Abstract
This study evaluated how dynamic compression induced changes in gene expression, tissue composition, and structural properties of the intervertebral disc using a rat tail model. We hypothesized that daily exposure to dynamic compression for short durations would result in anabolic remodeling with increased matrix protein expression and proteoglycan content, and that increased daily load exposure time and experiment duration would retain these changes but also accumulate changes representative of mild degeneration. Sprague-Dawley rats (n = 100) were instrumented with an Ilizarov-type device and divided into three dynamic compression (2 week-1.5 h/day, 2 week-8 h/day, 8 week-8 h/day at 1 MPa and 1 Hz) and two sham (2 week, 8 week) groups. Dynamic compression resulted in anabolic remodeling with increased matrix mRNA expression, minimal changes in catabolic genes or disc structure and stiffness, and increased glysosaminoglycans (GAG) content in the nucleus pulposus. Some accumulation of mild degeneration with 8 week-8 h included loss of annulus fibrosus GAG and disc height although 8-week shams also had loss of disc height, water content, and minor structural alterations. We conclude that dynamic compression is consistent with a notion of “healthy” loading that is able to maintain or promote matrix biosynthesis without substantially disrupting disc structural integrity. A slow accumulation of changes similar to human disc degeneration occurred when dynamic compression was applied for excessive durations, but this degenerative shift was mild when compared to static compression, bending, or other interventions that create greater structural disruption.
Keywords: intervertebral disc, disc degeneration, mechanobiology, animal model, biomechanics
Substantial socioeconomic problems that result from low back pain are often associated with intervertebral disc (IVD) degeneration.1 The etiology of disc degeneration is complex and multifactorial with heredity, mechanical loading, and nutrition all playing significant roles.2-7 These contributing factors are interactive and strongly affected by aging. For example, degradation of the molecular structure of the disc during aging can also render it more susceptible to mechanical injuries.4 Loading type, magnitude, duration, and frequency all influence cell metabolic responses and matrix remodeling.5,8,9 Mechanical loading may induce remodeling directly via tissue stresses that may predispose the matrix to damage or through alterations in the biosynthetic response due to mechanically altered biosynthesis of proteins and enzymes.8 IVD degeneration is manifested morphologically through a loss in disc height, decreased nucleus volume, and in a loss of distinction between nucleus pulposus (NP) and annulus fibrosus (AF). In more severe degeneration, a more extensive loss in IVD structural organization has been noted, with formation of clefts and tears in the AF.2 Degenerative changes on the biochemical level are noted first in the NP, with a loss of glysosaminoglycans (GAG) and a change in the ratio of collagen-1 and collagen-2; these changes are accompanied or initiated by increased synthesis and activation of matrix degrading enzymes such as matrix metalloproteinases (MMPs).10 Changes in the AF are less evident, but are characterized by changes in collagen matrix composition and organization.11 Biomechanically, IVD degeneration results in altered range of motion,12 and a variety of changes in mechanical properties of NP and AF.6
There are relatively few in vivo models of degeneration with cumulative trauma, nutritional, familial, or age-related factors as the initiating factor, but a relative preponderance of animal models of degeneration initiated by acute injury.13 For cumulative mechanical loading, static compression and bending applied in vivo to IVDs using rodent tail models were shown to cause IVD alterations representative of early degenerative changes.14-16 IVDs of dogs with sustained static compression showed some early signs of degeneration that were greatest when subjected to the highest force for the longest duration.17
Animal models demonstrated that short-term dynamic compression had stimulatory effects on matrix and enzyme mRNA levels while immobilization alone tended to downregulate matrix mRNA.8 Magnitude and frequency thresholds were also established suggesting that a maintenance stimulus (0.2 Hz, 0.2 MPa) exists at which the steady state of structural mRNA transcription is maintained, and that alterations above or below this level result in remodeling, repair or degeneration. The mRNA responses are extremely useful in establishing the rapid cellular response to mechanical stimulation, but may not be entirely predictive of protein level changes. Detecting IVD remodeling in response to physiologically relevant loads requires prolonged in vivo investigations.
The aim of this work was to evaluate how changes in gene expression, tissue composition, and structural properties occur and accumulate following short- and long-term dynamic compression. A previously established rat tail in vivo model18 and loading device19 were used because of the high levels of mechanical control on IVDs. The first experiment was performed to investigate effects of increasing daily exposure to dynamic compression for 2 weeks (sham, 1.5 h and 8 h). The second experiment was performed to investigate effects of prolonged experimental duration (2 weeks vs. 8 weeks) at 8 h/day exposure to dynamic compression. Discs were analyzed for gene expression, biochemical composition, mechanical properties, IVD height, and morphology. We hypothesized that daily exposure to dynamic compression would result in anabolic remodeling with increased matrix protein expression and proteoglycan content when applied for 1.5 h/day, and that increased daily load exposure time and experiment duration to 8 h/day would retain these changes but also accumulate mild degenerative changes. We use the term degeneration throughout to refer to remodeling changes that have similarities to those seen in human IVD degeneration.1,2 The magnitude (12.6 N) and frequency (1 Hz) of dynamic compression loading were chosen because they were suggestive of increased catabolic responses and demonstrated to be in the upper range of physiological stresses for rats in prior short-term studies.20 The load duration of 1.5 h per day was representative of daily exercise while 8 h per day was considered rigorous labor. The 8 week-8 h group was considered prolonged and rigorous for rats, especially when considering the rapid metabolic rates of rodents.
METHODS
Surgical Procedure
All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Vermont. Twelve-week-old Sprague-Dawley rats (skeletally mature) were instrumented with an Ilizarov-type device as previously described21 (Fig. 1). Briefly, under general anesthesia using xylazine (5-10 mg/kg rat) and ketamine (40-80 mg/kg rat) and application of subcutaneous analgesia (buprenorphine, 0.05 mg/kg rat), carbon fiber rings were attached to caudal vertebra c 8 and c 9, using sterile 0.8-mm Kirschner wires. Time of surgery was less than 30 min per animal, buprenorphine was administered 12, 24, and 36 h postoperatively and loading was initiated on postop day 3.
Figure 1.
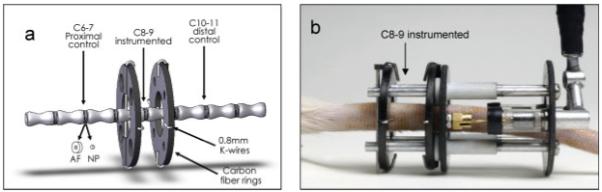
(a) Solid model of instrumented rat tail including control and instrumented levels and disc harvest involving separation of annulus fibrosus from nucleus pulposus. (b) Photograph of instrumented rat tail with pneumatic loading device. [Color scheme can be viewed in the online issue, which is available at http://www.interscience.wiley.com]
Loading Procedure
For all dynamic compression groups, a newly developed pneumatic loading apparatus (Fig. 1)19 applied sinusoidal compression loading with a peak magnitude of 12.6 N and a minimum at 0 N at a frequency of 1.0 Hz. IVD geometry was assumed circular with a diameter of 4 mm, corresponding to an effective stress of 1 MPa (approximately 300% body weight) which was previously shown to influence IVD cell mRNA expression.20 The wearable system eliminated the need for anesthesia during loading and permitted animals to move freely, eat, and drink. Sham animals had surgically attached rings and were kept under identical conditions but did not wear the loading apparatus and did not experience any loading except small magnitudes/durations during measurements of mechanical properties (see below).
Study Design
Animals were divided into the following groups:
2 Week-Sham (n = 20)
Rats subjected to same surgical procedure and conditions as the loaded 2 weeks animals but did not experience any loading.
2 Week-1.5 h (n = 20)
Rats subjected to 2 weeks of dynamic compression for 1.5 h per day, and sacrificed 24 h following the last loading procedure.
2 Week-8 h (n = 20)
Rats subjected to 2 weeks of dynamic compression for 8 h per day, and sacrificed 24 h following the last loading procedure.
8 Week-Sham (n = 20)
Rats subjected to same surgical procedure and conditions as the loaded 8 weeks animals but did not experience any loading.
8 Week-8 h (n = 20)
Rats subjected to 8 weeks of dynamic compression for 8 h per day and sacrificed 24 h following the last loading procedure.
In each group, animals were randomly assigned to gene expression analysis (n = 10), measurement of glycosaminoglycan (GAG) and water content (n = 5) or histology (n = 5). Animals assigned the GAG/water content and histology groups were also analyzed for measurement of IVD height and mechanical properties (phase angle, stiffness).
Measurement of Mechanical Properties and Disc Height
Mechanical properties were evaluated weekly using a loading device capable of applying force and measuring displacement.20 The test protocol was intended to provide rapid but consistent mechanical data in this in vivo study and consisted of a 20 s tare load at 1.875 N (i.e., ~0.15 MPa) followed by 280 s of sinusoidal loading between 1.875-12.6 N (~0.15-1.0 MPa) from which the complex (i.e., dynamic) stiffness and phase angle were calculated using software written in MATLAB (Mathworks, Natick, MA). Mechanical property measurements were made prior to the loading procedure with week 0 measurements being made following the 72 h surgical recovery period and prior to the first loading period.
IVD height was measured in animals undergoing mechanical property measurements at weeks 0, 2, 4, 6, and 8 using radiographs. Radiographs were taken immediately after gas anesthesia and before mechanical testing to minimize IVD swelling due to muscle relaxation. IVD height of instrumented levels (c 8-9) in loaded and sham animals were measured, and all radiographs used an aluminium step wedge for intensity corrections. Radiographs were scanned for image processing, and evaluated using an image analysis technique using software written in MATLAB involving semi-automated thresholding and edge detection, and a technique to minimize parallax error.15,22 For IVD height, week 0 measurements were made using the preoperative radiograph.
Gene Expression Analysis
Animals were sacrificed 24 h after the last loading cycle, and instrumented (c 8-9) as well as two control discs (c 6-7 and c 10-11) were harvested and flash frozen in liquid nitrogen. NP and AF were excised and prepared for real-time RT-PCR as previously described.20 Briefly, tissue was pulverized in liquid nitrogen and total RNA was isolated via Trizol extraction and purified by use of GenElute Mammalian Total RNA Kit (Sigma, St. Louis, MO). RNA was reverse transcribed using the Taqman Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Gene expression of aggrecan, collagen-1, collagen-2, TIMP-1, TIMP-3, MMP-2, MMP-3, MMP-13, and ADAMTS-4 was measured on the GeneAmp 7700 Sequence detection system using primers/probes previously published.18 Unpublished sequences are as follows: TIMP-1: fwd = acc tgg tta taa ggg cta aat tca tg, rv = att tcc cac agc gtc gaa tc, probe = tac cag cgt tat gag atc aag atg act aag atg. TIMP-3: fwd = cct ttg gca ctc tgg tct aca ct, rv = ctt tca gag gct tcc gtg tga, probe = aag caa atg aag atg tac cga gga ttc a. MMP2: Assay on demand, Applied Biosystems (Rn01538168_m1).
Relative gene expression was calculated using the comparative Ct method, as previously described,18 in which each of the nine genes was first normalized to 18S RNA [previously shown to be unaffected by loading,23 next normalized to the mRNA levels of the average of the two internal control discs (c 6-7 and c 10-11; Fig. 1)], and then presented as “fold change” in mRNA level in the instrumented disc relative to internal control. Loaded and sham animals were compared at their respective time points.
Histology Analysis
Motion segments (fresh) were fixed in 4% PFA for 5 days, decalcified in 10% EDTA for 14 days, divided in half with a mid-sagittal cut, embedded in paraffin, and subjected to routine sectioning. Sections were stained with alcian blue for proteoglycan and picosirius red for collagen, and analyzed under brightfield at 10× magnification.
Measurements of Water and GAG Content
Immediately after dissection from fresh motion segments, wet weight of NP and AF tissue samples were taken. Samples were lyophilized for 24 h, and dry weights and percent water content were determined. Lyophilized samples were rehydrated in 0.2 ml proteinase K (1 mg/ml in 50 mM Tris-HCl pH 7.6 from Sigma, St. Louis, MO) at 4°C and then incubated at 56°C for digestion for 5 days (adding 0.1 ml of fresh enzyme solution on days 2, 3, and 4). Solubilized individual disc samples were assayed for GAG content using the dimethyl-methylene blue (DMMB) method24 and averaged to obtain mean values.
Statistical Analysis
Effects of loading group on mRNA expression and biochemical measurements were analyzed by ANOVA with Fishers PLSD post hoc test to compare the loaded groups with their respective sham. For biochemical composition measurements, comparisons between shams and 8-h loaded groups of different durations were also made to track progression. Mechanical properties and IVD height measurements were analyzed using two-way ANOVA on load group and week with post hoc analyses using Fisher’s PLSD. All analyses were performed using StatView software (SAS Institute, Cary, NC) and a significance level of p < 0.05.
RESULTS
Animals tolerated surgical and loading procedures well with normal activity levels and behaviors, and maintained or slightly increased body weight during the study period. In some rats, minor inflammation around the pins could be noted, especially in the chronic loading group, which was treated by topical application of antiinflammatory medications.
Mechanical Properties (Fig. 2)
Figure 2.
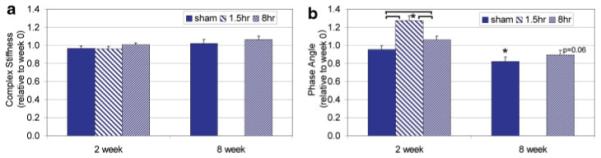
Mean±SEM of (a) complex stiffness and (b) phase angle normalized to week 0. Bracket indicates significant difference with p < 0.05 between loading group and sham at the same time point. *, Significant difference with p < 0.05 compared to week 0 within the same group. [Color scheme can be viewed in the online issue, which is available at http://www.interscience.wiley.com]
At 2 Weeks
There were no significant changes in complex stiffness between loaded and sham groups or with time. Phase angle was significantly increased after 2 weeks of loading for 1.5 h/day compared to sham and was also increased compared to day 0 values.
At 8 Weeks
No significant differences in complex stiffness were observed between loaded and sham animals or over time. Sham and 8-h loaded animals exhibited a loss in phase angle over time (compared to week 0), but loading was not different than sham. Average week 0 values for complex stiffness and phase angle were 53.4±7.1 N/mm and 4.8±0.6°, respectively.
Disc Height (Fig. 3)
Figure 3.
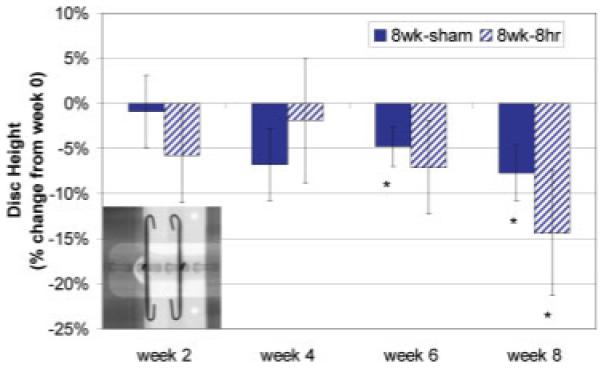
Mean±SEM relative IVD height change for the 8 week-8 h and sham groups measured from radiographs and expressed as percent change from preoperative conditions (week 0). Measurements were taken prior to daily loading regime following 16 h recovery. *, Significant with p < 0.05 relative to week 0. Inset: sample radiographic image. [Color scheme can be viewed in the online issue, which is available at http://www.interscience.wiley.com]
At 2 Weeks
No changes were observed after 2 weeks of loading (compared to shams) or compared to week 0.
At 8 Weeks
With an increase in time, disc height was decreased in loaded and sham animals compared to week 0. This effect was significant after 6 and 8 weeks. Average preoperative disc height was 0.82±0.03 mm.
Gene Expression
In general, larger fold changes in gene expression were observed in loaded NP tissue compared to loaded AF tissue. Loading effects on anabolic genes were more pronounced than on catabolic genes for NP. No changes in gene expression were observed for shams indicating that instrumentation did not affect mRNA levels (Fig. 4).
Figure 4.
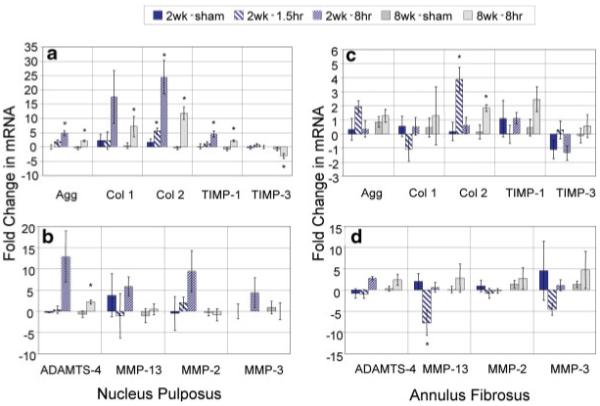
Mean±SEM fold change in mRNA levels relative to internal control levels from real-time RT-PCR for nine genes. Tissue was harvested 24 h after the last loading cycle. (a, c) Anabolic and (b, d) catabolic gene expression for nucleus pulposus and annulus fibrosus regions, respectively. *, Significant with p < 0.05 between loading group and sham. [Color scheme can be viewed in the online issue, which is available at http://www.interscience.wiley.com]
Nucleus Pulposus
At 2 Weeks
For most genes, largest alterations of mRNA expression were seen for the 2 week-8 h group compared to both 2 week-1.5 h and 8 week-8 h groups. In detail, aggrecan expression was significantly increased after 2 week-8 h (+4.9-fold). Collagen-1 expression was strongly stimulated for 2 week-8 h (+17.5-fold), but did not reach significance due to large variance. Similarly, collagen-2 expression was significantly stimulated at 2 week-1.5 h (+5.6-fold) and was strongly upregulated at 2 week-8 h (+24.4-fold, significant). A significant increase of TIMP-1 was seen for 2 week-8 h (+4.6-fold). No significant changes were observed for matrix enzymes, though increases were observed at 2 week-8 h in some animals.
At 8 Weeks
A similar pattern was seen at 8 weeks compared to 2 weeks, but effects were less pronounced. However, gene expression remained significantly altered after 8 weeks of loading, indicating that a steady state condition was reached. Aggrecan expression remained upregulated after 8 week-8 h daily loading (+2.1-fold). For collagen-1 expression, a significant +7.1-fold increase was found and collagen-2 stimulation was maintained (+11.8-fold). The anti-catabolic genes TIMP-1 and TIMP-3 were less influenced, but significant effects were seen for TIMP-1 (+2.2-fold) and TIMP-3(-3.1-fold). Effects on catabolic genes (ADAMTS-4, MMP-13, MMP-2, MMP-3) were less pronounced and showed high variation; a small but significant increase was seen for ADAMTS-4 expression for the 8 week-8 h group (±2.2 fold).
Annulus Fibrosus
At 2 Weeks
Aggrecan expression showed a trend towards stimulation at 2 week-1.5 h (+1.9-fold). While collagen-1 expression remained unchanged, collagen-2 was significantly increased at 2 week-1.5 h (+3.9-fold). With regard to catabolic enzymes, a decrease in MMP-13 for 2 week-1.5 h was observed (-7.7-fold).
At 8 Weeks
A minor upregulation of collagen-2 was seen at 8 week-8 h (+1.8-fold). TIMP-1 expression was slightly upregulated (+2.5-fold), but no changes were seen in TIMP-3. Similarly to NP, no significant effects were found for catabolic gene expression, though increases were observed in some animals relative to the sham.
Histology
Relatively small changes were seen in histological specimens for all groups (Fig. 5).
Figure 5.
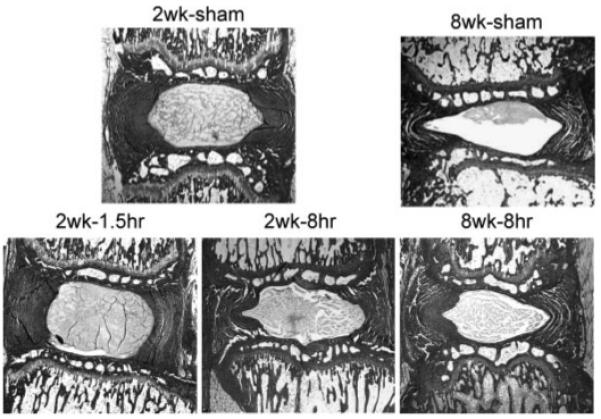
Histological sections of rat caudal IVDs stained with picosirius red and alcian blue (original magnification, ×10).
At 2 Weeks
Some loss of IVD height was observed in the 2 week-8 h group while the 2 week-1.5 h group had a large, prominent NP and retained IVD height.
At 8 Weeks
The histological preparation in the 8 week-sham and 8 week-8 h animals involved an extended decalcification that created some small-scale annular cracking in loaded and sham groups, and some loss of nuclear tissue in the sham. In this context, there was no obvious annular disruption in the loaded groups or sham groups, and no obvious annular damage that could be attributed to loading, although a loss of IVD height was observed.
Water Content (Fig. 6A)
Figure 6.
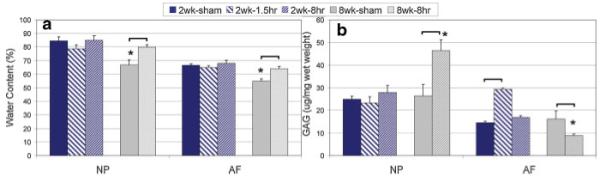
Mean±SEM of (a) water and (b) GAG contents. Bracket indicates significant difference with p < 0.05 between loading group and sham at the same time point. *, Significant difference between week 2 and week 8 within the same group. [Color scheme can be viewed in the online issue, which is available at http://www.interscience.wiley.com]
At 2 Weeks
No changes were observed in both 2-week groups in either NP or AF due to loading. However, water content was higher in 2 week-sham compared to 8 week-sham in NP and AF.
At 8 Weeks
In NP and AF, a statistically significant increase in water content was observed in 8 week-8 h groups compared to 8 week-sham.
GAG Content
GAG content was measured from individual NP and AF tissue samples, with an average size of 2.54±0.15 mg for NP and 7.96±0.64 mg for AF. In general, GAG content was approximately twofold higher in the NP than in the AF (Fig. 6B).
At 2 Weeks
There was no significant change in GAG due to loading in the NP, but a significant increase in the AF in the 2 week-1.5 h group (twofold).
At 8 Weeks
GAG content increased in the NP for 8 week-8 h compared to 8 week-sham. In contrast, the AF had a significant decrease in GAG for 8 week-8 h compared to 8 week-sham.
DISCUSSION
A rat tail model was used to test the hypotheses that 1) daily exposure to physiologic levels of dynamic compression would result in anabolic remodeling in the intervertebral disc and 2) increased daily exposure time and experiment duration would retain anabolic matrix protein expression but also induce processes similar to those seen for degenerated discs. This study also evaluated specific pathways of remodeling from gene expression changes to alterations on the composition and structural levels. Study 1 was a 2-week experiment that increased daily exposure time to dynamic compression from 1.5 h to 8 h. Study 2 increased experiment duration from 2 weeks to 8 weeks while maintaining the same 8-h daily exposure time.
Study 1
For the 2 week-1.5 h group, there was an increase in phase angle, an increase in anabolic gene expression (mainly collagen-2) and no changes in anti-catabolic genes or histology. For the AF, we additionally noticed an inhibition of MMP-13 expression and an increase in GAG content indicating anabolic remodeling. The 2 week-8 h group had similar results with larger increase in anabolic and anti-catabolic gene expression, but no other major changes. This leads to the conclusion that dynamic compression leads to anabolic remodeling even with increased daily exposure time.
Study 2
The 8 week-8 h group exhibited a general pattern of anabolic remodeling with an adaptation to a new steady state level. There was a notable decrease in TIMP-3 and a significant increase in ADAMTS-4 at 8 weeks in the NP that may indicate early degenerative changes. These changes were more apparent in the AF with loss of GAG and increase in water. Study 2 led to the conclusion that the disc responds anabolically to dynamic compression at high daily exposure rates, but that early signs of degeneration initiation and accumulation may begin with extended experiment duration, especially in the AF.
Pathways of Remodeling
For aggrecan, continued mechanical stimulation in study 2 resulted in a clear pathway towards anabolic remodeling,8 where significant upregulation on the mRNA level resulted in increased NP GAG content and an associated increase in water content in the 8 week-8 h group relative to 8 week-sham. A similar relationship between aggrecan mRNA and GAG was previously shown when applying hydrostatic pressure on isolated disc cells25,26 and static compression on cartilage explants.27 The significant and large upregulation of matrix proteins demonstrated in this study requires greater mRNA synthesis rates than upregulation of catabolic genes, considering the high baseline abundance of mRNA for aggrecan, collagen-1 and -2.21 Catabolic genes were generally expressed at low levels in this study. For catabolic genes, this remodeling pathway is further complicated by enzyme activation which is influenced by loading.28
Sham Effect
The sham group had a significant loss of IVD height and water content, a reduction in phase angle, and some minor histological changes. The lost water content and disc height suggest either an age-related change or disuse of the tail. The disuse theory is particularly likely since the 8 week-8 h group increased GAG and had a recovery of water content relative to the 8 week-sham animals. Unpublished results of intervertebral disc heights during growth and maturation in rats suggest that IVD heights do not decrease with maturation (I. Stokes: personal communication, 2008). However, human IVD height increases slightly with maturation and aging, although the amount is small compared to the overall increase in spine height due to vertebral growth.29,30 The literature also provides IVD heights that are larger than our published values,22,31 further supporting the disuse mechanism over aging.
A loss of IVD height is often considered among the most telling early signs of IVD degeneration. While the dynamic compression group had the largest loss of IVD height at 8 weeks, it was only reduced by about 15%, and it is noteworthy that the sham groups had significant loss of IVD height at earlier time points than the compression group. The reduction in IVD height of the compression group was not accompanied by a loss in water or GAG content in the NP, which may be explained by annular bulging or structural remodeling that was also detectable by phase angle changes. Accumulation of mild degeneration in the AF was detected by an increase in water content and loss of GAG content, which is suggestive of disruption in the collagen network, similar to changes observed in articular cartilage during early osteoarthritis.
The small size of the rat, the use of caudal IVDs, and the presence of notochordal cells in the rat NP are all limitations that reduce the relevance of this model to the human situation.32 Notochordal cells differ in morphology and function from chondrocyte-like cells found in the mature human NP.33,34 Notochordal cells are retained into maturity in the NP of the rat, pig, cat, rabbit, and some species of dogs, in contrast to humans that lose notochordal cells at very young ages in development.35,36 The large size of human IVDs and lack of notochordal cells diminishes the ability of human IVDs to repair,37 and we speculate that degenerative changes may accumulate less rapidly in rat IVDs than in human IVDs.
The high daily exposure to dynamic compression and long-term experimental duration applied to the rat tail IVDs in this study produced biochemical, biomechanical, and structural changes representative of mild and/or early accumulation of degeneration, however, it is likely that a more rapid accumulation of degeneration from mechanical loading may require immobilization, super-physiological load magnitudes or frequencies, endplate damage, and/or bending. Mechanically induced degeneration has been observed previously in rodent tail models. Static compression led to altered annular structure, increased apoptosis, decreased collagen-2 and aggrecan expression, and spinal instability after 1 week in a mouse model.16 Similarly, static bending induced cell death and phenotypic changes after 1 week, with no recovery even after 3 months.38 These tail models had less control over axial loading either because two motion segments were loaded (resulting in additional buckling) or because the loading device was less stiff than in the current study. Therefore, the degenerative changes observed in these prior studies may be associated with immobilization/static loading effects,6 as well as increased shear stresses resulting from increased bending moments.39 Static compression of 0.8 MPa applied to dog spines by Hutton et al. resulted in changes in the NP (reduced proteoglycan and collagen-2 and increased collagen-1), which can be interpreted as mild degeneration, but no changes in the AF.17 The reduced proteoglycan contrasts the increased GAG reported in the NP of the current study, providing further evidence that static compression induces greater changes than dynamic compression. Yet, both studies demonstrate that physiological compression loading does not result in accumulation of severe degenerative changes, even though large changes in mRNA expression may occur. Consequently, physical loading has the capacity to induce anabolic remodeling, as well as accelerate degenerative changes in very specific manners that may interact with other risk factors of degeneration including nutrition, genetics, and aging.
We conclude that dynamic compression is consistent with a notion of “healthy” loading that is able to maintain and/or promote matrix biosynthesis without substantially disrupting structural integrity of the IVD. However, dynamic compression can lead to the accumulation of mild degenerative changes, particularly in the AF, when applied at substantial daily levels for long-term durations. Based on our results and the literature, we infer that immobilization and bending are more damaging since they inhibit matrix biosynthesis and/or provide large shear strains that more substantially disrupt matrix structure. Current results demonstrated that cyclic compression had the ability to increase GAG content and restore water content, suggesting the potential that physical activity might be able to promote repair or postpone degenerative changes when applied to moderate levels. Based on the inability of dynamic compression to restore IVD height, we speculate that anabolic remodeling from dynamic compression alone would be insufficient to retain IVD integrity in diseased states or with large structural disruptions. This study provides baseline information on dynamic compression effects on rat tail IVD disc remodeling which may be compared with future studies distinguishing loading modes (e.g., torsion vs. compression), evaluating loading effects on transgenic animals, and evaluating biologic repair techniques.
ACKNOWLEDGMENTS
This research was supported by a grant from the National Institute of Health (R01 AR051146). The authors gratefully acknowledge Stephen Bell for the use of the Cardiology Research Lab, and Daniel Ackil for technical assistance.
REFERENCES
- 1.An HS, Anderson PA, Haughton VM, et al. Introduction: disc degeneration: summary. Spine. 2004;29:2677–2678. doi: 10.1097/01.brs.0000147573.88916.c6. [DOI] [PubMed] [Google Scholar]
- 2.Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine. 2006;31:2151–2161. doi: 10.1097/01.brs.0000231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- 3.Battie MC, Videman T. Lumbar disc degeneration: epidemiology and genetics. J Bone Joint Surg [Am] 2006;88(Suppl 2):3–9. doi: 10.2106/JBJS.E.01313. [DOI] [PubMed] [Google Scholar]
- 4.Hadjipavlou AG, Tzermiadianos MN, Bogduk N, et al. The pathophysiology of disc degeneration: a critical review. J Bone Joint Surg [Br] 2008;90:1261–1270. doi: 10.1302/0301-620X.90B10.20910. [DOI] [PubMed] [Google Scholar]
- 5.Setton LA, Chen J. Mechanobiology of the intervertebral disc and relevance to disc degeneration. J Bone Joint Surg [Am] 2006;88(Suppl 2):52–57. doi: 10.2106/JBJS.F.00001. [DOI] [PubMed] [Google Scholar]
- 6.Stokes IA, Iatridis JC. Mechanical conditions that accelerate intervertebral disc degeneration: overload versus immobilization. Spine. 2004;29:2724–2732. doi: 10.1097/01.brs.0000146049.52152.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urban JP, Smith S, Fairbank JC. Nutrition of the intervertebral disc. Spine. 2004;29:2700–2709. doi: 10.1097/01.brs.0000146499.97948.52. [DOI] [PubMed] [Google Scholar]
- 8.Iatridis JC, MacLean JJ, Roughley PJ, et al. Effects of mechanical loading on intervertebral disc metabolism in vivo. J Bone Joint Surg [Am] 2006;88(Suppl 2):41–46. doi: 10.2106/JBJS.E.01407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lotz JC, Chin JR. Intervertebral disc cell death is dependent on the magnitude and duration of spinal loading. Spine. 2000;25:1477–1483. doi: 10.1097/00007632-200006150-00005. [DOI] [PubMed] [Google Scholar]
- 10.Roughley PJ. Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine. 2004;29:2691–2699. doi: 10.1097/01.brs.0000146101.53784.b1. [DOI] [PubMed] [Google Scholar]
- 11.Gruber HE, Hanley EN., Jr. Observations on morphologic changes in the aging and degenerating human disc: secondary collagen alterations. BMC Musculoskelet Disord. 2002;3:9. doi: 10.1186/1471-2474-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mimura M, Panjabi MM, Oxland TR, et al. Disc degeneration affects the multidirectional flexibility of the lumbar spine. Spine. 1994;19:1371–1380. doi: 10.1097/00007632-199406000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Lotz JC. Animal models of intervertebral disc degeneration: lessons learned. Spine. 2004;29:2742–2750. doi: 10.1097/01.brs.0000146498.04628.f9. [DOI] [PubMed] [Google Scholar]
- 14.Court C, Colliou OK, Chin JR, et al. The effect of static in vivo bending on the murine intervertebral disc. Spine J. 2001;1:239–245. doi: 10.1016/s1529-9430(01)00056-0. [DOI] [PubMed] [Google Scholar]
- 15.Iatridis JC, Mente PL, Stokes IA, et al. Compression-induced changes in intervertebral disc properties in a rat tail model. Spine. 1999;24:996–1002. doi: 10.1097/00007632-199905150-00013. [DOI] [PubMed] [Google Scholar]
- 16.Lotz JC, Colliou OK, Chin JR, et al. Compression-induced degeneration of the intervertebral disc: an in vivo mouse model and finite-element study. Spine. 1998;23:2493–2506. doi: 10.1097/00007632-199812010-00004. [DOI] [PubMed] [Google Scholar]
- 17.Hutton WC, Toribatake Y, Elmer WA, et al. The effect of compressive force applied to the intervertebral disc in vivo. A study of proteoglycans and collagen. Spine. 1998;23:2524–2537. doi: 10.1097/00007632-199812010-00007. [DOI] [PubMed] [Google Scholar]
- 18.MacLean JJ, Lee CR, Grad S, et al. Effects of immobilization and dynamic compression on intervertebral disc cell gene expression in vivo. Spine. 2003;28:973–981. doi: 10.1097/01.BRS.0000061985.15849.A9. [DOI] [PubMed] [Google Scholar]
- 19.Stinnett-Donnelly J, MacLean J, Iatridis J. Removable precision device for in-vivo mechanical compression of rat tail intervertebral discs. J Med Devices. 2007;1:56–61. doi: 10.1115/1.2355692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maclean JJ, Lee CR, Alini M, et al. Anabolic and catabolic mRNA levels of the intervertebral disc vary with the magnitude and frequency of in vivo dynamic compression. J Orthop Res. 2004;22:1193–1200. doi: 10.1016/j.orthres.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Maclean JJ, Roughley PJ, Monsey RD, et al. In vivo intervertebral disc remodeling: kinetics of mRNA expression in response to a single loading event. J Orthop Res. 2008;26:579–588. doi: 10.1002/jor.20560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacLean JJ, Lee CR, Alini M, et al. The effects of short-term load duration on anabolic and catabolic gene expression in the rat tail intervertebral disc. J Orthop Res. 2005;23:1120–1127. doi: 10.1016/j.orthres.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 23.Lee CR, Grad S, Maclean JJ, et al. Effect of mechanical loading on mRNA levels of common endogenous controls in articular chondrocytes and intervertebral disk. Anal Biochem. 2005;341:372–375. doi: 10.1016/j.ab.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 25.Hutton WC, Elmer WA, Boden SD, et al. The effect of hydrostatic pressure on intervertebral disc metabolism. Spine. 1999;24:1507–1515. doi: 10.1097/00007632-199908010-00002. [DOI] [PubMed] [Google Scholar]
- 26.Hutton WC, Elmer WA, Bryce LM, et al. Do the intervertebral disc cells respond to different levels of hydro-static pressure? Clin Biomech (Bristol, Avon) 2001;16:728–734. doi: 10.1016/s0268-0033(01)00080-8. [DOI] [PubMed] [Google Scholar]
- 27.Ragan PM, Badger AM, Cook M, et al. Down-regulation of chondrocyte aggrecan and type-II collagen gene expression correlates with increases in static compression magnitude and duration. J Orthop Res. 1999;17:836–842. doi: 10.1002/jor.1100170608. [DOI] [PubMed] [Google Scholar]
- 28.Hsieh AH, Lotz JC. Prolonged spinal loading induces matrix metalloproteinase-2 activation in intervertebral discs. Spine. 2003;28:1781–1788. doi: 10.1097/01.BRS.0000083282.82244.F3. [DOI] [PubMed] [Google Scholar]
- 29.Pfirrmann CW, Metzdorf A, Elfering A, et al. Effect of aging and degeneration on disc volume and shape: a quantitative study in asymptomatic volunteers. J Orthop Res. 2006;24:1086–1094. doi: 10.1002/jor.20113. [DOI] [PubMed] [Google Scholar]
- 30.Stokes IA, Windisch L. Vertebral height growth predominates over intervertebral disc height growth in adolescents with scoliosis. Spine. 2006;31:1600–1604. doi: 10.1097/01.brs.0000222008.15750.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Connell GD, Vresilovic EJ, Elliott DM. Comparison of animals used in disc research to human lumbar disc geometry. Spine. 2007;32:328–333. doi: 10.1097/01.brs.0000253961.40910.c1. [DOI] [PubMed] [Google Scholar]
- 32.Alini M, Eisenstein SM, Ito K, et al. Are animal models useful for studying human disc disorders/degeneration? Eur Spine J. 2008;17:2–19. doi: 10.1007/s00586-007-0414-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erwin WM, Inman RD. Notochord cells regulate intervertebral disc chondrocyte proteoglycan production and cell proliferation. Spine. 2006;31:1094–1099. doi: 10.1097/01.brs.0000216593.97157.dd. [DOI] [PubMed] [Google Scholar]
- 34.Hunter CJ, Matyas JR, Duncan NA. The notochordal cell in the nucleus pulposus: a review in the context of tissue engineering. Tissue Eng. 2003;9:667–677. doi: 10.1089/107632703768247368. [DOI] [PubMed] [Google Scholar]
- 35.Hayes AJ, Benjamin M, Ralphs JR. Extracellular matrix in development of the intervertebral disc. Matrix Biol. 2001;20:107–121. doi: 10.1016/s0945-053x(01)00125-1. [DOI] [PubMed] [Google Scholar]
- 36.Hunter CJ, Matyas JR, Duncan NA. Cytomorphology of notochordal and chondrocytic cells from the nucleus pulposus: a species comparison. J Anat. 2004;205:357–362. doi: 10.1111/j.0021-8782.2004.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grunhagen T, Wilde G, Soukane DM, et al. Nutrient supply and intervertebral disc metabolism. J Bone Joint Surg [Am] 2006;88(Suppl 2):30–35. doi: 10.2106/JBJS.E.01290. [DOI] [PubMed] [Google Scholar]
- 38.Court C, Chin JR, Liebenberg E, et al. Biological and mechanical consequences of transient intervertebral disc bending. Eur Spine J. 2007;16:1899–1906. doi: 10.1007/s00586-007-0476-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Costi JJ, Stokes IA, Gardner-Morse M, et al. Direct measurement of intervertebral disc maximum shear strain in six degrees of freedom: motions that place disc tissue at risk of injury. J Biomech. 2007;40:2457–2466. doi: 10.1016/j.jbiomech.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


