Abstract
Membrane (lipid) rafts and caveolae, a subset of rafts, are cellular domains that concentrate plasma membrane proteins and lipids involved in the regulation of cell function. In addition to providing signaling platforms for G-protein-coupled receptors and certain tyrosine kinase receptors, rafts/caveolae can influence redox signaling. This review discusses molecular characteristics of and methods to study rafts/caveolae, determinants that contribute to the localization of molecules in these entities, an overview of signaling molecules that show such localization, and the contribution of rafts/caveolae to redox signaling. Of particular note is the evidence that endothelial nitric oxide synthase (eNOS), NADPH oxygenase, and heme oxygenase, along with other less well-studied redox systems, localize in rafts and caveolae. The precise basis for this localization and the contribution of raft/caveolae-localized redox components to physiology and disease are important issues for future studies. Antioxid. Redox Signal. 11, 1357–1372.
Introduction
The plasma membrane of cells has two key functions: (a) separation, via a lipid/protein barrier, of the intracellular and extracellular environments, and (b) provision of a cellular domain that can sense changes in the external environment and initiate events that alter cellular physiology (i.e., cell transport, cell-to- cell communication, and signal transduction). An essential aspect of those functional responses is the localization of molecules within the membrane that recognize external stimuli and then communication of the recognition events to constituents located in the plasma membrane or other cellular compartments. Initial ideas regarding the plasma membrane were advanced by the description in the early 1970s of the fluid mosaic model (151), which envisaged that plasma membranes were akin to lipid seas with floating proteins. Although a useful conceptualization, the fluid mosaic model had several limitations in terms of explaining function of the plasma membrane: for example, it did not define the physical nature of the “mosaics” nor did it recognize the complexity of organization of certain constituents, such as those involved in signaling pathways. The model largely ignored the existence of caveolae (“little caves”), flask-like invaginations of the plasma membrane (Fig.1), which had first been described in the 1950s (108, 172). Indeed, caveolae, along with membrane (lipid) rafts, the latter being regions without membrane indentations but that akin to caveolae, are enriched in lipids such as cholesterol and glycosphingolipids and provide a platform in the floating sea that anchors membrane proteins. Such proteins can recognize external stimuli and in addition, can transduce signals to modulate cellular activity. In the early 1990s, it was appreciated that caveolae were a specialized version of membrane/lipid rafts that contain the protein caveolin (shown to be important for producing the membrane invagination) and that rafts and caveolae share numerous biochemical characteristics, including detergent insolubility and decreased density (17, 29, 49). These entities are thus specialized regions of the plasma membrane that contribute to certain aspects of cell physiology, including the compartmentation of signal transduction events. This review will focus on a description of molecular/biochemical characteristics of membrane rafts/caveolae, methods to study these entities, properties that determine the localization of molecules in these microdomains, an overview of signaling entities known to localize to rafts/caveolae, and the contribution of rafts/caveolae to redox signaling.
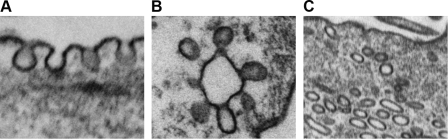
FIG. 1.
Electron microscopy of pulmonary artery smooth muscle cells depicting typical flask-like caveolar invaginations (A), grape-like clusters (B), or intracellular vesicles (caveosome, C).
Molecular/Biochemical Characteristics of Membrane Rafts/Caveolae
After the discovery of caveolae in the early 1950s (108, 172), studies over the next several decades were primarily limited to the ultrastructural assessment of the expression of caveolae or changes in their expression under various conditions. A substantial literature identified caveolae in a large number of cell types and changes in caveolae in pathologic settings (e.g., muscular dystrophy, hypertension, and ischemia) (1, 15, 103, 135, 136). Because they are not readily identifiable by microscopy, membrane/lipid rafts eluded such description. However, in the 1980–1990s plasma membrane rafts were recognized to share certain properties with caveolae. Filipin, a cholesterol marker, was shown to stain distinct, tightly clustered regions of plasma membranes, suggesting that such membranes are not homogenous mixtures of lipids, but instead, organized in a compartmental manner (145). Analysis of microsomes prepared from bovine luteal cell membranes revealed enrichment of sphingomyelin and cholesterol and decreased temperature sensitivity, results suggesting higher order organization of those membranes (42); such findings provided a conceptual framework for “lipid rafts” (67, 74).
More recent work involving the use of newer methodologies, including electrospray/mass spectrometric analysis, has revealed important difference in lipid composition in different portions of the plasma membrane (Table 1), such that the extracellular face is enriched in cholesterol, sphingolipids, and glycosphingolipids while ethanolamine-containing glycerophospholipids are predominantly found on the cytoplasmic face of the plasma membrane (121); differences in lipid profiles can be accentuated by methods used to isolate rafts (i.e., use of detergent vs. nondetergent methods) and rafts were shown to contain biologically active lipids and precursors such as arachidonic acid (121, 124). Rafts in polymorphonuclear leukocytes (PMN) contain lactosylceramide, a neutral glycosphingolipid, that helps regulate superoxide generation (60, 174). A key limitation of “component” analyses is the inability of the methods to unequivocally differentiate between lipid rafts and caveolae, since most methods used to study rafts and caveolae have been based on exploiting properties that depend on their (similar) lipid compositions. However, despite this limitation, considerable data reveal that not all portions of the plasma membrane are equivalent and that this inequality has functional consequences. Expression of these lipid entities is not restricted to mammalian cell membranes: plant membranes have such domains and these can be modified in their lipid content by outside stimuli (93). Such findings underscore the widespread expression of these membrane regions and their contribution to the regulation of cellular and organismal function.
Table 1.
Lipids Enriched in Membrane Rafts and/or Caveolae
| Cholesterol |
| Sphingomyelin |
| Glycosphingolipids |
| Ethanolamine glycerophospholipids |
| Phosphotidylserine |
| Arachidonic acid |
| Phosphatidylglucoside |
| Ceramide |
| Lactosylceramide |
As noted above, caveolae are considered to be a subset of lipid/membrane rafts and are 50–100 nm flask-like invaginations of the plasma membrane. Lipid rafts cannot be identified separately from other portions of the plasma membrane; their size is unclear and rafts of different sizes may exist: biochemical assessments of the diffusion of glycosylphosphatidylinositol (GPI)-linked proteins (68, 144, 147) have estimated a size between 100 and 200 nm. Subpopulations of rafts have been proposed, in part based on their size, constituents, and functional properties (92, 117). Rafts and caveolae are dynamic entities, forming and dissipating in response to various stimuli (159) and as a consequence of internalization, a clathrin (coated pit)-independent mechanism of endocytosis of plasma membrane constituents. Raft/caveolae-mediated endocytosis can facilitate transport of entities to other cellular regions and across the cell (transcytosis) (38, 91, 96, 101). Co-expression of the proteins flotillins 1 and 2 in rafts/caveolae may enhance the accumulation of intracellular vesicles and this assembly may be a common feature of rafts and caveolae (32). Caveolins can be secreted into the extracellular space and thereby, contribute to the regulation of survival and growth of tumors (7, 156, 157). Further studies are needed to determine the triggers and functional activities of caveolae and raft components in intracellular and extracellular locations.
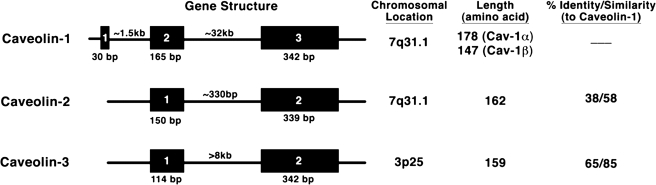
Caveolins (cavs) are structural proteins that provide an important, defining feature of caveolae and were initially thought to be specific markers of caveolae (79, 94, 97). The three different isoforms of cavs (Cav-1, -2, and -3) (170) are differentially expressed in cells: Cav-3 is restricted to skeletal, cardiac, and some smooth muscle, Cav-1 is more ubiquitously expressed, while expression of Cav-2 generally parallels that of Cav-1. Cav-1 and -2 share similar chromosomal location (7q31.1) whereas Cav-3 is located on a different chromosome (3p25) (26, 152). The cavs are highly conserved among species (170) with Cav-1 and Cav-3 sharing 65% identity and 85% similarity in protein sequence (Fig. 2). Transcriptional regulation occurs via sterol response elements for Cav-1 and an interaction of myogenin and inhibitor of transcription-2 (Id2) for Cav-3 (9, 30). Cav monomers range in molecular weight from 18 kDa to 24 kDa, with Cav-3 being the smallest and Cav-1 the largest (25, 170); the monomers are organized into multimers in the plasma membrane (94, 138, 140, 167). Cav-1 is expressed as two different mRNA and protein species (α and β isoforms) that derive from the same gene but have alternate start sites: the α form is full-length and the β form has a truncated amino terminus (76, 141). The participation of these two variants in homo/hetero-oligomers and specific impact on functional activities of cavs/caveolae are not clear. Studies with siRNA or knockout mice have revealed insights regarding the role of different cavs in caveolae formation and physiology (Table 2).
FIG. 2.
Gene structure of the three caveolin isoforms (human) as well as chromosome location, amino acid length, and sequence identity/similarity among the isoforms.
Table 2.
Caveolin Knockout Phenotypes
| Cav-1 KO | Cav-3 KO |
|---|---|
| • Caveolae present in heart and skeletal muscle | • Caveolae present in all organs except heart and skeletal muscle |
| Cardiac | |
| • Cardiac hypertrophy | • Cardiac hypertrophy |
| • Ischemia/reperfusion injury | • Ischemia/reperfusion injury |
| • Extracellular matrix | |
| Vascular | |
| • Reduced aortic contractile tone | |
| • Impaired angiogenic response | |
| • Neointimal hyperplasia | |
| • Altered microvascular permeability due to tight junction changes | |
| Pulmonary | |
| • Lung remodeling | |
| • Constricted alveolar spaces | |
| • Pulmonary hypertension | |
| Urogenital | |
| • Impaired renal calcium absorption | |
| • Enlarged seminal vesicles | |
| • Bladder hypertrophy | |
| Cancer | |
| • High sensitivity to carcinogens | |
| • Increased tumor permeability and growth | |
| Endocrine- Metabolic | |
| • Lipid abnormalities | • Lipid abnormalities |
| • Impaired lipolytic activity; altered lipid droplet architecture | • Decreased glucose uptake |
| • Accelerated mammary gland development | |
| • Exercise tolerance due to restrictive lung disease | |
| • Decreased glucose uptake | |
| Skeletal | |
| • Muscle abnormalities with tubular aggregation | • Muscle abnormalities with loss of dystrophin complex |
| Neuronal | |
| • Neuronal injury with increased infarct volume and apoptosis | |
| • Behavioral and motor defects |
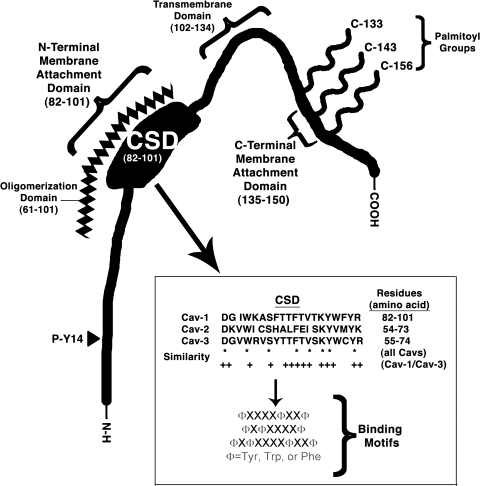
Caveolin proteins are highly structured and composed of four functional domains (Fig. 3). The caveolin scaffolding domain (CSD, amino acids 82–101 in Cav-1 and 55–74 in Cav-3) is a particularly important region within cavs because it is thought to mediate the interaction with various partners; the CSD contains consensus motifs comprised of aromatic ring-containing amino acids interspersed with other amino acids (16). By virtue of protein–protein interaction, which is thought to occur via hydrophobic–hydrophobic interaction between cavs and partners, cells enrich the expression of binding partners in caveolae. Cav-2 contains regions of similar homology, but unlike Cav-1 and Cav-3, does not have the ability to bind partners (16). An oligomerization domain (amino acids 61–101) partially overlaps with the CSD and facilitates oligomerization of cav monomers with one another (138). Cav-1 and -2 form hetero-oligomers and Cav-3 forms homo-oligomers or hetero-oligomers with Cav-1 (94, 138, 140, 167). The expression of Cav-1 and -3 appears to be mutually exclusive but the expression and localization of Cav-2 is dependent on Cav-1 (21, 109, 110, 112, 129). However, the expression of Cav-2 is not expendable: mice in which Cav-2 is deleted have a pulmonary hypertensive phenotype, even though such Cav-2 knockouts have similar expression of Cav-1 and formation of caveolae (130). Cavs are predicted to have a transmembrane domain (TMD, amino acids 102–134), which is a hydrophobic hairpin loop inserted in the cytoplasmic face of the plasma membrane that segments the carboxy and amino termini and makes both cytoplasmic (78, 170). Although it has been proposed that the TMD is not essential to membrane localization of cavs (18), other data indicate that amino and carboxy regions just proximal to the TMD and the CSD are key to such localization (142).
Fig. 3.
Domains on the caveolin (Cav-1) protein including sites of palmitoylation, C-terminal membrane attachment, transmembrane domain, N-terminal membrane attachment, caveolin scaffolding domain (CSD), oligomerization domain, and phosphorylation site. Inset details the CSD.
Cavs undergo covalent modification. Three C-terminal cysteine residues are each putative palmitoyalation sites (Cys133, 144, and 156) with Cys133 predominating; however, palmitoylation does not appear to be necessary for membrane localization and caveolae formation (20). Cav-1 (at Tyr14) is a phosphorylation target of the cellular oncogene Src (83, 134); Src-mediated phosphorylation alters the properties of Cav-1, including its interaction with extracellular matrix proteins (43, 44) and has other potential physiological functions (115). Recent data in our laboratory suggest that cavs are sumoylated (Fuhs and Insel, unpublished observations). Thus, cavs have a complex structure that influences their localization as well as their ability to bind various partners and to regulate cell function.
Studies of mice with knockout or transgenic overexpression of cavs implicate the expression of caveolin as both necessary and sufficient for the formation of caveolae. Recent studies have identified another protein, polymerase 1 and transcript release factor (PTRF, also called cavin), as a key component of caveolae. Electron microscopic and immunofluorescence studies indicate that this 60 kDa protein localizes in caveolae and is as abundant as caveolin in caveolar fractions (165). PTRF appears to be integral to the formation of caveolae: siRNA that decreases PTRF expression disrupts caveolae while expression of PTRF can be sufficient to form caveolae (54, 85). Other recent data reveal that exchange of cholesterol with its immediate precursor, desmosterol, changes the heterogeneity of caveolar structure, affinity of Cav-1 for sterols (i.e., less Cav-1 in caveolae) and the stability of Cav-1 oligomers (63). These data regarding PTRF and cholesterol–desmosterol exchange implicate factors other than cavs in the structure of caveolae. It will be of interest to determine how PTRF and cavs interact and whether one component is more important than the other in the formation of caveolae and in the regulation of cellular function including signal transduction events that localize to caveolae.
Approaches for the Study of Rafts and Caveolae
Use of appropriate methodology is critical for the assessment of rafts and caveolae. Indeed, some question the existence of rafts and caveolae as distinct cellular compartments (62, 80, 121). Others emphasize the heterogeneity of raft and caveolar domains (51, 92, 122). Several recent articles describe details for preparation of raft and caveolar microdomains (105, 113, 114). Table 3 summarizes several of these methods and their advantages and limitations.
Table 3.
Methodologies for the Study of Membrane Rafts and Caveolae
| Methods | Advantages | Disadvantages |
|---|---|---|
| Electron microscopy | Morphologically identifies caveolae High resolution with immunogold labeling |
Cannot identify lipid rafts Expensive, especially with use of gold labeling Time consuming Difficult to quantitate data |
| Immunofluorescence | Can co-localize proteins with rafts or
caveolae Can provide quantitative data |
Resolution is low because of the
50–200 nm length of
caveolae/rafts Relies on specificity of particular antibodies Unclear meaning of caveolins in noncaveolar locations |
| Immunoprecipitation | Can define specific binding partners (caveolin for caveolae; flotillin for rafts) | Relies on specificity of particular
antibodies Contamination with native antigen complicates analyses Problems with reproducibility and quantitation because of multiple wash steps |
| Density gradient fractionation | Separates rafts/caveolae from other portions of
membranes Inexpensive |
Cannot distinguish rafts from caveolae Detergent vs. nondetergent methods can yield different results |
| Proteomic methods | High-throughput Can characterize a broad spectrum of proteins found in rafts and caveolae |
Expensive Labor-intensive May be difficult to reproduce Need for large samples |
| Knockouts (KO) | Facilitates the study of whole animal/organ
function Commercial availability of Caveolin-1 KO mice |
Total body, lifetime loss of caveolins complicates data
interpretation Compensation may occur Difficult to obtain Caveolin-2 and Caveolin-3 KO mice No raft- KO is available |
| Pharmacologic agents (MBCD) | Inexpensive Easy treatment protocols |
Nonspecific (i.e, likely alters non-cav/raft membranes
and perhaps other cellular targets) Does not distinguish between rafts vs. caveolae |
The absence of a “gold standard” method to assess rafts and caveolae, and more specifically, the signaling components within them, has led to controversy regarding the validity of certain data and conclusions with respect to the role of rafts and caveolae in cell physiology, including signal transduction (58, 62, 80, 104, 106, 113, 114, 121, 125). An advantage of studying caveolae is that, unlike rafts, which are flat structures within membranes, caveolae have a distinct morphology that is readily identifiable by electron microscopy and more equivocally (given the <100 nm size of caveolae) by other microscopic techniques, for example, anti-caveolin antibodies and immunofluorescence. In the latter case, investigators often equate the expression of cavs with caveolae, although noncaveolar expression of cavs occurs and varying results have been noted if different anti-caveolin antibodies are used (51, 153). Antibody staining of the B subunit of cholera toxin, an exotoxin from V. cholerae that binds to GM1 ganglioside, a lipid enriched in rafts/caveolae, is another approach used to assess raft domains (48).
Biochemical analyses of rafts and caveolae, isolated by using membrane fractionation and density gradient centrifugation, exploit the greater buoyancy of rafts/caveolae that results from their enrichment in particular lipids. Such fractionation methods are used after cellular disruption by one of a variety of techniques, which may include nonionic detergents (84, 105). Data derived from such methods do not distinguish rafts from caveolae, although use of marker proteins (e.g., cavs) assists in defining caveolae. Flotillins (also called reggies) are proteins that define a pool (likely membrane rafts) of noncaveolar membranes that can, akin to caveolae, undergo endocytosis (5, 148). Co-immunoprecipitation of cavs and cav-localized components is another biochemical approach for the identification and assessment of such components.
Analyses of raft/caveolae domains by proteomic and lipidomic methods have not yet been widely used to evaluate such domains in a large number of cell types but studies to date suggest that there are different classes of these microdomains. It has been proposed that one subset of rafts is enriched in cholesterol–sphingomyelin–ganglioside–Cav-1/Src/EGFR (termed the “chol-raft”) and involved in cell signaling but when dysregulated, can alter cell function, such as by promoting cell transformation and tumor progression. Another type of raft (the “cer-raft”) is enriched with ceramide–sphingomyelin–ganglioside–Fas ligand/Ezrin and promotes apoptosis (92, 117). Such ideas imply that subcellular compartments may have higher order organization that tailors functional events (such as signal transduction cascades) to specific cellular regions in a dynamic manner (e.g., (99, 166)). Proteomic approaches should aid in defining differences in the composition of raft/caveolae microdomains, mechanisms by which proteins enter, are retained, and in some cases, exit such domains and post-translational modifications of raft/caveolae proteins (154, 155).
Additional ways to assess caveolae and rafts are the use of pharmacological approaches, knockdown strategies and knockout mice (55, 58, 113). Pharmacological approaches include the use of compounds that decrease the content of lipids that are enriched in rafts/caveolae (e.g., cholesterol and glycosphingolipids). Methyl-β-cyclodextrin (MβCD) is commonly used to deplete membranes of cholesterol; studies with MβCD should include an important control: MβCD combined with cholesterol, so as to eliminate a role for other effects of MβCD (179). Statins, through their ability to block cholesterol biosynthesis by inhibiting HMGCoA reductase, provide another way to disrupt rafts/caveolae (e.g., (116)). It is intriguing to consider whether certain effects of statins, for example in cardiovascular disorders (46), result from disruption of rafts/caveolae, although firm evidence for this idea has not yet been provided.
Techniques that alter expression of cavs have provided substantial data regarding cell regulation by cavs/caveolae. One approach to decrease cav expression is the use of siRNA. Mice that have knockout of one or more of the three cavs provide in vivo systems to evaluate cavs and caveolae (55, 113). However, full body (non-cell type specific) and lifetime elimination of cavs in such knockouts may lead to erroneous conclusions regarding their contribution to functional activities (58, 98, 159).
Use of multiple techniques to assess rafts/caveolae is preferred, since no one approach is ideal and complementary results from more than one technique lends greater confidence to conclusions regarding localization of components, functional roles of rafts/caveolae, and their contribution in physiologic and pathophysiologic settings.
Determinants of Localization of Components in Membrane Rafts/Caveolae
A large number of proteins and lipids, including receptors and post-receptor components, localize in rafts and/or caveolae, but the precise determinants of this localization are not known. Certain G-protein-coupled receptors (GPCR, also known as heptahelical receptors or 7-transmembrane, 7-TM, receptors, based on their membrane topology), receptor tyrosine kinases (RTK), and post-receptor signaling components show patterns of localization in those microdomains that differ among different cell types. The mechanism(s) for such cell-specific expression patterns is/are not known. Possible explanations include:
Protein–protein interaction
Interactions of particular proteins might be favored because of charge, size, and/or steric factors, but why such factors should differ in a cell type-selective manner is not clear. For GPCR, the ability to form oligomers with different composition may contribute to such localization (101). It has been proposed that the localization of RTK in membrane microdomains is attributable to protein–protein binding that occurs via sequences in the extracellular domain of the receptors; for the EGFR a 60-amino acid region mediates targeting to rafts/caveolae (120, 171).
Lipid–protein interaction
Lipid composition is important for rafts and caveolae with differences among different cell types and in the nature of the lipids in different portions of the plasma membrane (111, 134). Although such differences might contribute to changes in localization of proteins during states of altered lipid composition, or perhaps to cell-specific patterns of localization, direct evidence for this idea has not been provided. Lipid modification of proteins, in particular palmitoylation and myristoylation, contribute, for example, to the localization of G-protein signaling components in raft/caveolae domains (69, 127, 131, 132).
Caveolin-associated proteins
Cav-associated proteins might also contribute to differences in the localization of signaling molecules. As noted above, the CSD, a hydrophobic region in the cytoplasmic amino terminal tail that interacts with protein “partners” through hydrophobic interactions, has been proposed as a—or perhaps the—critical region by which signaling proteins interact with cavs (8, 13), but it is difficult to fathom how such a ∼20 amino acid domain (especially because it also, at least in part, overlaps with the oligomerization domain) accommodates such a large number and array of proteins (see below). Are there other regions on cavs that bind signaling proteins or is there a supramolecular assembly, whereby multiple signaling proteins create a “caveolin signaling particle,” akin to protein complexes involved in other cellular events (transcription, translation, secretion, etc.)? Use of proteomic methods to analyze cav-bound proteins in cells treated under various experimental conditions should prove useful in defining the existence (or not) of “caveolin signaling particles” and in defining the full range of protein partners in rafts/caveolae as well as changes in the amount and nature of such partners that occur in physiological states, with drug treatments or in disease. Proteomic methods show that a large number of proteins localize to caveolae (6, 24, 31, 88, 154), either by their interaction with the CSD or localization in the lipid microenvironment of caveolae.
An Overview of Signaling Entities That Localize to Rafts/Caveolae
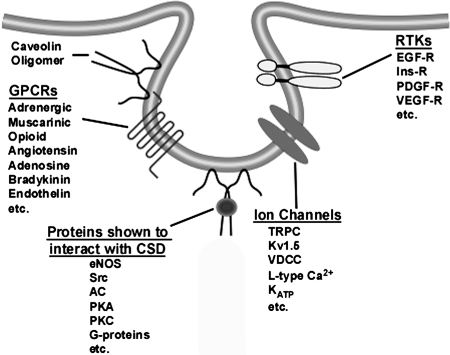
Many different types of molecular entities, including molecules involved in signal transduction, localize in raft and caveolar domains. The recent application of unbiased, comprehensive approaches (e.g., proteomics and lipidomics), has begun to reveal the full range of such entities (10, 31, 73, 88, 117, 155). Proteomic analyses suggest that many different classes of proteins are found in rafts/caveolae, including GPCR, RTK, and the most well-studied such protein, endothelial nitric oxide synthase [eNOS, NOS3 (22)]. Recent articles summarize findings related to signaling molecules in rafts/caveolae (59, 113, 114). Figure 4 describes some key examples. Future studies are expected to define the role of such localization in cell regulation by extracellular hormones, growth factors, etc., and differences in localization and functional roles of components in rafts vs. caveolae. The advantage of studying cav-localized components is that immunologic tools (e.g., antibodies that immunoprecipitate cavs and their partners) can be used to identify and isolate such components. Experimental techniques available for the study of rafts are limited and this limitation has led to rafts remaining controversial as entities in cells (62, 123); thus, future efforts that aid in the preparation of rafts should help in defining how the raft and caveolae “communities” differ.
FIG. 4.
Schematic depicting caveolae, caveolae–resident proteins, and proteins interacting with the caveolin scaffolding domain (CSD). AC, adenylyl cyclase; KATP, ATP-dependent potassium channel; eNOS, endothelial nitric oxide synthase; EGF-R, epidermal growth factor receptor; GPCR, G-protein coupled receptor; Ins-R, insulin receptor; PDGF-R, platelet-derived growth factor receptor; PKA, protein kinase A; PKC, protein kinase C; receptor tyrosine kinase (RTK); TRPC, transient receptor potential channel; VDCC, voltage-dependent calcium channel; VEGF-R, vascular endothelial growth factor receptor; Kv1.5, voltage-dependent potassium channel.
Impact of Rafts/Caveolae to Redox Signaling
It was once thought that the generation of reactive species (oxygen or nitrogen, ROS and RNS, respectively) resulted predominantly in damage to lipids, proteins, and DNA. However, with the discovery of the cellular production and actions of nitric oxide (NO) in the 1980s and more recently, of the signaling potential of molecules such as superoxide, hydroxyl radical, and carbon monoxide (CO), reactive species can be viewed in two distinct ways: either as nonspecific generation of such species or via a controlled burst of reactive molecules in a restricted space (such as caveolae or lipid rafts) that would have the potential for cellular regulation, such as by modifying signal transduction. The remainder of this review will focus on the regulation of localization and physiology of redox signaling by cavs/caveolae/lipid rafts. Specific enzyme systems, including nitric oxide synthase, NADPH oxidase, heme oxygenase, and certain less well studied enzymes, will be considered. We will also discuss the impact of association of cellular organelles with caveolae as a possible determinant in the generation of ROS and how these observations may relate to physiology and pathophysiology.
Endothelial Nitric Oxide Synthase (eNOS)
Among the binding partners of cavs, their interaction with eNOS has been the most extensively studied (37). Binding of eNOS to the CSD inhibits enzyme activity (41, 163). Regions on Cav-3 corresponding to the CSD on Cav-1 produce a similar suppression of eNOS activity, implying that this is a conserved binding and regulatory sequence motif in cavs (36). Loss of caveolin expression increases eNOS activity (128), consistent with the idea that the binding of eNOS to cavs negatively regulates this activity. The interaction of eNOS and cav, with enrichment in the microdomain associated with reduced enzyme activity, led to the “caveolar paradox,” whereby enrichment does not lead to enhanced activity (28). This paradox was resolved by evidence that eNOS is dually regulated: direct interaction with caveolin under basal conditions maintaining enzyme activity in an inactive state but enrichment of eNOS in caveolae providing a means for rapid, high-fidelity response upon stimulation (139).
Recent data have yielded new insights regarding the regulation of eNOS by cavs and caveolae (59); of particular note is the observation that endothelial-specific expression of eNOS and co-localization of eNOS with cavs in endothelial cells is important for NO-mediated vasodilation and decrease in blood pressure (27, 98). Recent data suggest that antioxidants such as resveratrol can enhance the generation of NO and that this enhancement depends upon the formation of an estrogen receptor alpha/Cav-1/c-Src complex that leads to increased phosphorylation and activity of eNOS in endothelial cells (75). Additionally, the flavonoid quercetin and wine polyphenols have been shown to improve endothelial function by enhancing the expression of Cav-1 and reducing the expression and activity of NADPH oxidase (and superoxide generation) in spontaneously hypertensive rats (86, 137). Local activation of eNOS can lead to S-nitrosylation of proteins (61) and eNOS can participate in the regulation of GPCR desensitization/internalization via S-nitrosylation of β-arrestin, whereby β-arrestin associates with eNOS, but upon agonist stimulation and NO generation the S-nitrosylation disassociates the eNOS-β-arrestin complex and targets the β- arrestin to clathrin (107). Such results suggest that the co-enrichment in microdomains of eNOS with signaling molecules may help to both initiate and turn off cellular signaling.
NADPH Oxidase (NOX)
NOX, a multicomponent enzyme system that generates ROS in the vasculature and immune system consists of membrane-bound (i.e., gp91phox [and its homologs in various cells: Nox1, Nox3, Nox4, and Nox5] and p22phox) and cytoplasmic subunits (i.e., p47phox, p67phox, and Rac1, a Rho-family GTP binding protein) (4, 12). NOX is thought to be inactive until cytoplasmic components are recruited to membrane raft domains, resulting in an active enzyme complex (161, 164). The NOX complex may be preassembled and functional in caveolae with enzyme activity enhanced by recruitment of additional components (173); an alternative suggestion is that the NOX complex is maintained in an inactive state in rafts but activated by disruption of rafts (47). Lipid rafts control the onset but not the maximal rate of NOX activity (146). Moreover, tobacco plants which when treated with cryptogein, a compound that initiates plant defense mechanisms, also recruit NOX to lipid rafts (93).
NOX plays an important role in cells of the immune system, PMN and macrophages. Proteinase 3, a serine protease, released by stimulated PMN localizes in lipid rafts with NOX complexes (33); this localization and NOX activity can be perturbed by MBCD, which, as noted above, removes cholesterol from raft domains (19). Mechanistic studies have revealed that interleukin-8 (IL-8) sequentially regulates the assembly of NOX in lipid rafts and primes the oxidant response to be activated by bacterial N-formyl peptides (fMLP): brief exposure to IL-8 (3 min) leads to a Bruton's tyrosine kinase (Btk) and extracellular regulated kinase (Erk 1/2)-mediated phosphorylation of p47phox and translocation of p47phox and Rac2 to lipid rafts; longer IL-8 incubation (15 min) enhances phosphorylation and translocation of p67phox to rafts, effects that treatment with MβCD attenuates (45). Mouse lungs perfused with fMLP-treated PMN prepared from Cav-1- knockout mice have reduced migration, adhesion, and generation of superoxide compared to PMN from wild-type mice (57). Exogenous expression of Cav-1 in COS-phox cells, COS7 cells engineered to express the NOX subunits, increases superoxide generation (57), further implicating Cav-1 in the regulation of NOX action.
Other cell types also localize NOX in lipid rafts/caveolae. In endothelial cells, various stimuli may form “lipid–raft platforms” that organize NOX components in ceramide-enriched lipid rafts (64, 82). Formation of such platforms can be initiated by cell death (pro-apoptotic) signals (i.e., Fas ligand, endostatin, and tumor necrosis factor alpha [TNFα]) and involves the assembly of larger complexes from individual raft units (177). In addition to pro-apoptotic signals, lipolysis of triglyceride-rich lipoproteins can promote aggregation of lipid rafts and enhance ROS production in endothelial cells, the latter effect being attenuated by NOX inhibitors (168). Cav-1 can serve as a sensor of shear stress in endothelial cells and as a consequence, regulate ROS-mediated signaling via NOX (90). Thus, numerous stimuli impact on rafts and modulate NOX in endothelial cells. In vascular smooth muscle Nox1 and Nox4 differentially localize to Cav-1-enriched regions and focal adhesions, respectively (53). In addition, Cav-1 appears to be necessary for Rac1 activation and subsequent activity of NOX in response to angiotensin II type 1 receptors (180). The numerous examples of caveolin–NOX interactions imply that such interactions are important for the regulation of cellular superoxide generation.
Heme Oxygenase (HO)
HO, which catalyzes the breakdown of heme to biliverdin, iron, and carbon monoxide (CO), exists as three membrane-bound isoforms: HO-1 which is inducible, HO-2 which is constitutively expressed, and HO-3 which is expressed but catalytically inactive (160). Among the products generated by HO, CO is of particular interest because it has signaling potential (70). CO was first shown to enhance long-term potentiation (149, 178) and later to be regulated by NO and potentially to modulate vascular function (23, 169). HO-1 in mesangial and endothelial cells interacts with Cav-1 and Cav-2 and localizes in caveolae in a manner regulated by various agents; akin to eNOS, HO-1 is negatively regulated by Cav-1 (66, 71). In vascular smooth muscle cells, loss of Cav-1 or HO-1 leads to increased neointimal formation and CO promotes antiproliferative effects through Cav-1, specifically its ability to activate p38 mitogen-activated protein kinase (72). Cav-1 KO mice are protected from hyperoxic damage in the lung as a consequence of increased expression and activity of HO-1, which is limited by Cav-1 expression (65). Such data suggest that cell-specific expression of cavs and interaction with partners, such as HO, helps determine responses that may be specific to a particular cell type.
Numerous enzymes that may interact with each other are housed in lipid rafts/caveolae, implying that such housing is likely not mutually exclusive. Compartmented generation of NO and superoxide by eNOS and NOX, respectively, may contribute to protein nitration on tyrosine residues; perturbation of raft/caveolae domains with cholesterol-sequestering agents dissociates those enzymes from the rafts and decreases their ability to generate reactive species and to nitrate proteins (173). In macrophages, HO-derived CO can prevent ROS-mediated recruitment of Toll-like receptor 4 (TLR-4) to lipid rafts by preferentially inhibiting NOX activity (100). Such data indicate an interplay among reactive species and their generating enzyme systems, which, at least in part, may derive from actions involving rafts/caveolae.
Less Well-Studied Redox Systems
Other pro- or anti-oxidant enzymes (e.g., superoxide dismutases, catalase, thioredoxin reductase, glutathione peroxidase, xanthine oxidase, and myeloperoxidase) have not been as well studied in terms of their interaction with caveolin and localization in lipid rafts/caveoale. One study suggested that xanthine oxidase-generated ROS is able to limit membrane localization of Cav-1 and affect eNOS activity (118). In vascular aging, caveolae-localized superoxide dismutase is decreased (162). Superoxide dismutase is also reported to be 6–9-fold concentrated in lipid raft domains of the pathogenic fungus Cryptococcus neoformans (150).
Recent data indicate that ROS balance may be critical for the formation and dissolution of rafts. In endothelial cells ROS activate acid sphingomyelinase to induce the formation and clustering of lipid raft redox platforms, an effect that is blocked by superoxide dismutase (176). In T lymphocytes, ROS promote the formation of rafts and this can be attenuated by scavenging of ROS (87). In hepatocytes, ethanol-induced oxidant stress has been shown to cluster lipid rafts, a response that can be reversed by antioxidants (102). Lipid peroxides can promote the generation of large rafts (3) and such an effect may help explain the clustering of membrane rafts observed in at least some of the studies described above. Additional data are required, especially in terms of defining the role of ROS and ROS modulating systems and of the regulation of cellular function by components within lipid rafts/caveolae. An additional system that requires further study in terms of rafts/caveolae is the generation and action of hydrogen sulfide (81) that can interact with redox systems and might be localized and regulated by rafts/caveolae.
Raft/Caveolae Association with Cellular Organelles
Recent data indicate that caveolae may form contacts with other cellular compartment so as to communicate membrane-derived signals to other parts of the cells. For example, smooth muscle cells can have “nanocontacts” between caveolae and the endoplasmic reticulum (40). Other data indicate that caveolins can be found in cells and intracellular regions that lack caveolae, suggesting roles for caveolins in addition to those that have been previously emphasized (51). Besides the oxidative enzyme systems described above, mitochondria are a major source of cellular oxidants, especially in the form of superoxide and peroxynitrite (39). Of note, mitochondria can be closely apposed to plasma membrane caveolae (personal observations, Fig. 5). Although the functional significance of such co-localization is not known, we hypothesize that caveolae localize certain enzymes that produce reductive species (e.g., eNOS/NO, HO/CO) that may modulate the function of mitochondria and/or that prevent plasma membrane action/damage from mitochondrial-derived oxidants. It is also possible that mitochondrially-generated ROS modulate raft/caveolar proteins, including proteins involved in signal transduction. Studies that investigate such possibilities will be of interest.
FIG. 5.
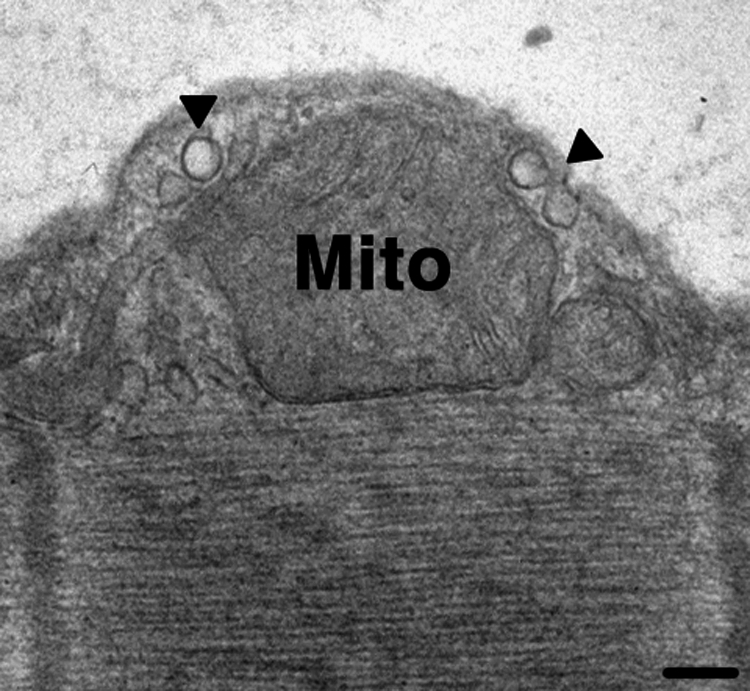
Electron micrograph showing the close apposition of caveolae (arrowheads) and mitochondria. Scale bar is equal to 100 nm.
Physiology and Pathophysiology of Lipid Raft/Caveolae-Associated Redox Signaling
Since many molecules that regulate cell function localize in lipid rafts and caveolae, rafts/caveolae can impact on numerous aspects of physiology and pathophysiology. Many reviews describe roles for rafts/caveolae in pathological settings (14, 35, 59, 89, 113). We focus our discussion on pathologies involving rafts/caveolae that are linked to redox signaling.
Native low-density lipoproteins (nLDL) can induce endothelial dysfunction; this dysfunction is associated with reduced interaction with heat shock protein 90 and nLDL-induced uncoupling of eNOS (126). In macrophages, oxidized LDL can derive from raft-derived ROS (via NOX), thereby increasing the potential for foam cell formation through oxidized LDL uptake and increased formation of ceramide-rich lipid rafts (143). Additionally, exposure of macrophages to high glucose concentration can increase oxidant stress, induce inducible NOS, and decrease Cav-1 expression and caveolae formation, ultimately resulting in increased apoptosis (50); apoptosis of macrophages in response to high glucose may lead to lipid deposition in vessel walls. Atherosclerotic plaque instability in humans is associated with reduced Cav-1 expression, suggesting that approaches to increase Cav-1 expression may be a means to enhance stabilization of such plaques (133).
The organization of rafts and caveolae in the vasculature has implications for pathophysiology in different types of hypertension (11, 59). Decreased expression of Cav and HO occurs in pulmonary hypertension in association with enhanced vascular cell proliferation and the development of hypertension (2). Similar effects have been observed in Cav-1 KO mice and attributed, at least in part, to a decrease in production of NO (109). However, endothelial-specific re-expression of Cav-1 can rescue the pulmonary hypertensive, as well as other cardiovascular abnormalities in Cav-1 KO mice (98). Other data indicate that unlike endothelial cells that have decreased cav/caveolae expression in primary pulmonary hypertension, pulmonary arterial smooth muscle cells have increased cav/caveolae expression, thus indicating that there are cell type-specific changes in this disease setting (116).
There is a decline of immune function with age that leads to a higher propensity for certain types of infections and secondary diseases. PMN from elderly subjects have a decline in receptor function as well as altered lipid raft expression (34), suggesting an association, and perhaps a causal relationship, between the expression of rafts and altered immune function in aging (158). In neurons, transmissible spongiform encephalopathies (i.e., prion diseases) result in neuronal loss due to accumulation of the prion protein PrP(Sc) and a recruitment of the prion protein/caveolin/Fyn (a Src isoform) complex that leads to hyperactivity of NOX and ROS-mediated injury (95, 119). These events may be the result of accumulation of protease-resistant N-terminal fragments of the prion protein that are associated with increased ROS and reduced superoxide dismutase and glutathione peroxidase (175). These observations suggest that altered caveolar/raft organization of redox signaling can impact on disease settings.
Recent studies have shown that cav and caveolae are integral for protection from neuronal ischemia and cardiac ischemic/reperfusion injury, two settings that involve ROS-induced injury. In neurons exposed to oxygen/glucose deprivation, the loss of Cav-1, either via siRNA or in neurons isolated from Cav-1 KO mice, eliminates protection, as assessed by assays of necrosis and apoptosis (52). In cardiac ischemia/reperfusion, loss of Cav-1 or -3 (56, 115) reduces the response of the heart to protective stimuli while mice with cardiac myocyte-specific Cav-3 expression are protected from ischemia/reperfusion (159). Recent studies suggest that there may be a link between ROS and downstream modulation of protective mediators via cav (77). It will be of interest to determine the cav-dependent mechanisms that regulate ROS so as to promote organ protection from ischemic damage.
Conclusions
Considerably more is known now about the functional contribution of rafts/caveolae than when caveolae were first recognized by electron microscopy ∼50 years ago. Newer data have led to the recognition that rafts/caveolae have a large number of protein and lipid “partners” that localize to these domains and that many of these partners have important roles in cellular regulation. Information remains limited regarding the precise determinants of raft/caveolae localization of proteins and lipids. The absence of universally accepted and broadly used means to assess rafts has hampered the accrual of information and conclusions regarding their roles in cell function. A full understanding of these roles—as well as those of caveolae—has yet to be discovered, especially in terms of interaction with components involved in redox signaling and the contribution of raft/caveolae-localized components to oxidative injury. Discoveries that define these roles have the potential to lead to new insights regarding a number of physiologic processes as well as important diseases—including certain infections, cardiovascular, neuropsychiatric, neoplastic, and inflammatory disorders.
Acknowledgments
Work in the authors' laboratories on the topic of this review is supported by grants from the American Heart Association, the Ellison Medical Foundation, and the National Institutes of Health.
Abbreviations
7, TM-7-transmembrane; Btk, Bruton's tyrosine kinase; Cav, caveolin; CO, carbon monoxide; CSD, caveolin scaffolding domain; EGFR, epidermal growth factor receptor; eNOS or NOS3, endothelial nitric oxide synthase; Erk, extracellular regulated kinase; fMLP, N-formyl peptide; GPCR, G-protein coupled receptor; GPI, glycosylphosphatidylinositol; HMGCoA, 3-hydroxy-3-methyl-glutaryl-CoA; HO, heme oxygenase; Id2, inhibitor of transcription-2; IL-8, interleukin-8; MβCD, methyl-β-cyclodextrin; nLDL, native low-density lipoproteins; No, nitric oxide; NOX, NADPH oxidase; RNS, reactive nitrogen species; ROS, reactive oxygen species; RTK, receptor tyrosine kinases; PMN, polymorphonuclear leukocytes; PTRF, polymerase 1 and transcript release factor; siRNA, small interfering ribonucleic acid; TLR, toll like receptor; TMD, transmembrane domain; TNFα, tumor necrosis factor alpha.
References
- 1. Abrahams PH. Day A. Allt G. Schwann cell plasma membrane changes induced by nerve crush. A freeze-fracture study. Acta Neuropathol. 1980;50:85–90. doi: 10.1007/BF00692856. [DOI] [PubMed] [Google Scholar]
- 2. Achcar RO. Demura Y. Rai PR. Taraseviciene–Stewart L. Kasper M. Voelkel NF. Cool CD. Loss of caveolin and heme oxygenase expression in severe pulmonary hypertension. Chest. 2006;129:696–705. doi: 10.1378/chest.129.3.696. [DOI] [PubMed] [Google Scholar]
- 3. Ayuyan AG. Cohen FS. Lipid peroxides promote large rafts: Effects of excitation of probes in fluorescence microscopy and electrochemical reactions during vesicle formation. Biophys J. 2006;91:2172–2183. doi: 10.1529/biophysj.106.087387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Babior BM. NADPH oxidase: An update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- 5. Babuke T. Tikkanen R. Dissecting the molecular function of reggie/flotillin proteins. Eur J Cell Biol. 2007;86:525–532. doi: 10.1016/j.ejcb.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 6. Banfi C. Brioschi M. Wait R. Begum S. Gianazza E. Fratto P. Polvani G. Vitali E. Parolari A. Mussoni L. Tremoli E. Proteomic analysis of membrane microdomains derived from both failing and non-failing human hearts. Proteomics. 2006;6:1976–1988. doi: 10.1002/pmic.200500278. [DOI] [PubMed] [Google Scholar]
- 7. Bartz R. Zhou J. Hsieh JT. Ying Y. Li W. Liu P. Caveolin-1 secreting LNCaP cells induce tumor growth of caveolin-1 negative LNCaP cells in vivo. Int J Cancer. 2008;122:520–525. doi: 10.1002/ijc.23142. [DOI] [PubMed] [Google Scholar]
- 8. Becher A. McIlhinney RA. Consequences of lipid raft association on G-protein-coupled receptor function. Biochem Soc Symp. 2005;72:151–164. doi: 10.1042/bss0720151. [DOI] [PubMed] [Google Scholar]
- 9. Bist A. Fielding PE. Fielding CJ. Two sterol regulatory element-like sequences mediate up-regulation of caveolin gene transcription in response to low density lipoprotein free cholesterol. Proc Natl Acad Sci USA. 1997;94:10693–10698. doi: 10.1073/pnas.94.20.10693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blonder J. Terunuma A. Conrads TP. Chan KC. Yee C. Lucas DA. Schaefer CF. Yu LR. Issaq HJ. Veenstra TD. Vogel JC. A proteomic characterization of the plasma membrane of human epidermis by high-throughput mass spectrometry. J Invest Dermatol. 2004;123:691–699. doi: 10.1111/j.0022-202X.2004.23421.x. [DOI] [PubMed] [Google Scholar]
- 11. Callera GE. Montezano AC. Yogi A. Tostes RC. Touyz RM. Vascular signaling through cholesterol-rich domains: implications in hypertension. Curr Opin Nephrol Hypertens. 2007;16:90–104. doi: 10.1097/MNH.0b013e328040bfbd. [DOI] [PubMed] [Google Scholar]
- 12. Cheng G. Cao Z. Xu X. van Meir EG. Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269:131–140. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 13. Chini B. Parenti M. G-protein coupled receptors in lipid rafts and caveolae: How, when and why do they go there? J Mol Endocrinol. 2004;32:325–338. doi: 10.1677/jme.0.0320325. [DOI] [PubMed] [Google Scholar]
- 14. Cohen AW. Hnasko R. Schubert W. Lisanti MP. Role of caveolae and caveolins in health and disease. Physiol Rev. 2004;84:1341–1379. doi: 10.1152/physrev.00046.2003. [DOI] [PubMed] [Google Scholar]
- 15. Costello BR. Shafiq SA. Freeze-fracture study of muscle plasmalemma in normal and dystrophic chickens. Muscle Nerve. 1979;2:191–201. doi: 10.1002/mus.880020307. [DOI] [PubMed] [Google Scholar]
- 16. Couet J. Li S. Okamoto T. Ikezu T. Lisanti MP. Identification of peptide and protein ligands for the caveolin-scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins. J Bio Chem. 1997;272:6525–6533. doi: 10.1074/jbc.272.10.6525. [DOI] [PubMed] [Google Scholar]
- 17. Danielsen EM. van Deurs B. A transferrin-like GPI-linked iron-binding protein in detergent-insoluble noncaveolar microdomains at the apical surface of fetal intestinal epithelial cells. J Cell Biol. 1995;131:939–950. doi: 10.1083/jcb.131.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Das K. Lewis RY. Scherer PE. Lisanti MP. The membrane-spanning domains of caveolins-1 and -2 mediate the formation of caveolin hetero-oligomers. Implications for the assembly of caveolae membranes in vivo. J Biol Chem. 1999;274:18721–18728. doi: 10.1074/jbc.274.26.18721. [DOI] [PubMed] [Google Scholar]
- 19. David A. Fridlich R. Aviram I. The presence of membrane Proteinase 3 in neutrophil lipid rafts and its colocalization with FcgammaRIIIb and cytochrome b558. Exp Cell Res. 2005;308:156–165. doi: 10.1016/j.yexcr.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 20. Dietzen DJ. Hastings WR. Lublin DM. Caveolin is palmitoylated on multiple cysteine residues. Palmitoylation is not necessary for localization of caveolin to caveolae. J Biol Chem. 1995;270:6838–6842. doi: 10.1074/jbc.270.12.6838. [DOI] [PubMed] [Google Scholar]
- 21. Drab M. Verkade P. Elger M. Kasper M. Lohn M. Lauterbach B. Menne J. Lindschau C. Mende F. Luft FC. Schedl A. Haller H. Kurzchalia TV. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–2452. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 22. Dudzinski DM. Michel T. Life history of eNOS: partners and pathways. Cardiovasc Res. 2007;75:247–260. doi: 10.1016/j.cardiores.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Durante W. Kroll MH. Christodoulides N. Peyton KJ. Schafer AI. Nitric oxide induces heme oxygenase-1 gene expression and carbon monoxide production in vascular smooth muscle cells. Circ Res. 1997;80:557–564. doi: 10.1161/01.res.80.4.557. [DOI] [PubMed] [Google Scholar]
- 24. Durr E. Yu J. Krasinska KM. Carver LA. Yates JR. Testa JE. Oh P. Schnitzer JE. Direct proteomic mapping of the lung microvascular endothelial cell surface in vivo and in cell culture. Nat Biotechnol. 2004;22:985–992. doi: 10.1038/nbt993. [DOI] [PubMed] [Google Scholar]
- 25. Engelman JA. Zhang X. Galbiati F. Volonté D. Sotgia F. Pestell RG. Minetti C. Scherer PE. Okamoto T. Lisanti MP. Molecular genetics of the caveolin gene family: implications for human cancers, diabetes, Alzheimer disease, and muscular dystrophy. Am J Hum Genet. 1998;63:1578–1587. doi: 10.1086/302172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Engelman JA. Zhang XL. Lisanti MP. Sequence and detailed organization of the human caveolin-1 and -2 genes located near the D7S522 locus (7q31.1). Methylation of a CpG island in the 5' promoter region of the caveolin-1 gene in human breast cancer cell lines. FEBS Lett. 1999;448:221–230. doi: 10.1016/s0014-5793(99)00365-8. [DOI] [PubMed] [Google Scholar]
- 27. Feron O. Belhassen L. Kobzik L. Smith TW. Kelly RA. Michel T. Endothelial nitric oxide synthase targeting to caveolae. Specific interactions with caveolin isoforms in cardiac myocytes and endothelial cells. J Biol Chem. 1996;271:22810–22814. doi: 10.1074/jbc.271.37.22810. [DOI] [PubMed] [Google Scholar]
- 28. Feron O. Kelly RA. The Caveolar Paradox: Suppressing, Inducing, and Terminating eNOS Signaling. Circ Res. 2001;88:129–131. doi: 10.1161/01.res.88.2.129. [DOI] [PubMed] [Google Scholar]
- 29. Fiedler K. Parton RG. Kellner R. Etzold T. Simons K. VIP36, a novel component of glycolipid rafts and exocytic carrier vesicles in epithelial cells. EMBO J. 1994;13:1729–1740. doi: 10.1002/j.1460-2075.1994.tb06437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fielding CJ. Bist A. Fielding PE. Caveolin mRNA levels are up-regulated by free cholesterol and down-regulated by oxysterols in fibroblast monolayers. Proc Natl Acad Sci USA. 1997;94:3753–3758. doi: 10.1073/pnas.94.8.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Foster LJ. De Hoog CL. Mann M. Unbiased quantitative proteomics of lipid rafts reveals high specificity for signaling factors. Proc Natl Acad Sci USA. 2003;100:5813–5818. doi: 10.1073/pnas.0631608100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Frick M. Bright NA. Riento K. Bray A. Merrified C. Nichols BJ. Coassembly of flotillins induces formation of membrane microdomains, membrane curvature, and vesicle budding. Curr Biol. 2007;17:1151–1156. doi: 10.1016/j.cub.2007.05.078. [DOI] [PubMed] [Google Scholar]
- 33. Fridlich R. David A. Aviram I. Membrane proteinase 3 and its interactions within microdomains of neutrophil membranes. J Cell Biochem. 2006;99:117–125. doi: 10.1002/jcb.20901. [DOI] [PubMed] [Google Scholar]
- 34. Fulop T. Larbi A. Douziech N. Fortin C. Guerard KP. Lesur O. Khalil A. Dupuis G. Signal transduction and functional changes in neutrophils with aging. Aging Cell. 2004;3:217–226. doi: 10.1111/j.1474-9728.2004.00110.x. [DOI] [PubMed] [Google Scholar]
- 35. Galbiati F. Razani B. Lisanti MP. Emerging themes in lipid rafts and caveolae. Cell. 2001;106:403–411. doi: 10.1016/s0092-8674(01)00472-x. [DOI] [PubMed] [Google Scholar]
- 36. Garcia–Cardena G. Martasek P. Masters BS. Skidd PM. Couet J. Li S. Lisanti MP. Sessa WC. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the nos caveolin binding domain in vivo. J Biol Chem. 1997;272:25437–25440. doi: 10.1074/jbc.272.41.25437. [DOI] [PubMed] [Google Scholar]
- 37. Garcia–Cardena G. Oh P. Liu J. Schnitzer JE. Sessa WC. Targeting of nitric oxide synthase to endothelial cell caveolae via palmitoylation: implications for nitric oxide signaling. Proc Natl Acad Sci USA. 1996;93:6448–6453. doi: 10.1073/pnas.93.13.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ge S. Song L. Serwanski DR. Kuziel WA. Pachter JS. Transcellular transport of CCL2 across brain microvascular endothelial cells. J Neurochem. 2008;104:1219–1232. doi: 10.1111/j.1471-4159.2007.05056.x. [DOI] [PubMed] [Google Scholar]
- 39. Ghafourifar P. Bringold U. Klein SD. Richter C. Mitochondrial nitric oxide synthase, oxidative stress and apoptosis. Biol Signals Recept. 2001;10:57–65. doi: 10.1159/000046875. [DOI] [PubMed] [Google Scholar]
- 40. Gherghiceanu M. Popescu LM. Electron microscope tomography: further demonstration of nanocontacts between caveolae and smooth muscle sarcoplasmic reticulum. J Cell Mol Med. 2007;11:1416–1418. doi: 10.1111/j.1582-4934.2007.00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ghosh S. Gachhui R. Crooks C. Wu C. Lisanti MP. Stuehr DJ. Interaction between caveolin-1 and the reductase domain of endothelial nitric-oxide synthase. Consequences for catalysis. J Biol Chem. 1998;273:22267–22271. doi: 10.1074/jbc.273.35.22267. [DOI] [PubMed] [Google Scholar]
- 42. Goodsaid–Zalduondo F. Rintoul DA. Carlson JC. Hansel W. Luteolysis-induced changes in phase composition and fluidity of bovine luteal cell membranes. Proc Natl Acad Sci USA. 1982;79:4332–4336. doi: 10.1073/pnas.79.14.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Grande–Garcia A. Del Pozo MA. Caveolin-1 in cell polarization and directional migration. Eur J Cell Biol. 2008;87:641–647. doi: 10.1016/j.ejcb.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 44. Grande–Garcia A. Echarri A. de Rooij J. Alderson NB. Waterman–Storer CM. Valdivielso JM. del Pozo MA. Caveolin-1 regulates cell polarization and directional migration through Src kinase and Rho GTPases. J Cell Biol. 2007;177:683–694. doi: 10.1083/jcb.200701006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Guichard C. Pedruzzi E. Dewas C. Fay M. Pouzet C. Bens M. Vandewalle A. Ogier-Denis E. Gougerot–Pocidalo MA. Elbim C. Interleukin-8-induced priming of neutrophil oxidative burst requires sequential recruitment of NADPH oxidase components into lipid rafts. J Biol Chem. 2005;280:37021–37032. doi: 10.1074/jbc.M506594200. [DOI] [PubMed] [Google Scholar]
- 46. Gullestad L. Oie E. Ueland T. Yndestad A. Aukrust P. The role of statins in heart failure. Fund Clin Pharmacol. 2007;21:35–40. doi: 10.1111/j.1472-8206.2007.00538.x. [DOI] [PubMed] [Google Scholar]
- 47. Han W. Li H. Villar VA. Pascua AM. Dajani MI. Wang X. Natarajan A. Quinn MT. Felder RA. Jose PA. Yu P. Lipid rafts keep NADPH oxidase in the inactive state in human renal proximal tubule cells. Hypertension. 2008;51:481–487. doi: 10.1161/HYPERTENSIONAHA.107.103275. [DOI] [PubMed] [Google Scholar]
- 48. Harder T. Scheiffele P. Verkade P. Simons K. Lipid domain structure of the plasma membrane revealed by patching of membrane components. J Cell Biol. 1998;141:929–942. doi: 10.1083/jcb.141.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Harder T. Simons K. Caveolae, DIGs, and the dynamics of sphingolipid-cholesterol microdomains. Curr Opin Cell Biol. 1997;9:534–542. doi: 10.1016/s0955-0674(97)80030-0. [DOI] [PubMed] [Google Scholar]
- 50. Hayashi T. Juliet PA. Miyazaki A. Ignarro LJ. Iguchi A. High glucose downregulates the number of caveolae in monocytes through oxidative stress from NADPH oxidase: implications for atherosclerosis. Biochim Biophys Acta. 2007;1772:364–372. doi: 10.1016/j.bbadis.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 51. Head BP. Insel PA. Do caveolins regulate cells by actions outside of caveolae? Trends Cell Biol. 2007;17:51–57. doi: 10.1016/j.tcb.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 52. Head BP. Patel HH. Tsutsumi YM. Hu Y. Mejia T. Mora RC. Insel PA. Roth DM. Drummond JC. Patel PM. Caveolin-1 expression is essential for N-methyl-D-aspartate receptor-mediated Src and extracellular signal-regulated kinase 1/2 activation and protection of primary neurons from ischemic cell death. FASEB J. 2008;22:828–840. doi: 10.1096/fj.07-9299com. [DOI] [PubMed] [Google Scholar]
- 53. Hilenski LL. Clempus RE. Quinn MT. Lambeth JD. Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24:677–683. doi: 10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]
- 54. Hill MM. Bastiani M. Luetterforst R. Kirkham M. Kirkham A. Nixon SJ. Walser P. Abankwa D. Oorschot VM. Martin S. Hancock JF. Parton RG. PTRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell. 2008;132:113–124. doi: 10.1016/j.cell.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hnasko R. Lisanti MP. The biology of caveolae: lessons from caveolin knockout mice and implications for human disease. Mol Interv. 2003;3:445–464. doi: 10.1124/mi.3.8.445. [DOI] [PubMed] [Google Scholar]
- 56. Horikawa YT. Patel HH. Tsutsumi YM. Jennings MM. Kidd MW. Hagiwara Y. Ishikawa Y. Insel PA. Roth DM. Caveolin-3 expression and caveolae are required for isoflurane-induced cardiac protection from hypoxia and ischemia/reperfusion injury. J Mol Cell Cardiol. 2008;44:123–130. doi: 10.1016/j.yjmcc.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hu G. Ye RD. Dinauer MC. Malik AB. Minshall RD. Neutrophil caveolin-1 expression contributes to mechanism of lung inflammation and injury. Am J Physiol Lung Cell Mol Physiol. 2008;294:L178–L186. doi: 10.1152/ajplung.00263.2007. [DOI] [PubMed] [Google Scholar]
- 58. Insel PA. Patel HH. Do studies in caveolin-knockouts teach us about physiology and pharmacology or instead, the ways mice compensate for 'lost proteins'? Br J Pharmacol. 2007;150:251–254. doi: 10.1038/sj.bjp.0706981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Insel PA. Patel HH. Membrane rafts and caveolae in cardiovascular signaling. Curr Opin Nephrol Hypertens. 2009;18:50–56. doi: 10.1097/MNH.0b013e3283186f82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Iwabuchi K. Prinetti A. Sonnino S. Mauri L. Kobayashi T. Ishii K. Kaga N. Murayama K. Kurihara H. Nakayama H. Yoshizaki F. Takamori K. Ogawa H. Nagaoka I. Involvement of very long fatty acid-containing lactosylceramide in lactosylceramide-mediated superoxide generation and migration in neutrophils. Glycoconj J. 2008;25:357–374. doi: 10.1007/s10719-007-9084-6. [DOI] [PubMed] [Google Scholar]
- 61. Iwakiri Y. Satoh A. Chatterjee S. Toomre DK. Chalouni CM. Fulton D. Groszmann RJ. Shah VH. Sessa WC. Nitric oxide synthase generates nitric oxide locally to regulate compartmentalized protein S-nitrosylation and protein trafficking. Proc Natl Acad Sci USA. 2006;103:19777–19782. doi: 10.1073/pnas.0605907103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jacobson K. Mouritsen OG. Anderson RG. Lipid rafts: at a crossroad between cell biology and physics. Nat Cell Biol. 2007;9:7–14. doi: 10.1038/ncb0107-7. [DOI] [PubMed] [Google Scholar]
- 63. Jansen M. Pietiainen VM. Polonen H. Rasilainen L. Koivusalo M. Ruotsalainen U. Jokitalo E. Ikonen E. Cholesterol substitution increases the structural heterogeneity of caveolae. J Biol Chem. 2008;283:14610–14618. doi: 10.1074/jbc.M710355200. [DOI] [PubMed] [Google Scholar]
- 64. Jin S. Zhang Y. Yi F. Li PL. Critical role of lipid raft redox signaling platforms in endostatin-induced coronary endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2008;28:485–490. doi: 10.1161/ATVBAHA.107.159772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Jin Y. Kim HP. Chi M. Ifedigbo E. Ryter SW. Choi AM. Deletion of caveolin-1 protects against oxidative lung injury via up-regulation of heme oxygenase-1. Am J Respir Cell Mol Biol. 2008;39:171–179. doi: 10.1165/rcmb.2007-0323OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jung NH. Kim HP. Kim BR. Cha SH. Kim GA. Ha H. Na YE. Cha YN. Evidence for heme oxygenase-1 association with caveolin-1 and -2 in mouse mesangial cells. IUBMB Life. 2003;55:525–532. doi: 10.1080/15216540310001620968. [DOI] [PubMed] [Google Scholar]
- 67. Karnovsky MJ. Kleinfeld AM. Hoover RL. Klausner RD. The concept of lipid domains in membranes. J Cell Biol. 1982;94:1–6. doi: 10.1083/jcb.94.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kenworthy AK. Petranova N. Edidin M. High-resolution FRET microscopy of cholera toxin B-subunit and GPI-anchored proteins in cell plasma membranes. Mol Biol Cell. 2000;11:1645–1655. doi: 10.1091/mbc.11.5.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kim HA. Kim KH. Lee RA. Expression of caveolin-1 is correlated with Akt-1 in colorectal cancer tissues. Exp Mol Pathol. 2006;80:165–170. doi: 10.1016/j.yexmp.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 70. Kim HP. Ryter SW. Choi AM. CO as a cellular signaling molecule. Annu Rev Pharmacol Toxicol. 2006;46:411–449. doi: 10.1146/annurev.pharmtox.46.120604.141053. [DOI] [PubMed] [Google Scholar]
- 71. Kim HP. Wang X. Galbiati F. Ryter SW. Choi AM. Caveolae compartmentalization of heme oxygenase-1 in endothelial cells. FASEB J. 2004;18:1080–1089. doi: 10.1096/fj.03-1391com. [DOI] [PubMed] [Google Scholar]
- 72. Kim HP. Wang X. Nakao A. Kim SI. Murase N. Choi ME. Ryter SW. Choi AM. Caveolin-1 expression by means of p38beta mitogen-activated protein kinase mediates the antiproliferative effect of carbon monoxide. Proc Natl Acad Sci USA. 2005;102:11319–11324. doi: 10.1073/pnas.0501345102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kiss E. Nagy P. Balogh A. Szollosi J. Matko J. Cytometry of raft and caveola membrane microdomains: From flow and imaging techniques to high throughput screening assays. Cytometry A. 2008;73:599–614. doi: 10.1002/cyto.a.20572. [DOI] [PubMed] [Google Scholar]
- 74. Klausner RD. Kleinfeld AM. Hoover RL. Karnovsky MJ. Lipid domains in membranes. Evidence derived from structural perturbations induced by free fatty acids and lifetime heterogeneity analysis. J Biol Chem. 1980;255:1286–1295. [PubMed] [Google Scholar]
- 75. Klinge CM. Wickramasinghe NS. Ivanova MM. Dougherty SM. Resveratrol stimulates nitric oxide production by increasing estrogen receptor alpha-Src-caveolin-1 interaction and phosphorylation in human umbilical vein endothelial cells. FASEB J. 2008;22:2185–2197. doi: 10.1096/fj.07-103366. [DOI] [PubMed] [Google Scholar]
- 76. Kogo H. Fujimoto T. Caveolin-1 isoforms are encoded by distinct mRNAs. Identification Of mouse caveolin-1 mRNA variants caused by alternative transcription initiation and splicing. FEBS Lett. 2000;465:119–123. doi: 10.1016/s0014-5793(99)01730-5. [DOI] [PubMed] [Google Scholar]
- 77. Koneru S. Penumathsa SV. Thirunavukkarasu M. Samuel SM. Zhan L. Han Z. Maulik G. Das DK. Maulik N. Redox regulation of ischemic preconditioning is mediated by the differential activation of caveolins and their association with eNOS and GLUT-4. Am J Physiol Heart Circ Physiol. 2007;292:H2060–H2072. doi: 10.1152/ajpheart.01169.2006. [DOI] [PubMed] [Google Scholar]
- 78. Krajewska WM. Maslowska I. Caveolins: Structure and function in signal transduction. Cell Mol Biol Lett. 2004;9:195–220. [PubMed] [Google Scholar]
- 79. Kurzchalia TV. Dupree P. Parton RG. Kellner R. Virta H. Lehnert M. Simons K. VIP21, a 21-kD membrane protein is an integral component of trans-Golgi-network-derived transport vesicles. J Cell Biol. 1992;118:1003–1014. doi: 10.1083/jcb.118.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lai EC. Lipid rafts make for slippery platforms. J Cell Biol. 2003;162:365–370. doi: 10.1083/jcb.200307087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Li L. Moore PK. Putative biological roles of hydrogen sulfide in health and disease: A breath of not so fresh air? Trends Pharmacol Sci. 2008;29:84–90. doi: 10.1016/j.tips.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 82. Li PL. Zhang Y. Yi F. Lipid raft redox signaling platforms in endothelial dysfunction. Antioxid Redox Signal. 2007;9:1457–1470. doi: 10.1089/ars.2007.1667. [DOI] [PubMed] [Google Scholar]
- 83. Li S. Seitz R. Lisanti MP. Phosphorylation of caveolin by src tyrosine kinases. The alpha-isoform of caveolin is selectively phosphorylated by v-Src in vivo. J Biol Chem. 1996;271:3863–3868. [PubMed] [Google Scholar]
- 84. Lingwood D. Simons K. Detergent resistance as a tool in membrane research. Nat Protoc. 2007;2:2159–2165. doi: 10.1038/nprot.2007.294. [DOI] [PubMed] [Google Scholar]
- 85. Liu L. Pilch PF. A critical role of cavin (polymerase I and transcript release factor) in caveolae formation and organization. J Biol Chem. 2008;283:4314–4322. doi: 10.1074/jbc.M707890200. [DOI] [PubMed] [Google Scholar]
- 86. Lopez–Sepulveda R. Jimenez R. Romero M. Zarzuelo MJ. Sanchez M. Gomez–Guzman M. Vargas F. O'Valle F. Zarzuelo A. Perez–Vizcaino F. Duarte J. Wine polyphenols improve endothelial function in large vessels of female spontaneously hypertensive rats. Hypertension. 2008;51:1088–1095. doi: 10.1161/HYPERTENSIONAHA.107.107672. [DOI] [PubMed] [Google Scholar]
- 87. Lu SP. Lin Feng MH. Huang HL. Huang YC. Tsou WI. Lai MZ. Reactive oxygen species promote raft formation in T lymphocytes. Free Radic Biol Med. 2007;42:936–944. doi: 10.1016/j.freeradbiomed.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 88. McMahon KA. Zhu M. Kwon SW. Liu P. Zhao Y. Anderson RG. Detergent-free caveolae proteome suggests an interaction with ER and mitochondria. Proteomics. 2006;6:143–152. doi: 10.1002/pmic.200500208. [DOI] [PubMed] [Google Scholar]
- 89. Michel V. Bakovic M. Lipid rafts in health and disease. Biol Cell. 2007;99:129–140. doi: 10.1042/BC20060051. [DOI] [PubMed] [Google Scholar]
- 90. Milovanova T. Chatterjee S. Hawkins BJ. Hong N. Sorokina EM. Debolt K. Moore JS. Madesh M. Fisher AB. Caveolae are an essential component of the pathway for endothelial cell signaling associated with abrupt reduction of shear stress. Biochim Biophys Acta. 2008;1783:1866–1875. doi: 10.1016/j.bbamcr.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Minshall RD. Malik AB. Transport across the endothelium: regulation of endothelial permeability. Handb Exp Pharmacol. 2006:107–144. doi: 10.1007/3-540-32967-6_4. [DOI] [PubMed] [Google Scholar]
- 92. Mishra S. Joshi PG. Lipid raft heterogeneity: An enigma. J Neurochem. 2007;103:135–142. doi: 10.1111/j.1471-4159.2007.04720.x. [DOI] [PubMed] [Google Scholar]
- 93. Mongrand S. Morel J. Laroche J. Claverol S. Carde JP. Hartmann MA. Bonneu M. Simon–Plas F. Lessire R. Bessoule JJ. Lipid rafts in higher plant cells: Purification and characterization of Triton X-100-insoluble microdomains from tobacco plasma membrane. J Biol Chem. 2004;279:36277–36286. doi: 10.1074/jbc.M403440200. [DOI] [PubMed] [Google Scholar]
- 94. Monier S. Parton RG. Vogel F. Behlke J. Henske A. Kurzchalia TV. VIP21-caveolin, a membrane protein constituent of the caveolar coat, oligomerizes in vivo and in vitro. Mol Biol Cell. 1995;6:911–927. doi: 10.1091/mbc.6.7.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Mouillet–Richard S. Schneider B. Pradines E. Pietri M. Ermonval M. Grassi J. Richards JG. Mutel V. Launay JM. Kellermann O. Cellular prion protein signaling in serotonergic neuronal cells. Ann NY Acad Sci. 2007;1096:106–119. doi: 10.1196/annals.1397.076. [DOI] [PubMed] [Google Scholar]
- 96. Mukherjee S. Tessema M. Wandinger–Ness A. Vesicular trafficking of tyrosine kinase receptors and associated proteins in the regulation of signaling and vascular function. Circ Res. 2006;98:743–756. doi: 10.1161/01.RES.0000214545.99387.e3. [DOI] [PubMed] [Google Scholar]
- 97. Murata M. Peranen J. Schreiner R. Wieland F. Kurzchalia TV. Simons K. VIP21/caveolin is a cholesterol-binding protein. Proc Natl Acad Sci USA. 1995;92:10339–10343. doi: 10.1073/pnas.92.22.10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Murata T. Lin MI. Huang Y. Yu J. Bauer PM. Giordano FJ. Sessa WC. Reexpression of caveolin-1 in endothelium rescues the vascular, cardiac, and pulmonary defects in global caveolin-1 knockout mice. J Exp Med. 2007;204:2373–2382. doi: 10.1084/jem.20062340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Nagatsuka Y. Hirabayashi Y. Phosphatidylglucoside: A new marker for lipid rafts. Biochim Biophys Acta. 2008;1780:405–409. doi: 10.1016/j.bbagen.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 100. Nakahira K. Kim HP. Geng XH. Nakao A. Wang X. Murase N. Drain PF. Wang X. Sasidhar M. Nabel EG. Takahashi T. Lukacs NW. Ryter SW. Morita K. Choi AM. Carbon monoxide differentially inhibits TLR signaling pathways by regulating ROS-induced trafficking of TLRs to lipid rafts. J Exp Med. 2006;203:2377–2389. doi: 10.1084/jem.20060845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Nichols B. Caveosomes and endocytosis of lipid rafts. J Cell Sci. 2003;116:4707–4714. doi: 10.1242/jcs.00840. [DOI] [PubMed] [Google Scholar]
- 102. Nourissat P. Travert M. Chevanne M. Tekpli X. Rebillard A. Le Moigne–Muller G. Rissel M. Cillard J. Dimanche–Boitrel MT. Lagadic–Gossmann D. Sergent O. Ethanol induces oxidative stress in primary rat hepatocytes through the early involvement of lipid raft clustering. Hepatology. 2008;47:59–70. doi: 10.1002/hep.21958. [DOI] [PubMed] [Google Scholar]
- 103. Oguchi K. Tsukagoshi H. An electron-microscopic study of the T-system in progressive muscular dystrophy (Duchenne) using lanthanum. J Neurol Sci. 1980;44:161–168. doi: 10.1016/0022-510x(80)90124-0. [DOI] [PubMed] [Google Scholar]
- 104. Ostrom RS. Insel PA. The evolving role of lipid rafts and caveolae in G protein-coupled receptor signaling: implications for molecular pharmacology. Br J Pharmacol. 2004;143:235–245. doi: 10.1038/sj.bjp.0705930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Ostrom RS. Liu X. Detergent and detergent-free methods to define lipid rafts and caveolae. Methods Mol Biol. 2007;400:459–468. doi: 10.1007/978-1-59745-519-0_30. [DOI] [PubMed] [Google Scholar]
- 106. Owen DM. Neil MA. French PM. Magee AI. Optical techniques for imaging membrane lipid microdomains in living cells. Semin Cell Dev Biol. 2007;18:591–598. doi: 10.1016/j.semcdb.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 107. Ozawa K. Whalen EJ. Nelson CD. Mu Y. Hess DT. Lefkowitz RJ. Stamler JS. S-nitrosylation of beta-arrestin regulates beta-adrenergic receptor trafficking. Mol Cell. 2008;31:395–405. doi: 10.1016/j.molcel.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Palade G. Fine structure of blood capillaries. J Appl Phys. 1953;24:1424. [Google Scholar]
- 109. Park DS. Cohen AW. Frank PG. Razani B. Lee H. Williams TM. Chandra M. Shirani J. Souza APD. Tang B. Jelicks LA. Factor SM. Weiss LM. Tanowitz HB. Lisanti MP. Caveolin-1 null (-/-) mice show dramatic reductions in life span. Biochemistry. 2003;42:15124–15131. doi: 10.1021/bi0356348. [DOI] [PubMed] [Google Scholar]
- 110. Park DS. Woodman SE. Schubert W. Cohen AW. Frank PG. Chandra M. Shirani J. Razani B. Tang B. Jelicks LA. Factor SM. Weiss LM. Tanowitz HB. Lisanti MP. Caveolin-1/3 double-knockout mice are viable, but lack both muscle and non-muscle caveolae, and develop a severe cardiomyopathic phenotype. Am J Pathol. 2002;160:2207–2217. doi: 10.1016/S0002-9440(10)61168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Park SC. Cho KA. Jang IS. Kim KT. Ryu SJ. Functional efficiency of the senescent cells: replace or restore? Ann NY Acad Sci. 2004;1019:309–316. doi: 10.1196/annals.1297.052. [DOI] [PubMed] [Google Scholar]
- 112. Parolini I. Sargiacomo M. Galbiati F. Rizzo G. Grignani F. Engelman JA. Okamoto T. Ikezu T. Scherer PE. Mora R. Rodriguez–Boulan E. Peschle C. Lisanti MP. Expression of caveolin-1 is required for the transport of caveolin-2 to the plasma membrane. Retention of caveolin-2 at the level of the golgi complex. J Biol Chem. 1999;274:25718–25725. doi: 10.1074/jbc.274.36.25718. [DOI] [PubMed] [Google Scholar]
- 113. Patel HH. Murray F. Insel PA. Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu Rev Pharmacol Toxicol. 2008;48:359–391. doi: 10.1146/annurev.pharmtox.48.121506.124841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Patel HH. Murray F. Insel PA. G-protein-coupled receptor-signaling components in membrane raft and caveolae microdomains. Handb Exp Pharmacol. 2008:167–184. doi: 10.1007/978-3-540-72843-6_7. [DOI] [PubMed] [Google Scholar]
- 115. Patel HH. Tsutsumi YM. Head BP. Niesman IR. Jennings M. Horikawa Y. Huang D. Moreno AL. Patel PM. Insel PA. Roth DM. Mechanisms of cardiac protection from ischemia/reperfusion injury: a role for caveolae and caveolin-1. FASEB J. 2007;21:1565–1574. doi: 10.1096/fj.06-7719com. [DOI] [PubMed] [Google Scholar]
- 116. Patel HH. Zhang S. Murray F. Suda RY. Head BP. Yokoyama U. Swaney JS. Niesman IR. Schermuly RT. Pullamsetti SS. Thistlethwaite PA. Miyanohara A. Farquhar MG. Yuan JX. Insel PA. Increased smooth muscle cell expression of caveolin-1 and caveolae contribute to the pathophysiology of idiopathic pulmonary arterial hypertension. FASEB J. 2007;21:2970–2979. doi: 10.1096/fj.07-8424com. [DOI] [PubMed] [Google Scholar]
- 117. Patra SK. Dissecting lipid raft facilitated cell signaling pathways in cancer. Biochim Biophys Acta. 2008;1785:182–206. doi: 10.1016/j.bbcan.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 118. Peterson TE. Poppa V. Ueba H. Wu A. Yan C. Berk BC. Opposing effects of reactive oxygen species and cholesterol on endothelial nitric oxide synthase and endothelial cell caveolae. Circ Res. 1999;85:29–37. doi: 10.1161/01.res.85.1.29. [DOI] [PubMed] [Google Scholar]
- 119. Pietri M. Caprini A. Mouillet–Richard S. Pradines E. Ermonval M. Grassi J. Kellermann O. Schneider B. Overstimulation of PrPC signaling pathways by prion peptide 106-126 causes oxidative injury of bioaminergic neuronal cells. J Biol Chem. 2006;281:28470–28479. doi: 10.1074/jbc.M602774200. [DOI] [PubMed] [Google Scholar]
- 120. Pike LJ. Growth factor receptors, lipid rafts and caveolae: An evolving story. Biochim Biophys Acta. 2005;1746:260–273. doi: 10.1016/j.bbamcr.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 121. Pike LJ. Lipid rafts: Bringing order to chaos. J Lipid Res. 2003;44:655–667. doi: 10.1194/jlr.R200021-JLR200. [DOI] [PubMed] [Google Scholar]
- 122. Pike LJ. Lipid rafts: Heterogeneity on the high seas. Biochem J. 2004;378:281–292. doi: 10.1042/BJ20031672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Pike LJ. Rafts defined: A report on the Keystone Symposium on lipid rafts and cell function. J Lipid Res. 2006;47:1597–1598. doi: 10.1194/jlr.E600002-JLR200. [DOI] [PubMed] [Google Scholar]
- 124. Pike LJ. Han X. Chung K–N. Gross RW. Lipid rafts are enriched in arachidonic acid and plasmenylethanolamine and their composition is independent of caveolin-1 expression: A quantitative electrospray ionization/mass spectrometric analysis. Biochemistry. 2002;41:2075–2088. doi: 10.1021/bi0156557. [DOI] [PubMed] [Google Scholar]
- 125. Pilch PF. Souto RP. Liu L. Jedrychowski MP. Berg EA. Costello CE. Gygi SP. Cellular spelunking: exploring adipocyte caveolae. J Lipid Res. 2007;48:2103–2111. doi: 10.1194/jlr.R700009-JLR200. [DOI] [PubMed] [Google Scholar]
- 126. Pritchard KA. Ackerman AW. Ou J. Curtis M. Smalley DM. Fontana JT. Stemerman MB. Sessa WC. Native low-density lipoprotein induces endothelial nitric oxide synthase dysfunction: role of heat shock protein 90 and caveolin-1. Free Radic Biol Med. 2002;33:52–62. doi: 10.1016/s0891-5849(02)00851-1. [DOI] [PubMed] [Google Scholar]
- 127. Ratajczak P. Damy T. Heymes C. Oliviero P. Marotte F. Robidel E. Sercombe R. Boczkowski J. Rappaport L. Samuel JL. Caveolin-1 and -3 dissociations from caveolae to cytosol in the heart during aging and after myocardial infarction in rat. Cardiovasc Res. 2003;57:358–369. doi: 10.1016/s0008-6363(02)00660-0. [DOI] [PubMed] [Google Scholar]
- 128. Razani B. Engelman JA. Wang XB. Schubert W. Zhang XL. Marks CB. Macaluso F. Russell RG. Li M. Pestell RG. Di Vizio D. Hou H., Jr. Kneitz B. Lagaud G. Christ GJ. Edelmann W. Lisanti MP. Caveolin-1 null mice are viable but show evidence of hyperproliferative and vascular abnormalities. J Biol Chem. 2001;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- 129. Razani B. Engelman JA. Wang XB. Schubert W. Zhang XL. Marks CB. Macaluso F. Russell RG. Li M. Pestell RG. Di Vizio D. Hou H., Jr. Knietz B. Lagaud G. Christ GJ. Edelmann W. Lisanti MP. Caveolin-1 null mice are viable but show evidence of hyper- proliferative and vascular abnormalities. J Biol Chem. 2001;276:38121–38138. doi: 10.1074/jbc.M105408200. [DOI] [PubMed] [Google Scholar]
- 130. Razani B. Wang XB. Engelman JA. Battista M. Lagaud G. Zhang XL. Kneitz B. Hou H., Jr. Christ GJ. Edelmann W. Lisanti MP. Caveolin-2-deficient mice show evidence of severe pulmonary dysfunction without disruption of caveolae. Mol Cell Biol. 2002;22:2329–2344. doi: 10.1128/MCB.22.7.2329-2344.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Razzaq TM. Ozegbe P. Jury EC. Sembi P. Blackwell NM. Kabouridis PS. Regulation of T-cell receptor signalling by membrane microdomains. Immunology. 2004;113:413–426. doi: 10.1111/j.1365-2567.2004.01998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Rodgers W. Farris D. Mishra S. Merging complexes: properties of membrane raft assembly during lymphocyte signaling. Trends Immunol. 2005;26:97–103. doi: 10.1016/j.it.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 133. Rodriguez–Feo JA. Hellings WE. Moll FL. De Vries JP. van Middelaar BJ. Algra A. Sluijter J. Velema E. van der Broek T. Sessa WC. De Kleijn DP. Pasterkamp G. Caveolin-1 influences vascular protease activity and is a potential stabilizing factor in human atherosclerotic disease. PLoS ONE. 2008;3:e2612. doi: 10.1371/journal.pone.0002612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Rothberg KG. Heuser JE. Donzell WC. Ying YS. Glenney JR. Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- 135. Sage MD. Jennings RB. Myocyte swelling and plasmalemmal integrity during early experimental myocardial ischemia in vivo. Scanning Microsc. 1988;2:477–484. [PubMed] [Google Scholar]
- 136. Sakata N. Ida T. Joshita T. Ooneda G. Scanning and transmission electron microscopic study on the cerebral arterial endothelium of experimentally hypertensive rats fed an atherogenic diet. Acta Pathol Jpn. 1983;33:1105–1113. doi: 10.1111/j.1440-1827.1983.tb02156.x. [DOI] [PubMed] [Google Scholar]
- 137. Sanchez M. Galisteo M. Vera R. Villar IC. Zarzuelo A. Tamargo J. Perez–Vizcaino F. Duarte J. Quercetin downregulates NADPH oxidase, increases eNOS activity and prevents endothelial dysfunction in spontaneously hypertensive rats. J Hypertens. 2006;24:75–84. doi: 10.1097/01.hjh.0000198029.22472.d9. [DOI] [PubMed] [Google Scholar]
- 138. Sargiacomo M. Scherer PE. Tang Z. Kübler E. Song KS. Sanders MC. Lisanti MP. Oligomeric structure of caveolin: implications for caveolae membrane organization. Proc Natl Acad Sci USA. 1995;92:9407–9411. doi: 10.1073/pnas.92.20.9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Sbaa E. Frerart F. Feron O. The double regulation of endothelial nitric oxide synthase by caveolae and caveolin: a paradox solved through the study of angiogenesis. Trends Cardiovasc Med. 2005;15:157–162. doi: 10.1016/j.tcm.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 140. Scherer PE. Lewis RY. Volonté D. Engelman JA. Galbiati F. Couet J. Kohtz DS. van Donselaar E. Peters P. Lisanti MP. Cell-type and tissue-specific expression of caveolin-2. Caveolins 1 and 2 co-localize and form a stable hetero-oligomeric complex in vivo. J Biol Chem. 1997;272:29337–29346. doi: 10.1074/jbc.272.46.29337. [DOI] [PubMed] [Google Scholar]
- 141. Scherer PE. Tang Z. Chun M. Sargiacomo M. Lodish HF. Lisanti MP. Caveolin isoforms differ in their N-terminal protein sequence and subcellular distribution. Identification and epitope mapping of an isoform-specific monoclonal antibody probe. J Biol Chem. 1995;270:16395–16401. doi: 10.1074/jbc.270.27.16395. [DOI] [PubMed] [Google Scholar]
- 142. Schlegel A. Lisanti MP. A molecular dissection of caveolin-1 membrane attachment and oligomerization. Two separate regions of the caveolin-1 C-terminal domain mediate membrane binding and oligomer/oligomer interactions in vivo. J Biol Chem. 2000;275:21605–21617. doi: 10.1074/jbc.M002558200. [DOI] [PubMed] [Google Scholar]
- 143. Schmitz G. Grandl M. Role of redox regulation and lipid rafts in macrophages during Ox-LDL-mediated foam cell formation. Antioxid Redox Signal. 2007;9:1499–1518. doi: 10.1089/ars.2007.1663. [DOI] [PubMed] [Google Scholar]
- 144. Schutz GJ. Kada G. Pastushenko VP. Schindler H. Properties of lipid microdomains in a muscle cell membrane visualized by single molecule microscopy. EMBO J. 2000;19:892–901. doi: 10.1093/emboj/19.5.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Severs NJ. Plasma membrane cholesterol in myocardial muscle and capillary endothelial cells. Distribution of filipin-induced deformations in freeze-fracture. Eur J Cell Biol. 1981;25:289–299. [PubMed] [Google Scholar]
- 146. Shao D. Segal AW. Dekker LV. Lipid rafts determine efficiency of NADPH oxidase activation in neutrophils. FEBS Lett. 2003;550:101–106. doi: 10.1016/s0014-5793(03)00845-7. [DOI] [PubMed] [Google Scholar]
- 147. Sheets ED. Lee GM. Simson R. Jacobson K. Transient confinement of a glycosylphosphatidylinositol-anchored protein in the plasma membrane. Biochemistry. 1997;36:12449–12458. doi: 10.1021/bi9710939. [DOI] [PubMed] [Google Scholar]
- 148. Shevchuk AI. Hobson P. Lab MJ. Klenerman D. Krauzewicz N. Korchev YE. Endocytic pathways: Combined scanning ion conductance and surface confocal microscopy study. Pflugers Arch. 2008;456:227–235. doi: 10.1007/s00424-007-0410-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Shinomura T. Nakao S. Mori K. Reduction of depolarization-induced glutamate release by heme oxygenase inhibitor: possible role of carbon monoxide in synaptic transmission. Neurosci Lett. 1994;166:131–134. doi: 10.1016/0304-3940(94)90468-5. [DOI] [PubMed] [Google Scholar]
- 150. Siafakas AR. Wright LC. Sorrell TC. Djordjevic JT. Lipid rafts in Cryptococcus neoformans concentrate the virulence determinants phospholipase B1 and Cu/Zn superoxide dismutase. Eukaryot Cell. 2006;5:488–498. doi: 10.1128/EC.5.3.488-498.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Singer SJ. Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science. 1972;175:720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- 152. Sotgia F. Minetti C. Lisanti MP. Localization of the human caveolin-3 gene to the D3S18/D3S4163/D3S4539 locus (3p25), in close proximity to the human oxytocin receptor gene. Identification of the caveolin-3 gene as a candidate for deletion in 3p-syndrome. FEBS Lett. 1999;452:177–180. doi: 10.1016/s0014-5793(99)00658-4. [DOI] [PubMed] [Google Scholar]
- 153. Sowa G. Pypaert M. Sessa WC. Distinction between signaling mechanisms in lipid rafts vs. caveolae. Proc Natl Acad Sci USA. 2001;98:14072–14077. doi: 10.1073/pnas.241409998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Sprenger RR. Horrevoets AJ. Proteomic study of caveolae and rafts isolated from human endothelial cells. Methods Mol Biol. 2007;357:199–213. doi: 10.1385/1-59745-214-9:199. [DOI] [PubMed] [Google Scholar]
- 155. Sprenger RR. Horrevoets AJ. The ins and outs of lipid domain proteomics. Proteomics. 2007;7:2895–2903. doi: 10.1002/pmic.200700189. [DOI] [PubMed] [Google Scholar]
- 156. Tahir SA. Yang G. Ebara S. Timme TL. Satoh T. Li L. Goltsov A. Ittmann M. Morrisett JD. Thompson TC. Secreted caveolin-1 stimulates cell survival/clonal growth and contributes to metastasis in androgen-insensitive prostate cancer. Cancer Res. 2001;61:3882–3885. [PubMed] [Google Scholar]
- 157. Tahir SA. Yang G. Goltsov AA. Watanabe M. Tabata K. Addai J. Fattah el MA. Kadmon D. Thompson TC. Tumor cell-secreted caveolin-1 has proangiogenic activities in prostate cancer. Cancer Res. 2008;68:731–739. doi: 10.1158/0008-5472.CAN-07-2668. [DOI] [PubMed] [Google Scholar]
- 158. Tomoiu A. Larbi A. Fortin C. Dupuis G. Fulop T., Jr Do membrane rafts contribute to human immunosenescence? Ann NY Acad Sci. 2007;1100:98–110. doi: 10.1196/annals.1395.008. [DOI] [PubMed] [Google Scholar]
- 159. Tsutsumi Y. Horikawa Y. Jennings M. Kidd M. Niesman I. Yokoyama U. Head B. Hagiwara Y. Ishikawa Y. Miyanohara A. Patel P. Insel P. Patel H. Roth D. Increased expression of caveolin-3 promotes ischemic preconditioning. Circulation. 2008;118:1979–1988. doi: 10.1161/CIRCULATIONAHA.108.788331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Unno M. Matsui T. Ikeda–Saito M. Structure and catalytic mechanism of heme oxygenase. Nat Prod Rep. 2007;24:553–570. doi: 10.1039/b604180a. [DOI] [PubMed] [Google Scholar]
- 161. Ushio–Fukai M. Localizing NADPH oxidase-derived ROS. Sci STKE. 2006;2006:re8. doi: 10.1126/stke.3492006re8. [DOI] [PubMed] [Google Scholar]
- 162. van der Loo B. Bachschmid M. Skepper JN. Labugger R. Schildknecht S. Hahn R. Mussig E. Gygi D. Luscher TF. Age-associated cellular relocation of Sod 1 as a self-defense is a futile mechanism to prevent vascular aging. Biochem Biophys Res Commun. 2006;344:972–980. doi: 10.1016/j.bbrc.2006.03.224. [DOI] [PubMed] [Google Scholar]
- 163. Venema VJ. Ju H. Zou R. Venema RC. Interaction of neuronal nitric-oxide synthase with caveolin-3 in skeletal muscle. Identification of a novel caveolin scaffolding/inhibitory domain. J Biol Chem. 1997;272:28187–28190. doi: 10.1074/jbc.272.45.28187. [DOI] [PubMed] [Google Scholar]
- 164. Vilhardt F. van Deurs B. The phagocyte NADPH oxidase depends on cholesterol-enriched membrane microdomains for assembly. EMBO J. 2004;23:739–748. doi: 10.1038/sj.emboj.7600066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Vinten J. Johnsen AH. Roepstorff P. Harpoth J. Tranum–Jensen J. Identification of a major protein on the cytosolic face of caveolae. Biochim Biophys Acta. 2005;1717:34–40. doi: 10.1016/j.bbamem.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 166. Viola A. Gupta N. Tether and trap: regulation of membrane-raft dynamics by actin-binding proteins. Nat Rev Immunol. 2007;7:889–896. doi: 10.1038/nri2193. [DOI] [PubMed] [Google Scholar]
- 167. Volonte D. McTiernan CF. Drab M. Kasper M. Galbiati F. Caveolin-1 and caveolin-3 form heterooligomeric complexes in atrial cardiac myocytes that are required for doxorubicin-induced apoptosis. Am J Physiol Heart Circ Physiol. 2008;294:H392–H401. doi: 10.1152/ajpheart.01039.2007. [DOI] [PubMed] [Google Scholar]
- 168. Wang L. Sapuri–Butti AR. Aung HH. Parikh AN. Rutledge JC. Triglyceride-rich lipoprotein lipolysis increases aggregation of endothelial cell membrane microdomains and produces reactive oxygen species. Am J Physiol Heart Circ Physiol. 2008;295:H237–H244. doi: 10.1152/ajpheart.01366.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Wang R. Wang Z. Wu L. Carbon monoxide-induced vasorelaxation and the underlying mechanisms. Br J Pharmacol. 1997;121:927–934. doi: 10.1038/sj.bjp.0701222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Williams TM. Lisanti MP. The caveolin proteins. Genome Biol. 2004;5:214. doi: 10.1186/gb-2004-5-3-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Yamabhai M. Anderson RG. Second cysteine-rich region of epidermal growth factor receptor contains targeting information for caveolae/rafts. J Biol Chem. 2002;277:24843–24846. doi: 10.1074/jbc.C200277200. [DOI] [PubMed] [Google Scholar]
- 172. Yamada E. The fine structure of the gall bladder epithelium of the mouse. J Biophys Biochem Cytol. 1955;1:445–458. doi: 10.1083/jcb.1.5.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Yang B. Rizzo V. TNF-alpha potentiates protein-tyrosine nitration through activation of NADPH oxidase and eNOS localized in membrane rafts and caveolae of bovine aortic endothelial cells. Am J Physiol Heart Circ Physiol. 2007;292:H954–H962. doi: 10.1152/ajpheart.00758.2006. [DOI] [PubMed] [Google Scholar]
- 174. Yoshizaki F. Nakayama H. Iwahara C. Takamori K. Ogawa H. Iwabuchi K. Role of glycosphingolipid-enriched microdomains in innate immunity: microdomain-dependent phagocytic cell functions. Biochim Biophys Acta. 2008;1780:383–392. doi: 10.1016/j.bbagen.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 175. Zeng F. Watt NT. Walmsley AR. Hooper NM. Tethering the N-terminus of the prion protein compromises the cellular response to oxidative stress. J Neurochem. 2003;84:480–490. doi: 10.1046/j.1471-4159.2003.01529.x. [DOI] [PubMed] [Google Scholar]
- 176. Zhang AY. Yi F. Jin S. Xia M. Chen QZ. Gulbins E. Li PL. Acid sphingomyelinase and its redox amplification in formation of lipid raft redox signaling platforms in endothelial cells. Antioxid Redox Signal. 2007;9:817–828. doi: 10.1089/ars.2007.1509. [DOI] [PubMed] [Google Scholar]
- 177. Zhang AY. Yi F. Zhang G. Gulbins E. Li PL. Lipid raft clustering and redox signaling platform formation in coronary arterial endothelial cells. Hypertension. 2006;47:74–80. doi: 10.1161/10.1161/01.HYP.0000196727.53300.62. [DOI] [PubMed] [Google Scholar]
- 178. Zhuo M. Small SA. Kandel ER. Hawkins RD. Nitric oxide and carbon monoxide produce activity-dependent long-term synaptic enhancement in hippocampus. Science. 1993;260:1946–1950. doi: 10.1126/science.8100368. [DOI] [PubMed] [Google Scholar]
- 179. Zidovetzki R. Levitan I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: evidence, misconceptions and control strategies. Biochim Biophys Acta. 2007;1768:1311–1324. doi: 10.1016/j.bbamem.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Zuo L. Ushio–Fukai M. Ikeda S. Hilenski L. Patrushev N. Alexander RW. Caveolin-1 is essential for activation of Rac1 and NAD(P)H oxidase after angiotensin II type 1 receptor stimulation in vascular smooth muscle cells: Role in redox signaling and vascular hypertrophy. Arterioscler Thromb Vasc Biol. 2005;25:1824–1830. doi: 10.1161/01.ATV.0000175295.09607.18. [DOI] [PubMed] [Google Scholar]