Abstract
Glucocorticoid-induced apoptosis is exploited for the treatment of hematologic malignancies. Innate and acquired resistance limits treatment efficacy; however, resistance mechanisms are not well understood. Previously, using WEHI7.2 murine thymic lymphoma cells, we found that increasing the resistance to hydrogen peroxide by catalase transfection or selection for hydrogen peroxide resistance caused glucocorticoid resistance. This suggests the possibility that increasing hydrogen peroxide sensitivity could sensitize the cells to glucocorticoids. In other cell types, increasing manganese superoxide dismutase (MnSOD) can increase intracellular hydrogen peroxide. The current study demonstrated that increased expression of MnSOD sensitized WEHI7.2 cells to glucocorticoid-induced apoptosis and hydrogen peroxide. Treatment of WEHI7.2 cells with the catalytic antioxidant Mn(III) meso-tetrakis(N-ethylpyridinium-2-yl)porphyrin (MnTE-2-PyP5+), a manganoporphyrin, mimicked the effects of increased MnSOD expression. MnTE-2-PyP5+ also sensitized WEHI7.2 cells to cyclophosphamide and inhibited cell growth; it had no effect on the WEHI7.2 cell response to doxorubicin or vincristine. In primary follicular lymphoma cells, MnTE-2-PyP5+ increased cell death due to dexamethasone. Treatment of H9c2 cardiomyocytes with MnTE-2-PyP5+ inhibited doxorubicin cytotoxicity. The profile of MnTE-2-PyP5+ effects suggests MnTE-2-PyP5+ has potential for use in hematologic malignancies that are treated with glucocorticoids, cyclophosphamide and doxorubicin.
Keywords: SOD2, AEOL 10113
Introduction
Glucocorticoids are effective in the treatment of lymphoid malignancies because of their ability to induce apoptosis. Unfortunately, innate and acquired resistance limits their efficacy. Progress has been made in defining critical events for glucocorticoid-induced apoptosis (reviewed in 1–3). Glucocorticoid-induced apoptosis requires binding of the steroid to a cytosolic receptor. The steroid-receptor complex translocates to the nucleus where it acts as a transcriptional activator and repressor of gene networks. This results in a complex and poorly understood interaction of signals that decides the life or death of the cell. Apoptosis then proceeds through the release of cytochrome c from the mitochondria and activation of caspases.
Clinically, some cases of glucocorticoid resistance have been traced to non-functional (or decreased) glucocorticoid receptors (4, 5); however, this mechanism may be relatively rare (5). Glucocorticoid-induced release of cytochrome c from the mitochondria appears to be controlled by the relative amount of pro-apoptotic and anti-apoptotic Bcl-2 family members (6–8). Inability to appropriately upregulate the pro-apoptotic Bcl-2 family member, Bim (9), or upregulation of anti-apoptotic Bcl-2 family members (10, 11) are also correlated with treatment failure in the clinic. Given the complexity of the signaling pathways that determine glucocorticoid sensitivity, multiple resistance mechanisms clearly exist. Additional characterization of the signaling phase of dexamethasone-induced apoptosis in model systems is continuing to identify other potential sources of resistance (reviewed in (2, 3).
Previous work in our laboratory using the WEHI7.2 murine thymic lymphoma cell model has shown that WEHI7.2 cells resistant to hydrogen peroxide (H2O2) via catalase transfection or selection for resistance to H2O2 are cross-resistant to dexamethasone (12, 13). The oxidative stress resistant cells show a delay or lack of cytochrome c release in response to dexamethasone treatment proportional to their expression of catalase, an enzyme that metabolizes H2O2 (12, 13). This suggests that H2O2 is a key signal in dexamethasone-induced apoptosis.
Based on these results, we hypothesized that increasing the intracellular production of H2O2 is a potential strategy for sensitizing the cells to dexamethasone-induced apoptosis. For this purpose, we chose to overexpress manganese superoxide dismutase (MnSOD). MnSOD is a mitochondrial enzyme that dismutes superoxide (O2 •-) forming H2O2 in the process (14). In other cell types, increasing MnSOD elevated intracellular H2O2 (14, 15). There is evidence from IM-9 multiple myeloma cells that increasing MnSOD increases dexamethasone sensitivity (16); however, gene dose of MnSOD in thymocytes did not alter dexamethasone sensitivity (17). In the current study, we tested the hypothesis that increasing MnSOD could sensitize WEHI7.2 cells to dexamethasone. By testing this hypothesis in a lymphoma cell line, we can determine whether manipulating MnSOD to increase glucocorticoid sensitivity could be a unique characteristic of lymphoid tumor cells that can be exploited for chemotherapy. We further tested to what extent we could replicate the MnSOD effects by pretreating the cells with a manganoporphyrin, Mn(III) meso-tetrakis(N-ethylpyridinium-2-yl)porphyrin (MnTE-2-PyP5+).
Materials and Methods
Reagents and drug treatments
MnTE-2-PyP5+ was provided by Aeolus Pharmaceuticals (Denver, CO). All other chemicals and drugs were purchased from Sigma Chemical Co. (St. Louis, MO) unless otherwise stated.
For the tissue culture cells, sensitivity to dexamethasone was determined by incubating cells in a final concentration of 1 µM dexamethasone in an ethanol vehicle (final concentration of ethanol = 0.01%) or vehicle alone. For MnTE-2-PyP5+ effects on drug or oxidant response, the cells were pretreated with the indicated concentrations of MnTE-2-PyP5+ 2 h prior to the addition of drugs or oxidants. For measurements of MnTE-2-PyP5+ effects on apoptosis and growth, the cells were treated with the indicated concentrations of MnTE-2-PyP5+ for 24 and 48 h, respectively.
Cell culture and transfections
The mouse thymic lymphoma WEHI7.2 parental cell line (18) was maintained as previously described (13). Stable WEHI7.2 clones overexpressing MnSOD were constructed by electroporation (13) with a pcDNA3.1 vector containing the human cDNA MnSOD sequence, a gift from Dr. Larry Oberley (University of Iowa, Iowa City, IA) (19); clones were isolated and maintained as previously described (13).
H9c2 cells, obtained from Dr. Qin Chen (University of Arizona, Tucson, AZ), are an immortalized clonal cell line derived from BD1X rat embryonic heart tissue (20). They do not beat in culture, but retain certain electrophysiological and biochemical properties of cardiac cells and can form myotubes upon confluency (20, 21). H9c2 cells were chosen for this study due to their frequent use as a doxorubicin cardiotoxicity model (e.g., 22). H9c2cells were maintained in DMEM-high glucose (Invitrogen, Carlsbad, CA), supplemented with 10% fetal bovine serum (Gemini Bio-products, Sacramento, CA) and 2 mM L-glutamine (Invitrogen), at 37°C in a 5% CO2 humidified environment until 60–85% confluence.
Primary follicular lymphoma cells
Two primary human tumor samples with the diagnosis of follicular lymphoma (FL) were obtained from the Arizona Lymphoma Tissue Repository in accordance with University of Arizona regulations for the use of primary human tissue. The cells were thawed and resuspended in Iscove’s Modified Dulbecco’s Medium (Invitrogen) with 20% fetal bovine serum (Gemini Bio-products) in the presence or absence of MnTE-2-PyP5+ and 750 µM dexamethasone. Drug concentrations were chosen based on the response in a human lymphoma-derived cell line. Viable B-cell content was analyzed prior to the addition of drugs and after incubation at 37°C in a 5% CO2 humidified environment for 24 h in the presence or absence of drugs. Cells were labeled with phycoerythrin-labeled anti-CD20 to identify B-cells (AbD Serotec, Oxford, UK) and 7.5 µg/ml propidium iodide, which stains dead cells, for 20 min. CD20 positive/propidium iodide negative cells were considered viable B-cells and measured by flow cytometry (EPICS XL-MCL, Beckman Coulter, Inc., Fullerton, CA). An isotype control was used for each run to gate out CD20 negative cells; debris was also gated out. At thaw, samples from patient 1 and patient 2 contained 49% and 40% viable B-cells, respectively. A minimum of 10,000 events were analyzed per sample. The percentage of viable B-cells in the sample containing no drugs was set to 100%.
Northern blots and immunoblots
MnSOD-transfected WEHI7.2 clones were screened for increased MnSOD expression by northern blots using cDNAs and protocols described previously (12, 23). Bands were quantitated using a Phosphorimager (Molecular Dynamics, Sunnyvale, CA) and the accompanying software. MnSOD expression was corrected for relative glyceraldehyde 3-phosphate expression and reported relative to the average expression in the Mneo1 and Mneo2 vector only transfectants.
MnSOD protein was quantitated by immunoblotting using anti-MnSOD, a gift from Dr. Larry Oberley (24), anti-β-actin (Sigma) and anti-rabbit Ig (GE Healthcare, Piscataway, NJ) or anti-mouse Ig (Pierce, Rockford, IL) as previously described (13). Bands were quantitated using a ScanJet 4C scanner (Hewlett Packard, Palo Alto, CA) and 1D analysis software (Kodak, Rochester, NY).
Enzyme activities
For catalase, samples were prepared and activity measured as previously described (12). For MnSOD, samples were prepared and activity measured using the method for total SOD activity as previously described (12) except that the samples were incubated with 50 mM diethyldithiocarbamate at 30°C for 1 h prior to dialysis to inactivate Cu,ZnSOD (25). Cellular protein was measured using the BCA Protein Assay Kit (Pierce) according to the manufacturer’s instructions. Enzyme activities were normalized to cellular protein.
EC50 measurements
Relative cell number was measured using the Cell Proliferation Kit II (Roche Diagnostics, Mannheim, Germany) (H2O2 only) or the Non-radioactive Cell Proliferation Assay (MTS) (Promega Corp., Madison, WI) according to the manufacturer’s protocol. The plates were read at 490 nm using a Microplate EL-311 or a Synergy HT plate reader (Bio-Tek Instruments, Winooski, VT). Fraction control absorbance was calculated as previously described (26). The EC50 was defined as the concentration at which the absorbance was 50% that of the control.
Viable cell number and apoptosis measurements
Viable cell number was determined by dye exclusion as previously described (13). For the MnSOD-transfected clones apoptosis was confirmed by morphologic examination using previously described criteria (13). The fraction of apoptotic cells, as measured by annexin V staining, was determined using the apoptosis detection kit (R & D Systems, Inc., Minneapolis, MN) as previously described (27).
Caspase-3 activity was measured using a colorimetric assay that depends on the cleavage of the synthetic caspase-3 specific substrate, Ac-DEVD-p-nitroanilide (pNA) (BIMOL International LTD, Bangkok, Thailand). Briefly, cells were lysed in: 10 mM Tris-HCl, pH 7.5; 100 mM NaCl; 1 mM EDTA; 0.01% Triton X-100 by sonication. The samples were clarified by centrifugation at 10,000×g for 10 min. An aliquot of the supernatant was first incubated in: 10 mM PIPES, pH 7.4; 2 mM EDTA; 0.01% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate hydrate (CHAPS); 5 mM DTT; 200 µM Ac-DEVD-pNA for 2 h and then the absorbance at 405 nm measured using a SynergyHT plate reader (Bio Tek Instruments, Inc.). Activity was normalized for cellular protein measured as described above.
Statistics
Means were compared using ANOVA or student’s t-tests, where appropriate, with the algorithms in Excel (Microsoft Corp., Redmond, WA). Means were considered significantly different when p ≤ 0.05.
Results
Construction and characterization of the MnSOD transfected clones
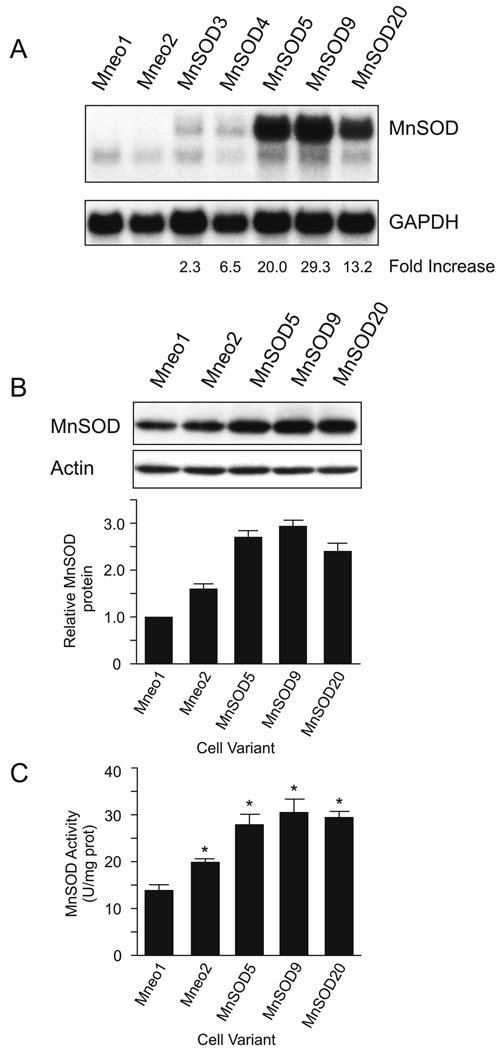
To determine the effect of MnSOD on dexamethasone-induced lymphoid cell apoptosis we first established WEHI7.2 cell clones with increased MnSOD by transfection. As shown in Figure 1A, we established several WEHI7.2 cell clones that have increased MnSOD expression at the mRNA level. Two vector only transfectants (Mneo1 and Mneo2) and three MnSOD-transfected clones (MnSOD5, MnSOD9 and MnSOD20) were selected for the current study. The MnSOD-transfected clones had significantly greater MnSOD protein levels than either of the two vector only transfected clones (Figure 1B). The MnSOD transfectants expressed 2.5–3 fold the Mneo1 MnSOD protein and were not significantly different from each other. MnSOD protein in the Mneo2 vector only transfected clone was also significantly higher than that in Mneo1, but still significantly lower than in the MnSOD-transfected clones. MnSOD activity increased with elevated MnSOD protein; the magnitude of the increase in activity was slightly less than that for the protein. (Figure 1C).
Figure 1.
Comparison of the MnSOD expression in the MnSOD-transfected and vector-transfected WEHI7.2 cells. A. Northern blot demonstrating MnSOD mRNA and the fold mRNA increase after correction for GAPDH expression. B. Representative immunoblots showing MnSOD and actin protein in selected clones. The lower panel indicates the average amount of MnSOD protein relative to that in Mneo1 quantitated from three independent blots. Representative full-length blots are presented in Supplementary Figure 1. C. MnSOD activity in the selected clones. Values represent the mean + S.E.M. (n=3). * denotes significantly different from Mneo1 values (p ≤ 0.05).
The enzymatic function of MnSOD is to metabolize O2 •-, forming H2O2 in the process (14). Cells that overexpress MnSOD may increase their ability to metabolize H2O2 because elevated MnSOD can increase the cellular H2O2 load (14, 28). Our previous studies indicate that resistance to H2O2 causes cross-resistance to dexamethasone (12, 13). Therefore, to interpret the phenotype of the MnSOD-transfected clones it was critical to understand their ability to metabolize H2O2. To assess the ability of the clones to metabolize H2O2, we measured catalase activity and the EC50 for H2O2. Catalase is the primary enzyme in WEHI7.2 cells that metabolizes H2O2. The EC50 for H2O2 was used to indicate the overall H2O2 sensitivity of the clones. Catalase activity in the MnSOD-transfected clones, vector only transfectants and the parental (WEHI7.2) cells was similar (Table 1) suggesting that the MnSOD-transfected clones had not upregulated catalase. The EC50 for H2O2 was either slightly (MnSOD5) or significantly (MnSOD9 and MnSOD20) lower than that in the WEHI7.2 and vector transfected (Mneo1) cells (Table 1). Thus, the H2O2 sensitivity of the MnSOD-transfected clones was greater than or equal to that in the WEHI7.2 cells. These data indicate that the MnSOD-transfected clones had not adapted to increased H2O2 production by upregulating components that would confer H2O2 resistance. These data are also consistent with elevated MnSOD increasing intracellular H2O2.
Table 1.
Response to H2O2.
| Cell Variant | Catalase activity (µmol H2O2/min/mg prot) |
EC50 H2O2 (% WEHI7.2 values) |
|---|---|---|
| WEHI7.2 | 14.64 ± 0.28 | 100.0 ± 2.3 |
| Mneo1 | 14.66 ± 0.37 | 95.9 ± 1.0 |
| Mneo2 | 12.54 ± 0.37 | |
| MnSOD5 | 13.77 ± 0.12 | 89.0 ± 3.9 |
| MnSOD9 | 12.54 ± 0.18 | 55.9 ± 7.6* |
| MnSOD20 | 13.76 ± 0.13 | 63.3 ± 9.8* |
denotes significantly different from WEHI7.2 and Mneo1 values (p ≤ 0.05). Values represent the mean ± S.E.M. (n = 3–6).
Increased MnSOD confers sensitivity to dexamethasone-induced apoptosis in WEHI7.2 cells
We hypothesized that increased MnSOD expression could sensitize WEHI7.2 cells to dexamethasone because of the ability of MnSOD to produce H2O2. Our hypothesis was based largely on our previous work showing that WEHI7.2 cells with increased catalase or selected for resistance to H2O2 are resistant to dexamethasone (12, 13). Recent data showing that increased MnSOD levels sensitize myeloma cells to dexamethasone (16) also suggested that lymphoma cells might have a similar response.
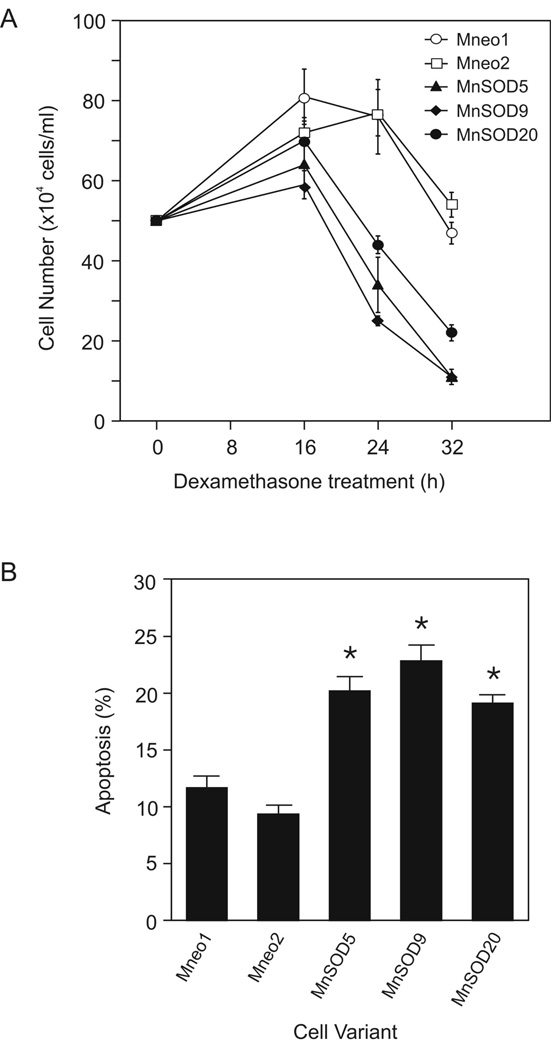
To test our hypothesis, we measured apoptosis in the MnSOD and vector only transfectants in three ways. As shown in Figure 2A, when we treated the MnSOD-transfected cells with dexamethasone we saw a faster loss of cells from the culture than in the vector only transfected cells. After 24 h in dexamethasone, MnSOD5 and MnSOD9 cell cultures had significantly fewer viable cells than the Mneo1 cell cultures. By 32 h after dexamethasone treatment, all three MnSOD transfectants had fewer viable cells than the control transfectants (p ≤ 0.05). The vector only transfectants showed kinetics of cell loss similar to that previously reported for the WEHI7.2 cells (12).
Figure 2.
Increased MnSOD protein increased sensitivity to dexamethasone-induced apoptosis. A. Number of viable cells in culture after the addition of dexamethasone. B. Percentage of apoptotic cells in culture 16 h after dexamethasone treatment. Cells that were annexin V positive and propidium iodide negative were considered apoptotic. The values have been corrected for the % apoptosis in vehicle-treated cell cultures. Values are the mean ± S.E.M. (n=3). * denotes significantly different from Mneo1 values (p ≤ 0.05). These are representative experiments which have been replicated.
Annexin V binding measurements showed that the MnSOD-transfected clone cultures had a greater amount apoptosis 16 h after dexamethasone treatment than the vector only transfectants (Figure 2B). Increased apoptosis due to dexamethasone in the MnSOD-transfected clones was also confirmed by morphologic examination of culture aliquots (data not shown). These data suggest that increased MnSOD expression is accelerating dexamethasone-induced apoptosis.
MnTE-2-PyP5+ decreases growth in WEHI7.2 cells
Our next step was to determine whether we could mimic the effects of MnSOD overexpression with a pharmacologic agent. For these studies, we used the manganoporphyrin, MnTE-2-PyP5+. We chose MnTE-2-PyP5+ because, in a cell-free assay, it dismutes O2 •- with a mechanism and thermodynamics similar to that of MnSOD and has a low affinity for H2O2 (29–31).
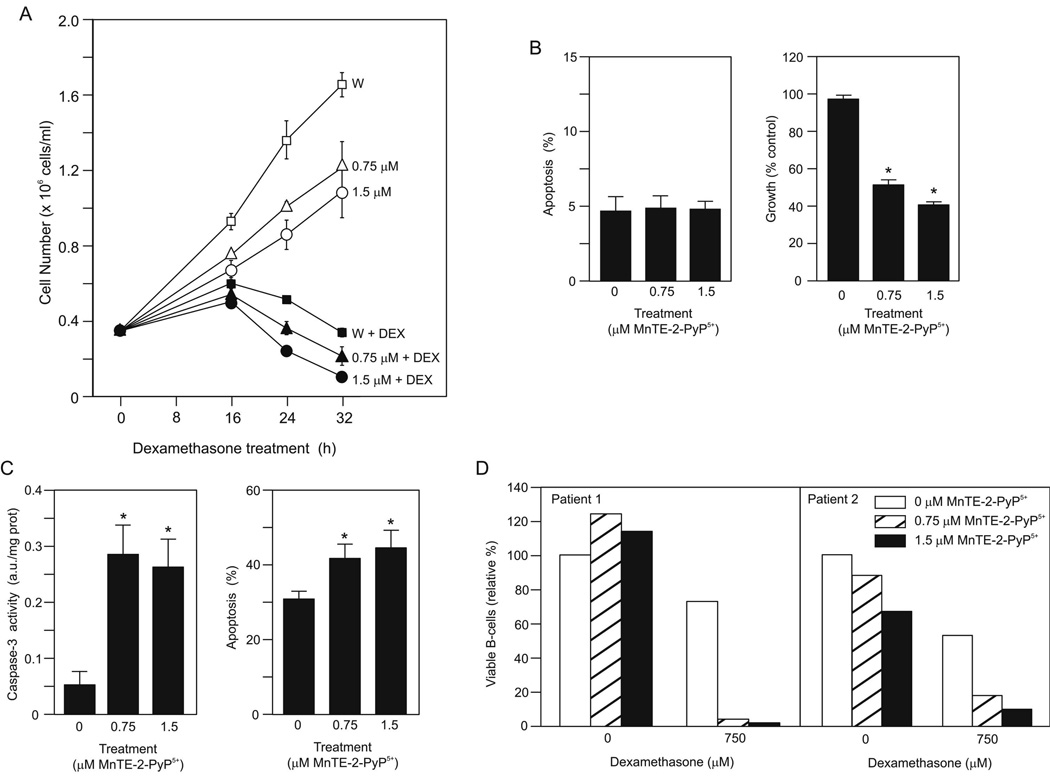
To provide an overview of the effects of MnTE-2-PyP5+ alone and in combination with dexamethasone we measured the number of WEHI7.2 cells in culture in the presence or absence of drugs by dye exclusion (Figure 3A). In the cultures treated with either concentration of MnTE-2-PyP5+ and vehicle, we saw lower cell numbers than in the WEHI7.2 cells treated with vehicle alone. WEHI7.2 cells treated with dexamethasone showed an initial increase in cell number followed by a loss of cells from the culture. In cultures of WEHI7.2 cells treated with the combination of dexamethasone and MnTE-2-PyP5+, cell numbers were slightly lower at 16 h than in the WEHI7.2 plus dexamethasone cultures. The rate of cell loss in the cultures from 16–32 h was significantly faster in the dexamethasone plus 1.5 µM MnTE-2-PyP5+ compared to dexamethasone only cultures (2.5 ± 0.2 × 104 cell/h vs. 1.9 ± 0.3 × 104 cell/h, respectively) (p ≤ 0.05). We confirmed the cell number data at the 24 h timepoint using the MTS assay which measures the number of metabolizing cells. Using WEHI7.2 cells treated with vehicle alone as the control MTS absorbance set at 100 ± 0.6 %, cultures treated with vehicle plus 0.75 or 1.5 µM MnTE-2-PyP5+ had significantly lower absorbances, 81.2 ± 3.0 or 64.3 ± 7.4%, respectively. WEHI7.2 cells treated with dexamethasone alone had 22.8 ± 1.4% control absorbance compared to 5.5 ± 1.4 or 2.7 ± 0.8% control absorbance for cells treated with dexamethasone plus 0.75 or 1.5 µM MnTE-2-PyP5+, respectively.
Figure 3.
MnTE-2-PyP5+ sensitized WEHI7.2 cells and primary follicular lymphoma cells to dexamethasone-induced apoptosis. A. Number of viable cells in the culture after a 2 h pretreatment with MnTE-2-PyP5+ and the addition of dexamethasone (DEX) or vehicle at time 0. W symbolizes WEHI7.2 cells without MnTE-2-PyP5+. This is a representative experiment, which has been replicated. B. (left panel) Percentage of apoptotic cells (annexin V positive and propidium iodide negative) in culture after a 24 h treatment with the indicated concentrations of MnTE-2-PyP5+. Values are mean + S.E.M (n = 6). (right panel) Cell growth in MnTE-2-PyP5+-treated cultures compared to control cells. Values represent the mean + S.E.M. (n = 36). * denotes significantly different from control (no MnTE-2-PyP5+ cells) (p ≤ 0.05). C. (left panel) Caspase-3 activity in cells after MnTE-2-PyP5+ pretreatment followed by an 8 h dexamethasone treatment. The values have been corrected for the caspase activity in vehicle-treated cell cultures. Values represent the mean + S.E.M (n = 4). (right panel) Percentage of apoptotic cells in culture after MnTE-2-PyP5+ pretreatment followed by a 28 h dexamethasone treatment. The values have been corrected for the % apoptosis in vehicle-treated cell cultures. Values represent the mean + S.E.M (n = 6). D. Relative % live B-cells remaining in culture 24 h after drug treatment. CD20 positive/propidium iodide negative cells were considered live B-cells. The value for the sample with no drugs for each patient was set to 100%.
The lower cell numbers in cultures treated with MnTE-2-PyP5+ and vehicle alone (Figure 3A) suggest that MnTE-2-PyP5+ alone has an effect on the WEHI7.2 cells. One possibility is that MnTE-2-PyP5+ itself causes apoptosis. To determine whether MnTE-2-PyP5+ causes apoptosis directly, we measured the percentage of annexin positive cells in culture after a 24 h MnTE-2-PyP5+ treatment. As shown in Figure 3B, MnTE-2-PyP5+ alone did not increase apoptosis. Further, we did not see an increase in propidium iodide positive cells in the cultures (data not shown); an increase in propidium iodide positive cells would be consistent with an increase in another type of cell death such as necrosis. A second possibility is that MnTE-2-PyP5+ decreases proliferation in WEHI7.2 cells as seen in other cell types (32, 33). When we compared growth in the presence of MnTE-2-PyP5+ we found that MnTE-2-PyP5+ inhibited growth in a dose dependent manner (Figure 3B).
MnTE-2-PyP5+ treatment sensitizes WEHI7.2 cells to dexamethasone-induced apoptosis
The ability of MnTE-2-PyP5+ to inhibit growth likely contributes to the decreased cell numbers seen in the cultures treated with dexamethasone and manganoporphyrin combined compared to dexamethasone treatment alone. However, if this is the sole explanation, the absorbances we would predict in the MTS assay for dexamethasone plus 0.75 or 1.5 µM MnTE-2-PyP5+ would be 18.5 and 14.6%, respectively. One possible explanation of the lower than predicted values is that the drug combination causes increased apoptosis compared to dexamethasone alone. In WEHI7.2 cells, treatment with 1 µM dexamethasone saturates the glucocorticoid receptors and causes apoptosis with reproducible kinetics (13). In these cultures, we see increases in annexin V positive cells at 16 h, caspase-3 activation and release of cytochrome c at 24 h, followed by loss of viable cells from the culture beginning at 24 h after the addition of dexamethasone (13, 27).
To determine whether MnTE-2-PyP5+ sensitizes cells to dexamethasone-induced apoptosis we tested for the appearance of increased amounts of apoptotic markers at earlier timepoints. We measured both the activation of caspase-3 and the percentage of annexin V positive cells. As shown in Figure 3C, eight hours after dexamethasone addition, caspase-3 activity was minimal in the cells treated with dexamethasone alone. The MnTE-2-PyP5+ pretreated cells had significantly increased caspase-3 activity compared to the dexamethasone-treated cells. The MnTE-2-PyP5+ pretreated cultures also showed a significant increase in the percentage of annexin V positive cells in the presence of dexamethasone compared to the cells treated with dexamethasone alone (Figure 3C). MnTE-2-PyP5+ pretreatment increased the annexin V positive cells at earlier timepoints; however, this was not significant. These measurements are corrected for the appropriate values in the vehicle-treated cells and thus reflect only the amount due to dexamethasone. Taken together the data indicate that pretreatment with MnTE-2-PyP5+ accelerates apoptosis due to dexamethasone in the WEHI7.2 cells; increased apoptosis likely contributes to the decreased cell numbers in the cultures treated with MnTE-2-PyP5+ and dexamethasone combined.
MnTE-2-PyP5+ sensitizes primary follicular lymphoma cells to dexamethasone-induced death
To determine whether MnTE-2-PyP5+ has a similar effect in primary tumor cells, we measured the effect of dexamethasone and MnTE-2-PyP5+ individually and combined on B-cell death in primary FL cells from two different patients (Figure 3D). As is typical of FL cells, the primary cells did not grow in culture, so the effect we measured was only on cell death. Treatment with MnTE-2-PyP5+ alone had no effect in one sample; however, the higher concentration decreased viable B-cells in patient 2. In both patient samples, dexamethasone alone decreased the viable B-cells. Adding MnTE-2-PyP5+ to dexamethasone increased B-cell death in both patient samples. These data indicate that MnTE-2-PyP5+ accelerates dexamethasone-induced cell death in primary tumor cells and suggests MnTE-2-PyP5+ has clinical potential for the treatment of lymphoma.
MnTE-2-PyP5+ pretreatment alters the oxidant and chemotherapeutic drug response in WEHI7.2 cells
To better understand the redox properties of MnTE-2-PyP5+ in the WEHI7.2 cells and interpret the effect of MnTE-2-PyP5+ on the chemotherapeutic drug response we measured the effect of MnTE-2-PyP5+ pretreatment on the response to H2O2 and paraquat. In cell-free assays, MnTE-2-PyP5+ has a low level of catalase activity (31). To determine whether MnTE-2-PyP5+ is contributing to H2O2 removal in the WEHI7.2 cells we measured the effect of MnTE-2-PyP5+ pretreatment on the EC50 for H2O2. As shown in Table 2A, pretreatment at the lower MnTE-2-PyP5+ dose did not alter H2O2 sensitivity; however, at the higher dose the cells were more sensitive to H2O2. This indicates that MnTE-2-PyP5+ is not acting as a catalase mimetic. It is more consistent with MnTE-2-PyP5+ acting as an oxidant under these conditions to sensitize WEHI7.2 cells to H2O2.
Table 2.
| Table 2A. Oxidant response (EC50 measurements) in WEHI7.2 cells treated with MnTE-2-PyP5+ | ||
|---|---|---|
| Treatment | H2O2 (µM) |
Paraquat (µM) |
| Control | 104.9 ± 5.4 | 44.6 ± 1.3 |
| 0.75 µM MnTE-2-PyP5+ | 102.8 ± 6.7 | 59.8 ± 1.6* |
| 1.5 µM MnTE-2-PyP5+ | 61.7 ± 1.7* | 63.7 ± 6.0* |
| Table 2B. Chemotherapeutic drug response (EC50 measurements) of WEHI7.2 cells pre-treated with MnTE-2-PyP5+ | |||
|---|---|---|---|
| Treatment | Cyclophosphamide (mM) |
Doxorubicin (nM) |
Vincristine (nM) |
| Control | 2.25 ± 0.10 | 10.3 ± 0.9 | 2.1 ± 0.1 |
| 0.75 µM MnTE-2-PyP5+ | 1.07 ± 0.08* | 9.6 ± 0.3 | 2.3 ± 0.2 |
| 1.5 µM MnTE-2-PyP5+ | 0.73 ± 0.04* | 10.3 ± 0.2 | 1.9 ± 0.2 |
denotes significantly different from untreated WEHI7.2 (control) cells (p ≤ 0.05). Values represent the mean ± S.E.M.; n = 3.
denotes significantly different from untreated WEHI7.2 (control) values (p ≤ 0.05). Values represent the mean ± S.E.M. (n=3 or 4).
Paraquat is a compound that redox cycles with a variety of oxidoreductases in cells to produce O2 •- (34, 35). Paraquat toxicity has been linked to its ability to produce superoxide because both increased SOD and treatment with superoxide dismutase mimetics reduce toxicity (36, 37). In WEHI7.2 cells, MnTE-2-PyP5+ pretreatment increased the EC50 for paraquat in a dose dependent manner indicating that MnTE-2-PyP5+ protected the WEHI7.2 cells from paraquat toxicity.
The data above suggest that MnTE-2-PyP5+ is a candidate chemotherapeutic agent in lymphoma treatment because it sensitizes the lymphoid cells to dexamethasone. To determine whether MnTE-2-PyP5+ would increase resistance to other commonly used lymphoma drugs, we tested MnTE-2-PyP5+ in combination with cyclophosphamide, doxorubicin and vincristine. These three drugs plus prednisone, a glucocorticoid, comprise the CHOP regimen, a standard protocol for treating diffuse large B-cell lymphoma (DLBCL) (38). These drugs are also used to treat other types of lymphoma (39). Testing MnTE-2-PyP5+ in combination with doxorubicin is particularly crucial because: 1) doxorubicin can redox cycle, similar to paraquat, to produce O2 •- (40–42); and 2) doxorubicin is a critical drug in lymphoma chemotherapy (43). As shown in Table 2B, MnTE-2-PyP5+ pretreatment increased the sensitivity of the cells to cyclophosphamide, but had no effect on doxorubicin or vincristine sensitivity.
MnTE-2-PyP5+ pretreatment protects H9c2 cardiomyocytes from doxorubicin-induced toxicity
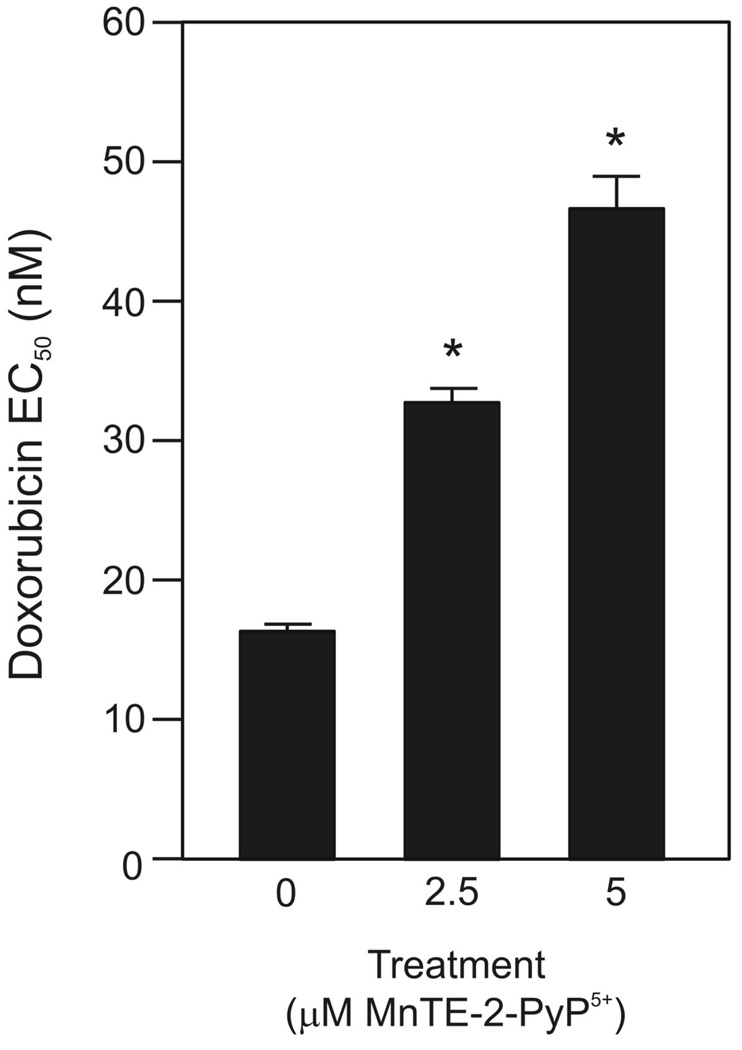
The clinical use of doxorubicin in lymphoma therapeutics is often limited by acute and chronic cardiotoxicity (44). A number of studies suggest that reactive oxygen species (ROS) produced by doxorubicin treatment play a key role in cardiotoxicity in cardiomyocyte cell culture and mouse models (e.g.(41, 42, 45) among others). Both increased MnSOD expression and treatment with other MnSOD mimetics are protective (41, 45). Given the redox properties of MnTE-2-PyP5+, we tested whether the EC50 for doxorubicin was altered by MnTE-2-PyP5+ pretreatment in H9c2 rat heart myocardiocytes. As shown in Figure 4, pretreatment with MnTE-2-PyP5+ protected H9c2 cells from doxorubicin-induced toxicity in a dose dependent manner.
Figure 4.
MnTE-2-PyP5+ protected H9c2 cardiomyocytes from doxorubicin toxicity. Doxorubicin EC50 measurements of H9c2 cells pretreated with 2.5 or 5 µM MnTE-2-PyP5+ for 2 h, followed by a 48 h doxorubicin treatment. Values represent the mean + S.E.M. (n=3). * denotes significantly different from control (no MnTE-2-PyP5+ cells) (p ≤ 0.05).
Discussion
These data suggest that increasing MnSOD or treating with a pharmacologic agent that mimics MnSOD effects has the potential to improve outcome in hematologic malignancies that are treated with glucocorticoids. In addition to accelerating dexamethasone-induced apoptosis in the WEHI7.2 and primary FL cells, MnTE-2-PyP5+ potentiated cyclophosphamide toxicity while inhibiting lymphoma cell growth and attenuating doxorubicin toxicity in H9c2 cardiomyocytes. This combination of responses suggests that MnTE-2-PyP5+ could benefit lymphoma patients who receive combined therapy which includes glucocorticoids, doxorubicin and cyclophosphamide.
Increasing MnSOD protein by genetic manipulation sensitizes the WEHI7.2 cells to dexamethasone. This effect in lymphoma cells is similar to the sensitization of multiple myeloma cells to dexamethasone when MnSOD is increased by transfection or treatment with demethylating agents (16). However, in thymocytes the gene dose of MnSOD (and resulting alterations in activity) did not track with dexamethasone sensitivity (17). This suggests the possibility that lymphoid tumor cells may respond differently from thymocytes in this regard, an attribute that could be exploited therapeutically. In DLBCL patient tumors we found that decreased MnSOD expression correlates with a poorer outcome (46). The loss of MnSOD may contribute to chemoresistance in these patients because standard therapy for DLBCL includes the glucocorticoid prednisone.
The ability of increased MnSOD protein to sensitize WEHI7.2 cells to dexamethasone-induced apoptosis fits a model whereby elevated MnSOD sensitizes the cells to glucocorticoids by increasing H2O2. This is supported by our observation that increased MnSOD sensitizes the cells to both H2O2 and dexamethasone. We also see a slight increase in ROS in the MnSOD-transfected cells (data not shown). MnSOD overexpression does increase H2O2 tone in other cell types (14, 28). In our model system, resistance to H2O2 results in resistance to glucocorticoids (12, 13), thus a sensitivity to H2O2 is expected to track with glucocorticoid sensitivity.
Although MnTE-2-PyP5+ treatment and increased MnSOD protein achieve similar endpoints, the chemical properties of MnTE-2-PyP5+ make it unlikely that the observed effects are solely due to the manganoporphyrin mimicking MnSOD enzymatic activity. In a cell-free assay, MnTE-2-PyP5+ has SOD activity that depends on the oxidation and reduction of the manganese in the center of the porphyrin ring (29). Due to the midpoint redox potential for this compound, the oxidation reaction and the reduction reaction are approximately equally likely to occur, i.e. similar thermodynamics to MnSOD (30). Unlike MnSOD, however, where the redox active manganese is surrounded by protein, the manganese in MnTE-2-PyP5+ is more exposed so that it is accessible to other cellular components (47). MnTE-2-PyP5+ will redox cycle with flavin oxidoreductases, i.e. complex I in the mitochondria and the cytochrome P450 complex, and use the reducing power of small molecule reductants such as ascorbate, glutathione and tetrahydrobiopterin (47–49). Thus, in a cell under oxidizing conditions MnTE-2-PyP5+ can act as an oxidant, directly oxidizing proteins such as NF-kB (50) or contributing to oxidative stress by depleting small molecule reductants.
MnTE-2-PyP5+ may potentiate dexamethasone-induced apoptosis in WEHI7.2 cells via several mechanisms. Dexamethasone treatment increases ROS and causes a more oxidized redox environment (51) and manuscript in preparation). MnTE-2-PyP5+ may act, in part, as an MnSOD mimetic further increasing intracellular H2O2 and accelerating apoptosis. In a more oxidized mileu, MnTE-2-PyP5+ can also be redox cycling and causing further oxidative stress. The H2O2 EC50 values in the presence of MnTE-2-PyP5+ indicate that MnTE-2-PyP5+ is not detoxifying H2O2; in the cultures with the higher MnTE-2-PyP5+ concentration, the cells are more sensitive to H2O2. This supports the hypothesis that in an oxidized environment MnTE-2-PyP5+ can enhance cell death in the WEHI7.2 cells. MnTE-2-PyP5+ may also be oxidizing NF-kB. Oxidizing NF-kB inactivates it by inhibiting its binding to DNA (50). Dexamethasone treatment alone inhibits NF-kB activity in lymphoid cells, in part by inhibiting NF-kB binding to DNA (52). Loss of NF-kB survival signals likely contributes to glucocorticoid-induced lymphocyte apoptosis (52). MnTE-2-PyP5+ may potentiate dexamethasone-induced apoptosis by accelerating NF-kB inhibition. Additional mechanisms are likely because of the complex signaling required for dexamethasone-induced apoptosis.
Inhibition of proliferation and potentiation of cyclophosphamide toxicity are two additional characteristics of MnTE-2-PyP5+ that would contribute to chemotherapeutic efficacy. In a mouse skin carcinogenesis model, MnTE-2-PyP5+ inhibits proliferation via inhibition of AP-1 (33). Inhibition of AP-1 or NF-kB (50) could certainly contribute to the antiproliferative effects of MnTE-2-PyP5+ in WEHI7.2 cells; however, the actual source of proliferation inhibition is unknown. Cyclophosphamide normally is bioactivated through hydroxylation by the cytochrome P450 system in the liver (53). The hydroxylated cyclophosphamide released by the liver is thought to be responsible for the cytotoxic effects of cyclophosphamide. In a cell free system MnTE-2-PyP5+ acts as a cytochrome P450 reductase mimetic and hydroxylates cyclophosphamide (53). The potentiation of cyclophosphamide toxicity is consistent with MnTE-2-PyP5+ acting as a cytochrome P450 reductase mimetic in the WEHI7.2 cells.
The ability of MnTE-2-PyP5+ to protect cardiomyocytes from doxorubicin toxicity could provide an added chemotherapeutic benefit because cardiotoxicity is a significant problem in the clinical use of doxorubicin (44). The effect of MnTE-2-PyP5+ on doxorubicin toxicity is cell type specific; MnTE-2-PyP5+ is protective in the H9c2 cardiomyocytes, but not in the WEHI7.2 cells. However, MnTE-2-PyP5+ protects WEHI7.2 cells from paraquat. Both paraquat and doxorubicin redox cycle with similar enzymes to produce O2 •- (34, 35, 42). This suggests that doxorubicin produces ROS in both cell types, but ROS are only involved in cardiomyocyte toxicity (40, 41, 45). There are at least two scenarios by which MnTE-2-PyP5+ pre-treatment can produce these results: 1) MnTE-2-PyP5+ could be scavenging the O2 •- produced by paraquat in the WEHI7.2 cells and doxorubicin in the H9c2 cells; or 2) because MnTE-2-PyP5+ redox cycles using the same enzymes as paraquat and doxorubicin, MnTE-2-PyP5+ may be competing with both compounds for reducing equivalents (47). By decreasing the redox cycling of paraquat and doxorubicin, MnTE-2-PyP5+ can prevent O2 •- generation. Inactivation of NF-kB by MnTE-2-PyP5+ could also play a role in cardiomyocyte protection because activation of NF-kB during doxorubicin treatment is proapoptotic in cardiomyocytes (54).
The profile of MnTE-2-PyP5+ effects: sensitizing lymphoid cells to dexamethasone and cyclophosphamide, not affecting the doxorubicin toxicity in the WEHI7.2 cells while protecting cardiomyocytes from doxorubicin and inhibiting proliferation suggests that MnTE-2-PyP5+ could be effective in DLBCL combined with CHOP. MnTE-2-PyP5+ also has potential for use in other hematologic malignancies that use glucocorticoids, especially multiple myeloma where there is decreased MnSOD and dexamethasone is standard treatment (16). These data further suggest that MnTE-2-PyP5+ could be investigated as a cardioprotectant for cancers treated with doxorubicin. The known chemistry of MnTE-2-PyP5+ suggests potential mechanisms by which some of the observed effects could occur. However, additional work is necessary to determine the molecular requirements for the MnTE-2-PyP5+ effect. In lymphoma, the heterogeneity of treatment response is a significant barrier to successful treatment. By further characterizing the mechanism of MnTE-2-PyP5+ action, it will be possible to identify the molecular profile of patients likely to benefit from treatment with MnTE-2-PyP5+.
Supplementary Material
Acknowledgment
We thank: Suzy Grayson for technical assistance, Debbie Sakiestewa for flow cytometry assistance; Dr. Larry Oberley for gifts of the MnSOD cDNA and antibody; Dr. Qin Chen for the H9c2 cells; the Arizona Lymphoma Tissue Repository, Dr. Lisa Rimsza and Betty Glinsmann-Gibson for providing the primary tumor samples and help with the analysis; and Dr. Remy Kachadourian for critical reading of the manuscript. Funding for this study comes from: the National Cancer Institute (CA-71768) (MMB) and (CA-09213) (MCJ); the Arizona Cancer Center Support Grant (CA-023074); and the U.S. Department of Defense (W81XWH-07-1-0550) (JDC).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Distelhorst CW. Recent insights into the mechanism of glucocorticosteroid-induced apoptosis. Cell Death Differ. 2002;9:6–19. doi: 10.1038/sj.cdd.4400969. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt S, Rainer J, Ploner C, Presul E, Riml S, Kofler R. Glucocorticoid-induced apoptosis and glucocorticoid resistance: molecular mechanisms and clinical relevance. Cell Death Differ. 2004;11 Suppl 1:S45–S55. doi: 10.1038/sj.cdd.4401456. [DOI] [PubMed] [Google Scholar]
- 3.Frankfurt O, Rosen ST. Mechanisms of glucocorticoid-induced apoptosis in hematologic malignancies: updates. Curr Opin Oncol. 2004;16:553–563. doi: 10.1097/01.cco.0000142072.22226.09. [DOI] [PubMed] [Google Scholar]
- 4.Hillmann AG, Ramdas J, Multanen K, Norman MR, Harmon JM. Glucocorticoid receptor gene mutations in leukemic cells acquired in vitro and in vivo. Cancer Res. 2000;60:2056–2062. [PubMed] [Google Scholar]
- 5.Bachmann PS, Gorman R, Mackenzie KL, Lutze-Mann L, Lock RB. Dexamethasone resistance in B-cell precursor childhood acute lymphoblastic leukemia occurs downstream of ligand-induced nuclear translocation of the glucocorticoid receptor. Blood. 2005;105:2519–2526. doi: 10.1182/blood-2004-05-2023. [DOI] [PubMed] [Google Scholar]
- 6.Wang Z, Malone MH, He H, McColl KS, Distelhorst CW. Microarray analysis uncovers the induction of the proapoptotic BH3-only protein Bim in multiple models of glucocorticoid-induced apoptosis. J Biol Chem. 2003;278:23861–23867. doi: 10.1074/jbc.M301843200. [DOI] [PubMed] [Google Scholar]
- 7.Rathmell JC, Lindsten T, Zong WX, Cinalli RM, Thompson CB. Deficiency in Bak and Bax perturbs thymic selection and lymphoid homeostasis. Nat Immunol. 2002;3:932–939. doi: 10.1038/ni834. [DOI] [PubMed] [Google Scholar]
- 8.Miyashita T, Reed JC. bcl-2 gene transfer increases relative resistance of S49.1 and WEHI7.2 lymphoid cells to cell death and DNA fragmentation induced by glucocorticoids and multiple chemotherapeutic drugs. Cancer Res. 1992;52:5407–5411. [PubMed] [Google Scholar]
- 9.Bachmann PS, Gorman R, Papa RA, Bardell JE, Ford J, Kees UR, et al. Divergent mechanisms of glucocorticoid resistance in experimental models of pediatric acute lymphoblastic leukemia. Cancer Res. 2007;67:4482–4490. doi: 10.1158/0008-5472.CAN-06-4244. [DOI] [PubMed] [Google Scholar]
- 10.Reed JC, Pellecchia M. Apoptosis-based therapies for hematologic malignancies. Blood. 2005;106:408–418. doi: 10.1182/blood-2004-07-2761. [DOI] [PubMed] [Google Scholar]
- 11.Gascoyne RD, Adomat SA, Krajewski S, Krajewska M, Horsman DE, Tolcher AW, et al. Prognostic significance of Bcl-2 protein expression and Bcl-2 gene rearrangement in diffuse aggressive non-Hodgkinx00027;s lymphoma. Blood. 1997;90:244–251. [PubMed] [Google Scholar]
- 12.Tome ME, Briehl MM. Thymocytes selected for resistance to hydrogen peroxide show altered antioxidant enzyme profiles and resistance to dexamethasone-induced apoptosis. Cell Death Differ. 2001;8:953–961. doi: 10.1038/sj.cdd.4400904. [DOI] [PubMed] [Google Scholar]
- 13.Tome ME, Baker AF, Powis G, Payne CM, Briehl MM. Catalase-overexpressing thymocytes are resistant to glucocorticoid- induced apoptosis and exhibit increased net tumor growth. Cancer Res. 2001;61:2766–2773. [PubMed] [Google Scholar]
- 14.Buettner GR, Ng CF, Wang M, Rodgers VG, Schafer FQ. A new paradigm: manganese superoxide dismutase influences the production of H2O2 in cells and thereby their biological state. Free Radic Biol Med. 2006;41:1338–1350. doi: 10.1016/j.freeradbiomed.2006.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodriguez AM, Carrico PM, Mazurkiewicz JE, Melendez JA. Mitochondrial or cytosolic catalase reverses the MnSOD-dependent inhibition of proliferation by enhancing respiratory chain activity, net ATP production, and decreasing the steady state levels of H(2)O(2) Free Radic Biol Med. 2000;29:801–813. doi: 10.1016/s0891-5849(00)00362-2. [DOI] [PubMed] [Google Scholar]
- 16.Hodge DR, Xiao W, Peng B, Cherry JC, Munroe DJ, Farrar WL. Enforced expression of superoxide dismutase 2/manganese superoxide dismutase disrupts autocrine interleukin-6 stimulation in human multiple myeloma cells and enhances dexamethasone-induced apoptosis. Cancer Res. 2005;65:6255–6263. doi: 10.1158/0008-5472.CAN-04-4482. [DOI] [PubMed] [Google Scholar]
- 17.van de Wetering CI, Coleman MC, Spitz DR, Smith BJ, Knudson CM. Manganese superoxide dismutase gene dosage affects chromosomal instability and tumor onset in a mouse model of T cell lymphoma. Free Radic Biol Med. 2008;44:1677–1686. doi: 10.1016/j.freeradbiomed.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris AW, Bankhurst AD, Mason S, Warner NL. Differentiated functions expressed by cultured mouse lymphoma cells. II. Theta antigen, surface immunoglobulin and a receptor for antibody on cells of a thymoma cell line. J Immunol. 1973;110:431–438. [PubMed] [Google Scholar]
- 19.Manna SK, Zhang HJ, Yan T, Oberley LW, Aggarwal BB. Overexpression of manganese superoxide dismutase suppresses tumor necrosis factor-induced apoptosis and activation of nuclear transcription factor-kappaB and activated protein-1. J Biol Chem. 1998;273:13245–13254. doi: 10.1074/jbc.273.21.13245. [DOI] [PubMed] [Google Scholar]
- 20.Kimes BW, Brandt BL. Properties of a clonal muscle cell line from rat heart. Exp Cell Res. 1976;98:367–381. doi: 10.1016/0014-4827(76)90447-x. [DOI] [PubMed] [Google Scholar]
- 21.Hescheler J, Meyer R, Plant S, Krautwurst D, Rosenthal W, Schultz G. Morphological, biochemical, and electrophysiological characterization of a clonal cell (H9c2) line from rat heart. Circ Res. 1991;69:1476–1486. doi: 10.1161/01.res.69.6.1476. [DOI] [PubMed] [Google Scholar]
- 22.Turakhia S, Venkatakrishnan CD, Dunsmore K, Wong H, Kuppusamy P, Zweier JL, et al. Doxorubicin-induced cardiotoxicity: direct correlation of cardiac fibroblast and H9c2 cell survival and aconitase activity with heat shock protein 27. Am J Physiol Heart Circ Physiol. 2007;293:H3111–H3121. doi: 10.1152/ajpheart.00328.2007. [DOI] [PubMed] [Google Scholar]
- 23.Siemankowski LM, Morreale J, Briehl MM. Antioxidant defenses in the TNF-treated MCF-7 cells: selective increase in MnSOD. Free Radic Biol Med. 1999;26:919–924. doi: 10.1016/s0891-5849(98)00273-1. [DOI] [PubMed] [Google Scholar]
- 24.Li JJ, Oberley LW, St Clair DK, Ridnour LA, Oberley TD. Phenotypic changes induced in human breast cancer cells by overexpression of manganese-containing superoxide dismutase. Oncogene. 1995;10:1989–2000. [PubMed] [Google Scholar]
- 25.Iqbal J, Whitney P. Use of cyanide and diethyldithiocarbamate in the assay of superoxide dismutases. Free Radic Biol Med. 1991;10:69–77. doi: 10.1016/0891-5849(91)90023-v. [DOI] [PubMed] [Google Scholar]
- 26.Efferth T, Briehl MM, Tome ME. Role of antioxidant genes for the activity of artesunate against tumor cells. Int J Oncol. 2003;23:1231–1235. [PubMed] [Google Scholar]
- 27.Tome ME, Lutz NW, Briehl MM. Overexpression of catalase or Bcl-2 delays or prevents alterations in phospholipid metabolism during glucocorticoid-induced apoptosis in WEHI7.2 cells. Biochim Biophys Acta. 2003;1642:149–162. doi: 10.1016/j.bbamcr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Ridnour LA, Oberley TD, Oberley LW. Tumor suppressive effects of MnSOD overexpression may involve imbalance in peroxide generation versus peroxide removal. Antioxid Redox Signal. 2004;6:501–512. doi: 10.1089/152308604773934260. [DOI] [PubMed] [Google Scholar]
- 29.Batinic-Haberle I, Benov L, Spasojevic I, Fridovich I. The ortho effect makes manganese(III) meso-tetrakis(N-methylpyridinium-2-yl)porphyrin a powerful and potentially useful superoxide dismutase mimic. J Biol Chem. 1998;273:24521–24528. doi: 10.1074/jbc.273.38.24521. [DOI] [PubMed] [Google Scholar]
- 30.Batinic-Haberle I, Spasojevic I, Hambright P, Benov L, Crumbliss AL, Fridovich I. Relationship among redox potentials, proton dissociation constants of pyrrolic nitrogens, and in vivo and in vitro superoxide dismutating activities of manganese(III) and iron(III) water-soluble porphyrins. Inorg Chem. 1999;38:4011–4022. [Google Scholar]
- 31.Kachadourian R, Johnson CA, Min E, Spasojevic I, Day BJ. Flavin-dependent antioxidant properties of a new series of meso-N,N'-dialkyl-imidazolium substituted manganese(III) porphyrins. Biochem Pharmacol. 2004;67:77–85. doi: 10.1016/j.bcp.2003.08.036. [DOI] [PubMed] [Google Scholar]
- 32.Moeller BJ, Batinic-Haberle I, Spasojevic I, Rabbani ZN, Anscher MS, Vujaskovic Z, et al. A manganese porphyrin superoxide dismutase mimetic enhances tumor radioresponsiveness. Int J Radiat Oncol Biol Phys. 2005;63:545–552. doi: 10.1016/j.ijrobp.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 33.Zhao Y, Chaiswing L, Oberley TD, Batinic-Haberle I, St Clair W, Epstein CJ, et al. A mechanism-based antioxidant approach for the reduction of skin carcinogenesis. Cancer Res. 2005;65:1401–1405. doi: 10.1158/0008-5472.CAN-04-3334. [DOI] [PubMed] [Google Scholar]
- 34.Day BJ, Patel M, Calavetta L, Chang LY, Stamler JS. A mechanism of paraquat toxicity involving nitric oxide synthase. Proc Natl Acad Sci U S A. 1999;96:12760–12765. doi: 10.1073/pnas.96.22.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cocheme HM, Murphy MP. Complex I is the major site of mitochondrial superoxide production by paraquat. J Biol Chem. 2008;283:1786–1798. doi: 10.1074/jbc.M708597200. [DOI] [PubMed] [Google Scholar]
- 36.Krall J, Bagley AC, Mullenbach GT, Hallewell RA, Lynch RE. Superoxide mediates the toxicity of paraquat for cultured mammalian cells. J Biol Chem. 1988;263:1910–1914. [PubMed] [Google Scholar]
- 37.Kachadourian R, Flaherty MM, Crumbliss AL, Patel M, Day BJ. Synthesis and in vitro antioxidant properties of manganese(III) beta-octabromo-meso-tetrakis(4-arboxyphenyl)porphyrin. J Inorg Biochem. 2003;95:240–248. doi: 10.1016/s0162-0134(03)00135-1. [DOI] [PubMed] [Google Scholar]
- 38.Fisher RI, Miller TP, O'Connor OA. Diffuse aggressive lymphoma. Hematology Am Soc Hematol Educ Program. 2004:221–236. doi: 10.1182/asheducation-2004.1.221. [DOI] [PubMed] [Google Scholar]
- 39.Perry MC, Anderson CM, Dorr VJ, Wilkes JD. Companion Handbook to the Chemotherapy Source Book. New York: Lippincott, Williams & Wilkins; 1999. [Google Scholar]
- 40.Gewirtz DA. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem Pharmacol. 1999;57:727–741. doi: 10.1016/s0006-2952(98)00307-4. [DOI] [PubMed] [Google Scholar]
- 41.Wang S, Konorev EA, Kotamraju S, Joseph J, Kalivendi S, Kalyanaraman B. Doxorubicin induces apoptosis in normal and tumor cells via distinctly different mechanisms. intermediacy of H(2)O(2)- and p53-dependent pathways. J Biol Chem. 2004;279:25535–25543. doi: 10.1074/jbc.M400944200. [DOI] [PubMed] [Google Scholar]
- 42.Kalivendi SV, Konorev EA, Cunningham S, Vanamala SK, Kaji EH, Joseph J, et al. Doxorubicin activates nuclear factor of activated T-lymphocytes and Fas ligand transcription: role of mitochondrial reactive oxygen species and calcium. Biochem J. 2005;389:527–539. doi: 10.1042/BJ20050285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jones SE, Grozea PN, Metz EN, Haut A, Stephens RL, Morrison FS, et al. Superiority of adriamycin-containing combination chemotherapy in the treatment of diffuse lymphoma: a Southwest Oncology Group study. Cancer. 1979;43:417–425. doi: 10.1002/1097-0142(197902)43:2<417::aid-cncr2820430203>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 44.Singal PK, Deally CM, Weinberg LE. Subcellular effects of adriamycin in the heart: a concise review. J Mol Cell Cardiol. 1987;19:817–828. doi: 10.1016/s0022-2828(87)80392-9. [DOI] [PubMed] [Google Scholar]
- 45.Yen HC, Oberley TD, Vichitbandha S, Ho YS, St Clair DK. The protective role of manganese superoxide dismutase against adriamycin-induced acute cardiac toxicity in transgenic mice. J Clin Invest. 1996;98:1253–1260. doi: 10.1172/JCI118909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tome ME, Johnson DB, Rimsza LM, Roberts RA, Grogan TM, Miller TP, et al. A redox signature score identifies diffuse large B-cell lymphoma patients with a poor prognosis. Blood. 2005;106:3594–3601. doi: 10.1182/blood-2005-02-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Day BJ, Kariya C. A novel class of cytochrome P450 reductase redox cyclers: cationic manganoporphyrins. Toxicol Sci. 2005;85:713–719. doi: 10.1093/toxsci/kfi108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Batinic-Haberle I, Spasojevic I, Fridovich I. Tetrahydrobiopterin rapidly reduces the SOD mimic Mn(III) ortho-tetrakis(N-ethylpyridinium-2-yl)porphyrin. Free Radic Biol Med. 2004;37:367–374. doi: 10.1016/j.freeradbiomed.2004.04.041. [DOI] [PubMed] [Google Scholar]
- 49.Ferrer-Sueta G, Hannibal L, Batinic-Haberle I, Radi R. Reduction of manganese porphyrins by flavoenzymes and submitochondrial particles: a catalytic cycle for the reduction of peroxynitrite. Free Radic Biol Med. 2006;41:503–512. doi: 10.1016/j.freeradbiomed.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 50.Tse HM, Milton MJ, Piganelli JD. Mechanistic analysis of the immunomodulatory effects of a catalytic antioxidant on antigen-presenting cells: implication for their use in targeting oxidation-reduction reactions in innate immunity. Free Radic Biol Med. 2004;36:233–247. doi: 10.1016/j.freeradbiomed.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 51.Tonomura N, McLaughlin K, Grimm L, Goldsby RA, Osborne BA. Glucocorticoid-induced apoptosis of thymocytes: requirement of proteasome-dependent mitochondrial activity. J Immunol. 2003;170:2469–2478. doi: 10.4049/jimmunol.170.5.2469. [DOI] [PubMed] [Google Scholar]
- 52.Novac N, Baus D, Dostert A, Heinzel T. Competition between glucocorticoid receptor and NFkappaB for control of the human FasL promoter. FASEB J. 2006;20:1074–1081. doi: 10.1096/fj.05-5457com. [DOI] [PubMed] [Google Scholar]
- 53.Spasojevic I, Colvin OM, Warshany KR, Batinic-Haberle I. New approach to the activation of anti-cancer pro-drugs by metalloporphyrin-based cytochrome P450 mimics in all-aqueous biologically relevant system. J Inorg Biochem. 2006;100:1897–1902. doi: 10.1016/j.jinorgbio.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 54.Wang S, Kotamraju S, Konorev E, Kalivendi S, Joseph J, Kalyanaraman B. Activation of nuclear factor-kappaB during doxorubicin-induced apoptosis in endothelial cells and myocytes is pro-apoptotic: the role of hydrogen peroxide. Biochem J. 2002;367:729–740. doi: 10.1042/BJ20020752. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.