Abstract
Objective
To validate serum neutrophil gelatinase-associated lipocalin (NGAL) as an early biomarker for acute kidney injury (AKI) in critically ill children with septic shock.
Design
Observational cohort study.
Setting
15 North American pediatric intensive care units (PICU).
Patients
A total of 143 critically ill children with SIRS or septic shock and 25 healthy controls.
Interventions
None.
Measurements and Main Results
Serum NGAL was measured during the first 24 hours of admission to the PICU. AKI was defined as a blood urea nitrogen (BUN) concentration > 100 mg/dL, serum creatinine > 2 mg/dL in the absence of pre-existing renal disease, or the need for dialysis. There was a significant difference in serum NGAL between healthy children (median 80 ng/mL, IQR 55.5-85.5 ng/mL), critically ill children with SIRS (median 107.5 ng/mL, IQR 89-178.5 ng/mL), and critically ill children with septic shock (median 302 ng/mL, IQR 151-570 ng/mL; p<0.001). AKI developed in 22 out of 143 (15.4%) critically ill children. Serum NGAL was significantly increased in critically ill children with AKI (median 355 ng/mL, IQR 166-1322 ng/mL) compared to those without AKI (median 186 ng/mL, IQR 98-365 ng/mL; p=0.009).
Conclusions
Serum NGAL is a highly sensitive, but nonspecific predictor of AKI in critically ill children with septic shock. Further validation of serum NGAL as a biomarker of AKI in this population is warranted.
Introduction
Acute kidney injury (AKI), formerly known as acute renal failure, continues to represent a very common and potentially devastating problem in critically ill children and adults. The reported incidence of AKI in this population varies greatly due to the lack of a standard, consensus definition. For example, AKI affects between 5% and 50% of critically ill patients in reported series (1-5). Unfortunately, the mortality and morbidity associated with AKI remain unacceptably high (up to 80% mortality in critically ill children and adults with multiple organ dysfunction syndrome, or MODS). While this dismal prognosis is partly attributable to other co-morbid conditions, recent studies have revealed that AKI may be an independent risk factor for mortality in both critically ill children (5-7) and adults (8-11). In addition, the treatment of AKI represents an enormous financial burden on society, with annual U.S. medical expenditures approaching $8 billion in adults alone (12). Currently, effective treatments to prevent AKI are lacking, and management is largely directed towards reversing the underlying cause (e.g. renal ischemia secondary to hypotension) and providing supportive care. Supportive care in the pediatric intensive care unit (PICU) has traditionally included optimizing fluid status and avoiding potentially nephrotoxic medications, as well as maintaining cardiorespiratory stability with vasoactive medications and mechanical ventilatory support. Renal replacement therapy (RRT) is currently the only available, proven therapy for critically ill children with AKI, and studies suggest that early initiation of RRT significantly improves survival in children with AKI secondary to septic shock and MODS (13-16).
Sepsis and its related syndromes account for significant morbidity and mortality in critically ill children, accounting for nearly 4,500 deaths and close to $2 billion per year in healthcare expenditures in the United States alone on an annual basis (17). Shock and subsequent multiple organ dysfunction remain significant risk factors for mortality in these patients . Sepsis remains a significant risk factor and one of the leading causes of AKI in critically ill children (3-6, 13, 14, 18-21).
In current clinical practice, AKI is typically diagnosed by measuring serum creatinine. However, it is well known that creatinine is an unreliable and insensitive indicator during early, acute changes in kidney function. First, serum creatinine concentrations may not change until about 50% of kidney function has already been lost. Second, serum creatinine does not accurately depict kidney function until a steady state has been reached, which may require several days (22). Thus, the use of serum creatinine significantly impairs our ability to both detect and quantify renal damage during the early, crucial stages of AKI when RRT may have the highest potential for improving outcome.
Serum neutrophil gelatinase-associated lipocalin (NGAL) is a highly sensitive, specific, and predictive, early biomarker for AKI in a wide range of different disease processes(12). For example, serum NGAL concentrations greater than 25 μg/L reliably predicted AKI in children at 2 hours following cardiopulmonary bypass with 70% sensitivity and 94% specificity (23). Therefore, we hypothesized that serum NGAL may also represent an early biomarker of AKI in critically ill children with septic shock.
Methods
Patients
All study subjects were drawn from a database generated by an ongoing, multi-insitutional research program focused on the genomics of pediatric SIRS and septic shock (24). We analyzed the serum samples and clinical data from all of the subjects enrolled in the database at the time of our analysis. The study protocol was approved by the individual Institutional Review Boards of each participating institution. Children < 10 years of age admitted to the PICU and meeting criteria for septic shock were eligible for the study. Sepsis and related syndromes (SIRS, sepsis, severe sepsis, and septic shock) were diagnosed according to the definitions of the American College of Chest Physicians/Society of Critical Care Medicine (25) modified specifically for pediatrics (26). Control patients were recruited from the participating institutions using the following exclusion criteria: any acute illness, a recent febrile illness (within 2 weeks), recent use of anti-inflammatory medications (within 2 weeks), or any history of chronic or acute disease associated with inflammation. The control patients were recruited from children referred to an outpatient phlebotomy laboratory for routine blood tests or from children with acyanotic congenital heart disease undergoing cardiac catheterization. Severity of illness was calculated using the PRISM III score (27). Organ failure was defined according to previously published criteria (28-30), and for the purposes of this study, AKI was defined as a blood urea nitrogen (BUN) concentration > 100 mg/dL, serum creatinine > 2 mg/dL in the absence of pre-existing renal disease, or the need for dialysis (28-30). Annotated clinical and laboratory data were collected daily while in the PICU. All study patients were followed for 28 days to determine survival. Clinical, laboratory, and biological data were entered and stored using a web-based database developed at our institution.
Sample Collection
After obtaining informed consent, blood samples were obtained within 24 hours of admission to the PICU into tubes without anticoagulants for collection of serum. A second blood sample was obtained on the third day of admission to the PICU. Samples were centrifuged at 2000 g for 30 min at 4°C and stored in 50 μL aliquots at −80°C in order to avoid multiple freeze-thaw cycles.
NGAL ELISA
The serum NGAL ELISA was performed as previously described (23) with some modifications. Briefly, microtiter plates pre-coated with a mouse monoclonal antibody raised against human NGAL (HYB211-05, AntibodyShop, Gentofte, Denmark) were blocked with buffer containing 1% BSA, coated with 100 μL of serum samples or standards (NGAL concentrations ranging from 1-1000 ng/mL, Randox Laboratories, Crumlin, UK), and incubated with a biotinylated monoclonal antibody against human NGAL (HYB211-01B, AntibodyShop) followed by avidin-conjugated HRP (Dako, Carpinteria, CA). TMB substrate (BD Biosciences, San Jose, CA) was added for color development, which was read after 30 min at 450 nm with a microplate reader (Benchmark Plus, BioRad, Hercules, CA). All measurements were made in triplicate. The inter- and intra-assay coefficient variations were 5-10%. The laboratory investigators were blinded to the sample sources and clinical outcomes until the end of the study.
Statistical Analysis
Data was analyzed using SigmaStat for Windows Version 3.11 software (Systat Software, Inc, San Jose, CA). Serum NGAL levels were expressed as median and interquartile range due the nonparametric nature of the data. The primary outcome variable was the development of AKI, as defined above. Categorical variables were compared using the Chi square test or Fischer's Exact test, as indicated. Continuous variables were compared using Mann-Whitney test and Kruskal-Wallis ANOVA with Dunn's post hoc test, as indicated. The associations between variables were assessed by Spearman rank order correlation analysis. In order to measure the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of serum NGAL for the prediction of AKI, receiver-operator characteristic (ROC) curves were generated and the area under the curve (AUC) was calculated (31). An AUC of 0.5 is no better than expected by chance, whereas a value of 1.0 signifies a perfect biomarker (Devarajan et al). Univariate regression analyses were performed to determine which factors were associated with increased risk of AKI. For purposes of model building, variables which were associated with increased risk of AKI at a p-value ≤ 0.10 were included in a list of potential independent risk factors in multivariate regression analysis, with AKI the dependent variable. A p value less than or equal to 0.05 was considered statistically significant.
Results
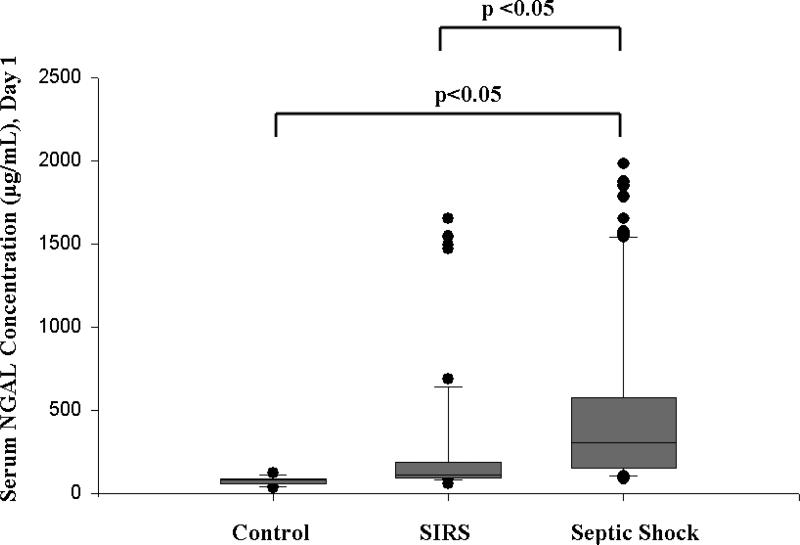
We prospectively measured serum NGAL concentrations during the first 24 hours of admission to the PICU in 143 critically ill children and 25 healthy children. Children in the healthy control group were younger than the children with either SIRS or septic shock, though the groups were otherwise similar, with the exception of a higher PRISM-III score, longer PICU LOS, increased number of organ failures, and higher mortality in the children with septic shock (Table 1). There was a significant difference in serum NGAL between healthy children (median 80 ng/mL, IQR 55.5-85.5 ng/mL), critically ill children with SIRS (median 107.5 ng/mL, IQR 89-178.5 ng/mL), and critically ill children with septic shock (median 302 ng/mL, IQR 151-570 ng/mL; p<0.001). In addition, serum NGAL was significantly increased in critically ill children with septic shock compared to critically ill children with SIRS, reflecting the greater severity of illness in these children (Figure 1).
Table 1.
Clinical characteristics of the study cohort.
| Control (n=25) | SIRS (n=53) | Septic Shock (n=90) | |
|---|---|---|---|
| Age, years (IQR) | 0.2 y (0, 5.9)*,# | 3.5 y (1.8, 7) | 2.2 y (0.8, 6.2) |
| Gender (M:F) | 13:12 | 35:18 | 58:32 |
| Race, No. (%) | |||
| White | 11 (44) | 38 (71.7) | 63 (70) |
| African-American | 9 (36) | 6 (11.3) | 18 (20) |
| Asian | 4 (16) | 7 (13.2) | 8 (8.9) |
| American Indian/Eskimo | 0 (0) | 0 (0) | 1 (1.1) |
| Hawaiian/Pacific Islander | 1 (4) | 2 (3.7) | 0 (0) |
| Median PRISM (IQR) | - | 10 (5, 14) | 16 (9.25, 22.75)* |
| Chronic Illness, No. (%) | - | 24 (45.3) | 38 (42.2) |
| Median No. of organ failures (IQR) | - | 1 (0, 2) | 2 (2, 3)* |
| Median PICU LOS (IQR) | - | 4.5 (2, 12.5) | 8 (4, 20)* |
| Non-survivors, No. (%) | 0 (0)# | 1 (1.9) | 11(12.2)* |
p<0.05 compared to SIRS
p<0.05 compared to Septic Shock
Figure 1. Box and whisker plot of serum NGAL concentrations measured on day 1 of admission to the PICU in 143 critically ill children versus 25 healthy children.
The vertical box represents the 25th percentile (bottom line), median (middle line), and 75th percentile (top line) values, while the error bars represent the 10th and 90th percentile values. The dots represent values outside the 10th and 90th percentile, respectively.
AKI developed in 22 out of 143 (15.4%) critically ill children - all but 4 of these critically ill children had septic shock. In general, critically ill children who developed AKI had a greater severity of illness as determined by PRISM score, median number of organ failures during the first week of hospitalization, and length of PICU stay compared to those critically ill children who did not develop AKI (Table 2). There was a non-signficant trend (p=0.08) for critically ill children with septic shock to develop AKI compared to critically ill children with SIRS. Consistent with the definition of AKI used in this cohort, serum creatinine was significantly increased in critically ill children with AKI (Table 2).
Table 2.
Clinical characteristics of critically ill children who developed AKI versus those who did not develop AKI
| AKI (n=22) | No AKI (n=121) | |
|---|---|---|
| Median Age, years (IQR) | 4.9 (1.4, 8.6) | 2.6 (1, 6) |
| Gender, M:F | 16:6 | 77:44 |
| Race, No. (%) | ||
| White | 15 (68.2) | 86 (71.1) |
| African-American | 5 (22.7) | 19 (15.7) |
| Asian | 2 (9.1) | 13 (10.7) |
| American Indian/Eskim | 0 (0) | 1 (<1) |
| Hawaiian/Pacific Islander | 0 (0) | 2 (1.7) |
| Median serum creatinine (mg/dL) (IQR) | 2.2 (1.2, 3.1)* | 0.5 (0.3, 0.7) |
| Median PRISM (IQR) | 22 (9, 27)# | 12 (7, 20.25) |
| Septic Shock, No. (%) | 18 (81.8) | 72(59.5) |
| Chronic Illness, No. (%) | 9 (40.9) | 53 (43.8) |
| Median No. of organ failures (IQR) | 3 (2, 4)* | 2 (1, 2) |
| Median PICU LOS (days) (IQR) | 17 (7, 28)# | 6 (3, 13) |
| Non-survivors, No. (%) | 4 (18.2) | 8 (6.6) |
p<0.001 AKI versus No AKI
p<0.01 AKI versus No AKI
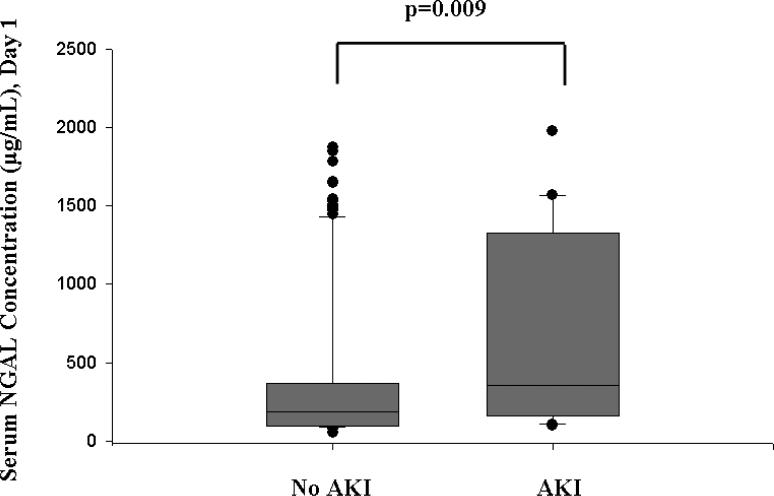
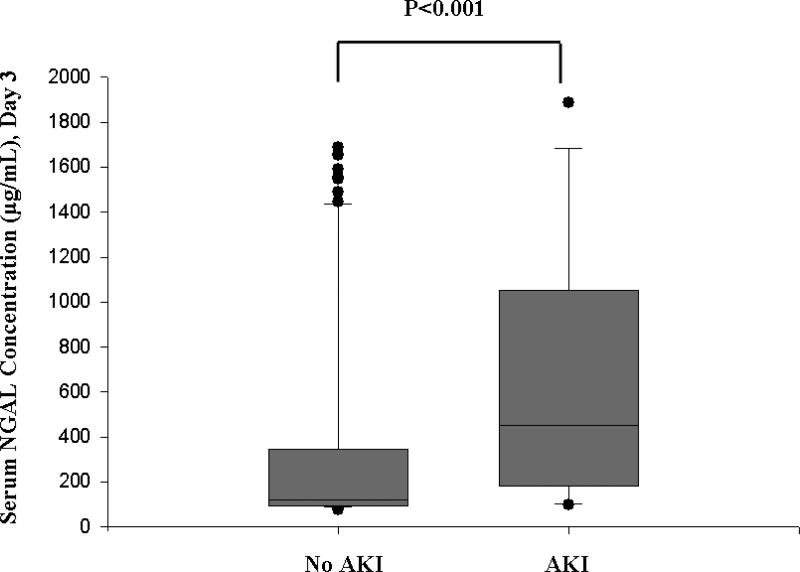
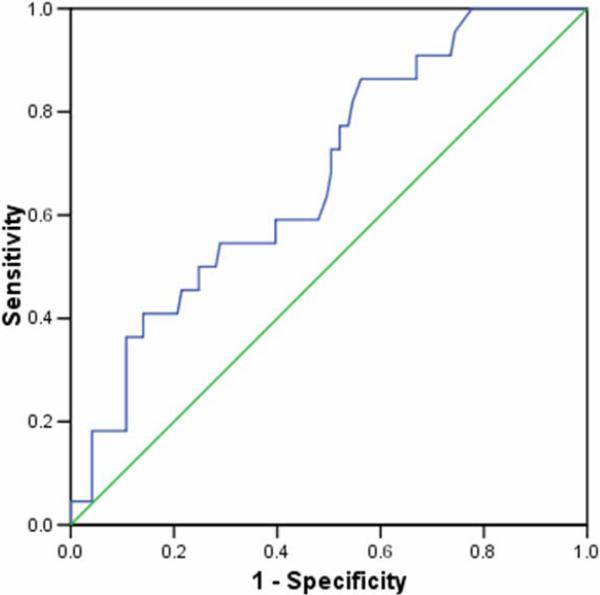
Serum NGAL did not correlate significantly with serum creatinine concentration on day 1 following PICU admission. AKI developed within the first 7 days of admission to the PICU (median 1 day following admission, range 1-6 days). Serum NGAL concentrations within 24 hours of PICU admission were significantly increased in these children (median, 355 ng/mL; interquartile range, 166-1322 ng/mL) compared to children who did not develop AKI (median, 186 ng/mL; interquartile range, 98-365 ng/mL; p=0.009) (Figure 2). A second sample was obtained on the third day of admission to the PICU in 113 critically ill children, representing 79.0% of the total cohort. Serum NGAL measured on day 1 did not correlate with the measurement performed on day 3 of admission to the PICU. However, the difference between critically ill children with AKI (median 450 ng/mL, IQR 185-980 ng/mL) and critically ill children without AKI (median 122 ng/mL, IQR 96-345 ng/mL; p<0.001) remained significant (Figure 3). Figure 4 shows the ROC curve of serum NGAL on admission to the PICU for the prediction of AKI. The AUC was 0.677 (95% C.I. 0.557, 0.786; p=0.008) with an optimal cutoff value of 139 ng/mL (sensitivity=86%, specificity=39%, PPV=39%, NPV=94%).
Figure 2. Box and whisker plot of serum NGAL concentrations measured on day 1 of admission to the PICU in critically ill children with AKI versus critically ill children without AKI.
The vertical box represents the 25th percentile (bottom line), median (middle line), and 75th percentile (top line) values, while the error bars represent the 10th and 90th percentile values. The dots represent values outside the 10th and 90th percentile, respectively.
Figure 3. Box and whisker plot of serum NGAL concentrations measured on day 3 of admission to the PICU in critically ill children with AKI versus critically ill children without AKI.
The vertical box represents the 25th percentile (bottom line), median (middle line), and 75th percentile (top line) values, while the error bars represent the 10th and 90th percentile values. The dots represent values outside the 10th and 90th percentile, respectively.
Figure 4.
Receiver-operating curve (ROC) analysis of serum NGAL concentrations measured on day 1 of admission to the PICU in critically ill children with AKI versus critically ill children without AKI.
Twelve children did not survive (28-day mortality, 8.4%). Four of the twenty-two critically ill children with AKI did not survive (mortality, 18.2%) versus 8 of 121 critically ill children without AKI (mortality 6.6%), though the difference was not significant (p=0.2). There were no significant differences in serum NGAL concentrations between survivors (median, 188 ng/mL, IQR 107-395 ng/mL) and non-survivors (median, 295 ng/mL, IQR 131-933 ng/mL; p=0.2).
Using univariate regression analysis, serum NGAL (p=0.007), the diagnosis of septic shock (p=0.048), serum creatinine (p<0.001), total number of organ failures (p<0.001), and PRISM score (p=0.04) were associated with an increased risk of AKI. However, upon subsequent multivariate analysis, only serum creatinine, Odds ratio 66.8, 95% C.I. 6.9 - 640.4 (p<0.001) and total number of organ failures, Odds ratio 14.3, 95% C.I. 2.7 – 74.3 (p=0.002) remained significant predictors of AKI.
Discussion
This is the first study of critically ill children with SIRS/septic shock to undergo prospective evaluation of serum NGAL as a biomarker for AKI. Herein we show that serum NGAL is significantly increased within the first 24 hours of admission to the PICU in critically ill children with septic shock compared to both healthy controls and critically ill children with SIRS. In addition, serum NGAL is significantly increased within the first 24 hours and remains increased at day 3 following admission to the PICU in critically ill children who develop AKI compared to critically children that do not develop AKI. Serum NGAL therefore appears to be a highly sensitive predictor of AKI in this population. In contrast to previous studies (5-7), the mortality rate in critically ill children with AKI was not significantly different than those critically ill children without AKI. The relatively low mortality rate in our overall cohort (8.4%) may have confounded this observation, and so the study may have been underpowered to detect a significant association between AKI and mortality.
NGAL is a 25 kDa protein that is expressed at very low concentrations in several human tissues, including the kidney, lungs, and gastrointestinal tract (32, 33). It is highly upregulated in injured epithelial cells. For example, increased NGAL concentrations have been found in the blood of patients with acute infections, as well as the bronchoalveolar lavage fluid of patients with lung disease (35). It is therefore not surprising that we found a significant difference in serum NGAL concentration between critically ill children with SIRS versus septic shock, even in the absence of AKI. In this regard, serum NGAL may also be a marker of MODS, similar to C-reactive protein, procalcitonin, or interleukin (IL)-6 (36, 37). Future studies are needed to address this question further, but it is tempting to speculate that serum NGAL could be used as a biomarker of severity of illness in critically ill children with septic shock and MODS. While an interesting question, at least in the current study, serum NGAL was not significantly different between survivors and non-survivors which may limit it utility as a marker for MODS.
NGAL is also highly upregulated in the epithelial cells of the proximal renal tubule following ischemia, and preclinical studies suggested that NGAL was easily detected in the urine relatively early in mouse and rat models of renal ischemia-reperfusion injury (34). Subsequently, several clinical studies have shown that urine and serum NGAL is a highly sensitive, specific, and predictive early biomarker for AKI in a wide range of different disease processes (23, 34, 38-41). In the current study, ROC analysis suggested that a serum NGAL cutoff value of 139 ng/mL within the first 24 hours of admission to the PICU is highly sensitive for predicting AKI, albeit with relatively poor specificity. However, serum NGAL was measured in critically ill children within the first 24 hours of admission to the PICU, which is not necessarily the first 24 hours of their disease process. In fact, the vast majority of these children developed AKI within the first 24 hours of admission to the PICU. We would therefore expect the serum NGAL concentrations to be much higher in critically ill children with septic shock and evolving kidney injury. The results of the current study support this argument.
Mishra et al (23) demonstrated that urine and serum NGAL concentrations at 2 hours following cardiopulmonary bypass were significantly predictive of AKI. Urine NGAL concentrations greater than 50 μg/L reliably predicted AKI in children at two hours following cardiopulmonary bypass, with 100% sensitivity and 98% specificity. While serum NGAL concentrations were not as robust in this study, concentrations greater than 25 μg/L had 70% sensitivity, 94% specificity, 82% positive predictive value, and 89% negative predictive value. Cardiopulmonary bypass represents a temporally predictable injury to the kidneys, and earlier detection of AKI may be more feasible in this population.. In the current study, serum NGAL in healthy children was much higher (median 80 ng/mL, IQR 55.5-85.5 ng/mL) than the serum NGAL in children with AKI in children following cardiopulmonary bypass. The serum NGAL measured in healthy controls in that study were consistenly less than 10 μg/L. These differences are likely related to the different techniques used to measure NGAL in the two studies. In support of this argument, the serum NGAL concentration of control patients in a recently published study which used the same technique as that of the current study ranged between 35-50 ng/mL(42).
Urine NGAL concentration, which was not measured in this cohort, may represent a more robust biomarker for AKI (23). Zappitelli et al (43) recently showed a correlation between urine NGAL concentration (standardized to urine creatinine concentration) and severity of AKI in 103 critically ill children, including children with sepsis. More importantly, urine NGAL concentration was an earlier marker of severe AKI compared to serum creatinine measurements. Finally, there is likely a range of values for urine and serum NGAL concentrations that lie along a continuum from reversible to irreversible AKI. This particular threshold is not currently known – therefore the threshold at which to institute CRRT and/or other therapeutic measures to prevent irreversible, permanent damage to the kidney is not currently known.
The lack of a standard, uniform, accepted definition of AKI has heretofore posed a significant limitation in advancing this field. For example, greater than 50 different definitions have been used in the AKI literature, making potential comparisons across studies and populations virtually impossible (44). The Acute Dialysis Quality Initiative (ADQI) group recently proposed the RIFLE (R=risk, I=injury, F=failure, L=loss, E=end-stage renal disease) criteria for AKI (45). These criteria have been validated in several different populations by several independent groups (46), including critically ill children (7). However, recent data suggest that even smaller changes in serum creatinine than those defined by the RIFLE criteria are associated with adverse outcome (46, 47). Consequently, the Acute Kidney Injury Network has proposed a new, revised classification that defines AKI as an abrupt (within 48 hours) reduction in kidney function as measured by an absolute increase in serum creatinine ≥ 0.3 mg/dL, a percentage increase in serum creatinine ≥ 50%, or documented oliguria (<0.5 mL/kg/hr) for more than 6 hours (46). Serum and urine NGAL may be a more robust biomarker of AKI using this new definition.
Conclusions
We conclude that serum NGAL is a highly sensitive predictor of acute kidney injury (AKI) in critically ill children with septic shock. The ability of biomarkers, such as NGAL to discern both the onset and resolution of AKI will further validate their use in the clinical setting and greatly enhance our understanding of AKI in the pediatric population. Further validation of serum and urine NGAL as biomarkers of AKI in this population will require a multicenter cohort study.
Acknowledgments
Members of the Genomics of Pediatric SIRS/Septic Shock Investigators: Thomas P. Shanley (C.S. Mott Children's Hospital at the University of Michigan, Ann Arbor, Michigan); Richard Lin (The Children's Hospital of Philadelphia, Philadelphia, PA); Geoffrey L. Allen (Children's Mercy Hospital, Kansas City, MO); Neal J. Thomas (Penn State Children's Hospital, Hershey, PA); Nancy M. Tofil (The University of Alabama at Birmingham, Birmingham, AL); Margaret Winkler (The University of Alabama at Birmingham, Birmingham, AL); Allan Doctor (St. Louis Children's Hospital, St. Louis, MO); Paul Checchia (St. Louis Children's Hospital, St. Louis, MO); Meena Kalyanaraman (Newark Beth Israel Medical Center, Newark, NJ); Scott Penfil (DuPont Hospital for Children, Wilmington, DE); Gwenn McLaughlin, M.D. (Jackson Memorial Hospital, Miami, FL); Cheri Landers, M.D. (Kentucky Children's Hospital, Lexington, KY); Gary Kohn, M.D. (Morristown Memorial Hospital, Morristown, NJ); Jose Gutierrez, M.D. (Pediatric Critical Care of Arizona, Phoenix, AZ); Douglas Willson (University of Virginia) and Steve Shane, M.D. (Washoe Medical Center, Reno, NV).
Supported by the National Institutes of Health, KO8 GM077432 (DSW), R21 DK070163 (PD), RO1 GM064619 and the Amanda Kanowitz Foundation (HRW)
References
- 1.Brivet FG, Kleinknecht DJ, Loirat P, et al. Acute renal failure in intensive care units - causes, outcome, and prognostic factors of hospital mortality: A prospective, multicenter study. French Study Group on Acute Renal Failure. Crit Care Med. 1996:192–198. doi: 10.1097/00003246-199602000-00003. [DOI] [PubMed] [Google Scholar]
- 2.de Mendonca A, Vincent JL, Suter PM, et al. Acute renal failure in the ICU: Risk factors and outcome evaluated by the SOFA score. Intensive Care Med. 2000:915–921. doi: 10.1007/s001340051281. [DOI] [PubMed] [Google Scholar]
- 3.Williams DM, Sreedhar SS, Mickell JS, et al. Acute kidney failure: A pediatric experience over 20 years. Arch Pediatr Adolesc Med. 2002:893–900. doi: 10.1001/archpedi.156.9.893. [DOI] [PubMed] [Google Scholar]
- 4.Hui-Stickle S, Brewer ED, Goldstein SL. Pediatric ARF epidemiology at a tertiary care center from 1999 to 2001. Am J Kidney Dis. 2005:96–101. doi: 10.1053/j.ajkd.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 5.Bailey D, Phan V, Litalien C, et al. Risk factors of acute renal failure in critically ill children: A prospective descriptive epidemiological study. Pediatr Crit Care Med. 2007:29–35. doi: 10.1097/01.pcc.0000256612.40265.67. [DOI] [PubMed] [Google Scholar]
- 6.Plotz FB, Hulst HE, Twist JW, et al. Effect of acute renal failure on outcome in children with severe septic shock. Pediatr Nephrol. 2005:1177–1181. doi: 10.1007/s00467-005-1946-1. [DOI] [PubMed] [Google Scholar]
- 7.Akcan-Arikan A, Zappitelli M, Loftis LL, et al. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007:1028–1035. doi: 10.1038/sj.ki.5002231. [DOI] [PubMed] [Google Scholar]
- 8.Clermont G, Acker CG, Angus DC, et al. Renal failure in the ICU: Comparison of the impact of acute renal failure and end-stage renal disease on ICU outcomes. Kidney Int. 2002:986–996. doi: 10.1046/j.1523-1755.2002.00509.x. [DOI] [PubMed] [Google Scholar]
- 9.Metnitz PG, Krenn CG, Steltzer H, et al. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med. 2002:2051–2058. doi: 10.1097/00003246-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Bagshaw SM, Mortis G, Doig CJ, et al. One-year mortality assessment in critically ill patients by severity of kidney dysfunction: A population-based assessment. Am J Kidney Dis. 2006:402–409. doi: 10.1053/j.ajkd.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality. A cohort analysis. JAMA. 1996:1489–1494. [PubMed] [Google Scholar]
- 12.Devarajan P. Emerging biomarkers of acute kidney injury. Contrib Nephrol. 2007:203–212. doi: 10.1159/000102085. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein SL, Currier H, Graf JM, et al. Outcome in children receiving continuous venovenous hemofiltration. Pediatrics. 2001:1309–1312. doi: 10.1542/peds.107.6.1309. [DOI] [PubMed] [Google Scholar]
- 14.Lowrie LH. Renal replacement therapies in pediatric multi-organ dysfunction syndrome. Pediatr Nephrol. 2000:6–12. doi: 10.1007/s004670050002. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein SL, Somers MJG, Baum MA, et al. Pediatric patients with multi-organ dysfunction syndrome receiving continuous renal replacement therapy. Kidney Int. 2005:653–658. doi: 10.1111/j.1523-1755.2005.67121.x. [DOI] [PubMed] [Google Scholar]
- 16.Foland JA, Fortenberry JD, Warshaw BL, et al. Fluid overload before continuous hemofiltration and survival in critically ill children: A retrospective analysis. Crit Care Med. 2004:1771–1776. doi: 10.1097/01.ccm.0000132897.52737.49. [DOI] [PubMed] [Google Scholar]
- 17.Watson RS, Carcillo JA, Linde-Zwirble WT, et al. The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med. 2003:695–701. doi: 10.1164/rccm.200207-682OC. [DOI] [PubMed] [Google Scholar]
- 18.Otukesh H, Hoseini R, Hooman N, et al. Prognosis of acute renal failure in children. Pediatr Nephrol. 2006:1873–1878. doi: 10.1007/s00467-006-0240-1. [DOI] [PubMed] [Google Scholar]
- 19.Arora P, Kher V, Rai PK, et al. Prognosis of acute renal failure in children: A multivariate analysis. Pediatr Nephrol. 1997:153–155. doi: 10.1007/s004670050247. [DOI] [PubMed] [Google Scholar]
- 20.Bunchman TE, McBryde KD, Mottes TE, et al. Pediatric acute renal failure: Outcome by modality and disease. Pediatr Nephrol. 2001:1067–1071. doi: 10.1007/s004670100029. [DOI] [PubMed] [Google Scholar]
- 21.Loza R, Estremadoyro L, Loza C, et al. Factors associated with mortality in acute renal failure (ARF) in children. Pediatr Nephrol. 2006:106–109. doi: 10.1007/s00467-005-2038-y. [DOI] [PubMed] [Google Scholar]
- 22.Moran SM, Myers BD. Course of acute renal failure studied by a model of creatinine kinetics. Kidney Int. 1985:928–937. doi: 10.1038/ki.1985.101. [DOI] [PubMed] [Google Scholar]
- 23.Mishra J, Dent C, Tarabish R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005:1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 24.Wong HR, Shanley TP, Sakthivel B, et al. Genome level expression profiles in pediatric septic shock indicate a role for altered zinc homeostasis in poor outcome. Physiol Genomics. 2007:146–155. doi: 10.1152/physiolgenomics.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992:864–874. [PubMed] [Google Scholar]
- 26.Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005:2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 27.Pollack MM, Patel KM, Ruttimann UE. PRISM III: An updated Pediatric Risk of Mortality score. Crit Care Med. 1996:743–752. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Wilkinson JD, Pollack MM, Glass NL, et al. Mortality associated with multiple organ system failure and sepsis in pediatric intensive care unit. J Pediatr. 1987:324–328. doi: 10.1016/s0022-3476(87)80448-1. [DOI] [PubMed] [Google Scholar]
- 29.Proulx F, Fayon M, Farrell CA, et al. Epidemiology of sepsis and multiple organ dysfunction syndrome in children. Chest. 1996:1033–1037. doi: 10.1378/chest.109.4.1033. [DOI] [PubMed] [Google Scholar]
- 30.Proulx F, Gauthier M, Nadeau D, et al. Timing and predictors of death in pediatric patients with multiple organ system failure. Crit Care Med. 1994:1025–1031. doi: 10.1097/00003246-199406000-00023. [DOI] [PubMed] [Google Scholar]
- 31.Hanley JA, McNeil BA. The meaning and use of the area under a receiver operating (ROC) curve. Radiology. 1982:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 32.Cowland JB, Borregaard N. Molecular characterization and pattern of tissue expression of the gene for neutrophil gelatinase-associated lipocalin from humans. Genomics. 1997:17–23. doi: 10.1006/geno.1997.4896. [DOI] [PubMed] [Google Scholar]
- 33.Kjeldsen L, Cowland JB, Borregaard N. Human neutrophil gelatinase-associated lipocalin and homologous proteins in rat and mouse. Biochim Biophys Acta. 2000:272–283. doi: 10.1016/s0167-4838(00)00152-7. [DOI] [PubMed] [Google Scholar]
- 34.Mishra J, Ma Q, Prada A, et al. Identification of NGAL as a novel urinary biomarker for ischemic injury. J Am Soc Nephrol. 2003:2534–2543. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 35.Xu S, Venge P. Lipocalins as biochemical markers of disease. Biochim Biophys Acta. 2000:298–307. doi: 10.1016/s0167-4838(00)00163-1. [DOI] [PubMed] [Google Scholar]
- 36.Stryjewski GR, Nylen ES, Bell MJ, et al. Interleukin-6, interleukin-8, and a raoid and sensitive assay for calcitonin precursors for the determination of bacterial sepsis in febrile neutropenic children. Pediatr Crit Care Med. 2005:129–135. doi: 10.1097/01.PCC.0000149317.15274.48. [DOI] [PubMed] [Google Scholar]
- 37.Han YY, Doughty LA, Kofos D, et al. Procalcitonin is persistently increased among children with poor outcome from bacterial sepsis. Pediatr Crit Care Med. 2003:21–25. doi: 10.1097/00130478-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Wagener G, Jan M, Kim M, et al. Association between increases in urinary neutrophil gelatinase-associated lipocalin and acute renal dysfunction after adult cardiac surgery. Anesthesiology. 2006:485–491. doi: 10.1097/00000542-200609000-00011. [DOI] [PubMed] [Google Scholar]
- 39.Mishra J, Mori K, Ma Q, et al. Neutrophil gelatinase-associated lipocalin: A novel early urinary biomarker for cisplatin nephrotoxicity. Am J Nephrol. 2004:307–315. doi: 10.1159/000078452. [DOI] [PubMed] [Google Scholar]
- 40.Trachtman H, Christen E, Cnaan A, et al. Urinary neutrophil gelatinase-associated lipocalin in D+HUS: A novel marker of renal injury. Pediatr Nephrol. 2006:989–994. doi: 10.1007/s00467-006-0146-y. [DOI] [PubMed] [Google Scholar]
- 41.Zappitelli M, Washburn K, Arikan AA, et al. Urine NGAL (uNGAL) is an early predictive biomarker of acute kidney injury (AKI) in critically ill children. J Am Soc Nephrol. 2006:404A. [Google Scholar]
- 42.Hirsch R, Dent C, Pfriem H, et al. NGAL is an early predictive biomarker of contrast-induced nephropathy in children. Pediatr Nephrol. 2007 doi: 10.1007/s00467-007-0601-4. Epub ahead of print. Accessed October 15, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Zappitelli M, Washburn KK, Ayse A, et al. Urine neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in critically ill children: A prospective cohort study. Crit Care. 2007 doi: 10.1186/cc6089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mehta RL, Chertow GM. Acute renal failure definitions and classification: Time for change? J Am Soc Nephrol. 2003:2178–2187. doi: 10.1097/01.asn.0000079042.13465.1a. [DOI] [PubMed] [Google Scholar]
- 45.Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure - Definition, outcome measures, animal models, fluid therapy, and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004:R204–R12. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007:1–8. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Praught ML, Shlipak MG. Are small changes in serum creatinine an important risk factor? Curr Opin Nephrol Hypertens. 2005:265–270. doi: 10.1097/01.mnh.0000165894.90748.72. [DOI] [PubMed] [Google Scholar]