Abstract
Exposure to accidental or deliberate radiation poses a threat to public health, proving lethal at higher doses in large part due to deleterious effects on marrow. In those cases, allogeneic hematopoietic cell transplantation (HCT) might be required to restore marrow function. Most radiation accident victims will have HLA-haploidentical relatives who could serve as HCT donors. Here, we assessed in a canine HCT model the total body irradiation (TBI) doses after which transplants might be required and successful engraftment would be possible. In an attempt at mimicking the logistical problems likely to exist after radiation accidents, 4-, 8- or 10-day intervals were placed between TBI and HCT. In order to keep the experimental readout simple, no graft-vs-host disease (GVHD) prevention was administered. All dogs transplanted after a 4-day delay following 700 or 920 cGy TBI successfully engrafted while virtually all those given 450 or 600 cGy rejected their grafts. Transplant delays of 8 and 10 days following 920 cGy TBI also resulted in successful engraftment in most dogs, while a delay of 8 days after 700 cGy resulted in virtually uniform graft failure. The time courses of acute GVHD and rates of granulocyte recovery in engrafting dogs were comparable among dogs regardless of the lengths of delay. In other studies, we showed that most dogs not given HCT survived 700 cGy TBI with intensive supportive care while those given 800 cGy TBI and higher died with marrow aplasia. Thus, DLA-haploidentical HCT was successful even when carried out 4, 8 or 10 days after TBI at or above radiation exposures where dogs survived with intensive care alone.
Keywords: Hematopoietic cell transplantation, Total body irradiation, Engraftment, Dogs
Introduction
Radiation poses a significant threat to public health through either accidental or deliberate exposures, proving lethal at higher doses in large part due to its deleterious effects on hematopoiesis. In those cases, allogeneic hematopoietic cell transplantation (HCT) might be required to restore marrow function. We previously showed that marrow grafts from DLA-identical canine littermates were beneficial in the setting of total body exposures to γ-radiation ranging from 450–1150 cGy, with most dogs surviving either with sustained allogeneic engraftment or eventual autologous marrow recovery after cellular support provided by transient allografts [1,2].
Realistically, most potential radiation accident victims will not have HLA-identical siblings who could serve as HCT donors, and there will not be sufficient time to search for suitably HLA-matched unrelated donors. However, it is safe to assume that virtually everyone will have HLA-haploidentical family members among parents, siblings, or children. Therefore, the current study evaluated in a canine model after which total body irradiation (TBI) exposures DLA-haploidentical HCT might be feasible and potentially useful. In order to mimic the logistical problems likely to exist after radiation accidents, 4-, 8-, and 10-day intervals were placed between TBI and HCT. In order to have a clear-cut study end point, no attempts were made to prevent graft-vs-host disease (GVHD), which therefore served as one of the readouts for successful allogeneic hematopoietic engraftment.
Materials and Methods
Dogs
Litters of random-bred dogs were either raised at the Fred Hutchinson Cancer Research Center (FHCRC) or purchased from commercial Class A vendors licensed by the US Department of Agriculture. The dogs weighed from 7.0 to 13.8 (median, 10.8) kg and were between 10 and 27 (median, 16.3) months old. All dogs were enrolled in a veterinary preventive medicine program that included routine anthelmintics and a standard immunization series [3]. The study was approved by the Institutional Animal Care and Use Committee at the FHCRC, which has been fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. Littermate donor and recipient pairs were selected on the basis of complete family studies showing haplo-identity for highly polymorphic major histocompatibility complex (MHC)-associated class I and II microsatellite markers [4] and for DLA-DRB1 alleles determined by direct sequencing [5].
TBI and HCT
TBI was delivered as a single dose at a rate of 7 cGy/min from a 4MEV and, more recently, a 6MEV linear accelerator (CLINAC 4/80 and CLINAC 600 C/D, respectively, Varian Associates, Palo Alto, CA). In one arm of the studies, recipient dogs were given 920, 700, 600 or 450 cGy TBI 4 days before HCT (Table 1). In another arm, recipient dogs were given 920 cGy and 700 cGy TBI 8 days before HCT. In a third study arm, recipients were given 920 cGy TBI 10 days before HCT. HCT consisted of intravenous infusions of marrow containing 1.5 to 7.5 (median, 3.8) × 108 nucleated cells per kilogram recipient body weight and 1.1 to 7.1 (median, 2.4)×108 peripheral blood buffy coat cells per kilogram body weight. The day of HCT was designated day 0. For comparison, previously published data on dogs given either 450 or 920 cGy TBI immediately preceding hematopoietic cell grafts from DLA-haploidentical littermates were also summarized (Table 2). For ease of interpretation of results, recipients were not given postgrafting immunosuppression for prevention of GVHD. Supportive care included prophylaxis with the oral antibiotic enrofloxacin from the day of TBI until the end of the study. Broader antibiotic coverage was administered when neutrophil counts declined to below 0.3 × 106/ml or fever developed. Intravenous fluids were administered when an excess of 8% dehydration occurred. Irradiated blood transfusions were given either when platelet counts declined below 0.5 ×106/ml or when petechiae and ecchymoses of skin and mucous membranes were observed.
Table 1.
Hematopoietic Cell Grafts From DLA-haploidentical Littermates Administered 4, 8 or 10 Days After TBI
| GVHD |
Survival (days) after |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | TBI dose (cGy) |
HCT delay (days) |
Recipient no. |
Granulocyte recovery* |
Histological | Clinical | Day of Onset after HCT |
Sustained allograft† |
Autologous marrow recovery |
Marrow cellularity‡ at autopsy (%) |
Origin of hematopoietic cells |
TBI | HCT | Causes of Death§ |
| 1 | 920 | 4 | G674 | Yes | S,G,L | S,G,L | 10 | Yes | No | 15 | Donor | 16 | 12 | ET1, GVHD |
| G658 | Yes | S,G,L | S,G,L | 8 | Yes | No | 20 | Donor | 14 | 10 | ET1, GVHD | |||
| G615 | Yes | S,G,L | SL | 11 | Yes | No | 25 | Donor | 18 | 14 | ET1, GVHD | |||
| G684 | Yes | S,G,L | S,L | 8 | Yes | No | 65 | Donor | 15 | 11 | ET1, GVHD | |||
| G520 | Yes | S,G,L | S,G,L | 10 | Yes | No | 25 | Donor | 15 | 11 | ET1, GVHD | |||
| 2 | 700 | 4 | G535 | Yes | S,G,L | S,L | 10 | Yes | No | 35 | Donor | 15 | 11 | ET1, GVHD |
| G679 | Yes | S,G,L | S,L | 15 | Yes | No | 52 | Donor | 22 | 18 | ET1, GVHD | |||
| G816 | Yes | S,G,L | S,L | 8 | Yes | No | 65 | Donor | 15 | 11 | ET1, GVHD | |||
| G687 | Yes | S,G,L | S,L | 9 | Yes | No | 50 | Donor | 15 | 11 | ET1, GVHD | |||
| G818 | Yes | S,G,L | S,L | 8 | Yes | No | 25 | Donor | 15 | 11 | ET1, GVHD | |||
| 3 | 600 | 4 | G793 | Yes | No | No | - | No | Yes | 100 | Host | 83 | 79 | ET2 |
| G817 | No | No | No | - | No | No | 2 | Host/ Donor | 44 | 40 | ET1, Aplasia | |||
| G805 | Yes | No | No | - | No | Yes | 45 | Host | 27 | 23 | ET2 | |||
| G801 | Yes | S,G,L | S,L | 9 | Yes | No | 35 | Donor | 14 | 10 | ET1, GVHD | |||
| G304 | Yes | No | No | - | No | Yes | 7.5 | Host | 48 | 44 | ET2 | |||
| 4 | 450 | 4 | G683 | Yes | No | No | - | No | Yes | NE | Host | 39 | 35 | ET2 |
| G650 | Yes | No | No | - | No | Yes | 75 | Host | 53 | 49 | ET1, Anemia | |||
| G681 | Yes | No | No | - | No | Yes | 75 | Host | 54 | 50 | ET2 | |||
| G578 | No | No | No | - | No | No | 0 | Host | 30 | 26 | Septicemia | |||
| 5 | 920 | 8 | G466 | Yes | S,G,L | S,G,L | 8 | Yes | No | 50 | Donor | 17 | 9 | ET1, GVHD |
| G762 | Yes | S,G,L | S,G,L | 8 | Yes | No | 50 | Donor | 17 | 9 | ET1, GVHD | |||
| G757 | Yes | S,G,L | S,G,L | 9 | Yes | No | 25 | Donor | 18 | 10 | ET1, GVHD | |||
| G976 | No | S | S,L | 7 | Yes | No | <5 | Donor | 15 | 7 | Septicemia, ET1, GVHD | |||
| 6 | 700 | 8 | H049 | No | S,G,L | S,G,L | 11 | No | No | 15 | Host | 19 | 11 | ET1, Pancytopenia |
| H064 | No | S,G,L | S,L | 6 | Yes | No | 15 | Donor | 16 | 8 | GVHD | |||
| H123 | Yes | No | No | - | No | Yes | 25 | Host | 43 | 35 | ET2 | |||
| H092 | No | No | No | - | No | No | 15 | Host | 34 | 26 | ET2 | |||
| H065 | No | No | No | - | No | No | 5 | Host | 21 | 13 | ET1, Septicemia | |||
| 7 | 920 | 10 | G991 | No | No | No | - | No | No | <5 | Host | 25 | 15 | ET1 |
| H013 | No | S,G,L | S,G,L | 7 | Yes | No | 15 | Donor | 18 | 8 | ET1, Septicemia | |||
| H031 | No | S,G,L | S,G,L | 7 | Yes | No | 15 | Donor | 18 | 8 | ET1, GVHD | |||
| G601 | Yes | S,G,L | S,G,L | 9 | Yes | No | 50 | Donor | 18 | 10 | ET1,GVHD | |||
| H112 | No | S,G,L | S,G,L | 8 | No | No | <5 | Host | 20 | 10 | ET1, Septicemia | |||
Increases of peripheral granulocyte counts to ≥1 × 106/ml after postirradiation nadirs
Sustained engraftment was demonstrated by persistence of donor cells in peripheral blood and donor-type hematopoiesis in bone marrow when assayed for variable number tandem repeat (VNTR) polymorphisms
Cell fat ratio.
Dogs were euthanized at the end of study because of poor clinical condition (ET1, GVHD), or when autologous recovery of host hematopoiesis had occurred (ET2).
Abbreviations: ET1, euthanized, poor condition; ET2, euthanized, end of study; GI, gastrointestinal tract; GPBMC, GCSF mobilized PBMC; L, liver; MBC, marrow, buffy coat; NE, not evaluated; S, skin; SCF, stem cell factor; S,G,L, skin, gut, liver.
Table 2.
Hematopoietic Cell Grafts from DLA-haploidentical Littermates Administered within Hours (Day 0) of TBI: Historical Data
| Group | TBI Dose (cGy) |
Source of Cells | No of Dogs |
Survival (days) |
Causes of Death | Reference | |||
|---|---|---|---|---|---|---|---|---|---|
| Studied | Sustained Grafts |
Acute GVHD |
Range | Median | |||||
| 8 | 920 | Marrow/BC | 6 | 6 | 6 | 8–13 | 10.5 | ET1; GVHD | [10] |
| 9 | 920 | GPBMC ± SCF | 9 | 9 | 9 | 7–12 | 7 | ET1; GVHD | [8] |
| 10 | 450 | GPBMC | 6 | 1 | 1 | 13–80 | 21 | ET1; GVHD(n=1) ET1 (n=2) ET2 (n=2) Pneumonia (n=1) |
[9] |
Abbreviations: BC, buffy coat; ET1, euthanized, poor condition; ET2, euthanized, end of study; GPBMC, GCSF mobilized PBMC; SCF, stem cell factor.
Engraftment/Chimerism
Hematopoietic engraftment was assessed by increases in granulocyte and platelet counts following post-irradiation nadirs, marrow histology from autopsy specimens, documentation of donor-type hematopoiesis in peripheral blood and marrow by variable number tandem repeat (VNTR) polymorphisms [6,7], and clinical and histologic evidence of GVHD.
Statistical Analysis
Logistic regression was used to develop a predictive model for the probability of engraftment as a function of the TBI dose and the delay between TBI and HCT.
Results
Table 1 summarizes the data for seven current groups of dogs, and Table 2 shows results in three historical groups of dogs. Dogs in groups 1–4 were given TBI 4 days before HCT. All five dogs given 920 cGy (Group 1) and all five given 700 cGy (Group 2) TBI showed prompt allogeneic engraftment. Sustained increases in granulocyte counts were seen after the TBI nadirs, which occurred between 4 and 5 days after HCT, respectively, with counts rising to an average of 1 × 106/ml 8 days after HCT. All ten dogs developed clinical and histological evidence of acute GVHD and were euthanized because of GVHD on medians of 15 days after TBI, and 11 days after HCT, respectively. Complete donor-type hematopoiesis was documented by VNTR assays of peripheral blood and bone marrow in all 10 dogs. In contrast, three of five dogs given 600 cGy (Group 3) rejected their allografts and survived with autologous marrow recovery. One dog (G817) remained pancytopenic, showed predominantly host but also some donor hematopoietic cells, and was euthanized on day 40 after HCT because of infection. One dog (G801) had sustained engraftment of donor cells and was euthanized 10 days after HCT because of severe GVHD. All four dogs given 450 cGy (Group 4), rejected their allografts and three of four showed autologous recovery. Two of the three were euthanized at the end of study and one was euthanized because of septicemia. The fourth dog (G650) rejected the allograft and was eventually euthanized on day 40 because of persisting low blood counts.
All four dogs given HCT 8 days after 920 TBI (Group 5) also had sustained engraftment of donor cells and developed both histological and clinical signs of GVHD. Granulocyte recoveries were seen in three of four dogs on average 7 days after HCT (15 days after TBI; Figure 1). All four were euthanized because of GVHD. In contrast, only 1 of 5 dogs given HCT 8 days after 700 cGy TBI (group 6) had sustained engraftment and GVHD, while four rejected; two of the latter four were euthanized after complete autologous recovery, one (H065) was euthanized because of sepsis, and one (h049) was euthanized because of infection from pancytopenia and with clinical evidence of GVHD, even though residual hematopoiesis was entirely of host origin. Three of five dogs given 920 TBI and HCT after a delay of 10 days (Group 7) had sustained engraftment of donor cells, developed GVHD and were euthanized. Their neutrophil changes are shown in Figure 1. Two dogs rejected the allografts, remained pancytopenic, and were euthanized because of septicemia/poor clinical condition.
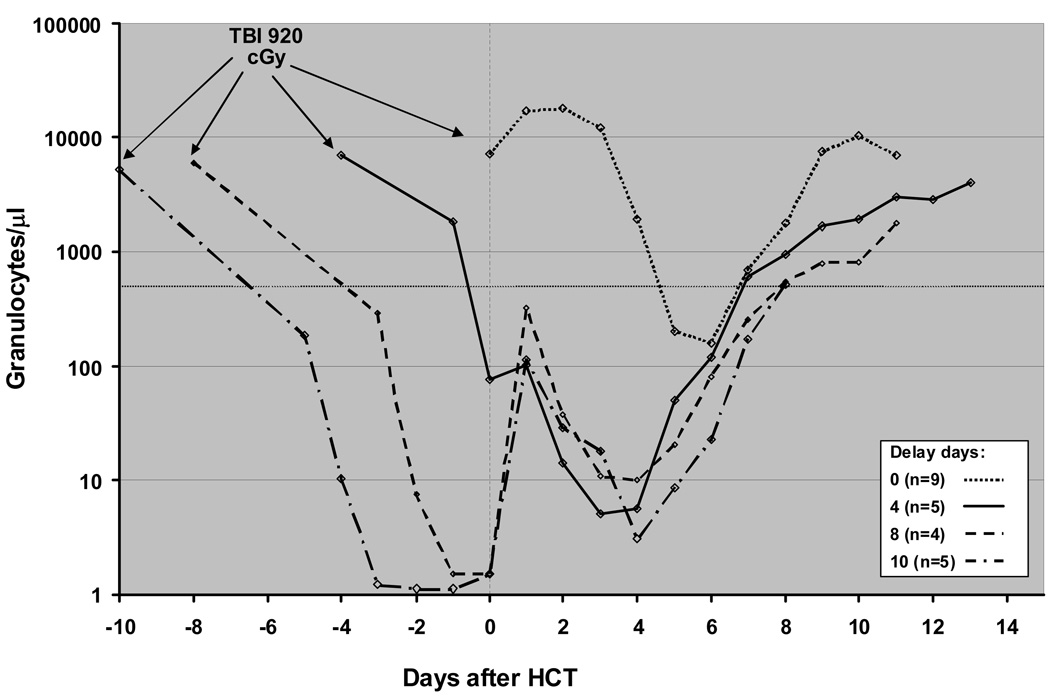
Figure 1.
Granulocyte changes in dogs given 920 cGy TBI and transplanted with marrow and buffy coat cells from DLA-haploidentical littermates after 0-, 4-, 8-, and 10-day delays. The median granulocyte counts for each group were determined and normalized to Day 0, the day of marrow and buffy coat infusions.
For comparison, Table 2 summarizes previous data in dogs given either 450 or 920 cGy TBI immediately before infusion of marrow/buffy coat cells or canine stem cell factor (SCF) and/or canine G-CSF-mobilized peripheral blood stem cells from DLA-haploidentical littermates [8–10]. Historically, G-CSF ± SCF mobilized blood cells have shown the same engraftment potential as the combination of marrow and buffy coat cells used in the present study. All 15 dogs treated with 920 cGy and immediate HCT (groups 8 and 9) had sustained donor engraftment and were euthanized because of GVHD; their survivals were comparable to those of dogs given HCT after 4 to 10-day delays. Only one of six dogs given 450 cGy TBI on the day of HCT (Group 9) had sustained engraftment and was euthanized on day 13 because of GVHD while five rejected their grafts and showed autologous marrow recovery. Four of the latter were euthanized at the end of study while one died of pneumonia on day 19. This outcome was comparable to that in dogs given 600 cGy TBI 4 days before HCT.
Peripheral blood granulocyte changes among dogs given 920 cGy TBI 4, 8 and 10 days before HCT (Groups 1, 5 and 7) were normalized with respect to day of HCT (Figure 1). The times to nadir and recovery of granulocytes were similar for dogs in the 3 groups. The immediate but transient neutrophil rises seen after delayed HCT were likely the result of transient production from committed granulocyte precursors contained in the marrow grafts. For comparison, Figure 1 also shows granulocyte changes in 9 dogs given HCT on day 0; note that, while the nadir is obviously delayed and higher, the recovery curve matches those in the other 3 groups of dogs. Full recovery of lymphocyte counts was precluded by the onset of GVHD and the need for euthanizing the dogs with the possible exception of the 8-day delay group in which the median lymphocyte counts increased to 800 cells/µl from less than 100 cells/µl 10 days after HCT (data not shown).
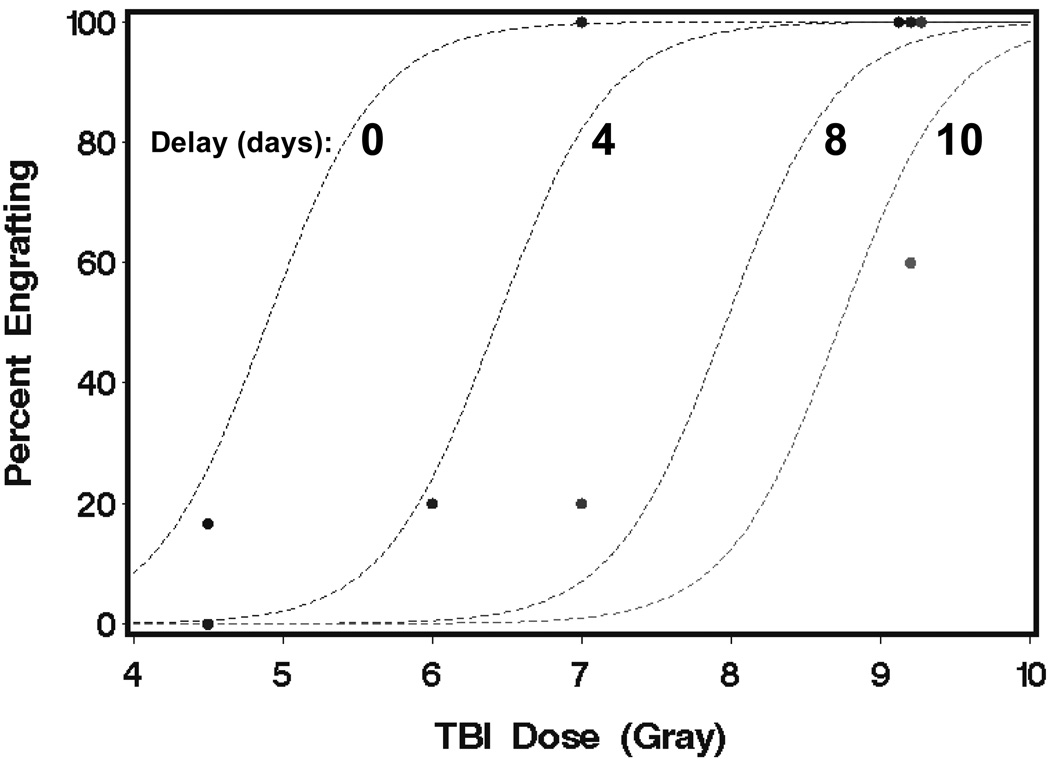
A logistic regression model was fit to the data from all of the groups except group 8 in order to develop a predictive model for the probability of engraftment as a function of TBI dose and the delay between TBI and HCT. The estimated probability of engraftment (p) from this model was expressed as p = exp(X) / (1 + exp(X)), where X = −13.1 + 2.68 × TBI dose (Gray) – 1.03 × Delay (days). Model predictions and observed data are shown in Figure 2. Consistent with radiobiological principles, the TBI doses required for uniform engraftment increased in direct relation to the increases in time intervals between TBI and HCT.
Figure 2.
Estimated probability of engraftment as a function TBI dose for delays of 0, 4, 8, and 10 days. The lines were the estimated percentages derived from the logistic regression model; points were the observed percentages from groups 1–7 and 9 in Table 1.
Discussion
In order to create a preclinical setting that mimicked the logistical problems likely to exist after radiation accidents, we focused on HCT donors among the immediate family which shared at least one MHC-haplotype with the accident victims, in this case, surrogates in the form of DLA-haploidentical littermates. In the case of human patients, MHC-haploidentical donors would include both parents, all children, and half of the siblings. MHC-haplodisparity raised two major issues: engraftment without rejection and, once the engraftment barrier had been crossed, GVHD.
With respect to engraftment, TBI is not only myeloablative but also immunosuppressive. Thus, at high TBI doses, host resistance to MHC-haploidentical hematopoietic grafts is weakened to a point where the immunological balance is shifted in favor of the grafts. Typically, TBI delivered as a single dose of 920 cGy was sufficient for uniform, sustained engraftment in dogs given MHC-haploidentical hematopoietic cell grafts within 24 hours of TBI when the immunosuppressive effect of radiation was at its peak [11,12]. Early studies on the effects of TBI on immune responses had shown regeneration of immunity as time elapsed after irradiation exposure [13–17]. When designing the current studies, we reasoned that, at the very earliest, an MHC-haploidentical donor might be identified and ready to deliver a hematopoietic cell graft 4 days after accidental radiation exposure of the recipient, although 4 days might be unrealistically short for mobilizing appropriate emergency care considering the recent experience after hurricane Katrina. Therefore, we also explored HCT 8 and 10 days after TBI. Encouragingly, the study showed virtually uniform engraftment of DLA-haploidentical hematopoietic cells transplanted 4 and 8 days after 920 cGy, the highest TBI dose studied, while only 3 of 5 dogs transplanted after a 10-day delay engrafted, suggesting the time limits for successful engraftment had been approached. The rates of neutrophil recovery in the three groups of dogs were not distinguishable from each other, or from those of "historical" control dogs given HCT on the day of TBI, when "normalized" for day of transplantation, suggesting normal function even when grafts were infused 10 days after TBI. Lowering the TBI dose to 700 cGy allowed for uniformly sustained grafts after a 4-day delay; however, all but one of the dogs rejected their grafts when infused after an 8-day delay. This finding was consistent with the notion of immunological recovery of host cells over time, as shown by the early radiobiological studies [13–17]. As we lowered the TBI dose to 600 cGy and 450 cGy, respectively, virtually all DLA-haploidentical grafts infused after 4 days were rejected, indicating inadequate host immunosuppression at these doses.
The 4-day minimum interval between radiation exposure and HCT precluded use of G-CSF-mobilized PBMC for which at least 5 days were required to transfer sufficient numbers of CD34+ progenitor/stem cells from marrow into blood [8]. Conversely, using marrow alone led to very high incidences (60–90%) of graft rejection in MHC-mismatched dogs, even when infused immediately after 920 cGy TBI [12,18]. This problem has been attributed to destruction of marrow grafts by relatively radio-resistant host natural killer cells [19]. Given these restrictions, we added donor buffy coat cells to the marrow inoculum which, as shown already years ago [11,12], helped overcome the host natural killer cell barrier, presumably through a shift in the immunological balance toward the donor by high numbers of infused lymphocytes [12,20].
A number of studies have evaluated the effects of varying time intervals between TBI and infusion of hematopoietic grafts. Virtually all studies were done in inbred rodents. One of the first, published in 1961 [21], found that 30-day survivals after syngeneic grafts declined from 92% to 50% as the time interval was increased from 1 to 8 days. Vos et al. [22], grafting rat marrow into mice, also reported declining 30-day survivals from 85% to 25% when increasing the time interval from 1 to 4 days. Fiala et al. saw best results when murine grafts were carried out 1 day after TBI and worse outcomes after 4 days [23]. In contrast, more recent studies of marrow transplantation in inbred H2-mismatched mice suggested that a 4 day delay compared to no delay improved engraftment at doses of 500 and 600 cGy but not at 700 cGy TBI [24]. The authors explained this counter-intuitive finding by a significant increase in the inflammatory cytokine IL-6 directly after TBI which, somehow, impaired engraftment early after TBI and which declined over time.
Another study in H2-incompatible mice showed both a dramatic reduction in GVHD and improvement in survival from 0% to 60% when the time interval from TBI to transplantation was increased from 0 to 4 days [25]. The uniformly poor outcome of transplantation on day 0 was attributed to cytokine release ("cytokine storm") induced by TBI [26–28] which "caused or aggravated host tissue damage and modulated MHC antigen expression," thereby leading to lethal GVHD. The authors postulated that by delaying the transplant by 4 days, the cytokine reaction cycle would be interrupted, thereby reducing tissue damage and GVHD incidence and mortality.
In contrast to the observations in mice, current and past results in randombred dogs failed to show attenuation of GVHD with increasing time interval between TBI and HCT. Regardless of whether grafts were infused on days 0, 4, 8 or even 10 after 920 cGy TBI, all dogs promptly developed GVHD which was fatal within 8–14 days of HCT. Extending the reasoning used to explain the murine study results [24,25], we would expect a TBI-induced "cytokine storm" to have subsided by days 4, 8, and 10 when current grafts were infused. Yet, acute GVHD occurred as fast and was as severe in dogs transplanted 4–10 days after TBI as in those given their hematopoietic grafts within hours of TBI. Clearly, in this large, randombred animal model, the degrees of histoincompatibility between donors and recipients were the major determinants for the tempo of immunological reactions leading to GVHD, regardless of the timing of transplantation after TBI.
How to explain the profound effect of the "cytokine storm" on outcomes of murine HCT and the apparent lack of effect in the canine model? Is it possible that inbred mice, kept in relatively pathogen-free barrier facilities, were especially responsive to radiation insults in generating cytokines, while dogs (and also human patients) have been continuously exposed to bacterial, fungal and viral antigens with the result that those exposures overshadowed the effect of the additional TBI exposure?
As already indicated, the current study did not attempt to prevent GVHD. Earlier studies had shown that antimetabolites, such as methotrexate or mycophenolate mofetil, given either alone or combined with calcineurin inhibitors could ameliorate or even prevent GVHD [29–32]. Yet other studies in dogs conditioned with 920 cGy TBI showed facilitation of engraftment of DLA-nonidentical marrow with a short course of postgrafting methotrexate [33], presumably by hindering the proliferation of radio-resistant host natural killer cells [19]. Similarly, combining mycophenolate mofetil with a calcineurin inhibitor facilitated engraftment of DLA-identical marrow after suboptimal TBI doses [34]. Conceivably, therefore, the use of postgrafting immunosuppression in the current model might not only extend the time threshold for uniform engraftment, say, after 920 cGy TBI beyond 8 days, but, moreover, allay GVHD and assure long-term survival.
Current study results have to be seen in the context of what can be achieved with state-of-the-art supportive care without HCT in this canine model. We reported previously uniform endogenous recovery and survival in dogs given 200 cGy TBI without marrow “rescue,” while most dogs given 400 cGy TBI died as a consequence of pancytopenia [35,36]. Most recently, perhaps owing to more advanced antibiotics and more intensive fluid and electrolyte support, most dogs exposed to up to 700 cGy TBI survived, while those given 800 cGy TBI eventually were euthanized because of complications from pancytopenia (G. Georges et al., unpublished observations). Taken together, results of the current study and those in dogs not given hematopoietic grafts suggested that optimal supportive care could save individuals exposed up to a maximum of 700 cGy TBI, while DLA-haploidentical HCT were successful at doses of 700 cGy or higher. It must be remembered, however, that these data were generated using a relatively low dose rate of 7 cGy/min. We know from earlier studies in dogs that higher dose rates generated different toxicity profiles on the hematopoietic system, the gut, and slow-responding tissues [36,37]. Dose rates experienced in radiation accidents might vary widely, and higher dose rates should be evaluated in the current preclinical model.
In conclusion, most DLA-haploidentical HCT was successful even after 4-, 8- and 10-day intervals from TBI doses at and above levels where dogs could be saved by intensive supportive care.
Acknowledgments
We thank Eustacia Zellmer, Patrice Stroup, Carol Loretz, Tiffany Miwongtum, and Diane Stone for their technical help in conducting the studies; Michele Spector, DVM, Alix Joslyn, and technicians of the animal health resources for their excellent care of and dedication to the dogs; Michael A. Harkey, PhD, Ludmilla Golubev, and the FHCRC Clonal Analysis Core for the VNTR analysis; Drs Beard, Burroughs, Diaconescu, Gerull, Jochum, Kerbauy, Mielcarek, Nash, Parker, Thakar, and Wang who participated in the weekend treatments; and Bonnie Larson, Helen Crawford and Sue Carbonneau for help with manuscript preparation.
This work was supported in part by NIH grants AI067770, CA015704, HL036444, and DK056465, National Institutes of Health, Bethesda, MD, USA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Storb R, Raff RF, Appelbaum FR, et al. Comparison of fractionated to single-dose total body irradiation in conditioning canine littermates for DLA-identical marrow grafts. Blood. 1989;74:1139–1143. [PubMed] [Google Scholar]
- 2.Storb R, Raff RF, Appelbaum FR, et al. What radiation dose for DLA-identical canine marrow grafts? Blood. 1988;72:1300–1304. [PubMed] [Google Scholar]
- 3.Burroughs L, Mielcarek M, Little M-T, et al. Durable engraftment of AMD3100-mobilized autologous and allogeneic peripheral blood mononuclear cells in a canine transplantation model. Blood. 2005;106:4002–4008. doi: 10.1182/blood-2005-05-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagner JL, Burnett RC, DeRose SA, Francisco LV, Storb R, Ostrander EA. Histocompatibility testing of dog families with highly polymorphic microsatellite markers. Transplantation. 1996;62:876–877. doi: 10.1097/00007890-199609270-00032. [DOI] [PubMed] [Google Scholar]
- 5.Wagner JL, Works JD, Storb R. DLA-DRB1 and DLA-DQB1 histocompatibility typing by PCR-SSCP and sequencing (Brief Communication) Tissue Antigens. 1998;52:397–401. doi: 10.1111/j.1399-0039.1998.tb03063.x. [DOI] [PubMed] [Google Scholar]
- 6.Yu C, Ostrander E, Bryant E, Burnett R, Storb R. Use of (CA)n polymorphisms to determine the origin of blood cells after allogeneic canine marrow grafting. Transplantation. 1994;58:701–706. [PubMed] [Google Scholar]
- 7.Hilgendorf I, Weirich V, Zeng L, et al. Canine haematopoietic chimerism analyses by semiquantitative fluorescence detection of variable number of tandem repeat polymorphism. Veterinary Research Communications. 2005;29:103–110. doi: 10.1023/b:verc.0000047486.01458.c5. [DOI] [PubMed] [Google Scholar]
- 8.Sandmaier BM, Storb R, Santos EB, et al. Allogeneic transplants of canine peripheral blood stem cells mobilized by recombinant canine hematopoietic growth factors. Blood. 1996;87:3508–3513. [PubMed] [Google Scholar]
- 9.Sandmaier BM, Fukuda T, Gooley T, Yu C, Santos EB, Storb R. Dog leukocyte antigenhaploidentical stem cell allografts after anti-CD44 therapy and reduced-intensity conditioning in a preclinical canine model. Exp Hematol. 2003;31:168–175. doi: 10.1016/s0301-472x(02)01022-6. [DOI] [PubMed] [Google Scholar]
- 10.Raff RF, Storb R, Graham T, et al. Succinyl acetone plus methotrexate as GVHD prophylaxis in DLA-haploidentical canine littermate marrow grafts (Letter) Transplantation. 1992;54:947–948. doi: 10.1097/00007890-199211000-00040. [DOI] [PubMed] [Google Scholar]
- 11.Storb R, Epstein RB, Bryant J, Ragde H, Thomas ED. Marrow grafts by combined marrow and leukocyte infusions in unrelated dogs selected by histocompatibility typing. Transplantation. 1968;6:587–593. doi: 10.1097/00007890-196807000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Weiden PL, Storb R, Graham TC, Sale GE, Thomas ED. Resistance to DLA-nonidentical marrow grafts in lethally irradiated dogs. Transplant Proc. 1977;9:285–288. [PubMed] [Google Scholar]
- 13.Makinodan T, Friedberg BH, Tolbert MG, Gengozian N. Relation of secondary antigen injection to time of irradiation on antibody production in mice. J Immunol. 1959;83:184–188. [PubMed] [Google Scholar]
- 14.Taliaferro WH. Radiation and Immune Mechanisms. New York, NY: Academic Press; 1964. U.S. Atomic Energy Commission. [Google Scholar]
- 15.Makinodan T. Encyclopedia of Medical Radiology, Part 2 Radiation Biology. Berlin: Springer-Verlag; 1966. Changes in immunobiological processes caused by radiation; pp. 303–333. [Google Scholar]
- 16.Makinodan T, Gengozian N. Effect of radiation on antibody formation. In: Hollaender A, editor. Radiation Protection and Recovery. New York, NY: Pergamon, Press; 1960. pp. 316–351. [Google Scholar]
- 17.Simic MM. Antibody Formation in Irradiated Rats. Beograd: Boris Kidric Institute of Nuclear Sciences; 1965. [Google Scholar]
- 18.Storb R, Deeg HJ. Failure of allogeneic canine marrow grafts after total body irradiation: Allogeneic “resistance” vs transfusion induced sensitization. Transplantation. 1986;42:571–580. doi: 10.1097/00007890-198612000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Raff RF, Deeg HJ, Loughran TP, Jr, et al. Characterization of host cells involved in resistance to marrow grafts in dogs transplanted from unrelated DLA-nonidentical donors. Blood. 1986;68:861–868. [PubMed] [Google Scholar]
- 20.Deeg HJ, Storb R, Weiden PL, et al. Abrogation of resistance to and enhancement of DLA-nonidentical unrelated marrow grafts in lethally irradiated dogs by thoracic duct lymphocytes. Blood. 1979;53:552–557. [PubMed] [Google Scholar]
- 21.Unsgaard B. Optimal time for marrow injection in mice after total body irradiation. Acta Radiologica. 1961;56:296–304. doi: 10.3109/00016926109172824. [DOI] [PubMed] [Google Scholar]
- 22.Vos O, Crouch BG, van Bekkum D. The interval between irradiation and bone-marrow transplantation. Int J Radiat Biol. 1961;3:337–349. doi: 10.1080/09553006114550401. [DOI] [PubMed] [Google Scholar]
- 23.Fiala J, Viktora L, Urbancova J. A suitable time interval between lethal irradiation of mice and transplantation of bone marrow [German] Blut. 1972;24:166–173. doi: 10.1007/BF01631464. [DOI] [PubMed] [Google Scholar]
- 24.Xu H, Exner BG, Chilton PM, et al. A delay in bone marrow transplantation after partial conditioning improves engraftment. Transplantation. 2004;77:819–826. doi: 10.1097/01.tp.0000116414.66171.81. [DOI] [PubMed] [Google Scholar]
- 25.Xun CQ, Tsuchida M, Thompson JS. Delaying transplantation after total body irradiation is a simple and effective way to reduce acute graft-versus-host disease mortality after major H2 incompatible transplantation. Transplantation. 1997;64:297–302. doi: 10.1097/00007890-199707270-00021. [DOI] [PubMed] [Google Scholar]
- 26.Antin JH, Ferrara JLM. Cytokine dysregulation and acute graft-versus-host disease. Blood. 1992;80:2964–2968. [PubMed] [Google Scholar]
- 27.Holler E, Kolb HJ, Mittermuller J, et al. Modulation of acute graft-versus-host-disease after allogeneic bone marrow transplantation by tumor necrosis factor alpha (TNF alpha) release in the course of pretransplant conditioning: role of conditioning regimens and prophylactic application of a monoclonal antibody neutralizing human TNF alpha (MAK 195F) Blood. 1995;86:890–899. [PubMed] [Google Scholar]
- 28.Schwaighofer H, Kernan NA, O'Reilly RJ, et al. Serum levels of cytokines and secondary messages after T-cell-depleted and non-T-cell-depleted bone marrow transplantation: influence of conditioning and hematopoietic reconstitution. Transplantation. 1996;62:947–953. doi: 10.1097/00007890-199610150-00013. [DOI] [PubMed] [Google Scholar]
- 29.Storb R, Epstein RB, Graham TC, Thomas ED. Methotrexate regimens for control of graft-versus-host disease in dogs with allogeneic marrow grafts. Transplantation. 1970;9:240–246. doi: 10.1097/00007890-197003000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Deeg HJ, Storb R, Weiden PL, et al. Cyclosporin A and methotrexate in canine marrow transplantation: engraftment, graft-versus-host disease, and induction of tolerance. Transplantation. 1982;34:30–35. doi: 10.1097/00007890-198207000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Storb R, Raff RF, Appelbaum FR, et al. FK506 and methotrexate prevent graft-versus-host disease in dogs given 9.2 Gy total body irradiation and marrow grafts from unrelated DLA-nonidentical donors. Transplantation. 1993;56:800–807. doi: 10.1097/00007890-199310000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Yu C, Seidel K, Nash RA, et al. Synergism between mycophenolate mofetil and cyclosporine in preventing graft-versus-host disease among lethally irradiated dogs given DLA-nonidentical unrelated marrow grafts. Blood. 1998;91:2581–2587. [PubMed] [Google Scholar]
- 33.Deeg HJ, Sale GE, Storb R, et al. Engraftment of DLA-nonidentical bone marrow facilitated by recipient treatment with anti-class II monoclonal antibody and methotrexate. Transplantation. 1987;44:340–345. doi: 10.1097/00007890-198709000-00003. [DOI] [PubMed] [Google Scholar]
- 34.Storb R, Yu C, Wagner JL, et al. Stable mixed hematopoietic chimerism in DLA-identical littermate dogs given sublethal total body irradiation before and pharmacological immunosuppression after marrow transplantation. Blood. 1997;89:3048–3054. [PubMed] [Google Scholar]
- 35.Storb R, Raff RF, Graham T, et al. Marrow toxicity of fractionated versus single dose total body irradiation is identical in a canine model. Int J Radiat Oncol Biol Phys. 1993;26:275–283. doi: 10.1016/0360-3016(93)90207-c. [DOI] [PubMed] [Google Scholar]
- 36.Storb R, Raff RF, Graham T, et al. Dose rate-dependent marrow toxicity of TBI in dogs and marrow sparing effect at high dose rate by dose fractionation. Biol Blood Marrow Transplant. 1999;5:155–161. doi: 10.1053/bbmt.1999.v5.pm10392961. [DOI] [PubMed] [Google Scholar]
- 37.Storb R, Raff R, Deeg HJ, et al. Dose rate-dependent sparing of the gastrointestinal tract by fractionated total body irradiation in dogs given marrow autografts. Int J Radiat Oncol Biol Phys. 1998;40:961–966. doi: 10.1016/s0360-3016(97)00913-9. [DOI] [PubMed] [Google Scholar]