Abstract
Lysophosphatidic acid (LPA), a component of mildly-oxidized LDL and the lipid rich core of atherosclerotic plaques, elicits platelet activation. LPA is the ligand of G protein-coupled receptors (GPCR) of the EDG family (LPA1–3) and the newly identified LPA 4–7 subcluster. LPA4, LPA5 and LPA7 increase cellular cAMP levels that would induce platelet inhibition rather than activation. In the present study we quantified the mRNA levels of the LPA1–7 GPCR in human platelets and found a rank order LPA4=LPA5>LPA7>LPA6=LPA2>>LPA1>LPA3. We examined platelet shape change using a panel of LPA receptor subtype-selective agonists and antagonists and compared them with their pharmacological profiles obtained in heterologous LPA1–5 receptor expression systems. Responses to different natural acyl and alkyl species of LPA, and octyl phosphatidic acid analogs, alpha-substituted phosphonate analogs, N-palmitoyl-tyrosine phosphoric acid, N-palmitoyl-serine phosphoric acid were tested. All of these compounds elicited platelet activation and also inhibited LPA-induced platelet shape change after pre-incubation, suggesting that receptor desensitization is likely responsible for the inhibition of this response. Fatty acid free albumin (10 µM) lacking platelet activity completely inhibited platelet shape change induced by LPA with an IC50 of 1.1 µM but had no effect on the activation of LPA1,2,3,&5 expressed in endogenously non-LPA-responsive RH7777 cells. However, albumin reduced LPA4 activation and shifted the dose-response curve to the right. LPA5 transiently expressed in RH7777 cells showed preference to alkyl-LPA over acyl-LPA that is similar to that in platelets. LPA did not increase cAMP levels in platelets. In conclusion, our results with the pharmacological compounds and albumin demonstrate that LPA does not induce platelet shape change simply through activation of LPA1–5, and the receptor(s) mediating LPA-induced platelet activation remains elusive.
Keywords: lysophosphatidic acid, G protein-coupled receptor, agonist, antagonist, albumin
Introduction
Oxidative modifications of LDL and platelet activation are the central events in the pathogenesis of atherosclerosis and cardiovascular disease [1,2]. Oxidatively modified LDL, present in the circulation or exposed after rupture of atherosclerotic plaques to blood cells, is known to stimulate platelets [3,4]. Platelets, once activated, change their shape, aggregate, and become procoagulant, leading to the formation of a platelet- and fibrin-rich intravascular thrombus, which can precipitate acute ischemic syndromes such as unstable angina, myocardial infarction, and stroke [3,5]. Lysophosphatidic acid (LPA) has been identified as a biologically active lipid in mildly-oxidized LDL (mox-LDL), human atherosclerotic plaques, and supernatant of activated platelets [2,6,7,8,9]. It is also known that serum LPA level is significantly elevated in patients with acute myocardial infarction [10]. Importantly, oxidatively modified LDL such as mox-LDL and the lipid-rich core of human atherosclerotic plaques have been shown to stimulate platelets through the activation of LPA receptors [2,6].
LPA is predominantly associated with serum albumin [11] and acts through various sets of specific G protein-coupled receptors (GPCRs) in an autocrine and paracrine fashion [8,12,13]. The LPA receptor gene products are expressed in most mammalian tissues with spatially and temporally regulated expression patterns. LPA receptors can be divided into two subfamilies. One is composed of three members, LPA1, LPA2, LPA3, belonging to the Endothelial Differentiation Gene (EDG-) subfamily of GPCRs [14]. The second subfamily consists of recently identified LPA receptors: LPA4 (GPR23), LPA5 (GPR92), LPA6 (GPR87), and LPA7 (P2Y5) [15,16,17,18] which are structurally more related to the purinoreceptor (P2Y) cluster of GPCRs.
Platelets express mRNA for the LPA1–5 receptors [19,20,21]; the expression of LPA receptors at the protein level is unknown due to the lack of specific antibodies. Although LPA-induced platelet activation is inhibited by the LPA1 and LPA3 receptor antagonist dioctylglycerol pyrophosphate (DGPP) (8:0), as well as either N-palmitoyl-serine phosphoric acid (NPSPA) or N-palmitoyl-tyrosine phosphoric acid (NPTPA), both of which activate several LPA receptors [7,22], the LPA responses in platelets (alkyl-LPA being potent than acyl-LPA) are not consistent with the pharmacological properties of LPA1 and LPA3 receptors [6,23,24]. Therefore, the receptor(s) through which LPA stimulates platelets remain unidentified. Here we applied a comprehensive set of recently identified LPA receptor agonists and antagonists to evaluate their effects in cells individually expressing LPA1–5 and compared those with platelet responses to LPA. We have also evaluated the effect of serum albumin that inhibits LPA-induced platelet activation on LPA-GPCR individually expressed in endogenously non-responsive RH7777 cells. The present findings underline the complexity of LPA responsiveness in human platelets revealing a mismatch with the individual pharmacological properties of LPA1/2/3/4/5.
Materials and methods
Materials
The LPA receptor agonists and antagonists were purchased either from Avanti Polar Lipids (Alabaster, AL) or synthesized as previously described [25,26]. ADP, apyrase (A-6535), fatty acid free bovine serum albumin (FAF-BSA), acetylsalicylic acid were obtained from Fluka (Taufkirchen, Germany). The enzyme immunoassay kit for cyclic adenosine monophosphate (cAMP) measurement was from Assay Designs, distributed by Biotrend (Cologne, Germany). DNase I, ThermoScript RT-PCR System for First-Strand cDNA Synthesis were purchased from Invitrogen (Carlsbad, CA). RT2 Real-Time SYBR Green/ROX kit was from SuperArray (Frederick, MD).
Agonist and Antagonist preparation
LPA species were either dissolved and diluted in ethanol, or else after ethanol evaporation reconstituted in FAF-BSA buffer (20 mM HEPES, 138 mM NaCl, FAF-BSA 0.25 mM, at a LPA/BSA ratio of 4:1). The pharmacological compounds were dissolved and used in methanol or in FAF-BSA buffer (compound/BSA ratio of 4:1), as described [26].
Preparation of Washed Human Platelets for Activation Studies
Blood was obtained by venipuncture from healthy male and female donors using 3.13% natrium citrate as an anticoagulant. Venous blood was drawn and collected into plastic tubes containing natrium citrate in a 1:10 anticoagulant to blood ratio. All studies involving human subjects were conducted in accordance with the Declaration of Helsinki following the protocols approved by the ethical committee of LMU Munich. The whole blood was centrifuge at 180g for 20 min at 24°C. The supernatant (platelet-rich plasma) was aspirated, taking care not to disturb the blood/plasma interface and PRP was incubated in the presence of acetylsalicylic acid (1mM) and apyrase (0.3 U/mL) for 15 min at 37°C. Subsequently, citric acid (9 mM) and EDTA (5 mM) were added and PRP was centrifuged at 800 g for 20 min at 24°C. The platelets were washed in buffer B (20 mM HEPES, 138 mM NaCl, 2.9 mM KCl, 1 mM MgCl2, 0.36 mM NaH2PO4, 0.3 U /mL apyrase; pH 6.2). The platelet pellet was then washed once with buffer B containing apyrase (0.3U/mL). The final platelet pellet was resuspended at a concentration of 400,000/µL in buffer C (20 mM HEPES, 138 mM NaCl, 2.9 mM KCl, 1 mM MgCl2, 0.36 mM NaH2PO4, 5 mM glucose, 0.6 U/mL apyrase; pH 7.4).
Measurement of Platelet Shape Change
For measurement of shape change, human platelets were isolated and incubated at 37°C with various concentrations of the LPA receptor agonists and antagonists, FAF-BSA or vehicle control before exposure to LPA. Shape change was measured by the decrease in light transmission of the stirred (1100 rpm) platelet suspension as described previously [6]. To analyze agonist effect of the compounds, washed platelets were incubated at 37°C for 2 minutes and stimulated with different concentration of compounds dissolved in methanol or FAF-BSA buffer (at a lipid:BSA molar ratio 4:1). For analysis of the antagonist effect of the compounds, washed platelets were incubated with different concentrations of the compounds at 37°C for 30 minutes or shorter and stimulated with 20nM acyl-LPA 16:0.
Measurement of platelet cAMP
Platelets were pretreated with aspirin, washed, and resuspended in buffer (400,000/µL) containing apyrase as described above. Platelet suspensions were incubated at 37°C while stirring before exposure to LPA (0.02- 40 µM) or iloprost (50 nM) for 1 minute. Levels of cAMP were determined with an enzyme immunoassay kit from Assay Designs GmBH according to the instructions of the manufacturer.
Isolation of mRNA from Human Platelets
Two units of platelet rich plasma (∼505 ml containing ∼57 ml ACD each expired for human use 1 day prior) were put through a Purecell PL high efficiency leukocyte reduction filter for platelets (PL6T from Pall Biomedical Products Company in East Hills, NY). The leukocyte reduced filtrate was then mixed with an equal volume of buffer consisting of 138mM NaCl, 3.3mM NaH2PO4, 2.9mM KCl, 1mM MgCl2, 20mM HEPES, 1mg/ml glucose, containing 15% of 0.8% citric acid, 2.2% Na-citrate2H2O, 2.45% glucose and 1µM PGE1 (Sigma-Aldrich, St. Louis, MO). The sample was centrifuged at 3000 × rpm for 10 min at room temperature and the supernatant was carefully removed and discarded. The platelet pellet was transferred to 2ml Eppendorf tubes and 1ml of TRIZol reagent (Invitrogen, Carlsbad.CA) was added. RNA was extracted according to the protocol provided by the manufacturer. The RNA pellet was dissolved in 15 µl DEPC-treated water yielding ∼50 µg per unit of platelets.
Quantitative PCR of LPA Receptor mRNA in Human Platelets
One µg of total RNA was digested with DNase I and used for the subsequent synthesis of cDNA using the First Strand Synthesis kit as recommended by the manufacturer. The following primer pairs were used: LPA1: (forward) GTCTTCTGGGCCATTTTCAA and (reverse) TCATAGTCCTCTGGCGAACA; LPA2: (forward) GGGCCAGTGCTACTACAACG and (reverse) ACCAGCAGATTGGTCAGCA ; LPA3: (forward) GAAGCTAATGAAGACGGTGATGA and (reverse) AGCAGGAACCACCTTTTCAC ; LPA4: (forward) TCTGGATCCTAGTCCTCAGTGG and (reverse) CCAGACACGTTTGGAGAAGC ; LPA5: (forward) CGCCATCTTCCAGATGAAC and (reverse) TAGCGGTCCACGTTGATG ; LPA6: (forward) AAATCCAGCAGGCAATTCAT and (reverse) CCCTGATGCTCTGGTTATGTT ; LPA7: (forward) TCTGGCAATTGTCTACCCATT and (reverse) TCAAAGCAGGCTTCTGAGG ; β-actin: (forward) TTCTACAATGAGCTGCGTGTG and (reverse) GGGGTGTTGAAGGTCTCAAA. The primer sets were designed with a melting temperature of 59–61°C. Amplicon size was 50–200 bases. Amplification was performed for 40 cycles at 94ºC/15 sec and 60ºC /60 sec using an ABI Model 7300 Real Time PCR machine (Foster City, CA). Quantitative values were obtained from the threshold cycle value (Ct), which is the point where a significant increase of fluorescence is first detected. The transcript number of human β-actin was quantified as an internal RNA control, and each sample was normalized on the basis of its β-actin content. The relative gene expression level of each gene was then normalized to LPA1 gene (calibrator). Final results, expressed as N-fold difference in gene expression relative to β-actin and LPA1, termed N, were calculated as: N = 2(Ct gene - Ct calibrator) (http://dorakmt.tripod.com/genetics/realtime.html), where Ct values of the gene and calibrator were determined by subtracting the average Ct value of a target gene from the corresponding Ct value of the β-actin gene.
Measurement of LPA-elicited Ca2+transients
Wild type McArdle rat hepatoma (RH7777, from ATCC) cells do not respond to LPA with changes in [Ca2+]i. RH7777 cell lines individually expressing either LPA1, LPA2, LPA3 or LPA5 receptors and Chinese hamster ovary (CHO) cell line were used to examine agonism and antagonism of the compounds. CHO cells stably expressing either vector or LPA4 were a kind gift from Dr. Takao Shimizu (University of Tokyo, Tokyo, Japan).
Stable transformants of LPA1/2/3 receptors; RH7777 cells stably expressing each receptor were plated onto poly-L-lysine (PLL, 0.1 mg/ml)-coated black-wall clear-bottom 96- well plates (Corning Incorporated Life Sciences, Acton, MA) at a density of 5 × 104 cells/well and cultured overnight. The following day, the culture medium was replaced with modified Krebs buffer (120 mM NaCl, 5 mM KCl, 0.62 mM MgSO4, 1.8 mM CaCl2, 10 mM HEPES, 6 mM glucose, pH 7.4), and the cells were serum starved for 6 h. Subsequently, cells were loaded with Fura-2 AM (Invitrogen, Carlsbad, CA) for 35 min in modified Krebs buffer containing 2% (v/v) pluronic acid. Stable transformants of LPA4; CHO cells stably expressing either vector or LPA4 were plated non-coated 96 well plates at a density of 4 × 104 cells/ well and cultured overnight. The following day, cells were loaded with Fura-2 AM for 1 h in modified Krebs buffer containing 2% (v/v) pluronic acid and 2.5mM probenecid.
For transient transfection of LPA5, RH7777 cells in 10 cm dish at a density of 2 × 106 were transfected with 2 µg of plasmid DNA with Effectene (Qiagen, Valencia, CA) according to the manufacturer's instructions for 24 h, then replated onto PLL-coated 96- well plates at a density of 5 × 104 cells/well and cultured overnight. The following day, the culture medium was then replaced with modified Krebs buffer, and the cells were serum starved for 4 h. Subsequently, cells were loaded with Fura-2 AM for 30 min in modified Krebs buffer containing 2% (v/v) pluronic acid, rinsed with Krebs buffer and changes in the intracellular Ca2+ concentration were monitored by determining the ratio of emitted light intensities at 520 nm in response to excitation at 340 and 380 nm using FLEXstation II (Molecular Devices, Sunnyvale, CA). Each well was monitored for 80–120s. For testing agonist activity of the compounds, the test compounds were added automatically after 15 s of baseline measurement. To determine antagonist properties, varying concentrations of the compounds were mixed with constant concentration of LPA and responses were monitored. Each test was performed in quadruplicate. CHO cells endogenously express LPA1, therefore, to access the effect of LPA4 in CHO cells, the response in vector-transfected cells were subtracted from the response in LPA4-transfected cells.
Statistical Analysis
Significant difference was determined by the Student’s test at a P value of 0.05. IC50 values were calculated by fitting a sigmoid function to data points by using the nonlinear curve-fitting feature of KaleidaGraph (Synergy Software, Essex Junction, VT).
Results
Expression of LPA Receptors mRNAs in Human Platelets
We applied real-time PCR to quantify the abundance of LPA1–7 mRNAs in purified human platelets. We used platelets isolated and pooled from four healthy human donors. The platelets used for mRNA extraction have been depleted of white blood cells and red blood cells using Purecell PL membranes. The purified platelet preparation was stained with May-Grunwald Giemsa stain and non-platelet cells were counted. The preparation contained less than 0.01% white blood cells and was considered highly pure for platelets. The abundance of LPA receptor RNA arbitrarily normalized to LPA1 is shown in figure 1. The rank order of abundance was LPA4 = LPA5 >LPA7> LPA6 = LPA2 >> LPA1 > LPA3. These results suggest that LPA receptors of the purinergic cluster represent the most abundant number of transcripts in human platelets.
Figure 1.
Relative abundance of LPA receptor transcripts in purified human platelets determined by quantitative real-time PCR.
The effect of short chain octyl-serinediamide phosphates on platelets and LPA4,5 receptors
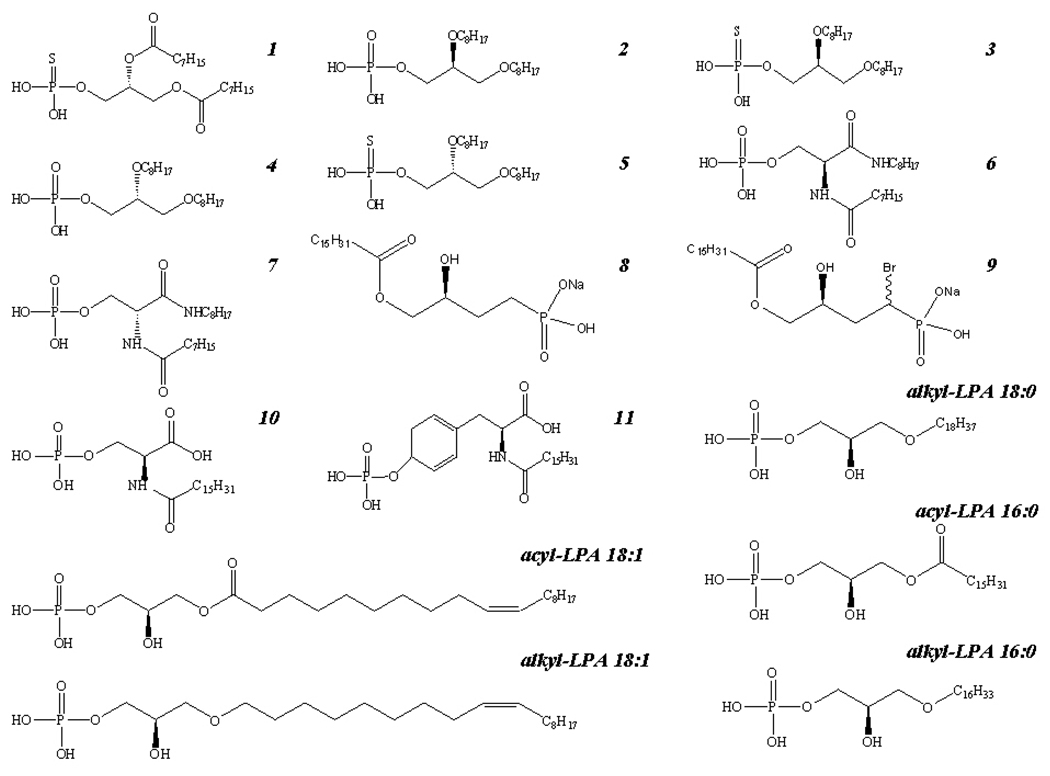
Durgam and colleagues [26] have synthesized and partially characterized analogs of phosphatidic acid (PA) 1–7 (Figure 2). Compounds 2, 3, and 6, 7 had no agonist activity, but potent antagonist effects on LPA1 and LPA3 expressed in RH7777 cells; compounds 4 and 5 had agonistic activity on LPA1–3 , but no antagonistic activity; compound 1 was a mixed LPA2 agonist/ LPA1,3 antagonist (Figure 2; Table I). We expanded the characterization of these compounds to human platelets that express very low copies of LPA1 and LPA3 (Figure1). The compounds were dissolved in either methanol or FAF-BSA buffer and tested for agonist and LPA-antagonist activity on human platelets. Methanol at the highest concentration tested (0.5% V/V) had no effect on LPA-mediated platelet activation (Figure 3A), whereas the FAF-BSA buffer (5µM) inhibited the LPA-response (Figure 3B). Nonetheless, the agonist activity of the compounds was independent of the type of vehicle used and the maximal efficacy of the drugs (Emax) was not significantly changed (Figure 3A and B; data not shown). Due to the interfering effect of 5 µM FAF-BSA with LPA-induced platelet activation, the compounds were dissolved in methanol for further analysis. In contrast to the results in heterologous expression system, all seven compounds induced platelet activation, and after 30 min incubation, inhibited the LPA-induced platelet shape change in a concentration-dependent manner (Table I). The EC50 values of the compounds were much higher than that of LPA and showed no correlation with their potency established at LPA1–3. Surprisingly, the LPA3 selective antagonist compound 7 was maximally active in inducing platelet shape change with a very low EC50 (∼550 nM) even though LPA3 was the least abundant transcript in platelets (Table I, Figure 1–Figure 3). These data reveal a structure-activity relationship (SAR) in platelets that is not consistent with that of LPA1, LPA2, and LPA3.
Figure 2.
Chemical structure of the compounds used in the present study.
Table I.
Effect of PA 8:0 analogs on intracellular Ca2+ transients of LPA1–5 transfected cells [25] and on platelet shape change
| HETEROLOGOUS EXPRESSION SYSTEMS | PLATELETS | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| PA(8:0) Analogs |
LPA receptor |
Agonist activity |
Antago nist activity |
Agonist activity | Antagonist activity | ||||
|
aEmax % |
EC50 µM |
IC50 µM |
bEmax % |
EC50 µM |
cImax % |
IC50 µM |
n | ||
| 1 | |||||||||
| LPA1/LPA3 | LPA1 | NE | 0.68 | ||||||
| Antagonist | LPA2 | 58 | 6.33 | NE | 69±12 | 1.77±0.22 | 76±18 | 2.92±1.73 | 3 |
| LPA2 | LPA3 | NE | 0.011 | ||||||
| Agonist | |||||||||
| 2 | LPA1 | NE | 1.58 | ||||||
| LPA1/LPA3 | LPA2 | NE | NE | 38±18 | 5.91±2.62 | 72±9 | 1.7±1.5 | 3 | |
| Antagonist | LPA3 | NE | 0.14 | ||||||
| 3 | LPA1 | NE | 0.14 | ||||||
| LPA1/LPA3 | LPA2 | NE | NE | ||||||
| Antagonist | LPA3 | NE | 0.184 | 69±13 | 1.79±0.95 | 91±9 | 0.55±0.37 | 3 | |
| LPA5 | LPA4 | NE | NE | ||||||
| Agonist | LPA5 | 92 | NS | NE | |||||
| 4 | LPA1 | 57 | 3.26 | NE | |||||
| LPA1/LPA3 | LPA2 | NE | NE | 57±4 | 2.43±1.1 | 93±4 | 0.35±0.1 | 3 | |
| Agonist | LPA3 | 109 | 0.16 | NE | |||||
| 5 | LPA1 | 30.6 | 0.69 | NE | |||||
| LPA1–3 | LPA2 | 27 | 5.72 | NE | |||||
| Agonist | LPA3 | 109 | 0.003 | NE | 48±10 | 0.39±0.11 | 87±13 | 0.21±0.16 | 4 |
| LPA5 | LPA4 | NE | NE | ||||||
| Agonist | LPA5 | 97 | NS | NE | |||||
| 6 | LPA1 | NE | NE | ||||||
| select.LPA3 | LPA2 | NE | NE | 68±12 | 8.4±0.74 | 80±2 | 8.2±0.96 | 3 | |
| Antagonist | LPA3 | NE | 0.414 | ||||||
| 7 | LPA1 | NE | NE | ||||||
| select.LPA3 | LPA2 | NE | NE | ||||||
| Antagonist | LPA3 | NE | 0.935 | 110±9 | 0.55±0.18 | 95±5 | 2.6±0.4 | 3 | |
| LPA5 | LPA4 | NE | NE | ||||||
| Agonist | LPA5 | 36 | NS | NE | |||||
Note: Emax = maximal efficacy of the drug/maximal efficacy of LPA 18:1; NE= no effect; NS = the response did not saturate at the highest concentration tested (10 µM).
Emax= (maximal shape change induced by drug/ shape change induced by 20 nM acyl-LPA 16:0)x100. Shape change induced by 20–25 nM LPA was 89±11 % (mean SD, n=22) of maximal, and set to 100 %. Values are mean ± SD from different experiments with different platelet donors. Inhibitory activity was tested 30 min after addition of the phospholipids, when platelets had reached again their discoid shape (Figure 3A, right tracings).
Imax= maximal inhibition of LPA-induced shape change tested by 20 µM of PA analogs.
Figure 3. Effect of LPA3 antagonist (7) on platelet.
Suspensions of washed human platelets were incubated with solvent or 20 µM 7 dissolved A, in methanol or B, in albumin buffer (lipid:BSA 4:1) for 30 min before exposure to acyl-LPA 16:0 (20 nM). Shape change was recorded as decrease of light transmission. Tracings shown are representative for 3 experiments.
Since the compound 4 an apparent LPA1/LPA3 agonist, was found to be a weak agonist, and had a higher antagonistic than agonistic potency (IC50 0.35±0.1 µM; EC50 2.43±1.1 µM) (Figure 2; Table I), further experiments were carried out with this compound to distinguish whether platelet inhibition by 4 was due to LPA receptor desensitization or antagonism. Concentrations of 4 that did not induce shape change were chosen, and incubated with platelets for various times (5 sec, 5 min, 15 min) before addition of LPA. No inhibition was found after 5 sec pre-incubation but shape change was inhibited progressively at longer pre-incubation times, consistent with a mechanism of receptor desensitization rather than a LPA receptor antagonistic effect (Figure 4).
Figure 4. The inhibitory effect of a LPA1/3 agonist (compound 4) on platelets is pre-incubation time dependent.
A concentration of 0.2 µM compound 4 that itself did not induce shape change was incubated for different time periods before exposure to acyl-LPA 16:0 (20nM).
As the compounds 3, 5, 7 induced platelet shape change with higher potency, we also determined their action on LPA4 and LPA5 receptors. All compounds had no effect on LPA4 activation and were weak agonists of LPA5 (Table I). These data indicate that LPA5 could be involved in LPA-induced platelet shape change.
The effect of N-palmitoyl amino phosphoric acids on LPA responses in platelets and heterologously expressed LPA1–5
We and others have shown previously that compounds 10 NPSPA and 11 NPTPA (Figure 2) effectively induce platelet shape change and aggregation; however, after preincubation inhibit platelet responses to LPA [6,27,28]. NPSPA was slightly more potent than NPTPA, the EC50 and IC50 of these phospholipids were in a similar range (between 0.2 and 0.3 µM) [6]. These compounds were reported not to inhibit the EDG family LPA receptors [22]. Here we tested both compounds for their effects on LPA1–5 (Table II). NPSPA was a weak agonist of the EDG family LPA receptors, had no effect on LPA4 and was a partial agonist of LPA5. Also, NPTPA was inactive on LPA4 and in the micromolar range was partial agonist of LPA5. In contrast to NPSPA, NPTPA weakly inhibited LPA1 with a Ki of 533 nM and had no agonist or antagonist effect on LPA2, LPA3. Like in the case of the PA analogs, we could not draw a clear correlation between their pharmacological profile and the expression of the LPA receptor found in platelets.
Table II.
Effect of NPSPA NPTPA on LPA responses of heterologously expressed LPA1–5
| Compound | LPA1 | LPA2 | LPA3 | LPA4 | LPA5 | |
|---|---|---|---|---|---|---|
| NPSPA | Partial agonist (Emax= 63%) |
Partial agonist (Emax= 46%) |
Partial agonist (Emax= 81%) |
No effect | Partial agonist (Emax= 23%) |
|
| EC50= 0.74 µM | EC50= 11.9 µM | EC50= 0.73 µM | EC50= 0.42 µM | |||
| NPTPA | Antagonist | No effect | No effect | No effect | Partial agonist (Emax= 72.0%) |
|
| Ki= 0.53 µM | EC50 - NS | |||||
Note: Emax = (maximal efficacy of compound/maximal efficacy of LPA 18:1)×100
EC50 = concentration eliciting half maximal activation
Ki = inhibitory binding constant
NS= the response did not saturate at the highest concentration tested
The effect of LPA4 receptor agonist and antagonist compounds on platelets and LPA5
Jiang et al. reported the initial characterization of a series of α-substituted phosphonate analogs of LPA and the first two compounds 8 and 9 (Figure 2) with LPA4 receptor agonist and antagonist properties, respectively [25]. Here we have expanded the characterization of the compounds to include LPA5 and also assessed their actions in platelets. Both compounds 8 and 9 were weak agonists of LPA5 yielding 59% and 45% activation of the maximum response to LPA 18:1 at 10 µM, respectively (Table III). Both α-substituted phosphonate analogs activated platelets and inhibited LPA-induced platelet shape change after 30 min pre-incubation. Interestingly, these two phosphonate analogs were more active than the PA analogs as shown by their lower EC50 values (Table III). These data indicate that these analogs are not specific for LPA4 and also suggest that not LPA4 but LPA5 could be involved in LPA-elicited platelet shape change. We have also examined the agonist properties of alkyl-LPAs relative to acyl-LPAs at LPA4 expressed heterologously in CHO cells and found that the alkyl-ether-analogs were weaker agonists than the acyl-analogs with an equal number of hydrocarbons (Table III).
Table III.
Effect of acyl and alkyl LPAs and α-substituted phosphonate analogs of LPA on intracellular Ca2+ transients of LPA1–5 transfected cells and platelet shape change
| HETEROLOGOUS EXPRESSION SYSTEMS | PLATELET | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Analogs | LPA receptor |
Agonist activity |
Antago nist activity |
Agonist activity | Antagonist activity |
||||
|
aEmax % |
EC50 µM |
IC50 µM |
bEmax % |
EC50 nM |
cImax % |
IC50 nM |
n | ||
| Acyl LPA 18:1 | LPA1 | 100 | 0.130 | ||||||
| LPA2 | 100 | 0.003 | |||||||
| LPA3 | 100 | 0.081 | 100 | 9±2.9 | 4 | ||||
| LPA4 | 100 | 0.245 | |||||||
| LPA5 | 100 | 0.015 | |||||||
| Alkyl LPA 18:1 | LPA1 | 100 | 1.445 | ||||||
| LPA2 | 108 | 0.100 | |||||||
| LPA3 | 63 | dNS | 100 | 1.85±0.7 | 4 | ||||
| LPA4 | 98 | 0.303 | |||||||
| LPA5 | 97 | 0.002 | |||||||
| 8 | LPA1 | NE | PA | ||||||
| LPA4,5 | LPA2 | NE | 2.59 | ||||||
| Agonist | LPA3 | NE | 2.56 | 78±9 | 280±40 | 81±5 | 900±110 | 3 | |
| LPA1–3 | LPA4 | 29 | 5.4 | NE | |||||
| Antagonist | LPA5 | 59 | dNS | ||||||
| 9 | LPA1 | NE | 1.5 | ||||||
| LPA1–4 | LPA2 | NE | 1.42 | ||||||
| Antagonist | LPA3 | NE | 1.16 | 73±9 | 810±370 | 80±9 | 300±150 | 3 | |
| LPA5 | LPA4 | NE | 0.27 | ||||||
| Agonist | LPA5 | 45 | dNS | ||||||
Note: Emax = (maximal efficacy of compound/maximal efficacy of LPA 18:1)x100; NE= no effect; PA=partial antagonist with 39±3.2% inhibition of 200 nM LPA response at the highest concentration (30µM) tested.
Emax=(maximal shape change induced by drug/ shape change induced by 20 nM acyl-LPA 16:0)×100. Values are mean ± SD from different experiments with different platelet donors. Inhibitory activity was tested 30 min after addition of the phospholipids.
Imax= maximal inhibition of LPA-induced shape change tested by 10 µM of analogs.
NS = the response did not saturate at 10 µM the highest concentration tested preventing the determination of EC50
LPA5 Receptor and Platelet Activation Shows Similar SAR
Recently GPR92 has been identified as a new LPA receptor (LPA5) and we have been able to test the SAR of this receptor in RH7777 using the Ca2+ mobilization assay [16]. In platelets and in LPA5–transfected RH7777 cells a similar rank order of activation was found: alkyl-LPA 18:1 ≥ alkyl-LPA 16:0 > acyl-LPA 18:1 >> alkyl-LPA 18:0 (Table IV; Figure 5; data not shown). This striking similarity in the SAR argues for the possible involvement of LPA5 receptor in mediating LPA-induced platelet shape change.
Table IV.
Activities of different molecular species of LPA
| LPA species | Platelets | LPA5 transiently transfected RH7777 cells |
|---|---|---|
| EC50 nM |
EC50 nM |
|
| acyl-LPA 18:1 | 9 ±2.9 | 15.2±5.4 |
| alkyl-LPA 16:0 | 4.6±0.98 | 4.1±2.1 |
| alkyl-LPA 18:0 | 20.17±17.5 | 69.9±21.4 |
| alkyl-LPA 18:1 | 1.85±0.7 | 2.1±0.9 |
Note: EC50 values were calculated from concentration-response curves induced by various LPA species in platelets (n=4) and LPA5 receptor transiently transfected RH7777 cells (mean ± SD).
Figure 5. Dose-response curves induced by various LPA species.
Suspensions of washed human platelets were stirred at 37°C for 2 minutes and then exposed to increasing concentrations of LPA species. Shape change was measured by the decrease in light transmission in a LABOR aggregometer®. Values represent the mean ± SD from 4 different experiments with different platelet donors.
The effect of FAF-BSA on LPA-induced platelet shape change
In serum, LPA is predominantly associated with serum albumin [11]. In order to investigate the effect of FAF-BSA on LPA-induced platelet shape change, we determined the dose-response curves for acyl-LPA 16:0 dissolved in ethanol or FAF-BSA buffer. As shown in Figure 6A, the presence of FAF-BSA (molar LPA: BSA ratio, 4:1) had only a small inhibitory effect on platelet activation induced by LPA, which was not significant (p>0.05). However, pre-incubation of platelets with FAF-BSA at a concentration of 2.5 µM shifted the dose-response curve of LPA to the right (Figure 6B). LPA (1 µM) could overcome the inhibition of FAF-BSA. FAF-BSA inhibited platelet shape change regardless of whether acyl- or alkyl-LPA was used (data not shown).
Figure 6. Effect of albumin on LPA-mediated shape change.
A, Dose-response curves comparing the effect of LPA dissolved in ethanol (dotted line) and in albumin buffer (LPA:BSA 4:1) (solid line) on platelet shape change (mean SD, n=3). B, BSA inhibits platelet shape change in a competitive manner. Platelets were incubated with BSA (2.5 µM) (solid line) for 1 min before exposure to increasing concentration of LPA (in albumin buffer), n=3. C, BSA inhibits LPA-induced platelet shape change in a dose-dependent manner. Washed platelets were incubated with different concentrations of BSA for 1 min before exposure to 20nM of LPA (in albumin buffer), n=6. D, BSA reduced LPA-initiated platelet shape change in a time-independent manner. Incubation of platelets was performed with BSA (2.5 µM) for different time periods before exposure to 20nM of LPA (in albumin buffer), n=3. Values represent the mean ± SD. *<0.05 vs. control.
To quantify the inhibitory effect of albumin on LPA-induced platelet shape change, we pre-incubated platelets with increasing concentrations of FAF-BSA (0.1–5µM) before addition of 20 nM acyl-LPA 18:1 (dissolved in FAF-BSA buffer). As shown in Figure 6C, FAF-BSA dose-dependently decreased the LPA-elicited shape change response which was complete at 5µM concentration. The calculated IC50 of FAF-BSA was 1.15±0.37 µM (mean SD, n=6). Furthermore, we investigated whether the inhibitory effect of FAF-BSA required pre-incubation with the platelets. As shown in figure 6D, inhibition of LPA-induced shape change by FAF-BSA was independent of the duration of pre-incubation time. This suggests that the inhibitory effect might be due to albumin’s avidity for LPA or due to albumin modulation of platelet LPA receptors.
The effect of FAF-BSA on LPA1–5 receptor activation
To evaluate the effect of albumin on the LPA receptors and draw a parallel between the activation of platelet LPA receptors and that of LPA1–5, we tested the effect of albumin on these heterologously expressed receptors. In contrast to the FAF-BSA-inhibition of LPA-induced platelet activation, the pre-incubation of FAF-BSA (15µM) for 5 min did not inhibit activation of LPA1, LPA2, LPA3 and LPA5 receptors in the heterologous expression system (Figure 7). However, LPA4 activation was inhibited by 40% in the presence of 15µM FAF-BSA and the dose-response curve of LPA was shifted to the right (Figure 7).
Figure 7. Effect of albumin on LPA1–5 activation in heterologous expression systems.
The effect of BSA (15µM, 5min pre-incubation) on LPA receptor activation induced by LPA was examined by measuring the transient increase of intracellular [Ca2+] in Fura-2-loaded RH7777 cells expressing human LPA1–3 and LPA5, or CHO cells expressing LPA4 (mean ± SD).
The effect of LPA on cAMP levels in platelet
It has been shown that LPA4 and LPA5 activation in addition to eliciting Ca2+ transients induces an increase of intracellular cAMP [16,29,30]. In the present study we investigated whether LPA might also induce the activation of Gs that leads to an increase of cAMP levels in platelets. Levels of cAMP in unstimulated platelets were 5.83±1.26 pmol/108 platelets (Figure 8). In platelets treated with a large concentration range of acyl-LPA 18:1 (0.02 – 40 µM) and for different times (30 s, 1 min, 5 min, 15 min) no significant change in cAMP was found (p>0.05) (Figure 8; data not shown). The prostacyclin-analog, iloprost (50 nM), elicited a robust increase in cAMP (Figure 8). Also acyl-LPA 16:0 and alkyl-LPA 16:0 did not affect platelet cAMP levels (data not shown). Previously we have found that LPA added subsequently to iloprost did not change the cAMP levels stimulated by this prostaglandin analog [31]. Therefore, platelet LPA receptors are unlikely to couple to Gi and Gs.
Figure 8. Effect of LPA on cAMP levels in platelet.
Suspensions of washed human platelets were at 37°C for 2 minutes and were exposed to increasing concentration of LPA (0.02 µM – 40 µM) or iloprost (50nM) for 1 minute. Levels of cAMP were determined with an enzyme immunoassay kit. Values represent the mean ± SD from different experiments with different platelet donors (n=3).
Discussion
LPA accumulating in atherosclerotic plaque and oxidatively modified LDL activates human platelets [2,6,7]. Although, alkyl glycerophosphate, commonly known as alkyl-LPA, is active at subnanomolar concentrations making it a very potent platelet activating mediator, the receptor(s) mediating this effect remain unknown [6]. The LPA response in platelets shows some distinct features – preference to alkyl-LPA over acyl-LPA, sensitivity to albumin, SAR of inhibitors – that could not be reconciled with the EDG family of LPA receptors. The EDG family shows clear preference for acyl forms of LPA and little inhibition by albumin, although LPA3 shows some sensitivity to this protein carrier [32]. In light of the recent discovery of a new subcluster of LPA receptors with high degree of sequence homology to the purinergic GPCR, in the present paper we extended a comprehensive analysis of SAR to LPA1–5. The expression of some receptors of the purinergic cluster has been recently reported in human platelets [20]. While this work was nearing completion, LPA6 and LPA7 were reported [17,18]. Our initial attempts to obtain Ca2+ transients from LPA6 and LPA7 transfected RH7777 cells were unsuccessful so we have omitted it from our functional assays. Nevertheless, we found LPA6 and LPA7 transcripts in human platelets at a level similar to that of LPA2. The role of LPA6 or LPA7 and other not yet reported LPA receptors will have to be addressed in future studies.
The main finding of this study is that the antagonists that have been developed to the EDG family LPA1–3 receptors and to LPA4 act as agonists for platelet activation. Unexpectedly, some of them, for example the selective LPA3 receptor antagonist octyl serine diamide compound 7, were more effective in inducing platelet shape change than inhibiting LPA3. This is in sharp contrast with our earlier analysis of the SAR of platelet responses in which we proposed that LPA1 and LPA3 activation triggers LPA-induced platelet activation [6]. The present observations supported by the low abundance of LPA1 and LPA3 transcripts in human platelets necessitates the revision of this working hypothesis and argues against that the LPA1 or LPA3 receptors mediate LPA-induced platelet shape change. Although knockout mice are available to these two receptor subtypes, direct analysis of their platelet responses is not possible because rodent platelets do not respond to LPA [33].
By comparing the biological effect of three molecular LPA species, it has been previously shown that alkyl-LPA species are more potent at inducing platelet activation than acyl-LPA [6,34]. The present study confirms in part these observations. Our experiments demonstrated that the LPA5 receptor had pharmacological properties similar to LPA-induced platelet activation arguing for the possible involvement of LPA5 receptor in mediating platelet shape change. However, it has been shown that LPA5 activation induces an increase of intracellular cAMP in LPA5 transfected HeLa HF1 and B103 cells [16,29], and an increase of cAMP mediates platelet inhibition. Similarly, LPA7 has been shown to couple to the elevation of cAMP [18]. We did not observe an increase of cAMP upon LPA stimulation of platelets. Very recently, Pamuklar and colleagues have noted that platelets of ∼20% of individuals failed to aggregate to LPA and showed an increase of platelet cAMP to LPA (1µM), which correlated with a higher expression of LPA4 transcripts as compared to the LPA responsive platelet donors. These authors suggested that LPA stimulation of a receptor coupled to cAMP increase could prevent LPA induced platelet aggregation in the non-responsive donors [21]. We also observed previously donor-dependent variations of LPA-induced platelet aggregation in blood and PRP. However, LPA-induced platelet shape change in blood and washed cell suspensions was donor-independent [31]. Our results showing no measurable changes in cAMP and platelet shape change upon LPA-stimulation do not support this hypothesis.
Tokumura et al. have shown that albumin can dose-dependently inhibit LPA-induced platelet aggregation [34]. However, its effect on LPA-induced platelet shape change has not been examined systematically. In our study, we found that FAF-BSA inhibited platelet shape change induced by LPA bound to BSA in a competitive manner and independently of incubation time. It is unlikely that this inhibitory effect can be explained by the property of BSA to bind LPA, since platelet shape change was induced by LPA in complex with BSA at a molar ratio of 4:1. Albumin binds LPA with stoichiometry of 3 moles LPA to 1 mole of BSA [11,35] and we showed that LPA in complex with albumin (ratio LPA:BSA 4:1) activated platelets with similar potency as LPA dissolved in 70 % ethanol (final concentration < 0.07%). Therefore, it seems more plausible that albumin modulates LPA receptors. Indeed, Hama et al. showed that the inhibitory effect of albumin occurs in a receptor specific manner, and Sf9 cells expressing the LPA3 receptor are more sensitive to BSA than LPA1 and LPA2 expressing cells [32]. However, we found that albumin had no effect on LPA3 receptor activation in transfected RH7777 cells, which might be explained by the differences in the heterologous expression systems used. The lack of effect of albumin on LPA1, LPA2, LPA3 and LPA5 receptor activation in heterologous expression system further support our conclusion that these receptors cannot simply account for eliciting LPA-induced platelet shape change.
We also found that activation of LPA4 receptor was significantly inhibited by a low concentration of albumin (15µM), raising the possibility that LPA4 could play a role in LPA-mediated platelet activation. This would be supported by our Q-PCR data and those by others showing that LPA4 and LPA5 transcripts are much more abundant in human platelets than LPA1 and LPA3 transcripts [20,21]. However, our experiments with the compound 9, a LPA4 antagonist, which stimulated platelets, argue against an involvement of LPA4 in LPA-induced platelet activation.
In summary, the present results provide a comprehensive analysis of the pharmacological properties of the LPA-induced platelet shape changes, and indicate that LPA activates platelets not simply by either one of the LPA1–5 receptors, but perhaps through a new LPA receptor such as LPA6, LPA7 or a yet unidentified GPCR. They point to the need of further studies on this pathophysiologically important question.
Acknowledgments
The study was supported by grants from the August-Lenz-Stiftung, from the Deutsche Forschungsgemeinschaft (the Graduate Program “Vascular Biology in Medicine” GRK 438; to A.L.K; P.G.; the grant Si 274/9), and by grants of the Bayern University (the Graduate Program of the Bavarian Eliteförderungsgesetz (BayEFG; to A.L. K.), and the LMU (“Förderprogramm für Forschung und Lehre of the LMU Munich” ;FöFoLe N⍛ 541), NIH HL790007, HL92160, and CA92160 (to GT).
References
- 1.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 2.Siess W, Tigyi G. Thrombogenic and atherogenic activities of lysophosphatidic acid. J Cell Biochem. 2004;92:1086–1094. doi: 10.1002/jcb.20108. [DOI] [PubMed] [Google Scholar]
- 3.Holvoet P, Vanhaecke J, Janssens S, Van de Werf F, Collen D. Oxidized LDL and malondialdehyde-modified LDL in patients with acute coronary syndromes and stable coronary artery disease. Circulation. 1998;98:1487–1494. doi: 10.1161/01.cir.98.15.1487. [DOI] [PubMed] [Google Scholar]
- 4.Weidtmann A, Scheithe R, Hrboticky N, Pietsch A, Lorenz R, Siess W. Mildly oxidized LDL induces platelet aggregation through activation of phospholipase A2. Arterioscler Thromb Vasc Biol. 1995;15:1131–1138. doi: 10.1161/01.atv.15.8.1131. [DOI] [PubMed] [Google Scholar]
- 5.Sevanian A, Hwang J, Hodis H, Cazzolato G, Avogaro P, Bittolo-Bon G. Contribution of an in vivo oxidized LDL to LDL oxidation and its association with dense LDL subpopulations. Arterioscler Thromb Vasc Biol. 1996;16:784–793. doi: 10.1161/01.atv.16.6.784. [DOI] [PubMed] [Google Scholar]
- 6.Rother E, Brandl R, Baker DL, Goyal P, Gebhard H, Tigyi G, Siess W. Subtype-selective antagonists of lysophosphatidic Acid receptors inhibit platelet activation triggered by the lipid core of atherosclerotic plaques. Circulation. 2003;108:741–747. doi: 10.1161/01.CIR.0000083715.37658.C4. [DOI] [PubMed] [Google Scholar]
- 7.Siess W, Zangl KJ, Essler M, Bauer M, Brandl R, Corrinth C, Bittman R, Tigyi G, Aepfelbacher M. Lysophosphatidic acid mediates the rapid activation of platelets and endothelial cells by mildly oxidized low density lipoprotein and accumulates in human atherosclerotic lesions. Proc Natl Acad Sci U S A. 1999;96:6931–6936. doi: 10.1073/pnas.96.12.6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gardell SE, Dubin AE, Chun J. Emerging medicinal roles for lysophospholipid signaling. Trends Mol Med. 2006;12:65–75. doi: 10.1016/j.molmed.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Sano T, Baker D, Virag T, Wada A, Yatomi Y, Kobayashi T, Igarashi Y, Tigyi G. Multiple mechanisms linked to platelet activation result in lysophosphatidic acid and sphingosine 1-phosphate generation in blood. J Biol Chem. 2002;277:21197–21206. doi: 10.1074/jbc.M201289200. [DOI] [PubMed] [Google Scholar]
- 10.Chen X, Yang XY, Wang ND, Ding C, Yang YJ, You ZJ, Su Q, Chen JH. Serum lysophosphatidic acid concentrations measured by dot immunogold filtration assay in patients with acute myocardial infarction. Scand J Clin Lab Invest. 2003;63:497–503. doi: 10.1080/00365510310003265. [DOI] [PubMed] [Google Scholar]
- 11.Tigyi G, Miledi R. Lysophosphatidates bound to serum albumin activate membrane currents in Xenopus oocytes and neurite retraction in PC12 pheochromocytoma cells. J Biol Chem. 1992;267:21360–21367. [PubMed] [Google Scholar]
- 12.Siess W. Athero- and thrombogenic actions of lysophosphatidic acid and sphingosine-1-phosphate. Biochim Biophys Acta. 2002;1582:204–215. doi: 10.1016/s1388-1981(02)00173-7. [DOI] [PubMed] [Google Scholar]
- 13.Meyer zu Heringdorf D, Jakobs KH. Lysophospholipid receptors: signalling, pharmacology and regulation by lysophospholipid metabolism. Biochim Biophys Acta. 2007;1768:923–940. doi: 10.1016/j.bbamem.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 14.Chun J, Goetzl EJ, Hla T, Igarashi Y, Lynch KR, Moolenaar W, Pyne S, Tigyi G. International Union of Pharmacology. XXXIV. Lysophospholipid receptor nomenclature. Pharmacol Rev. 2002;54:265–269. doi: 10.1124/pr.54.2.265. [DOI] [PubMed] [Google Scholar]
- 15.Noguchi K, Ishii S, Shimizu T. Identification of p2y9/GPR23 as a novel G protein-coupled receptor for lysophosphatidic acid, structurally distant from the Edg family. J Biol Chem. 2003;278:25600–25606. doi: 10.1074/jbc.M302648200. [DOI] [PubMed] [Google Scholar]
- 16.Lee CW, Rivera R, Gardell S, Dubin AE, Chun J. GPR92 as a new G12/13- and Gq-coupled lysophosphatidic acid receptor that increases cAMP, LPA5. J Biol Chem. 2006;281:23589–23597. doi: 10.1074/jbc.M603670200. [DOI] [PubMed] [Google Scholar]
- 17.Tabata K, Baba K, Shiraishi A, Ito M, Fujita N. The orphan GPCR GPR87 was deorphanized and shown to be a lysophosphatidic acid receptor. Biochem Biophys Res Commun. 2007;363:861–866. doi: 10.1016/j.bbrc.2007.09.063. [DOI] [PubMed] [Google Scholar]
- 18.Pasternack SM, von Kugelgen I, Aboud KA, Lee YA, Ruschendorf F, Voss K, Hillmer AM, Molderings GJ, Franz T, Ramirez A, Nurnberg P, Nothen MM, Betz RC. G protein-coupled receptor P2Y5 and its ligand LPA are involved in maintenance of human hair growth. Nat Genet. 2008;40:329–334. doi: 10.1038/ng.84. [DOI] [PubMed] [Google Scholar]
- 19.Motohashi K, Shibata S, Ozaki Y, Yatomi Y, Igarashi Y. Identification of lysophospholipid receptors in human platelets: the relation of two agonists, lysophosphatidic acid and sphingosine 1-phosphate. FEBS Lett. 2000;468:189–193. doi: 10.1016/s0014-5793(00)01222-9. [DOI] [PubMed] [Google Scholar]
- 20.Amisten S, Braun OO, Bengtsson A, Erlinge D. Gene expression profiling for the identification of G-protein coupled receptors in human platelets. Thromb Res. 2007 doi: 10.1016/j.thromres.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 21.Pamuklar Z, Lee JS, Cheng HY, Panchatcharam M, Steinhubl S, Morris AJ, Charnigo R, Smyth SS. Individual Heterogeneity in Platelet Response to Lysophosphatidic Acid. Evidence for a Novel Inhibitory Pathway. Arterioscler Thromb Vasc Biol. 2008 doi: 10.1161/ATVBAHA.107.151837. [DOI] [PubMed] [Google Scholar]
- 22.An S, Bleu T, Zheng Y, Goetzl EJ. Recombinant human G protein-coupled lysophosphatidic acid receptors mediate intracellular calcium mobilization. Mol Pharmacol. 1998;54:881–888. doi: 10.1124/mol.54.5.881. [DOI] [PubMed] [Google Scholar]
- 23.Fujiwara Y, Sardar V, Tokumura A, Baker D, Murakami-Murofushi K, Parrill A, Tigyi G. Identification of residues responsible for ligand recognition and regioisomeric selectivity of lysophosphatidic acid receptors expressed in mammalian cells. J Biol Chem. 2005;280:35038–35050. doi: 10.1074/jbc.M504351200. [DOI] [PubMed] [Google Scholar]
- 24.Bandoh K, Aoki J, Taira A, Tsujimoto M, Arai H, Inoue K. Lysophosphatidic acid (LPA) receptors of the EDG family are differentially activated by LPA species. Structure-activity relationship of cloned LPA receptors. FEBS Lett. 2000;478:159–165. doi: 10.1016/s0014-5793(00)01827-5. [DOI] [PubMed] [Google Scholar]
- 25.Jiang G, Xu Y, Fujiwara Y, Tsukahara T, Tsukahara R, Gajewiak J, Tigyi G, Prestwich GD. alpha-Substituted Phosphonate Analogues of Lysophosphatidic Acid (LPA) Selectively Inhibit Production and Action of LPA. ChemMedChem. 2007;2:679–690. doi: 10.1002/cmdc.200600280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Durgam GG, Tsukahara R, Makarova N, Walker MD, Fujiwara Y, Pigg KR, Baker DL, Sardar VM, Parrill AL, Tigyi G, Miller DD. Synthesis and pharmacological evaluation of second-generation phosphatidic acid derivatives as lysophosphatidic acid receptor ligands. Bioorg Med Chem Lett. 2006;16:633–640. doi: 10.1016/j.bmcl.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 27.Liliom K, Bittman R, Swords B, Tigyi G. N-palmitoyl-serine and N-palmitoyl-tyrosine phosphoric acids are selective competitive antagonists of the lysophosphatidic acid receptors. Mol Pharmacol. 1996;50:616–623. [PubMed] [Google Scholar]
- 28.Sugiura T, Nakane S, Kishimoto S, Waku K, Yoshioka Y, Tokumura A, Hanahan DJ. Occurrence of lysophosphatidic acid and its alkyl ether-linked analog in rat brain and comparison of their biological activities toward cultured neural cells. Biochim Biophys Acta. 1999;1440:194–204. doi: 10.1016/s1388-1981(99)00127-4. [DOI] [PubMed] [Google Scholar]
- 29.Kotarsky K, Boketoft A, Bristulf J, Nilsson NE, Norberg A, Hansson S, Owman C, Sillard R, Leeb-Lundberg LM, Olde B. Lysophosphatidic acid binds to and activates GPR92, a G protein-coupled receptor highly expressed in gastrointestinal lymphocytes. J Pharmacol Exp Ther. 2006;318:619–628. doi: 10.1124/jpet.105.098848. [DOI] [PubMed] [Google Scholar]
- 30.Lee CW, Rivera R, Dubin AE, Chun J. LPA(4)/GPR23 is a lysophosphatidic acid (LPA) receptor utilizing G(s)-, G(q)/G(i)-mediated calcium signaling and G(12/13)-mediated Rho activation. J Biol Chem. 2007;282:4310–4317. doi: 10.1074/jbc.M610826200. [DOI] [PubMed] [Google Scholar]
- 31.Haseruck N, Erl W, Pandey D, Tigyi G, Ohlmann P, Ravanat C, Gachet C, Siess W. The plaque lipid lysophosphatidic acid stimulates platelet activation and platelet-monocyte aggregate formation in whole blood: involvement of P2Y1 and P2Y12 receptors. Blood. 2004;103:2585–2592. doi: 10.1182/blood-2003-04-1127. [DOI] [PubMed] [Google Scholar]
- 32.Hama K, Bandoh K, Kakehi Y, Aoki J, Arai H. Lysophosphatidic acid (LPA) receptors are activated differentially by biological fluids: possible role of LPA-binding proteins in activation of LPA receptors. FEBS Lett. 2002;523:187–192. doi: 10.1016/s0014-5793(02)02976-9. [DOI] [PubMed] [Google Scholar]
- 33.Schumacher KA, Classen HG, Spath M. Platelet aggregation evoked in vitro and in vivo by phosphatidic acids and lysoderivatives: identity with substances in aged serum (DAS) Thromb Haemost. 1979;42:631–640. [PubMed] [Google Scholar]
- 34.Tokumura A, Sinomiya J, Kishimoto S, Tanaka T, Kogure K, Sugiura T, Satouchi K, Waku K, Fukuzawa K. Human platelets respond differentially to lysophosphatidic acids having a highly unsaturated fatty acyl group and alkyl ether-linked lysophosphatidic acids. Biochem J. 2002;365:617–628. doi: 10.1042/BJ20020348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thumser AE, Voysey JE, Wilton DC. The binding of lysophospholipids to rat liver fatty acid-binding protein and albumin. Biochem J. 1994;301(Pt 3):801–806. doi: 10.1042/bj3010801. [DOI] [PMC free article] [PubMed] [Google Scholar]