Abstract
Under different circumstances, tumors can inhibit or activate macrophage (Mϕ) effector functions. We studied the mechanisms of tumor-Mϕ interactions leading to Mϕ activation. The results show that L5178Y mouse T-cell lymphoma cells can prime naïve mouse Mϕ to subsequent LPS stimulation, resulting in increased NO production and anti-lymphoma effects in vitro. L5178Y cells, but not naïve splenocytes, primed Mϕ respond to ligation of TLR4 but not TLR9. L5178Y-primed Mϕ incubated with LPS showed down-regulation of CD40 and up-regulation of NKG2D expression. While L5178Y T cell lymphoma cells prime naïve mouse Mϕ, mouse A20 B cell lymphoma, B16 melanoma, or NIH-3T3 fibroblasts, and human Jurkat T cell lymphoma, Daudi B cell lymphoma, or M21 melanoma tumor cells lines all failed to prime mouse Mϕ. Neither L5178Y-conditioned supernatants nor co-culture of Mϕ and L5178Y cells in transwells resulted in priming, indicating that direct L5178Y cell-Mϕ contact was needed. Several receptor-ligand pairs are reciprocally expressed on Mϕ and L5178Y cell membranes and can be potentially involved in Mϕ priming. Of these, the CD40-CD154 pair played the most important role, as blocking the interaction of these molecules substantially reduced in vitro Mϕ priming. Furthermore, simultaneous blocking of interactions between CD40–CD154, NKG2D–H60, and CD18–ICAM-1/-2 led to complete abrogation of Mϕ-mediated NO secretion and complete inhibition of Mϕ-mediated tumor cell cytostasis. The priming of Mϕ to LPS with L5178Y cells was also observed in vivo. These results suggest that contact with certain tumor cells via CD40, NKG2D, and CD18 molecules on the Mϕ may facilitate Mϕ-mediated anti-tumor immune surveillance.
Keywords: Monocytes/macrophages, Innate immunity, Tumor recognition
Introduction
Activation of cells of the innate immune system, including professional antigen-presenting cells, with vaccine adjuvants strongly influences the efficacy of adaptive immunity induced by vaccination (1). Furthermore, cells of the innate immune system are also the first line of antitumor defense capable of early recognition and killing of autologous cells with aberrant immunophenotypes. NK cells and macrophages (Mϕ) are both involved in tumor immune defense (2–4).
An array of stimulatory receptors on naive NK cells allows for recognition of cancer cells with altered surface expression of MHC class I antigens (MHC-I) or MHC-I – like structures, such as H60, MULT1, and Rae-1 proteins in mice, and MHC-I chain-related proteins (MICA and MICB) and UL16-binding proteins (ULPB) in humans, which results in NK cell activation (5–10). This effect frequently requires mutual co-ligation of multiple activating receptors and adhesion molecules on NK cells (11,12) and can further be augmented by stimulation of NK cells via cytokines or Toll-like receptors (TLRs) (13). Notably, activation of secretory and cytolytic functions may require different patterns of NK cell co-stimulation (11,13). Interactions involved in cancer cell recognition by naïve Mϕ are less well characterized. Several studies demonstrated roles for MHC-I and MHC-I – like molecules in regulation of Mϕ activation (10,14–17). The CD11b/CD18 complex (also known as LFA-1) on Mϕ has also been shown to regulate Mϕ-mediated tumor cell cytolysis by facilitating anchoring of the ICAM+-tumor cell targets to activated Mϕ (18,19). Mϕ also express CD40 on their membranes and therefore could be stimulated via CD40 ligation. Indeed, we have recently reported that in vitro and in vivo stimulation of Mϕ with agonistic anti-CD40 monoclonal antibody (αCD40) results in Mϕ activation that could be synergistically amplified via TLR4 by bacterial LPS or via TLR9 by class-B CpG-containing oligodeoxynucleotides (CpG) (20,21). These αCD40-stimulated Mϕ could secrete NO, TNF-α, IL-12, and IFN-γ, and mediate anti-tumor effects in vitro and in vivo against CD40-negative tumors (20,21).
In several experiments pertaining to our previous studies, we observed that culturing naïve Mϕ in vitro with L5178Y T cell lymphoma cells in the presence of low concentrations of LPS resulted in secretion of NO and induced tumor cytostasis. Notably, Mϕ and L5178Y cells cultured separately did not secrete any detectable NO in the presence of LPS. This led to the hypothesis that tumor cells can sensitize naïve Mϕ via TLR4. In this study, we investigated the mechanisms of this L5178Y cell-mediated priming of naïve Mϕ and found that it required direct L5178Y cell-Mϕ interaction and involved simultaneous ligation of CD40, NKG2D, and CD18 on the Mϕ surface, with the CD40-CD154 interaction playing the most important role. This cross-talk also led to reciprocal alterations of the immunophenotype of both Mϕ and L5178Y cells and resulted in inhibition of L5178Y cell proliferation in vitro. The priming of Mϕ with L5178Y cells was also demonstrated in vivo, suggesting that Mϕ activation can be a mechanism of immunological lymphoma surveillance.
Material and Methods
Mice
Six to ten week old C57BL/6 mice (Harlan Sprague Dawley, Madison, WI, The Jackson Laboratory, Bar Harbor, ME, Taconic, Germantown, NY), DBA/2, BALB/c, A/J mice (all from Taconic), and CD40−/− mice (strain B6.129P2-CD40tm1Kik/J) (Jackson laboratories) were housed, cared for, and used in accordance with the Guide for Care and Use of Laboratory Animals (NIH publication 86-23, National Institutes of Health, Bethesda, MD, 1985).
Tumor cell lines
Mouse L5178Y T-cell lymphoma (DBA/2 origin), EL4 T-cell lymphoma (C57BL/6), YAC-1 thymoma (A/Sn), A20 B-cell lymphoma (BALB/c), B16 melanoma (C57BL/6), and NIH-3T3 fibroblast (BALB/c) cell lines were grown in RPMI-1640 complete cell culture medium supplemented with 10% FBS (Sigma-Aldrich, St. Louis, MO), 2 mM L-glutamine, 100 U/ml penicillin/streptomycin, and 0.5 µM 2-ME (Invitrogen Life Technologies, Carlsbad, CA) at 37°C in a humidified 5% CO2 atmosphere. Human Jurkat T-cell lymphoma, Daudi B-cell lymphoma, and M21 melanoma cell lines were grown in RPMI-1640 complete cell culture medium, as formulated above, but without 2-ME. Mouse fibroblast L cell line (ATCC ID: CRL-2648), and CD40L or control CD32 (FcR) - transfected L cells (CD40L-L and CD32-L, respectively), provided by Dr. Erik Ranheim, UW Department of Pathology and Laboratory Medicine, were cultured in RPMI 1640 complete medium with G418 (400 µg/ml). In separate experiments, we used paraformaldehyde-fixed L5178Y cells (PF-L5178Y) prepared by exposing L5178Y cells to 2% paraformaldehyde in PBS on ice for 1 hr, followed by 5 separate cell wash cycles with complete cell culture medium. The fixed cells were kept in ice-cold medium for 24 to allow for complete elimination of any contaminating paraformaldehyde. Cell death was confirmed microscopically by staining with 1% eosin.
Splenocyte preparation
Splenocytes were prepared from whole spleens pooled from 3–4 C57BL/6 or DBA/2 mice by processing the spleens to a single cell suspension, followed by lysis of erythrocytes by hypotonic shock.
In vitro Mϕ-mediated tumor cell proliferation inhibition
Peritoneal cells (PC) were obtained from naïve mice by peritoneal cavity lavage. Total PC were seeded in 96-microwell flat-bottom cell-culture clusters (3×106 viable large leukocytes/ml, 0.1 ml/well, unless otherwise indicated). Ninety minutes (min) later, non-adherent cells were removed from the culture by repeated pipetting. This protocol yields a relatively pure population of mature Mϕ, based on 98% expression of F4/80 on CD11b+ cells. The resultant adherent Mϕ were thereafter incubated with tumor cells (5×105/ml, 0.05 ml/well, unless otherwise indicated) or splenocytes for 24–48 hours (hr) in medium with or without 10 ng/ml of LPS from Salmonella enteritidis (Sigma-Aldrich) or 5 µg/ml CpG1826 (Coley Pharmaceuticals, Wellesley, MA) in the final volume of 0.2 ml.
In some experiments, Mϕ were activated with recombinant mouse IFN-γ (Roche) or agonistic anti-CD40 mAb (αCD40) (20, 21). The FGK 45.5 hybridoma producing αCD40 was a gift from Dr. F. Melchers (Basel Institute for Immunology, Basel, Switzerland). αCD40 was obtained from ascites of nude mice injected with the hybridoma cells and enriched for IgG by ammonium sulfate precipitation.
In selected experiments, 10 µg/ml of functional grade blocking mAbs to NKG2D (191004, R&D Systems, Minneapolis, MN), CD154 (MR1), CD18 (M18/2), MHC-I (34-1-2S, reacts with both H-2Kd and H-2Dd), (all from eBioscience, San Diego, CA.), alone or in various combinations, were added into Mϕ cultures at the initiation of the experiment, but 30 min before tumor cells and LPS were added to the cultures. In the setting where αCD154 mAb was used, L5178Y cells were additionally pre-coated with 10 µg/ml αCD154 mAb or control IgG on ice for 30 min, and added to Mϕ cultures without additional washing.
To measure cell proliferation, the cells were pulsed with 1 µCi/well of 3H-Thymidine (3H-TdR) for the last 6 hr, and retained radioactivity was counted by β-scintillation of total cells harvested from the wells onto glass fiber filters (Packard Instrument), using the Packard Matrix 9600 Direct Beta Counter (Packard Instrument). Under these conditions Mϕ incorporate negligible amounts of 3H-TdR (20), enabling 3H-TdR to reflect the level of proliferation of the tumor cells. Inhibition of proliferation was calculated as c = [(a-b)/a] x 100, where c is inhibition index; a is mean value of incorporated 3H-TdR into tumor cells or splenocytes cultured in triplicates in medium without Mϕ in the absence or presence of LPS and CpG; b is mean value of incorporated 3H-TdR into tumor cells or splenocytes cultured in triplicates in medium with Mϕ in the absence or presence of LPS and CpG.
Transwell experiments
In separate experiments, we used the Trasnswell® system (Costar-3460, 12-macrowell polystyrene plates with 12 mm, 0.4 µm pore size polyester membrane transwell chambers [TW-chamber]). Total PC at a concentration of 3×106 large leukocytes/ml, 1.0 ml/well were plated into wells for 90 min before non-adherent cells were removed, and 1.45 ml of complete cell culture medium was replaced. L5178Y cells (5×105/ml, 0.5 ml/well) were added either into the wells or into the TW-chamber. Under certain experimental conditions, L5178Y cells were added both in the wells and in the transwell chamber. Finally, LPS (0.4 µg/ml, 0.05 ml/well) was added in the well to achieve the final concentration of 10 ng/ml in 2.0 ml. After 24 hr of co-culture, the TW-chambers were removed from the wells and placed into 15 ml centrifuge conical tubes. To harvest L5178Y cells from the TW-chamber, the polyester membrane was delicately perforated by the tip of a 1 ml pipette, and L5178Y cells were washed off by repeated pipetting of cell culture medium through the transwell chamber. In the same wells, non-adherent L5178Y cells, cultured together with adherent Mϕ, were harvested from the bottom of the macrowells by gentle pipetting and repeated aspiration of the cell culture supernatants. This technique yielded more than 95% of total L5178 cells cultured in the well, with less than 2% of contaminating Mϕ, detached from plastic by pipetting. Finally, the harvested L5178Y cells (from the TW-chambers or the bottom of the wells) were spun down, the pellet was resuspended in 0.3 ml of the cell culture medium, and the cells were re-seeded in 96-microwell round-bottom cell-culture clusters in triplicates, 0.1 ml/well. Inhibition of L5178Y cell proliferation was measured with 3H-TdR as described above.
Nitric oxide detection
Mϕ were cultured with tumor cells as described above. At different time points, the cell culture supernatants were collected without disturbing the cell monolayers. In experiments involving the transwell system, the supernatants were taken from both the well and the TW-chamber. Nitrite accumulation in the cell-culture supernatants was determined by using the Griess reagent (Sigma-Aldrich) as described (20).
Tumor cell medium conditioning
L5178Y cells (5×105 cells/ml, 20 ml) were cultured in complete cell culture medium, with or without 10 ng/ml LPS, at 37°C in a humidified 5% CO2 atmosphere. At 24 hr, cell cultures were harvested and cells pelleted by centrifugation at 453 × g for 20 min. Cell culture supernatants were collected and centrifuged for at 3220 × g for 60 min, and the resultant supernatants were subsequently used in in vitro experiments.
Tumor cell testing for Mycoplasma spp. contamination
L5178Y, YAC-1, and B16 tumor cell lines were tested for contamination with Mycoplasma spp. as described (22). This method is sensitive for M. hyorhinis, M. arginini, M. fermentans, M. orale, M. pirum, M. hominis, M. salivarium, and M. laidlawii. Madin Darby bovine kidney (MDBK) cells were used as the indicator cell line. To confirm results of the above test, the cells were also cultured for 96 hr on the PPLO Mycoplasma agar, and the formed colonies were counted.
In vivo L5178Y lymphoma-mediated Mϕ priming
DBA/2 mice (n=3) were injected i.p. with 5×105 L5178Y cells in 1 ml of PBS. Control group of mice was injected with 1 ml of PBS without tumor cells. Twenty four hrs later, animals were injected i.v with 20 ng of LPS in 0.2 ml of PBS or with 0.2 ml of PBS (control). At 48 hrs of experiment, animals were euthanized, and then PC were collected by lavage and seeded in 6-well plates in complete RPMI medium to allow for purification of Mϕ by adhesion (20).
Flow cytometry analysis
Adhesion-purified peritoneal Mϕ from C57BL/6 mice were harvested as described above, and resuspended in ice-cold PBS + 2% FCS. Mϕ (1×105/sample) were labeled with FITC-conjugated αCD11 (M1/70), APC-conjugated αF4/80 (BM8), and PE-conjugated αGr1 (RB6-8C5), all from eBioscience. Analysis was based on co-expression of F4/80 and Gr1 on CD11b+ viable Mϕ. he analysis was preformed on FACSCalibur flow cytometer with CellQuest software (BD, Franklin Lakes, NJ).
In separate experiments, adherent Mϕ (3×106 cell/well) and L5178Y cells (2.5×105 cell/well) were cultured separately or together in medium with or without 10 ng/ml LPS in 12-macrowell polystyrene plates. At 12 (for analysis of L5178Y cells) or 24 (for analysis of Mϕ) hr, non-adherent L5178Y cells were harvested by repeated gentle pipetting, and adherent Mϕ were harvested by harsh pipetting of the cells following pretreatment with 5% EDTA (Sigma-Aldrich). Cells were pelleted and resuspended in the flow cytometry buffer. All cells were labeled with APC-conjugated αF4/80 to enable flow cytometric discrimination of F4/80− L5178Y cells from F4/80+ Mϕ. In addition, B16 and EL4 tumor cells were labeled with FITC-conjugated αH2Kb (AF6-88.5, BD), and L5178Y, A20, and NIH-3T3 cells were labeled with FITC-conjugated αH2Kd (SF1-1.1, BD), FITC-conjugated αICAM-1 (KAT-1, eBioscience), FITC-conjugated αICAM-2 (3C4, BD), αCD154 (MR1, eBioscience), PE-conjugated αH60 (205326, R&D). Separately, Mϕ were labeled with PE-conjugated αCD40 (1C10), PE-conjugated αNKG2D (CX5), FITC-conjugated αCD18 (M18/2), FITC-conjugated αMHC class II-(M5/114.15.2), FITC-conjugated αCD80 (16-10A1), FITC-conjugated αCD86 (GL1), all from eBioscience. Non-specific staining of Mϕ with αNKG2D mAb was prevented by pretreating Mϕ with the functional grade purified anti-mouse CD16+CD32 FcR block from eBioscience (clone 93, rat IgG2a) prior to staining with the anti-NKG2D mAb. Immunophenotyping of L5178Y cells was based on gating on viable F4/80− cells, whereas analysis of Mϕ was based on gating on viable F4/80+ cells. The analysis was preformed on FACSCalibur flow cytometer with CellQuest (BD) or FlowJo (TreeStar, Ashland, OR) software. Results presented as Mean Fluorescence Intensity (MFI) ratio calculated as value of MFI of staining with specific mAb divided by value of MFI of staining with isotype-matched control IgG; MFI ratio equals 1 when an antigen is not expressed. This approach allows for comparison of the same parameter but in different experimental conditions.
In in vivo Mϕ-priming experiment, freshly collected PC were re-suspended in flow cytometry buffer and stained with αF4/80-APC and α-NKG2D-PE, or α-MHC-II-FITC, α-CD80-FITC and α-CD86-FITC. Analysis of Mϕ phenotype was preformed by gating on viable F4/80+ cells. In another approach, collected PC were cultured for 9 hr in complete medium supplemented with monensin, depleted of non-adherent cells, harvested with 5% EDTA in PBS as described above, and resuspended in flow cytometry buffer followed by staining with α-F4/80-APC mAb for 40 min on ice. After staining with α-F4/80-APC mAb, cells were washed in ice-cold flow cytometry buffer, fixed, and permeabilized as previously described (20,21), and then stained with αIL4-PE (11B11), αIL10-PE (JES5-16E3), αIFN-γ-PE (XMG1.2), αTNF-α-PE (MP6-XT22), or αIL12-PE (C17.8) mAbs. Analysis was performed by gating on F4/80+ cells.
In vivo tumoristasis assay
CFSE-labeled L5178Y cells (5×105/ml) were injected i.p. into DBA/2 mice (n=3/group). Twenty-four hours after CFSE+L5178Y cell implantation the mice were treated i.v. with 0.2 ml PBS with or without 20 ng LPS. After 48 hr, mice were euthanized, resident peritoneal cells + lymphoma cells collected from each mouse of the group and pooled, and F4/80−CFSE+L5178Y were tested by flow cytometry for CFSE fluorescence. In parallel, CFSE+L5178Y cells (5×105/ml, 0.05 ml/well) were cultured in vitro in triplicates with naïve DBA/2 Mϕ as described in In vitro Mϕ-mediated tumor cell proliferation inhibition assay on page 6. After 48 hr, cell cultures were collected by pipetting and pooled into one analysis sample for each experimental group, and F4/80−CFSE+L5178Y were tested by flow cytometry for CFSE fluorescence. As a proliferation suppression control, L5178Y cells were γ-irradiated by 60Co γ-source.
Statistical analysis
A two-tailed Student’s t-test was used to determine significance of differences between experimental and relevant control values within one experiment. Statistical analysis of inhibition of tumor cell proliferation under different experimental conditions was performed on original 3H-TdR-incorporation values, which were also used to calculate % of inhibition.
Results
L5178Y lymphoma cells but not other tumor cell lines prime Mϕ to LPS
Based on our initial incidental observation (unpublished) that L5178Y lymphoma cells primed C57BL/6 Mϕ to LPS, we hypothesized that tumor cells can sensitize naïve Mϕ to stimulation via TLR4. Freshly isolated adhesion-purified peritoneal cells from naïve C57BL/6 mice contained 96% of CD11b+F4/80+Gr1− Mϕ (Fig. 1A). These Mϕ were cultured in vitro with mouse L5178Y or EL4, or human Jurkat T-cell lymphoma cells; mouse A20 or human Daudi B-cell lymphoma cells; mouse B16 or human M21 melanoma cells, or mouse 3T3-NIH fibroblast cells in medium with or without LPS. Following 42 hr of co-culture, we tested the cell cultures for NO concentration in the supernatant (Fig. 1B), as well as inhibition of 3H-TdR incorporation into the tumor cells (Fig. 1C). The results of this experiment show that only L5178Y cells, but not any of the other cells tested, were able to prime Mϕ to LPS, as measured by production of NO and anti-lymphoma effects in vitro. This priming effect of L5178Y cells on Mϕ was similar using PC Mϕ harvested from syngeneic DBA/2 and allogeneic C57BL/6 (but not from BALB/c or A/J) mouse strains obtained from various vendors as summarized in Table 1. In contrast, EL4 cells primed neither allogeneic (DBA/2) nor syngeneic (C57BL/6) Mϕ (Fig. 1B,C and data not shown).
Figure 1. L5178Y lymphoma cells prime naïve mouse Mϕ to LPS, resulting in NO production and anti-proliferative effects in vitro.
Flow cytometric profile of PC (A). PC obtained from naïve C57BL/6 mice contained ~35% of CD11b+ cells (Ai). Following 90 min of adhesion, non-adherent PC were removed from cultures by repeated pipetting. The resultant adherent population consisted of 99% CD11b+ Mϕ (Aii,). These adherent cells were comprised of 96% F4/80+ Gr1− Mϕ (Aiii). Naïve adherent C57BL/6 Mϕ were cultured for 24 hr with different mouse and human tumor cell lines in medium with or without LPS. Results are presented as concentration of NO metabolites (µM) in the supernatants (B) or % of inhibition of 3H-TdR incorporation into tumor cells (C). In this experiment, the proliferation of L5178Y cells in medium alone at 24 hrs was 16.5 ± 0.23 × 103 counts of 3H-TdR; in other experiments where cultures were assayed at 48 hrs, counts for L5178Y cells in medium were 70–110 × 103. * -negligible value.
Table 1. Comparison of Mϕ from different strains of mice.
Peritoneal Mϕ (3×l05 cells/0.1 ml), taken from naive mice of different strains obtained from different vendors, were cultured for 24 hr with 2.5×l04/0.1 ml L5178Y cells in medium with or without 10 ng/ml of LPS. After 24 hr of co-culture, NO concentrations (µM) in the cell culture supernatants, as well as inhibition (%) of L5178Y cell 3H-TdR incorporation, were evaluated as described in the Materials and Methods section. ND - not detectable: value is below the detection limit.
| Mouse strain |
Nitrite (µM) | Inhibition (%) | ||
|---|---|---|---|---|
| Medium | LPS | Medium | LPS | |
| C57BL/6Tac | ND | 53.1 ± 2.02 | 6.2 | 46.8 |
| C57BL/6Hln | 0.08 ± 0.08 | 51.9 ± 1.6 | 11.6 | 43.6 |
| C57BL/6Jax | ND | 47.8 ± 3.4 | 13.2 | 46.5 |
| DBA/2Tac | 1.2 ± 0.6 | 51.2 ± 1.1 | 9.4 | 41.4 |
| Balb/cTac | ND | ND | 8.0 | 0.3 |
| A/JTac | 1.8 ± 1.8 | ND | 10.1 | 7.3 |
L5178Y lymphoma cells but not normal splenocytes prime naïve Mϕ to LPS but not CpG
We tested if culturing naive Mϕ with L5178Y lymphoma cells would result in Mϕ priming to CpG as well as LPS, as we have previously reported that both LPS and CpG could trigger CD40 ligation-primed Mϕ (21). Mϕ from C57BL/6 mice were cultured for 48 hr with either L5178Y cells or splenocytes from the same donor mice (to further test if the observed priming effect is specific for L5178Y tumor cells), in culture medium with or without LPS or CpG. As shown in Fig. 2A, naïve Mϕ cultured in medium failed to respond to LPS or CpG as measured by NO production. In contrast, Mϕ were found to secrete a substantial amount of NO when cultured with L5178Y cells but not with normal splenocytes, and only in response to LPS but not CpG. This pattern of Mϕ -secretory activity induced by co-culture with L5178Y cells directly correlated with inhibition of L5178Y cell proliferation (Fig. 2B), whereas the anti-proliferative effect of Mϕ primed with splenocytes was substantially weaker and not reproducible in every experiment.
Figure 2. L5178Y cells induce dose-dependent priming of naïve Mϕ to LPS but not CpG.
Adherent C57BL/6 Mϕ were cultured for 48 hr alone or with 2.5×104 well of L5178Y lymphoma cells or autologous splenocytes (A, B), or various numbers of L1578Y cells (as shown in the legend box) in a separate experiment (E, F), in medium with or without LPS (10 ng/ml, as well as 0.1 and 1000 ng/ml in E and F) or CpG (5 µg/ml). Alternatively (C, D), C57BL/6 Mϕ were cultured with 2.5×104 well of L5178Y lymphoma cells or splenocytes from DBA/2 mice for 24 hr, followed by thorough removal of L5178Y cells and splenocytes and placement of 2.5×104/well of new L5178Y cells or B16 melanoma cells, with or without 10 ng/ml LPS, for another 24 hr,. At 42 hr, cell culture supernatants were harvested for the Griess nitrite test, and 1 µCi 3H-TdR was added for 6 hr to the cultures to measure L5178Y cell or splenocyte proliferation. Results are presented as concentration of NO metabolites (µM) in the supernatants (A, C) or % of inhibition of 3H-TdR incorporation into splenocytes (B) or L5178Y lymphoma cells (B, D). *-negligible values. The symbols (in C and D) correspond to the p values for each bar as compared to the previous bar to its left: for C †p=0.0032; ‡p=0.0114; §p=0.0978; ¶p=0.0034; #p=0.0325; @p=0.5572; for D ‡p=0.0221; §p=0.388; #p=0.838; @p=0.958; p values for D were calculated by comparing with Mean ± SEM values of original 3H-TdR incorporation counts. The experiments are representative of 3 separate experiments with similar results.
To test if L5178Y cell-mediated priming followed by LPS stimulation would enable Mϕ to be cytotoxic to tumor cells other than L5178Y, we primed C57BL/6 Mϕ with L5178Y cells or splenocytes from DBA/2 mice (so that lymphoma cells and splenocytes would be of the same H-2d haplotype) for 24 hr in complete medium. L5178Y cells or splenocytes were then removed and replaced by freshly-prepared L5178Y cells or B16 cells for another 24 hr period, with or without LPS (Fig. 2C,D). As in the previous experiment (Fig. 2A,B), L5178Y cells but not splenocytes from naïve donors could prime Mϕ to respond to LPS, resulting in cytotoxicity not only against the priming cell line (L5178Y), but also against the B16 melanoma cell line, which does not possess the priming ability (Fig. 1B,C).
NO production by Mϕ cultured with L5178Y cells in the presence of LPS correlated with the number of L5178Y cells present in culture (Fig. 2E). The greater the number of L5178Y cells in the culture, the higher the concentration of NO detected in the supernatants when using 10 ng/ml LPS. Increasing the amount of LPS in the cell culture medium to 1000 ng/ml induced NO secretion even in the absence of L5178Y cells; addition of L5178Y cells did further boost NO secretion, but did not show cell-dose sensitivity in the range tested. Notably, L5178Y cells themselves did not produce any NO in response to LPS or CpG (not shown), which suggests that this NO was produced by activated Mϕ. The pattern of Mϕ-mediated anti-proliferative effects in these same cultures (Fig. 2F) corresponded approximately to NO production (Fig. 2E). These results demonstrate that L5178Y cells, but not normal splenocytes, can augment sensitivity of naïve Mϕ to LPS, but not CpG, that results in in vitro NO release and anti-tumor effects.
Contact with L5178Y cells must precede or occur simultaneously with LPS stimulation in the course of activation of Mϕ
Next we tested if priming signals provided by L5178Y cells, and stimulation by LPS, need to occur in a certain sequence in order to activate cytotoxic Mϕ. Thus, L5178Y cells and LPS were added to Mϕ cultures either simultaneously (Fig. 3A–C) at the beginning of the experiment, or L5178Y cells were added at the beginning of the experiment and LPS was added at 24 hr (Fig. 3D–F), or LPS was added at the beginning of the experiment and L5178Y cells were added at 24 hr of the course of experiment (Fig. 3G–I). The overall duration of the experiment was 48 hrs. Results of this experiment demonstrate that Mϕ exposed simultaneously to both L5178Y cells and LPS were able to secrete progressively higher amounts of NO, as measured at 24 hr (Fig. 3A) and 42 hr (Fig. 3B) of the experiment, as well as to inhibit L5178Y cell proliferation at the end of the experiment (Fig. 3C). The magnitude of these effects depended upon the number of L5178Y cells present in the cultures. At the same time, Mϕ cultured with L5178Y cells added at 1 hr but without LPS did not secrete any NO or inhibit L5178Y cell proliferation (the 2–4 columns to the left in Fig. 3A,B, C). Similarly, when Mϕ were exposed to L5178Y cells only, i.e. without LPS, for the first 24 hr, they secreted only negligible amounts of NO detected at 24 hr (Fig. 3D). However, when LPS was added at 24 hr to the culture of Mϕ and L5178Y cells, Mϕ rigorously responded to LPS stimulation and secreted a high amount of NO 18 hr later (at 42 hr of the experiment) (Fig. 3E) that was comparable to the amount of NO secreted by Mϕ simultaneously exposed to both L5178Y cells and LPS for 42 hr (Fig. 3B). These Mϕ were also able to inhibit L5178Y cell proliferation (Fig. 3F), similar to the results shown in Fig. 2C. In contrast, when Mϕ were first stimulated with LPS for 24 hrs and then exposed to L5178Y cells for another 24 hr, they secreted only negligible amounts of NO at both 24 (Fig. 3G) and 42 hr (Fig. 3H), and were unable to suppress L5178Y cell proliferation (Fig. 3I). These results indicate that contact of Mϕ with L5178Y cells need to precede or occur simultaneously with LPS stimulation when these two stimuli are combined to activate Mϕ.
Figure 3. Contact of Mϕ with L5178Y lymphoma cells must precede or occur simultaneously with LPS stimulation to achieve Mϕ activation.
Adherent C57BL/6 Mϕ were cultured in vitro for 48 hr alone or in the presence of various numbers (as shown in the legend box) of L5178Y lymphoma cells. L5178Y cells were added to Mϕ 1 hr after beginning the experiment (A–C, D–F) or 24 hr after beginning the experiment (G–I). Similarly, LPS was added to Mϕ-L5178Y cell cultures 1 hr after beginning the experiment (A–C, G–I) or 24 hr after beginning the experiment (D–F). Cell culture supernatants were taken at 24 hr (A,D,G) (just before L5178Y cells (G) or LPS (D) were added to Mϕ cultures) or at 42 hr (B,E,H), before 1 µCi 3H-TdR was added to the cell cultures. Results are presented as concentration of NO metabolites (µM) in the supernatants (A,B; D,E; G,H) or % of inhibition of 3H-TdR incorporation into L5178Y lymphoma cells (C,F,I). *-negligible values. The experiments are representative of three separate experiments with similar results.
Incubation with L5178Y tumor cells induces alteration of Mϕ immunophenotype
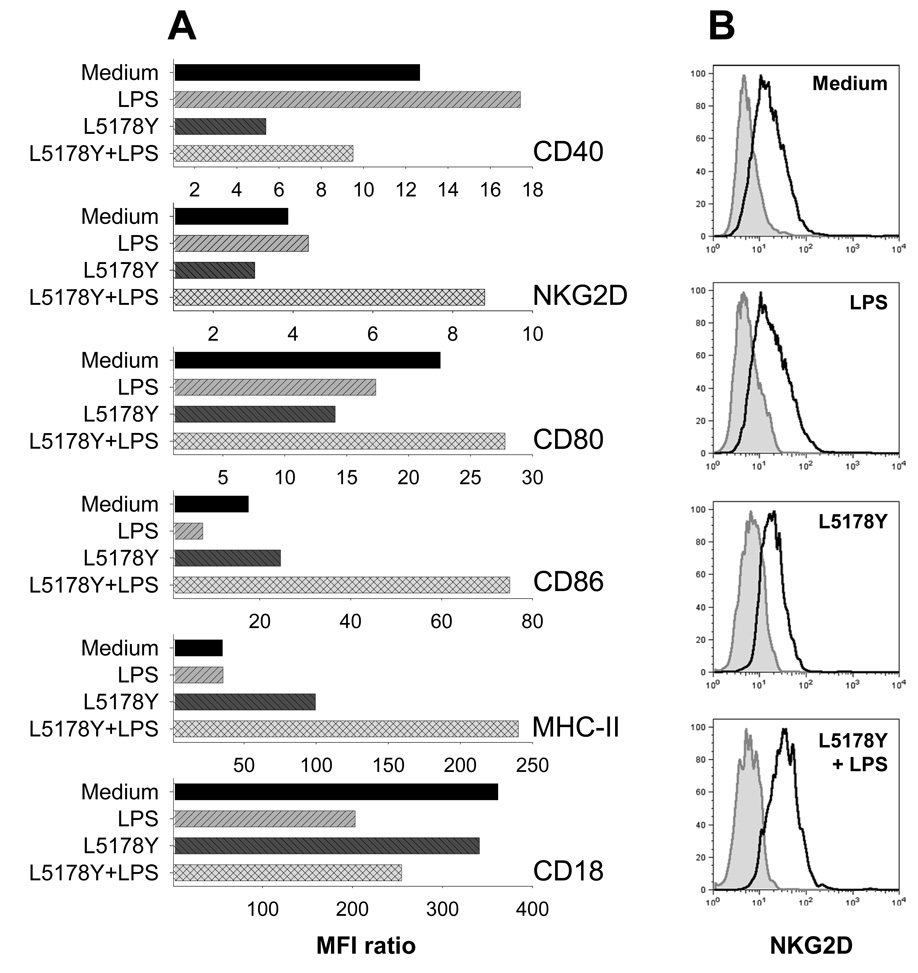
In addition to testing Mϕ abilities to produce NO and suppress tumor cell proliferation, we determined if Mϕ exposure to L5178Y cells and LPS had an effect on Mϕ immunophenotype. Adherent naïve Mϕ were cultured with or without L5178Y cells in the absence or presence of LPS. In our previous experiments (Fig. 3A) we found that co-culture of Mϕ with L5178Y cells and LPS for 24 hr led to Mϕ activation and production of a substantial amount of NO. Therefore, after 24 hr of co-culture, the culture supernatants and the L5178Y cells (growing non-adherently) were removed by repeated gentle pipetting and aspiration. Following this, the adherent Mϕ were additionally washed with warm PBS and harvested by vigorous pipetting with PBS containing EDTA for flow cytometric analysis. Expression of CD40, NKG2D, TLR9, CD18, MHC class II, CD80, and CD86 antigens by Mϕ was evaluated. The results (Fig. 4A) show that culturing Mϕ with L5178Y cells plus LPS suppressed expression of CD40 (7–25% in three separate experiments) and CD18 (17–29%), but induced substantial upregulation (44–127%) of expression of NKG2D (Figs. 4A,B), CD80 (16–23%), CD86 (101–328%) and MHC-II (201–585%) on Mϕ.
Figure 4. Priming of Mϕ by L5178Y cells results in alterations of Mϕ immunophenotype.
Naïve C57Bl/6 Mϕ were cultured for 24 h with or without L5178Y lymphoma cells in medium with or without LPS; Mϕ were then tested by flow cytometry for expression of different surface or intracytoplasmic molecules. Functional Grade Purified anti-mouse CD16+32 FcR block from eBioscience (clone 93, rat IgG2a) was used to pre-coat cells for 30 min on ice followed by washing of the cells. A. Results are presented as MFI ratios calculated as described in the Materials and Methods section. *-negligible values. The experiments are representative of three separate experiments with similar results. B. Representative histograms showing NKG2D expression on Mϕ. These correspond to the data shown for NKG2D expression in Fig. 4A. Dark grey peak – isotype-matched IgG control staining. Solid light grey line with grey fill – staining with anti-NKG2D mAb. MFI values for Mϕ stained with isotype-matched control IgG vs. anti-NKG2D mAb are 6.67 vs. 25.83 (Medium), 6.25 vs. 27.39 (LPS), 7.44 vs. 22.62 (L5178Y), and 6.99 vs. 55.02 (L5178Y+LPS).
The magnitude of NKG2D expression by Mϕ has remained somewhat controversial, probably because of the low amounts of NKG2D expressed on the cell surface of macrophages. Fig 4B displays representative histograms of NKG2D expression on naïve and manipulated Mϕ. αNKG2D mAb stained Mϕ, but not irrelevant cells such as B16 melanoma [MFI values for B16 cells stained with isotype control IgG vs. αNKG2D mAb were 5.27 vs. 5.95, respectively (data not shown)].
Alterations of immunophenotype of L5178Y lymphoma cells exposed to Mϕ
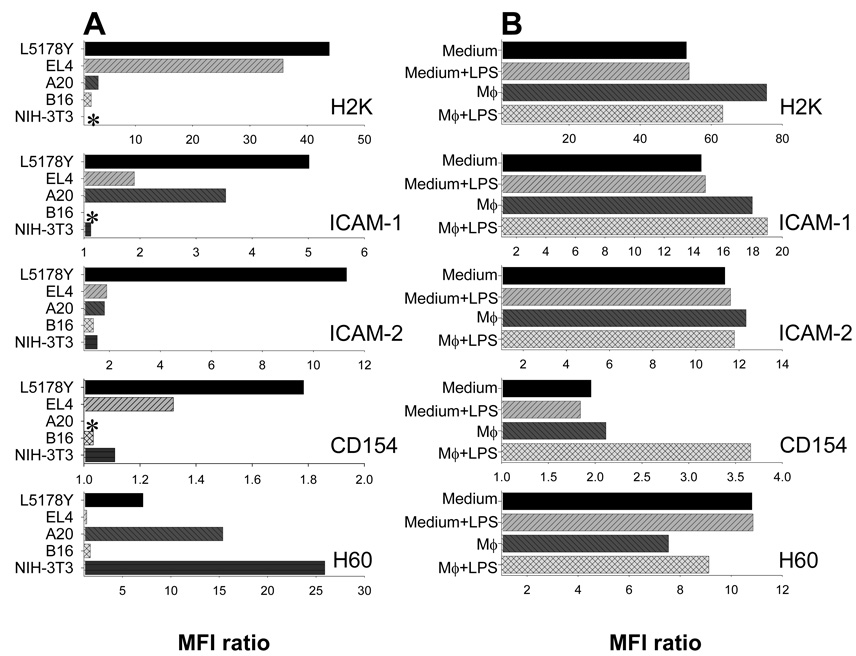
As L5178Y tumor cells in the presence of LPS altered the phenotype of Mϕ (Fig. 4), we hypothesized that exposure to activated Mϕ may result in phenotypic changes of tumor cells. First, L5178Y, EL4, A20, B16, and 3T3-NIH mouse cell lines (cultured in medium alone) were tested by flow cytometry for expression of MHC-I (H2), ICAM-1, ICAM-2, CD154, and H60 antigens (Fig. 5A), which were previously shown to be involved in a cross-talk with immune effectors. The results of this experiment suggest that no two cell lines were similar in the pattern and magnitude of expression of the tested surface antigens. Only L5178Y cells were found to express detectable levels of all the antigens at the time of analysis. Hence, the phenotype of L5178Y cells was established as MHC-I+/ICAM-1+/ICAM-2+/CD154+/H60+.
Figure 5. L5178Y lymphoma cells have unique immunophenotype that can be altered by exposure to Mϕ.
A, L5178Y and EL4 T-cell lymphoma cell lines, A20 B-cell lymphoma cell line, B16 melanoma cell line, and NIH-3T3 fibroblast cell line, cultured in medium alone, were tested by flow cytometry for expression of different surface antigens. Results are presented as MFI ratios calculated as described in Materials and Methods section. B, L5178Y lymphoma cells were cultured alone or in the presence of naïve C57BL/6 Mϕ in medium, with or without LPS. After 12 hr of co-culture, non-adherent cells were harvested and tested for expression of different antigens on F4/80-negative viable L5178Y lymphoma cells by flow cytometry. The experiments are representative of three separate experiments with similar results.
We tested if exposure of L5178Y cells to Mϕ (with or without LPS) would result in alterations of this immunophenotype. L5178Y cells were cultured either alone, as shown in Fig. 4A, or with adherent Mϕ, in medium with or without LPS. We did this analysis at 12 hr rather than at 24 hr (as done for Mϕ), to avoid manipulations with dead or dying L5178Y cells undergoing apoptosis. Following 12 hr of co-culture, L5178Y cells were harvested and the immunophenotype was evaluated (Fig 5B). The expression of MHC-I (H2k) and ICAM-1 was minimally increased following co-culture with Mϕ, and the expression of H60 was slightly downregulated with or without LPS. No regulatory effect on expression of ICAM-2 was documented. Interestingly, the expression of CD154 nearly doubled, provided that L5178Y cells were cultured with Mϕ and LPS. Notably, culture with LPS alone induced no alterations of L5178Y cell immunophenotype. Hence, cross-talk between Mϕ and L5178Y cells results in reciprocal alterations in immunophenotype of both Mϕ (Fig. 4) and L5178Y cells (Fig. 5B), and priming of Mϕ to LPS via TLR4, that ultimately leads to activation of anti-tumor Mϕ (Fig. 1B,C; Fig. 2).
The cross-talk between Mϕ and L5178Y cells requires direct cell-to-cell contact
We determined whether direct Mϕ-tumor cell contact is required for the effects observed in the previous experiments. We used the transwell (TW) in vitro system that allows for exchange of soluble secretory products, but not direct cell membrane contacts between Mϕ and L5178Y cells cultured in the same well. Mϕ were adhered to the bottom of the well, and L5178Y cells placed either together with Mϕ at the bottom of the well or in the TW-chamber. In some wells, L5178Y cells were placed both on the bottom of the well and in the TW-chamber. It was found that Mϕ could secrete substantial amounts of NO (Fig. 6A), and mediate in vitro anti-lymphoma effects (Fig. 6B) only when both Mϕ and L5178Y cells were co-cultured in close proximity, but not when they were separated by the semi-permeable membrane. When L5178Y cells were present both on the bottom of the well and in the TW-chamber, the LPS-activated anti-proliferative effect was mediated only towards those L5178Y cells that were cultured in proximity to Mϕ (Fig. 6B), despite apparent equilibrium of the NO (and presumably other cytotoxic factors secreted by activated Mϕ) in both compartments of the TW system (Fig. 6A). Only viable but not paraformaldehyde-fixed L5178Y cells triggered Mϕ activation, suggesting that either secretory activity or membrane fluidity and surface ligand re-arrangements of L5178Y cells were involved in the cross-talk with Mϕ.
Figure 6. Priming of Mϕ by L5178Y lymphoma requires direct tumor cell-Mϕ contact.
Naïve adherent C57BL/6 Mϕ were placed on the bottom of the wells in the transwell (TW) plates. Viable or paraformaldehyde fixed (PF)-L5178Y lymphoma cells were cultured either together with Mϕ (bottom), or placed in the TW-chamber, in the presence or absence of LPS. In a separate group, Mϕ were placed on the bottom of the wells and L5178Y cells were added both to the bottom of the well and into the TW-chamber. In this group, supernatants for NO detection were collected (A), and L5178Y cells (B) were removed for 3H-TdR incorporation evaluation either from the bottom of the wells (dashed rectangle) or from the TW-Chamber (dotted rectangle). Results are presented as concentration of NO metabolites (µM) in the supernatants (A) or % of inhibition of 3H-TdR incorporation into tumor cells (B). C, Naïve adherent Mϕ were cultured (with or without LPS) either with L5178Y lymphoma cells or in L5178Y cell-conditioned medium, or in L5178Y+LPS-conditioned medium. Results are presented as concentration of NO metabolites (µM) in the supernatants at 24 hr of experiment. *-negligible values. N/T-not tested as the PF-L5178Y cells did not incorporate 3H-TdR. The experiments are representative of three separate experiments with similar results.
To clarify a possible role of L5178Y cell-secreted factors in the Mϕ-L5178Y cross-talk, we used the L5178Y cell-conditioned supernatants from these lymphoma cell cultures, grown for 24 hr in medium with or without LPS. As shown in Fig. 6C, only L5178Y cells but not L5178Y cell-derived secretory products resulted in Mϕ activation and NO production. Hence, these findings demonstrate that direct and active cross-talk between naïve Mϕ and viable L5178Y cells is required for polarization of naïve Mϕ into immune effectors with cytotoxic properties.
Priming of Mϕ by L5178Y cells involves concurrent ligation of CD40, CD18, and NKG2D
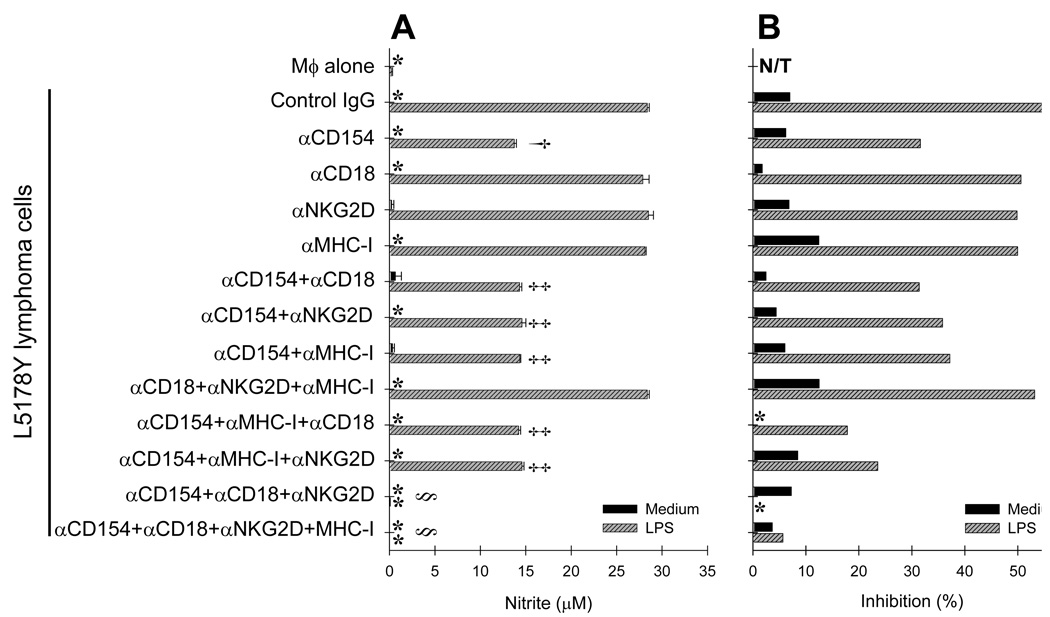
Multiple receptor-ligand interactions were previously shown to be involved in recognition of tumor cells by various immune effectors. As shown in Fig. 5A, L5178Y cells express CD154, ICAM-1, ICAM-2, and H60, which are known to be the functional ligands to CD40, CD18, and NKG2D receptors, respectively, expressed by Mϕ (Fig. 4). Therefore, we tested if these receptor-ligand pairs are involved in the Mϕ-L5178Y cell cross-talk. To exclude potential contribution of MHC-I mismatch between Mϕ and L5178Y cells, we used naïve Mϕ from DBA/2 mice, which were previously found to be sensitive to the priming effect by L5178Y cells (Table 1). To investigate the roles of CD40–CD154, NKG2D–H60, and CD18–ICAM-1/-2 interactions in the Mϕ-L5178Y cell cross-talk, we cultured Mϕ and L5178Y cells for 24 hr in medium with or without LPS and in the presence of blocking mAbs to the receptors or ligands. These blocking mAbs were used alone or in different combinations. Isotype-matched IgGs were used as control. When a combination of blocking mAbs was tested, αMHC-I (specific for both H-2Kd and H-2Dd) was used both as a control for potential ADCC via Fc receptors on Mϕ, and as a negative control, as it would bind to both the Mϕ and the L5178Y cells, but not directly interact with the specific receptor-ligand pairs that are recognized by the blocking mAbs. When a single blocking mAb was used (αCD154), it was found that interference with CD40–CD154 interactions reduced NO production (Fig. 7A) and in vitro anti-proliferative effects by approximately 50% (Fig. 7B). In contrast, blocking single interactions via CD18–ICAM-1/-2 (αCD18) or NKG2D–H60 (αNKG2D) had no distinct inhibitory effect on Mϕ priming. These mAbs did not activate Mϕ, thereby ruling out that their cross-linking by Fc receptors had a significant agonistic effect. The three-mAb combinations of αCD154 with either αCD18 or with αNKG2D and αMHC-I mAbs did not add to the effect of αCD154 mAb alone. However, simultaneous blocking of CD154, CD18, and NKG2D antigens on Mϕ (using the three-mAb combination of αCD154, αCD18, and αNKG2D) led to complete abrogation of Mϕ priming and anti-lymphoma effects in vitro (Fig. 7). This experiment was repeated three times with similar results.
Figure 7. L5178Y cell-mediated priming of Mϕ involves engagement of CD40, NKG2D, and CD18 on Mϕ.
Naïve Mϕ from syngeneic DBA/2 mice were cultured for 24 hr alone or with L5178Y cells in medium with or without LPS and in the presence of either control isotype-matched IgG or blocking mAbs against CD154, NKG2D, CD18, and MHC-I, used alone or in various combinations. Results are presented as concentration of NO metabolites (µM) in the cell culture supernatants (A) or % of inhibition of 3H-TdR incorporation into L5178Y lymphoma cells (B). *-negligible values. N/T-not tested. The experiments are representative of three separate experiments with similar results. †p=0.00064; ‡p>0.05; §p=0.00031 The symbols † corresponds to p value of αCD40 treatment bar compared to control IgG treatment bar; symbols ‡ correspond to p value of either combined treatment bars compared to αCD40 treatment bar; symbols § correspond to p values of αCD40+αCD18+αNKG2D or αCD40+αCD18+αNKG2D+αMHC-I treatments compared to any other combined treatments.
CD40-CD40L interaction is important for Mϕ-priming by tumor cells
In the next series of experiments, the role of CD40-CD40L interaction in Mϕ-priming by tumor cells was further addressed. We cultured ICAM-1+ICAM-2+H60+CD154+ L5178Y cells, ICAM-1+ICAM-2−H60+CD154− A20 cells, or ICAM-1−ICAM-2−H60−CD154− B16 cells with naïve Mϕ in medium with or without LPS in the presence of agonistic αCD40 (Fig. 8). In this experiment, we chose to use low (1 and 5 µg/ml) concentrations of αCD40, which we previously found to induce no or very little Mϕ activation in vitro (20). Figure 8A shows that L5178Y cells, but not A20 and B16 cells, could prime Mϕ in the presence of LPS but in the absence of exogenous αCD40. Adding αCD40 at 1 µg/ml to these cultures caused Mϕ activation in the presence of A20 cells but not B16 cells, nor did it further enhance the priming induced by L5178Y cells. Adding αCD40 at 5 µg/ml did not induce priming of Mϕ by B16 cells, but increased Mϕ-priming ability of both L5178Y and A20 cells, as shown by augmented NO production (in the presence of L5178Y cells) and tumoristasis (of both L5178Y and A20 cells). Hence, an additional signal provided by exogenous αCD40 was sufficient to enable Mϕ priming by CD154−ICAM-1+ICAM-2−H60+ A20 cells, but was not enough to enable Mϕ priming by CD154−ICAM-1−ICAM-2−H60− B16 cells, consistent with the hypothesis that ligation of CD40, CD18, and NKG2D receptors is required for priming of Mϕ. Exogenous αCD40 also augmented Mϕ priming by CD154+ICAM-1+ICAM-2+H60+ L5178Y cells, but only at the higher dose.
Figure 8. Role of CD40-–CD40L interaction in priming of Mϕ by tumor cells.
A. Peritoneal Mϕ from naïve C57BL/6 mice were cultured for 24 hr with L5178Y, A20, and B16 tumor cells in medium with or without 10 ng/ml LPS and agonistic αCD40 mAb, used at concentrations shown in X axis. Tumor cells and αCD40 mAb were added to Mϕ cultures 6 hr before LPS to enable Mϕ priming. At 24 hr, Mϕ activation was evaluated by measuring the cell culture supernatant NO metabolite content (upper panel) and inhibition of 3H-TdR incorporation into tumor cells (lower panel). The amount of NO secreted by Mϕ stimulated with LPS and αCD40 mAb but without tumor cells was negligible and is omitted from the figure.
B–E. Peritoneal Mϕ from CD40+/+ (B,C) and CD40−/− (D,E) C57BL/6 mice were cultured for 24 hr with or without L5178Y cells in medium with LPS, αCD40 mAb and mouse IFN-γ. Tumor cells, αCD40 mAb and IFN-γ were added to Mϕ cultures 6 hr before LPS to enable Mϕ priming. At 24 hr of co-culture, Mϕ activation was evaluated by measuring supernatant NO metabolite content (B,D) and inhibition of 3H-TdR incorporation into the tumor cells (C,E). *-value is below the detection limit. N/T - not tested. Mϕ cultured with L5178Y cells without LPS, with or without αCD40 mAb or IFN-γ, secreted negligible amounts of NO and demonstrated no antitumor effect (not shown).
The importance of CD40-CD40L interaction for Mϕ priming was confirmed using Mϕ from CD40−/− mice (Fig. 8B–E). The priming effect of L5178Y cells and αCD40, used alone or in combination, on CD40−/−Mϕ was either abrogated (Fig. 8D) or substantially reduced (Fig. 8E). However, CD40−/−Mϕ were readily primed by IFN-γ, which served as a positive control demonstrating the ability of these Mϕ to become activated via other mechanisms.
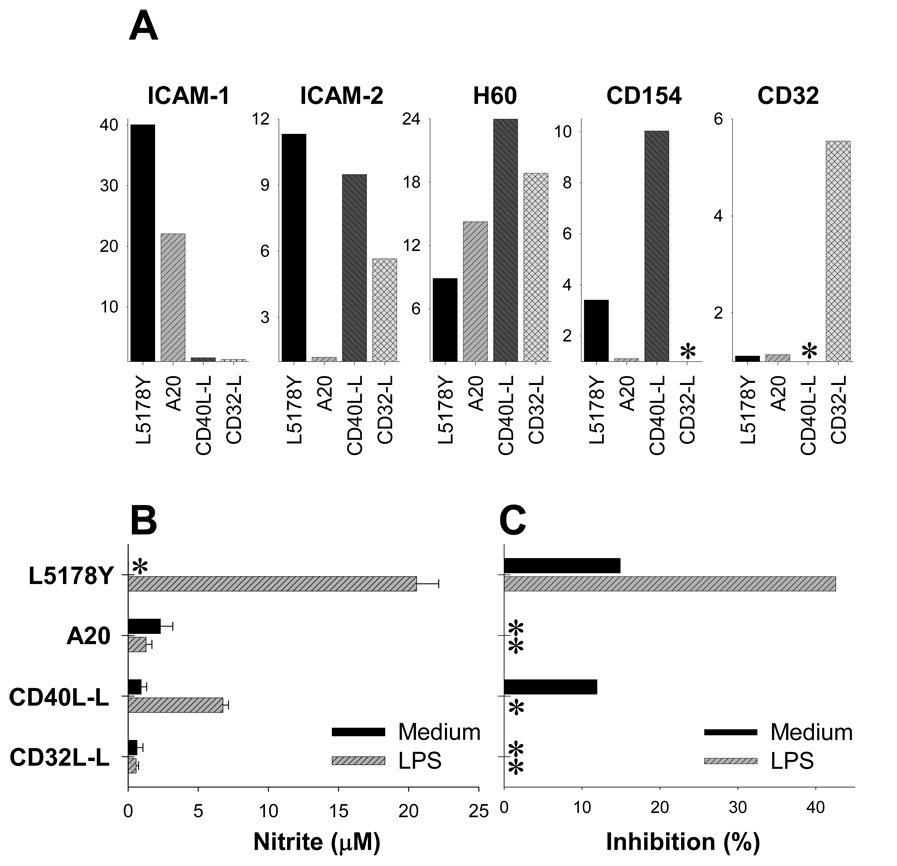
To further clarify role of CD40-CD40L interaction in priming of Mϕ by tumor cells, we used mouse L fibroblasts transfected with CD40L or CD32 (transfection control). In preliminary experiments, CD40L-L cells were confirmed to stimulate proliferation of mouse splenic CD19+ B cells (not shown). These CD40L-L and CD32-L lines also expressed ICAM-2 and H60, thereby showing phenotypic similarity with L5178Y cells (Fig. 9A). However, in contrast to L5178Y cells, CD40L-L cells could not effectively prime Mϕ to subsequent stimulation with LPS, which was shown by a limited NO production (Fig. 9B) and absence of cytostasis (Fig. 9C).
Figure 9. CD154+ICAM-I/II+H60+ L5178Y lymphoma cells but not CD154+ICAM-II+H60+ L-fibroblasts prime Mϕ.
A. Lymphoid L5178Y and A20 and non-lymphoid fibroblast CD40L-L and CD32-L cells lines were tested by flow cytometry for expression of ICAM-1, ICAM-2, H60, CD154, and CD32 surface antigens. Results are presented as MFI ratio values, as described in the Materials and Methods. B,C. Naïve adherent C57BL/6 Mϕ were cultured for 24 hr with L5178Y, A20, CD40L-L, and CD32-L cell lines in medium with or without LPS, and Mϕ activation was measured. Results are presented as concentration of NO metabolites (µM) in the supernatants (B) or % of inhibition of 3H-TdR incorporation into L5178Y, A20, CD40L-L, and CD32-L cells (C). * - negligible value.
However, when CD40L-L cells were mixed together with a small number of viable L5178Y cells (0.5–1×104 cells/well) that would be otherwise insufficient to prime Mϕ (Fig. 1–3), Mϕ became activated and were found to secrete NO (Fig. 10A). This synergistic priming effect was observed only when L5178Y cells were combined with CD40L-L but not CD32-L cells, suggesting that the interaction of CD40L on tumor cells with CD40 on Mϕ is required for the priming. Interestingly, A20 cells were also able to provide co-stimulation to enable CD40L-L cells, but not CD32-L cells, to prime Mϕ (Fig. 10B). These results suggest that co-stimulation by L5178Y and A20 cells does not require CD154 expression “in cis”, as A20 cells are CD154-negative (Fig 9A), but might depend on the expression of ICAM-I or soluble factors secreted by lymphoma cells.
Figure 10. Synergistic effect of CD40L-L fibroblasts and L5178Y or A20 lymphoma cells on Mϕ priming.
A,B. Peritoneal Mϕ (~3×105 cells/well) from naïve C57BL/6 mice were cultured for 24 hr in medium or in the presence of CD40L-L or CD32-L cells (2.5×104 cells/well), with our without LPS in medium. At 1 hr of experiment, different numbers of L5178Y (A) or A20 (B) cells were added to some Mϕ-L cell cultures. At 24 hr, Mϕ activation was evaluated by measuring concentration of NO metabolites in cell culture supernatants.
C,D. Peritoneal Mϕ (~3×105 cells/well) from CD40+/+ (C) and CD40−/− (D) C57BL/6 mice were cultured for 24 hr in medium with or without LPS and in the presence of L5178Y cells or CD40L-L cells (1×104 cells/well), added to Mϕ separately or mixed together. At 24 hr, Mϕ activation was evaluated by measuring concentration of NO metabolites in cell culture supernatants.
To confirm the importance of CD40-CD40L interaction for efficient priming of Mϕ in this test model, we stimulated Mϕ from CD40+/+ and CD40−/− mice with CD40L-L and L5718Y cells (1×104 cells/well), alone or in combination. Similarly to the results shown in Figure 8B–E, the priming of Mϕ with a combination of these tumor cells was substantially reduced in CD40−/− mice compared to CD40+/+ mice, as shown by decreased NO production (Fig. 10C). Together with the findings presented above, these results demonstrate that the Mϕ priming we observe with the tumor cells tested here is a consequence of tumor cell expression of CD40L, ICAM-I, and H60.
L5178Y cells prime mouse naïve Mϕ in vivo
In the next series of experiments, we tested whether the priming of Mϕ with L5178Y lymphoma cells observed in vitro can also take place in vivo. L5178Y cells (5×105) were implanted i.p. into naïve DBA/2 mice, followed by i.v. injection of 20 ng LPS 24 hrs later. This dose was used to achieve an endotoxin concentration in vivo similar to that used in our in vitro experiments (10 ng/ml): the mice used in the experiments, weighing about 30 gram each, have a blood volume of approximately 2 ml. Three other groups of mice received L5178Y cells alone, LPS alone, or PBS (control). After 48 hr, PC were harvested, and Mϕ were tested for production of IL4, IL10, IFN-γ, TNF-α, and IL12p40, as well as alterations of expression of NKG2D, CD80, CD86, and MHC-II surface antigens (Fig. 11A).
Figure 11. L5178Y cells and LPS synergize in activation of Mϕ in vivo.
A. DBA/2 mice (n=3/group) were injected i.p. with L5178Y cells (5×105/ml) or PBS (control). Twenty-four hrs later, the mice were treated i.v. with 0.2 ml PBS with or without 20 ng LPS. At 48 hr of experiment, mice were euthanized, resident PC collected from each mouse of the experimental group and pooled, and F4/80+ Mϕ tested by flow cytometry for production of IL4, IL10, IFN-γ, TNF-α and IL12 or expression of NKG2D, CD80, CD86, and MHC-II. On histograms: grey filled peaks – staining with isotype-matched control IgG, open black peak – staining with specific mAb. Numbers are MFI ratios calculated as described in Materials and Methods.
B. Proliferation of CFSE-labeled L5178Y cells is suppressed in DBA/2 mice after systemic administration of low amount of LPS. CFSE-labeled L5178Y cells (5×105/ml) were injected i.p. into DBA/2 mice (n=3/group). Twenty-four hours after tumor cell implantation the mice were treated i.v. with 0.2 ml PBS with or without 20 ng LPS. After 48 hr, mice were euthanized, resident peritoneal cells + tumor cells collected from each mouse of the group and pooled, and F4/80−CFSE+L5178Y were tested by flow cytometry for CFSE fluorescence. In parallel, CFSE-labeled L5178Y cells (5×105/ml, 0.05 ml/well) were cultured in vitro in triplicates with naïve DBA/2 Mϕ as described in Fig. 3D–F. After 48 hr, cell cultures were collected by pipetting and pooled into one analysis sample for each experimental group, and F4/80−CFSE+L5178Y were tested by flow cytometry for CFSE fluorescence. As a proliferation suppression control, L5178Y cells were γ-irradiated. Results are presented as CFSE Mean Fluorescence Intensity.
Injection of L5178Y cells alone slightly augmented production of IFN-γ (42%) and TNF-α (40%), as well as significantly upregulated MHC-II (900%) by Mϕ. LPS alone augmented secretion of IFN-γ (125%), TNF-α (80%), and IL10 (100%), MHC-II (108%) and slightly upregulated NKG2D (23%). However, in animals that received both L5178Y implantation and LPS, Mϕ were found to secrete significant amounts of IL10 (218%), IFN-γ (617%), and TNF-α (970%) and upregulated CD86 (30.6%), and MHC-II (1088%), whereas expression of CD80 (8.7%) and secretion of IL4 (14%) and IL12 (13%) was almost unaltered. Although the level of NKG2D expression on mouse Mϕ from the L5178Y+LPS group was 27% higher than on Mϕ from control pre-treatment and L5178Y-only recipient groups (MFI ratio: 2.8 vs. 2.2 vs. 1.6), it was not different (3.7% increase) from the levels of expression on Mϕ from the LPS-only experimental group (MFI ratio: 2.8 vs. 2.7).
In parallel (Fig. 11B), we tested the in vivo anti-tumor effects of Mϕ primed to LPS by L178Y cells, and found that proliferation of CFSE-labeled L5178Y cells injected i.p. was suppressed in DBA/2 mice after i.v. administration of low amount of LPS. Thus, CFSE+L5178Y cells unrestrictedly proliferated in control, PBS-treated animals, and by the end of experiment retained only 4.5% of CFSE fluorescence as compared to CFSE+L5178Y cells that were kept in medium in vitro and were γ-irradiated prior to incubation to permanently suppress their proliferation. At the same time, CFSE+L5178Y from animals that were challenged with LPS 24 hr after CFSE+L5178Y implantation were found to retain ~20% of the amount of CFSE as compared to control, γ-irradiated cells. Similar results were seen with CFSE+L5178Y cells cultured with naïve Mϕ in vitro. Together, these results show that L5178Y cells can prime naïve Mϕ in vivo to subsequent stimulation with LPS, which results in Mϕ activation, as revealed by phenotypic alterations, change of cytokine expression, and anti-tumor activity.
Discussion
In this study, we demonstrate that Mϕ can recognize L5178Y tumor cells. Previous studies indicated that isolated ligation of CD40, NKG2D, and CD18 can be involved in activation of Mϕ (17,20,23). Our current study dissects a functional hierarchy of these interactions in a polyvalent cross-talk between tumor cells and Mϕ. Even though CD154 is expressed in relatively low amounts on L1578Y cells (Fig. 5 A), its role in priming of Mϕ via CD40 was found to have a much greater stimulatory impact on Mϕ than Mϕ cross-linking via CD18 by ICAM-1 and ICAM-2, which are highly expressed on L5178Y cells. Similarly, whereas isolated blockage of NKG2D–H60 interactions had no noticeable effect on Mϕ in vitro secretory and anti-tumor activities, it was required, in addition to blockage of CD40–CD154 and CD18–ICAM-1/-2, for abrogation of Mϕ activation by L5178Y cells. Thus, the Mϕ-tumor cell cross-talk that ultimately leads to induction of anti-tumor Mϕ involves concurrent engagement of multiple receptor-ligand pairs.
It also appears that tumor cells should simultaneously express several stimulatory ligands in order to induce Mϕ activation. In this regard, normal syngeneic mouse splenocytes (essentially comprised of CD3+ T cells [~35%], B220+ B cells [~40%], CD11b+ mononuclear cells [~10%], and CD49b+ NK cells [5–7%]) did not prime Mϕ, in contrast to priming by L5178Y cells. Whereas most of the splenocytes expressed ICAM-1 and ICAM-2 (in a very heterogeneous fashion), only a small fraction (mostly CD4+ T cells, less than 15% of total naïve splenocytes) expressed CD 154 (and only at very low levels), and no splenocytes expressed ligands of NKG2D (data not shown). Activation of splenocytes with LPS and CpG to produce IL12 and IFN-γ (24–26) might facilitate Mϕ activation and induce moderate levels of anti-tumor effects in vitro without involvement of the CD40/NKG2D/CD18-dependent mechanism; however, in our in vitro experiments, splenocytes did not trigger NO production by Mϕ. Indeed, culturing Mϕ with 1×104 L5178Y cells led to only marginal activation of Mϕ (Fig 1B–E, Fig 2), whereas culturing Mϕ with 2.5–5×104 L5178Y cells led to robust activation of Mϕ.
Several reports have previously demonstrated that the Mycoplasma arginini – infected L5178Y and YAC-1 cell lines could activate thioglycollate-elicited peritoneal Mϕ in the presence of LPS or exogenous IFN-γ to mediate in vitro anti-tumor effects via NO- and TNF-α-dependent mechanisms (28,29). Results of those studies differed in the sensitivity of the model to the suppressive effects of PG-E2, but both agreed that the priming effects could be transferred by the tumor cell-conditioned supernatants or mediated by paraformaldehyde-fixed tumor cells. In our study, we tested L5178Y cell line for Mycoplasma spp. contamination, and found no evidence for infection. The absence of Mycoplasma spp. in L5178Y cells was also confirmed at a DNA level by using fluorescent probes (data not shown). Most importantly, our results, unlike those published by Young et al. (28), and Ribeiro-Dias et al. (29), suggest that priming of Mϕ required direct cell contact with viable L5178Y cells; Mϕ could not be primed by either L5178Y cell-conditioned supernatants (Fig. 5C) or fixed L5178Y cells. Furthermore, separation of Mϕ and viable L5178Y cells by the transwell also abrogated priming of Mϕ. Whereas we cannot exclude a contribution of soluble, LPS-induced tumor cell-derived factors, such as IL-1 (30), our results suggest that membrane-bound, but not soluble factors are important for induction of cytotoxic Mϕ in our in vitro model. The inability of fixed L5178Y cells to prime Mϕ might also be due to potential toxic effects of fixed L5178Y cells on Mϕ (such as leaching of paraformaldehyde). However, our preliminary data argue against this, because co-culture of fixed L5178Y cells with viable L5178Y cells did not affect proliferation of viable L5178Y cells as measured by 3H-TdR incorporation (data not shown). Complete absence of Mϕ priming by fixed L5178Y cells in our in vitro experiments could be explained by denaturation of CD154 subunits by paraformaldehyde, or by inability of fixed L5178Y cells to upregulate CD154 expression upon contact with Mϕ in the presence with LPS, which might be important for compensation of CD40-downregulation on L5178Y-primed Mϕ. Lastly, loss of L5178Y cell membrane fluidity and inability of PF-L5178Y cells to mobilize CD154-, H60- or ICAM-1- and ICAM-2-containing membrane rafts to the site of direct contact with Mϕ might also lead to this loss of Mϕ-priming properties, provided that the cells express no other priming substances such as those associated with Mycoplasma spp. contamination (28,29).
Despite high concentrations of NO produced by L5178Y cell + LPS-stimulated Mϕ, L5178Y cell proliferation in the TW-chamber was not suppressed when co-cultured with L5178Y cell + LPS-stimulated Mϕ in the bottom of the TW-chamber system. In contrast, direct contact with Mϕ resulted in substantial anti-tumor effects, suggesting membrane-mediated death signaling. In this regard, we found only very limited expression of membrane-bound TNF-α, FasL, and TRAIL on the surface of Mϕ after their exposure to L5178Y cells and LPS (data not shown). The potential roles of other factors in L5178Y + LPS-stimulated Mϕ-mediated cytotoxicity remain to be clarified.
Flow cytometric analysis of the immunophenotype of Mϕ and L5178Y cells revealed that exposure of these cells to each other results in changes in expression of some of the antigens forming certain receptor-ligand pairs. Hence, a relative decrease (7–25%) of CD40 expression on Mϕ coincided with upregulation (31–88%) of CD154 on L5178Y cells, after these cells were cultured in the presence of LPS. Similarly, upregulation (44–127%) of NKG2D on Mϕ coincided with a slight decrease (11–16%) in expression of H60 on L5178Y cells. The observed alterations of the CD40–CD154 pair expression could favor recognition of L5178Y cells via CD154. The decreased expression of H60 on L5178Y cells following contact with Mϕ is in agreement with the observation by Bui et al. (31) that type I IFN down-regulated H60 in some tumors, and could be associated with mechanisms of immune evasion (32); yet we observed upregulation of NKG2D expression on L5178Y lymphoma-primed Mϕ in the presence of LPS (Fig 4). Unlike the increased MHC-I we have noted on tumor cells escaping from NK cell effects (33), there was no substantive change in MHC-I expression on L5178Y cells cultured with Mϕ (Fig.5B). Treatment of L5178Y cells with αMHC-I mAb did not alter secretory and anti-proliferative functions of Mϕ (Fig. 7). In agreement with this, we have recently demonstrated that αCD40-induced Mϕ-mediated in vivo anti-tumor effects can be effectively induced against both highly-and weakly-immunogenic tumors, independent of MHC-I expression (34).
Although the expression of NKG2D and its potential role in Mϕ activation and Mϕ–mediated killing has been controversial (17, 35), our data document upregulation of cell surface NKG2D on Mϕ when cultured together with L5178Y cells and LPS. Furthermore, we demonstrate the abrogation of Mϕ activation in these cultures when Mϕ–based CD40, CD18, and NKG2D molecules are simultaneously prevented from recognizing their respective ligands. These data are consistent with the demonstration that mouse Mϕ activated by LPS and IFN-γ upregulate NKG2D and these activated Mϕ kill tumor cells that express adenovirus E1A in an NKG2D-dependent manner (J. Routes, Medical College of WI, manuscript in preparation, personal communication).
In a variety of immunological reactions, Mϕ can serve as antigen-presenting cells providing stimuli for T cells by MHC class II-associated exogenous antigens as well as via a number of co-stimulatory molecules. However, engagement of certain cell surface molecules on Mϕ causes reciprocal signaling, resulting in Mϕ activation. Several Mϕ membrane-associated surface molecules, including CD40, MHC class II, and CD18, could be involved in this reciprocal stimulation associated with modulation of signaling pathways, production of cytokines, and changes of immunophenotype. Frequently, ligation of more than one type of molecule on the surface of Mϕ is required for induction of cytokines. For example, it was shown that interaction of Mϕ with T cells via CD40–CD154 leads to accumulation of IL-12p40 in Mϕ; at the same time, additional contacts via the MHC class II/TCR pair was needed to trigger Mϕ to produce IL-12p35, thus enabling production of functional IL-12p70 (36–38). Our results demonstrate that L5178Y lymphoma cells express high amounts of H60 as well as ICAM-1 and ICAM-2. Thus, it is possible that H60 and ICAM-1/-2 molecules could synergize with CD154 in Mϕ activation. The potential mechanisms of these additional interactions between Mϕ and L5178Y cells remain to be clarified, but could range from a simple anchoring of the ICAM+-tumor cells to the surface of CD18+-Mϕ (18) (to facilitate cross-talk via CD40-CD154 and NKG2D-H60 pairs) to an active recruitment of a Toll/IL-1 receptor family-like cascade to modulate TLR-signaling (39). The latter effect on adapter proteins of TLR-signaling pathways might partially explain selective sensitivity of L5178Y-primed Mϕ to LPS but not CpG, although the most likely explanation is that peritoneal mouse Mϕ readily express TLR4 (40), whereas TLR9 expression is negligible without proper stimulation (21).
Alternatively, the ability to prime Mϕ may be restricted to the cells of a certain embryonic origin. Thus, L5178Y cells and A20 cells of lymphoid origin could prime Mϕ [for A20 cells, presence of the exogenous αCD40 (Fig. 8A) or other CD40L-expressing cells (Fig. 10B) was required], whereas B16 melanoma cells (neural crest origin) and mouse fibroblast L cells (mesenchymal origin) did not have this ability even in the presence of exogenous αCD40 (B16, Fig. 8A) or after transfection with functional CD40L (CD40L-L, Fig. 9). L5178Y and A20 cells have also been able to facilitate priming by other CD154+ cells (CD40L-L), but this co-stimulatory ability has been lost when cells were paraformaldehyde-fixed (not shown). Importantly, this effect was independent from the CD154-expressing status of these lymphoma cells, as A20 cells are CD154-negative (Fig. 5A). Hence, it remains possible that some soluble factors or other surface co-stimulatory molecules expressed by lymphoid cells in the process of initial cross-talk between tumor cells and Mϕ, are involved in the process of Mϕ priming.
The conclusions made from in vitro experiments were consistent with data obtained in our in vivo tumor model. Intraperitoneal implantation of L5178Y cells resulted in alterations of phenotype of resident Mϕ (upregulation of MHC-II and downregulation of CD80 and CD86), as well as expression of IFN-γ and TNF-α, but not IL4 or IL10 (Fig. 11). It is unclear whether these immunological changes are solely due to interaction of peritoneal Mϕ with L5178Y cells, or if this is a result of the cross-talk between Mϕ and tumor cells in the presence of very low, “physiological” concentrations of circulating endotoxin. However, when small doses of LPS were given to the tumor cell recipients, Mϕ expressed much higher levels of MHC-II, CD80, CD86, IFN-γ, and TNF-α than after the treatment with L5178Y cells or LPS alone. Surprisingly, there was a significant increase of production of IL10, which is known to play an important role in regulation of Mϕ activation. Discrepancy in magnitude of Mϕ phenotypic changes seen in in vitro vs. in vivo models could be explained by overall differences in experimental conditions. Thus, in in vitro experiments (Fig. 4) Mϕ and L5178Y cells were in constant contact for the entire duration of the experiment, and the volume of distribution of Mϕ-derived and potentially L5178Y cell-derived soluble factors was confined to 0.2 ml. On the other hand, in the in vivo experiment interactions between Mϕ and L5178Y cells could be less durable and the volume of distribution of soluble factors was much larger and potentially affected by physiologic metabolic processes. Even if the overall phenotypic changes are less pronounced in vivo, they could be considered as proof of principle of active Mϕ-L5178Y cell cross-talk resulting in phenotypic and functional alterations of Mϕ.
Efficient tumor recognition at the early stage of tumor progression might be a critical component of cancer immune surveillance. The state of “dormant” cancer exemplifies immune-mediated tumor growth restriction in the absence of active exogenous immunostimulatory interventions. L5178Y lymphoma in DBA/2 mice has been used for decades to study this biological phenomenon (41–44). Both cytotoxic T cells and Mϕ are involved in maintaining L5178Y lymphoma in the dormant non-progressive stage of disease (42–44). In the present study, we suggest an additional mechanism of this immunological lymphoma surveillance: L5178Y cell-induced priming of Mϕ to endotoxin, which is naturally present in biological fluids in minute concentrations (45), might be involved in Mϕ activation and result in anti-lymphoma effects in vivo. A number of reports demonstrate that human T cell lymphomas, as well as other cancers, can express CD154 (46–51). Whether human Mϕ can recognize autologous cancer cells via similar mechanisms, shown here for L5178Y cells, remains to be determined.
Acknowledgements
The authors thank Drs. Jacquelyn A. Hank, Jacek Gan, and Zane C. Neal of The University of Wisconsin, and Dr. Jack Routes of The Medical College of Wisconsin, for helpful discussions.
The authors also thank Mrs. Patricia Sharp, Department of Pathobiological Sciences of The UW School of Veterinary Medicine, for testing the tumor cell lines for Mycoplasma spp. contamination, and Dr. Qingli Wu, Department of Pathology and Laboratory Medicine at UW, for helping with FlowJo flow cytometry analysis.
Non-standard abbreviations used
- Mϕ
macrophages
- PC
peritoneal cells
- 3H-TdR
3H-thymidine
- PF
paraformaldehyde-fixed
- TW
transwell
Footnotes
This work was supported by The National Institutes of Health Grants CA87025 and CA032685 (to PMS) and AI066897 (to LLL), grants from the Midwest Athletes Against Childhood Cancer Fund (to PMS, ALR and INB), and the grant from the UW Cure Kids Cancer Coalition (INB and PMS).
LLL is an American Cancer Society Research Professor.
References
- 1.Blattman JN, Greenberg PD. Cancer immunotherapy: a treatment for the masses. Science. 2004;305:200–205. doi: 10.1126/science.1100369. [DOI] [PubMed] [Google Scholar]
- 2.Chang CC, Ferrone S. NK cell activating ligands on human malignant cells: molecular and functional defects and potential clinical relevance. Semin. Cancer. Biol. 2006;16:383–392. doi: 10.1016/j.semcancer.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 3.Lamagna C, Aurrand-Lions M, Imhof BA. Dual role of macrophages in tumor growth and angiogenesis. J. Leukoc. Biol. 2006;80:705–713. doi: 10.1189/jlb.1105656. [DOI] [PubMed] [Google Scholar]
- 4.Greeneltch KM, Schneider M, Steinberg SM, Liewehr DJ, Stewart TJ, Liu K, Abrams SI. Host immunosurveillance controls tumor growth via IFN regulatory factor-8 dependent mechanisms. Cancer. Res. 2007;67:10406–10416. doi: 10.1158/0008-5472.CAN-07-1228. [DOI] [PubMed] [Google Scholar]
- 5.Bottino C, Castriconi R, Moretta L, Moretta A. Cellular ligands of activating NK receptors. Trends Immunol. 2005;26:221–226. doi: 10.1016/j.it.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Coudert JD, Held W. The role of the NKG2D receptor for tumor immunity. Semin. Cancer. Biol. 2006;16:333–343. doi: 10.1016/j.semcancer.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Hayakawa Y, Smyth MJ. Innate immune recognition and suppression of tumors. Adv. Cancer. Res. 2006;95:293–322. doi: 10.1016/S0065-230X(06)95008-8. [DOI] [PubMed] [Google Scholar]
- 8.Cerwenka A, Baron JL, Lanier LL. Ectopic expression of retinoic acid early inducible-1 gene (RAE-1) permits natural killer cell-mediated rejection of a MHC class I-bearing tumor in vivo. Proc. Natl. Acad. Sci. USA. 2001;98:11521–11526. doi: 10.1073/pnas.201238598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liao NS, Bix M, Zijlstra M, Jaenisch R, Raulet D. MHC class I deficiency: susceptibility to natural killer (NK) cells and impaired NK activity. Science. 1991;253:199–202. doi: 10.1126/science.1853205. [DOI] [PubMed] [Google Scholar]
- 10.Diefenbach A, Hsia JK, Hsiung MY, Raulet DH. A novel ligand for the NKG2D receptor activates NK cells and macrophages and induces tumor immunity. Eur. J. Immunol. 2003;33:381–391. doi: 10.1002/immu.200310012. [DOI] [PubMed] [Google Scholar]
- 11.Bryceson YT, March ME, Ljunggren HG, Long EO. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood. 2006;107:159–166. doi: 10.1182/blood-2005-04-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vĕtvicka V, Hanikýrová M, Vĕtvicková J, Ross GD. Regulation of CR3 (CD11b/CD18)-dependent natural killer (NK) cell cytotoxicity by tumour target cell MHC class I molecules. Clin. Exp. Immunol. 1999;115:229–235. doi: 10.1046/j.1365-2249.1999.00800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Girart MV, Fuertes MB, Domaica CI, Rossi LE, Zwirner NW. Engagement of TLR3, TLR7, and NKG2D regulate IFN-gamma secretion but not NKG2D-mediated cytotoxicity by human NK cells stimulated with suboptimal doses of IL-12. J. Immunol. 2007;179:3472–3479. doi: 10.4049/jimmunol.179.6.3472. [DOI] [PubMed] [Google Scholar]
- 14.Bertschmann M, Käsermann D, Keller R. MHC class-I antigen deficiency, malignancy and susceptibility of P815 mastocytoma to NK and macrophage killing. Int. J. Cancer. 1990;46:739–744. doi: 10.1002/ijc.2910460431. [DOI] [PubMed] [Google Scholar]
- 15.Nomi H, Tashiro-Yamaji J, Miura-Takeda S, Shimizu T, Azuma H, Ueda H, Katsuoka Y, Kubota T, Yoshida R. Infiltration of H-2d-specific cytotoxic macrophage with unique morphology into rejection site of allografted meth A (H-2d) tumor cells in C57BL/6 (H-2b) mice. Microbiol. Immunol. 2007;51:297–306. doi: 10.1111/j.1348-0421.2007.tb03911.x. [DOI] [PubMed] [Google Scholar]
- 16.Cerwenka A, Lanier LL. NKG2D ligands: unconventional MHC class I-like molecules exploited by viruses and cancer. Tissue Antigens. 2003;61:335–343. doi: 10.1034/j.1399-0039.2003.00070.x. [DOI] [PubMed] [Google Scholar]
- 17.Diefenbach A, Jamieson AM, Liu SD, Shastri N, Raulet DH. Ligands for the murine NKG2D receptor: expression by tumor cells and activation of NK cells and macrophages. Nat. Immunol. 2000;1:119–126. doi: 10.1038/77793. [DOI] [PubMed] [Google Scholar]
- 18.Strassmann G, Springer TA, Somers SD, Adams DO. Mechanisms of tumor cell capture by activated macrophages: evidence for involvement of lymphocyte function-associated (LFA)-1 antigen. J. Immunol. 1986;136:4328–4333. [PubMed] [Google Scholar]
- 19.Singh AR, Sodhi A. LFA-1-dependent tumoricidal activity of cisplatin-treated macrophages. Immunol. Cell. Biol. 1998;76:343–349. doi: 10.1046/j.1440-1711.1998.00751.x. [DOI] [PubMed] [Google Scholar]
- 20.Buhtoiarov IN, Lum HD, Berke G, Paulnock DM, Sondel PM, Rakhmilevich AL. CD40 ligation activates murine macrophages via an IFN-gamma-dependent mechanism resulting in tumor cell destruction in vitro. J. Immunol. 2005;174:6013–6022. doi: 10.4049/jimmunol.174.10.6013. [DOI] [PubMed] [Google Scholar]
- 21.Buhtoiarov IN, Lum HD, Berke G, Sondel PM, Rakhmilevich AL. Synergistic activation of macrophages via CD40 and TLR9 results in T cell independent antitumor effects. J. Immunol. 2006;176:309–318. doi: 10.4049/jimmunol.176.1.309. [DOI] [PubMed] [Google Scholar]
- 22.McGarrity GJ, Steiner T, Vanaman V. Detection of Mycoplasmal Infection of Cell Cultures by DNA Fluorochrome Staining. In: Tully JG, Razin S, editors. Methods in Mycoplasmology Vol. 2 Diagnostic Mycoplasmology. New York: Academic Press; 1983. [Google Scholar]
- 23.Matsuno R, Aramaki Y, Arima H, Adachi Y, Ohno N, Yadomae T, Tsuchiya S. Contribution of CR3 to nitric oxide production from macrophages stimulated with high-dose of LPS. Biochem. Biophys. Res. Commun. 1998;244:115–119. doi: 10.1006/bbrc.1998.8231. [DOI] [PubMed] [Google Scholar]
- 24.Varma TK, Lin CY, Toliver-Kinsky TE, Sherwood ER. Endotoxin-induced gamma interferon production: contributing cell types and key regulatory factors. Clin. Diagn. Lab. Immunol. 2002;9:530–543. doi: 10.1128/CDLI.9.3.530-543.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hino A, Igarashi O, Tagawa YI, Iwakura Y, Nariuchi H. Interferon gamma priming is not critical for IL-12 production of murine spleen cells. Cytokine. 2000;12:12–20. doi: 10.1006/cyto.1999.0515. [DOI] [PubMed] [Google Scholar]
- 26.Hochrein H, Shortman K, Vremec D, Scott B, Hertzog P, O'Keeffe M. Differential production of IL-12, IFN-alpha, and IFN-gamma by mouse dendritic cell subsets. J. Immunol. 2001;166:5448–5455. doi: 10.4049/jimmunol.166.9.5448. [DOI] [PubMed] [Google Scholar]
- 27.Hitoshi Y, Mita S, Tominaga A, Kikuchi Y, Sonoda E, Takatsu K, Watanabe Y. Interferon-gamma inhibits the proliferation but not the differentiation of murine B cells in response to IL-5. Int. Immunol. 1989;1:185–190. doi: 10.1093/intimm/1.2.185. [DOI] [PubMed] [Google Scholar]
- 28.Yang G, Coffman FD, Wheelock EF. Characterization and purification of a macrophage-triggering factor produced in Mycoplasma arginini-infected L5178Y cell cultures. J. Immunol. 1994;153:2579–2591. [PubMed] [Google Scholar]
- 29.Ribeiro-Dias F, Russo M, Marzagão Barbuto JA, Fernandes do Nascimento FR, Timenetsky J, Jancar S. Mycoplasma arginini enhances cytotoxicity of thioglycollate-elicited murine macrophages toward YAC-1 tumor cells through production of NO. J. Leukoc. Biol. 1999;65:808–814. [PubMed] [Google Scholar]
- 30.Uchimura E, Watanabe N, Kobayashi Y. Modulation by lipopolysaccharide of inflammatory cytokine production by two T cell lines. Cytokine. 1997;9:727–733. doi: 10.1006/cyto.1997.0230. [DOI] [PubMed] [Google Scholar]
- 31.Bui JD, Carayannopoulos LN, Lanier LL, Yokoyama WM, Schreiber RD. IFN-dependent down-regulation of the NKG2D ligand H60 on tumors. J Immunol. 2006;176:905–913. doi: 10.4049/jimmunol.176.2.905. [DOI] [PubMed] [Google Scholar]
- 32.Lodoen MB, Abenes G, Umamoto S, Houchins JP, Liu F, Lanier LL. The cytomegalovirus m155 gene product subverts natural killer cell antiviral protection by disruption of H60-NKG2D interactions. J. Exp. Med. 2004;200:1075–1081. doi: 10.1084/jem.20040583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neal ZC, Imboden M, Rakhmilevich AL, Kim KM, Hank JA, Surfus J, Dixon JR, Lode HN, Reisfeld RA, Gillies SD, Sondel PM. NXS2 murine neuroblastomas express increased levels of MHC class I antigens upon recurrence following NK-dependent immunotherapy. Cancer. Immunol. Immunother. 2004;53:41–52. doi: 10.1007/s00262-003-0435-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rakhmilevich AL, Buhtoiarov IN, Malkovsky M, Sondel PM. CD40 ligation in vivo can induce T cell independent antitumor effects even against immunogenic tumors. Cancer. Immunol. Immunother. 2008 doi: 10.1007/s00262-007-0447-4. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diefenbach A, Hsia JK, Hsiung MY, Raulet DH. A novel ligand for the NKG2D receptor activates NK cells and macrophages and induces tumor immunity. Eur J Immunol. 2003;33:381–391. doi: 10.1002/immu.200310012. [DOI] [PubMed] [Google Scholar]
- 36.Kato T, Hakamada R, Yamane H, Nariuchi H. Induction of IL-12 p40 messenger RNA expression and IL-12 production of macrophages via CD40-CD40 ligand interaction. J. Immunol. 1996;156:3932–3938. [PubMed] [Google Scholar]
- 37.Yamane H, Kato T, Nariuchi H. Effective stimulation for IL-12 p35 mRNA accumulation and bioactive IL-12 production of antigen-presenting cells interacted with Th cells. J. Immunol. 1999;162:6433–6441. [PubMed] [Google Scholar]
- 38.Kennedy MK, Picha KS, Fanslow WC, Grabstein KH, Alderson MR, Clifford KN, Chin WA, Mohler KM. CD40/CD40 ligand interactions are required for T cell-dependent production of interleukin-12 by mouse macrophages. Eur. J. Immunol. 1996;26:370–378. doi: 10.1002/eji.1830260216. [DOI] [PubMed] [Google Scholar]
- 39.Shi C, Zhang X, Chen Z, Robinson MK, Simon DI. Leukocyte integrin Mac-1 recruits toll/interleukin-1 receptor superfamily signaling intermediates to modulate NF-kappaB activity. Circ. Res. 2001;89:859–865. doi: 10.1161/hh2201.099166. [DOI] [PubMed] [Google Scholar]
- 40.Lum HD, Buhtoiarov IN, Berke G, Paulnock DM, Sondel P, Rakhmilevich AL. In vivo CD40 ligation can induce T cell-independent antitumor effects that involve macrophages. J. Leuk. Biol. 2006;79:1181–1192. doi: 10.1189/jlb.0405191. [DOI] [PubMed] [Google Scholar]
- 41.Weinhold KJ, Goldstein LT, Wheelock EF. The tumor dormant state. Quantitation of L5178Y cells and host immune responses during the establishment and course of dormancy in syngeneic DBA/2 mice. J. Exp. Med. 1979;149:732–744. doi: 10.1084/jem.149.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wheelock EF, Robinson MK, Truitt GA. Establishment and control of the L5178Y-cell tumor dormant state in DBA/2 mice. Cancer Metastasis Rev. 1982;1:29–44. doi: 10.1007/BF00049479. [DOI] [PubMed] [Google Scholar]
- 43.Chen LP, Suzuki Y, Liu CM, Wheelock EF. Maintenance and cure of the L5178Y murine tumor-dormant state by interleukin 2: dependence of interleukin 2 on induced interferon-gamma and on tumor necrosis factor for its antitumor effects. Cancer. Res. 1990;50:1368–1374. [PubMed] [Google Scholar]
- 44.Liu CM, Suzuki Y, Chen LP, Okayasu T, Calkins CE, Wheelock EF. Maintenance and cure of the L5178Y murine tumor-dormant state by interleukin 2: in vivo and in vitro effects. Cancer. Res. 1990;50:1361–1367. [PubMed] [Google Scholar]
- 45.Anikhovskaya IO Oparina, Yakovleva M, Yakovlev M. Intestinal endotoxin as a universal factor of adaptation and pathogenesis of general adaptation syndrome. Human Physiology. 2006;32:200–203. [PubMed] [Google Scholar]
- 46.Storz M, Zepter K, Kamarashev J, Dummer R, Burg G, Häffner AC. Coexpression of CD40 and CD40 ligand in cutaneous T-cell lymphoma (mycosis fungoides) Cancer. Res. 2001;61:452–454. [PubMed] [Google Scholar]
- 47.Carbone A, Gloghini A, Zagonel V, Aldinucci D, Gattei V, Degan M, Improta S, Sorio R, Monfardini S, Pinto A. The expression of CD26 and CD40 ligand is mutually exclusive in human T-cell non-Hodgkin's lymphomas/leukemias. Blood. 1995;86:4617–4626. [PubMed] [Google Scholar]
- 48.Inghirami G, Lederman S, Yellin MJ, Chadburn A, Chess L, Knowles DM. Phenotypic and functional characterization of T-BAM (CD40 ligand)+ T-cell non-Hodgkin's lymphoma. Blood. 1994;84 866-782. [PubMed] [Google Scholar]
- 49.Voorzanger-Rousselot N, Blay JY. Coexpression of CD40 and CD40L on B lymphoma and carcinoma cells: an autocrine anti-apoptotic role. Leuk Lymphoma. 2004;45:1239–1245. doi: 10.1080/1042819032000159834. [DOI] [PubMed] [Google Scholar]
- 50.Cantaluppi V, Deregibus MC, Biancone L, Deambrosis I, Bussolati B, Albini A, Camussi G. The expression of CD154 by Kaposi's sarcoma cells mediates the anti-apoptotic and migratory effects of HIV-1-TAT protein. Int. J. Immunopathol. Pharmacol. 2006;19:81–96. [PubMed] [Google Scholar]
- 51.Bussolati B, Russo S, Deambrosis I, Cantaluppi V, Volpe A, Ferrando U, Camussi G. Expression of CD154 on renal cell carcinomas and effect on cell proliferation, motility and platelet-activating factor synthesis. Int. J. Cancer. 2002;100:654–661. doi: 10.1002/ijc.10545. [DOI] [PubMed] [Google Scholar]