Abstract
The neuroregulatory activities of PMS-601, a platelet activating factor antagonist, were investigated in laboratory and animal models of HIV-1 encephalitis (HIVE). For the former, PMS-601 reduced monocyte-derived macrophage pro-inflammatory factors, multinucleated giant cell (MGC) formation, and neuronal loss independent of antiretroviral responses. PMS-601 treatment of HIVE severe combined immunodeficient mice showed reduced microgliosis, MGC formation and neurodegeneration. These observations support the further development of PMS-601 as an adjunctive therapy for HIV-1 associated neurocognitive disorders.
Keywords: platelet activating factor, PMS-601, human immunodeficiency virus, multinucleated giant cell, nuclear factor kappa beta, proline-rich tyrosine kinase 2
1. Introduction
The prevalence of human immunodeficiency virus (HIV)-1-associated neurocognitive disorders (HAND) continues to rise in HIV-1 infected people despite widespread use of antiretroviral therapies (ART) (Letendre et al., 2007). HAND parallels increases in patient life span (Boisse et al., 2008), the emergence of resistant viral phenotypes, drug toxicities, incomplete ART blood-brain barrier penetration and sustained mononuclear phagocytes (MP; blood borne macrophages and microglia) viral reservoirs in the brain as sources for continuous HIV-1 infection (Charpentier et al., 2004; Deeks et al., 2005; Roquebert et al., 2006; Tozser, 2001). All have hampered drug successes. Sustained neuroinflammatory responses and the secretion of viral and cellular neurotoxins by immune competent and HIV-1 infected brain MP in the face of restricted viral growth in the brain now characterize HAND pathobiology (Buscemi et al., 2007; Dhillon et al., 2008; Herbein et al., 2008; Okamoto et al., 2005). HIV-1 infected and activated brain MP secrete reactive oxygen species and pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF- α), interleukin-1® (IL-1β) and IL-8, which can affect neural function (Janelsins et al., 2008; Stone and Behan, 2007; Viviani et al., 2006; Xiong et al., 2003). Of the putative cellular toxins, those linked to the arachidonic acid pathway are considered among the most significant in affecting the production and release of other neurotoxins or in directly inducing cytotoxicities (Fang et al., 2008; Kwon et al., 2005; Sang et al., 2005). Indeed, several of the leukotrienes, HETEs, prostaglandins and platelet activating factor (PAF) affect MP inflammatory responses seen in a broad range of systemic degenerative and nervous system disorders (Bate et al., 2006a; Bate et al., 2006b; Ichiyama et al., 2009; Rey et al., 1998; Schuhmann et al., 2003; Smith et al., 2001).
Of the metabolites of arachidonic acid, PAF has broad effects on innate immunity and is a known participant in HAND pathobiology (Del Sorbo et al., 2001; Del Sorbo et al., 1999; Perry et al., 1998; Tong et al., 2001). This includes PAF’s abilities to affect MP chemotaxis (Del Sorbo et al., 1999), neurotoxicity (Ryan et al., 2008) and cellular metabolism (Belanger et al., 2008). HIV-1 replication is enhanced by PAF (Herbein and Khan, 2008; Herbein et al., 2008; Lima et al., 2006). PAF leads to increased TNF-α MP secretion activating both the classical nuclear factor kappa beta (NF-κB) and mitogen activated protein kinase (MAPK) pathways resulting in IL-1, 6, 10 and TNF-α production (Kihara et al., 2005; Lee et al., 2005; Muller-Ladner et al., 2002). TNF-α affects HIV-1 replication and as a result enhances the concentration of viral and cellular toxins as well as PAF regulation (Sneddon et al., 2006). Previous studies performed by others and within our laboratories demonstrated potent effects of PAF antagonists on neuroinflammatory responses induced by HIV-1 encephalitis (HIVE) in models of human disease (Gelbard et al., 1994; Persidsky et al., 2001; Tong et al., 2001).
With this in mind, we investigated the neuroregulatory properties of PMS-601, a PAF receptor antagonist, in laboratory and animal HIVE models. Herein, we demonstrate decreased HIV-1 infected monocyte-derived macrophages (MDM) activation as evidenced by cytoskeletal modulation and decreased multinucleated giant cell (MGC) formation. Attenuation of protein kinase C (PKC) -glcycogen synthase kinase 3® (GSK3β), NF-κB and MAPK signaling cascades resulted in decreased neurotoxicity to primary murine cortical neurons exposed to HIV-1 culture supernatants. We also show that PMS-601 is neuroprotective, both directly and indirectly, for primary murine cortical neurons treated with supernatants from HIV-1 infected MDM. In vivo, a substantial decrease in microglial activation as well as neuroprotective responses were observed.
2. Materials and methods
2.1. Human monocyte isolation, cultivation and HIV-1 infection
Human peripheral blood mononuclear cells were obtained by leukopheresis from HIV-1, 2 and hepatitis B seronegative donors, and monocytes were purified by countercurrent centrifugal elutriation. Monocytes were cultured with 10% heat-inactivated pooled human serum, 1% glutamine (Sigma Chemical Co., St. Louis, MO), 10 µg/mL ciproflaxin (Sigma) and 1,000 U/mL highly purified recombinant human macrophage colony stimulating factor (MCSF) in Dulbecco’s Modified Eagle’s Medium (DMEM) (Invitrogen, Grand Island, NY) for 7 days. After 7 days MDM were infected with HIV-1ADA at a multiplicity of infection of 0.01 (Gendelman et al., 1988). Cells were maintained with half media exchanges every other day. PMS-601 was added to MDM cultures 24 hr before, at or 4 hr after HIV-1ada infection, and culture supernatants and cells assayed every other day for 10 days for reverse transcriptase (RT) activity and cellular viability by mitochondrial activity, respectively. The latter was determined by reduction of tetrazolium salt 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Kovacs et al., 2004). For RT activity measures, 5 µL of sample was incubated with a reaction mixture consisting of 0.05% nonidet P-50 (Sigma) and [3H]dTTP (2 Ci/mmol; Amersham, Arlington Heights, IL) in Tris-HCl buffer (pH 7.9) for 24 hr. Radiolabeled nucleotides were precipitated on paper filters in an automatic cell harvester (Skatron, Sterling, VA), and incorporated activity was measured by liquid scintillation spectroscopy (Kalter et al., 1991). Number of MGC were counted after fixation in 1:1 acetone:methanol and staining with Mayer’s hematoxylin. Total numbers of MGC were determined by counting cells with ≥5 nuclei (total MGC), and total numbers of nuclei/cell (MGC index) were also assessed. Data were quantified using 4 wells per group and 2 separate experiments with 2 separate donors.
2.2. PMS-601 preparation
PMS-601 was prepared as a 500 mM stock solution in DMSO. Fresh dilutions were made in media for a working stock concentration of 10 mM with serial dilutions ranging between 10 nM to 10 mM PMS-601 for laboratory studies. For rodent studies, drug was dissolved in DMSO and then diluted in sterile distilled water with intermittent heating and gentle mixing until a 10% DMSO solution was obtained.
2.3. Mouse cortical neurons (MCN) cultures and neurotoxicity assays
Cultures were harvested from the cerebral hemisphere of embryonic day 17 mice and cultured as described previously (Zheng et al., 1999). Primary MCN were plated in poly-d-lysine-coated 96-well plates at a density of 3 × 104 neurons/well. Human MDM were simultaneously infected with HIV-1ADA and treated with PMS-601 as described above. MDM were washed extensively on day 4 to remove drug, and media replaced with neural basal media. Supernatants were harvested on day 5 and placed onto MCN at a ratio of 1:5 (supernatant:neural basal media) for 24 hr to assess numbers of viable neurons (Zheng et al.,1999) using anti-MAP2 (MAP-2) (neural cell bodies, axons and dendrites; 1:1000) (Chemicon International, Temecula, CA) and anti-NeuN (NeuN) (1:100) (Chemicon International) antibodies and fluorescent-labeled, Alexa Fluor® 488 (green) and 594 (red) (Molecular Probes Inc., Eugene, OR) secondary rabbit or mouse antibodies (1:1000 dilutions). Lactate dehydrogenase (LDH) present in culture supernatants of treated-neurons was determined using a commercial kit (Roche, Indianopolis, IN). Data from neuronal immunocytochemistry and LDH assays were quantified using 4 wells/group for 3 separate donors. MCN were cultured then treated with PMS-601 in neurobasal media or 2 µM staurosporine (Chemicon International) for 24 hr. MCN were exposed to drug with or without HIV-1 infected MDM conditioned media for 24 hr. Viable neurons and LDH levels were quantified from MCN cultured in 6 or 12 wells/group from 3 separate experiments.
2.4. Ca2+ detection by Alizarin Red S staining
One hundred thousand MDM were cultured in 96-well plates and treated with PMS-601 at time of infection. Drug concentrations were maintained for 5 days with media changes every other day. MDM were fixed with 50% ethanol, washed and stained for 5 min with Alizarin Red S (1% aqueous solution, GFS Chemicals, Inc., Columbus, OH), used in clinical histology to visualize free Ca2+ in cytosol. Cells were washed 5 times with distilled water to wash off excess Alizarin Red S solution and covered with 100 µM of distilled water. Each well was read at 450 nm on a spectrometer plate reader (Friedman et al., 2006).
2.5. In situ ELISA for cell signaling measures
Cell signaling measurements were adapted from standard protocols (Versteeg et al., 2000). Briefly, MDM were cultured at a density of 105 in 96-well plates and infected as previously described. MDM were treated with PMS-601 at time of infection with appropriate drug concentrations maintained for 5 days with half media changes every other day. At day 5, cells were fixed using 1:1 acetone:methanol for subsequent exposure to primary antibodies or 4% paraformaldehyde for treatment with AlexaFluor® 546 Phalloidin (Invitrogen), then washed 3 times with Tris buffered saline (TBS). Cells were permeabilized using 0.025% Triton X-100 (Sigma) in TBS for 3 min, then washed 4 times using TBS. AlexaFluor® 546 Phalloidin (Invitrogen, Carlsbad, CA) was used as the detector and cells were allowed to take up the dye for 20 min. Cells were then washed 4 times with distilled water and mean fluorescent intensity (MFI) read at the appropriate wavelength. Cells fixed in 1:1 acetone:methanol were washed with TBS 4 times and blocked in 5% bovine serum albumin in TBS for 1 hr. Cells were incubated with primary antibody, at appropriate concentrations in blocking solution overnight at 4° C while shaking. Cells were washed 3 times with TBS and incubated with appropriate secondary fluorescent antibodies (1:1000 dilutions) to anti-rabbit and anti-mouse Alexa Fluor® 488 (green) and 594 (red) (Molecular Probes Inc.) for 1 hr shaking in the dark. Cells were washed 4 times with distilled water then distilled water was used to cover the wells. Plates were read using a fluorescent plate reader and MFI acquired at 519 or 615 nM. Primary antibodies used were those directed against p-p38 (Thr180/Tyr182) (1:1000, Cell Signaling Technology, Inc., Danvers, MA), p38 (1:1000, Cell Signaling Technology, Inc.), ERK1/2 (1:1000, Cell Signaling Technology, Inc.), phosphorylated- (p-) ERK1/2 (Thr202/Tyr204) (1:1000, Cell Signaling Technology, Inc.), JNK (1:1000) (Cell Signaling Technology, Inc.), p-JNK (Thr183/Tyr185) (1:2000) (Cell Signaling Technology, Inc.), p-PKC (Ser643/676) (1:1000, Cell Signaling Technology, Inc.), p50 (1;1000) (Biosource, Camarillo, CA), p65 (1:1000, Biosource), p-NF-κB (Ser529) (1:1000, Biosource), GSK3® (1:1000, BD Biosciences, San Jose, CA), p-GSK3β (Ser9) (1:1000, Cell Signaling Technology, Inc.), Pyk2 (1:1000, Cell Signaling Technology, Inc.), and p-Pyk2 (Tyr402) (1:1000, Cell Signaling Technology, Inc). Alexa Fluor® 546 Phalloidin (1:40, Invitrogen) was used to quantify filamentous (F)-actin. Each group contained 6 wells. Data are representative of 3 experiments using 3 donors. All results are expressed as MFI ± standard error of the mean (SEM) and are comparable to uninfected MDM as having a MFI of 1.0 ± SEM.
2.6. SCID HIVE mice
Four week old male C.B-17/IcrCrl-SCIDbr (CB17/scid) mice were purchased from Charles River Laboratory (Wilmington, WA). Animals were maintained in sterile microisolator cages under pathogen-free conditions in the Laboratory of Animal Medicine at the University of Nebraska Medical Center in accordance with ethical guidelines for the care of laboratory animals set forth by the National Institutes of Health. HIV-1ADA-infected MDM (1.5 × 105 cells in 5 µL) were stereotactically injected intracranially (i.c.) into caudate-putamen after 1 day of viral infection and referred to as HIVE mice (Persidsky et al., 1996; Tyor et al., 1993). Ten and 12 animals were included in the vehicle-only and drug treated groups, respectively. Animals were treated daily by intraperitoneal injection for 14 d with vehicle-only or 750 mg/kg/day PMS-601 1 d after HIV-1ada MDM i.c. injection.
2.7. Histopathology and image analyses
Brain tissue was collected at time of sacrifice, fixed in 4% phosphate-buffered paraformaldehyde and embedded in paraffin. Immunohistochemistry was carried out as previously described (Limoges et al., 2001). Anti-HIV-1 p24 (clone Kal-1; Dako, Carpinteria, CA) was used to determine virus infected human MDM for each mouse. Anti-vimentin intermediate filaments (clone VIM 3B4; Boehringer Mannheim, Indianapolis, IN) were used for detection of human cells in mouse brains. Mouse microglia were detected by antibodies to Iba-1 (WAKO, Osaka, Japan) and astrocytes with antibodies to glial fibrillary acidic protein (GFAP; Dako). Anti-NeuN, anti-MAP-2 (both from Chemicon International Inc., Temecula, CA), and anti-light and heavy chain (200kD) neurofilament (Dako) were used for the cell body, dendrite, cell body and process and axon, respectively, detection of neurons. Appropriate secondary antibody polymers (Dako), the Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA) or diaminobenzidine (DAB) kit (Dako) were used to complete the immunohistochemical tests. All sections were counterstained with Mayer’s hematoxylin. The numbers of human MDM and HIV-1 p24 antigen-positive cells were counted with a Nikon Microphot-FXA microscope. All obtained images were imported into Image-Pro Plus, v. 4.0 (Media Cybernetics, Silver Spring, MD) for quantification of levels of GFAP, Iba-1, MAP-2 and NeuN staining.
2.8. Real time polymerase chain reaction (PCR)
HIV RNA levels in the ipsilateral (site of injection) and contralateral brain were determined by real time PCR with ABI 7000 prism (Perkin-Elmer, Applied Biosystems, Foster City, CA). Primers and probes used were previously described (Poluektova et al., 2004) and are forward, 5’-ACA TCA AGC CAT GCA ATT −3’; reverse, 5’- ATC TGG CCT GGT GCA ATA GG −3’; and probe (FAM), 5’- CAT CAA TGA GGA AGC TGC AGA ATG GGA TAG A −3’ (TAMRA). cDNA was further amplified using primers and probe at 50°C for 2 min, 95°C for 10 min, and 40 cycles at 95°C for 15 sec and 60°C for 1 min. Separate GAPDH amplifications were used as an endogenous control to ensure that equal amounts of RNA were used. GAPDH primers are: forward, 5’-GAA GGT GAA GGT CGG AGT C-3’; reverse, 5’-GAA GAT GGT GAT GGG ATT TC-3’; and probe, 5’–CAA GCT TCC CGT TCT CAG CCC. Other primers used were Assays on demand TNF-α (Applied Biosystems) and PAF receptor (Invitrogen, Carlsbad, CA).
2.9. Statistical analysis
Data are expressed as mean ± SEM. Analysis of variance (ANOVA) was used to assess differences between experimental groups and Student t-test was used to determine differences between individual groups. Statistical significance was set at P < 0.05.
3. Results
3.1. PMS-601 anti-inflammatory, antiretroviral and cytotoxicity for human MDM
3.1.1. Anti-retroviral activities and cytotoxity
PMS-601 reduced progeny virus formation in HIV-1Ba-L infected MDM (Serradji et al., 2000). This was seen starting at concentrations from 1 µM. Here RT activity, measured by radio-labeled thymidine 3H incorporation, was decreased by 46% when compared to untreated virus-infected MDM (P <0.05, Fig. 1A). Interesting, at 1 mM, RT reductions were comparable at 42% (P<0.01) without changes in cell viability. PMS-601 treatment did not change MDM viability, measured by MTT, when administered from 10 nM to 1 mM concentrations (data not shown).
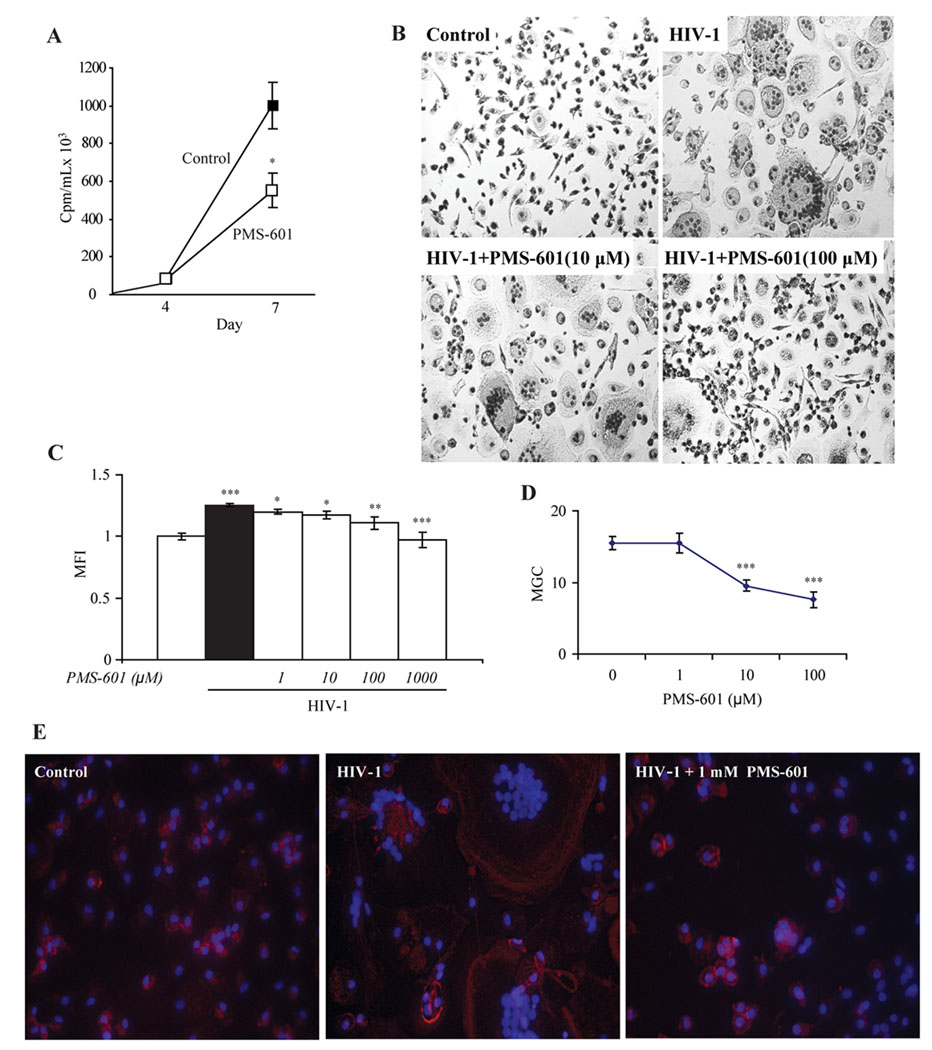
Figure 1.
Effects on viral replication and cytoskeletal organization in HIV-1ADA infected MDM by PMS-601. A Viral replication at 4 and 7 d in infected human MDM and infected human MDM treated with 10 µM PMS-601 normalized to cell viability using MTT assay. B MDM were infected with HIV-1ADA and cultured for 5 days with drug concentrations maintained throughout infection. Cells were fixed using 4% paraformaldehyde and stained with Mayer’s hematoxylin. Microphotographs were taken from each quadrant with multiple wells per group. MGC with ≥5 nuclei were counted. C Graph of levels of F-actin stained with philloidin in human MDM treated with increasing concentrations of PMS-601 as measured by MFI in in situ ELISA assay. Each group has 6 replicates. D Graph of number of MGC with increasing concentrations of PMS-601. E Fluorescent microscopy pictures of philloidin (red) and DAPI (blue) in human MDM infected with HIV-1ADA for 5 days treated with PMS-601. Experiments are representative of 3 replicate assays performed with MDM from 3 different donors. Data are expressed as means ± S.E.M. * P≤0.05 ** P≤0.01 *** P≤0.001
3.1.2. MGC and cytoskeletal transformation
The mechanisms for PMS-601 induced viral inhibition remains incomplete (Martin et al., 2000; Serradji et al., 2000; Serradji et al., 2004). Herein, we found that PMS-601 reduced HIV-1 induced MGC formation, index and numbers. MDM infected with HIV-1ADA and treated with 10 and 100 µM PMS-601 showed a 38 and 51% reduction in the formation of MGC (P<0.0001), respectively. At 1mM PMS-601 MGC was rare (Fig. 1D). Readily observable differences in numbers and size of MGC in control, virus-infected and PMS-601 and virus-infected MDM were also observed (Fig. 1B).
Reduction of viral replication was not associated with changes in viral co-receptor expression including CD4, CCR5 and CXCR4 (Bailer et al., 2000). One possible endpoint was that cytoskeletal rearrangement, which could affect viral particle assembly (Chertova et al., 2006) would be induced by PMS-601. We hypothesized that F-actin could be modulated by PMS-601. Thus we used phalloidin to visualize F-actin and showed that HIV-1ADA infected MDM induced a 25% increase (P<0.00001) of phalloidin when compared to uninfected control MDM. In contrast, PMS-601 treatment of virus-infected MDM showed a significant dose dependent decrease in phalloidin staining beginning at 1 µM (Fig. 1C). Fluorescent photomicrographs of phalloidin show differences in cell size and localization of phalloidin in control, infected and virus-infected MDM treated with drug (Fig. 1E).
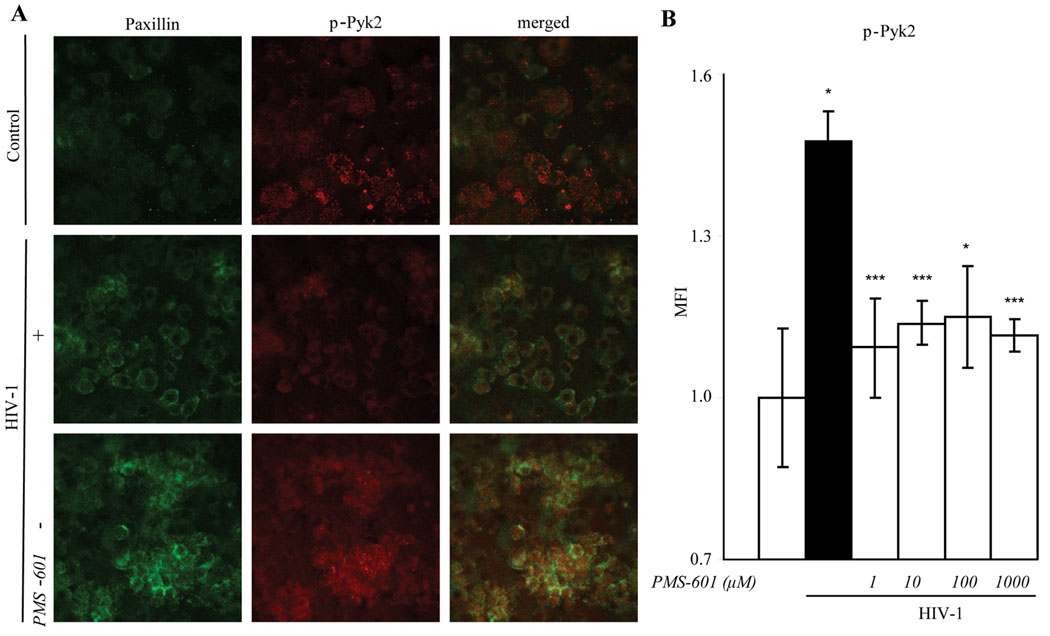
We next theorized that changes observed in cytoskeletal rearrangement resulting in decreased MGC formation might be linked to anchoring of the cytoskeleton to the plasma membrane. Proline-rich kinase 2 (Pyk2), a non receptor tyrosine kinase also known to be upstream of signaling cascades (Pandey et al., 1999), is involved in cytoskeletal anchoring. Total Pyk-2 and phosphorylated (p)-Pyk2 levels were thus examined by in situ ELISA. MDM were treated with or without drug at time of infection, and cultures were maintained for 5 days thereafter. HIV-1ada infection of MDM induced total Pyk2 when compared to uninfected cells (38%; P<0.05). Drug did not affect total Pyk2 in HIV-1ADA infected MDM or in uninfected MDM. Levels of p-Pyk2 were increased in HIV-1ADA infected MDM when compared with uninfected cells (48%; P<0.05). Treatment with drug significantly decreased p-Pyk2 to near control levels starting at 1 µM concentration (Fig. 2B). Uninfected MDM treated with PMS-601 showed no significant differences in p-Pyk2 when compared to MDM alone (data not shown). Phosphorylated Pyk2 is known to co-localize with paxillin (Duong and Rodan, 2000). Confocal microscopy revealed significant increases of p-Pyk2 in HIV-1ADA infected MDM compared to MDM (Fig. 2A). Treatment with 100 µM and 1 mM concentrations PMS-601 showed decreased p-Pyk2 (Fig. 2B).
Figure 2.
Phosphorylation of Pyk-2 is decreased with increasing concentrations of PMS-601 in HIV-1ADA infected MDM. A Confocal microscopy of human MDM infected with HIV-1ADA for 5 d and then stained for anti-paxillin (green) and anti-phosphorylated Pyk-2 (red); images merged. B Graph of phosphorylated Pyk2 (p-Pyk2) in HIV-1ADA infected human MDM treated with increasing concentrations of PMS-601 as measured by MFI using in situ ELISA assay. In situ ELISA experiments are representative of 6 replicate assays performed with MDM from 3 different donors. Data are expressed as means ± S.E.M. * P≤0.05 *** P≤0.001
3.1.3. Cell signaling
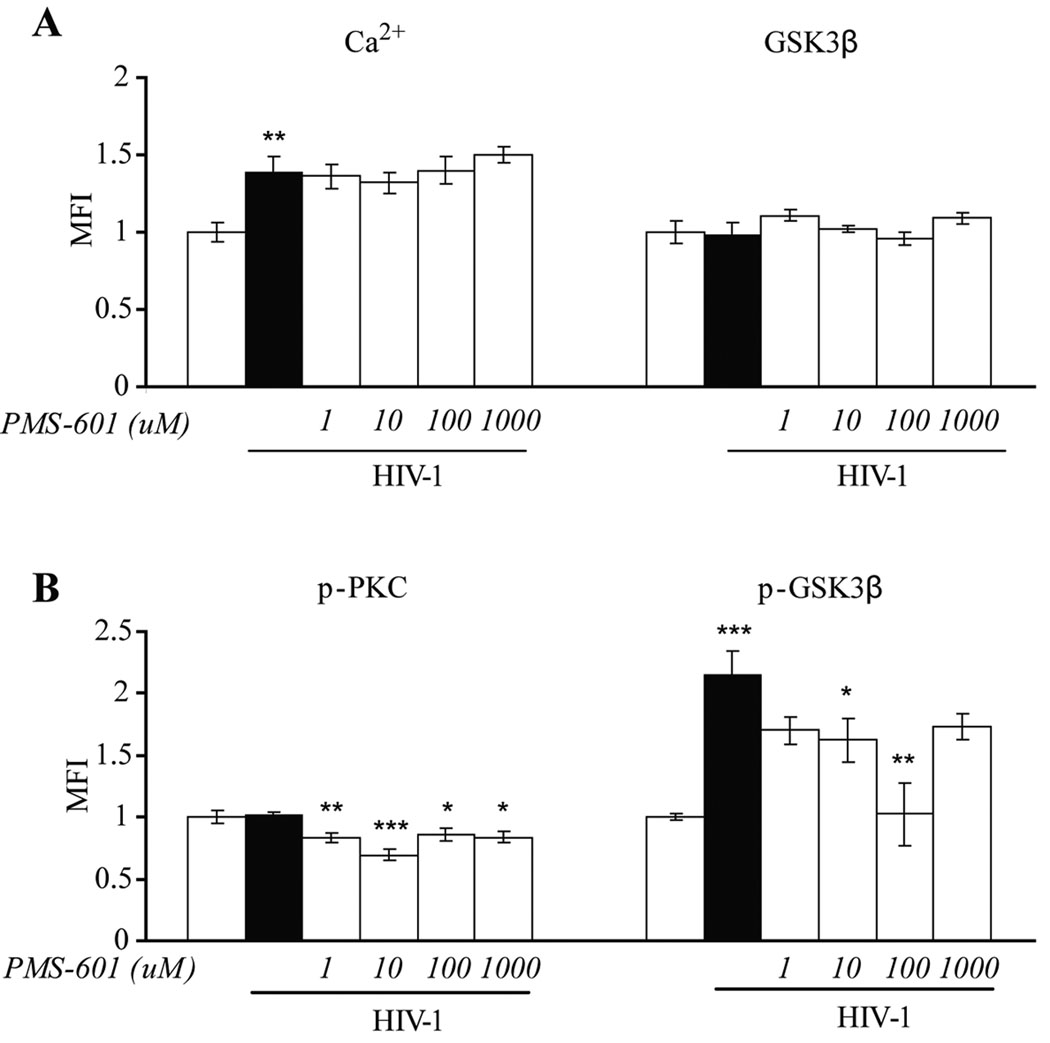
PMS-601 is known to diminish PAF, TNF-α, regulated upon activation normal T cell expressed and secreted (RANTES) and CCL5α/β (Martin et al., 2000). All of these except TNF-α, signal through G-protein coupled receptors. Signaling through Gαi/s leads to activation of transcription factors, while through Gαq leads to both activation of transcription factors and cytoskeletal rearrangements (Chakraborty, 2001; Lattin et al., 2007). Ca2+ is involved as a second messenger after activation of phospholipase C (PKC), inositol triphosphophate (IP3) and diacylglycerol (DAG) by Gαq. We identified free calcium in the MDM cytoplasm using the Alizarin Red S 1% aqueous solution assay. High concentrations of Ca2+ are bound to cell proteins. The assay binds to free Ca2+; and we examined cultures, not individual cells. HIV-1ADA infected MDM showed an increase of 39% in free Ca2+ when compared to control cells (P<0.01); treatment with drug failed to reduce Ca2+ (Fig. 3A). Pyk2 is phosphorylated in a Ca2+ -dependent manner (Tokiwa et al., 1996). We observed a decrease in p-Pyk2 when Ca2+ was high in virus-infected MDM providing evidence that the phosphorylation of Pyk2 is not Ca2+-dependent. Uninfected and drug-treated MDM showed no significant differences in free Ca2+ levels compared to MDM alone.
Figure 3.
PMS-601 treatment of HIV-1ADA infected MDM attenuates phosphorylated PKC and phosphorylated GSK3® without altering free Ca2+ or total GSK3® levels. A Graphs of levels of free Ca2+ using Alizarin Red S assay, total GSK3® levels using in situ ELISA assay in control MDM and HIV-1ADA infected MDM treated with increasing concentrations of PMS-601. B Graph of phosphorylated PKC (p-PKC) and phosphorylated GSK3® (p-GSK3®) using in situ ELISA assay in control MDM and HIV-1ADA infected MDM treated with increasing concentrations of PMS-601. In situ ELISA assays were measured by MFI. Experiments are representative of 6 replicate assays performed with MDM from 3 different donors. Data are expressed as means ± S.E.M. * P≤0.05 ** P≤0.01 *** P≤0.001
PKC, an enzyme activated by Ca2+ (Tsoukas et al., 2001). Because Ca2+ was not decreased, we examined p-PKC by in situ ELISA. No differences were found in levels of p-PKC between uninfected and virus-infected MDM (P=0.86). However, a significant decrease in p-PKC starting at 1 µM drug (17%; P <0.01) with the greatest decrease seen at 10 µM (31%; P <0.001) in virus-infected MDM. Statistically significant decreases were observed up to 1 mM drug concentrations (Fig. 3B). Uninfected MDM treated with increasing concentrations of drug resulted in no statistical differences in p-PKC compared to MDM alone.
Activation of GSK3β in a cytokine-rich environment is PKC mediated PKC (Vilimk and Duronio, 2006) and leads to stimulation of MAPK and NF-κB pathways (Grimes and Jope, 2001; Kim et al., 2003). HIV-1 infected MDM produce cytokines albeit at low levels. As PMS-601 decreases p-PKC we next determined levels of total and p-GSK3β by in situ ELISA. No differences were observed in total GSK3β between HIV1-infected MDM when compared to MDM alone (P=0.86). Treatment of virus-infected MDM with PMS-601, at a range of concentrations, showed no differences in total GSK3β levels when compared to untreated virus-infected MDM (Fig. 3A). Phosphorylated GSK3β (p-GSK3β) was increased in HIV-1 infected MDM when compared to uninfected cells (115%; P<0.001). Decreases in levels of p-GSK3β were observed with concentrations starting at 1 µM PMS-601 with significant decreases seen at 10 µM (30%; P<0.05) and 100 µM (52%; P<0.01) PMS-601 concentrations (Fig. 3B). No significant differences were seen in levels of total GSK3β or p-GSK3β in uninfected MDM treated with increasing concentrations of drug when compared to MDM alone.
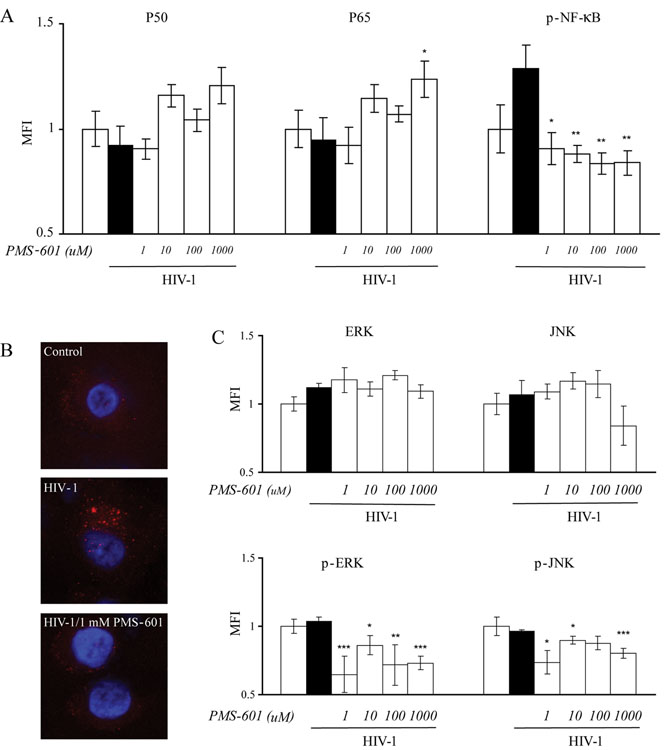
Activated GSK3β upregulates NF-κB (Martin et al., 2005). Because decreased levels of p-GSK3β were observed, we determined if PMS-601 affected NF-κB. Levels of p50, p65 and p-NF-κB were evaluated using an in situ ELISA. Levels of total p50 and p65 were not statistically different between uninfected and virus-infected MDM treated or not treated with drug (Fig. 4A). Levels of p-NF-κB were increased in virus-infected MDM when compared to control MDM, but this difference was not significant (P=0.11). PMS-601 treatment of infected MDM resulted in decreased p-NF-κB compared to HIV-1 ADA infected MDM at all drug concentrations including 1µM (30%; P<0.05), 10 µM (31%; P<0.01), 100 µM (35%; P<0.01) and 1 mM (35%; P<0.01) (Fig. 4A). Confocal micrographs showed localization of p-NF-κB principally in the cytosol of drug-treated and virus-infected MDM (Fig. 4B). Uninfected MDM treated with drug at similar concentrations showed no significant differences in levels of total p50 and p65 or p-NF-κB when compared to uninfected MDM.
Figure 4.
Effects of PMS-601 on the NF-| B pathway activation and MAPK pathway. A Graphs of levels of p50, and p65 and phosphorylated NF-| B (p- NF-| B) in human MDM treated with increasing concentrations of PMS-601 as measured by MFI using in situ ELISA assay. B Confocal microscopy of anti-p-NF-| B in control human MDM, human MDM infected with HIV-1ADA and human MDM infected with HIV-1ADA treated with 1 mM PMS-601. C Graphs of levels of total ERK, total JNK, phosphorylated ERK (p-ERK) and phosphorylated JNK (p-JNK) in control human MDM and HIV-1ADA infected human MDM treated with increasing concentrations of PMS-601 as measured by MFI using in situ ELISA assay. In situ ELISA experiments are representative of 6 replicate assays performed with MDM from 3 different donors. Data are expressed as means ± S.E.M. * P≤0.05 ** P≤0.01 *** P≤0.001
Activated GSK3β, tumor necrosis factor receptor (TNFR 1 and 2) can activate the MAPK pathway (Grimes and Jope, 2001; Lee et al., 2005). PMS-601 decreases p-GSK3β. These findings led us to evaluate the effect of PMS-601 on MAPK. Levels of ERK1/2, p-ERK1/2, JNK, p-JNK, p38 and phosphorylated p38 (p-p38) were assayed by in situ ELISA. No significant differences were found for levels of total ERK1/2, JNK or p38 when compared between infected or uninfected MDM. Treatment with PMS-601 did not show differences between cell groups with or without virus infection for total ERK1/2, JNK or p38. Phosphorylated ERK1/2 (p-ERK1/2) levels were comparable between uninfected and virus-infected MDM (P=0.58). Treatment of infected MDM with 1 µM (37%; P<0.001) drug resulted in significant decreases but was not changed at higher (1 mM) PMS-601 concentrations. Levels of phosphorylated-JNK (p-JNK) were not different between infected and control MDM (P=0.62). Levels of p-JNK in infected MDM decreased in response to drug with significant decreases observed at 1 µM (25% P<0.05) and 1 mM (18%; P<0.01, Fig. 4C). p-p38 showed no difference between uninfected and infected groups or among PMS-601 treated groups (data not shown). Levels of total JNK, p38, p-JNK, p-ERK1/2 and p-p38 were not significantly different in uninfected MDM treated with increasing concentrations of drug when compared with MDM. Interestingly, an increase in total ERK in uninfected MDM treated with 10 µM (P<0.05) and 1 mM (P=0.01) of drug was seen when compared to uninfected MDM.
3.1.4. Neuroprotective activities
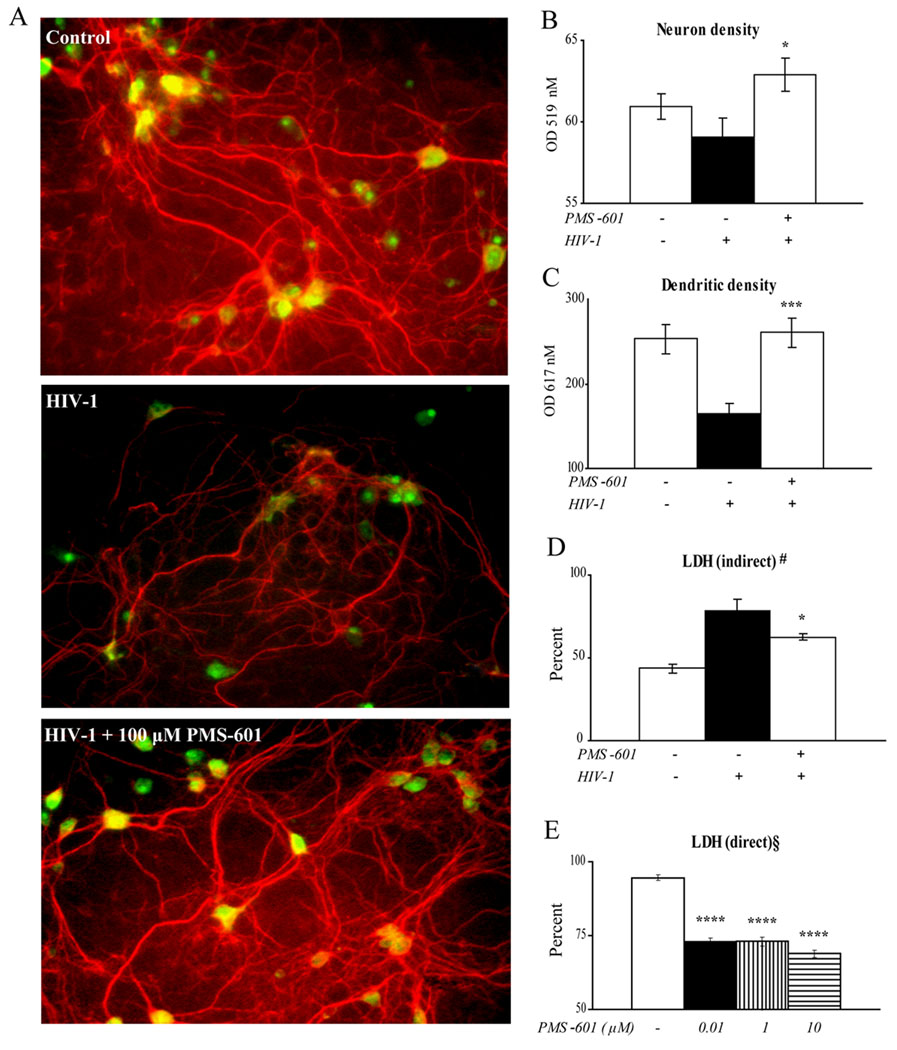
As PMS-601 readily crosses the blood brain barrier (Serradji et al., 2004) we determined if PMS-601 was directly neurotoxic. Cellular viability of primary mouse cortical neurons (MCN) cultures treated with drug for 24 hr was measured using an LDH release assay. Control neurons treated with vehicle for drug resulted in 8.71% ± 0.20 LDH. Treatment with drug in concentrations ranging from 10 nM to 10 mM did not result in any significant changes in viability (data not shown). PAF is a neurotoxin secreted by virus-infected MDM (Gelbard et al., 1994). MCN treated with supernatant from HIV-1ADA infected MDM alone resulted in 94.62% ± 0.88 LDH. Significant neuroprotection was seen in neurons treated with HIV-1ADA infected MDM supernatant and PMS-601 at 10 nM (73.18% ± 1.13; P<0.00001). This neuroprotection was observed for all drug treated groups (Fig. 5E).
Figure 5.
HIV-1ADA infected human MDM mediated neurotoxicity. Human MDM were treated with PMS-601 at time of HIV-1ADA infection. Cultures were subject to medium changes every other day with respective drug concentrations. On day 4, cultures were washed with PBS and media replaced with neurobasal media without PMS-601. Supernatants were harvested 24 hr later and placed on 10 d MCN cultures for 24 hr. A Fluorescent microscopy was used to visualize differences (anti-MAP-2, red; anti-NeuN, green). Pictures are representative of control MCN in neurobasal media, MCN incubated with neurobasal media from HIV-1ADA infected human MDM or MCN incubated with neurobasal media from HIV-1ADA infected human MDM treated with 100 µM PMS-601. Graphs of (B) neuron density (anti-NeuN; green), (C) dendritic density (anti-MAP-2; red), and (D) indirect neurotoxicity shown by lactate dehydrogenase (LDH) secreted by neurons as a measure of cellular viability. (E) PMS-601 elicits direct neuroprotection against HIV-1 infected MDM supernatants. Graph of LDH from 10 d MCN after 24 hr simultaneous treatment to HIV-1 infected MDM supernatants and PMS-601. Experiments are representative of 6 replicate assays performed with MDM from 3 different donors. Data are expressed as means ± S.E.M. * P≤0.05 ** P≤0.001 *** P≤0.00001 **** P≤0.000001
Thus, PMS-601 is neuroprotective when neurons are treated with supernatants from HIV-1ADA infected MDM when assayed for LDH. As PMS-601 also modulates the secretion of cytokines, we hypothesized this effect might contribute to diminished neurotoxicity. To directly assess neurotoxic MDM and drug responses, supernatants were collected from human MDM treated at the time of HIV-1ADA infection with either 0 nM (vehicle) or 100 µM concentrations of PMS-601 and cultured for 5 days with drug. To preclude direct PMS-601 affects on neurons, MDM were washed extensively with phosphate-buffered saline (PBS) on day 4, and media was replaced with neural basal media with no drug present. MDM conditioned media was harvested on day 5 and placed on MCN for 24 hours. Neurotoxicity was assessed by measuring dual MAP-2 and NeuN immunostaining. MCN cultured in neural basal media showed cells were distributed through the culture dish. A high density of dendritic branchpoints and long neuritic processes were seen with prominent cell bodies. Cultures exposed to HIV-1ADA conditioned MDM media exhibited a loss of connected processes, lower density of dendritic branch points and shorter, blunted neurites. MDM conditioned media from drug treated cultures reversed these neurotoxic effects. PMS-601 treated HIV-1ADA supernatant exposed MCN neurites and dendritic processes showed long processes in high density similar to controls (Fig. 5A). MCN treated with supernatants from infected MDM treated with 100 µM drug showed statistically significant increases in MAP-2 expression compared to MCN treated with supernatants from infected MDM (260.50 ± 17.19 and 164.38 ± 11.87, respectively (P<0.001, Fig. 5C). MCN treated with supernatants from infected MDM treated with 100 µM drug showed statistically significant increases in NeuN compared to MCN treated supernatants from untreated infected MDM (62.87 ± 1.02 and 59.05 ± 1.15, respectively (P<0.01) (Fig. 5B). LDH levels in the extracellular milieu also confirmed neuronal protection. LDH concentrations were decreased in primary cortical neuron cultures treated with supernatants from infected MDM treated with 100 µM drug as compared to MCN treated with supernatants from untreated infected MDM (62.68 ± 1.83 and 78.79 ± 0.66, respectively (P<0.05) (Fig. 5D).
3.2. SCID HIVE mice
We used our rodent HIVE model to evaluate drug effects (Persidsky et al., 2001). Briefly, SCID mice were stereotactically injected into the basal ganglia with HIV-1ADA infected MDM. Pathological changes observed paralleled those seen in human HIVE and included the formation of MGC, astrocytosis and neuronal dropout. Anti-retroviral and anti-inflammatory activities were evaluated using immunohistochemistry and real time PCR in HIVE SCID mice after administration of PMS-601. Animals were treated with vehicle-only or 750 mg/kg/d drug by daily intraperitoneal (i.p.) injection for 14 days (5 mice/vehicle-only treated group; 6 mice/drug treated group for immunohistochemistry and for RT-PCR).
Changes in astrocyte morphology surrounding the lesion site were evaluated using GFAP staining quantified using 3 sections from each animal at or adjacent to the lesion site. GFAP staining intensities were quantified by determining GFAP positive area as a percentage of total area per microscopic field. GFAP percentage was 19.89 ± 0.47 and 22.58 ± 0.69 for PMS-601 and vehicle-only treated mice, respectively (P<0.01). GFAP expression was assessed by RT-PCR, and we observed 1.393 ± .48 and 5.166 ± 1.11 SEM for drug treated mice as compared to vehicle-only treated animals (P≤0.05).
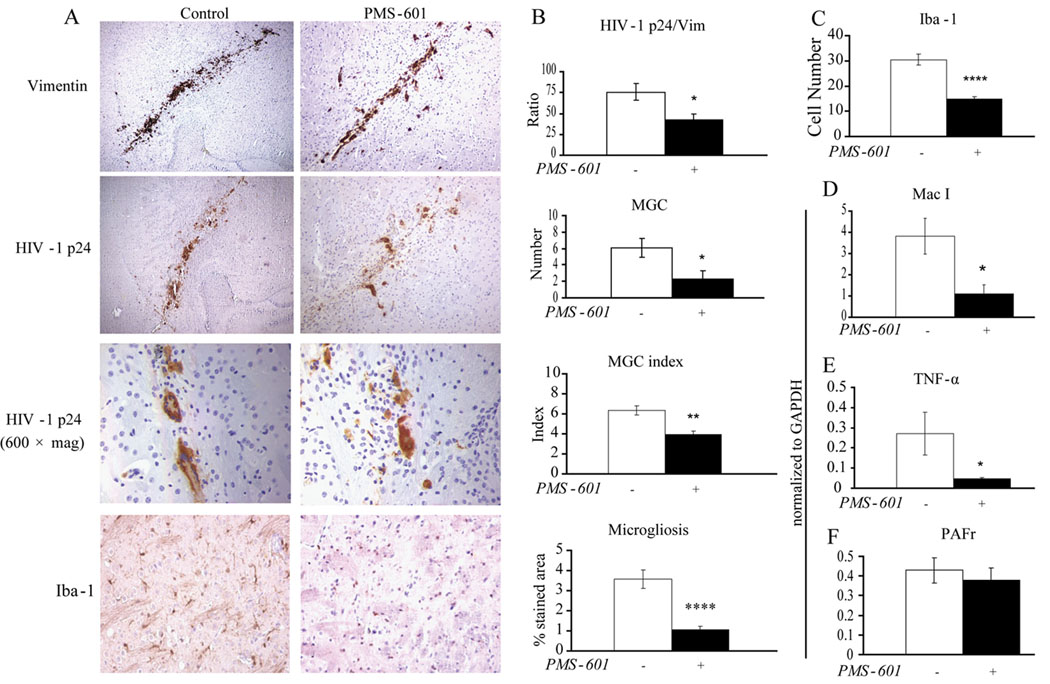
Activation of microglia was determined using serial brain sections stained for anti-Iba-1 (Fig. 6A). Microglia stained area was calculated as a percentage of the area of the microscopic field of view. Iba-1 percentage was 1.06 ± 0.17 and 3.56 ± 0.46 for PMS-601 and vehicle treated mice, respectively (P<0.00001, Fig. 6B). To determine if the difference in Iba-1 stained area percentage between both groups was due to a decrease in overall cell number or decrease in area per cell, all anti-Iba-1 positive cells were counted in the same microscopic fields. We observed 14.91 ± 0.85 (P<0.00001) microglia in each field of view for drug treated animals compared with 30.45 ± 2.18 for vehicle-only treated animals (Fig. 6C). Mac-1 RNA levels were assessed by RT-PCR, and we observed 1.09 ± 0.42 and 3.81 ± 0.85 for PMS-601 treated mice as compared to vehicle-only treated animals (P≤0.05, Fig. 6D).
Figure 6.
PMS-601 affects HIV-1ADA infection and microglial response in HIVE SCID mouse model. SCID mice were injected with HIV-1ADA infected human MDM and then treated with 750 mg/kg/d PMS-601 for 14 days. (A) Photomicrographs of immunohistochemistry of brain sections for anti-vimentin showing engraftment, anti-HIV-1-p24 showing infection, and MGC, and anti-Iba-1 showing microglial response in HIVE mice treated with vehicle-only or PMS-601. (B) Graphs of ratio of anti-HIV-1-p24 positive cells to anti-vimentin positive cells, MGC, MGC nuclei index and microgliosis. Microgliosis was quantified using color densitometry. (C) Graphs of anti-Iba-1 positive cells from HIVE mice treated with vehicle-only or PMS-601. Pictures used to count the number of anti-Iba-1 positive cells were the same pictures used to quantify microgliosis. Graphs of (D) Mac-1 mRNA expression, (E) TNF-〈 mRNA expression and (F) PAF receptor mRNA expression in the ipsilateral side from HIVE mice treated with vehicle-only or PMS-601. * P≤0.05 ** P≤0.01 **** P≤0.0001
MGC are one of the distinguishing features of HIVE. We examined MGC by both number and the number of cells comprising each MGC as measured by nuclei in each MGC (index). We found 2.33 ± 0.93. MGC for each lesion in drug treated animals as compared to 6.10 ± 1.17 MGC for vehicle only treated animals (P<0.05) (Fig. 6A–B). The number of cells comprising each MGC was assessed, 3.90 ± 0.33 and 6.31 ± 0.46 cells for drug- and vehicle-treated animals, respectively (P<0.01, Fig. 6B).
HIV-1ADA infected and uninfected MDM were determined by anti-vimentin and anti-HIV p-24 in 5 µM thick tissue slices. Total numbers of HIV-1 p24 and vimentin stained cells in and surrounding the lesion were counted. Two serial tissue slices were counted for vimentin followed by two serial slices counted for HIV-1 p24 (Fig. 6A). The mean number of vimentin-positive cells per slice was 58.50 ± 9.06 for PMS-601 treated animals and 72.40 ± 5.31 for vehicle only animals. No statistical significance was found for PMS-601 effects on MDM engraftment. HIV-p24 positive cells were counted and were 27.88 ± 11.60 for PMS-601 treated animals and 54.533 ± 8.41 for vehicle-only treated animals. It was found that 47.7% and 75.3% MDM were infected in the PMS-601 and vehicle only treated animals respectively (P<0.05, Fig. 6B). Treatment with PMS-601 significantly attenuated replication of HIV-1.
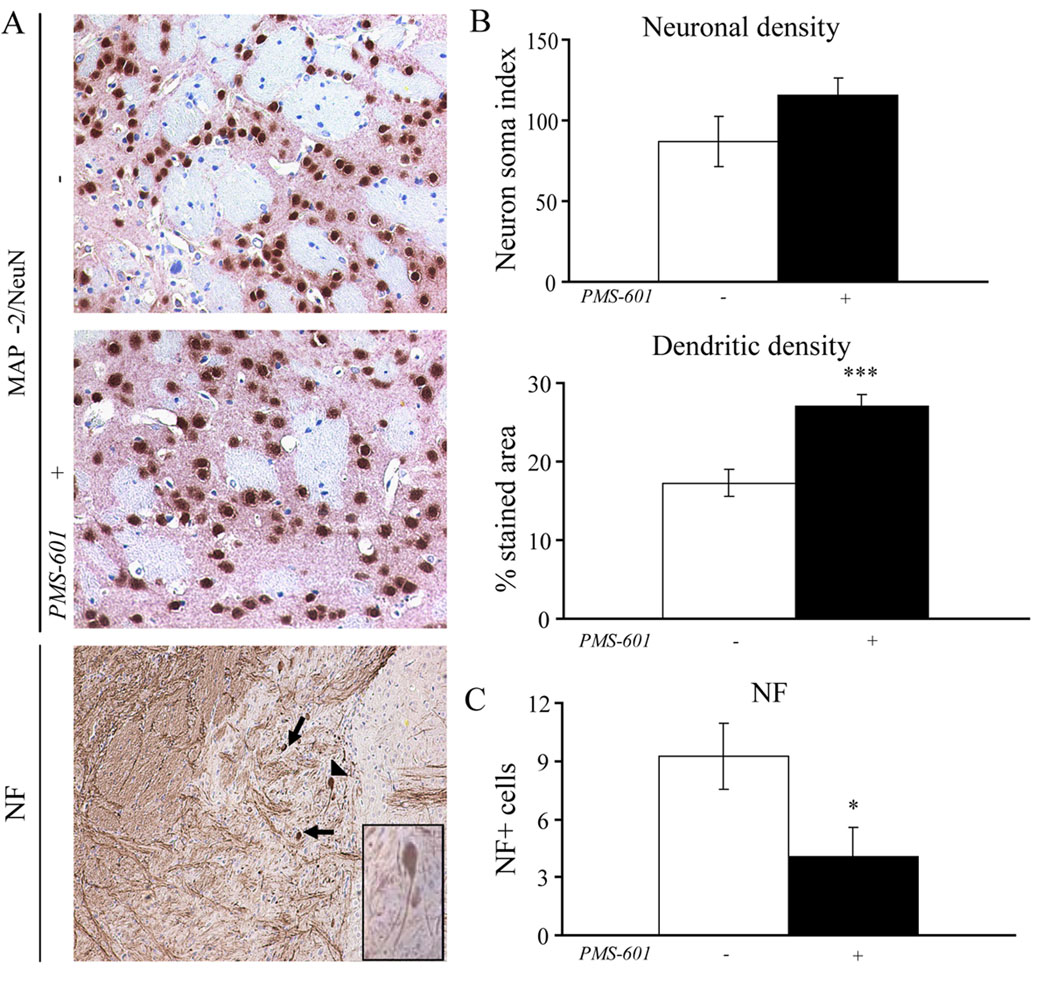
Neuroprotection was assessed in HIVE mice by the relative immunostaining of anti-NeuN and anti-MAP-2 antibodies, as well as anti-light and anti-heavy chain neurofilament antibodies in the same area as used for analyses of astroglial and microglial activation. Analyses of MAP-2/NeuN and neurofilament positive staining showed a significant difference in mice treated with PMS-601 in comparison to vehicle-only treated animals. Neural loss was assessed by counting neurons, stained with NeuN, surrounding the lesion area (Fig. 7A). Animals treated with PMS-601 had 115.25 ± 10.83 neurons as compared with 87.20 ± 15.56 neurons in vehicle-only treated mice (Fig. 7B). Dendritic processes, stained with anti-MAP-2 in the same field as neurons were counted, were quantified as a percentage of the area of the entire microscopic field (20×). Several brain sections were used to determine dendritic process loss surrounding the lesion area. Dendritic loss was significantly decreased in animals receiving PMS-601 (27.08 ± 1.37) compared to vehicle-only treated animals (17.24 ± 1.74 (P<0.001, Fig. 7B). Neuron soma-localized neurofilament was assessed by positive staining of anti-light and anti-heavy chain neurofilament antibodies and counted manually (Fig. 7A). We observed 4.09 ± 1.45 soma localized neurofilament immunostaining in drug treated mice as compared to 9.23 ± 1.70 in vehicle-only treated mice (P<0.05, Fig. 7C).
Figure 7.
PMS-601 is neuroprotective in vivo. Photomicrographs of anti-MAP-2 (pink) and anti-NeuN (brown) for (A) control HIVE mice, PMS-601 treated HIVE mice, and heavy and light chain NF in control HIVE mice. Arrow head in picture is for inset in lower right corner. Inset: Phosphorylated neurofilament the cell has failed to transport from the soma in the dendrites. B Graphs of neuronal density (NeuN) and dendritic density (MAP-2) in HIVE mice treated with vehicle-only or PMS-601. C Graph of number of neurons which visually have failed to move phosphorylated heavy and light chain neurofilament from the soma. * P≤0.05 *** P≤0.001
We next performed RT-PCR to determine TNF-α mRNA expression in the brains of HIVE mice. TNF-α expression levels were significantly decreased in drug treated animals as compared to vehicle-only treated animals, 0.04 ± 0.01 and 0.27 ± 0.10 respectively (P<0.05, Fig. 6D). Since PMS-601 is a PAF receptor antagonist, we wanted to know if PAF receptor expression changed due to drug treatment. RT-PCR was used to assess PAF receptor mRNA expression levels in the brains of HIVE mice. PAF receptor expression levels were 0.37 ± 0.06 for drug treated as compared to 0.42 ± 0.63 for vehicle treated animals (P=0.6, Fig. 6D).
4. Discussion
We show in this report that PMS-601 can modulate a broad range of neuroinflammatory responses linked to HIVE and HAND. In laboratory and a rodent model of HIVE, the drug demonstrates potent inhibition of pro-inflammatory pathways, microglial responses, MGC formation and viral growth. Most importantly, it affords substantial neuroprotection. In laboratory studies PMS-601 inhibit both NF-κB and MAPK pathways that offer insights into the drug’s mechanism of action (Table 1).
Table 1.
Summary of changes in levels of cell signaling molecules in MDM treated with increasing concentrations of PMS-601.
| MDM | HIV-1-infected PMS-601 treated concentrations |
|||||
|---|---|---|---|---|---|---|
| Uninfected | HIV-1 | 1µM | 10µM | 100µM | 1mM | |
| SIGNALING MOLECULES | ||||||
| Ca2++ | 1.0 ± 0.06 | 1.39 ± 0.09 | 1.32 ± 0.06 | 1.36 ± 0.08 | 1.40 ± 0.08 | 1.50 ± 0.05 |
| (P <0.01) | (P = 0.50) | (P = 0.79) | (P = 0.97) | (P = 0.37) | ||
| GSK3 ® | 1.0 ± 0.07 | 0.98 ± 0.08 | 1.11 ± 0.03 | 1.02 ± 0.01 | 0.96 ± 0.86 | 1.09 ± 0.03 |
| (P = 0.86) | (P = 0.20) | (P = 0.63) | (P = 0.86) | (P - 0.26) | ||
| Phosphorylated | 1.0 ± 0.02 | 2.15 ± 0.18 | 1.70 ± 0.11 | 1.62 ± 0.17 | 1.02 ± 0.25 | 1.73 ± 0.10 |
| GSK3 ® | (P <0.001) | (P = 0.07) | (P = 0.08) | (P <0.01) | (P = 0.08) | |
| NFκB Pathway | ||||||
| p50 | 1.0 ± 0.08 | 0.95 ± 0.10 | 0.92 ± 0.08 | 1.14 ± 0.06 | 1.07 ± 0.03 | 1.23 ± 0.09 |
| (P = 0.70) | (P = 0.85) | (P = 0.15) | (P = 0.30) | (P = 0.06) | ||
| p65 | 1.0 ± 0.08 | 0.92 ± 0.09 | 0.91 ± 0.04 | 1.16 ± 0.05 | 1.04 ± 0.05 | 1.20 ± 0.08 |
| (P = 0.53) | (P = 0.89) | (P = 0.06) | (P = 0.29) | (P <0.05) | ||
| Phosphorylated | 1.0 ± 0.11 | 1.29 ± 0.11 | 0.90 ± 0.07 | 0.87 ± 0.04 | 0.83 ± 0.04 | 0.83 ± 0.05 |
| NF-| B | (P = 0.10) | (P <0.05) | (P <0.01) | (P <0.01) | (P <0.01) | |
| MAPK Pathway | ||||||
| ERK1/2 | 1.0 ± 0.05 | 1.12 ± 0.03 | 1.17 ± 0.09 | 1.11 ± 0.05 | 1.21 ± 0.03 | 1.09 ± 0.05 |
| (P = 0.08) | (P = 0.58) | (P = 0.88) | (P = 0.08) | (P = 0.67) | ||
| Phosphorylated | 1.0 ± 0.05 | 1.03 ± 0.03 | 0.65 ± 0.13 | 0.86 ± 0.06 | 0.72 ± 0.14 | 0.73 ± 0.04 |
| ERK1/2 | (P = 0.58) | (P <0.001) | (P <0.05) | (P = 0.01) | (P <0.001) | |
| JNK | 1.0 ± 0.08 | 1.07 ± 0.10 | 1.09 ± 0.05 | 1.17 ± 0.06 | 1.14 ± 0.09 | 0.84 ± 0.14 |
| (P = 0.61) | (P = 0.86) | (P = 0.42) | (P = 0.61) | (P = 0.22) | ||
| Phosphorylated | 1.0 ± 0.06 | 0.97 ± 0.01 | 0.74 ± 0.08 | 0.90 ± 0.03 | 0.88 ± 0.05 | 0.80 ± 0.03 |
| JNK | (P = 0.063) | (P < 0.05) | (P = 0.05) | (P = 0.11) | (P <0.001) | |
Colored keys:  – upregulated in HIV-1 infected MDM,
– upregulated in HIV-1 infected MDM,  – downregulated by PMS-601 in virus-infected MDM
– downregulated by PMS-601 in virus-infected MDM
It is noteworthy that PMS-601 treated virus-infected MDM undergo marked morphological changes. Why this occurs is unknown. However, formation of macrophage MGC commonly occurs as a result of the cell’s response to contain microbial infections, stress, immune activation or environmental changes. MGC are also frequently observed during chronic fungal and mycobacterial infections, as well as during viral replication (Helming et al., 2009; Lopez-Balderas et al., 2007; Matucci et al., 2008; Zhu and Friedland, 2006). Microbes can often reside in MGC for prolonged time periods that span months or years. Inhibition of giant cell formation may also reflect deactivation of immunity and would be an advantage to the host cell in its abilities to facilitate movement into tissues or to contain HIV-1 growth (Baruzzi et al., 2008). Such changes would favor the host cell over the virus and may be in part responsible for the restriction of viral infection seen by PMS-601 (Ibarrondo et al., 2001). One of the pathological hallmarks of HAND is MGC formation (Masliah et al., 1996; Navia et al., 1986). MGC may also contribute to the diversity of HIV-1 subtypes as up to 2000 proviral DNA copies may be harbored by a single MGC (Koenig et al., 1986; Steain et al., 2008).
One possible mechanism for the antiviral effects of PMS-601 resides in its modulation of specific cell signaling pathways. These are independent of PAF-acetyl hydrolase. Although this enzyme is present in brain and peripheral tissues including cells of macrophage lineage PMS-601’s activities is as an antagonist of the PAF receptor and distinct from the anti-inflammatory responses seen in the current report. Phosphorylated Pyk2 and F-actin may be involved in cell fusion. Pyk2 localizes at the cell membrane and one of its functions is to help anchor the cytoskeleton to the plasma membrane (Mitra et al., 2005). Phosphorylated Pyk2 also interacts with Rho/Rac pathway leading to changes in migration, adhesion and the cytoskeleton of the cell (Zhu et al., 2008; Gismondi et al., 1997; Worthylake and Burridge, 2003; Rumsey et al., 2001; Rodriguez-Fernandez et al., 1999). Pyk2 can be phosphorylated in the presence of Ca2+ (Li et al., 1998) but it also has been shown that TNF-α can lead to phosphorylation of Pyk2 in a Ca2+-independent manner in neutrophils (Tokiwa et al., 1996). We speculate this mechanism may be present in MDM, possibly because decreased MGC formation may be due to lower levels of chemotactic and inflammatory factors, which in turn affect alterations in cellular activation, cytoskeletal modulation and ultimately cell fusion.
We posit that use of PMS-601 could be developed as an adjunctive therapy for HAND and possibly for other neurodegenerative disorders where neuroinflammatory responses play a prominent role. Indeed, misfolded and aggregated proteins which underlie the progression of Alzheimer’s and Parkinson’s diseases and amyotrophic lateral sclerosis show persistent neuroinflammatory responses that could be controlled by drugs such as PMS-601. In all these conditions, microglial activation underlies disease progression. Persistent HIV-1 infected and activated microglia, perivascular macrophages and infiltrating monocytes release arachidonic acid metabolites, cytokines, reactive oxygen species and quinolinic acid among various other substances affecting neurotoxic activities (Persidsky et al., 1997; Kadiu et al., 2005; Lipton, 1998). The marked abilities of PMS-601 to attenuate such cellular neurotoxic responses justifies further efforts to investigate its utility for HAND therapies.
Although inflammation may be advantageous for remodeling of tissue, chronic inflammation as seen in neurodegenerative diseases leads to degenerative activities (Liew et al., 2005; Nathan, 2002; Wells et al., 2005). This is certainly true for HAND that is characterized by interrelationships between brain macrophage and microglial viral and cellular neurotoxins with cognitive, behavioral and/or motor dysfunctions. This often progresses into broad neurological dysfunction but remains a diagnosis of exclusion after opportunistic infections and cancers are excluded (Villa et al., 1996). The pathological hallmark of HAND is HIVE and is characterized by astrocytosis, myelin pallor in the gray matter and the accumulation of virus-infected MP and neuronal loss (Masliah et al., 1996). This has changed in recent years with the advent of highly active antiretroviral therapies (Bhaskaran et al., 2008; Mellgren et al., 2007; Tozzi et al., 2007). Nonetheless, disease incidence remains on the rise and morbidity is commonplace during advanced disease (Letendre et al., 2007; Tozzi et al., 2007). Importantly, the brain harbors virus within infected MP despite antiretroviral treatments (Deeks et al., 2005; Tozser, 2001) and even limited viral infection could contribute to inflammation and neurodegeneration. PMS-601 readily crosses the blood brain barrier and can modulate the inflammatory secretions of MP (Martin et al., 2000; Serradji et al., 2004). Although PMS-601 would not treat the underlying cause of HAND, it might positively affect decreased disease progression by modulating neuroinflammatory responses. Certainly, developing adjunct therapies targeting cells and pathways involved in the inflammatory response to be administered in parallel with antiretrovirals would positively affect the disease course.
We believe the effects of PMS-601, a PAF receptor antagonist are derived from the inhibition of PAF to bind to and activate its receptor but additionally and ultimately, by subsequent inhibition of cytokine synthesis and secretion. HIV-1 infected monocytes and activated macrophages secrete high levels of TNF-α and PAF (Perry et al., 1998; Lee et al., 2005). PAF, a pleiotropic molecule with many normal functions, can be a potent neurotoxin in part by up-regulating expression of TNF-α (Kihara et al., 2005; Lee et al., 2005; Gelbard et al., 1994). Although PMS-601 crosses the blood brain barrier, the mechanism of how PMS-601 exerts both its anti-HIV and anti-PAF activities in the brain are currently unknown. PMS-601 has previously been shown to significantly decrease levels of TNF-α, MIP-1α, MIP-1β and RANTES in LPS-stimulated monocytes (Martin et al., 2000). All of these cytokines have been shown to contribute to the inflammatory environment either as chemoattractants for immune cells or through up-regulation of the NF-κB and/or MAPK pathways (Montecucco et al., 2008; Ryan et al., 2008). PMS-601 has previously been shown to have an anti-PAF activity at 8 µM and an anti-HIV activity at 11µM (Serradji et al., 2000). The modest anti-retroviral effect may be due to inhibiting PAF action that subsequently decreases production of inflammatory molecules such as TNF-α and PAF, which have been shown to enhance viral replication of HIV-1 (Devadas et al., 2004; Lima et al., 2006). PKC can phosphorylate HIV Nef for increased viral transcription (Wolf et al., 2008). HIV Tat has been shown to activate PKC in macrophage and monocytes leading to activation of MAPK p38 and ERK1/2 as well as the NF-κB pathway (Leghmari et al., 2008a; Leghmari et al., 2008b; Leghmari et al., 2008c). Interestingly, it has also been shown that the inhibition of PKC activation can also lead to HIV-1 viral transcription at early viral entry time points (Warrilow et al., 2006). HIV gp120 can activate the PI3K pathway (Francois and Klotman, 2003) that includes PKC. When cells are in a cytokine-stimulated environment, the phosphorylation of GSK3β is mainly carried out by PKC (Vilimek and Duronio, 2006).
In closing, PAF plays a pivotal role in inflammation. Unarguably, it is one of the most potent inflammatory molecules that are endogenously expressed. PAF and TNF-α induce one another in a vicious autocrine and paracrine regulatory loop (Maestre et al., 1990). Moreover, PAF can induce a number of other cytokines that could play equal to or greater roles in diseases as does TNF-α. PAF is paramount in inducing activation of the arachidonic acid pathway resulting in lipid mediators of inflammation such as leukotrienes (Boyce, 2008). By inhibiting PAF action, parts of the arachidonic acid pathway are curtained and the lipid-mediated inflammatory cascade halted (Cuschieri et al., 2002; Dent et al., 2000; Schaloske et al., 2005). The effect of PMS-601 on the arachidonic acid pathway and resulting inflammation still needs to be investigated. Blocking PAF also inhibits cytokine synthesis and secretion involved in subsequent signaling cascades leading to decreased inflammation. We speculate the changes we observe in cytoskeletal modification, signaling and inflammatory cascades are related to the altered secretory environment that PMS-601 creates as a PAF receptor antagonist. Our observations of reduced viral replication, phosphorylation of signaling molecules and inflammatory cascades, and cytoskeletal rearrangement by a compound which readily crosses the blood brain barrier make PMS-601 an extremely attractive drug for HAND.
Acknowledgements
We thank Ms. Robin Taylor for outstanding administrative and computer support and Nan Gong for MCN isolations and culture. Michael Jacobson and. Janice A. Taylor support for confocal microscopy is much appreciated. National Institute of Health grants P01MH645702, R37 NS36126, P01 NS31492, 2R01 NS034239, P20RR 15635, P20RR 21937, P20 DA026146 and P01 NS43985 supported this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bailer RT, Lee B, Montaner LJ. IL-13 and TNF-alpha inhibit dual-tropic HIV-1 in primary macrophages by reduction of surface expression of CD4, chemokine receptors CCR5, CXCR4 and post-entry viral gene expression. Eur J Immunol. 2000;30:1340–1349. doi: 10.1002/(SICI)1521-4141(200005)30:5<1340::AID-IMMU1340>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Baruzzi A, Caveggion E, Berton G. Regulation of phagocyte migration and recruitment by Src-family kinases. Cell Mol Life Sci. 2008;65:2175–2190. doi: 10.1007/s00018-008-8005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bate C, Kempster S, Williams A. Platelet-activating factor antagonists protect amyloid-beta damaged neurons from microglia-mediated death. Neuropharmacology. 2006a;51:173–181. doi: 10.1016/j.neuropharm.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Bate C, Kempster S, Williams A. Prostaglandin D2 mediates neuronal damage by amyloid-beta or prions which activates microglial cells. Neuropharmacology. 2006b;50:229–237. doi: 10.1016/j.neuropharm.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Belanger C, Elimam H, Lefebvre J, Borgeat P, Marleau S. Involvement of endogenous leukotriene B4 and platelet-activating factor in polymorphonuclear leucocyte recruitment to dermal inflammatory sites in rats. Immunology. 2008;124:295–303. doi: 10.1111/j.1365-2567.2007.02767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskaran K, Mussini C, Antinori A, Walker AS, Dorrucci M, Sabin C, Phillips A, Porter K. Changes in the incidence and predictors of human immunodeficiency virus-associated dementia in the era of highly active antiretroviral therapy. Ann Neurol. 2008;63:213–221. doi: 10.1002/ana.21225. [DOI] [PubMed] [Google Scholar]
- Boisse L, Gill MJ, Power C. HIV infection of the central nervous system: clinical features and neuropathogenesis. Neurol Clin. 2008;26:799–819. doi: 10.1016/j.ncl.2008.04.002. x. [DOI] [PubMed] [Google Scholar]
- Boyce JA. Eicosanoids in asthma, allergic inflammation, and host defense. Curr Mol Med. 2008;8:335–349. doi: 10.2174/156652408785160989. [DOI] [PubMed] [Google Scholar]
- Buscemi L, Ramonet D, Geiger JD. Human immunodeficiency virus type-1 protein Tat induces tumor necrosis factor-alpha-mediated neurotoxicity. Neurobiol Dis. 2007;26:661–670. doi: 10.1016/j.nbd.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty P. G-protein-mediated signaling and its control in macrophages and mammalian cells. Crit Rev Microbiol. 2001;27:1–8. doi: 10.1080/20014091096666. [DOI] [PubMed] [Google Scholar]
- Charpentier C, Dwyer DE, Mammano F, Lecossier D, Clavel F, Hance AJ. Role of minority populations of human immunodeficiency virus type 1 in the evolution of viral resistance to protease inhibitors. J Virol. 2004;78:4234–4247. doi: 10.1128/JVI.78.8.4234-4247.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chertova E, Chertov O, Coren LV, Roser JD, Trubey CM, Bess JW, Jr, Sowder RC, 2nd, Barsov E, Hood BL, Fisher RJ, Nagashima K, Conrads TP, Veenstra TD, Lifson JD, Ott DE. Proteomic and biochemical analysis of purified human immunodeficiency virus type 1 produced from infected monocyte-derived macrophages. J Virol. 2006;80:9039–9052. doi: 10.1128/JVI.01013-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuschieri J, Gourlay D, Bulger E, Garcia I, Jelacic S, Maier RV. Platelet-activating factor priming of inflammatory cell activity requires cellular adherence. Surgery. 2002;132:157–166. doi: 10.1067/msy.2002.125170. [DOI] [PubMed] [Google Scholar]
- Deeks SG, Hoh R, Neilands TB, Liegler T, Aweeka F, Petropoulos CJ, Grant RM, Martin JN. Interruption of treatment with individual therapeutic drug classes in adults with multidrug-resistant HIV-1 infection. J Infect Dis. 2005;192:1537–1544. doi: 10.1086/496892. [DOI] [PubMed] [Google Scholar]
- Del Sorbo L, Arese M, Giraudo E, Tizzani M, Biancone L, Bussolino F, Camussi G. Tat-induced platelet-activating factor synthesis contributes to the angiogenic effect of HIV-1 Tat. Eur J Immunol. 2001;31:376–383. doi: 10.1002/1521-4141(200102)31:2<376::aid-immu376>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Del Sorbo L, DeMartino A, Biancone L, Bussolati B, Conaldi PG, Toniolo A, Camussi G. The synthesis of platelet-activating factor modulates chemotaxis of monocytes induced by HIV-1 Tat. Eur J Immunol. 1999;29:1513–1521. doi: 10.1002/(SICI)1521-4141(199905)29:05<1513::AID-IMMU1513>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Dent G, Munoz NM, Zhu X, Ruhlmann E, Magnussen H, Leff AR, Rabe KF. Involvement of protein tyrosine kinases in activation of human eosinophils by platelet-activating factor. Immunology. 2000;100:231–237. doi: 10.1046/j.1365-2567.2000.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devadas K, Hardegen NJ, Wahl LM, Hewlett IK, Clouse KA, Yamada KM, Dhawan S. Mechanisms for macrophage-mediated HIV-1 induction. J Immunol. 2004;173:6735–6744. doi: 10.4049/jimmunol.173.11.6735. [DOI] [PubMed] [Google Scholar]
- Dhillon N, Zhu X, Peng F, Yao H, Williams R, Qiu J, Callen S, Ladner AO, Buch S. Molecular mechanism(s) involved in the synergistic induction of CXCL10 by human immunodeficiency virus type 1 Tat and interferon-gamma in macrophages. J Neurovirol. 2008;14:196–204. doi: 10.1080/13550280801993648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong LT, Rodan GA. PYK2 is an adhesion kinase in macrophages, localized in podosomes and activated by beta(2)-integrin ligation. Cell Motil Cytoskeleton. 2000;47:174–188. doi: 10.1002/1097-0169(200011)47:3<174::AID-CM2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Fang KM, Chang WL, Wang SM, Su MJ, Wu ML. Arachidonic acid induces both Na+ and Ca2+ entry resulting in apoptosis. J Neurochem. 2008;104:1177–1189. doi: 10.1111/j.1471-4159.2007.05022.x. [DOI] [PubMed] [Google Scholar]
- Francois F, Klotman ME. Phosphatidylinositol 3-kinase regulates human immunodeficiency virus type 1 replication following viral entry in primary CD4+ T lymphocytes and macrophages. J Virol. 2003;77:2539–2549. doi: 10.1128/JVI.77.4.2539-2549.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman MS, Long MW, Hankenson KD. Osteogenic differentiation of human mesenchymal stem cells is regulated by bone morphogenetic protein-6. J Cell Biochem. 2006;98:538–554. doi: 10.1002/jcb.20719. [DOI] [PubMed] [Google Scholar]
- Gelbard HA, Nottet HS, Swindells S, Jett M, Dzenko KA, Genis P, White R, Wang L, Choi YB, Zhang D, et al. Platelet-activating factor: a candidate human immunodeficiency virus type 1-induced neurotoxin. J Virol. 1994;68:4628–4635. doi: 10.1128/jvi.68.7.4628-4635.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman HE, Orenstein JM, Martin MA, Ferrua C, Mitra R, Phipps T, Wahl LA, Lane HC, Fauci AS, Burke DS, et al. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J Exp Med. 1988;167:1428–1441. doi: 10.1084/jem.167.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gismondi A, Bisogno L, Mainiero F, Palmieri G, Piccoli M, Frati L, Santoni A. Proline-rich tyrosine kinase-2 activation by beta 1 integrin fibronectin receptor cross-linking and association with paxillin in human natural killer cells. J Immunol. 1997;159:4729–4736. [PubMed] [Google Scholar]
- Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol. 2001;65:391–426. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- Helming L, Winter J, Gordon S. The scavenger receptor CD36 plays a role in cytokine-induced macrophage fusion. J Cell Sci. 2009;122:453–459. doi: 10.1242/jcs.037200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbein G, Khan KA. Is HIV infection a TNF receptor signalling-driven disease? Trends Immunol. 2008;29:61–67. doi: 10.1016/j.it.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Herbein G, Varin A, Larbi A, Fortin C, Mahlknecht U, Fulop T, Aggarwal BB. Nef and TNFalpha are coplayers that favor HIV-1 replication in monocytic cells and primary macrophages. Curr HIV Res. 2008;6:117–129. doi: 10.2174/157016208783884985. [DOI] [PubMed] [Google Scholar]
- Ibarrondo FJ, Choi R, Geng YZ, Canon J, Rey O, Baldwin GC, Krogstad P. HIV type 1 Gag and nucleocapsid proteins: cytoskeletal localization and effects on cell motility. AIDS Res Hum Retroviruses. 2001;17:1489–1500. doi: 10.1089/08892220152644197. [DOI] [PubMed] [Google Scholar]
- Ichiyama T, Hasegawa M, Hashimoto K, Matsushige T, Hirano R, Furukawa S. Cysteinyl leukotrienes induce macrophage inflammatory protein-1 in human monocytes/macrophages. Int Arch Allergy Immunol. 2009;148:147–153. doi: 10.1159/000155745. [DOI] [PubMed] [Google Scholar]
- Janelsins MC, Mastrangelo MA, Park KM, Sudol KL, Narrow WC, Oddo S, LaFerla FM, Callahan LM, Federoff HJ, Bowers WJ. Chronic neuron-specific tumor necrosis factor-alpha expression enhances the local inflammatory environment ultimately leading to neuronal death in 3xTg-AD mice. Am J Pathol. 2008;173:1768–1782. doi: 10.2353/ajpath.2008.080528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadiu I, Glanzer JG, Kipnis J, Gendelman HE, Thomas MP. Mononuclear phagocytes in the pathogenesis of neurodegenerative diseases. Neurotox Res. 2005;8:25–50. doi: 10.1007/BF03033818. [DOI] [PubMed] [Google Scholar]
- Kalter DC, Nakamura M, Turpin JA, Baca LM, Hoover DL, Dieffenbach C, Ralph P, Gendelman HE, Meltzer MS. Enhanced HIV replication in macrophage colony-stimulating factor-treated monocytes. J Immunol. 1991;146:298–306. [PubMed] [Google Scholar]
- Kihara Y, Ishii S, Kita Y, Toda A, Shimada A, Shimizu T. Dual phase regulation of experimental allergic encephalomyelitis by platelet-activating factor. J Exp Med. 2005;202:853–863. doi: 10.1084/jem.20050660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Lee JE, Kim MJ, Cho EG, Cho SG, Choi EJ. Glycogen synthase kinase 3 beta is a natural activator of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase kinase 1 (MEKK1) J Biol Chem. 2003;278:13995–14001. doi: 10.1074/jbc.M300253200. [DOI] [PubMed] [Google Scholar]
- Koenig S, Gendelman HE, Orenstein JM, Dal Canto MC, Pezeshkpour GH, Yungbluth M, Janotta F, Aksamit A, Martin MA, Fauci AS. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986;233:1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- Kovacs AD, Chakraborty-Sett S, Ramirez SH, Sniderhan LF, Williamson AL, Maggirwar SB. Mechanism of NF-kappaB inactivation induced by survival signal withdrawal in cerebellar granule neurons. Eur J Neurosci. 2004;20:345–352. doi: 10.1111/j.1460-9568.2004.03493.x. [DOI] [PubMed] [Google Scholar]
- Kwon KJ, Jung YS, Lee SH, Moon CH, Baik EJ. Arachidonic acid induces neuronal death through lipoxygenase and cytochrome P450 rather than cyclooxygenase. J Neurosci Res. 2005;81:73–84. doi: 10.1002/jnr.20520. [DOI] [PubMed] [Google Scholar]
- Lattin J, Zidar DA, Schroder K, Kellie S, Hume DA, Sweet MJ. G-protein-coupled receptor expression, function, and signaling in macrophages. J Leukoc Biol. 2007;82:16–32. doi: 10.1189/jlb.0107051. [DOI] [PubMed] [Google Scholar]
- Lee C, Tomkowicz B, Freedman BD, Collman RG. HIV-1 gp120-induced TNF-{alpha} production by primary human macrophages is mediated by phosphatidylinositol-3 (PI-3) kinase and mitogen-activated protein (MAP) kinase pathways. J Leukoc Biol. 2005;78:1016–1023. doi: 10.1189/jlb.0105056. [DOI] [PubMed] [Google Scholar]
- Leghmari K, Bennasser Y, Bahraoui E. HIV-1 Tat protein induces IL-10 production in monocytes by classical and alternative NF-kappaB pathways. Eur J Cell Biol. 2008a;87:947–962. doi: 10.1016/j.ejcb.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Leghmari K, Bennasser Y, Tkaczuk J, Bahraoui E. HIV-1 Tat protein induces IL-10 production by an alternative TNF-alpha-independent pathway in monocytes: role of PKC-delta and p38 MAP kinase. Cell Immunol. 2008b;253:45–53. doi: 10.1016/j.cellimm.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Leghmari K, Contreras X, Moureau C, Bahraoui E. HIV-1 Tat protein induces TNF-alpha and IL-10 production by human macrophages: differential implication of PKC-betaII and -delta isozymes and MAP kinases ERK1/2 and p38. Cell Immunol. 2008c;254:46–55. doi: 10.1016/j.cellimm.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Letendre S, Ances B, Gibson S, Ellis RJ. Neurologic complications of HIV disease and their treatment. Top HIV Med. 2007;15:32–39. [PubMed] [Google Scholar]
- Li X, Hunter D, Morris J, Haskill JS, Earp HS. A calcium-dependent tyrosine kinase splice variant in human monocytes. Activation by a two-stage process involving adherence and a subsequent intracellular signal. J Biol Chem. 1998;273:9361–9364. doi: 10.1074/jbc.273.16.9361. [DOI] [PubMed] [Google Scholar]
- Liew FY, Xu D, Brint EK, O'Neill LA. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5:446–458. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- Lima RG, Moreira L, Paes-Leme J, Barreto-de-Souza V, Castro-Faria-Neto HC, Bozza PT, Bou-Habib DC. Interaction of macrophages with apoptotic cells enhances HIV Type 1 replication through PGE2, PAF, and vitronectin receptor. AIDS Res Hum Retroviruses. 2006;22:763–769. doi: 10.1089/aid.2006.22.763. [DOI] [PubMed] [Google Scholar]
- Limoges J, Poluektova L, Ratanasuwan W, Rasmussen J, Zelivyanskaya M, McClernon DR, Lanier ER, Gendelman HE, Persidsky Y. The efficacy of potent anti-retroviral drug combinations tested in a murine model of HIV-1 encephalitis. Virology. 2001;281:21–34. doi: 10.1006/viro.2000.0758. [DOI] [PubMed] [Google Scholar]
- Lipton SA. Neuronal injury associated with HIV-1: approaches to treatment. Annu Rev Pharmacol Toxicol. 1998;38:159–177. doi: 10.1146/annurev.pharmtox.38.1.159. [DOI] [PubMed] [Google Scholar]
- Lopez-Balderas N, Huerta L, Villarreal C, Rivera-Toledo E, Sandoval G, Larralde C, Lamoyi E. In vitro cell fusion between CD4(+) and HIV-1 Env(+) T cells generates a diversity of syncytia varying in total number, size and cellular content. Virus Res. 2007;123:138–146. doi: 10.1016/j.virusres.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Maestre C, Zarco P, Gomez-Guerrero C, Gonzalez E, Herrero-Beaumont G, Braquet M, Egido J. Cooperation between tumor necrosis factor (TNF) and platelet-activating factor (PAF) in the inflammatory response. J Lipid Mediat. 1990;2 Suppl:S151–S159. [PubMed] [Google Scholar]
- Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 2005;6:777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M, Serradji N, Dereuddre-Bosquet N, Le Pavec G, Fichet G, Lamouri A, Heymans F, Godfroid JJ, Clayette P, Dormont D. PMS-601, a new platelet-activating factor receptor antagonist that inhibits human immunodeficiency virus replication and potentiates zidovudine activity in macrophages. Antimicrob Agents Chemother. 2000;44:3150–3154. doi: 10.1128/aac.44.11.3150-3154.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Ge N, Achim CL, DeTeresa R, Wiley CA. Patterns of neurodegeneration in HIV encephalitis. J NeuroAIDS. 1996;1:161–173. doi: 10.1300/j128v01n01_08. [DOI] [PubMed] [Google Scholar]
- Matucci A, Rossolillo P, Baroni M, Siccardi AG, Beretta A, Zipeto D. HLA-C increases HIV-1 infectivity and is associated with gp120. Retrovirology. 2008;5:68. doi: 10.1186/1742-4690-5-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellgren A, Price RW, Hagberg L, Rosengren L, Brew BJ, Gisslen M. Antiretroviral treatment reduces increased CSF neurofilament protein (NFL) in HIV-1 infection. Neurology. 2007;69:1536–1541. doi: 10.1212/01.wnl.0000277635.05973.55. [DOI] [PubMed] [Google Scholar]
- Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- Montecucco F, Steffens S, Burger F, Da Costa A, Bianchi G, Bertolotto M, Mach F, Dallegri F, Ottonello L. Tumor necrosis factor-alpha (TNF-alpha) induces integrin CD11b/CD18 (Mac-1) up-regulation and migration to the CC chemokine CCL3 (MIP-1alpha) on human neutrophils through defined signalling pathways. Cell Signal. 2008;20:557–568. doi: 10.1016/j.cellsig.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Muller-Ladner U, Gay RE, Gay S. Role of nuclear factor kappaB in synovial inflammation. Curr Rheumatol Rep. 2002;4:201–207. doi: 10.1007/s11926-002-0066-1. [DOI] [PubMed] [Google Scholar]
- Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- Navia BA, Cho ES, Petito CK, Price RW. The AIDS dementia complex: II. Neuropathology. Ann Neurol. 1986;19:525–535. doi: 10.1002/ana.410190603. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Wang X, Baba M. HIV-1-infected macrophages induce astrogliosis by SDF-1alpha and matrix metalloproteinases. Biochem Biophys Res Commun. 2005;336:1214–1220. doi: 10.1016/j.bbrc.2005.08.251. [DOI] [PubMed] [Google Scholar]
- Pandey P, Avraham S, Kumar S, Nakazawa A, Place A, Ghanem L, Rana A, Kumar V, Majumder PK, Avraham H, Davis RJ, Kharbanda S. Activation of p38 mitogen-activated protein kinase by PYK2/related adhesion focal tyrosine kinase-dependent mechanism. J Biol Chem. 1999;274:10140–10144. doi: 10.1074/jbc.274.15.10140. [DOI] [PubMed] [Google Scholar]
- Perry SW, Hamilton JA, Tjoelker LW, Dbaibo G, Dzenko KA, Epstein LG, Hannun Y, Whittaker JS, Dewhurst S, Gelbard HA. Platelet-activating factor receptor activation. An initiator step in HIV-1 neuropathogenesis. J Biol Chem. 1998;273:17660–17664. doi: 10.1074/jbc.273.28.17660. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Buttini M, Limoges J, Bock P, Gendelman HE. An analysis of HIV-1-associated inflammatory products in brain tissue of humans and SCID mice with HIV-1 encephalitis. J Neurovirol. 1997;3:401–416. doi: 10.3109/13550289709031186. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Limoges J, McComb R, Bock P, Baldwin T, Tyor W, Patil A, Nottet HS, Epstein L, Gelbard H, Flanagan E, Reinhard J, Pirruccello SJ, Gendelman HE. Human immunodeficiency virus encephalitis in SCID mice. Am J Pathol. 1996;149:1027–1053. [PMC free article] [PubMed] [Google Scholar]
- Persidsky Y, Limoges J, Rasmussen J, Zheng J, Gearing A, Gendelman HE. Reduction in glial immunity and neuropathology by a PAF antagonist and an MMP and TNFalpha inhibitor in SCID mice with HIV-1 encephalitis. J Neuroimmunol. 2001;114:57–68. doi: 10.1016/s0165-5728(00)00454-9. [DOI] [PubMed] [Google Scholar]
- Poluektova L, Gorantla S, Faraci J, Birusingh K, Dou H, Gendelman HE. Neuroregulatory events follow adaptive immune-mediated elimination of HIV-1-infected macrophages: studies in a murine model of viral encephalitis. J Immunol. 2004;172:7610–7617. doi: 10.4049/jimmunol.172.12.7610. [DOI] [PubMed] [Google Scholar]
- Rey A, M'Rini C, Sozzani P, Lamboeuf Y, Beraud M, Caput D, Ferrara P, Pipy B. IL-13 increases the cPLA2 gene and protein expression and the mobilization of arachidonic acid during an inflammatory process in mouse peritoneal macrophages. Biochim Biophys Acta. 1998;1393:244–252. doi: 10.1016/s0005-2760(98)00080-0. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Fernandez JL, Gomez M, Luque A, Hogg N, Sanchez-Madrid F, Cabanas C. The interaction of activated integrin lymphocyte function-associated antigen 1 with ligand intercellular adhesion molecule 1 induces activation and redistribution of focal adhesion kinase and proline-rich tyrosine kinase 2 in T lymphocytes. Mol Biol Cell. 1999;10:1891–1907. doi: 10.1091/mbc.10.6.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roquebert B, Malet I, Wirden M, Tubiana R, Valantin MA, Simon A, Katlama C, Peytavin G, Calvez V, Marcelin AG. Role of HIV-1 minority populations on resistance mutational pattern evolution and susceptibility to protease inhibitors. Aids. 2006;20:287–289. doi: 10.1097/01.aids.0000202650.03279.69. [DOI] [PubMed] [Google Scholar]
- Rumsey LM, Teague RM, Benedict SH, Chan MA. MIP-1alpha induces activation of phosphatidylinositol-3 kinase that associates with Pyk-2 and is necessary for B-cell migration. Exp Cell Res. 2001;268:77–83. doi: 10.1006/excr.2001.5272. [DOI] [PubMed] [Google Scholar]
- Ryan SD, Harris CS, Carswell CL, Baenziger JE, Bennett SA. Heterogeneity in the sn-1 carbon chain of platelet-activating factor glycerophospholipids determines pro-or anti-apoptotic signaling in primary neurons. J Lipid Res. 2008;49:2250–2258. doi: 10.1194/jlr.M800263-JLR200. [DOI] [PubMed] [Google Scholar]
- Sang N, Zhang J, Marcheselli V, Bazan NG, Chen C. Postsynaptically synthesized prostaglandin E2 (PGE2) modulates hippocampal synaptic transmission via a presynaptic PGE2 EP2 receptor. J Neurosci. 2005;25:9858–9870. doi: 10.1523/JNEUROSCI.2392-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaloske RH, Provins JW, Kessen UA, Dennis EA. Molecular characterization of the lipopolysaccharide/platelet activating factor- and zymosan-induced pathways leading to prostaglandin production in P388D1 macrophages. Biochim Biophys Acta. 2005;1687:64–75. doi: 10.1016/j.bbalip.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Schuhmann MU, Mokhtarzadeh M, Stichtenoth DO, Skardelly M, Klinge PM, Gutzki FM, Samii M, Brinker T. Temporal profiles of cerebrospinal fluid leukotrienes, brain edema and inflammatory response following experimental brain injury. Neurol Res. 2003;25:481–491. doi: 10.1179/016164103101201896. [DOI] [PubMed] [Google Scholar]
- Serradji N, Bensaid O, Martin M, Kan E, Dereuddre-Bosquet N, Redeuilh C, Huet J, Heymans F, Lamouri A, Clayette P, Dong CZ, Dormont D, Godfroid JJ. Structure-activity relationships in platelet-activating factor (PAF). 10. From PAF antagonism to inhibition of HIV-1 replication. J Med Chem. 2000;43:2149–2154. doi: 10.1021/jm9911276. [DOI] [PubMed] [Google Scholar]
- Serradji N, Martin M, Bensaid O, Cisternino S, Rousselle C, Dereuddre-Bosquet N, Huet J, Redeuilh C, Lamouri A, Dong CZ, Clayette P, Scherrmann JM, Dormont D, Heymans F. Structure-activity relationships in platelet-activating factor. 12. Synthesis and biological evaluation of platelet-activating factor antagonists with anti-HIV-1 activity. J Med Chem. 2004;47:6410–6419. doi: 10.1021/jm040860g. [DOI] [PubMed] [Google Scholar]
- Smith DG, Guillemin GJ, Pemberton L, Kerr S, Nath A, Smythe GA, Brew BJ. Quinolinic acid is produced by macrophages stimulated by platelet activating factor, Nef and Tat. J Neurovirol. 2001;7:56–60. doi: 10.1080/135502801300069692. [DOI] [PubMed] [Google Scholar]
- Sneddon AA, McLeod E, Wahle KW, Arthur JR. Cytokine-induced monocyte adhesion to endothelial cells involves platelet-activating factor: suppression by conjugated linoleic acid. Biochim Biophys Acta. 2006;1761:793–801. doi: 10.1016/j.bbalip.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Steain MC, Wang B, Saksena NK. The possible contribution of HIV-1-induced syncytia to the generation of intersubtype recombinants in vitro. Aids. 2008;22:1009–1017. doi: 10.1097/QAD.0b013e3282f82b6c. [DOI] [PubMed] [Google Scholar]
- Stone TW, Behan WM. Interleukin-1beta but not tumor necrosis factor-alpha potentiates neuronal damage by quinolinic acid: protection by an adenosine A2A receptor antagonist. J Neurosci Res. 2007;85:1077–1085. doi: 10.1002/jnr.21212. [DOI] [PubMed] [Google Scholar]
- Tokiwa G, Dikic I, Lev S, Schlessinger J. Activation of Pyk2 by stress signals and coupling with JNK signaling pathway. Science. 1996;273:792–794. doi: 10.1126/science.273.5276.792. [DOI] [PubMed] [Google Scholar]
- Tong N, Sanchez JF, Maggirwar SB, Ramirez SH, Guo H, Dewhurst S, Gelbard HA. Activation of glycogen synthase kinase 3 beta (GSK-3beta) by platelet activating factor mediates migration and cell death in cerebellar granule neurons. Eur J Neurosci. 2001;13:1913–1922. doi: 10.1046/j.0953-816x.2001.01572.x. [DOI] [PubMed] [Google Scholar]
- Tozser J. HIV inhibitors: problems and reality. Ann N Y Acad Sci. 2001;946:145–159. doi: 10.1111/j.1749-6632.2001.tb03909.x. [DOI] [PubMed] [Google Scholar]
- Tozzi V, Balestra P, Bellagamba R, Corpolongo A, Salvatori MF, Visco-Comandini U, Vlassi C, Giulianelli M, Galgani S, Antinori A, Narciso P. Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. J Acquir Immune Defic Syndr. 2007;45:174–182. doi: 10.1097/QAI.0b013e318042e1ee. [DOI] [PubMed] [Google Scholar]
- Tsoukas CD, Grasis JA, Ching KA, Kawakami Y, Kawakami T. Itk/Emt: a link between T cell antigen receptor-mediated Ca2+ events and cytoskeletal reorganization. Trends Immunol. 2001;22:17–20. doi: 10.1016/s1471-4906(00)01795-6. [DOI] [PubMed] [Google Scholar]
- Tyor WR, Power C, Gendelman HE, Markham RB. A model of human immunodeficiency virus encephalitis in scid mice. Proc Natl Acad Sci U S A. 1993;90:8658–8662. doi: 10.1073/pnas.90.18.8658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versteeg HH, Nijhuis E, van den Brink GR, Evertzen M, Pynaert GN, van Deventer SJ, Coffer PJ, Peppelenbosch MP. A new phosphospecific cell-based ELISA for p42/p44 mitogen-activated protein kinase (MAPK), p38 MAPK, protein kinase B and cAMP-response-element-binding protein. Biochem J. 2000;350(Pt 3):717–722. [PMC free article] [PubMed] [Google Scholar]
- Vilimek D, Duronio V. Cytokine-stimulated phosphorylation of GSK-3 is primarily dependent upon PKCs, not PKB. Biochem Cell Biol. 2006;84:20–29. doi: 10.1139/o05-154. [DOI] [PubMed] [Google Scholar]
- Villa G, Solida A, Moro E, Tavolozza M, Antinori A, De Luca A, Murri R, Tamburrini E. Cognitive impairment in asymptomatic stages of HIV infection. A longitudinal study. Eur Neurol. 1996;36:125–133. doi: 10.1159/000117228. [DOI] [PubMed] [Google Scholar]
- Viviani B, Gardoni F, Bartesaghi S, Corsini E, Facchi A, Galli CL, Di Luca M, Marinovich M. Interleukin-1 beta released by gp120 drives neural death through tyrosine phosphorylation and trafficking of NMDA receptors. J Biol Chem. 2006;281:30212–30222. doi: 10.1074/jbc.M602156200. [DOI] [PubMed] [Google Scholar]
- Warrilow D, Gardner J, Darnell GA, Suhrbier A, Harrich D. HIV type 1 inhibition by protein kinase C modulatory compounds. AIDS Res Hum Retroviruses. 2006;22:854–864. doi: 10.1089/aid.2006.22.854. [DOI] [PubMed] [Google Scholar]
- Wells CA, Ravasi T, Hume DA. Inflammation suppressor genes: please switch out all the lights. J Leukoc Biol. 2005;78:9–13. doi: 10.1189/jlb.1204710. [DOI] [PubMed] [Google Scholar]
- Wolf D, Giese SI, Witte V, Krautkramer E, Trapp S, Sass G, Haller C, Blume K, Fackler OT, Baur AS. Novel (n)PKC kinases phosphorylate Nef for increased HIV transcription, replication and perinuclear targeting. Virology. 2008;370:45–54. doi: 10.1016/j.virol.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Worthylake RA, Burridge K. RhoA and ROCK promote migration by limiting membrane protrusions. J Biol Chem. 2003;278:13578–13584. doi: 10.1074/jbc.M211584200. [DOI] [PubMed] [Google Scholar]
- Xiong H, Boyle J, Winkelbauer M, Gorantla S, Zheng J, Ghorpade A, Persidsky Y, Carlson KA, Gendelman HE. Inhibition of long-term potentiation by interleukin-8: implications for human immunodeficiency virus-1-associated dementia. J Neurosci Res. 2003;71:600–607. doi: 10.1002/jnr.10503. [DOI] [PubMed] [Google Scholar]
- Zheng J, Thylin MR, Ghorpade A, Xiong H, Persidsky Y, Cotter R, Niemann D, Che M, Zeng YC, Gelbard HA, Shepard RB, Swartz JM, Gendelman HE. Intracellular CXCR4 signaling, neuronal apoptosis and neuropathogenic mechanisms of HIV-1-associated dementia. J Neuroimmunol. 1999;98:185–200. doi: 10.1016/s0165-5728(99)00049-1. [DOI] [PubMed] [Google Scholar]
- Zhu X, Boetticher E, Wang L, Duan Y, Learoyd J, Leff AR. Proline-rich tyrosine kinase 2 regulates spreading and migration of eosinophils after beta2-integrin adhesion. Am J Respir Cell Mol Biol. 2008;39:263–269. doi: 10.1165/rcmb.2008-0047OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XW, Friedland JS. Multinucleate giant cells and the control of chemokine secretion in response to Mycobacterium tuberculosis. Clin Immunol. 2006;120:10–20. doi: 10.1016/j.clim.2006.01.009. [DOI] [PubMed] [Google Scholar]