Abstract
We have previously reported that breast cancer cells which overexpress HER2 produce higher levels of VEGF than cells with low levels of HER2. This study tested the hypothesis that dual targeting of the VEGF (with VEGF-Trap) and HER2 (with trastuzumab) pathways would result in greater growth inhibition of HER2-overexpressing breast cancer xenografts than either agent alone. In this study we found that human and murine endothelial cells expressed high levels of VEGF receptors (VEGFR1, VEGFR2, & VEGFR3). VEGF-Trap decreased levels of secreted VEGF derived from both human and murine cells and effectively blocked VEGF-induced tyrosine phosphorylation of VEGFR2. VEGF-Trap as a single treatment inhibited tumor microvessel density (MVD), tumor vasculature, cell proliferation, and tumor growth of BT474 xenografts in a dose-dependent manner from 2.5 mg/kg to 25 mg/kg. VEGF-Trap decreased levels of both human VEGF and PlGF protein in vivo. Trastuzumab as a single agent effectively inhibited BT474 tumor growth in a dose-dependent manner, associated with a decrease in human VEGF, tumor MVD and tumor cell proliferation. Treatment with a combination of VEGF-Trap (2.5-10 mg/kg) and trastuzumab (1 mg/kg) produced significantly greater inhibition of BT474tumor growth than either individual agent, associated with greater inhibition of tumor MVD and tumor cell proliferation. Thus, VEGF-Trap in combination with trastuzumab produces superior growth inhibition of tumor xenografts which overexpress HER2, which may result from inhibition of both tumor angiogenesis and proliferation. Similar mechanisms may contribute to the clinical anti-tumor activity of trastuzumab in combination with inhibitors of VEGF signaling pathway in women with breast cancers which overexpress HER2.
Keywords: HER2, trastuzumab, VEGF, breast cancer, angiogenesis
INTRODUCTION
The human epidermal growth factor receptor 2 (HER2, also known as ErbB-2 or c-neu) is overexpressed in 20-30% of cases of human breast cancer.1 Specific targeting of HER2 with trastuzumab (Herceptin®), a humanized monoclonal antibody against HER2, dramatically enhances the anti-tumor activity of chemotherapy.2,3 Previous studies, including our own, have demonstrated that trastuzumab inhibits proliferation of breast cancer cells by upregulating the cyclin dependent kinase inhibitor p27Kip1 and inducing G1 cell cycle arrest.4-8 Other mechanisms may contribute to the activity of trastuzumab, including inhibition of tumor angiogenesis and impaired antibody-dependent cellular cytotoxicity (ADCC) activity.9,10
Vascular endothelial growth factor (VEGF) signaling utilizes six ligands (VEGF-A, -B, -C, -D, -E, & placental growth factor) and three receptors (VEGFR1, VEGFR2 & VEGFR3).11 VEGF-A (also known as VEGF), and VEGFR2 are primarily responsible for blood vessel formation in cancer.11 At least five different isoforms (VEGF121, VEGF145, VEGF165, VEGF189 and VEGF206) are generated by alternative splicing of the VEGF gene, of which VEGF121, VEGF165, and VEGF189 are most frequently expressed.12 VEGF is a potent pro-angiogenic factor that not only stimulates endothelial cell proliferation, migration and survival, but also increases vascular permeability.11 Promotion of angiogenesis is a well-known prerequisite for tumor growth, invasion, and metastasis.11 VEGF expression is positively regulated by HER2 signaling.13-16 In our own studies, forced expression of HER2 upregulates VEGF levels, whereas inhibition of HER2 signaling with trastuzumab or HER2 siRNA decreases VEGF levels through downregulating the PI3K activity.9 VEGF levels have correlated with HER2 expression in clinical specimens of breast cancer and patients with high levels of VEGF tend to have worse clinical outcome.17-18 Thus, both preclinical and clinical studies have linked VEGF expression and HER2 signaling in human breast cancers. High levels of VEGF may contribute to the poor prognosis and aggressive behavior associated with breast cancers that overexpress HER2.
A number of monoclonal antibodies and small molecule inhibitors have been developed to target either VEGF ligands (e.g. bevacizumab and VEGF-Trap) or their receptors (e.g. sunitinib and sorafenib).19 VEGF-Trap is a humanized decoy protein that combines the Fc portion of human IgG1 with principal extracellular ligand-binding domains of VEGFR1 and VEGFR2.20 VEGF-Trap has high binding affinity for human and mouse VEGF and PlGF.21,22 VEGF-Trap can inhibit tumor growth in murine and rat transplant models, as well as human xenografts of melanoma20, glioma20, rhabdomyosarcoma20, pancreatic cancer21, Wilms’ tumor22, Ewing’s sarcoma23, ovarian cancer24, glioblastoma25, and renal cell carcinoma26, Phase I clinical trials have demonstrated that VEGF-Trap can improve systemic symptoms and generate radiographic responses in patients with advanced solid malignancies.21
Although clinical trials are currently evaluating a combination of trastuzumab and bevacizumab,27 few, if any, reports have explored mechanisms underlying the interaction of trastuzumab and VEGF-Trap in xenograft model. In this report, we have tested the efficacy of dual targeting HER2 signaling with trastuzumab and VEGF pathway with VEGF-Trap in the BT474 breast cancer xenograft model.
MATERIALS AND METHODS
Cell culture
T47D, BT549, MDAMB435, MDAMB231, MDAMB453, AU565, and SKBr3 breast cancer cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA) and grown in complete media containing RPMI 1640 (Media Preparation Core Facility, M. D. Anderson Cancer Center) supplemented with 10% fetal bovine serum (FBS, Sigma). BT474, MCF-7, murine embryonic fibroblasts (MEF), and 2H11 cell lines were purchased from ATCC and grown in Dulbecco’s modified Eagle’s medium supplemented with 10% FBS. HCC1569, HCC1954, HCC202, and ZR7530 breast cancer cell lines were kindly provided by Drs. Joe W. Gray and Richard M. Neve and were cultured in RPMI 1640 media supplemented with 10% FBS. SUM-190 and SUM-225 breast cancer cell lines were kindly provided by Dr. Stephen P. Ethier and were cultured in serum free Ham’s F12 medium (Invitrogen, Carlsbad, CA) supplemented with bovine serum albumin (0.5 μg/ml), 5 mM ethanolamine, 10 mM HEPES; 5μg/ml transferrin, 50uM NaSeO3, 5 μg/ml insulin; and 1μg/ml hydrocortisone (Sigma). Human umbilical vascular endothelial cells (HUVEC) and normal human dermal fibroblasts (NHDF) were purchased from PromoCell (Heidelberg, Germany). HUVECs were cultured in endothelial cell growth medium containing 0.02 ml/ml of FBS, 1.0 ng/ml of basic fibroblast growth factor (bFGF), 0.004 ml/ml of endothelial cell growth supplement, 0.1 ng/ml of epidermal growth factor, and 1.0 μg/ml hydrocortisone (PromoCell). NHDFs were cultured in Fibroblast Growth Medium containing 1.0 ng/ml of bFGF and 5.0 μg/ml of insulin (PromoCell). All cell lines were tested for mycoplasma and other pathogens and found to be free from contamination.
Reagents
VEGF-Trap and human Fc control of immunoglobin G (hFc) were provided by Regeneron Pharmaceuticals (Tarrytown, NY). Trastuzumab and bevacizumab were purchased from Genentech through the M D Anderson Pharmacy. An antibody to CD31 was purchased from BD Pharmingen (San Diego, CA). An antibody to Ki67 (cat# PP249) was obtained from Biocare Medical (Concord, CA). Antibodies recognizing phosphor-VEGFR2 (Y1175) and total VEGFR2 were purchased from Cell Signaling (Danvers, MA). Recombinant VEGF was purchased from Sigma. Human immunoglobin G (hIgG) purified from plasma of patients with myelomas was obtained from Calbiochem and dialyzed against sterile cold phosphate-buffered saline (PBS) to eliminate sodium azide. An antibody to HER2 (for Western blotting) was purchased from Oncogene Research Products (Cambridge, MA).
Quantitative reverse-transcription polymerase chain reaction (QRTPCR) analysis
Total RNA isolation and RT were performed as described previously.28 TaqMan QRTPCR was performed with an ABI Prism 7900HT Sequence Detection System using TaqMan® universal PCR master mix (Applied Biosystems Incorporated or ABI, Foster City, CA) as described previously.28 TaqMan primer sets used for mouse VEGF, human VEGF, mouse VEGFR1/R2/R3 were assay ID no. Mm00437304_m1, Hs00173626_m1, Mm00438980_m1, Mm01222419_m1, and Mm00433337_m1, respectively (ABI). Human GAPDH gene was used as an endogenous control (ABI, Cat# 4326317E). A SYBR Green QRTPCR assay was also used to detect human VEGFRs and VEGF isoforms. Oligonucleotide sequences of these primer sets were: human VEGFR1 (forward 5′-tctcacaca tcgacaaaccaataca-3′, reverse 5′-ggtagcagtacaattgaggacaaga-3′); human VEGFR2 (forward 5′-gcaggggacagagggacttg-3′, reverse 5′-gaggccatcgctgcactca-3′); human VEGFR3 (forward 5′-gacagctacaagtacgagcatctg-3′, reverse 5′-cgttcttgcagtcgagcagaa-3′); human VEGF121 (forward 5′-ccctgatgagatcgagtacatctt-3′, reverse 5′-gcctcggcttgtcacatttt-3′); human VEGF165 (forward 5′-ccctgatgagatcgagtacatctt-3′, reverse 5′-agcaaggcccacagggattt-3′); human VEGF189 (forward 5′-ccctgatgagatcgagtacatctt-3′, reverse 5′-aacgctccaggacttataccg-3′); human GAPDH: forward (5′-cgtcttcaccaccatggaga-3′, reverse 5′-cggccatcacgccacagttt-3′). Primers were synthesized in Sigma-Genosis (Houston, TX). PCR was in a total volume of 30μl containing 15μl of 2× Universal PCR Master mix (ABI), 3μL of SYBR Green 10×, 20μM of each forward and reverse primer and 1μl of each cDNA sample. Amplifications were carried out in triplicate in ABI7900HT 96-well microtiter plates (ABI). Thermal cycling conditions for the ABI7900HT were as follows: one cycle of 95 °C for 5 min, followed by 40 cycles of 95 °C for 10s and 59 °C for 40s; and a melting curve analysis consisting of 95 °C for 15s, 59 °C for 15s, followed by increasing temperature 1 °C per 2s until 95 °C and 95 °C for 15s. The melting curves were used to ensure there was not any non-specific amplification.
Cell proliferation assay
A crystal violet cell growth assay was used to assess anchorage-dependent cell proliferation as described previously.7
Enzyme-linked immunosorbent assays (ELISA)
Levels of human and mouse VEGF, P1GF and bFGF in cell supernatants or xenograft lysates were measured by Quantikine kits from R&D Systems (Minneapolis, MN) according to the manufacturer’s protocols.
VEGF-Trap in vitro assay
To assess the effect of VEGF-Trap on secreted VEGF of human origin, NHDF at 60% confluency were cultured in 6-well plates and were treated with either VEGF-Trap (2 μg/ml), bevacizumab (2 μg/ml), trastuzumab (2 μg/ml), or control hIgG (2 μg/ml), control hFc (2 μg/ml) for 72hrs. Supernatants were then collected and assessed for human VEGF by ELISA as described above. Similarly, to assess the effect of VEGF-Trap on secreted VEGF of murine origin, MEF at 50% confluency were cultured in 6-well plates and were treated with either VEGF-Trap (2 μg/ml), bevacizumab (2 μg/ml), or control hFc (2 μg/ml) for 72 hrs. Supernatants were then collected and assayed for murine VEGF by ELISA. To assess the ability of VEGF-Trap to block the VEGF-induced signaling, HUVEC at 80% confluency in 35mm culture dishes were starved in DMEM media without serum and antibiotics for 6 hrs. Cells were replenished with 1ml of new starved media and then stimulated with or without human VEGF (10 ng/ml) for 5 min in the presence of VEGF-Trap (2 μg/ml) or hFc (2 μg/ml). Supernatants were collected and assayed for human VEGF by ELISA, while cells were directly dissolved in situ with RIPA buffer for Western blotting with VEGFR2 antibodies.
BT474 xenografts in nude mice
BT474 xenografts in nude mice were established as described in our previous report.9 Once the tumors became palpable (mean size of 0.2 ~ 0.3 mm3 at day 12 after injection), mice were treated intraperitoneally (IP) with trastuzumab, subcutaneously with VEGF-Trap, or respective control agents twice a week. Tumors were collected immediately after scarifice to extract RNA and protein, to freeze in liquid nitrogen and to fix in formalin. Tumor sizes and body weights were measured twice a week by the same observer. The tumor volume in mm3 was calculated as described previously.9 Experiments with nude mice were repeated twice with similar results.
Immunohistochemistry (IHC) and Hematoxylin-Eosin (HE) staining procedures
Frozen tissues were used for IHC staining of CD31 as described previously.9 Formalin-fixed paraffin-embedded sections were used for IHC staining of Ki67 (used at 1:200 dilution overnight at 4°C). HE staining was routinely used to check for cell morphology and carried out according to a standard protocol provided by Poly Scientific (Bay shore, NY).
Immunofluorescence Staining for CD31
Frozen sections were fixed in cold acetone for 15 min, blocked with protein blocker for 30 min at room temperature, then incubated with CD31 antibody (1:500, Pharmingen, San Diego, CA), overnight, followed by incubation with Alexa 594-conjugated anti-rat antibody (1:1000, Invitrogen, Eugene, OR) for 1 hr at room temperature. After washing with PBS, sections were counterstained with Hoechst for 2 min and mounted.
Lectin Perfusion and Tumor Vascular Staining
Mice were anesthetized and injected intravenously with 100 μl of fluorescein Lycopersicon esculentum lectin (Vector Laboratories). Ten minutes later, we perfused mice through the ascending aorta with 4% paraformaldehyde for 2 min. Tumors were extracted, placed in 4% paraformaldehyde for another 2 hours followed by immersion in 30% sucrose/PBS overnight. Tumor samples were then embedded in OCT, and cryostat sectioned for visualization using confocal fluorescence microscopy.
Statistical Analysis
All experiments were repeated at least three times on different occasions. The results are presented as the mean ± 95% confidence intervals for all values. A paired Student’s t tests or ANOVA testing was used to compare the differences among groups, with statistical significance considered if P ≤0.05. All statistical tests and corresponding P values were two sided.
RESULTS
Human and mouse endothelial cells express exceptionally high levels of VEGFR1, VEGFR2 and VEGFR3
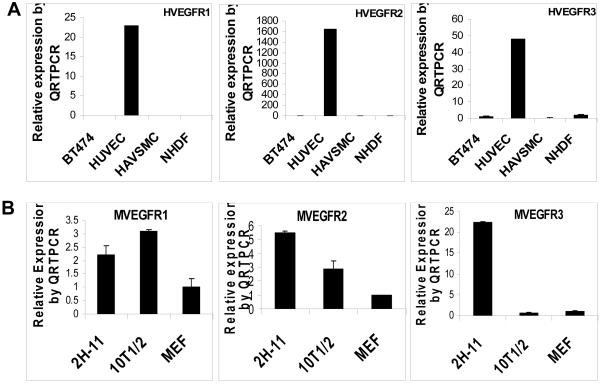
Our previous studies demonstrated that VEGF levels were greatly elevated in breast cancer cells that overexpress HER2 when measured both in vivo and in vitro.9 To identify cells capable of responding to VEGF, we have measured the expression of the three VEGF receptors in endothelial cells, pericyte-like cells, fibroblasts, and breast cancer cells. As shown in Figure 1A, only HUVECs expressed very high levels of all three VEGFRs, measured by QRTPCR. Compared to breast cancer cells (BT474), HAVSMCs and NHDFs, HUVECs expressed about 1600 times higher levels of VEGFR2, about 50 times higher levels of VEGFR3, and about 22 times higher levels of VEGFR1 (Fig. 1A). Similarly, a simian virus 40 T antigen-transformed mouse lymphoid endothelial cell line 2H-11.29 was found to express high levels of murine VEGFRs (Fig. 1B). 2H-11 cells expressed about 22 times higher levels of murine VEGFR3 and 5 times higher levels of mouse VEGFR2 when compared to MEFs (Fig. 1B). Mouse pericyte-like cells (10T1/2) also expressed VEGFR1 and VEGFR2 (Fig. 1B). Substantial expression of VEGFRs in human and murine endothelial cells indicates that endothelial cells in tumor environment may heavily depend on VEGF for cell proliferation and survival.
Fig. 1. Human and mouse endothelial cells express high levels of VEGFR1, VEGFR2 and VEGFR3.
(A), QRTPCR analysis of human VEGFR1, VEGFR2 and VEGFR3 in human breast cancer cells (BT474), human endothelial cells (HUVEC), human pericyte-like cells (HAVSMC) and human fibroblasts (NHDF). (B), QRTPCR analysis of murine VEGFR1, VEGFR2 and VEGFR3 in mouse endothelial cells (2H-11), mouse pericyte-like cells (10T1/2) and mouse fibroblasts (MEF).
Human and mouse pericyte-like cells and fibroblasts express high levels of VEGF ligands
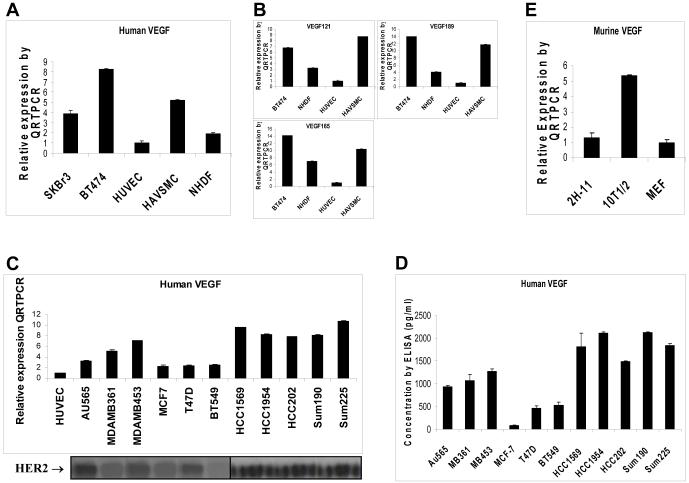
To identify sources of the VEGFs, we have used QRTPCR to measure levels of human-specific VEGFs and mouse-specific VEGF in endothelial cells, pericyte-like cells, fibroblasts, and breast cancer cells. As shown in Figure 2A, human pericyte-like cells (HAVSMC) and human fibroblasts (NHDF) expressed higher levels of human-specific VEGF than did human endothelial cells (HUVEC). Three major isoforms of human VEGFs (VEGF121, VEGF165, and VEGF189) were further measured in these cell types. As shown in Figure 2B, all isoforms of VEGF121, VEGF165, and VEGF189 were overexpressed in HAVSMC and NHDF, while HUVECs expressed only low levels of VEGF ligands. Similarly, mouse pericyte-like cells (10T1/2), but not mouse endothelial 2H-11 cells, expressed high levels of mouse-specific VEGF (Fig. 2D). MEF fibroblasts expressed low levels of VEGF similar to 2H-11 (Fig. 2D). These data suggest that the pericytes and fibroblasts produce significant amount of VEGF, whereas endothelial cells do not produce high levels of VEGF for autocrine growth stimulation.
Fig. 2. Human breast cancer cells, human and mouse pericyte-like cells, and human and mouse fibroblasts express high levels of VEGF ligands.
(A), Human-specific VEGF mRNA measured by QRTPCR in human breast cancer cells (BT474 & SKBr3), human endothelial cells (HUVEC), human pericyte-like cells (HAVSMC), and human fibroblasts (NHDF). (B), Human-specific VEGF isoforms measured by QRTPCR. (C), Human-specific VEGF mRNA measured by QRTPCR in an additional 12 human breast cancer cell lines. HUVEC was used as a comparison. HER2 levels of these cell lines were revealed by Western blotting. (D), Levels of human-specific VEGF protein in cultured supernatants were measured by ELISA in these 12 human breast cancer cell lines described in (C). (E), Murine-specific VEGF mRNA measured by QRTPCR in mouse pericyte-like cells (10T1/2), mouse endothelial 2H-11 cells and mouse fibroblasts (MEF).
Human breast cancer cells that overexpress endogenous HER2 express high levels of VEGF ligands
In our previous study, regular RT-PCR and ELISA had been used to demonstrate that forced expression of HER2 in MCF7 and T47D breast cancer cells that originally express low levels of HER2 upregulated VEGF levels.9 To determine whether this observation can be generalized to breast cancer cell lines that overexpress endogenous HER2, an additional 13 human breast cancer cell lines that express different levels of HER2 were analyzed for human-specific VEGF expression by QRTPCR. As shown in Figure 2A, human SKBr3 and BT474 breast cancer cells that overexpress HER2 expressed higher levels of human-specific VEGF than did HUVECs. Isoforms of VEGF121, VEGF165, and VEGF189 were all overexpressed in BT474 cells (Fig. 2B). All cell lines that overexpess HER2 (AU565, MDAMB361, MDAMB453, HCC1569, HCC1954, HCC202, SUM190, and SUM225) have much higher levels of VEGF (Fig. 2C). Breast cancer cell lines that express low levels of HER2 (MCF7, T47D, BT549, and MDAMB435) had low VEGF expression (Fig. 2C). These results were further supported by ELISA data that showed high VEGF secretion in HER2-overexpressing cell lines and low VEGF secretion in cell lines with low or none HER2 (Fig. 2D). These data suggest that the human breast cancer cells that overexpress HER2 produce high levels of VEGF.
VEGF-Trap effectively sequesters endogenous and exogenous VEGF and blocks VEGF-induced tyrosine phosphorylation of VEGFR
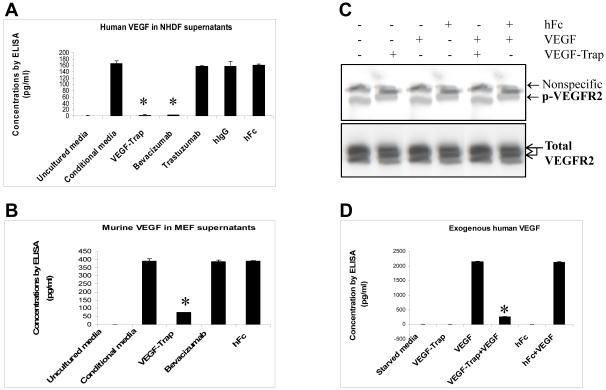
To test the ability of VEGF-Trap to sequester human VEGF, NHDFs were treated with VEGF-Trap, hFc (negative control), or bevacizumab (positive control). As shown in Figure 3A, VEGF-Trap at concentration of 2μg/ml was sufficient to reduce secreted human VEGF levels in supernatants of NHDFs, while control hFc at the same concentration did not. As expected, bevacizumab at 2μg/ml also decreased secreted VEGF levels in supernatants (Fig. 3A). Trastuzumab and its hIgG did not decrease VEGF (Fig. 3A). To test the ability of VEGF-Trap to sequester murine VEGF, MEFs were treated with VEGF-Trap, bevacizumab, or control hFc. As shown in Figure 3B, only VEGF-Trap, but not bevacizumab or control hFc decreased levels of murine VEGF. Thus, VEGF-Trap was capable of sequestering both human and murine VEGF protein. To test the ability of VEGF-Trap to block VEGF-induced signaling, starved-HUVEC were stimulated with VEGF for 5 min in the presence of VEGF-Trap or hFc. As shown in Figure 3C, VEGF (10 ng/ml) robustly activated VEGFR2 as indicated by tyrosine 1175 phosphorylation. VEGF-Trap completely blocked VEGF-induced VEGFR2 phosphorylation, whereas control hFc did not (Fig. 3C). As shown in Figure 3D, VEGF-Trap sequestered most of the exogenous VEGF added in the supernatants, whereas hFc did not (Fig. 3D). High levels of free VEGF corresponded to activation of VEGFR2 (Fig. 3D). These data demonstrated that VEGF-Trap sequesters endogenous and exogenous VEGF and blocks VEGF-induced tyrosine phosphorylation of VEGFR.
Fig. 3. VEGF-Trap sequesters endogenous and exogenous VEGF and blocks VEGF-induced tyrosine phosphorylation of VEGFR.
(A), Measurement of human-specific VEGF protein by ELISA. NHDF at 60% confluency were cultured and treated as described in Materials and Methods. Uncultured complete medium was used as a blank control. *, P < 0.01 compared to conditional media group. (B), Measurement of mouse-specific VEGF protein by ELISA. MEF at 50% confluency were cultured and treated as described in Materials and Methods. Uncultured complete medium was used as a blank control. *, P < 0.01 versus conditional media group. (C), VEGFR phosphorylation blocked by VEGF-Trap. HUVEC at 80% confluency were cultured and treated as described in Materials and Methods. Western blotting was performed using a phosphor-VEGFR2 (Y1175) rabbit monoclonal antibody and a total VEGFR2 monoclonal antibody. (D). Sequestration of VEGF by VEGF-Trap. While cell component was used for Western blotting as described in (C), supernatants were collected and assayed for human VEGF by ELISA, *, P < 0.01 compared to VEGF-treated group.
VEGF-Trap decreases levels of human VEGF and PlGF and inhibits tumor growth of BT474 breast cancer xenografts in vivo
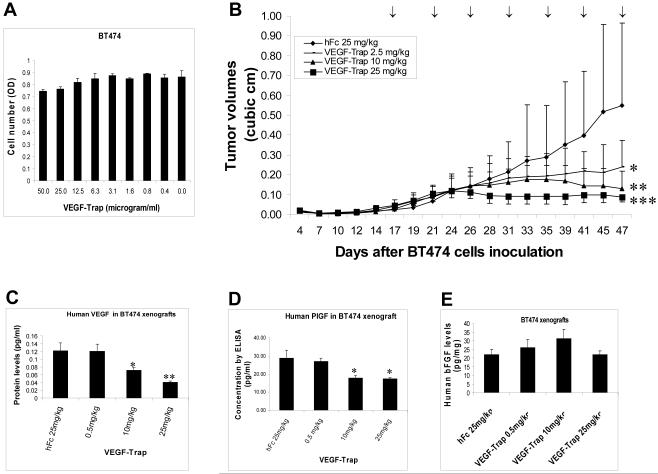
Effect of VEGF-Trap as a single treatment on BT474 breast cancer cells that overexpress HER2 was first tested in vitro. As shown in Figure 4A, VEGF-Trap had a nominal effect on cell proliferation of BT474 cells in vitro, with about 10% growth inhibition at the highest concentration of 50 μg/ml. Effect of VEGF-Trap as a single treatment on BT474 breast cancer cells was further tested in vivo. Pilot experiments in the BT474 xenograft model indicated that VEGF-Trap at 0.5 - 1 mg/kg had minimal effects on tumor growth (Data not shown). Thus three dose levels of VEGF-Trap (2.5, 10, and 25 mg/kg) were subsequently used in xenograft experiments. As shown in Figure 4B, VEGF-Trap at doses of 2.5 to 25 mg/kg was able to inhibit BT474 tumor growth in a dose-dependent manner. VEGF-Trap at the highest tested dose of 25 mg/kg completely blocked the growth of BT474 tumors (Fig. 3C). ELISA was used to measure levels of human VEGF and PlGF derived from BT474 xenograft lysates. As shown in Figure 4C, VEGF-Trap decreased the levels of human VEGF protein in a dose-dependent fashion. PlGF levels were also suppressed by VEGF-Trap treatment (Fig. 4D). Reduction of the VEGF and PlGF levels by VEGF-Trap was specific since the levels of bFGF in the same samples were not significantly affected by VEGF-Trap treatment (Fig. 4E). These data indicate that VEGF-Trap decreases both human VEGF and PlGF protein in vivo, and inhibits tumor growth of BT474 breast cancer xenografts.
Fig. 4. VEGF-Trap decreases levels of human VEGF and PlGF and inhibits tumor growth of BT474 breast cancer xenografts in vivo.
(A), Effect of VEGF-Trap on anchorage-dependent cell growth of BT474 breast cancer cells. BT474 cells were seeded on 96-well plates and treated with VEGF-Trap at a series of concentrations for 72 hr. Crystal violet staining was used to determine the numbers of viable cells. (B), VEGF-Trap suppresses tumor growth of BT474 breast cancer xenografts that overexpress HER2. BT474 xenografts in nu/nu mice were established as described in Materials and Methods. Mice were treated with subcutaneous injection of three different doses of VEGF-Trap (2.5, 10, 25 mg/kg), or control hFc (25 mg/kg), twice a week as indicated by arrows. Tumor volumes were expressed as mean ± SD from 5 mice in each group. *, P < 0.05, versus control hFc group since day 35. **, P < 0.05, versus control hFc group since day 31. ***, P < 0.05, versus control hFc group since day 28. (C), Measurement of human-specific VEGF protein by ELISA in lysates from BT474 xenografts treated with VEGF-Trap. *, P < 0.05, versus the hFc group. **, P < 0.01, versus the hFc group. (D), Measurement of human-specific PlGF levels by ELISA in lysates from VEGF-Trap-treated xenografts. The same samples as described in (C) were assayed for PlGF by an ELISA kit from R&D. (E), Measurement of human-specific bFGF protein by ELISA.
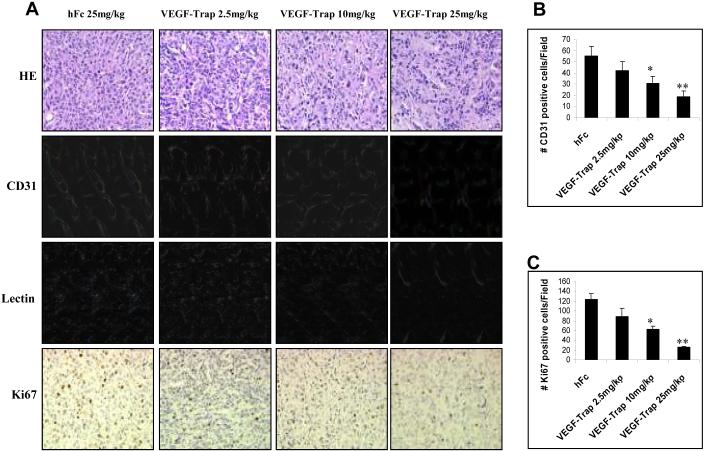
VEGF-Trap inhibits microvessel density, tumor vasculature, and tumor growth of breast cancer xenografts
Effect of VEGF-Trap on microvessel density (CD31 staining), tumor vasculature (lectin perfusion) and tumor proliferation (Ki67 staining) were studied in BT474 xenografts that were treated with VEGF-Trap. As shown in Figure 5A, VEGF-Trap decreased the densities and the sizes of blood vessels in a dose-dependent manner, which was illustrated by the CD31 immunofluorescence staining. Average numbers of CD31-positive cells per field (under 200 X magnification) decreased in a dose-dependent manner from 55 in hFc-treated controls, to 19 in tumors treated with the highest dose of VEGF-Trap (25 mg/kg) (Fig. 5B). CD31-stained results were further confirmed by FITC-lectin perfusion assay, which directly outlines tumor vasculature. Fluorescent staining clearly showed that VEGF-Trap decreased the densities and network of tumor microvessels in a dose-dependent fashion (Fig. 5A). Only scant and short vessels were seen in the tumor treated with 25mg/kg of VEGF-Trap (Fig. 5A). As shown in Figure 5A, the brown immunostaining of Ki67 antigen that localized in nuclei was strongly positive in hFc-treated xenografts. The average numbers of Ki67-positive cells decreased from 123 in hFc-treated controls, to 26 in tumors treated with the highest dose of VEGF-Trap (25 mg/kg) (Fig. 5C). H&E staining was used to delineate the cell types within VEGF-Trap-treated tumors. Fewer cancer cells and greater deposition of fibrinoid material were seen in tumors treated with higher doses of VEGF-Trap (Fig. 5A). Collectively, these results demonstrate that VEGF-Trap as a single treatment inhibits microvessel density, tumor vasculature, and cancer cell proliferation in BT474 breast cancer xenografts.
Fig. 5. VEGF-Trap inhibits microvessel density, tumor vasculature, and tumor growth of BT474 breast cancer xenografts.
(A), BT474 xenografts in nu/nu mice were established as described in Figure 3. Tumors were resected, fresh frozen or fixed in formalin. Immunofluorescence staining of CD31 marker, fluorescence-labeled lectin, HE staining, and Ki67 IHC staining were performed as described in Materials and Methods. (B), Quantitation of CD31-positive cells in hFc- and VEGF-Trap-treated tumors. Results are shown as the number (mean ± SD) of positive cells/field (200 X). Each data point was derived from three independent tumors. Six fields were counted per tumor. Except indicated otherwise, this quantitative method was also applied to the IHC of CD31and Ki67. An asterisk denotes p = 0.01 versus hFc control. ** denotes p = 0.008. (C), Quantitation of Ki67-positive cells in hFc- and VEGF-Trap-treated tumors. An asterisk denotes p = 0.009. ** denotes p = 0.004.
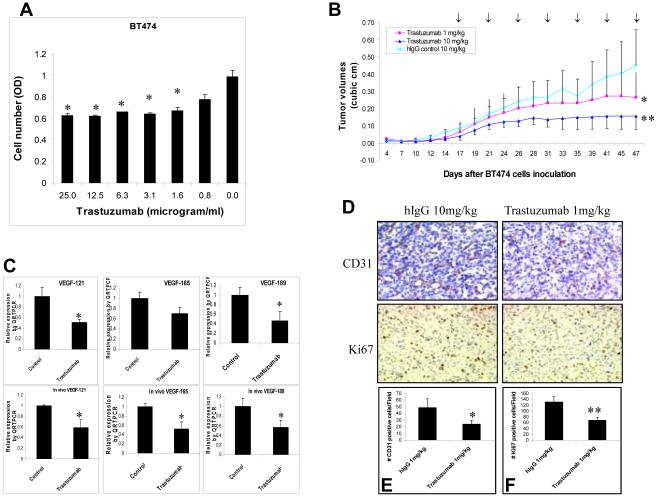
Trastuzumab decreases human tumor VEGF and tumor growth in vivo and vitro
In vitro, trastuzumab was able to inhibit proliferation of BT474 breast cancer cells by approximately 40% in the range of concentrations of 2 to 25 μg/ml (Fig. 6A). In vivo, trastuzumab dramatically inhibited tumor growth of BT474 breast cancer xenografts in a dose-dependent manner (Fig. 6B). Trastuzumab (1 mg/kg) suppressed BT474 tumor growth by about 40%, whereas higher dose trastuzumab at 10 mg/kg inhibited tumor growth by about 65% (Fig. 6B). Trastuzumab at the highest dose tested (25 mg/kg) completely blocked BT474 tumor growth (Data not shown). Thus, there existed a discrepancy of trastuzumab efficacy in vitro and in vivo. The ability of trastuzumab to inhibit both cell proliferation and tumor angiogenesis in vivo may account for this discrepancy. Indeed, trastuzumab decreased levels of all three major isoforms of human VEGF in BT474 cancer cells both in vitro and in vivo (Fig. 6C). Trastuzumab also decreased microvessel density detected by the CD31 staining (Fig. 6D). Average numbers of CD31-positive cells decreased from 49 in hFc-treated controls to 24 in tumors treated with trastuzumab (Fig. 6E). Trastuzumab also decreased the number of Ki67 positive cells and the intensity of nuclear staining (Fig. 6D). The average numbers of Ki67-positive cells decreased from 131 in hFc-treated controls to 68 in tumors treated with trastuzumab (Fig. 6F). Taken together, these results demonstrate that trastuzumab inhibits tumor proliferation, human VEGF expression, and microvessel density.
Fig. 6. Trastuzumab decreases human tumor VEGF and tumor growth in vivo and vitro.
(A), Growth inhibition of BT474 cells by trastuzumab in vitro. BT474 cells were seeded in 96-well plates in triplicate and treated for 72 hrs with different concentrations of trastuzumab as indicated. A crystal violet assay was used to assess the cell viability. *, p < 0.05, compared to the untreated control. (B), Trastuzumab suppresses tumor growth of BT474 breast cancer xenografts in vivo. BT474 xenografts were established in nu/nu mice as described in Materials and Methods. Mice were treated intraperitoneally twice a week with different doses of trastuzumab (1 and 10 mg/kg) or control hIgG (10 mg/kg). *, p < 0.05, versus the hIgG control at day 47. **, p < 0.01, versus the hIgG control at day 47. (C), Measurement of human-specific VEGF isoforms by QRTPCR in samples from in vitro cell culture (upper panel) and from in vivo BT474 xenografts (lower panel). *, P < 0.05, versus control hIgG. (D) - (F), CD31 and Ki67 staining in xenograft tumors. CD31 and Ki67 IHC staining (D) were performed as described in Materials and Methods. CD31- (E) and Ki67- (F) positive cells were counted in hFc- and VEGF-Trap-treated tumors. An asterisk denotes p = 0.01. ** denotes p = 0.02.
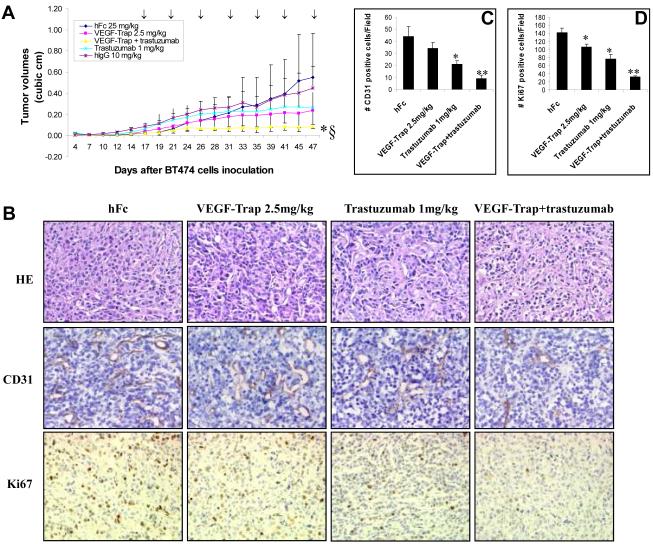
VEGF-Trap plus trastuzumab treatment results in greater inhibition of tumor microvessel density, cell proliferation, and tumor growth in vivo than either individual treatment
BT474 xenografts in nude mice were treated either with VEGF-Trap (2.5mg/kg) alone, trastuzumab (1mg/kg) alone, VEGF-Trap and trastuzumab combination, or control agents. As shown in Figure 7A, VEGF-Trap alone or trastuzumab alone moderately suppressed BT474 tumor growth. Combination of VEGF-Trap and trastuzumab dramatically inhibited BT474 tumor growth more than either agent alone (Fig. 7A). Compared to either agent alone or to controls, treatment with the combination produced a striking and superior inhibition of BT474 tumor growth (p=0.008). hFc, hIgG, or hFc plus hIgG had no greater effect than saline on growth of BT474 xenografts (Fig. 7A & data not shown). Combined treatment significantly decreased the microvessel density as indicated by CD31 staining (Fig. 7B). The average numbers of CD31-positive cells decreased from 34 in VEGF-Trap-treated tumors and 21 in trastuzumab-treated tumors to 9 in combination-treated tumors (Fig. 6C). Combined treatment also decreased cell proliferation as indicated by Ki67 staining (Fig. 7B). Average numbers of Ki67-positive cells decreased from 106 in VEGF-Trap-treated tumors and 77 in trastuzumab-treated tumors to 32 in combination-treated tumors (Fig. 6C). Thus, VEGF-Trap in combination with trastuzumab exerted greater inhibition of BT474 breast cancer xenografts than did either treatment alone through better inhibition of both tumor angiogenesis and cell proliferation.
Fig. 7. VEGF-Trap plus trastuzumab treatment produces greater inhibition of tumor microvessel density, cell proliferation, and tumor growth in vivo than either agent alone.
(A), Trastuzumab in combination with VEGF-Trap suppresses tumor growth of BT474 xenografts more effectively than either agent used alone. Mice were treated twice a week 1) intraperitoneally with trastuzumab (1 mg/kg) alone, 2) subcutaneously with VEGF-Trap (2.5mg/kg) alone, 3) with a combination of trastuzumab and VEGF-Trap, 4) intraperitoneally with hIgG (10 mg/kg) or 5) subcutaneously with hFc (25 mg/kg). * denotes combination versus trastuzumab alone or VEGF-Trap alone (p= 0.02). § denotes combination versus control hIgG or control hFc (p= 0.008). (B), HE, CD31 and Ki67 staining in xenograft tumors. (C), Quantitation of CD31-positive cells in tumors. An asterisk denotes p = 0.01. ** denotes p = 0.005. (D), Quantitation of Ki67-positive cells in tumors. An asterisk denotes p < 0.05. ** denotes p = 0.008.
DISCUSSION
This is the first report to demonstrate in vivo that dual targeting of HER2 with trastuzumab and VEGF with VEGF-Trap produces superior growth inhibition of HER2-positive human breast cancer. The results presented here suggest that dual targeting of HER2 signaling with trastuzumab and of VEGF pathway with VEGF-Trap could provide a better therapeutic regimen than trastuzumab alone for patients with breast cancers that overexpress HER2.
Molecular mechanisms underlying the inhibition of breast cancer xenograft growth by trastuzumab and VEGF-Trap in combination were explored in this study. Better inhibition of both tumor angiogenesis and tumor proliferation were found (Fig. 7). Greater inhibition of tumor angiogenesis generated by the combined treatment might result from additive blockade of VEGF. VEGF-Trap effectively blocked extracellular VEGF derived from both cancer cells and host cells (Fig. 3). HUVECs could be particularly susceptible to VEGF-Trap treatment due to their VEGFR levels (Fig. 1). Indeed, even low doses of VEGF-Trap were able to inhibit endothelial cells in vivo as indicated by CD31 staining (Fig. 5). On the other hand, trastuzumab decreases tumor VEGF through the PI3K pathway in HER2-overexpressing cancer cells.9,30-32 Trastuzumab can also increases anti-angiogenic factor (thrombospordin-1) and inhibits additional pro-angiogenic factors such as transforming growth factor-α, angiopoietin-1, plasminogen-activator inhibitor-1 and interleukin-8.9,30 Therefore, trastuzumab could have synergistic interaction with VEGF-Trap to suppress tumor angiogenesis through modulation of multiple angiogenic factors. In this sense, human breast cancers that overexpress HER2 may be the ideal targets for dual therapy with agents that inhibit VEGF and HER2.
Combinations of FDA-approved anti-VEGF agents (bevacizumab, sorafenib or sunitinib) with chemotherapy has already proven more active than chemotherapy alone in solid cancers, notably colorectal and renal cell carcinomas.19,33 Bevacizumab significantly increased response rates in heavily pretreated breast cancer patients, but there was no significant improvement in progression-free survival (PFS) or overall survival (OS).34 However, more recent data suggested that the addition of bevacizumab to paclitaxel treatment as first line therapy was associated with a significantly higher response rate and longer PFS in patients with metastatic breast cancer.19 It is anticipated that combination of bevacizumab and paclitaxel might improve the OS as well.19 Our preclinical results with murine xenografts presented here support the use of anti-VEGF agents in combination with trastuzumab to treat breast cancer patients whose tumors overexpress HER2. There are some difference between VEGF-Trap and bevacizumab. First, VEGF-Trap is reported to exhibit approximately 50-fold higher binding affinity for VEGF ligands than does anti-VEGF antibody such as bevacizumab.20,21 Second, VEGF-Trap can block another angiogenic factor PlGF except VEGF as shown in Figure 4. Third, VEGF-Trap is smaller than bevacizumab and may more easily penetrated into some areas that bigger antibody is prevented such as brain. Therefore, VEGF-Trap in combination with chemotherapy may provide better therapeutic efficacy than the combination of bevacizumab and chemotherapy.
Trastuzumab exhibits greater anti-cancer activity in vivo than in vitro. In our studies, trastuzumab inhibited growth of BT474 breast cancer cells by approximately 40% (Fig. 6A) and no additional growth suppression was observed at levels greater than 2 μg/ml (Fig. 6A). In vivo, trastuzumab effectively inhibited growth of BT474 xenografts in a dose-dependent fashion (Fig. 5B) and at the highest dose level tested, trastuzumab completely blocked BT474 xenograft growth. Similarly, VEGF-Trap as a single agent produced more profound growth inhibition in vivo than did in vitro (Fig. 4). The combination of VEGF-Trap and trastuzumab also did not generate meaningful inhibitory effects on VEGF production and growth in vitro (Data not shown). The mechanisms underlying differences in vitro and in vivo are not fully understood, but angiogenesis and antibody dependent cellular cytotoxicity may both contribute. In cell culture, there are no vessels with endothelial cells or pericytes, no fibroblasts and no inflammatory infiltrate. In addition, antibody-dependent cellular cytotoxicity (ADCC), may also contribute to the ability of trastuzumab to inhibit tumor growth in vivo.10,35 Loss or blockade of the FcγRIII receptor on leukocytes has been shown to severely impair the anti-tumor effect of trastuzumab in vivo.36 indicating involvement of Fc-receptor-dependent mechanisms. Similar effects of ADCC have been documented in the treatment of lymphoma and leukemia with the humanized anti-CD20 antibody rituximab.37
Acknowledgements
We are grateful to Regeneron Pharmaceuticals (Tarrytown, NY) for supplying VEGF-Trap and hFc. We sincerely thank Drs. Joe W. Gray and Richard M. Neve at Lawrence Berkeley National Laboratory (Berkeley, CA), and Dr. Stephen P. Ethier at the Barbara Ann Karmanos Cancer Institute (Detroit, MI) for generously providing breast cancer cell lines. The Media Preparation Core Facility and Animal Core Facility were supported in part by the Cancer Center Support Grant CA16672 from NCI. These studies were supported in part by grants from NCI-CA39930 and the Zarrow Foundation.
Footnotes
This work was supported in part by the Zarrow Foundation, grant CA39930, and grant P50CA 083639 from the National Cancer Institute.
REFERENCES
- 1.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 2.Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17:2639–2648. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 3.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 4.Le XF, McWatters A, Wiener J, Wu JY, Mills GB, Bast RC., Jr. Anti-HER2 antibody and heregulin suppress growth of HER2-overexpressing human breast cancer cells through different mechanisms. Clin Cancer Res. 2000;6:260–270. [PubMed] [Google Scholar]
- 5.Lane HA, Motoyama AB, Beuvink I, Hynes NE. Modulation of p27/Cdk2 complex formation through 4D5-mediated inhibition of HER2 receptor signaling. Ann Oncol. 2001;12(Suppl 1):S21–22. doi: 10.1093/annonc/12.suppl_1.s21. [DOI] [PubMed] [Google Scholar]
- 6.Yakes FM, Chinratanalab W, Ritter CA, King W, Seelig S, Arteaga CL. Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt Is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res. 2002;62:4132–4141. [PubMed] [Google Scholar]
- 7.Le XF, Claret FX, Lammayot A, Tian L, Deshpande D, LaPushin R, et al. The role of cyclin-dependent kinase inhibitor p27Kip1 in anti-HER2 antibody-induced G1 cell cycle arrest and tumor growth inhibition. J Biol Chem. 2003;278:23441–23450. doi: 10.1074/jbc.M300848200. [DOI] [PubMed] [Google Scholar]
- 8.Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117–127. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 9.Wen XF, Yang G, Mao W, Thornton A, Liu J, Bast RC, Jr, et al. HER2 signaling modulates the equilibrium between pro- and anti-angiogenic factors via distinct pathways: implications for HER2-targeted antibody therapy. Oncogene. 2006;25:6986–96. doi: 10.1038/sj.onc.1209685. [DOI] [PubMed] [Google Scholar]
- 10.Cooley S, Burns LJ, Repka T, Miller JS. Natural killer cell cytotoxicity of breast cancer targets is enhanced by two distinct mechanisms of antibody-dependent cellular cytotoxicity against LFA-3 and HER2/neu. Exp Hematol. 1999;27:1533–41. doi: 10.1016/s0301-472x(99)00089-2. [DOI] [PubMed] [Google Scholar]
- 11.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 12.Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes JC, et al. The human gene for vascular endothelial growth factor: multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991;266:11947–11954. [PubMed] [Google Scholar]
- 13.Klos KS, Wyszomierski SL, Sun M, Sun M, Tan M, Zhou X, et al. ErbB2 increases vascular endothelial growth factor protein synthesis via activation of mammalian target of rapamycin/p70S6K leading to increased angiogenesis and spontaneous metastasis of human breast cancer cells. Cancer Res. 2006;66:2028–2037. doi: 10.1158/0008-5472.CAN-04-4559. [DOI] [PubMed] [Google Scholar]
- 14.Yen L, You XL, Al Moustafa AE, Batist G, Hynes NE, Mader S, et al. Heregulin selectively upregulates vascular endothelial growth factor secretion in cancer cells and stimulates angiogenesis. Oncogene. 2000;19:3460–3469. doi: 10.1038/sj.onc.1203685. [DOI] [PubMed] [Google Scholar]
- 15.Laughner E, Taghavi P, Chiles K, Mahon PC, Semenza GL. HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alphA), synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol. 2001;21:3995–4004. doi: 10.1128/MCB.21.12.3995-4004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loureiro RM, Maharaj AS, Dankort D, Muller WJ, D’Amore PA. ErbB2 overexpression in mammary cells upregulates VEGF through the core promoter. Biochem Biophys Res Commun. 2005;326:455–465. doi: 10.1016/j.bbrc.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 17.Yang W, Klos K, Yang Y, Smith TL, Shi D, Yu D. ErbB2 overexpression correlates with increased expression of vascular endothelial growth factors A, C, and D in human breast carcinoma. Cancer. 2002;94:2855–61. doi: 10.1002/cncr.10553. [DOI] [PubMed] [Google Scholar]
- 18.Konecny GE, Meng YG, Untch M, Wang HJ, Bauerfeind I, Epstein M, et al. Association between HER-2/neu and vascular endothelial growth factor expression predicts clinical outcome in primary breast cancer patients. Clin Cancer Res. 2004;10:1706–1716. doi: 10.1158/1078-0432.ccr-0951-3. [DOI] [PubMed] [Google Scholar]
- 19.Schneider BP, Sledge GW., Jr. Drug insight: VEGF as a therapeutic target for breast cancer. Nat Clin Pract Oncol. 2007;4:181–189. doi: 10.1038/ncponc0740. [DOI] [PubMed] [Google Scholar]
- 20.Holash J, Davis S, Papadopoulos N, Croll SD, Ho L, Russell M, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002;99:11393–11398. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rudge JS, Thurston G, Davis S, Papadopoulos N, Gale N, Wiegand SJ, et al. VEGF trap as a novel antiangiogenic treatment currently in clinical trials for cancer and eye diseases, and VelociGene-based discovery of the next generation of angiogenesis targets. Cold Spring Harbor Symp Quant Biol. 2005;70:411–418. doi: 10.1101/sqb.2005.70.052. [DOI] [PubMed] [Google Scholar]
- 22.Huang J, Frischer JS, Serur A, Kadenhe A, Yokoi A, McCrudden KW, et al. Regression of established tumors and metastases by potent vascular endothelial growth factor blockade. Proc Natl Acad Sci USA. 2003;100:7785–7790. doi: 10.1073/pnas.1432908100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dalal S, Berry AM, Cullinane CJ, Mangham DC, Grimer R, Lewis IJ, et al. Vascular endothelial growth factor: a therapeutic target for tumors of the Ewing’s sarcoma family. Clin Cancer Res. 2005;11:2364–2378. doi: 10.1158/1078-0432.CCR-04-1201. [DOI] [PubMed] [Google Scholar]
- 24.Lu L, Hofmann J, Holash J, Yancopoulos GD, Sood AK, Jaffe RB. Vascular endothelial growth factor trap combined with paclitaxel strikingly inhibits tumor and ascites, prolonging survival in a human ovarian cancer model. Clin Cancer Res. 2005;11:6966–6971. doi: 10.1158/1078-0432.CCR-05-0910. [DOI] [PubMed] [Google Scholar]
- 25.Wachsberger PR, Burd R, Cardi C, Thakur M, Daskalakis C, Holash J, et al. VEGF trap in combination with radiotherapy improves tumor control in U87 glioblastoma. Int J Radiat Oncol Biol Phys. 2007;67:1526–1537. doi: 10.1016/j.ijrobp.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Verheul HM, Hammers H, van Erp K, Wei Y, Sanni T, Salumbides B, et al. Vascular endothelial growth factor trap blocks tumor growth, metastasis formation, and vascular leakage in an orthotopic murine renal cell cancer model. Clin Cancer Res. 2007;13:4201–4208. doi: 10.1158/1078-0432.CCR-06-2553. [DOI] [PubMed] [Google Scholar]
- 27.Pegram MD, Reese DM. Combined biological therapy of breast cancer using monoclonal antibodies directed against HER2/neu protein and vascular endothelial growth factor. Semin Oncol. 2002;29(3 Suppl 11):29–37. doi: 10.1053/sonc.2002.34053. [DOI] [PubMed] [Google Scholar]
- 28.Le XF, Lammayot A, Gold D, Lu Y, Mao W, Chang T, et al. Genes affecting the cell cycle, growth, maintenance, and drug sensitivity are preferentially regulated by anti-HER2 antibody through phosphatidylinositol 3-kinase-AKT signaling. J Biol Chem. 2005;280:2092–2104. doi: 10.1074/jbc.M403080200. [DOI] [PubMed] [Google Scholar]
- 29.O’Connell KA, Edidin M. A mouse lymphoid endothelial cell line immortalized by simian virus 40 binds lymphocytes and retains functional characteristics of normal endothelial cells. J Immunol. 1990;144:521–525. [PubMed] [Google Scholar]
- 30.Izumi Y, Xu L, di Tomaso E, Fukumura D, Jain RK. Tumour biology: herceptin acts as an anti-angiogenic cocktail. Nature. 2002;416:279–80. doi: 10.1038/416279b. [DOI] [PubMed] [Google Scholar]
- 31.Klos KS, Zhou X, Lee S, Zhang L, Yang W, Nagata Y, et al. Combined trastuzumab and paclitaxel treatment better inhibits ErbB-2-mediated angiogenesis in breast carcinoma through a more effective inhibition of Akt than either treatment alone. Cancer. 2003;98:1377–85. doi: 10.1002/cncr.11656. [DOI] [PubMed] [Google Scholar]
- 32.Spiridon CI, Guinn S, Vitetta ES. A comparison of the in vitro and in vivo activities of IgG and F(ab’)2 fragments of a mixture of three monoclonal anti-Her-2 antibodies. Clin Cancer Res. 2004;10:3542–51. doi: 10.1158/1078-0432.CCR-03-0549. [DOI] [PubMed] [Google Scholar]
- 33.Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3:24–40. doi: 10.1038/ncponc0403. [DOI] [PubMed] [Google Scholar]
- 34.Miller KD, Chap LI, Holmes FA, Cobleigh MA, Marcom PK, Fehrenbacher L, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005;23:792–799. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 35.Kono K, Takahashi A, Ichihara F, Sugai H, Fujii H, Matsumoto Y. Impaired antibody-dependent cellular cytotoxicity mediated by herceptin in patients with gastric cancer. Cancer Res. 2002;62:5813–5817. [PubMed] [Google Scholar]
- 36.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 37.Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–758. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]