Summary
Cannabinoids have been shown to cause CB1-receptor dependent anticonvulsant activity in both in vivo and in vitro models of status epilepticus (SE) and acquired epilepsy (AE). It has been further demonstrated in these models that the endocannabinoid system functions in a tonic manner to suppress seizure discharges through a CB1-receptor dependent pathway. Although acute cannabinoid treatment has anticonvulsant activity, little is known concerning the effects of prolonged exposure to CB1 agonists and development of tolerance on the epileptic phenotype. This study was carried out to evaluate the effects of prolonged exposure to the CB1 agonist WIN55,212-2 on seizure activity in a hippocampal neuronal culture model of low-Mg2+ induced spontaneous recurrent epileptiform discharges (SREDs). Following low-Mg2+ induced SREDs, cultures were returned to maintenance media containing 10, 100 or 1000 nM WIN55,212-2 from 4 to 24 hours. Whole-cell current-clamp analysis of WIN55,212-2 treated cultures revealed a concentration-dependent increase in SRED frequency. Immunocytochemical staining revealed that WIN55,212-2 treatment induced a concentration-dependent down-regulation of the CB1 receptor in neuronal processes and at both glutamatergic and GABAergic presynaptic terminals. Prolonged exposure to the inactive enantiomer WIN55,212-3 in low-Mg2+ treated cultures had no effect on the frequency of SREDs or CB1 receptor staining. The results from this study further substantiate a role for a tonic CB1 receptor-dependent endocannabinoid regulation of seizure discharge and suggest that prolonged exposure to cannabinoids results in the development of tolerance to the anticonvulsant effects of cannabinoids and an exacerbation of seizure activity in the epileptic phenotype.
Keywords: cannabinoid, endocannabinoid, CB1, tolerance, seizure, culture, neuronal
1. Introduction
The brain endocannabinoid system plays a key role in regulation of many neuronal processes associated with both physiological and pathological conditions (Alger, 2006; Di Marzo et al., 1998; Mackie and Stella, 2006). Modulation of the endocannabinoid system has been shown to be protective in a number of in vivo and in vitro models of neuronal injury (reviewed in: Consroe, 1998; Mechoulam et al., 2002; Micale et al., 2007), including studies on seizures and epilepsy (Blair et al., 2006; Consroe and Wolkin, 1977; Karler and Turkanis, 1981; Monory et al., 2006; Wallace et al., 2003; Wallace et al., 2002). The central effects of cannabinoids work primarily through activation of the type 1 cannabinoid (CB1) receptor which is a Gi/o-coupled G-protein coupled receptor (GPCR) (Devane et al., 1988; Howlett, 1995; Matsuda et al., 1990). The CB1 receptor is widely distributed throughout the brain and is one of the most abundant GPCRs in the CNS (Egertova and Elphick, 2000; Herkenham et al., 1991).
GPCRs readily undergo agonist-induced receptor desensitization (uncoupling) and (or) internalization, followed by either degradation (downregulation) or recycling back to the plasma membrane (Hanyaloglu and von Zastrow, 2008). Prolonged administration of cannabinoids in vivo result in the development of tolerance as indicated by a progressive decrease in their pharmacological efficacy (Gonzalez et al., 2005; Martin et al., 2004) which, in the CNS, is primarily attributed to CB1 receptor desensitization or downregulation (Breivogel et al., 2003; McKinney et al., 2008; Sim-Selley and Martin, 2002; Sim-Selley et al., 2006). Additionally, tolerance to cannabinoids in vitro has been observed in cultured neurons as a result of either agonist-induced internalization (Coutts et al., 2001) or desensitization (Lundberg et al., 2005) of the CB1 receptor.
Previous work from our laboratory has demonstrated that exogenous cannabinoids are acutely anticonvulsant by CB1 receptor activation (Blair et al., 2006; Deshpande et al., 2007a; Wallace et al., 2003; Wallace et al., 2001) and that antagonism of the CB1 receptor in the epileptic condition results in an exacerbation of seizure activity (Deshpande et al., 2007b; Wallace et al., 2003; Wallace et al., 2002). In addition, long-term changes in hippocampal CB1 receptor expression and function have been observed in pilocarpine-induced AE in the rat (Falenski et al., 2007). These findings suggest that in the epileptic condition, alterations in the endocannabinoid system contribute to abatement of seizure activity via CB1 receptor activation. Although acute treatment with cannabinoids causes CB1-receptor dependent anticonvulsant activity, little is known concerning the effects of prolonged exposure to CB1 receptor agonists on the epileptic phenotype.
In the current study, we set out to investigate the effects of prolonged exposure to the cannabimimetic WIN55,212-2 (+WIN) on epileptiform seizure activity and CB1 receptor protein expression in the well established hippocampal neuronal culture (HNC) model of acquired epilepsy (AE) (Sombati and DeLorenzo, 1995). The HNC model of AE is well suited to investigate the pharmacodynamics of CB1 receptor agonists by allowing direct analysis of molecular mechanisms and electrophysiological evaluation of neurons undergoing spontaneous recurrent epileptiform discharges (SREDs) (Blair et al., 2004; Carter et al., 2006; Deshpande et al., 2007b; Sombati and DeLorenzo, 1995). Following prolonged exposure to +WIN (10–1000 nM), neurons underwent patch clamp recordings and immunocytochemical analysis to evaluate SRED activity and CB1 receptor expression, respectively. The results indicate that chronic treatment of SRED cultures with +WIN causes a decrease in CB1 receptor expression and the development of tolerance to the anticonvulsant effects of cannabinoids in this model. Understanding the effects of prolonged cannabinoid administration in the epileptic phenotype may prove important for more optimal therapeutic targeting of the endocannabinoid system in neurological disorders.
2. Materials and Methods
2.1. Materials
WIN55,212-3 (−WIN) and WIN55,212-2 (+WIN) were purchased from Sigma Chemical (St. Louis, MO). SR141716A was supplied through the NIDA Chemical Synthesis and Drug Supply Program. Stocks (1 mM) of −WIN, +WIN and SR141716A were made up in dimethyl sulfoxide that were then diluted at a minimum of 1:1000 to a final working concentration in the physiological bath recording solution (pBRS; see composition below).
2.2. Hippocampal neuronal culture
Primary mixed hippocampal cultures were prepared as described previously by our laboratory with slight modifications (Sombati and DeLorenzo, 1995). The experimental protocols were approved by Virginia Commonwealth University IACUC and conformed to the National Institutes of Health guide for the care and use of Laboratory animals. Briefly, hippocampal cells were prepared from 2-day postnatal Sprague-Dawley rats (Harlan, Frederick, MD) and plated at a density of 2.0 × 104 cells/cm2 onto a glial support layer previously plated onto poly-L-lysine (0.05 mg/ml) coated Lab-Tek two-well cover glass chambers or 35 mm cell culture dishes (Nunc, Naperville, IL). Cultures were maintained at 37°C in a 5% CO2/95% air atmosphere and fed twice weekly with Minimal Essential Media (MEM) with Earle’s Salts (Invitrogen Corp., San Diego, CA) enriched with N3 supplement containing 25 mM HEPES buffer (pH 7.4), 2 mM L-Glutamine, 3 mM Glucose, 100 µg/ml transferrin, 5 µg/ml insulin, 100 µM putrescine, 3 nM sodium selenite, 200 nM progesterone, 1 mM sodium pyruvate, 0.1% ovalbumin, 0.2 ng/ml triiodothyroxine, 0.4 ng/ml corticosterone and supplemented with a glial bed-condition media (20%). Unless otherwise noted, reagents were purchased from Sigma-Aldrich Co. (St. Louis, MO).
Immunocytochemical evaluation of this hippocampal culture preparation using antiserum against either α-CaM kinase II or GAD-67 demonstrates that the contribution of glutamatergic and GABAergic neuronal phenotypes is 65.3±3.25% and 28.6±2.7% respectively. GAD-67 (GABAergic) staining was generally observed on the somatodendritic regions of large neurons that revealed either bipolar or multiple dendritic processes extending out, while α-CaM kinase II (glutamatergic) staining was limited to smaller pyramidal shaped neurons. These findings are in agreement with previous studies utilizing this preparation of rat primary hippocampal neuronal cultures (Cao et al., 1996; Hoch and Dingledine, 1986).
2.3. Induction of SREDs by Low-Mg2+ treatment of hippocampal neuronal cultures
After two weeks, cultures were utilized for experimentation. Maintenance media were replaced with pBRS with or without MgCI2 containing (in mM): 145 NaCI, 2.5 KCI, 10 HEPES, 2 CaCI2, 10 glucose, 0.002 glycine, pH 7.3, and osmolarity adjusted to 325mOsm with sucrose. Thus, low-Mg2+ treatment was carried out with pBRS without added MgCI2, while sham controls were treated with pBRS containing 1 mM MgCI2. Unless indicated as low-Mg2+ treatment, experimental protocols in this study utilized pBRS containing 1 mM MgCI2.
Continuous epileptiform high-frequency bursts (SE) were induced by exposing neuronal cultures to pBRS without added MgCI2 (low-Mg2+). The SE continued until pBRS containing 1 mM MgCI2 was added back to the cultures. A 3 h exposure of SE was employed to induce AE with SREDs in the HNC model using established procedures (Sombati and DeLorenzo, 1995). Briefly, after removal of maintenance media, cells were washed gently with 3 × 1.5 ml of pBRS (±1 mM MgCI2) and then allowed to incubate in this solution at 37°C under 5% CO2 /95% air. For the HNC model of AE, at the end of treatment with either sham control (1 mM MgCI2) or low-Mg2+ (without added MgCI2) conditions for 3 hours, cultures were restored to the physiological concentration (1mM) of MgCI2 by washing gently with 3 × 1.5 ml of MEM at 37°C, returned to maintenance feed and incubated at 37°C under 5% CO2 /95% air. For acute cannabinoid treatment, control and SRED cultures were exposed to +WIN or −WIN (1000 nM) and evaluated by whole-cell current-clamp (see protocol below). CB1 receptor antagonism was carried out with co-application of SR141716A (1000 nM) and +WIN (1000 nM) to cultures (Blair et al., 2006). For cultures undergoing prolonged cannabinoid exposure, following 3 h of treatment and wash described above, cultures were then returned to maintenance feed containing varying concentrations of +WIN or −WIN (0–1000 nM)for 4, 12 and 24 h prior to experimental manipulation. The neurons exposed to this 3 h treatment with low Mg2+ pBRS manifested SREDs for the life of the neurons in culture.
2.4. Immunocytochemistry
Following experimental manipulation, neuronal cultures on two-well cover glass chambers were fixed in 4% paraformaldehyde in PBS. Fixed cultures were blocked and permeabilized in SuperBlock® blocking buffer (Pierce, Rockford IL) containing 0.2% Triton X-100 for 60 min at room temperature, followed by a 3 h incubation with rabbit antiserum to the C-terminal tail of CB1 (1:5000) (Egertova and Elphick, 2000) in SuperBlock® blocking buffer containing 0.1% Triton X-100 (SBBT). Additional immunocytochemical stains were undertaken to determine the degree of CB1 receptor localized at both inhibitory GABAergic and excitatory glutamatergic neuronal processes in this neuronal culture preparation using colocalization analysis of CB1 staining with rabbit antiserum to the vesicular GABA transporter (VGAT: 2 ug/ml in SBBT, 16h 4°C; Millipore, Billerica, MA) or guinea pig antiserum to the vesicular glutamate transporter 1 (VGLUT1: 1:500 in SBBT, 16h 4°C; Millipore) respectively in control hippocampal neuronal cultures. For neuronal phenotype characterization, separate immunocytochemical stains of somatic regions were carried out with 3h incubations with either mouse antiserum against α-CaM kinase II (10 µg/ml, clone 6G9; Biomol, Plymouth Meeting, PA) or mouse antiserum against GAD-67 (1:1000, clone 1G 10.2; Millipore) to identify either glutamatergic or GABAergic cultured neurons respectively. For single antibody immunocytochemical staining, after washing four times in PBS containing 0.1% Triton X-100 (PBST), cultures were then incubated with either anti-rabbit or anti-mouse fluorochrome-conjugated secondary antibodies (1:200, Alexa Fluor® 488 or 594; Invitrogen Corp., Eugene, OR) in SBBT for 60 min. After four PBST washes, stained samples were counterstained for 5 min with DAPI (300 nM in PBS). Stained cultures were washed three times in PBS, coated with ProLong Gold® anti-fade agent (Invitrogen Corp., Eugene, OR) and cover slipped. Control experiments without primary antibodies were carried out in an identical manner. Stained cultures were evaluated with a fluorescent IX70 inverted microscope with a 20X objective (Olympus America, Center Valley, PA) and excitation/emission filters for visualization of TxRed, FITC and DAPI. Digital images (16-bit grayscale) were acquired with a Hamamatsu ORCA-ER camera (Hamamatsu Photonics, Japan) using IPLab image processing software (BD Biosciences, Franklin Lakes, NJ).
For double-immunocytochemical staining with the two rabbit primary antibodies, cultures were first incubated in rabbit anti-CB1 (1:5000 in SBBT, 3h), washed and incubated with a monovalent Fab fragment secondary antibody (biotin-SP-AffiniPure Fab fragment goat-anti-rabbit IgG; 1:100 in SBBT, 1h). Utilization of the monovalent Fab fragment secondary antibody allows for only one antigen binding site, thus not providing for binding to the second rabbit primary antibody and resulting in false cross-reactivity. Cultures were then washed and incubated in FITC-streptavidin (5 ug/ml in SBBT, 1h), followed by a wash and then incubation in biotin (0.05% in PBST, 1h) to saturate all free sites on the FITC-streptavidin. Following wash, CB1 stained cultures were then incubated in rabbit anti-VGAT (2 ug/ml in SBBT, 16h 4°C), washed and incubated in biotin-SP-AffiniPure goat-anti-rabbit IgG (1:100 in SBBT, 1h) followed by wash and incubation in Texas red-streptavidin (5 ug/ml in SBBT, 1h). For double staining with CB1 and VGLUT1, secondary antibodies used were biotin-SP-AffiniPure donkey-anti-rabbit IgG and biotin-SP-AffiniPure donkey-anti-guinea pig IgG followed by FITC and Texas red-streptavidin respectively. For all double-immunocytochemical staining, secondary antibodies and streptavidin conjugates were purchased form Jackson Immunoresearch (West Grove, PA). Appropriate no primary antibody controls were carried out to confirm no cross-reactivity between first and second rabbit antisera.
Quantitative analysis of CB1 receptor immunofluorescent stain was evaluated using IPLab image processing software. Grayscale images were corrected for background and segmented to allow for isolation of specific CB1 receptor immunostaining from non-specific fluorescence. For each acquired field obtained from the experimental and control cultures, CB1 receptor staining of neuronal processes was measured over four separate rectangular regions of interest (ROIs) to obtain an average of the total sum of all segmented pixel intensity values within the ROI. All settings and measurement parameters (immunofluorescent excitation/emission, camera exposure duration, background correction, segmentation and size and dimensions of ROIs) for both experimental and control groups were kept constant throughout. Pixel intensity values for each acquired field were then normalized to a percent of mean of the control group. Outliers were determined using the Grubb’s ESD method. Normalized values of CB1 receptor staining for control and experimental cultures were pooled from four independent studies and then underwent parametric statistical analysis and plotted using SigmaPlot/SigmaStat analysis software 9.01 (SPSS Inc., Chicago, III).
For colocalization analysis, double-immunostained cultures were evaluated using a Leica TCS-SP2 confocal laser scanning microscope with a 63×/1.4 n.a. oil objective in sequential scan mode acquisition (Leica Microsystems Inc., Bannockburn, IL). Colocalization analysis of 16-bit confocal scans was carried out using ImageJ (NIH, public domain; Colocalization Threshold plug-in: authors Tony Collins and Wayne Rasband) to calculate Mander’s colocalization coefficient (above threshold) for each channel (tM1: CB1; tM2: VGAT or VGLUT1) and Pearson’s coefficient (above threshold) following automatic image background correction and threshold determination. Further image processing for representative figures was carried out with Adobe Photoshop (Adobe Systems Inc., San Jose, CA).
2.5. Whole-Cell Current-Clamp recordings in low-Mg2+ treated hippocampal neuronal cultures
Whole-cell current-clamp recordings were performed using previously established procedures in our laboratory (Blair et al., 2006; Sombati and DeLorenzo, 1995). Briefly, hippocampal cultures were mounted on the stage of an inverted microscope (Nikon Diaphot, Japan) and then studied using the whole-cell current-clamp recording procedure. To optimize success of recording from pyramidal neurons, phase-bright cells were selected based on both size and pyramidal shaped soma. For cultures undergoing prolonged exposure to +WIN, neurons were maintained in specific concentrations of agonist throughout the recording duration. Patch electrodes with a resistance of 2–4 MΩ were pulled on a Brown-Flaming P-80C electrode puller (Sutter Instruments, Novato, CA), fire polished and filled with a solution containing (in mM) 140 K+gluconate, 1 MgCI2 and 10 Na-HEPES, pH 7.2, osmolarity adjusted to 310 ± 5 mOsm with sucrose. Data were digitized (Digidata 1322A; Molecular Devices, Sunnyvale, CA) and transferred to videotape using a PCM device (Neurocorder, New York, NY) and then played back on a DC-500 Hz chart recorder (Astro-Med Dash II, Warwick, Rl). Intracellular recordings were carried out using an Axopatch 200B amplifier (Molecular Devices) in whole-cell current-clamp mode. All recordings were performed at I = 0 settings and no current was injected at any time during the recording.
2.6. SRED frequency Analysis
For duration of exposure and concentration response analysis, suppression of SREDs was determined as a percent decrease in frequency over increasing concentrations of +WIN. Analysis of SREDs frequency for each recorded neuron was carried out over 60 min and determined by counting individual epileptiform events that had discreet onset and termination and consisted of multiple individual paroxysmal depolarization shifts (PDSs). Wholecell current-clamp frequency analysis was carried out on multiple hippocampal cultured neurons at each point within both duration of exposure and concentration range of +WIN. Mean frequencies were then represented as a percent inhibition from control frequency (SREDs frequency in the absence of +WIN). Data underwent parametric statistical analysis and was plotted using SigmaPlot/SigmaStat analysis software 9.01 (SPSS Inc., Chicago, III).
3. Results
3.1. The cannabimimetic WIN55,212-2 is acutely anticonvulsant in the HNC model of SREDs via a CB1 receptor mechanism
To evaluate the anticonvulsant activity of the cannabimimetic compound +WIN, whole-cell current clamp analysis was utilized with the HNC model of SREDs previously developed in our laboratory (Sombati and DeLorenzo, 1995). To induce SREDs, cultures at 14 DIV were exposed to Mg2+-free media for three hours and then returned to a maintenance media containing Mg2+. Shortly thereafter, low-Mg2+ treated cultures developed SREDs which then persisted for the life of neurons in culture (Sombati and DeLorenzo, 1995). For whole-cell current clamp recordings, neurons were chosen using the criteria of both size and pyramidal morphology. Immunocytochemical staining has revealed that typically smaller pyramidal shaped neurons are glutamatergic in nature (see Materials and Methods). Recordings from age matched sham treated (+Mg2+) control neurons revealed “normal” baseline activity, displaying spontaneously occurring action potentials and with no development of SRED activity (Fig. 1A). One day following 3 hr of low-Mg2+ treatment, recording from a representative neuron demonstrated an “epileptic” phenotype as evident by the presence of three individual SREDs or “seizure” events (Fig 1B). Each SRED demonstrated a succinct onset and termination and was comprised of multiple paroxysmal depolarization shifts (PDSs) each of which was overlaid with poly spikes. The presence of PDSs is a pathophysiological characteristic similar to what is observed in clinical epilepsy (Lothman et al., 1991).
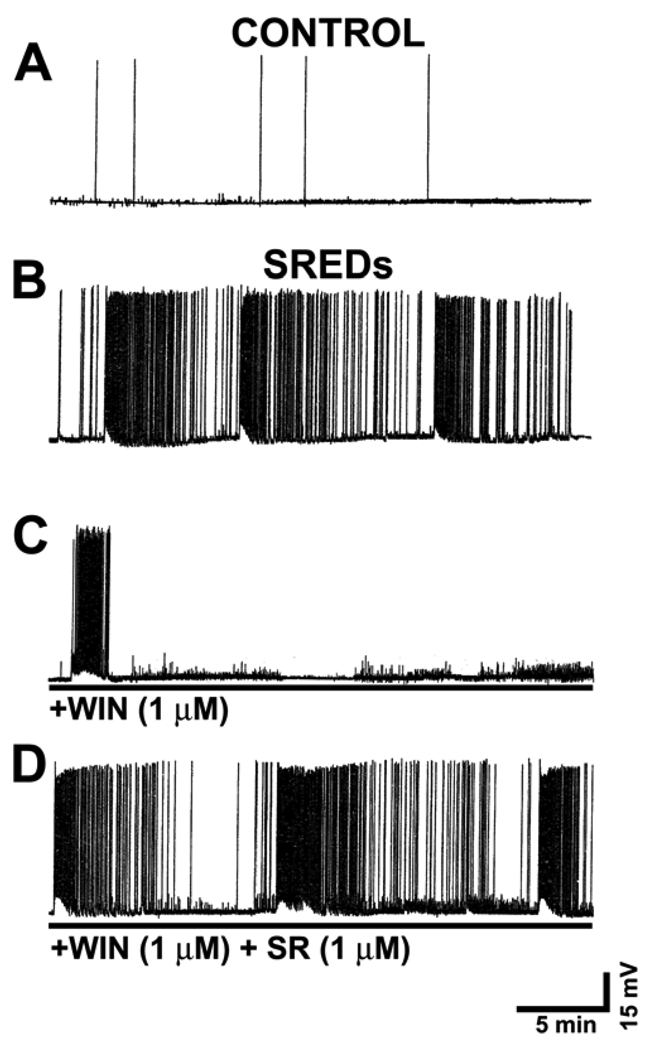
Fig 1. Acute WIN55,212-2 treatment confers anticonvulsant activity in a CB1-dependent manner.
Hippocampal pyramidal neurons underwent whole-cell current-clamp recordings 1 day following a 3 h exposure to Mg2+-free solution. A. A representative recording from a control (+Mg2+ solution) treated pyramidal neuron demonstrating basal activity as indicated by the presence of intermittent spontaneous action potentials. B. A representative recording from an “epileptic” neuron 1day following exposure to 3h of Mg2+-free media. The pathophysiological state of epileptiform activity in this in vitro preparation is evident by the presence of three independent SREDs. C. Treatment of “epileptic” cultures with the cannabimimetic WIN55,212-2 (1 µM; +WIN) suppressed seizure activity. D. Co-application of the specific CB1 receptor antagonist SR141716A (1 µM) blocked the anticonvulsant effect of +WIN (1 µM).
The cannabinoid receptor agonist WIN55,212-2 suppressed SREDs stereoselectively in the HNC model of AE. Employing electrophysiological analysis of seizure activity in a representative low-Mg2+ treated neuron, the active enantiomer +WIN (1000 nM) acutely suppressed epileptiform activity as demonstrated by the presence of only one SRED (Fig 1C). Previous work from our laboratory has demonstrated that +WIN acutely inhibits SRED activity with an EC50 = 0.85 µM and produced total suppression at 3 µM (Blair et al., 2006). Conversely, exposure of low-Mg2+ treated cultures to the inactive enantiomer −WIN (1000 nM) had no effect on the expression or frequency of SREDs in “epileptic” cultures (data not shown) (Blair et al., 2006). These results demonstrate that only the active enantiomer of the cannabimimetic WIN55,212 demonstrated anticonvulsant activity in the HNC model of SREDs. To determine if +WIN was suppressing SRED activity by activation of the CB1 receptor, the specific receptor antagonist SR141617A (SR) was co-applied with +WIN and low-Mg2+ treated neurons were recorded in the whole-cell current-clamp mode. The CB1 receptor antagonist SR (1000 nM) blocked the ability of +WIN to suppress SRED activity (Fig 1D). SRED frequency analysis for experimental conditions represented in Fig 1B–D revealed that addition of +WIN • (LM+WIN; 1000 nM) significantly suppressed SREDs by 49.0±1.3 % (percent of low-Mg2+ control ±SEM), while the co-application of SR (1000 nM) with +WIN (LM+WIN+SR) showed no significant change from low-Mg2+ (LM) control (one-way ANOVA, p≤ 0.001; Holm-Sidak post-hoc, p≤ 0.025, LM+WIN vs. LM or LM+WIN+SR; n = 3,5 and 3 for LM, LM+WIN and LM+WIN+SR respectively). Thus, the stereoselectivity of +WIN to inhibit SREDs and the antagonism of this effect by SR confirmed that the anticonvulsant effect of +WIN in this in vitro model of epileptiform activity occurs via a CB1 receptor mediated mechanism.
3.2. Prolonged exposure to WIN55,212-2 results in a concentration-dependent increase in frequency of SREDs and decrease in CB1 receptor expression in low-Mg2+ treated hippocampal neuronal cultures
To evaluate the effects of prolonged +WIN exposure on SRED activity, whole-cell current-clamp recording was carried out on ‘epileptic’ neurons that were exposed to varying concentrations of +WIN for 24 hours. Immediately following three hours of Mg2+-free treatment, cultures were returned to maintenance media containing +WIN at concentrations which included 10 nM, 100 nM and 1000 nM. Exposure of low-Mg2+ treated cultures to +WIN for 24 hours resulted in a concentration-dependent increase in frequency of SREDs when compared to low-Mg2+ treated cultures without +WIN exposure (Fig. 2A). The increase in frequency of SREDs was accompanied by a shorter intra-SRED interval. At the highest +WIN concentration of 1000 nM, the increase in frequency of SREDs was nearing a state of continuous epileptiform (SE-like) seizure activity. At all concentrations of +WIN exposure, sham control (Mg2+-containing) treated cultures showed no change in electrophysiological properties as recorded in whole-cell current-clamp mode when compared to controls not exposed to agonist (data not shown). For each neuron analyzed, whole-cell current-clamp recordings were evaluated for 60 min and the frequency of SREDs was determined. Prolonged exposure of epileptic cultures to 100 nM and 1000 nM +WIN resulted in significant increases in SRED frequency to 215.2±18.3% and 239.6±31.2% of control (epileptic no +WIN) respectively, while exposure to 10 nM resulted in a marginal but non-significant increase in frequency (Fig. 3A).
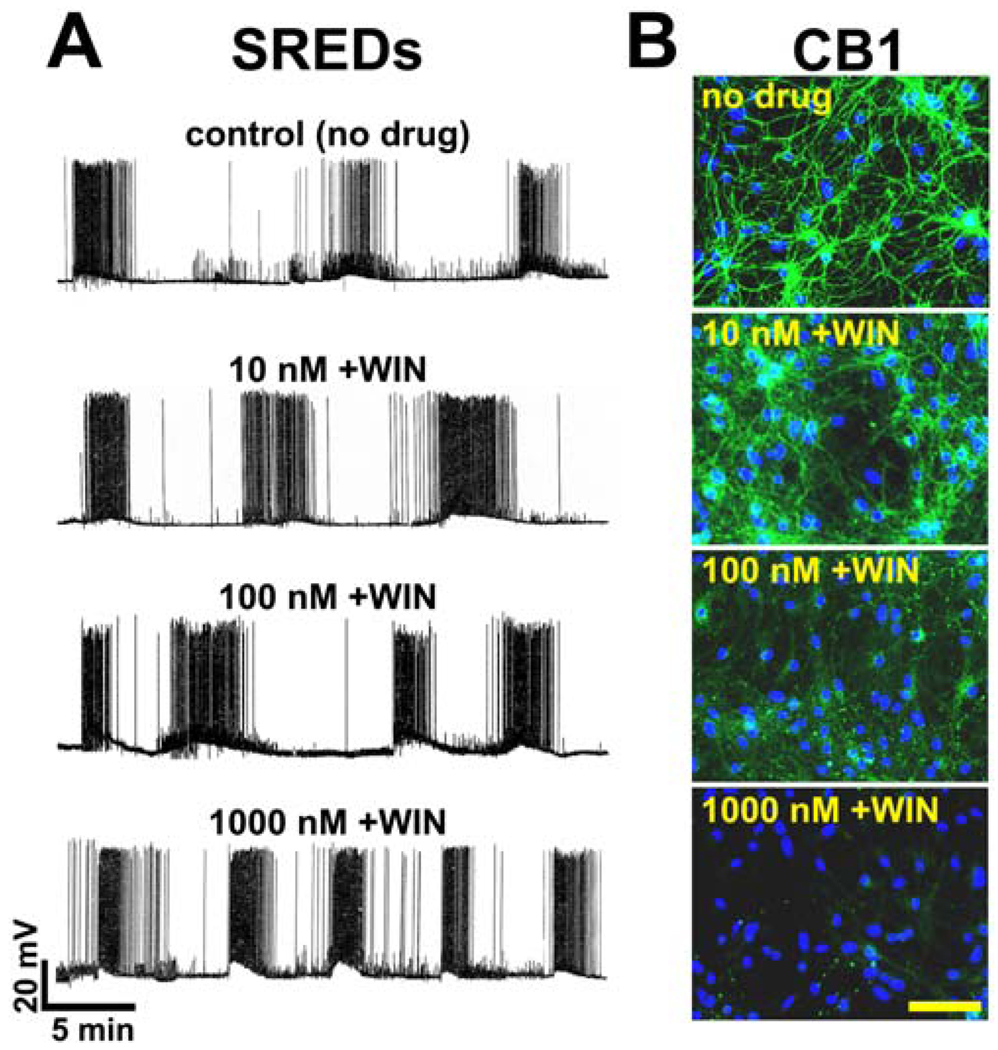
Fig 2. Prolonged exposure of epileptic cultures to WIN55,212-2 results in a concentration-dependent increase in SRED frequency and down-regulation of CB1 receptor expression.
Following 3 hours of low Mg2+ treatment to induce SREDs, epileptic cultures were exposed to varying concentrations (0-1000 nM) of WIN 55,212-2 (+WIN) for 24 hours and then underwent electrophysiological and immunocytochemical analysis. A. (top trace) Representative whole-cell current-clamp recording from an epileptic (no drug) control neuron displaying three independent SREDs. Prolonged exposure to +WIN at 10 nM, 100 nM and 1000 nM causes a concentration-dependent increase in freguency of SREDs. B. (top panel) Representative image of an epileptic (no drug) control neuronal culture showing CB1 receptor staining (FITC-green) throughout the neuronal processes. Prolonged exposure of epileptic cultures to +WIN results in a concentration-dependent decrease in staining for CB1receptor in neuronal processes. Nuclear staining of DNA with DAPI (blue) revealed that prolonged exposure to increasing concentrations of +WIN had no effect on neuronal culture density (scale = 100 microns).
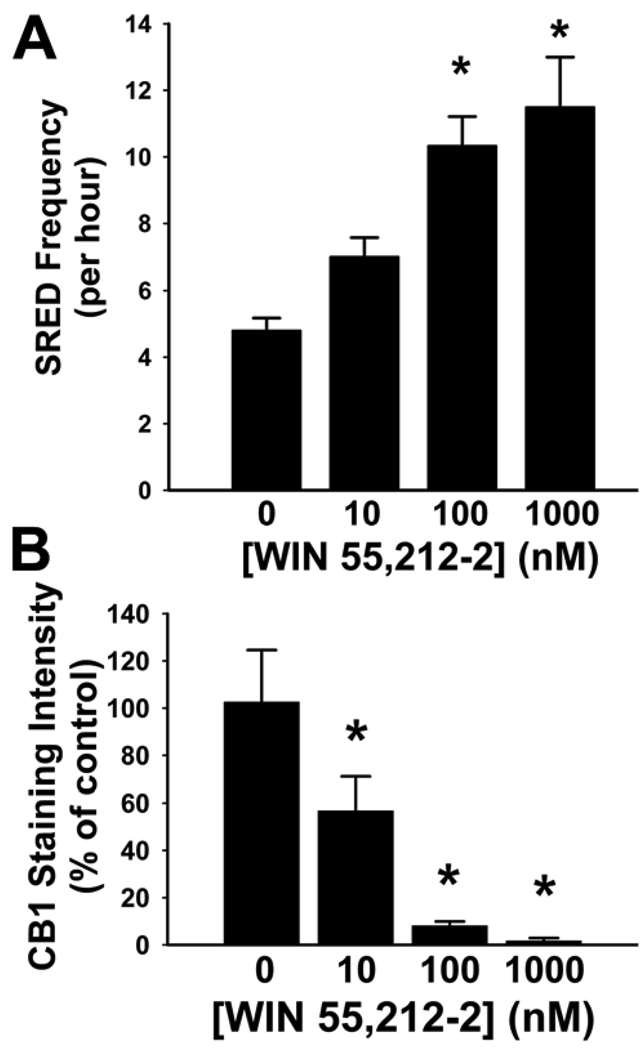
Fig 3. Statistical evaluation of SRED frequency and CB1 receptor expression in epileptic cultures following 24 hour exposure to varying concentrations of WIN55.212-2.
Following 3 hours of Mg2+-free exposure to induce SREDs, epileptic cultures were exposed to varying concentrations (0-1000 nM) of WIN55,212-2 (+WIN) for 24 hours and then underwent statistical analysis of both SRED frequency and CB1 receptor staining. A. Electrophysiological traces were evaluated over 60 min, and the frequency of SREDs was determined for each neuron recorded. Exposure of epileptic cultures to 100 nM or 1000 nM +WIN resulted in significant increases in frequency to 215.2±18.3% and 239.6±31.2% respectively. Exposure to 10 nM +WIN resulted in no significant increase in frequency of SREDs (one-way ANOVA, p=0.003; Holm-Sidak post-hoc,- *, p≤ 0.05, n = 5, 3, 3, 6 for 0.0, 10, 100 and 1000 nM +WIN respectively). B. Quantification of CB1 receptor staining intensity in epileptic cultures following 24h exposure to varying concentrations of +WIN. Grayscale images were segmented for analysis of specific CB1 stain and pixel intensity values were measured for each neuronal culture plate evaluated (see Materials and Methods). Exposure of epileptic cultures to 10 nM,100 nM and 1000 nM +WIN resulted in significant decreases in CB1 receptor stain intensity of 43.2±14.3 %, 91.8 ±1.7 % and 98.2± 1.1 % respectively (one-way ANOVA, p≤0.001; Duncan’s post-hoc,- *, p≤ 0.05, n = 5–7). Values in A and B are percent of control±S.E.M.
To evaluate the effect of prolonged exposure to +WIN on CB1 receptor expression, epileptic cultures were exposed to a concentration range of +WIN (0–1000 nM) for 24 h and then evaluated for receptor protein expression by immunocytochemical staining with an antibody directed against the intracellular C-terminal tail of the CB1 receptor. Prior to immunocytochemical analysis, plates were evaluated for the presence or absence of SRED activity. This antibody has been previously characterised in neuronal tissue and has been shown to recognize CB1 receptors at both GABAergic and glutamatergic terminals (Monory et al., 2006). Prolonged exposure to +WIN induced a concentration-dependent downregulation of the CB1 receptor throughout the neuronal processes (Fig 2B). The 10nM and 100 nM concentrations of +WIN resulted in a decrease in CB1 receptor staining, with the latter concentration inducing a higher degree of receptor downregulation. Exposure to +WIN 1000 nM resulted in a total loss of CB1 receptor staining throughout the neuronal processes (Fig 2B). Counterstaining with DAPI (blue) reveals that neither the induction of SREDs (epileptic) nor WIN55,212-2 exposure had any observable effect on cell culture densities (Fig 2B).
Quantification and statistical analysis of immunocytochemical stains for CB1receptor throughout the neuronal processes revealed that prolonged exposure of epileptic cultures to +WIN resulted in a significant downregulation of the receptor in a concentration-dependent manner when compared to epileptic (control) cultures treated with the inactive enantiomer WIN55,212-3 (Fig 3B). Although exposure to 10 nM +WIN resulted in a marginal but non-significant decrease in CB1 receptor levels, prolonged exposure to both the 100 nM and 1000 nM concentrations of +WIN resulted in significant decreases in CB1 receptor staining of 91.8 ±1.7 % and 98.2± 1.1 % respectively (Fig 3B). These findings indirectly correlate with the concentration-dependent effect of prolonged +WIN exposure on SRED frequency (Fig 3A) further affirming a causal relationship between these two observations.
3.3. Degree of increase in SRED frequency and decrease in CB1 receptor expression is dependent on both concentration and duration of WIN55,212-2 exposure
To temporally evaluate the +WIN-induced down-regulation of the CB1 receptor and loss of its anticonvulsant effects in this preparation, epileptic cultures were exposed to varying concentrations of +WIN over a time course of exposure duration and evaluated for SRED frequency and CB1 receptor expression levels. Epileptic cultures were treated with +WIN 10 nM or 1000 nM and evaluated following 4 and 12 h of exposure. Although acute treatment of epileptic cultures with 1000 nM +WIN produces total suppression of SRED activity (Fig 1), within 4h of continuous exposure, SRED frequency had recovered to a level slightly below that of control (no treatment) epileptic cultures suggesting a loss of the anticonvulsant effect (Fig 4A). In addition, 4h exposure to 10 nM +WIN had no significant effect on SRED frequency as compared to epileptic control cultures (Fig 4A). Evaluation of CB1 receptor levels following 4h exposure to +WIN revealed that both the 10 nM and 1000 nM treatments resulted in substantial down-regulation of the receptor that was 42.3±12.3% and 36.6.1±6.5% from control respectively (Fig 4B). Thus, 4h of exposure of epileptic cultures to 10 nM or 1000 nM +WIN resulted in approximately a 40% downregulation of the CB1 receptor, but had no effect on SRED frequency.
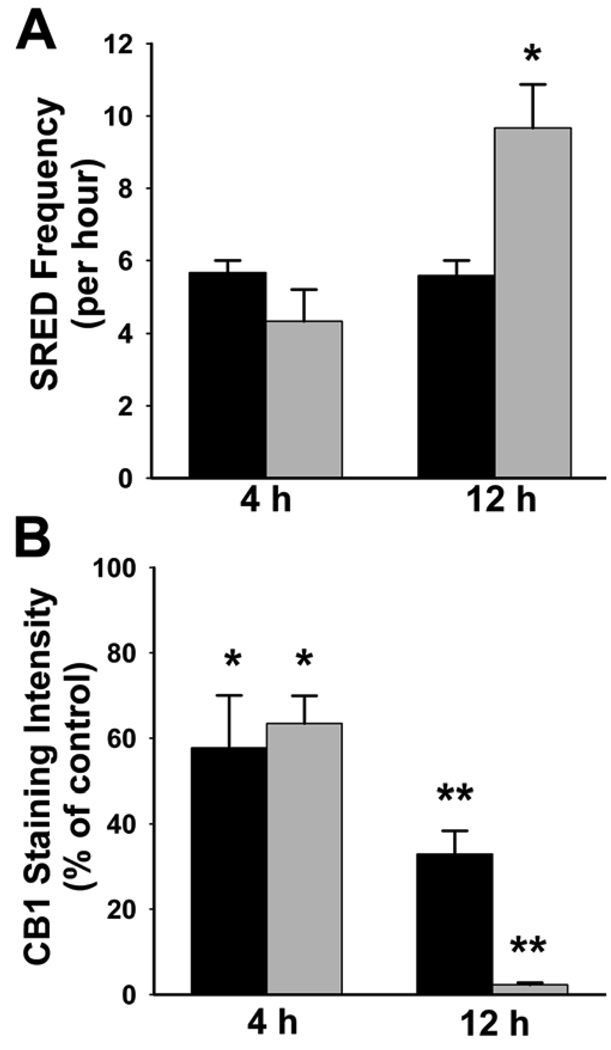
Fig 4. WIN55,212-2-induced increase in SRED frequency and down-regulation of the CB1 receptor in epileptic cultures is dependent on duration and concentration of agonist exposure.
Following 3 hours of Mg2+-free exposure to induce SREDs, epileptic cultures were exposed for 4 and 12h to WIN55,212-2 (+WIN) at 10 nM (black bars) and 1000 nM (grey bars), and then underwent statistical evaluation for both SRED frequency and intensity of CB1 receptor staining. A. Electrophysiological traces were evaluated over 60 min, and the frequency of SREDs was determined for each neuron recorded. Exposure of epileptic cultures for 4h to either 10 nM_or 1000 nM +WIN resulted in no significant change in SRED frequency. Following 12h exposure to +WIN, a significant increase of 200±25% in SRED frequency was observed at the 1000 nM concentration, while no change was evident at the 10 nM +WIN (one-way ANOVA, p=0.007; Holm-Sidak post-hoc,- *, p≤ 0.05, n = 3). B. Quantification of CB1 receptor staining intensity in epileptic cultures exposed for 4 and 12h to +WIN at 10 nM (black bars) and 1000 nM (grey bars) concentrations. Exposure for 4h at both 10 nM and 1000 nM +WIN resulted in significant decreases of 42.3±12.3% and 36.6±6.5% in CB1 receptor staining respectively. Following 12h exposure to +WIN resulted in significant decreases in CB1 receptor staining of 67.1±5.5% and 97.7±0.5% for both the 10 nM and 1000 nM concentrations respectively (one-way ANOVA, p≤ 0.001; Bonferroni t-test,- *, p≤ 0.005, **, p≤ 0.001, n = 30, 7, 9, 5, 10 for pooled (no +WIN) controls, 4h/10 nM, 4h/1000 nM, 12h/10 nM and12h/1000 nM respectively). Values in A and B are percent of control±S.E.M.
Following 12h exposure of epileptic cultures to +WIN, the SRED frequency remained unchanged at the 10 nM concentration while exposure to 1000 nM +WIN resulted in a significant increase to 200±25% of epileptic control (no treatment) cultures (Fig 4A). The increase in SRED frequency following 12h exposure to 1000 nM +WIN coincided with a dramatic reduction in CB1 receptor staining that was reflected by a significant decrease of 97.7±0.5% from epileptic controls (Fig 4B). However, 12h exposure of epileptic cultures to 10 nM +WIN had no effect on SRED frequency, but resulted in a significant decrease in CB1 receptor levels that was 67.1±5.5% from epileptic controls (no treatment) (Fig 4B). Thus, these findings demonstrate that loss of the anticonvulsant effect of +WIN in this preparation is dependent on both duration and concentration of agonist exposure, and that the +WIN-induced down-regulation of the CB1 receptor must reach a critical level for ‘tolerance’ to occur.
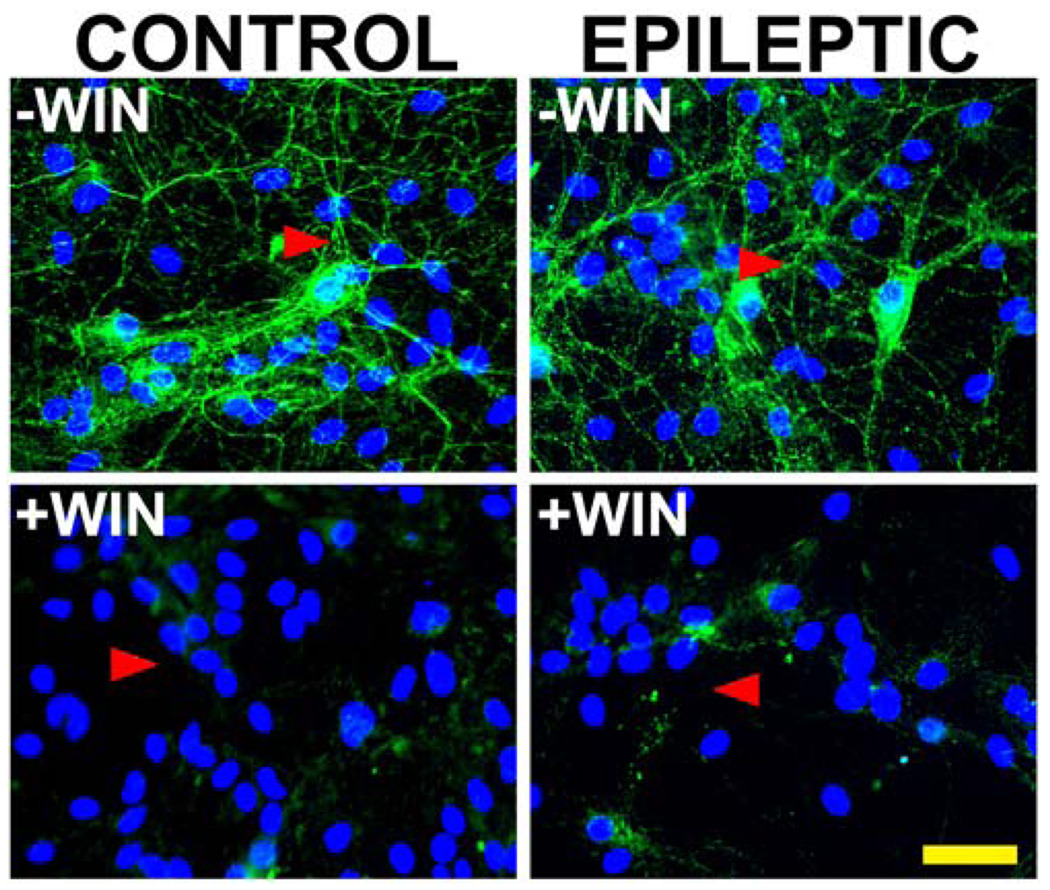
3.4. Prolonged exposure to WIN55,212-2 (1000 nM) results in a decrease in CB1 receptor expression in both control and epileptic hippocampal neuronal cultures
To determine the effect of prolonged exposure of hippocampal neuronal cultures to cannabinoids on expression of the CB1 receptor in sham control and epileptic (SREDs) neuronal cultures, immunocytochemical staining was carried out with an antibody directed against the intracellular C-terminal tail of the CB1 receptor following a 24 h exposure to either the active isomer WIN55,212-2 (+WIN; 1000 nM) or its inactive enantiomer WIN55,212-3 (−WIN; 1000 nM). Sham control and low-Mg2+ treated (SREDs) cultures showed a total loss of CB1 receptor staining throughout the neuronal processes following 24 h exposure to +WIN, while exposure to the inactive isomer –WIN had no effect on levels of staining for CB1 receptor (Fig. 5). Thus, prolonged exposure of either sham control or SRED cultures to the active isomer WIN55,212-2 results in a dramatic down-regulation of CB1 receptor levels throughout the neuronal processes. In the epileptic (SRED) cultures, this agonist-induced downregulation was accompanied by not only the development of ‘tolerance’ to the anticonvulsant effect of CB1 receptor activation, but also a marked exacerbation of SRED activity above non-treated epileptic cultures (Fig 2, Fig 3 and Fig 4). Although sham control neuronal cultures also showed a dramatic down-regulation of the CB1 receptor with prolonged exposure to +WIN, no changes in electrophysiological properties (SREDs) were observed when compared to naïve (untreated) cultures.
Fig 5. Prolonged exposure to the cannabimimetic WIN55,212-2 results in down-regulation of the CB1 receptor in control and epileptic cultures.
Immunocytochemical staining for the CB1 receptor in sham control and epileptic (SREDs) hippocampal neuronal cultures following 24 h exposure to potent the cannabimimetic WIN55,212-2 (1000 nM; +WIN) or its inactive enantiomer WIN55,212-3 (1000 nM; −WIN). In cultures exposed to the inactive isomer –WIN (upper panels), CB1 receptor staining (FITC-green) was present throughout a neuronal network of processes (arrowheads) with no discernable differences between control and epileptic. Prolonged exposure to the potent cannabimimetic +WIN (lower panels) resulted in a pronounced down-regulation of the CB1 receptor (arrowheads) equally in both control and epileptic hippocampal cultures. Nuclear staining of DNA with DAPI (blue) revealed that neither the low-Mg2+ treatment or prolonged +WIN exposure had any effect on neuronal culture density (scale = 50 microns).
3.5. The CB1 receptor is localized at both GABAergic and glutamatergic processes in hippocampal neuronal cultures
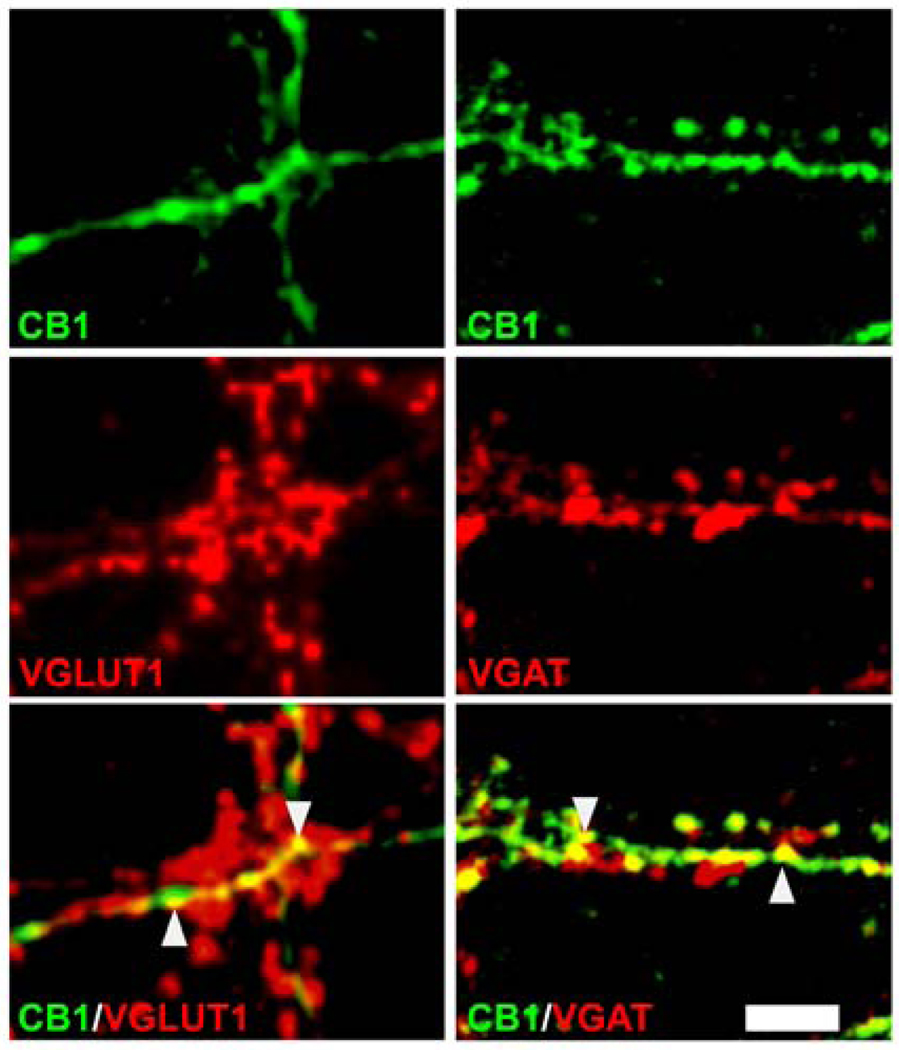
The CB1 receptor has been shown to play an important role in regulating presynaptic function at both excitatory and inhibitory terminals (Cao et al., 1996; Lundberg et al., 2005; Marsicano et al., 2003; Monory et al., 2006). To evaluate the degree of localization of the CB1 receptor at GABAergic and glutamatergic presynaptic terminals in hippocampal neuronal cultures, double-immunocytochemical staining was carried out for the CB1 receptor with either the vesicular GABA transporter (VGAT) or the vesicular glutamate transporter 1 (VGLUT1) out in control cultures. VGAT and VGLUT1 antibodies intensely label GABAergic and glutamatergic presynaptic terminals respectively. Confocal laser scanning microscopy of double-stained cultures revealed that the CB1 receptor was localized at both GABAergic and glutamatergic presynaptic nerve terminals in this hippocampal culture preparation. Punctate CB1 receptor staining (FITC green) was shown to overlap with staining for both VGAT and VGLUT1 (Texas red) as indicated by the yellow ‘merged’ signal throughout the neuronal processes (Fig. 6). Analysis of confocal scans of double-stained cultures revealed that CB1 and VGAT have considerable colocalization with a Pearson’s coefficient of 0.763±0.029 and Mander’s coefficients tM1 = 0.589±0.027 and tM2 = 0.488±0.017 for CB1 and VGAT respectively (n = 5). The highest degree of colocalization for either CB1 or VGAT with each other in all fields evaluated was 63.3%, indicating that experimental conditions used for double-immunocytochemical staining with the two rabbit primary antibodies (see Methods) prevented any false double-immuno-positive staining. Additionally, CB1 and VGLUT1 demonstrated substantial colocalization with a Pearson’s coefficient of 0.67±0.02 and Mander’s coefficients of tM1 = 0.362±0.043 and tM2 = 0.337±0.033 for CB1 and VGLUT1 respectively (n = 11). The above findings demonstrate that the CB1 receptor localized at both GABAergic and glutamatergic presynaptic terminals. Therefore, pharmacodynamic modulation of the CB1 receptor can result in changes in both inhibitory and excitatory synaptic transmission in the hippocampal neuronal culture preparation.
Fig. 6. The CB1 receptor is localized at both GABAergic and glutamatergic terminals in hippocampal neuronal cultures.
Double- immunofluorescent analysis demonstrates colocalization of CB1 receptor staining with both VGAT and VGLUT1 staining in control hippocampal neuronal cultures. High magnification confocal micrographs reveal punctate CB1 receptor staining (green top panels) along the length of several neuronal processes. Secondary immunofluorescent analyses for VGLUT1 or VGAT (red -lower adjacent panels left and right) demonstrate specific punctate presynaptic terminal staining along the neuronal processes denoting either glutamtergic or GABAergic presynaptic terminals respectively. Merged images of CB1 receptor staining with either VGLUT1 (bottom left) or VGAT (bottom right) reveal overlapping stains (yellow arrowheads) indicative of colocalization. Micrographs are representative of a minimum of 5 samples pooled from at least 2 independent experiments. (Scale bar = 5 microns).
4. Discussion
Prolonged exposure to the CB1 receptor agonist WIN55,212-2 (+WIN) resulted in a loss of its anticonvulsant activity against SREDs in the HNC model of AE. Furthermore, this ‘tolerance’ effect was dependent on concentration and duration of +WIN exposure as directly reflected by increased SRED frequency and degree of CB1 receptor down-regulation. Acute exposure to the cannabimimetic +WIN (1000 nM) produced anticonvulsant activity against SREDs in the HNC model of AE, which was blocked in the presence of the specific receptor antagonist SR141716A (1 µM), demonstrating a CB1 receptor-dependent mechanism of seizure suppression (Fig 1). These results are in agreement with earlier findings from this laboratory showing that CB1 receptor activation produced total suppression of SRED and SE activity following acute application of +WIN at 3 µM and 5 µM respectively (Blair et al., 2006).
Prolonged (24 h) exposure of epileptic cultures to +WIN over a concentration range of 10–1000 nM resulted in the development of ‘tolerance’ to its anticonvulsant effect with a concentration-dependent increase in frequency of SREDs (Fig 2A). Evaluation of SRED frequency revealed a significant increase from epileptic (no +WIN) controls following prolonged exposure to +WIN at the 100 and 1000 nM concentrations (Fig 3A). Control (non-epileptic) cultures showed no change from baseline electrophysiological activity following prolonged exposure to +WIN at all concentrations. Immunocytochemical staining of CB1 revealed that following 24 h exposure of epileptic cultures to varying concentrations (10–1000 nM) of +WIN resulted in a concentration-dependent decrease in staining throughout the neuronal processes as compared to epileptic control (no agonist exposure) cultures (Fig 2B). Analysis of immunocytochemical staining revealed a significant +WIN-induced down-regulation of the CB1 receptor (Fig 3B). The findings of this study further demonstrate a temporal association with duration of +WIN exposure, decrease in CB1 receptor staining and increased SRED frequency, further substantiating a causal relationship between receptor down-regulation and development of ‘tolerance’ in this preparation (Fig 4A and B). Sham control (+Mg2+-treated) neuronal cultures that underwent +WIN-induced downregulation of CB1 receptor levels (Fig 5) showed no change in whole-cell current-clamp recordings when compared to untreated controls. Thus, the time and concentration-dependent loss of CB1 receptor staining in +WIN treated epileptic cultures indirectly correlates with SRED frequency, which strongly associates receptor downregulation with the development of ‘tolerance’. This ‘tolerant’ effect is unique to the epileptic condition in that control (non-epileptic) cultures showing an equal degree of +WIN-induced loss of CB1 receptor expression showed no indication of seizure activity or increased neuronal excitability. Further immunofluorescent colocalization analysis revealed that the CB1 receptor is localized at both GABAergic and glutamatergic presynaptic terminals indicating that the localization of this receptor is in a position to presynaptically regulate inhibitory and excitatory neurotransmission. This is the first study to demonstrate an increase in seizure activity in association with the development of ‘tolerance’ to a cannabinoid. The dramatic downregulation of the CB1 receptor and the increase in frequency of SREDs in epileptic cultures following prolonged exposure to +WIN indicates that the development of ‘tolerance’ to exogenous cannabinoids may result in a deregulation of compensatory mechanisms responsible for control of seizure discharge in this phenotype.
It is well established that repeated and prolonged administration of cannabinoids experimentally results in the development of tolerance to their pharmacological effects (Gonzalez et al., 2005; Martin et al., 2004). Cannabinoids with varying agonist efficacy may induce tolerance to their effects via different pharmacodynamic mechanisms which include desensitization and (or) downregulation of the CB1 receptor (Breivogel et al., 2003; Sim-Selley and Martin, 2002). Additionally, the degree, agonist efficacy and timing of these adaptations have been demonstrated to vary regionally throughout the brain (McKinney et al., 2008; Sim-Selley and Martin, 2002; Sim-Selley et al., 2006). The hippocampus, a limbic structure that has been shown to be involved in the development and maintenance of seizure discharges, highly expresses CB1 receptors and has been shown to undergo rapid adaptation following repeated administration of cannabinoids (Breivogel et al., 1999; McKinney et al., 2008). The cannabimimetic +WIN is a full agonist at the CB1 receptor and has been shown to primarily induce CB1 receptor internalization in hippocampal neuronal cultures following exposure to high concentrations or for prolonged periods of time (Coutts et al., 2001; Martini et al., 2007). Following +WIN-induced internalization, the CB1 receptor can follow one of two routes and either be recycled back to the plasma membrane or directed towards the degradative lysozomal pathway depending on exposure duration and concentration of agonist (Coutts et al., 2001; Hsieh et al., 1999; Martini et al., 2007; Wu et al., 2008). Our findings demonstrate that the full agonist +WIN resulted in a downregulation of the CB1 receptor in this HNC preparation that was dependent on duration and concentration of agonist exposure. Although receptor desensitization cannot be ruled out, the downregulation observed in the present study is more likely the result of receptor internalization/degradation in light of findings from previous studies evaluating the effects of prolonged +WIN exposure (Coutts et al., 2001; Wu et al., 2008) and the total loss of CB1 receptor staining observed in this study (Fig 2 and Fig 4). Thus, agonist efficacy, duration of exposure and dose can result in different degrees of cannabinoid-induced adaptation/tolerance which may be capable of undermining the regulatory role of the endogenous cannabinoid system in the brain.
In vitro hippocampal culture preparations have demonstrated desensitization of cannabinoid mediated inhibition of glutamatergic synaptic transmission following prolonged exposure to the cannabinoids Δ9-THC and +WIN (Kouznetsova et al., 2002; Lundberg et al., 2005). In addition, tolerance to the antinociceptive effects of cannabinoids has also been observed following repeated and prolonged exposure (Tappe-Theodor et al., 2007). Previous studies demonstrated that repeated administration of Δ9-THC to electrically kindled rats resulted in an attenuation of its effects on kindling, indicating the development of behavioral tolerance (Fried and Mclntyre, 1973). The current study demonstrated utilizing an in vitro model of SREDs that exposure of epileptic cultures to the cannabimimetic +WIN over time resulted in a concentration-dependent increase in SRED frequency. At the highest concentration of +WIN (1000 nM) exposure for 24h, the increase in SRED frequency was nearing a state of continuous (SE-like) seizure activity and was associated with a significant downregulation of the CB1 receptor throughout the neuronal processes.
Previous findings from our lab have shown that pharmacological blockade of the CB1 receptor in both in vivo (Wallace et al., 2003) and in vitro (Deshpande et al., 2007b) models of AE results in the immediate conversion from spontaneous recurrent seizure activity to high-frequency seizure (SE-like) discharges. Thus, these previous findings along with the results from the present study suggest that in the epileptic phenotype, pharmacological suppression of CB1 receptor function by either antagonism or agonist-induced receptor downregulation results in exacerbation of seizure activity. The present findings not only show evidence of the development of a ‘tolerance’ effect to the anticonvulsant properties of +WIN exposure, but an exacerbation of seizure activity above that of untreated epileptic (control) cultures.
The endocannabinoid system plays an important role in the CNS through its regulatory action on many physiological processes some of which include neuroexcitability, cognition, appetite, and pain perception (Di Marzo et al., 1998; Elphick and Egertova, 2001; Howlett et al., 2004). As a result of its broad regulatory function within the brain, much research has focused on how regulating the endocannabinoid system may show promise towards the development of therapeutic interventions for a number of neuronal disorders (Consroe, 1998; Mackie, 2006). Modulation of the endocannabinoid system by exogenous cannabinoid receptor agonists and antagonists or regulation of the cellular machinery supporting endocannabinoid-mediated neurotransmission has been shown to produce protective/restorative effects in models of neurotoxicity (Abood et al., 2001; Docagne et al., 2007; Kim et al., 2006; Marsicano et al., 2003; Zani et al., 2007; Zhuang et al., 2005). One limitation to the therapeutic use of cannabinoid compounds in select pathologies has been the development of tolerance to their effects (Martin et al., 2004; McKinney et al., 2008). This has been clearly demonstrated experimentally in studies demonstrating a loss of the antinociceptive properties (analgesic tolerance) of cannabinoids (Smith et al., 2007; Tappe-Theodor et al., 2007). Interestingly, recent findings have shown that the combination of cannabinoids with ultra-low doses of CB1 receptor antagonists can prevent the onset of analgesic tolerance (Paquette et al., 2007). This pharmacological approach to modulate CB1 receptor-dependent transmission for the chronic suppression of neuronal hyper-excitability associated with epilepsy is attractive, and warrants further investigations.
Although external/pharmacological manipulation of the endocannabinoid system shows much promise in therapeutic intervention, there is also increasing evidence demonstrating that plasticity of this system occurs in a number of neuronal disorders, and that this ‘remodeling’ of the endocannabinoid system is functioning in a compensatory/protective role within these neuropathologies (Bisogno and Di Marzo, 2007). In light of the adaptability of the endocannabinoid system which ensues following repeated and prolonged administration of cannabinoid compounds, agonist-induced desensitization/downregulation of target proteins could cause maladaptive repercussions in a neuropathological phenotype. Our results demonstrate that prolonged exposure of epileptic cultures to the cannabimimetic +WIN results in concentration-dependent downregulation of CB1 receptor levels and exacerbation of seizure activity in this model of AE.
ACKNOWLEDGEMENTS
We would like to thank Drs. Dawn S. Carter and Katherine W. Falenski for their editorial contributions and Elisa C. Attkisson for neuronal culture work. The NIDA Chemical Synthesis and Drug Supply Program provided through the Department of Pharmacology and Toxicology at Virginia Commonwealth University. Confocal microscopy was performed at the VCU - Dept. of Neurobiology & Anatomy Microscopy Facility, supported, in part, with funding from NIH-NINDS Center core grant (5P30NS047463). This work was supported by National Institute of Neurological Disorders and Stroke Grants (R01-NS051505 and R01-NS052529 to RJD) and a National Institute of Drug Abuse Grant (P50DA005274 to BRM and RJD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abood ME, Rizvi G, Sallapudi N, McAllister SD. Activation of the CB1 cannabinoid receptor protects cultured mouse spinal neurons against excitotoxicity. Neurosci Lett. 2001;309:197–201. doi: 10.1016/s0304-3940(01)02065-1. [DOI] [PubMed] [Google Scholar]
- Alger BE. Not too excited? Thank your endocannabinoids. Neuron. 2006;51:393–395. doi: 10.1016/j.neuron.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Di Marzos V. Short- and long-term plasticity of the endocannabinoid system in neuropsychiatric and neurological disorders. Pharmacol Res. 2007;56:428–442. doi: 10.1016/j.phrs.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Blair RE, Deshpande LS, Sombati S, Falenski KW, Martin BR, DeLorenzo RJ. Activation of the cannabinoid type-1 receptor mediates the anticonvulsant properties of cannabinoids in the hippocampal neuronal culture models of acquired epilepsy and status epilepticus. J Pharmacol Exp Ther. 2006;317:1072–1078. doi: 10.1124/jpet.105.100354. [DOI] [PubMed] [Google Scholar]
- Blair RE, Sombati S, Lawrence DC, McCay BD, DeLorenzo RJ. Epileptogenesis causes acute and chronic increases in GABAA receptor endocytosis that contributes to the induction and maintenance of seizures in the hippocampal culture model of acquired epilepsy. J Pharmacol Exp Ther. 2004;310:871–880. doi: 10.1124/jpet.104.068478. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Vogt LJ, Sim-Selley LJ. Chronic delta9-tetrahydrocannabinol treatment produces a time-dependent loss of cannabinoid receptors and cannabinoid receptor-activated G proteins in rat brain. J Neurochem. 1999;73:2447–2459. doi: 10.1046/j.1471-4159.1999.0732447.x. [DOI] [PubMed] [Google Scholar]
- Breivogel CS, Scates SM, Beletskaya IO, Lowery OB, Aceto MD, Martin BR. The effects of delta9-tetrahydrocannabinol physical dependence on brain cannabinoid receptors. Eur J Pharmacol. 2003;459:139–150. doi: 10.1016/s0014-2999(02)02854-6. [DOI] [PubMed] [Google Scholar]
- Cao Y, Wilcox KS, Martin CE, Rachinsky TL, Eberwine J, Dichter MA. Presence of mRNA for glutamic acid decarboxylase in both excitatory and inhibitory neurons. Proc Natl Acad Sci U S A. 1996;93:9844–9849. doi: 10.1073/pnas.93.18.9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter DS, Haider SN, Blair RE, Deshpande LS, Sombati S, DeLorenzo RJ. Altered calcium/calmodulin kinase II activity changes calcium homeostasis that underlies epileptiform activity in hippocampal neurons in culture. J Pharmacol Exp Ther. 2006;319:1021–1031. doi: 10.1124/jpet.106.110403. [DOI] [PubMed] [Google Scholar]
- Consroe P. Brain cannabinoid systems as targets for the therapy of neurological disorders. Neurobiol Dis. 1998;5:534–551. doi: 10.1006/nbdi.1998.0220. [DOI] [PubMed] [Google Scholar]
- Consroe P, Wolkin A. Cannabidiol--antiepileptic drug comparisons and interactions in experimentally induced seizures in rats. J Pharmacol Exp Ther. 1977;201:26–32. [PubMed] [Google Scholar]
- Coutts AA, Anavi-Goffer S, Ross RA, MacEwan DJ, Mackie K, Pertwee RG, Irving AJ. Agonist-induced internalization and trafficking of cannabinoid CB1 receptors in hippocampal neurons. J Neurosci. 2001;21:2425–2433. doi: 10.1523/JNEUROSCI.21-07-02425.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande LS, Blair RE, Ziobro JM, Sombati S, Martin BR, Delorenzo RJ. Endocannabinoids block status epilepticus in cultured hippocampal neurons. Eur J Pharmacol. 2007a;558:52–59. doi: 10.1016/j.ejphar.2006.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande LS, Sombati S, Blair RE, Carter DS, Martin BR, DeLorenzo RJ. Cannabinoid CB1 receptor antagonists cause status epilepticus-like activity in the hippocampal neuronal culture model of acquired epilepsy. Neurosci Lett. 2007b;411:11–16. doi: 10.1016/j.neulet.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devane WA, Dysarz FA, 3rd, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34:605–613. [PubMed] [Google Scholar]
- Di Marzo V, Melck D, Bisogno T, De Petrocellis L. Endocannabinoids: endogenous cannabinoid receptor ligands with neuromodulatory action. Trends Neurosci. 1998;21:521–528. doi: 10.1016/s0166-2236(98)01283-1. [DOI] [PubMed] [Google Scholar]
- Docagne F, Muneton V, Clemente D, Ali C, Loria F, Correa F, Hernangomez M, Mestre L, Vivien D, Guaza C. Excitotoxicity in a chronic model of multiple sclerosis: Neuroprotective effects of cannabinoids through CB1 and CB2 receptor activation. Mol Cell Neurosci. 2007;34:551–561. doi: 10.1016/j.mcn.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Egertova M, Elphick MR. Localisation of cannabinoid receptors in the rat brain using antibodies to the intracellular C-terminal tail of CB. J Comp Neurol. 2000;422:159–171. doi: 10.1002/(sici)1096-9861(20000626)422:2<159::aid-cne1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Elphick MR, Egertova M. The neurobiology and evolution of cannabinoid signalling. Philos Trans R Soc Lond B Biol Sci. 2001;356:381–408. doi: 10.1098/rstb.2000.0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falenski KW, Blair RE, Sim-Selley LJ, Martin BR, DeLorenzo RJ. Status epilepticus causes a long-lasting redistribution of hippocampal cannabinoid type 1 receptor expression and function in the rat pilocarpine model of acquired epilepsy. Neuroscience. 2007;146:1232–1244. doi: 10.1016/j.neuroscience.2007.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried PA, Mclntyre DC. Electrical and behavioral attenuation of the anti-convulsant properties of delta 9-TNC following chronic administrations. Psychopharmacologia. 1973;31:215–227. doi: 10.1007/BF00422512. [DOI] [PubMed] [Google Scholar]
- Gonzalez S, Cebeira M, Fernandez-Ruiz J. Cannabinoid tolerance and dependence: a review of studies in laboratory animals. Pharmacol Biochem Behav. 2005;81:300–318. doi: 10.1016/j.pbb.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Hanyaloglu AC, von Zastrow M. Regulation of GPCRs by endocytic membrane trafficking and its potential implications. Annu Rev Pharmacol Toxicol. 2008;48:537–568. doi: 10.1146/annurev.pharmtox.48.113006.094830. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch DB, Dingledine R. GABAergic neurons in rat hippocampal culture. Brain Res. 1986;390:53–64. doi: 10.1016/0165-3806(86)90151-3. [DOI] [PubMed] [Google Scholar]
- Howlett AC. Pharmacology of cannabinoid receptors. Annu Rev Pharmacol Toxicol. 1995;35:607–634. doi: 10.1146/annurev.pa.35.040195.003135. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Porrino LJ. Cannabinoid physiology and pharmacology: 30 years of progress. Neuropharmacology. 2004;47 Suppl 1:345–358. doi: 10.1016/j.neuropharm.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Hsieh C, Brown S, Derleth C, Mackie K. Internalization and recycling of the CB1 cannabinoid receptor. J Neurochem. 1999;73:493–501. doi: 10.1046/j.1471-4159.1999.0730493.x. [DOI] [PubMed] [Google Scholar]
- Karler R, Turkanis SA. The cannabinoids as potential antiepileptics. J Clin Pharmacol. 1981;21 doi: 10.1002/j.1552-4604.1981.tb02624.x. 437S‒448S. [DOI] [PubMed] [Google Scholar]
- Kim SH, Won SJ, Mao XO, Jin K, Greenberg DA. Molecular mechanisms of cannabinoid protection from neuronal excitotoxicity. Mol Pharmacol. 2006;69:691–696. doi: 10.1124/mol.105.016428. [DOI] [PubMed] [Google Scholar]
- Kouznetsova M, Kelley B, Shen M, Thayer SA. Desensitization of cannabinoid-mediated presynaptic inhibition of neurotransmission between rat hippocampal neurons in culture. Mol Pharmacol. 2002;61:477–485. doi: 10.1124/mol.61.3.477. [DOI] [PubMed] [Google Scholar]
- Lothman EW, Bertram EH, Stringer JL. Functional anatomy of hippocampal seizures. Prog.Neurobiol. 1991;37:1–82. doi: 10.1016/0301-0082(91)90011-o. [DOI] [PubMed] [Google Scholar]
- Lundberg DJ, Daniel AR, Thayer SA. Delta(9)-Tetrahydrocannabinol-induced desensitization of cannabinoid-mediated inhibition of synaptic transmission between hippocampal neurons in culture. Neuropharmacology. 2005 doi: 10.1016/j.neuropharm.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Mackie K. Cannabinoid receptors as therapeutic targets. Annu Rev Pharmacol Toxicol. 2006;46:101–122. doi: 10.1146/annurev.pharmtox.46.120604.141254. [DOI] [PubMed] [Google Scholar]
- Mackie K, Stella N. Cannabinoid receptors and endocannabinoids: evidence for new players. AAPS J. 2006;8:E298–E306. doi: 10.1007/BF02854900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, Azad SC, Cascio MG, Gutierrez SO, van der Stelt M, Lopez-Rodriguez ML, Casanova E, Schutz G, Zieglgansberger W, Di Marzo V, Behl C, Lutz B. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302:84–88. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- Martin BR, Sim-Selley LJ, Selley DE. Signaling pathways involved in the development of cannabinoid tolerance. Trends Pharmacol Sci. 2004;25:325–330. doi: 10.1016/j.tips.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Martini L, Waldhoer M, Pusch M, Kharazia V, Fong J, Lee JH, Freissmuth C, Whistler JL. Ligand-induced down-regulation of the cannabinoid 1 receptor is mediated by the G-protein-coupled receptor-associated sorting protein GASP1. FASEB J. 2007;21:802–811. doi: 10.1096/fj.06-7132com. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- McKinney DL, Cassidy MP, Collier LM, Martin BR, Wiley JL, Selley DE, Sim-Selley LJ. Dose-related differences in the regional pattern of cannabinoid receptor adaptation and in vivo tolerance development to delta9-tetrahydrocannabinol. J Pharmacol Exp Ther. 2008;324:664–673. doi: 10.1124/jpet.107.130328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Spatz M, Shohami E. Endocannabinoids and neuroprotection. Sci STKE. 2002:RE5. doi: 10.1126/stke.2002.129.re5. [DOI] [PubMed] [Google Scholar]
- Micale V, Mazzola C, Drago F. Endocannabinoids and neurodegenerative diseases. Pharmacol Res. 2007;56:382–392. doi: 10.1016/j.phrs.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Monory K, Massa F, Egertova M, Eder M, Blaudzun H, Westenbroek R, Kelsch W, Jacob W, Marsch R, Ekker M, Long J, Rubenstein JL, Goebbels S, Nave KA, During M, Klugmann M, Wolfel B, Dodt HU, Zieglgansberger W, Wotjak CT, Mackie K, Elphick MR, Marsicano G, Lutz B. The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron. 2006;51:455–466. doi: 10.1016/j.neuron.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette JJ, Wang HY, Bakshi K, Olmstead MC. Cannabinoid-induced tolerance is associated with a CB1 receptor G protein coupling switch that is prevented by ultra-low dose rimonabant. Behav Pharmacol. 2007;18:767–776. doi: 10.1097/FBP.0b013e3282f15890. [DOI] [PubMed] [Google Scholar]
- Sim-Selley LJ, Martin BR. Effect of chronic administration of R-(+)-[2,3-Dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo[1,2,3-de]-1,4-b enzoxazinyl]-(1-naphthalenyl)methanone mesylate (WIN55,212-2) or delta(9)-tetrahydrocannabinol on cannabinoid receptor adaptation in mice. J Pharmacol Exp Ther. 2002;303:36–44. doi: 10.1124/jpet.102.035618. [DOI] [PubMed] [Google Scholar]
- Sim-Selley LJ, Schechter NS, Rorrer WK, Dalton GD, Hernandez J, Martin BR, Selley DE. Prolonged recovery rate of CB1 receptor adaptation after cessation of long-term cannabinoid administration. Mol Pharmacol. 2006;70:986–996. doi: 10.1124/mol.105.019612. [DOI] [PubMed] [Google Scholar]
- Smith PA, Selley DE, Sim-Selley LJ, Welch SP. Low dose combination of morphine and delta9-tetrahydrocannabinol circumvents antinociceptive tolerance and apparent desensitization of receptors. Eur J Pharmacol. 2007;571:129–137. doi: 10.1016/j.ejphar.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sombati S, DeLorenzo RJ. Recurrent spontaneous seizure activity in hippocampal neuronal networks in culture. J.Neurophysiol. 1995;73:1706–1711. doi: 10.1152/jn.1995.73.4.1706. [DOI] [PubMed] [Google Scholar]
- Tappe-Theodor A, Agarwal N, Katona I, Rubino T, Martini L, Swiercz J, Mackie K, Monyer H, Parolaro D, Whistler J, Kuner T, Kuner R. A molecular basis of analgesic tolerance to cannabinoids. J Neurosci. 2007;27:4165–4177. doi: 10.1523/JNEUROSCI.5648-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MJ, Blair RE, Falenski KW, Martin BR, DeLorenzo RJ. The endogenous cannabinoid system regulates seizure frequency and duration in a model of temporal lobe epilepsy. J Pharmacol Exp Ther. 2003;307:129–137. doi: 10.1124/jpet.103.051920. [DOI] [PubMed] [Google Scholar]
- Wallace MJ, Martin BR, DeLorenzo RJ. Evidence for a physiological role of endocannabinoids in the modulation of seizure threshold and severity. Eur J Pharmacol. 2002;452:295–301. doi: 10.1016/s0014-2999(02)02331-2. [DOI] [PubMed] [Google Scholar]
- Wallace MJ, Wiley JL, Martin BR, DeLorenzo RJ. Assessment of the role of CB1 receptors in cannabinoid anticonvulsant effects. Eur J Pharmacol. 2001;428:51–57. doi: 10.1016/s0014-2999(01)01243-2. [DOI] [PubMed] [Google Scholar]
- Wu DF, Yang LQ, Goschke A, Stumm R, Brandenburg LO, Liang YJ, Hollt V, Koch T. Role of receptor internalization in the agonist-induced desensitization of cannabinoid type 1 receptors. J Neurochem. 2008;104:1132–1143. doi: 10.1111/j.1471-4159.2007.05063.x. [DOI] [PubMed] [Google Scholar]
- Zani A, Braida D, Capurro V, Sala M. Delta9-tetrahydrocannabinol (THC) and AM 404 protect against cerebral ischaemia in gerbils through a mechanism involving cannabinoid and opioid receptors. Br J Pharmacol. 2007;152:1301–1311. doi: 10.1038/sj.bjp.0707514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang SY, Bridges D, Grigorenko E, McCloud S, Boon A, Hampson RE, Deadwyler SA. Cannabinoids produce neuroprotection by reducing intracellular calcium release from ryanodine-sensitive stores. Neuropharmacology. 2005;48:1086–1096. doi: 10.1016/j.neuropharm.2005.01.005. [DOI] [PubMed] [Google Scholar]