscript number 19568 from the Scripps Research Institute. This work was supported by NIH R01 awards AI052351, AI027028 and AI077607 (to JLW) and AI074862 (to JKW).
Footnotes
Conflict of interest.
The authors declare that there are no conflicts of interest.
References
- 1.Holmes C, Stanford WL. Concise review: stem cell antigen-1: expression, function, and enigma. Stem Cells. 2007;25:1339–1347. doi: 10.1634/stemcells.2006-0644. [DOI] [PubMed] [Google Scholar]
- 2.Yutoku M, Grossberg AL, Pressman D. A cell surface antigenic determinant present on mouse plasmacytes and only about half of mouse thymocytes. J Immunol. 1974;112:1774–1781. [PubMed] [Google Scholar]
- 3.Rock KL, Reiser H, Bamezai A, McGrew J, Benacerraf B. The LY-6 locus: a multigene family encoding phosphatidylinositol-anchored membrane proteins concerned with T-cell activation. Immunol Rev. 1989;111:195–224. doi: 10.1111/j.1600-065x.1989.tb00547.x. [DOI] [PubMed] [Google Scholar]
- 4.Shevach EM, Korty PE. Ly-6: a multigene family in search of a function. Immunol Today. 1989;10:195–200. doi: 10.1016/0167-5699(89)90324-1. [DOI] [PubMed] [Google Scholar]
- 5.Murali-Krishna K, Ahmed R. Cutting edge: naïve T cells masquerading as memory cells. J Immunol. 2000;165:1733–1737. doi: 10.4049/jimmunol.165.4.1733. [DOI] [PubMed] [Google Scholar]
- 6.Palfree RG, Dumont FJ, Hammerling U. Ly-6A.2 and Ly-6E.1 molecules are antithetical and identical to MALA-1. Immunogenetics. 1986;23:197–207. doi: 10.1007/BF00373821. [DOI] [PubMed] [Google Scholar]
- 7.Toulon M, Palfree RG, Palfree S, Dumont FJ, Hammerling U. Ly-6 A/E antigen of murine T cells is associated with a distinct pathway of activation. Requirements for interferon and exogenous interleukin 2. Eur J Immunol. 1988;18:937–942. doi: 10.1002/eji.1830180616. [DOI] [PubMed] [Google Scholar]
- 8.van de Rijn M, Heimfeld S, Spangrude GJ, Weissman IL. Mouse hematopoietic stem-cell antigen Sca-1 is a member of the Ly-6 antigen family. Proc Natl Acad Sci USA. 1989;86:4634–4638. doi: 10.1073/pnas.86.12.4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Bragt MP, Ciliberti N, Stanford WL, de Rooij DG, van Pelt AM. LY6A/E (SCA-1) expression in the mouse testis. Biol Reprod. 2005;73:634–638. doi: 10.1095/biolreprod.105.040303. [DOI] [PubMed] [Google Scholar]
- 10.Yeh ET, Reiser H, Benacerraf B, Rock KL. The expression, function, and ontogeny of a novel T cell-activating protein, TAP, in the thymus. J Immunol. 1986;137:1232–1238. [PubMed] [Google Scholar]
- 11.Spangrude GJ, Aihara Y, Weissman IL, Klein J. The stem cell antigens Sca-1 and Sca-2 subdivide thymic and peripheral T lymphocytes into unique subsets. J Immunol. 1988;141:3697–3707. [PubMed] [Google Scholar]
- 12.Bamezai A, Palliser D, Berezovskaya A, McGrew J, Higgins K, Lacy E, Rock KL. Regulated expression of Ly-6A.2 is important for T cell development. J Immunol. 1995;154:4233–4239. [PubMed] [Google Scholar]
- 13.Yang L, Kobie JJ, Mosmann TR. CD73 and Ly-6A/E distinguish in vivo primed but uncommitted mouse CD4 T cells from type 1 or type 2 effector cells. J Immunol. 2005;175:6458–6464. doi: 10.4049/jimmunol.175.10.6458. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Joe G, Hexner E, Zhu J, Emerson SG. Host-reactive CD8+ memory stem cells in graft-versus-host disease. Nat Med. 2005;11:1299–1305. doi: 10.1038/nm1326. [DOI] [PubMed] [Google Scholar]
- 15.Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111:837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- 16.Yu XZ, Anasetti C. Memory stem cells sustain disease. Nat Med. 2005;11:1282–1283. doi: 10.1038/nm1205-1282. [DOI] [PubMed] [Google Scholar]
- 17.Rock KL, Yeh ET, Gramm CF, Haber SI, Reiser H, Benacerraf B. TAP, a novel T cell-activating protein involved in the stimulation of MHC-restricted T lymphocytes. J Exp Med. 1986;163:315–333. doi: 10.1084/jem.163.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marmor MD, Bachmann MF, Ohashi PS, Malek TR, Julius M. Immobilization of glycosylphosphatidylinositol-anchored proteins inhibits T cell growth but not function. Int Immunol. 1999;11:1381–1393. doi: 10.1093/intimm/11.9.1381. [DOI] [PubMed] [Google Scholar]
- 19.Ivanov V, Fleming TJ, Malek TR. Regulation of nuclear factor-kappa B and activator protein-1 activities after stimulation of T cells via glycosylphosphatidylinositol-anchored Ly-6A/E. J Immunol. 1994;153:2394–2406. [PubMed] [Google Scholar]
- 20.Malek TR, Ortega G, Chan C, Kroczek RA, Shevach EM. Role of Ly-6 in lymphocyte activation. II. Induction of T cell activation by monoclonal anti-Ly-6 antibodies. J Exp Med. 1986;164:709–722. doi: 10.1084/jem.164.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henderson SC, Kamdar MM, Bamezai A. Ly-6A.2 expression regulates antigen-specific CD4+ T cell proliferation and cytokine production. J Immunol. 2002;168:118–126. doi: 10.4049/jimmunol.168.1.118. [DOI] [PubMed] [Google Scholar]
- 22.Stanford WL, Haque S, Alexander R, Liu X, Latour AM, Snodgrass HR, Koller BH, Flood PM. Altered proliferative response by T lymphocytes of Ly-6A (Sca-1) null mice. J Exp Med. 1997;186:705–717. doi: 10.1084/jem.186.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanson P, Mathews V, Marrus SH, Graubert TA. Enhanced green fluorescent protein targeted to the Sca-1 (Ly-6A) locus in transgenic mice results in efficient marking of hematopoietic stem cells in vivo. Exp Hematol. 2003;31:159–167. doi: 10.1016/s0301-472x(02)01021-4. [DOI] [PubMed] [Google Scholar]
- 24.Ito CY, Li CY, Bernstein A, Dick JE, Stanford WL. Hematopoietic stem cell and progenitor defects in Sca-1/Ly-6A-null mice. Blood. 2003;101:517–523. doi: 10.1182/blood-2002-06-1918. [DOI] [PubMed] [Google Scholar]
- 25.Dumont FJ, Coker LZ. Interferon-alpha/beta enhances the expression of Ly-6 antigens on T cells in vivo and in vitro. Eur J Immunol. 1986;16:735–740. doi: 10.1002/eji.1830160704. [DOI] [PubMed] [Google Scholar]
- 26.Dumont FJ, Dijkmans R, Palfree RG, Boltz RD, Coker L. Selective up-regulation by interferon-gamma of surface molecules of the Ly-6 complex in resting T cells: the Ly-6A/E and TAP antigens are preferentially enhanced. Eur J Immunol. 1987;17:1183–1191. doi: 10.1002/eji.1830170816. [DOI] [PubMed] [Google Scholar]
- 27.Malek TR, Danis KM, Codias EK. Tumor necrosis factor synergistically acts with IFN-gamma to regulate Ly-6A/E expression in T lymphocytes, thymocytes and bone marrow cells. J Immunol. 1989;142:1929–1936. [PubMed] [Google Scholar]
- 28.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 29.Oxenius A, Bachmann MF, Zinkernagel RM, Hengartner H. Virus-specific MHC-class II-restricted TCR-transgenic mice: effects on humoral and cellular immune responses after viral infection. Eur J Immunol. 1998;28:390–400. doi: 10.1002/(SICI)1521-4141(199801)28:01<390::AID-IMMU390>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 30.Zajac AJ, Blattman JN, Murali-Krishna K, Sourdive DD, Suresh M, Altman JD, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slifka MK, Whitton JL. Functional avidity maturation of CD8+ T cells without selection of higher affinity TCR. Nat Immunol. 2001;2:711–717. doi: 10.1038/90650. [DOI] [PubMed] [Google Scholar]
- 32.Kagi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen KJ, Podack ER, Zinkernagel RM, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 33.Walsh CM, Matloubian M, Liu CC, Ueda R, Kurahara CG, Christensen JL, Huang MT, Young JD, Ahmed R, Clark WR. Immune function in mice lacking the perforin gene. Proc Natl Acad Sci USA. 1994;91:10854–10858. doi: 10.1073/pnas.91.23.10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kagi D, Seiler P, Pavlovic J, Ledermann B, Burki K, Zinkernagel RM, Hengartner H. The roles of perforin- and Fas-dependent cytotoxicity in protection against cytopathic and noncytopathic viruses. Eur J Immunol. 1995;25:3256–3262. doi: 10.1002/eji.1830251209. [DOI] [PubMed] [Google Scholar]
- 35.Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kondrack RM, Harbertson J, Tan JT, McBreen ME, Surh CD, Bradley LM. Interleukin 7 regulates the survival and generation of memory CD4 cells. J Exp Med. 2003;198:1797–1806. doi: 10.1084/jem.20030735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flanagan K, Modrusan Z, Cornelius J, Chavali A, Kasman I, Komuves L, Mo L, Diehl L. Intestinal epithelial cell up-regulation of LY6 molecules during colitis results in enhanced chemokine secretion. J Immunol. 2008;180:3874–3881. doi: 10.4049/jimmunol.180.6.3874. [DOI] [PubMed] [Google Scholar]
- 38.Pflugh DL, Maher SE, Bothwell AL. Ly-6 superfamily members Ly-6A/E, Ly-6C, and Ly-6I recognize two potential ligands expressed by B lymphocytes. J Immunol. 2002;169:5130–5136. doi: 10.4049/jimmunol.169.9.5130. [DOI] [PubMed] [Google Scholar]
- 39.Chen HC, Frissora F, Durbin JE, Muthusamy N. Activation induced differential regulation of stem cell antigen-1 (Ly-6A/E) expression in murine B cells. Cell Immunol. 2003;225:42–52. doi: 10.1016/j.cellimm.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 40.Kumar KR, Zhu J, Bhaskarabhatla M, Yan M, Mohan C. Enhanced expression of stem cell antigen-1 (Ly-6A/E) in lymphocytes from lupus prone mice correlates with disease severity. J Autoimmun. 2005;25:215–222. doi: 10.1016/j.jaut.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 41.Whitmire JK, Benning N, Whitton JL. Cutting Edge: Early IFN-γ signaling directly enhances primary antiviral CD4+ T cell responses. J Immunol. 2005;175:5624–5628. doi: 10.4049/jimmunol.175.9.5624. [DOI] [PubMed] [Google Scholar]
- 42.Pircher H, Burki K, Lang R, Hengartner H, Zinkernagel RM. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 1989;342:559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- 43.Whitmire JK, Tan JT, Whitton JL. Interferon-γ acts directly on CD8+ T cells to increase their abundance during virus infection. J Exp Med. 2005;201:1053–1059. doi: 10.1084/jem.20041463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muller U, Steinhoff U, Reis LFL, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- 45.Foster B, Prussin C, Liu F, Whitmire JK, Whitton JL. Detection of Intracellular Cytokines by Flow Cytometry. In: Coligan JE, Kruisbeek AM, Marguiles DH, Shevach EM, Strober W, editors. Current Protocols in Immunology. John Wiley & Sons; 2007. pp. 1–21. [DOI] [PubMed] [Google Scholar]