Abstract
The maintenance of the intervertebral disc extracellular matrix is regulated by mechanical loading, nutrition, and the accumulation of matrix proteins and cytokines that are affected by both aging and degeneration. Evidence suggests that cellular aging may lead to alterations in the quantity and quality of extracellular matrix produced. The aims of this study were to examine the role of loading and maturation (a subset of aging), and the interaction between these two factors in intervertebral disc cell gene expression and biosynthesis in a controlled 3D culture environment. Cells were isolated from young (4–6 months) and mature (18–24 months) bovine caudal annulus fibrosus and nucleus pulposus tissue. Isolated cells were seeded into alginate and dynamically compressed for 7 days at either 0.1, 1, or 3 Hz or maintained as a free-swelling control. After 7 days, DNA and sulfated glycosaminoglycan contents were analyzed along with real time, quantitative reverse transcription-polymerase chain reaction analysis for collagen types I and II, aggrecan, and matrix metalloproteinase-3 gene expression. Results suggest that maturation plays an important role in intervertebral disc homeostasis and influences the cell response to mechanical loading. While isolated intervertebral disc cells responded to mechanical compression in 3D culture, the effect of loading frequency was minimal. Altered cellular phenotype and biosynthesis rates appear to be an attribute of the cell maturation process, potentially independent of changes in cellular microenvironment associated with lost nutrition and disc degeneration. Mature cells may have a decreased capacity to create or retain extracellular matrix components in response to mechanical loading compared to young cells.
Keywords: intervertebral disc, aging, mechanical loading, alginate, gene expression
Mechanical stimulation has been demonstrated to affect cell metabolism and gene expression in the intervertebral disc (IVD) in vivo,1–3 in situ,4 and in vitro.5–7 However, differences in culture systems, methods of load application, and cellular phenotypes between species have complicated comparison of results between studies. Cellular aging also has demonstrated effects in articular cartilage,8 IVD,9,10 and bone,11 among other tissues.12,13 Investigation of the interaction between mechanical stimulation and cellular aging has potential implications for the understanding of the mechanism of IVD degeneration, and for future repair strategies, such as tissue engineering and cell therapy.
The response of the IVD to dynamic compression has been reported in vivo; however, limited literature exists on the effects of dynamic compression in vitro. The isolation of IVD cells from their surrounding matrix is advantageous for mechanotransduction studies as it allows for a consistent load to be applied to every cell. Tissue matrix mechanical properties can vary, and evidence of increased pericellular matrix stiffness with aging and disease in chondrocytes14 points to changes in the ability of an applied load to be transduced to the cell level. The primary method used to apply mechanical stimulation to IVD cells in vitro thus far has been hydrostatic pressure, although static compression has also been studied.15 In chondrocytes, however, compressive loads have been applied dynamically to examine a range of biological responses.16–19 Interestingly, while chondrocytes have been shown to respond to both frequency and duration of an applied deformational load,16–19 the IVD has been shown to respond primarily to the magnitude of an applied hydrostatic load.6,7 Further investigation of applied deformational load in the IVD is needed, as hydrostatic pressure may fail to simulate in vivo cell mechanical stimulation pathways by neglecting cell strain, and static and dynamic compression have been shown to have dramatically different effects.20
It is difficult to differentiate the cellular response to aging from that of exposure to a degenerative environment because the two are often coupled. Decreased cellular function and altered synthesis of extracellular matrix components have been demonstrated in articular chondrocytes with normal aging,8 reducing both the quantity and quality of repaired matrix after damage. A recent study on IVD cells demonstrated increasing incidence of cellular senescence correlated with increased disc degeneration, potentially indicating that fewer cells populate the matrix following necrosis and/or apoptosis,21 again leading to diminished capacity for repair. A shift in cell phenotype in the nucleus pulposus (NP) also occurs in some species, with some (pig, rat, rabbit) retaining notochordal cells into maturity, whereas others (human, cow, sheep) lack this cell type.22
This study was composed of three aims. Aim 1 addressed whether tissue donor age affects IVD cell synthesis and gene expression in 3D alginate culture. Bovine IVD tissues, from young (4–6 months) and mature (18–24 months) caudal discs, previously shown to compare well with IVD tissue from humans aged < 15 years and 15–40 years, respectively,23 were used to ensure relative homogeneity in genetic, nutritional, and other environmental factors. Cells isolated from tissue of a greater age (mature cells) were hypothesized to have a reduced capacity for recreating extracellular matrix, with reduced DNA and sulfated glycosaminoglycan (sGAG) contents, and lower expression of anabolic genes, such as collagen types I and II and aggrecan. Aim 2 sought to determine whether dynamic compression loading would affect IVD cell synthesis and gene expression in a frequency-dependent manner. Based on evidence from earlier studies,1,5,24 increasing compression frequency was hypothesized to increase the accumulation of extracellular matrix and DNA synthesis, and to increase anabolic gene expression, consistent with findings in chondrocytes.18,25 Aim 3 was to determine the interaction between aging and dynamic loading frequency. We hypothesized that decreases in cell metabolism associated with age could be counteracted by an increased cell metabolism brought about by mechanical stimulation, with similar GAG and DNA contents achieved at higher frequencies between young and mature tissue-derived cells, and similar patterns of gene expression.
Methods
IVDs were removed from five young (4–6 months) and five mature (18–24 months) bovine tails. NP and annulus fibrosus (AF) tissue were separated by careful dissection and placed in washing medium (high glucose DMEM, 10% fetal bovine serum [FBS], 200 U/mL penicillin and streptomycin, 0.50 μg/mL amphotericin-B, 10% FBS, 50 μg/mL ascorbic acid, and 0.5% v/v 5 M NaCl and 0.4 M KCl to adjust medium osmolarity) for 4–6 days followed by cell isolation through enzymatic digestion consisting of 1 h of pronase (0.2%) and 8–10 h of collagenase type IV (0.2% for AF, 0.125% for NP) at 37°C with constant agitation. The resulting cell suspensions were passed through a 70 μm mesh sieve and washed twice with phosphate buffered saline (PBS). The cells from all five mature or all five young tails were pooled and a one-time expansion at a high cell density (8 × 106 cells/mm2) was performed when necessary to achieve adequate starting numbers of cells. The duration of monolayer expansion was less than 9 days, with medium changes every 3 to 4 days. The whole experiment was repeated once (for a total of 10 mature and 10 young tails used).
Alginate gel constructs were created in 96-well plates using a slow set technique,26 with cells seeded at a density of 4 × 106 cells/mL. The slow set technique, which allows for control over geometry and uniform cell seeding of the alginate-cell construct, utilizes calcium carbonate as the source of calcium ions to crosslink the alginate and form a hydrogel. When mixed with slowly hydrolyzing d-glucono-δ-lactone, calcium ions are liberated from the calcium carbonate at a controlled rate, allowing for homogeneous dispersion of both crosslinking ions and cells prior to gelation, producing a structurally uniform gel with homogeneous cell distribution. All gels were created with a consistent volume of alginate-cell suspension resulting in consistently reproducible construct geometry (height of 3.27 ± 0.15 mm and diameter of 5.82 ± 0.22 mm). After curing, gels were placed into mechanical stimulation test dishes with culture medium (high glucose DMEM, 100 U/mL penicillin and streptomycin, 0.25 μg/mL amphotericin-B, 10% FBS, 50 μg/mL ascorbic acid, and 0.5% v/v 5 M NaCl and 0.4 M KCl to adjust medium osmolarity to be similar to that in the IVD in situ) and allowed to equilibrate overnight in a 5% O2 incubator.
Mechanical stimulation consisted of 2 h of daily compressive strain from 0.065 to 0.392 mm from initial loading platen to alginate gel contact. The initial gel height averaged 3.27 mm, which translates the applied displacements to 2–12% of the initial alginate gel height. Stimulation was applied for 7 days at one of three frequencies (0.1, 1, or 3 Hz) using a previously described loading device.18 Additionally, an unstimulated (free-swelling) control was maintained. After 7 days of loading, gels were harvested for analysis. For all groups, gels were maintained at 37°C, 5% O2. Media was changed every 3–4 days, and 1 mL was saved at −80°C for later analysis.
Nitrite concentrations were measured from aliquots of cell culture medium saved at each medium change using the Griess reaction (Promega, Madison, WI). Standard curves were generated with unused culture medium. For each 50 μL sample of culture medium, 50 μL of sulfanilamide was added and allowed to incubate for 5–10 min away from light. Then 50 μL of N-1-napthylethylenediamine dihydrochloride (NED) solution was added to the wells and again incubated 5–10 min protected from light. Sample and standard curve absorbance was analyzed at 550 nm on a microplate reader. Resulting molar concentrations were normalized to the number of gel constructs present in each dish.
Gels were dissociated by the addition of 1 mL of a 55 mM sodium citrate solution. Cell viability was checked using 1 μM calcein-AM and 1 μM ethidium homodimer-1 (LIVE/DEAD kit; Invitrogen, Carlsbad, CA) in PBS, which was incubated for 15 min before visualization. The remaining dissociated gel was centrifuged and the supernatant carefully removed from the separated pellet and stored. Pellets and supernatants were digested (300 μg/mL papain, 10 mM l-cystine, 10 mM EDTA, and 100 mM sodium acetate) at 60°C overnight. Both pellet and supernatant digests were analyzed for DNA content and sGAG content. DNA content was determined using a Picogreen assay kit (Picogreen; Sigma, St. Louis, MO). sGAG content was determined with 1,9-dimethyl-methylene blue (DMMB) adjusted to a pH of 1.5 to minimize alginate interference. Chondroitin-6-sulfate was used to generate the standard curve.
Real time, quantitative reverse transcription-polymerase chain reaction (qRT-PCR) was performed on dissociated gels (n = 5 per group). After RNA isolation and cDNA transcription, gene expression of 18S rRNA, aggrecan, collagen types I and II, and matrix metalloproteinase (MMP3) was analyzed using bovine gene-specific primers and SYBR Green. Transcript levels were normalized to that of the 18S rRNA housekeeping gene.
Statistical analyses were performed for each of the hypotheses. First, a one way ANOVA (p < 0.05) followed by a Bonferroni post-hoc test was performed for loading effects (LOAD, hypothesis 1) by comparing each loaded group with the age-matched control and again for aging effects (AGE, hypothesis 2) by comparing data between ages at matched condition points. Finally, a two-way ANOVA was performed to address the interaction between aging and loading effects (AGE*LOAD) again followed by a Bonferroni post-hoc test.
Results
Viability was maintained throughout the experiment with >93% of cells viable after culturing and loading. Results between repeated experiments (each set of five pooled animals) were consistent, and results are presented as an average ± SEM of all samples.
DNA content was greater in mature NP cell constructs (286.2 ± 65.4 ng) versus young NP cells (68.2 ± 6.4 ng) (p = 0.02), and was not significantly affected by loading in the NP. No significant statistical interaction between age and load was found in DNA content in the NP. In the AF, DNA content increased in young AF cells (181.7 ± 21.5 ng) versus mature AF cells (103.3 ± 10.0 ng) (p < 0.0001), and was also affected by loading (p < 0.0001), with pair-wise increases in the 0.1 Hz (156.5 ± 28.8 ng) and 3 Hz (182.4 ± 18.6 ng) loading groups versus controls (87.8 ± 18.6 ng) and 1 Hz loading (93.3 ± 9.2 ng). A statistical interaction between age and load was also found (p < 0.0001).
GAG content in the NP was not significantly different between ages (p > 0.7) or between loading groups (p > 0.7), and no interaction was seen between the groups (p > 0.7). In the AF, an effect of age was observed, with higher GAG contents in mature (43.5 ± 1.62 μg) than in young AF constructs (37.6 ± 1.84 μg) (p = 0.05). No significant effect of loading or relationship between groups was observed (p > 0.4).
When the sGAG content was normalized to the matched DNA content of each construct (Fig. 1), the value was greater in young NP constructs (0.656 ± 0.047 μg/ng) versus mature NP constructs (0.228 ± 0.031 μg/ng) (p < 0.0001). No effect of loading was found in the NP (p = 0.2); however, a statistical interaction between age and load was observed in the NP (p < 0.0001). In the AF, a higher sGAG/DNA content was observed in mature AF constructs (0.445 ± 0.028 μg/ng) compared to young AF constructs (0.26 ± 0.025 μg/ng) (p < 0.001). The 3 Hz load group had lower sGAG/DNA (0.25 ± 0.022 μg/ng) compared to all other loading groups (0.4 ± 0.031 μg/ng) (p < 0.0001). Furthermore, a significant interaction between age and load was found (p < 0.001).
Figure 1.
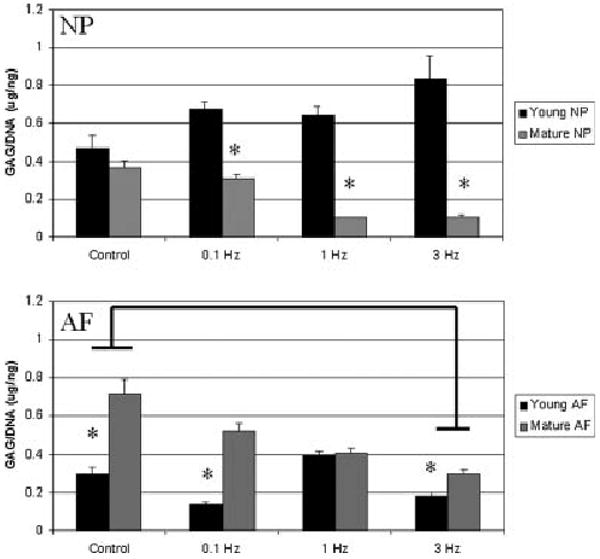
sGAG/DNA content (μg/ng) of cell–alginate constructs derived from the nucleus pulposus (top) and annulus fibrosus (bottom) after 7 days in culture under mechanical stimulation. Values shown are mean ± SEM. Results for cells derived from young tissue are shown in black, mature cells in grey. Asterisks indicate significantly lower amounts between age groups; bars indicate significant differences between loading groups. sGAG/DNA content was reduced in mature NP cells versus young NP cells, and increased in mature AF cells versus young AF cells.
Nitrite concentrations (Fig. 2) in the medium containing both the AF and NP cell constructs remained consistent, without significant differences between young and mature constructs, and between loading groups. No significant interaction between age and load was found for either cell type.
Figure 2.

Nitrite concentrations (μM) in medium normalized to the number of constructs in each well of the nucleus pulposus (top) and annulus fibrosus (bottom) after 7 days in culture under mechanical stimulation. Values shown are mean ± SEM. Results for cells derived from young tissue are shown in black, mature cells in grey. No significant differences were found between groups.
In the NP (Fig. 3), expression of collagen types I and II increased significantly in mature cells as compared to young cells (p < 0.03). No significant effect of loading was observed (p = 0.1). However, a significant relationship between age and loading was observed (p < 0.001), with expression of collagen types I and II decreasing in young cells and increasing in mature cells with increasing loading frequency. Aggrecan gene expression in the NP was not significantly affected by age or loading, and no significant interaction was found between the two (p > 0.05). MMP3 gene expression was significantly affected by age (p < 0.02) and by loading (p < 0.05), with a significant interaction observed between the two (p < 0.05). In the AF (Fig. 4), collagen type I expression was significantly affected by aging and 3 Hz loading, and a significant interaction was also observed between the two (p < 0.001). Aggrecan expression was affected by aging and loading at 1 and 3 Hz (p < 0.05). Expression of collagen type II, aggrecan, and MMP3 were all affected by aging and 1 and 3 Hz loading (p < 0.001) in the annulus.
Figure 3.

qRT-PCR analysis of gene expression in cultures of nucleus pulposus cells under mechanical stimulation. Results for cells derived from young tissue are shown in black, mature cells in grey. Values shown are mean ± SEM. Asterisks indicate significantly lower amounts between age groups; bars indicate significant differences between loading groups. Expression of collagen types I and II was increased in mature cells versus young cells. MMP3 expression was increased in response to 0.1 and 1 Hz loading in young NP cells, and was decreased in mature NP cells.
Figure 4.

qRT-PCR data for annulus fibrosus cells under mechanical stimulation. Results for cells derived from young tissue are shown in black, mature cells in grey. Values shown are mean ± SEM. Asterisks indicate significantly lower amounts between age groups; bars indicate significant differences between loading groups. Expression of collagen types I and II, aggrecan, and MMP3 was significantly decreased in young AF versus mature AF cells. Loading at 1 and 3 Hz significantly decreased expression of collagen type II and aggrecan, while significantly increasing MMP3 expression. Collagen type I was only increased at 3 Hz loading.
Discussion
This study examined the effects of cell maturation (as a model of aging) and loading frequency on extracellular matrix production and gene expression of isolated IVD cells in 3D gel culture. Young and mature IVD cells remained viable and mechanically responsive in 3D alginate culture. Generally, anabolic gene expression was increased in mature cells and catabolic gene expression of MMP3 was decreased. However, less sGAG/DNA production was observed in mature cells than in young cells. Therefore, increasing age increased the anabolic and decreased the catabolic gene expression of IVD cells, but this shift was not reflected in terms of the level of sGAG production. Loading effects were typically frequency independent, indicating that the application of mechanical stimulation had a similar effect regardless of the frequency with which it was applied. Overall, aging was a dominating factor over loading. A significant interaction between age and loading was observed in some cases, particularly in the AF where loading had very distinct anabolic and catabolic responses for mature and young cells.
This study is among the first to examine the response of isolated intervertebral cells to dynamic compression. Increased hydrostatic loading frequencies have been shown to decrease DNA content in the NP6 and increase DNA content in the AF, leading to a decreased sGAG/DNA content.24 Similarly, this study observed greater DNA contents in the mature NP and the young and mature AF, which further translated into reduced sGAG/DNA contents, suggesting that these cells proliferate at a higher rate than they produce extracellular matrix. In the young NP, however, an increase in the sGAG/DNA value was noted with loading, possibly indicating an age-related change in the ability for these cells to produce and accumulate sGAG in response to loading. Expression levels of aggrecan and collagen type II have also been shown to be up-regulated with dynamic hydrostatic loading.5 In this study, an up-regulation of collagen type II was observed in mature NP cells, but not in young NP, or any AF cells. In addition, an up-regulation of collagen type I was observed in mature AF and NP cells, possibly indicating a difference in the cell response to compression versus hydrostatic loading, but not in the young NP or AF, again indicating an age-related change in the cellular response to loading.
Alginate has been shown to be a suitable culture system for IVD NP cells,27,28 but may15,27,29 or may not30 be appropriate for AF cells. No indication of phenotype shift in the AF cells was seen in this study, with levels of gene expression for collagen type I and aggrecan maintained in control samples after 7 days relative to day zero (data not shown). While a decrease in IVD cell viability in alginate has been previously reported, 30,27 no apparent increase in cell death was noted in this study. It should be noted that in this study the duration of monolayer expansion was also kept to a minimum to ensure phenotype maintenance.28
This was a displacement-controlled loading experiment with compression frequency being varied and load duration remaining constant. Consequently, both strain rate and duty cycle varied with the frequency effects (360 cycles for 0.1Hz, 3,600 cycles for 1Hz, 10,800 cycles for 3 Hz). Interestingly, however, very few frequency effects were detected, suggesting that the cell strain had larger effects on IVD cells than strain rate or duty cycle in alginate. This is inconsistent with the second hypothesis of the current study, and with studies performed with chondrocytes,18,25 but agrees with previous results following the application of hydrostatic pressure to isolated IVD cells, where loading amplitude was found to more strongly affect cell biosynthesis than frequency.6,7 Further study of chondrocytes and IVD cells is warranted at different loading amplitudes and frequencies to confirm these striking differences between their signaling responses to dynamic compression.
Prior finite element analysis on uniform agarose constructs under similar conditions demonstrated that axial strains, radial strains, and radial fluid flux are present as expected in this unconfined compression configuration.18 It is notable that for agarose, the radial strain and pressurization fields were uniform throughout the constructs but fell to zero immediately adjacent to the outer free edge. We assume similar behaviors for alginate as modulus is similar to agarose,26 although a full set of material properties for alginate gels created using the methods in this study is not available. However, the relative ratio of hydrostatic pressure to matrix strain is likely to be in contrast to that occurring in the highly viscoelastic in vivo IVD tissue matrix, so we further interpret these findings as cell strain effects in 3D culture rather than a true simulation of in vivo compression.
Based on these results, we conclude that maturation plays an important role in IVD homeostasis and also influences the cell response to externally applied stimulation by mechanical loading. This finding in bovine IVD cells over a course of 14–20 months should be further investigated in human cells, where the course of maturation into aging is not only longer in duration, but may also be accompanied by a shift from notochordal to more chondrocytic cell types in the NP. While isolated bovine IVD cells respond to mechanical loading, the effect of the loading frequency was minimal, and mature cells may also have a decreased capacity to create or retain extracellular matrix components in response to mechanical stimulation compared to young cells. In vivo, age-related changes in composition and structure of the IVD and endplate reduce nutritional transport to influence cell biosynthesis and phenotype. Likewise, age-related stiffening of pericellular matrix may alter the transmission of an applied load to the cell. The removal of the cells from their in vivo environment and resuspension into alginate allowed the two cell ages (mature and young) to have similar physicochemical signals with equal nutritional availability and similar mechanical signals because the pericellular matrix was removed by the pronase-collagenase digestion. Therefore, the age-related changes observed in this study were solely the result of cellular maturation and not due to altered disc nutrition or pericellular matrix stiffening. However, it is possible that the cellular maturation observed is associated with age-related alterations to the physicochemical microenvironment of the cell, and plasticity of cellular responses following longer duration culture is a relevant area for future study. These results indicate that altered cell phenotype and biosynthesis rates are attributes of normal cell maturation processes, consistent with previous studies in different tissues,8 and suggest that aging effects are potentially independent of changes in cellular microenvironment associated with degeneration and decreases in cell nutrition.
Acknowledgments
This study was sponsored by an NIH Grant (R01AR051146) and the Intramural Research Program of the NIH, and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (Z01 AR41131).
References
- 1.MacLean JJ, Lee CR, Grad S, et al. Effects of immobilization and dynamic compression on intervertebral disc cell gene expression in vivo. Spine. 2003;28:973–981. doi: 10.1097/01.BRS.0000061985.15849.A9. [DOI] [PubMed] [Google Scholar]
- 2.MacLean JJ, Lee CR, Alini M, et al. The effects of short-term load duration on anabolic and catabolic gene expression in the rat tail intervertebral disc. J Orthop Res. 2005;23:1120–1127. doi: 10.1016/j.orthres.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 3.Maclean JJ, Lee CR, Alini M, et al. Anabolic and catabolic mRNA levels of the intervertebral disc vary with the magnitude and frequency of in vivo dynamic compression. J Orthop Res. 2004;22:1193–1200. doi: 10.1016/j.orthres.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Haschtmann D, Stoyanov JV, Ferguson SJ. Influence of diurnal hyperosmotic loading on the metabolism and matrix gene expression of a whole-organ intervertebral disc model. J Orthop Res. 2006;24:1957–1966. doi: 10.1002/jor.20243. [DOI] [PubMed] [Google Scholar]
- 5.Wuertz K, Urban JP, Klasen J, et al. Influence of extracellular osmolarity and mechanical stimulation on gene expression of intervertebral disc cells. J Orthop Res. 2007;25:1513–1522. doi: 10.1002/jor.20436. [DOI] [PubMed] [Google Scholar]
- 6.Kasra M, Merryman WD, Loveless KN, et al. Frequency response of pig intervertebral disc cells subjected to dynamic hydrostatic pressure. J Orthop Res. 2006;24:1967–1973. doi: 10.1002/jor.20253. [DOI] [PubMed] [Google Scholar]
- 7.Kasra M, Goel V, Martin J, et al. Effect of dynamic hydrostatic pressure on rabbit intervertebral disc cells. J Orthop Res. 2003;21:597–603. doi: 10.1016/S0736-0266(03)00027-5. [DOI] [PubMed] [Google Scholar]
- 8.Martin JA, Buckwalter JA. Aging, articular cartilage chondrocyte senescence and osteoarthritis. Biogerontology. 2002;3:257–264. doi: 10.1023/a:1020185404126. [DOI] [PubMed] [Google Scholar]
- 9.Taylor TK, Melrose J, Burkhardt D, et al. Spinal biomechanics and aging are major determinants of the proteoglycan metabolism of intervertebral disc cells. Spine. 2000;25:3014–3020. doi: 10.1097/00007632-200012010-00008. [DOI] [PubMed] [Google Scholar]
- 10.Kandel RA, Hamilton D, Seguin C, et al. An in vitro tissue model to study the effect of age on nucleus pulposus cells. Eur Spine J. 2007;16:2166–2173. doi: 10.1007/s00586-007-0467-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perrien DS, Akel NS, Dupont-Versteegden EE, et al. Aging alters the skeletal response to disuse in the rat. Am J Physiol Regul Integr Comp Physiol. 2007;292:R988–996. doi: 10.1152/ajpregu.00302.2006. [DOI] [PubMed] [Google Scholar]
- 12.Vannini N, Pfeffer U, Lorusso G, et al. Endothelial cell aging and apoptosis in prevention and disease: E-selectin expression and modulation as a model. Curr Pharm Des. 2008;14:221–225. doi: 10.2174/138161208783413248. [DOI] [PubMed] [Google Scholar]
- 13.Dudhia J, Scott CM, Draper ER, et al. Aging enhances a mechanically-induced reduction in tendon strength by an active process involving matrix metalloproteinase activity. Aging Cell. 2007;6:547–556. doi: 10.1111/j.1474-9726.2007.00307.x. [DOI] [PubMed] [Google Scholar]
- 14.Alexopoulos LG, Setton LA, Guilak F. The biomechanical role of the chondrocyte pericellular matrix in articular cartilage. Acta Biomater. 2005;1:317–325. doi: 10.1016/j.actbio.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Yan W, Setton LA. Static compression induces zonal-specific changes in gene expression for extracellular matrix and cytoskeletal proteins in intervertebral disc cells in vitro. Matrix Biol. 2004;22:573–583. doi: 10.1016/j.matbio.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Lee DA, Bader DL. Compressive strains at physiological frequencies influence the metabolism of chondrocytes seeded in agarose. J Orthop Res. 1997;15:181–188. doi: 10.1002/jor.1100150205. [DOI] [PubMed] [Google Scholar]
- 17.Kim YJ, Sah RL, Grodzinsky AJ, et al. Mechanical regulation of cartilage biosynthetic behavior: physical stimuli. Arch Biochem Biophys. 1994;311:1–12. doi: 10.1006/abbi.1994.1201. [DOI] [PubMed] [Google Scholar]
- 18.Mauck RL, Byers BA, Yuan X, et al. Regulation of cartilaginous ECM gene transcription by chondrocytes and MSCs in 3D culture in response to dynamic loading. Biomech Model Mechanobiol. 2007;6:113–125. doi: 10.1007/s10237-006-0042-1. [DOI] [PubMed] [Google Scholar]
- 19.Urban JP. The chondrocyte: a cell under pressure. Br J Rheumatol. 1994;33:901–908. doi: 10.1093/rheumatology/33.10.901. [DOI] [PubMed] [Google Scholar]
- 20.Wang DL, Jiang SD, Dai LY. Biologic response of the intervertebral disc to static and dynamic compression in vitro. Spine. 2007;32:2521–2528. doi: 10.1097/BRS.0b013e318158cb61. [DOI] [PubMed] [Google Scholar]
- 21.Gruber HE, Ingram JA, Norton HJ, et al. Senescence in cells of the aging and degenerating intervertebral disc: immunolocalization of senescence-associated beta-galactosidase in human and sand rat discs. Spine. 2007;32:321–327. doi: 10.1097/01.brs.0000253960.57051.de. [DOI] [PubMed] [Google Scholar]
- 22.Hunter CJ, Matyas JR, Duncan NA. Cytomorphology of notochordal and chondrocytic cells from the nucleus pulposus: a species comparison. J Anat. 2004;205:357–362. doi: 10.1111/j.0021-8782.2004.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demers CN, Antoniou J, Mwale F. Value and limitations of using the bovine tail as a model for the human lumbar spine. Spine. 2004;29:2793–2799. doi: 10.1097/01.brs.0000147744.74215.b0. [DOI] [PubMed] [Google Scholar]
- 24.Reza AT, Nicoll SB. Hydrostatic pressure differentially regulates outer and inner annulus fibrosus cell matrix production in 3D scaffolds. Ann Biomed Eng. 2008;36:204–213. doi: 10.1007/s10439-007-9407-6. [DOI] [PubMed] [Google Scholar]
- 25.Buschmann MD, Gluzband YA, Grodzinsky AJ, et al. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J Cell Sci. 1995;108:1497–1508. doi: 10.1242/jcs.108.4.1497. [DOI] [PubMed] [Google Scholar]
- 26.Kuo CK, Ma PX. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: part 1. Structure, gelation rate and mechanical properties. Biomaterials. 2001;22:511–521. doi: 10.1016/s0142-9612(00)00201-5. [DOI] [PubMed] [Google Scholar]
- 27.Kluba T, Niemeyer T, Gaissmaier C, et al. Human anulus fibrosis and nucleus pulposus cells of the intervertebral disc: effect of degeneration and culture system on cell phenotype. Spine. 2005;30:2743–2748. doi: 10.1097/01.brs.0000192204.89160.6d. [DOI] [PubMed] [Google Scholar]
- 28.Wang JY, Baer AE, Kraus VB, et al. Intervertebral disc cells exhibit differences in gene expression in alginate and monolayer culture. Spine. 2001;26:1747–1751. doi: 10.1097/00007632-200108150-00003. discussion 1752. [DOI] [PubMed] [Google Scholar]
- 29.Baer AE, Wang JY, Kraus VB, et al. Collagen gene expression and mechanical properties of intervertebral disc cell-alginate cultures. J Orthop Res. 2001;19:2–10. doi: 10.1016/S0736-0266(00)00003-6. [DOI] [PubMed] [Google Scholar]
- 30.Horner HA, Roberts S, Bielby RC, et al. Cells from different regions of the intervertebral disc: effect of culture system on matrix expression and cell phenotype. Spine. 2002;27:1018–1028. doi: 10.1097/00007632-200205150-00004. [DOI] [PubMed] [Google Scholar]


