Abstract
Using background data that live vaccines against several viral pathogens are effective in inducing life-long protection against disease, we undertook studies in macaques to determine the duration of protection that two live SHIV vaccines could induce against AIDS. Earlier studies had established that macaques immunized once with a live vaccine and challenged 6 months later were protected, and that other macaques given two sequential inoculations of live vaccines were protected for at least one year. Protection was associated with persistence of the vaccine viruses. In this study, we sought to determine whether the duration of protection in macaques given a single inoculation of replication competent live vaccines would extend beyond three years. Two groups of four rhesus macaques were inoculated with two live SHIV vaccines, respectively. The viruses replicated transiently in all animals but at the 3 year time point, PCR analysis of PBMC did not detect DNA of either virus in any of the animals, and all were negative for CMI responses in the blood. All 8 animals succumbed to disease when challenged with pathogenic viruses.
INTRODUCTION
The well known biological properties of HIV that include its preferential infection in HIV-specific CD4 T cells (Douek et al., 2003), its propensity for undergoing continuous mutation (Nickle et al., 2003) and the long incubation period of disease (Lassen et al., 2004) have created major road blocks for development and evaluation of safety, immunogenicity, and efficacy of a vaccine that could prevent AIDS. The macaque models of HIV pathogenesis using SIV and SHIV strains of virus in different species of macaques have provided important insights into the feasibility of developing an efficacious vaccine against disease (Igarashi et al., 1999; Joag et al., 1996; Luciw et al., 1995; Reimann et al., 1996a; Reimann et al., 1996b). Use of this animal model system has made conditions for development and assessment of efficacy relatively simple and has clearly established the principle that it is possible to develop an efficacious vaccine for use in humans, even though it has not been possible as yet to develop an efficacious vaccine that could predictably prevent infection in macaques with pathogenic challenge viruses (Johnston and Fauci, 2007).
Efficacy of long term protection against well known human viral pathogens such as measles, polio, mumps, small pox viruses provided the initial major thrust for efforts to develop a live vaccine against HIV. However, the data from macaques showing that attenuated SIVmac239 which proved to be highly efficacious in preventing AIDS in adult macaques was still pathogenic in infant animals (Baba et al., 1995) greatly dampened enthusiasm for pursuing development of such vaccines for use in humans. Despite this, live vaccine studies in macaques are still being pursued using SIV and SHIV vaccines (Amara et al., 2005; Desrosiers, 1998; Johnson and Desrosiers, 1998; Johnson et al., 1997; Koff et al., 2006) because such vaccines have proven to be efficacious in adult animals and still safe in young animals even after considerable effort to bring out the pathogenic potential of such viruses . Such efforts have included IV inoculation of animals at birth and observation for more than six years (Smith, unpublished observation), IC inoculation (Smith et al., 2002) and serial passage of the virus in young animals (Mackay et al., 2002) all without pathological effects.
Deletion of accessory genes from pathogenic SIV provided the first method for development of live vaccines against macaque lentiviral pathogens (Daniel et al., 1992; Johnson and Desrosiers, 1998; Wyand et al., 1999; Wyand et al., 1996). Use of this concept has been exploited for development of SIV and later, safe SHIV vaccines (Abel et al., 2003; Amara et al., 2005; Desrosiers, 1998; Hu, 2005; Joag et al., 1998b; Johnson and Desrosiers, 1998; Johnson et al., 1997; Koff et al., 2006; Silverstein et al., 2000). The process resulted in development of viruses that have attenuated replication potential and pathogenicity, thereby making them candidates for live virus vaccines against AIDS. These SHIV agents have been used to test proof of concept hypotheses about mechanisms of vaccine-induced protection, identification of immunological correlates or protection, and duration of the protection following immunization (Joag et al., 1998b; Johnson et al., 1997; Kumar et al., 2002; Wyand et al., 1999). Studies on the SHIV vaccines showed that the viruses replicated productively for a few weeks, after which they persisted in a minimally productive state for an indefinite period (Joag et al., 1998b; Kumar et al., 2002; Mackay et al., 2004; Silverstein et al., 2000). Control of productive replication was associated with development of CMI responses (Johnson et al., 1997; Kumar et al., 2001; Kumar et al., 2002; Silverstein et al., 2000) similar to the responses associated with control of HIV in humans (Pantaleo and Koup, 2004).
In one of our previously reported studies (Joag et al., 1998b), we had immunized two cohorts of six macaques with ΔvpuΔnef SHIV-4 (Vacc-I) and ΔvpuSHIVppc (Vacc-II), respectively. The parental viruses from which the vaccines were derived varied greatly in replication competence in macaques, SHIV-4, being poor (Joag et al., 1996; Mackay et al., 2002), and SHIVppc, being highly competent. Vacc-I replicated poorly, similar to the parental virus, SHIV-4, and viral DNA was found in only 4 of the 6 animals six months later. Similar to its parent, SHIVppc, Vacc-II replicated vigorously and established latent infection in all six animals. The 12 animals were challenged vaginally 6 months after immunization with pathogenic SHIVKU1, a virus that underwent massive replication in non-immunized animals, and within two weeks, caused near complete elimination of CD4+T cells. Nearly all such animals developed fatal AIDS within one year (Joag et al, 1998b). The 2 animals in cohort 1 (Vacc-I group) that lacked vaccine viral DNA in PBMC developed a disease pattern similar to that seen in the control animals (Joag et al., 1998b). The remaining 10 immunized animals all became infected with the challenge virus as indicated by infectivity in PBMC and plasma, but this was only transient. Animals that lacked infectivity in PBMC still had cell-associated viral DNA (Silverstein et al., 2000) indicating that both vaccine and challenge viruses had established persistent low grade infections. The vaccine viruses had thus induced protection against acute disease but not against infection by the challenge virus. Control of pathogenic virus was associated with strong CMI responses. However, these responses waned with time, in concert with minimal replication of either virus. Studies on these animals for the next six years showed that the animals eliminated one or the other of their “latent” viral genomes at different time points varying from 1 year to 5 years. Elimination of pathogenic virus and retention of the vaccine virus left animals in a permanently healthy state (Silverstein et al., 2000; Mackay et al., 2004). However, elimination of the vaccine virus but retention of the pathogenic virus was invariably associated with activation of the pathogenic virus and onset of AIDS (Mackay et al., 2004).
In a second study (Kumar et al., 2002), we sought to determine whether macaques that were immunized first with Vacc-I, followed by immunization with Vacc-II, would develop a more limited period of productive replication of Vacc-II, but yet still benefit from the protection induced by Vacc-II, as had been seen in the study reported above (Joag et al., 1998b). This report (Kumar et al., 2002) showed that the immunized macaques did develop an extremely limited period of replication of Vacc-II, but when challenged 58 weeks later with a mixture of three viruses, SHIV89.6P, SHIVKU2 (homologous with the vaccine viruses), and SIVmac17E, all of the animals were protected against disease (Kumar et al., 2002). Thus, persistence of Vacc-II virus must have been responsible for protection against disease.
In the present study, we focused the question on duration of protection following a single inoculation of replication-competent vaccine viruses, and asked whether the umbrella of protection induced by Vacc-II, and a derivative of Vacc-II, Vacc-III, would extend beyond three years. Vacc-II that had been used in the previous studies was used to inoculate four rhesus macaques, and Vacc-III, a replica of Vacc-II except that nef was deleted from the genome, was used to inoculate four other rhesus macaques. Replication-competence and safety of the Vacc-III virus was confirmed in earlier studies (Mackay et al., 2002; Smith et al., 2002). None of the animals were protected when challenged at three years with the three viruses described above. This established a new principle with expectations from live vaccine viruses. Classically, these types of vaccines have been known to confer life-long protection against disease. In the case of lentiviruses such as HIV however, protection from disease even by a safe and efficacious live vaccine may be of considerably shorter duration than expected.
RESULTS AND DISCUSSION
Following the observation that Vacc-II could induce protection for at least six months prior to challenge, we undertook another study in which we used a double immunization protocol of Vacc-I followed 4 months later by Vacc-II to ask three questions: (i) Would the productive replication of Vacc-II be modulated in macaques previously immunized with Vacc-I? (ii) Would Vacc-II induce the same level of protection in these animals as it had in the earlier study reported by Joag? (Joag et al., 1998b) and (iii) Would the induced protection extend to heterologous viruses such as SHIV89.6P and SIVmac17E? Studies on the animals prior to challenge showed that replication of Vacc-II was greatly restricted, but the animals nevertheless developed potent CMI responses that were still present at the time of challenge 58 weeks later. The pathogenic viruses had replicated transiently followed by control that lasted 74 weeks (Kumar et al., 2002). Having shown that the Vacc-I/Vacc-II protocol could induce protection for as long as one year, we now sought to determine whether a single inoculation with replication competent vaccine virus, Vacc-II and Vacc-III, (Vacc-III being Vacc-II that had been attenuated further by deletion of nef) would induce protection that would last at least three years.
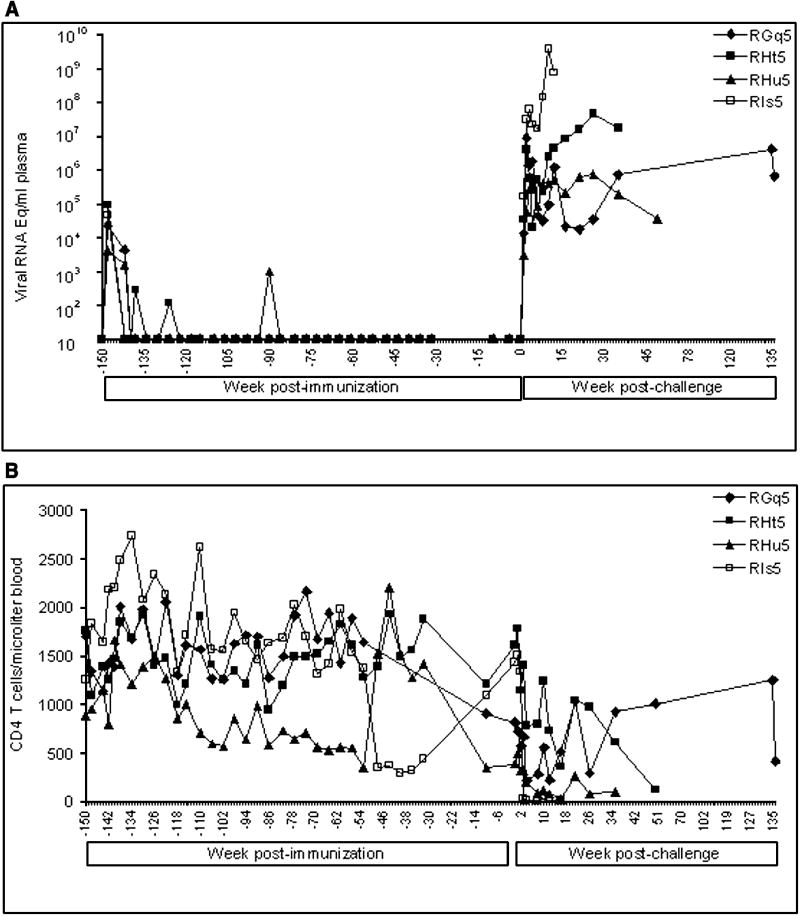
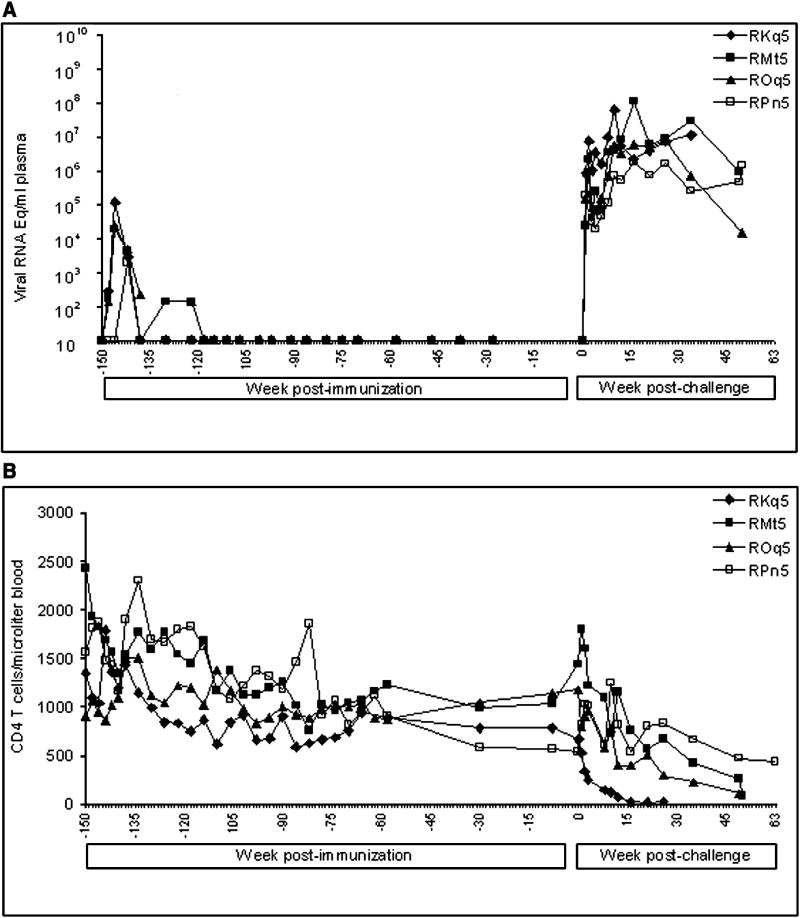
In this new study, we inoculated four rhesus macaques orally with Vacc-II and another four with Vacc-III. These animals, along with two non-immunized macaques, were then challenged rectally three years after immunization with the same three viruses that had been used in the previously reported study (Kumar et al., 2002). Plasma viral RNA loads and CD4 T cell counts in these animals are shown in Fig-1 and 2, respectively. Both vaccine viruses replicated productively in all eight macaques. Peak viral RNA concentrations, ranging between 2×103 and 1.2×105 RNA copies/ml plasma, were established by four weeks post inoculation. The virus replication then became undetectable in plasma within 3 months after administration of Vacc-II or III. However, sporadic spurts of virus replication evidenced by presence of viral RNA in plasma were seen occasionally in some of the animals during the first year following immunization. At the time of challenge at three years, none of the animals had vaccine virus RNA in plasma (Fig-1 and 2), and PBMCs lacked antiviral T cells measured by ELISPOT (Table-I). The animals were challenged rectally with the three-virus combination at Year 3. After pathogenic challenge, the virus-specific T cells became detectable in peripheral circulation within a week suggesting development of virus-specific cellular immune response. However, we can not rule out the possibility of these cells being present in secondary lymphoid organs at the time of challenge which could have migrated to peripheral circulation after challenge.
FIG. 1.
Plasma viral RNA concentrations and CD4+ T cell counts in macaques immunized with Vacc-II and challenged with the three virus cocktail of SHIVKU-2, SHIV89.6P, and SIVMACR71/17E. Plasma samples were collected at different time points and total viral RNA concentrations was measured in real time RT-PCR using SIVgag primers. The viral RNA concentrations are expressed as RNA copy numbers per milliliter of plasma. The CD4+ T cell counts were monitored by staining whole blood with a mixture of MAbs directed against CD3, CD4, and CD8 molecules. The absolute number of CD4+ T cells/μl of blood was calculated by multiplying the percentage of the lymphocyte subset with the absolute number of lymphocytes/μl of blood from complete blood count (CBC).
FIG. 2.
Plasma viral RNA concentrations and CD4+ T cell counts in macaques immunized with Vacc-III and challenged with the three virus cocktail of SHIVKU-2, SHIV89.6P, and SIVMACR71/17E. Measurements identical to those summarized in Figure 1 were used here.
Table-I.
Virus-specific cellular response in macaques immunized with Vac-IIa or Vac-III and challenged with cocktail virus
| Macaque | Virus-specific T cells/106 PBMC on weeks post-challenge |
||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 3 | 8 | 12 | 16 | 21 | |
| Vac-II | |||||||
| RGq5 | 0 | 10 | 235 | 530 | 760 | 1070 | 295 |
| RHt5 | 0 | 220 | 20 | 30 | 110 | 75 | 0 |
| RHu5 | 0 | 25 | 125 | 130 | 145 | 280 | 15 |
| RIs5 | 0 | 15 | 125 | 35 | 5b | ||
| Vacc-III | |||||||
| RKq5 | 0 | 385 | 135 | 230 | 100 | 110 | 25 |
| RMt5 | 0 | 35 | 425 | 85 | 160 | 415 | 540 |
| ROq5 | 0 | 45 | 225 | 70 | 45 | 240 | 170 |
| RPn5 | 0 | 70 | 485 | 350 | 205 | 325 | 215 |
The ELISPOT results are presented as number of virus-specific cells/million PBMC
The animal was euthanized at week 14, post-challenge
Following rectal infusion of the three challenge viruses into the eight vaccinated and two unvaccinated animals, systemic infections became established in all of the animals as evidenced by detection of viral RNA in plasma (Fig-1 and 2). Unlike our previous study where all vaccinated animals eventually controlled virus replication at one point or other (Kumar et al., 2002), the pathogenic viruses in this study continued to replicate in animals in both vaccine groups until the animals progressed to clinical disease, at which time they were euthanized (Fig-1 and 2).
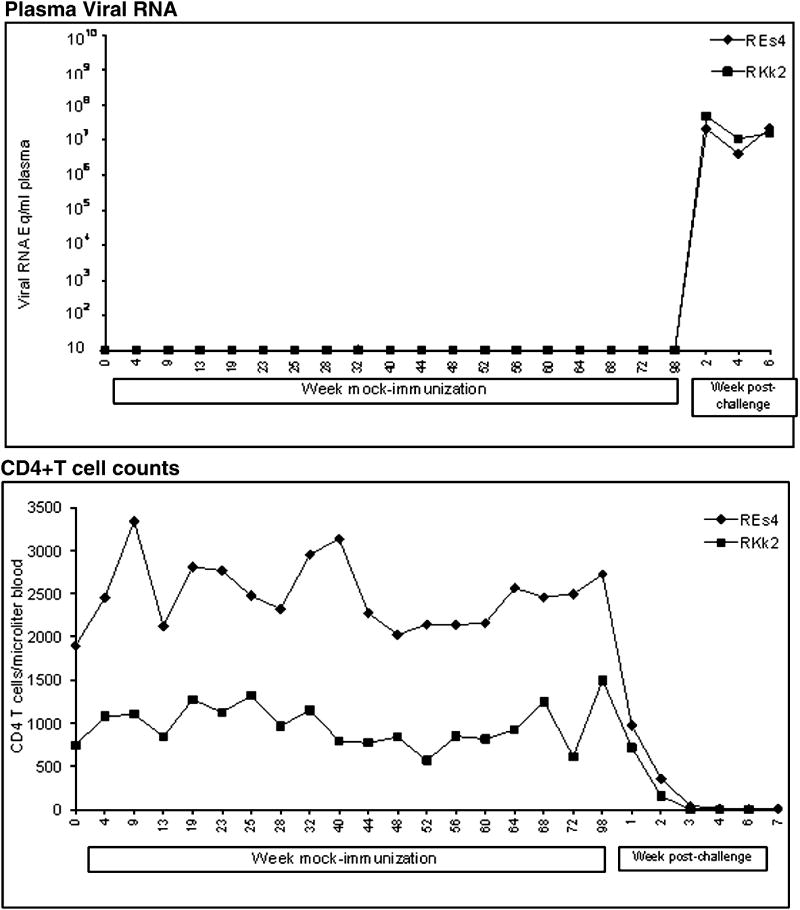
The animals were monitored for the rapidity of development of SIV/SHIV-induced AIDS. The control animals progressed to clinical AIDS and lost >25% body weight within 6 weeks after challenge. Both animals developed neurological signs (hyper-reactivity, movement-coordination, hand tremor and balance). These animals showed high virus replication and massive CD4 loss in the blood (Fig-3). They were euthanized at weeks 6 and 7 and showed meningitis, mild encephalitis, and severe depletion of CD4+ T cells from lymphoid tissues (data not shown). On the other hand the animals in both vaccine groups survived for more than one year (Fig-1 and 2), suggesting that the vaccines had induced a measure of protection against clinical disease. Compared to the virus control animals, the challenged vaccinates had an increased survival time by almost 9-fold.
FIG. 3.
Viral RNA concentrations and CD4+ T cell counts in two unvaccinated macaques that were challenged with the three-virus cocktail. Measurements were similar to those summarized in Figure 1.
In addition to developing high viral RNA concentrations in plasma, the animals developed progressive loss of CD4+ T cells. Examination of plasma by env-specific RT-PCR prior to necropsy of the animals showed that SIV was present in all animals, and that SHIVKU2 and SHIV89.6 were present in only some of the animals (Table-III). Examination of tissues from the animals showed that there was no trace of vaccine viral DNA [truncated vpu (Silverstein et al., 2000)], except for one macaque, RKq5, that had vaccine viral DNA in the brain (Table-II). Analysis of tissues exemplified in studies on RKq5 and RMt5 showed a wide distribution of the three viruses which were identified by env-specific sequences of the three viruses (Table-II). There was no particular pattern of distribution of the three viruses. However, in general, lymph node and spleen were specifically targeted by the three viruses, compared to the CNS, where there was minimal infection.
Table-III.
Persistence of challenge viruses in the plasma of challenged macaques
| Animal name | SHIVKU-2 | SHIV89.6P | SIV17E/71BR |
|---|---|---|---|
| RGq5 | - | - | + |
| RHt5 | + | - | + |
| RHu5 | - | - | + |
| RIs5 | + | + | + |
| RMt5 | - | + | + |
| RKq5 | + | + | + |
| RPn5 | - | - | + |
| ROq5 | - | + | + |
The presence of individual challenge virus in the plasma of macaques at the time of death
Table-II.
Viral DNA distribution in plasma, lung, spleen and 3 brain regions of macaques immunized with Vac-III and challenged with pathogenic SHIVs and SIV
| Macaque RKq5 | Challenge vpu | Vaccine vpu | KU2 env | 89.6 env | SIV 17E env |
|---|---|---|---|---|---|
| Virus in plasma | not done | not done | + | + | + |
| Lung | + | - | + | + | + |
| Spleen | + | - | - | + | + |
| Lymph Node | + | - | + | + | + |
| Occipetal Cortex | - | - | - | - | - |
| Parietal Cortex | + | + | - | - | - |
| Basal Ganglia | + | + | - | - | - |
| Macaque RMt5 | Challenge vpu | Vaccine vpu | KU2 env | 89.6 env | SIV 17E env |
| Virus in plasma | not done | not done | - | + | + |
| Lung | + | - | - | - | - |
| Liver | + | - | + | - | + |
| Spleen | + | - | + | + | + |
| Lymph Node | + | - | - | + | + |
| Occipetal Cortex | - | - | - | - | - |
| Parietal Cortex | - | - | - | - | - |
| Basal Ganglia | - | - | - | - | + |
In summary, our findings indicated that the transient productive infection caused by a single inoculation of the live attenuated vaccine viruses failed to induce protection against disease at three years. In earlier studies, protection correlated with continuous sporadic bursts of virus replication that presumably contributed to maintenance of protective CMI responses (Daniel et al., 1992; Daniel et al., 1985; Desrosiers, 1998; Evans et al., 2005; Gauduin et al., 2006; Johnson and Desrosiers, 1998; Johnson et al., 1997). In the animals reported in this study, there was no evidence of such bursts of virus replication between Years 2 and 3. In an earlier report (Mackay et al., 2004), we have previously shown that elimination of the vaccine virus was associated with activation of pathogenic virus. In the present study, lack of persistence of the vaccine virus in the plasma and cell-mediate immune response in the blood could have been a possible reason for the susceptibility of the animals to disease.
MATERIALS AND METHODS
Viruses
The construction of Vacc-II, Vacc-III and derivation of SHIVKU-2 have been previously described (Joag et al., 1998a; Kumar et al., 2002; Mackay et al., 2002). Vaccine-II was derived from a non-pathogenic virus SHIVPPC by deleting 60 bp from vpu (Joag et al., 1996) whereas Vacc-III was derived by deleting vpu and nef from SHIVPPC (Mackay et al., 2002). SHIVKU-2 was derived from non-pathogenic SHIV-4 first by passaging into pigtailed macaques (Joag et al., 1996) followed by passage in rhesus macaques (Joag et al., 1998a). SHIV89.6P was kindly provided by Dr. N. Letvin (Reimann et al., 1996a). SIVmacR71/17E is a M tropic and neurovirulent virus (Sharma et al., 1992). Stock preparations of these viruses were propagated in macaque mitogen-stimulated PBMC and viral titers were determined in C8166 cells.
Vaccination of macaques and challenge with SHIVKU-2, SHIV89.6P, and SIVmacR71/17E
Ten 3-5 year old rhesus macaques (Macaca mulatta) were obtained and maintained in the AAALAC-approved Animal Facility of the first in Yerkes Primate Center, Atlanta, GA followed by transportation to University of Kansas Medical Center where they were housed in AAALAC-approved facility for post-challenge observation. These animals were divided into 3 groups of 4, 4 and 2 macaques. First 2 groups were orally inoculated with 1 ml of Vacc-II and III, respectively. Third group of 2 animals were administered equal volume Hank’s buffered salt solution (HBSS). The vaccinated and control animals were challenged twice, one day apart, by intrarectal route with a mixture of undiluted SHIVKU-2, SHIV89.6P, and SIVmacR71/17E stocks. The Vacc-II and Vacc-III animals were challenged 148 and 140 weeks post-immunization, respectively. The animals were bled regularly to monitor CD4+ T cell profiles and plasma viral loads.
Flow cytometry
Lymphocyte subset cell profiles were determined by staining for CD3, CD4 and CD8 cell surface markers using the whole blood lysis technique (Wyand et al., 1996). Briefly, 10 μl of the antibody mix against CD3, CD4, and CD8 (Becton Dickinson) was added to 100 μl of whole blood and incubated for one hour in the dark. Lysing solution (Becton Dickinson) was then added and the samples were incubated for another 10 min at room temperature. Stained cells were fixed with 0.5% paraformaldehyde and analyzed in a flow cytometer (Becton Dickinson FACS Calibur).
Total viral load measurements
Plasma viral RNA levels were determined by real-time reverse transcriptase PCR (ABI Prism 7700-sequence detection system, Perkin-Elmer) essentially as described previously (Mackay et al., 2002). Briefly RNA samples were subjected to real time RT-PCR using gag primers and a 5’-FAM- and 3’-TAMARA- labeled Taqman probe homologous to SIVmac239 gag, which is identical in both vaccine and 3 challenge viruses. Viral RNA copy numbers were calculated per ml plasma.
Differential detection of challenge virus in plasma of vaccinated macaques
Total RNA was extracted from plasma samples collected at the time of necropsy and used as template in a RT-PCR reaction using primers and conditions described previously (Kumar et al., 2002). Following the second round of amplification, a 5-μl aliquot was electrophoresed on a 1.5% agarose gel, and the bands visualized by staining with ethidium bromide. Different proviral DNA were identified using a set of primers specific for vaccine and different challenge virus. The primer sequence and PCR condition has been reported in our earlier publication (Kumar et al., 2002).
ELISPOT Assay
We measured viral antigen-specific cells in PBMC in an ELISPOT assay using their ability to secrete IFN-γ. The assay was performed on weeks 0, 1, 3 8, 12, 16 and 21 after pathogenic challenge. This assay used overlapping peptides encompassing the full length HIV Env (Cat no. 6451), Tat (Cat no. 5138) and Rev (Cat no. 6445) as well as SIVmac239 Gag (Cat no. 6204) and Nef (Cat no. 6206) proteins, provided by NIH AIDS Research and Reference Reagent Program. Env and Gag peptides were dissolved into 10 and 5 pools respectively whereas other 3 set of peptides were dissolved into individual pools with final concentration of 1 mg/ml. The peptide stocks were stored at -80°C. High binding Immobilon-P plates (Billerica, MA) were coated overnight at 4°C with 50 μl/well of 5 μg/ml of anti-monkey IFN-γ Abs (mAb G2-4, Mabtech Stockholm, Sweden). The unbound antibodies were removed next by washing 4 times with PBS. The wells were blocked with 10% fetal bovine serum (FBS). Fifty μl mixture of 5 peptide pools were added to each well except positive and negative control wells that received Con-A and FBS, respectively. 105 PBMC were added to each wells in triplicate for 6 hrs incubation at 37°C, followed by washing with PBS-Tween. The wells were incubated again with another anti-monkey biotinylated IFN-γ Abs (7-B6-1, Mabtech Stockholm, Sweden). The wells were then washed and 50 μl of Vectastain (Vector Laboratories, Buringame, CA) was added. The reaction was developed using 100 μl/well Nova-Red for 4 minutes. The plate was washed with tap water and spots were counted using a stereomicroscope. The negative controls (un-stimulated PBMC in the assay) showed 1-3 positive spots every time. These numbers were subtracted from total number of spots obtained in the wells containing peptide-stimulated PBMC.
Acknowledgments
We thank Zhuang Li for help in animal studies and Istvan Adany in flow cytometry. This work was supported by Public Health Service grants NS040238, RR006753, AI051220, P20RR016443, DA015013 and P51RR000165.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel K, Compton L, Rourke T, Montefiori D, Lu D, Rothaeusler K, Fritts L, Bost K, Miller CJ. Simian-human immunodeficiency virus SHIV89.6-induced protection against intravaginal challenge with pathogenic SIVmac239 is independent of the route of immunization and is associated with a combination of cytotoxic T-lymphocyte and alpha interferon responses. J Virol. 2003;77(5):3099–118. doi: 10.1128/JVI.77.5.3099-3118.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amara RR, Patel K, Niedziela G, Nigam P, Sharma S, Staprans SI, Montefiori DC, Chenareddi L, Herndon JG, Robinson HL, McClure HM, Novembre FJ. A combination DNA and attenuated simian immunodeficiency virus vaccine strategy provides enhanced protection from simian/human immunodeficiency virus-induced disease. J Virol. 2005;79(24):15356–67. doi: 10.1128/JVI.79.24.15356-15367.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba TW, Jeong YS, Pennick D, Bronson R, Greene MF, Ruprecht RM. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science. 1995;267(5205):1820–5. doi: 10.1126/science.7892606. [DOI] [PubMed] [Google Scholar]
- Daniel MD, Kirchhoff F, Czajak SC, Sehgal PK, Desrosiers RC. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science. 1992;258(5090):1938–41. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- Daniel MD, Letvin NL, King NW, Kannagi M, Sehgal PK, Hunt RD, Kanki PJ, Essex M, Desrosiers RC. Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science. 1985;228(4704):1201–4. doi: 10.1126/science.3159089. [DOI] [PubMed] [Google Scholar]
- Desrosiers RC. Will there be a live-attenuated HIV vaccine available for human safety trials by the year 2000? Interview by Gordon Nary. J Int Assoc Physicians AIDS Care. 1998;4(11):22–3. [PubMed] [Google Scholar]
- Douek DC, Picker LJ, Koup RA. T cell dynamics in HIV-1 infection. Annu Rev Immunol. 2003;21:265–304. doi: 10.1146/annurev.immunol.21.120601.141053. [DOI] [PubMed] [Google Scholar]
- Evans DT, Bricker JE, Sanford HB, Lang S, Carville A, Richardson BA, Piatak M, Jr, Lifson JD, Mansfield KG, Desrosiers RC. Immunization of macaques with single-cycle simian immunodeficiency virus (SIV) stimulates diverse virus-specific immune responses and reduces viral loads after challenge with SIVmac239. J Virol. 2005;79(12):7707–20. doi: 10.1128/JVI.79.12.7707-7720.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauduin MC, Yu Y, Barabasz A, Carville A, Piatak M, Lifson JD, Desrosiers RC, Johnson RP. Induction of a virus-specific effector-memory CD4+ T cell response by attenuated SIV infection. J Exp Med. 2006;203(12):2661–72. doi: 10.1084/jem.20060134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu SL. Non-human primate models for AIDS vaccine research. Curr Drug Targets Infect Disord. 2005;5(2):193–201. doi: 10.2174/1568005054201508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi T, Endo Y, Englund G, Sadjadpour R, Matano T, Buckler C, Buckler-White A, Plishka R, Theodore T, Shibata R, Martin M. Emergence of a highly pathogenic simian/human immunodeficiency virus in a rhesus macaque treated with anti-CD8 mAb during a primary infection with a nonpathogenic virus. Proc Natl Acad Sci U S A. 1999;96(24):14049–54. doi: 10.1073/pnas.96.24.14049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joag SV, Li Z, Foresman L, Stephens EB, Zhao LJ, Adany I, Pinson DM, McClure HM, Narayan O. Chimeric simian/human immunodeficiency virus that causes progressive loss of CD4+ T cells and AIDS in pig-tailed macaques. J Virol. 1996;70(5):3189–97. doi: 10.1128/jvi.70.5.3189-3197.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joag SV, Li Z, Wang C, Jia F, Foresman L, Adany I, Pinson DM, Stephens EB, Narayan O. Chimeric SHIV that causes CD4+ T cell loss and AIDS in rhesus macaques. J Med Primatol. 1998a;27(23):59–64. doi: 10.1111/j.1600-0684.1998.tb00227.x. [DOI] [PubMed] [Google Scholar]
- Joag SV, Liu ZQ, Stephens EB, Smith MS, Kumar A, Li Z, Wang C, Sheffer D, Jia F, Foresman L, Adany I, Lifson J, McClure HM, Narayan O. Oral immunization of macaques with attenuated vaccine virus induces protection against vaginally transmitted AIDS. J Virol. 1998b;72(11):9069–78. doi: 10.1128/jvi.72.11.9069-9078.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RP, Desrosiers RC. Protective immunity induced by live attenuated simian immunodeficiency virus. Curr Opin Immunol. 1998;10(4):436–43. doi: 10.1016/s0952-7915(98)80118-0. [DOI] [PubMed] [Google Scholar]
- Johnson RP, Glickman RL, Yang JQ, Kaur A, Dion JT, Mulligan MJ, Desrosiers RC. Induction of vigorous cytotoxic T-lymphocyte responses by live attenuated simian immunodeficiency virus. J Virol. 1997;71(10):7711–8. doi: 10.1128/jvi.71.10.7711-7718.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston MI, Fauci AS. An HIV vaccine--evolving concepts. N Engl J Med. 2007;356(20):2073–81. doi: 10.1056/NEJMra066267. [DOI] [PubMed] [Google Scholar]
- Koff WC, Johnson PR, Watkins DI, Burton DR, Lifson JD, Hasenkrug KJ, McDermott AB, Schultz A, Zamb TJ, Boyle R, Desrosiers RC. HIV vaccine design: insights from live attenuated SIV vaccines. Nat Immunol. 2006;7(1):19–23. doi: 10.1038/ni1296. [DOI] [PubMed] [Google Scholar]
- Kumar A, Lifson JD, Li Z, Jia F, Mukherjee S, Adany I, Liu Z, Piatak M, Sheffer D, McClure HM, Narayan O. Sequential immunization of macaques with two differentially attenuated vaccines induced long-term virus-specific immune responses and conferred protection against AIDS caused by heterologous simian human immunodeficiency Virus (SHIV(89.6)P) Virology. 2001;279(1):241–56. doi: 10.1006/viro.2000.0695. [DOI] [PubMed] [Google Scholar]
- Kumar A, Mukherjee S, Shen J, Buch S, Li Z, Adany I, Liu Z, Zhuge W, Piatak M, Jr, Lifson J, McClure H, Narayan O. Immunization of macaques with live simian human immunodeficiency virus (SHIV) vaccines conferred protection against AIDS induced by homologous and heterologous SHIVs and simian immunodeficiency virus. Virology. 2002;301(2):189–205. doi: 10.1006/viro.2002.1544. [DOI] [PubMed] [Google Scholar]
- Lassen K, Han Y, Zhou Y, Siliciano J, Siliciano RF. The multifactorial nature of HIV-1 latency. Trends Mol Med. 2004;10(11):525–31. doi: 10.1016/j.molmed.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Luciw PA, Pratt-Lowe E, Shaw KE, Levy JA, Cheng-Mayer C. Persistent infection of rhesus macaques with T-cell-line-tropic and macrophage-tropic clones of simian/human immunodeficiency viruses (SHIV) Proc Natl Acad Sci U S A. 1995;92(16):7490–4. doi: 10.1073/pnas.92.16.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay GA, Liu Z, Singh DK, Smith MS, Mukherjee S, Sheffer D, Jia F, Adany I, Sun KH, Dhillon S, Zhuge W, Narayan O. Protection against late-onset AIDS in macaques prophylactically immunized with a live simian HIV vaccine was dependent on persistence of the vaccine virus. J Immunol. 2004;173(6):4100–7. doi: 10.4049/jimmunol.173.6.4100. [DOI] [PubMed] [Google Scholar]
- Mackay GA, Niu Y, Liu ZQ, Mukherjee S, Li Z, Adany I, Buch S, Zhuge W, McClure HM, Narayan O, Smith MS. Presence of Intact vpu and nef genes in nonpathogenic SHIV is essential for acquisition of pathogenicity of this virus by serial passage in macaques. Virology. 2002;295(1):133–46. doi: 10.1006/viro.2002.1368. [DOI] [PubMed] [Google Scholar]
- Nickle DC, Shriner D, Mittler JE, Frenkel LM, Mullins JI. Importance and detection of virus reservoirs and compartments of HIV infection. Curr Opin Microbiol. 2003;6(4):410–6. doi: 10.1016/s1369-5274(03)00096-1. [DOI] [PubMed] [Google Scholar]
- Pantaleo G, Koup RA. Correlates of immune protection in HIV-1 infection: what we know, what we don’t know, what we should know. Nat Med. 2004;10(8):806–10. doi: 10.1038/nm0804-806. [DOI] [PubMed] [Google Scholar]
- Reimann KA, Li JT, Veazey R, Halloran M, Park IW, Karlsson GB, Sodroski J, Letvin NL. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J Virol. 1996a;70(10):6922–8. doi: 10.1128/jvi.70.10.6922-6928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann KA, Li JT, Voss G, Lekutis C, Tenner-Racz K, Racz P, Lin W, Montefiori DC, Lee-Parritz DE, Lu Y, Collman RG, Sodroski J, Letvin NL. An env gene derived from a primary human immunodeficiency virus type 1 isolate confers high in vivo replicative capacity to a chimeric simian/human immunodeficiency virus in rhesus monkeys. J Virol. 1996b;70(5):3198–206. doi: 10.1128/jvi.70.5.3198-3206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma DP, Zink MC, Anderson M, Adams R, Clements JE, Joag SV, Narayan O. Derivation of neurotropic simian immunodeficiency virus from exclusively lymphocytetropic parental virus: pathogenesis of infection in macaques. J Virol. 1992;66(6):3550–6. doi: 10.1128/jvi.66.6.3550-3556.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein PS, Mackay GA, Mukherjee S, Li Z, Piatak M, Jr, Lifson JD, Narayan O, Kumar A. Pathogenic simian/human immunodeficiency virus SHIV(KU) inoculated into immunized macaques caused infection, but virus burdens progressively declined with time. J Virol. 2000;74(22):10489–97. doi: 10.1128/jvi.74.22.10489-10497.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MS, Niu Y, Li Z, Adany I, Pinson DM, Liu ZQ, Berry T, Sheffer D, Jia F, Narayan O. Systemic infection and limited replication of SHIV vaccine virus in brains of macaques inoculated intracerebrally with infectious viral DNA. Virology. 2002;301(1):130–5. doi: 10.1006/viro.2002.1548. [DOI] [PubMed] [Google Scholar]
- Wyand MS, Manson K, Montefiori DC, Lifson JD, Johnson RP, Desrosiers RC. Protection by live, attenuated simian immunodeficiency virus against heterologous challenge. J Virol. 1999;73(10):8356–63. doi: 10.1128/jvi.73.10.8356-8363.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyand MS, Manson KH, Garcia-Moll M, Montefiori D, Desrosiers RC. Vaccine protection by a triple deletion mutant of simian immunodeficiency virus. J Virol. 1996;70(6):3724–33. doi: 10.1128/jvi.70.6.3724-3733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]