Summary
When attention is directed to a region of space, visual resolution at that location flexibly adapts, becoming sharper to resolve fine-scale details or coarser to reflect large-scale texture and surface properties [1]. By what mechanism does attention improve spatial resolution? An improved signal-to-noise ratio (SNR) at the attended location contributes [2], due to retinotopically-specific signal gain [3–10]. Additionally, attention could sharpen position tuning at the neural population level, so that adjacent objects activate more distinct regions of visual cortex. A dual mechanism involving both signal gain and sharpened position tuning would be highly efficient at improving visual resolution, but there is no direct evidence that attention can narrow the position tuning of population responses. Here, we compared the spatial spread of the fMRI BOLD response for attended versus ignored stimuli. The activity produced by adjacent stimuli overlapped less when subjects were attending at their locations versus attending elsewhere, despite a stronger peak response with attention. Our results show that even as early as primary visual cortex (V1), spatially-directed attention narrows the tuning of population-coded position representations.
Results
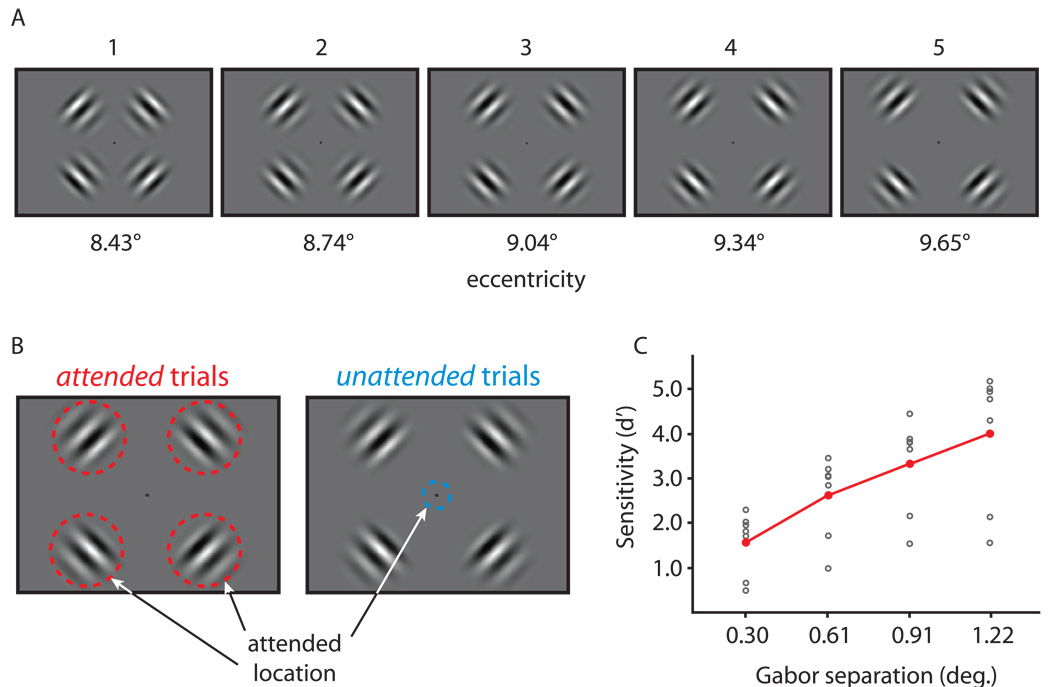
To test whether spatially-directed attention narrows population position tuning, we parametrically varied the position of a stimulus in an fMRI experiment and measured the degree of overlap in the neural activity patterns produced by adjacent stimuli. The stimuli were four flickering (7.5 Hz) Gabor patches, one in each visual quadrant. In five conditions, the Gabors were positioned at five different eccentricities ranging from 8.43 to 9.65 degrees from fixation (Fig. 1A). These five conditions, and a sixth fixation baseline condition, were presented in randomly ordered 10 second blocks during each 6 minute scanning run. In separate runs, subjects were instructed to attend to the Gabors to determine their eccentricity (attended trials) or to ignore the Gabors and attend to fixation for a counting task (unattended trials; Fig. 1B). During attended trials, subjects reported the Gabors’ eccentricity in a five alternative forced choice (5AFC) task (Fig. 1C) while maintaining attention at the Gabors’ positions to detect two small patterns presented randomly during each block. Subjects reported whether the two patterns were the same or different at the end of each block (group performance was 86.6 % correct +/− 3% sd). During unattended trials, subjects counted the occurrences of two different textures that appeared around the fixation point and reported which appeared most often during the block (group performance was 76.4 % correct +/− 4% sd). Besides the task instructions, the stimuli were identical for attended and unattended trials.
Figure 1.
Experimental stimuli and psychophysical performance. A) On each trial, four Gabor patches (0.38 cycles/deg, with 1.66 deg sd Gaussian envelopes) were presented at 8.430, 8.735, 9.039, 9.343, or 9.647 degrees from fixation. The Gabors flickered in counterphase at 7.5 Hz for the duration of each 10s block. B) In separate trials, subjects either attended to the fixation point to perform a counting task, or attended to the surrounding Gabors to judge their eccentricity and perform a texture matching task (see Supplemental Experimental Procedures for details). The same stimuli were presented on both types of trials – the only difference between the two was the locus of attention. Keeping the loci of attention in attended and unattended trials distinct, along with preventing subjects from achieving ceiling performance on the eccentricity judgment task, motivated the peripheral presentation of the Gabor stimuli. C) On attended trials, subjects made a 5AFC discrimination on the eccentricity of the Gabors. Sensitivity (d') is plotted against the spatial separation between Gabor centroids (individual subject data is plotted in gray, averaged group data is plotted in red). The positive slope indicates that while subjects often mistook the presented eccentricity for an adjacent one, they rarely mistook it for an eccentricity that was three or four increments away. Thus, our stimuli sampled in the dynamic range of subjects' psychophysical discrimination.
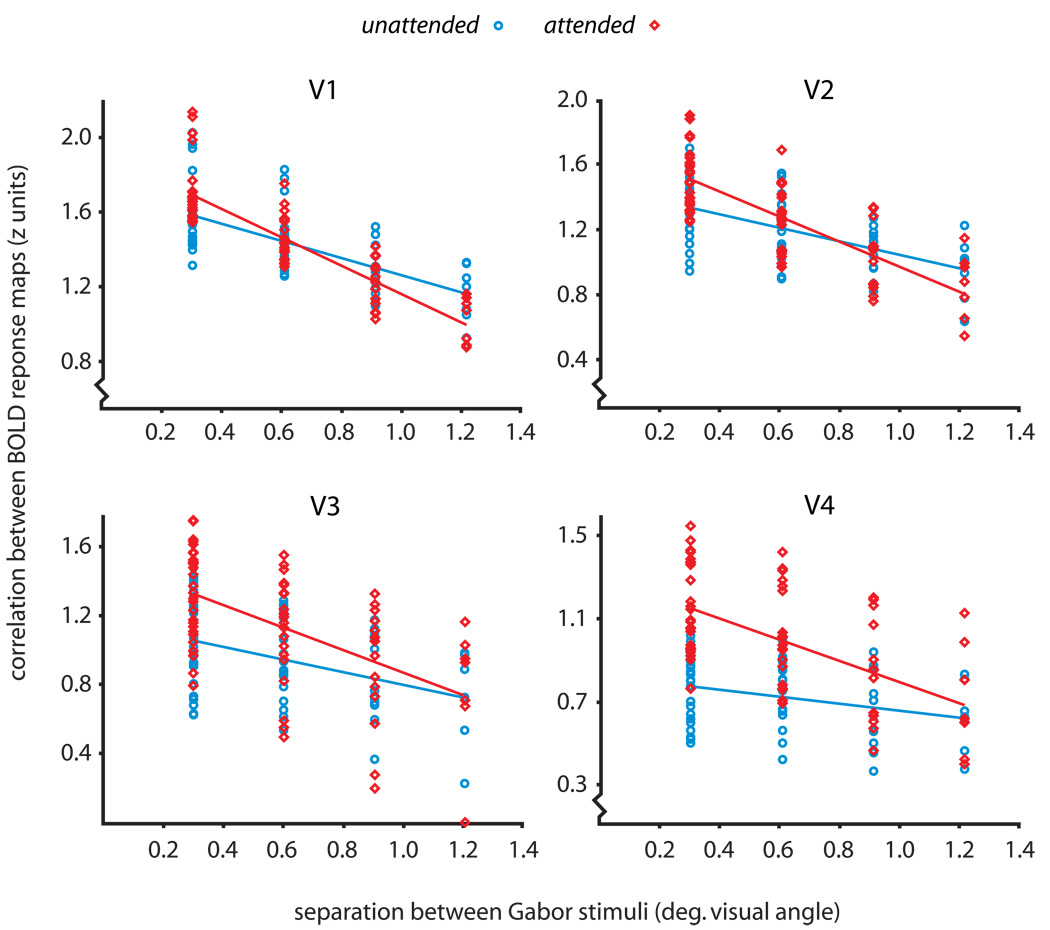
Psychophysical studies indicate that spatially-directed attention can improve visual resolution to facilitate the performance of fine-scaled tasks [1]. To complement these behavioral results, we tested for improved spatial coding in early visual areas with attention, using a cross-correlational pattern analysis [11, 12]. For each subject, we generated five maps of BOLD response by contrasting the five stimulus positions with the baseline. Within independently localized ROIs for areas V1- V4, we cross-correlated the five BOLD maps, obtaining a correlation for each possible pairing of maps. We then plotted these correlations as a function of the physical separation between the stimuli that yielded each pair of BOLD maps, producing a position discrimination plot (Fig. 2; see Supplemental Experimental Procedures for details). A negative slope on the position discrimination plot indicates that stimuli produce more distinct loci of activation in the ROI as they move further apart in the visual field. Figure 2 shows data from all subjects, plotted separately for attended and unattended runs. In every visual area, attention significantly improved the precision of spatial coding, yielding a steeper slope on the position discrimination plot (Z = 6.07 for V1, Z = 5.54 for V2, Z = 5.36 for V3, Z = 6.92 for V4; all p < 0.001). Thus, consistent with psychophysical results [1, 13–15], attention improved the precision of retinotopic encoding in the cortex by representing fine position differences with more substantial changes in the pattern of BOLD response.
Figure 2.
Position discrimination plots for V1 through V4. Each plot shows the relationship between the retinal proximity of a pair of stimulus conditions (x axis) and the correlation between their resulting patterns of BOLD response (y axis). A negative linear trend in the correlations reflects the fact that stimuli presented in close proximity to each other produced highly overlapping regions of BOLD response within the ROI being tested, whereas stimuli presented at more distant locations produced more distinct patterns of activation. We analyzed the unthresholded BOLD response within each ROI in order to include position information carried by nonsignificant and negative voxels [12, 29]. We averaged each subject’s correlations across runs to produce ten Fisher z scores per subject, and plotted all subjects’ points together to produce group position discrimination plots for areas V1 through V4. Attended trials are shown in red, and unattended trials are shown in blue. We fit separate linear regressions to the attended and unattended data to quantify the precision of position coding; each regression included a random effects variable to account for plotting multiple subjects together (see Supplemental Experimental Procedures). The linear fit for the attended data was significantly steeper than that for the unattended data in each visual area (the least significant difference was for area V3, Z = 5.36, p < 0.001), indicating that attention improved the precision of retinotopic encoding in each of V1 through V4.
The improved precision of position coding with attention could result from signal gain alone, which improves the SNR in the BOLD response, or from a combination of signal gain and narrowed population position tuning, which would produce more spatially distinct activity regions for adjacent stimuli. To resolve this, we examined correlations at the largest (1.22 deg.) separation on the position discrimination plots. While the SNR improvement afforded by a BOLD gain would increase the measured correlations, position tuning narrowing would work conversely, decreasing the correlations by reducing the overlap in the regions of BOLD response stimuli produced. This expected pattern holds across the different types of gain that have been distinguished in the attention literature (see Supplemental Figure 1); here, signal gain refers to a multiplicative vertical scaling of the BOLD response profile. A decrease in the measured correlations with attention could only be due to the influence of narrowed position tuning, and such a decrease would be most prominent at the largest stimulus separation, where the corresponding regions of activity are already most distinct.
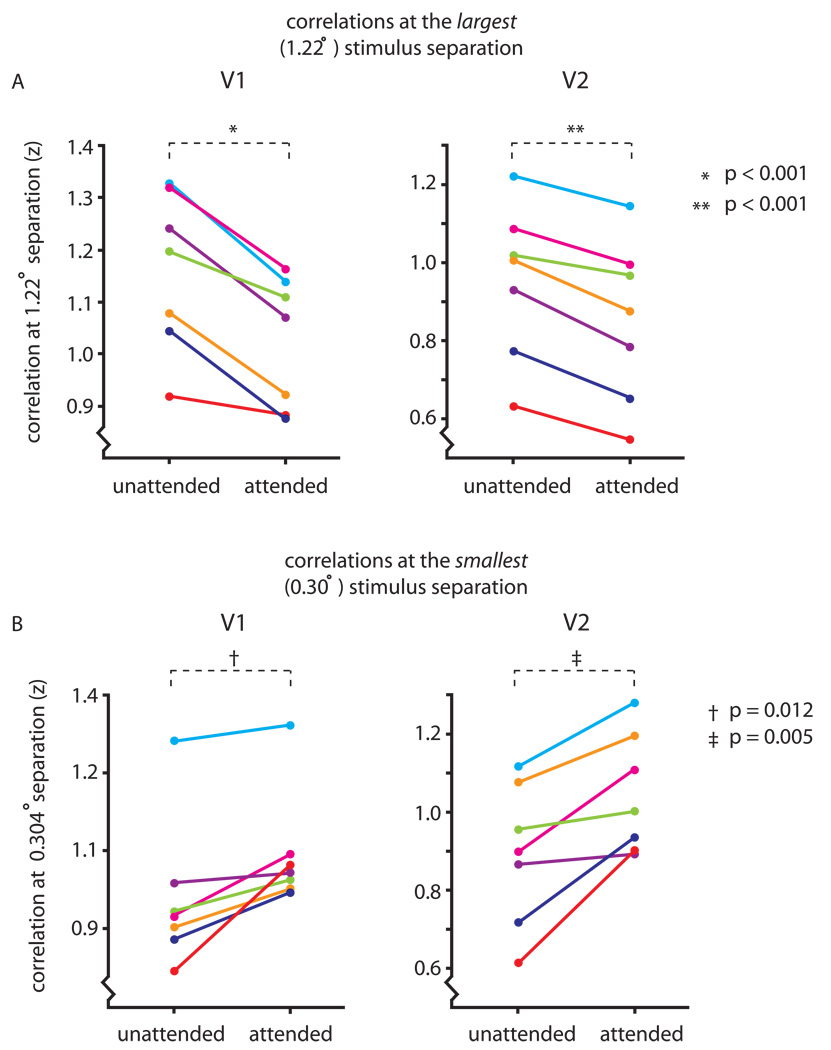
Figure 3A compares the correlations, at 1.22 deg separation, for the attended and unattended trials in areas V1 and V2. In every subject, attending to the Gabor patches significantly reduced the correlation between their resulting patterns of activity (paired t-tests; t(6) = 5.04, p < 0.001 for V1; t(6) = 5.69, p < 0.001 for V2). Because signal gain cannot be responsible for these reduced correlations, these data show that attention reduced the spatial spread of the BOLD response around the attended stimuli.
Figure 3.
Attention reduced the overlap between adjacent patterns of activity, while increasing the peak BOLD response. A) To evaluate whether attention narrowed position tuning in the BOLD response, we examined the correlation between the patterns of activity produced by the most foveal and most eccentric stimuli. Each subject’s z scores for the 1.22 degree stimulus separation are shown separately for attended and unattended conditions (each color is one subject). In both V1 and V2, the correlations were significantly lower for the attended stimuli, indicating a reduction in the spatial spread of the BOLD response with attention (paired t test; t(6) = 5.04, p < 0.001 for V1; t(6) = 5.69, p < 0.001 for V2). B) To test for an increased peak BOLD response with attention, we examined the correlations at the smallest stimulus separation (.304 degrees). As in panel A, subjects’ z scores are plotted separately for attended and unattended conditions in V1 and V2. For every subject, the correlation increased with attention in both V1 and V2, indicating a robust signal gain with attention. These increases were significant in both areas (paired t-tests; t(6) = 3.57, p = 0.012 for V1; t(6) = 4.32, p = 0.005 for V2).
We made the same comparison for the stimuli at the two most foveal positions, separated by only 0.304 deg. If attention increased the peak amplitude of the BOLD response in addition to narrowing its spatial spread, we would expect increased correlations with attention for very closely spaced stimuli due to an increase in the signal, relative to noise (in the extreme case of stimuli presented in the same position, the correlation between the two patterns of response would track SNR independently of position tuning narrowing). In fact, at this smallest stimulus separation, the correlations for all subjects increased significantly with attention (Fig. 3B; paired t-tests; t(6) = 3.57, p = 0.012 for V1; t(6) = 4.32, p = 0.005 for V2). We independently verified that attention increased the peak BOLD response by comparing the maximum responses in V1 and V2 in the attended and unattended conditions (paired t-tests; t(6) = 3.23, p = 0.018 for V1; t(6) = 3.90, p = 0.008 for V2). This confirms that correlations can simultaneously reveal both signal gain and position tuning narrowing in the BOLD response.
The decrease in correlation at the largest stimulus separation found in V1 and V2 was not found in V3 or V4. However, the regression lines for the attended and unattended data intersect well above the horizontal axis in the plots for both areas (at (1.27, 0.70) for V3, and (1.40, 0.59) for V4), suggesting that measuring at larger stimulus separations would likely reveal the same decrease in correlation with attention as in V1 and V2. Coarser retinotopy in V3 and V4 compared with V1 and V2, and a stronger gain effect of attention at later stages in the cortical hierarchy [16, 17], likely underlie the need to sample at larger stimulus separations in V3 and V4.
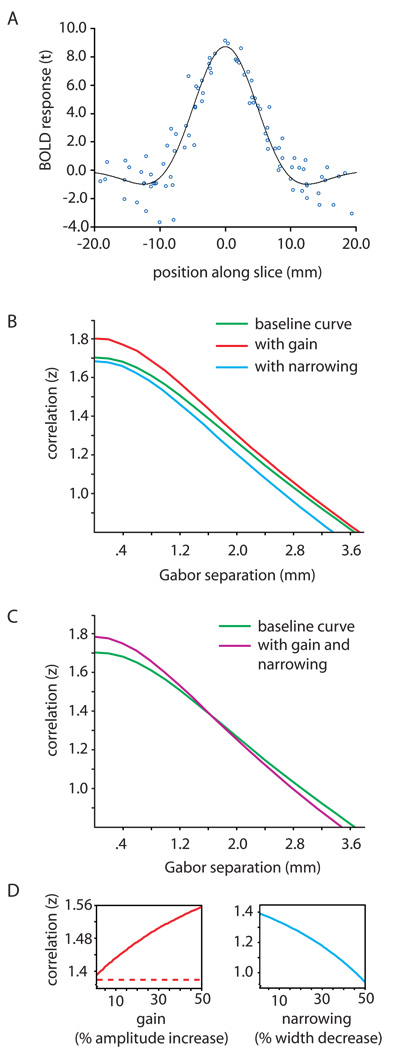
To better understand how attention-related tuning and gain are reflected in the position discrimination plots, we simulated the correlation analysis with a modeled BOLD response profile (Fig. 4A). Figure 4B shows a green baseline curve reflecting correlation as a function of separation between patterns of BOLD, along with curves simulating a 10% signal gain (red) and a 10% narrowing of population position tuning (blue). Both the red and blue curves are steeper than the baseline curve, indicating more precise position coding; importantly, signal gain only increased correlations, whereas narrowed position tuning only decreased correlations. This pattern reflects the fact that signal gain improves position coding by increasing SNR, while narrowed position tuning improves position coding by making the BOLD response patterns more distinct. The purple curve in Figure 4C, the result of simultaneous gain and tuning narrowing, matches the characteristic crossover pattern in the position discrimination plots for areas V1 and V2, revealing evidence for both attentional mechanisms at work.
Figure 4.
Modeling the effects of signal gain and position tuning narrowing. A) We obtained a BOLD response curve for use in subsequent simulations by measuring the BOLD signal along slices on each subject’s inflated cortex (see Supplemental Experimental Procedures). Data from all subjects were plotted together and normalized by peak amplitude to generate a prototypical BOLD response profile for use in modeling. These data were fit with a difference-of-Gaussians function (black curve), and 200 points were sampled evenly along this curve for use in subsequent simulations. B) Using the resulting BOLD response curve, we produced a simulated position discrimination plot (like those in Fig. 2), sampling on a continuous range of stimulus separations (green curve). To produce this plot, in a Monte Carlo simulation, we added realistic noise (SNR = 15.33; see Supplemental Experimental Procedures) to two copies of the prototype BOLD response curve, and varied the offset between them, computing the correlation at each separation. To model the effects of gain and narrowing on the position discrimination plot, we repeated the same Montecarlo simulation after adding a 10% amplitude gain (red curve) and, separately, a 10% width narrowing (blue curve) to the BOLD response profiles. The application of signal gain increased the correlation between the simulated BOLD response patterns at every separation along the abscissa, while narrowing the BOLD response profiles decreased the correlation at every separation; both gain and tuning narrowing made the slope of the curve steeper. C) Simultaneously applying both gain and narrowing to the simulated BOLD response profiles produced the purple curve. This curve crosses the baseline (green) curve in the same fashion that the attended and unattended regression lines cross in the position discrimination data presented in Figure 2. D) To model the effects across a broad range of signal gain strengths and levels of position tuning narrowing, we performed a similar Monte Carlo simulation as above, this time holding the separation between the two simulated BOLD response curves constant, and instead varying the gain level (left plot in red) or the curve width (right plot in blue) over a wide range of values. We separately tested the effects of response gain, activity gain, and additive gain (see Supplemental Figure 1): response gain and activity gain yielded the same monotonically increasing curve (solid red), while additive gain left the correlations unchanged at all levels (dashed red). Narrowing the curve widths always decreased their correlation (blue curve).
Could signal gain alone ever decrease the correlation between two BOLD response profiles? In an additional simulation, we fixed the separation between two modeled BOLD response curves and measured the correlation between the curves over a range of signal gain levels. Vertically scaling the BOLD response by a positive factor (response gain or activity gain) always increased the correlation between the two curves (solid red curve in Fig. 4D), whereas rigidly shifting the curves upward (additive gain) had no effect on the correlations (dashed red line in Fig. 4D). There was no instance in which signal gain of any type reduced the correlation between the patterns of modeled BOLD response (see Supplemental Figure 2 for a proof that both response and activity gain always increase a preexisting positive correlation between two response patterns). In a complementary simulation, we adjusted the BOLD response curve widths over a broad range; narrowing the curves always reduced the correlation between the two profiles (blue curve in Fig. 4D).
One concern is that subjects may have attended to a stimulus feature of the Gabors (e.g., orientation) rather than its location. To ensure the position tuning narrowing we measured was due to spatially-directed attention, in a control experiment we presented broadband noise patches rather than Gabors (Supplemental Fig. 3A). The results for two subjects (Supplemental Fig. 3C) were consistent with the main experiment, revealing significant position tuning narrowing with attention (a decrease in the correlations at the largest separation in attended vs. unattended trials; t = 2.92, p = 0.02 in V1, t = 3.27, p = 0.01 in V2). Because these noise stimuli contained no dominant spatial frequency or orientation to which subjects could attend (Supplemental Fig. 3B), we are confident that the position tuning narrowing observed is due to spatially-directed attention.
To complement the main analysis, we directly examined the BOLD response along slices through the peak activations in V1 in each subject (Supplemental Figure 4). We fit a difference of Gaussian curve to each measured BOLD response profile and compared the width parameters of the positive lobes for attended and unattended conditions to test for narrowing of the BOLD response with attention. A 3-way (position x attention x subject) ANOVA revealed a significant main effect of attention on curve width (F = 10.24, p = 0.002); attended curves were significantly narrower than unattended curves. We also found that attention significantly increased the curve amplitude parameters (F = 12.57, p < 0.001), consistent with the simultaneous signal gain and decreased spread of the BOLD response found using the correlational approach.
This analysis verified that attention acted over the entire BOLD response profile, rather than just a restricted portion. If attentional effects were localized to a subregion of the stimulus, one or both of its edges along the slices should be free of attentional modulation. We tested for an attentional effect at the negative “dips” at the edges of the BOLD response profiles, and found that attention significantly reduced the responses at both edges (F = 8.28, p = 0.006 at the foveal edge; F = 11.13, p = 0.001 at the eccentric edge). Thus, there was significant attentional modulation over the entire extent of the BOLD stimulus representation. This also shows that the narrowed position tuning is not simply due to a positive signal gain that drops off as a function of distance from the center of the spotlight.
We also tested for a difference in the curve phase parameters between the attended and unattended conditions. Attention had no effect on the peak positions of the BOLD response profiles (F = 0.19, p = 0.67), and there was no significant attention x stimulus position interaction, which might have indicated shifts in different directions for different stimulus positions (F = 0.003, p ≈ 1.0). Attention narrowed the BOLD response spread without significantly shifting its distribution. This slice-based approach allowed us to directly visualize the BOLD response’s shape along a single dimension, complementing and supporting the more powerful multivariate analysis that took into account the changes in the BOLD response pattern in all dimensions simultaneously.
Discussion
Our results show that spatially-directed attention improves retinotopic coding precision in V1 through V4 by boosting signal amplitude and by narrowing position tuning at the neural population level. This is consistent with an emerging pattern of results in the feature tuning domain: attention narrows the tuning of population responses [18, 19], even when tuning for the same features shows little or no change at the single-unit level [6, 20]. This makes sense, because psychophysical resolution ultimately relies on population coding [21, 22].
What mechanism underlies the narrowing effect that spatial attention has on population position tuning? Is it at the single unit level? Several studies show that a V4 neuron’s response when two stimuli were positioned inside its receptive field selectively reflected the attended stimulus, suggestive of shrinking receptive fields with attention [23] [24, 25]. However, this has only been established in extrastriate areas, where narrowed position tuning could result from a spatially specific gain in V1, even if position tuning remained unchanged there [26]. Whether attention can narrow the position tuning of single cells in V1 remains unresolved, but narrowed single-unit position tuning could shrink the spread of the population response.
Still, spatial attention need not narrow position tuning at the single-unit level to yield narrowed population position tuning—in a coarse coding framework, the most precise population codes are achieved by larger, highly-overlapping receptive fields [27]. As such, increasing the number of active units at the attended location while decreasing them in the surrounding region would decrease the spread of the BOLD response we measured while providing a higher resolution population code at the attended location by increasing RF overlap there. Such an attentional mechanism, which has heterogeneous effects across the extent of the attentional “spotlight,” could narrow population position tuning while leaving single-unit tuning unchanged.
From a perception standpoint, an attentional mechanism that modulates visual resolution by controlling both signal gain and population position tuning is far more efficient than one using signal gain alone. For many attentionally demanding tasks, performance benefits from the greatest resolution the visual system can achieve; some tasks, such as texture perception [14], are better performed at lower resolutions. The attentional system’s challenge, then, is not simply to achieve the maximum possible resolution but the broadest possible dynamic range of resolutions. The ability to adjust population position tuning affords the attentional system dynamic control of visual resolution over a broad and continuous range, even if signal gain is held constant or manipulated independently to subserve additional functions such as coordinate frame transformations [28]. Thus, a dual gain and tuning mechanism effectively maximizes the flexibility and dynamic range of visual resolution.
Supplementary Material
Acknowledgements
We thank Erica Veinsreideris, David Bressler, Elizabeth Louie, and Nicole Spotswood.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yeshurun Y, Montagna B, Carrasco M. On the flexibility of sustained attention and its effects on a texture segmentation task. Vision Res. 2008;48:80–95. doi: 10.1016/j.visres.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu ZL, Dosher BA. External noise discriminates mechanisms of attention. In: Itti L, Rees G, Tsotsos JK, editors. Neurobiology of attention. Academic Press; 2004. pp. 448–453. [Google Scholar]
- 3.Motter BC. Focal attention produces spatially selective processing in visual cortical areas V1, V2, and V4 in the presence of competing stimuli. J Neurophysiol. 1993;70:909–919. doi: 10.1152/jn.1993.70.3.909. [DOI] [PubMed] [Google Scholar]
- 4.Spitzer H, Desimone R, Moran J. Increased attention enhances both behavioral and neuronal performance. Science. 1988;240:338–340. doi: 10.1126/science.3353728. [DOI] [PubMed] [Google Scholar]
- 5.Haenny PE, Schiller PH. State dependent activity in monkey visual cortex. I. Single cell activity in V1 and V4 on visual tasks. Experimental brain research. Experimentelle Hirnforschung. 1988;69:225–244. doi: 10.1007/BF00247569. [DOI] [PubMed] [Google Scholar]
- 6.McAdams CJ, Maunsell JH. Effects of attention on orientation-tuning functions of single neurons in macaque cortical area V4. J Neurosci. 1999;19:431–441. doi: 10.1523/JNEUROSCI.19-01-00431.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Somers DC, Dale AM, Seiffert AE, Tootell RB. Functional MRI reveals spatially specific attentional modulation in human primary visual cortex. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:1663–1668. doi: 10.1073/pnas.96.4.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tootell RB, Hadjikhani N, Hall EK, Marrett S, Vanduffel W, Vaughan JT, Dale AM. The retinotopy of visual spatial attention. Neuron. 1998;21:1409–1422. doi: 10.1016/s0896-6273(00)80659-5. [DOI] [PubMed] [Google Scholar]
- 9.Brefczynski JA, DeYoe EA. A physiological correlate of the 'spotlight' of visual attention. Nat Neurosci. 1999;2:370–374. doi: 10.1038/7280. [DOI] [PubMed] [Google Scholar]
- 10.Gandhi SP, Heeger DJ, Boynton GM. Spatial attention affects brain activity in human primary visual cortex. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:3314–3319. doi: 10.1073/pnas.96.6.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer J, Whitney D. Precise discrimination of object position in the human pulvinar. Hum Brain Mapp. 2007 doi: 10.1002/hbm.20485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bressler D, Spotswood N, Whitney D. Negative BOLD fMRI response in the visual cortex carries precise stimulus-specific information. PLoS ONE. 2007;2:e410. doi: 10.1371/journal.pone.0000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balz GW, Hock HS. The effect of attentional spread on spatial resolution. Vision Res. 1997;37:1499–1510. doi: 10.1016/s0042-6989(96)00296-9. [DOI] [PubMed] [Google Scholar]
- 14.Yeshurun Y, Carrasco M. Attention improves or impairs visual performance by enhancing spatial resolution. Nature. 1998;396:72–75. doi: 10.1038/23936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeshurun Y, Carrasco M. Spatial attention improves performance in spatial resolution tasks. Vision Res. 1999;39:293–306. doi: 10.1016/s0042-6989(98)00114-x. [DOI] [PubMed] [Google Scholar]
- 16.Maunsell JHR. The Role of Attention in Visual Cerebral Cortex. In: Werner JS, LM Chalupa, editors. The visual neurosciences. Cambridge, Mass: MIT Press; 2004. pp. 1538–1545. [Google Scholar]
- 17.Treue S. Neural correlates of attention in primate visual cortex. Trends Neurosci. 2001;24:295–300. doi: 10.1016/s0166-2236(00)01814-2. [DOI] [PubMed] [Google Scholar]
- 18.Martinez-Trujillo JC, Treue S. Feature-based attention increases the selectivity of population responses in primate visual cortex. Curr Biol. 2004;14:744–751. doi: 10.1016/j.cub.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 19.Murray SO, Wojciulik E. Attention increases neural selectivity in the human lateral occipital complex. Nat Neurosci. 2004;7:70–74. doi: 10.1038/nn1161. [DOI] [PubMed] [Google Scholar]
- 20.Treue S, Martinez Trujillo JC. Feature-based attention influences motion processing gain in macaque visual cortex. Nature. 1999;399:575–579. doi: 10.1038/21176. [DOI] [PubMed] [Google Scholar]
- 21.Pouget A, Dayan P, Zemel R. Information processing with population codes. Nature reviews. 2000;1:125–132. doi: 10.1038/35039062. [DOI] [PubMed] [Google Scholar]
- 22.Purushothaman G, Bradley DC. Neural population code for fine perceptual decisions in area MT. Nat Neurosci. 2005;8:99–106. doi: 10.1038/nn1373. [DOI] [PubMed] [Google Scholar]
- 23.Moran J, Desimone R. Selective attention gates visual processing in the extrastriate cortex. Science. 1985;229:782–784. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- 24.Luck SJ, Chelazzi L, Hillyard SA, Desimone R. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J Neurophysiol. 1997;77:24–42. doi: 10.1152/jn.1997.77.1.24. [DOI] [PubMed] [Google Scholar]
- 25.Treue S, Maunsell JH. Attentional modulation of visual motion processing in cortical areas MT and MST. Nature. 1996;382:539–541. doi: 10.1038/382539a0. [DOI] [PubMed] [Google Scholar]
- 26.Maunsell JH, McAdams CJ. Effects of attention on neuronal response properties in visual cerebral cortex. In: Gazzaniga MS, editor. The New Cognitive Neurosciences. Cambridge, Mass: MIT Press; 2000. pp. 315–324. [Google Scholar]
- 27.Eurich CW, Schwegler H. Coarse coding: calculation of the resolution achieved by a population of large receptive field neurons. Biological cybernetics. 1997;76:357–363. doi: 10.1007/s004220050349. [DOI] [PubMed] [Google Scholar]
- 28.Salinas E, Abbott LF. Invariant visual responses from attentional gain fields. J Neurophysiol. 1997;77:3267–3272. doi: 10.1152/jn.1997.77.6.3267. [DOI] [PubMed] [Google Scholar]
- 29.Norman KA, Polyn SM, Detre GJ, Haxby JV. Beyond mind-reading: multi-voxel pattern analysis of fMRI data. Trends Cogn Sci. 2006;10:424–430. doi: 10.1016/j.tics.2006.07.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.