Abstract
Integrins are cell surface receptors for extracellular matrix proteins and play a key role in cell survival, proliferation, migration and gene expression. Integrin signaling has been shown to be deregulated in several types of cancer, including prostate cancer. This review is focused on integrin signaling pathways known to be deregulated in prostate cancer and known to promote prostate cancer progression.
Keywords: Focal adhesion kinase, PTEN, PI 3-kinase/AKT, Ras/Raf/MAPK, cdc2, survivin, Bcl-2
Introduction
Prostate cancer is a significant burden in western countries and has been predicted to account for more than 28,660 deaths and 186,320 new cases in 2008 [1]. Prostate cancer development proceeds through a series of defined states. These include prostatic intraepithelial neoplasia (PIN); high-grade PIN lesions, which usually develop prior to invasive cancer; androgen-sensitive invasive cancer and an androgen-independent castration-resistant state [2, 3]. The current therapies for prostate cancer involve surgery, androgen ablation, or the blockade of the androgen receptor; however, a significantly high percentage of treated prostate cancers eventually grows, despite either castration levels of androgen or the presence of anti-androgens. For these patients, radiation therapy is the only treatment available. Still, a large number of patients relapse.
Integrins are cell surface receptors for extracellular matrix proteins and play a key role in cell survival, proliferation, migration and gene expression. Integrin signaling has been shown to be deregulated in several types of cancer, including prostate cancer. In prostate cancer, tumor cells have a different surrounding matrix than normal cells; thus changes in the integrin profile may be functionally relevant and contribute to aberrant intracellular signaling [4–8]. Several studies have associated deregulation of integrin expression with the progression of prostate cancer to an advanced stage (Table 1) [4, 8–11]. This article reviews the literature on the major signaling pathways activated by integrins and their deregulation in prostate cancer.
Table 1.
Deregulated expression of integrin subunits in human prostate cancer and metastasis
| Up-Regulated | |||
|---|---|---|---|
| Subunit | Adenocarcinoma | Metastasis | References |
| α6 | unknown | ↑ | Knox et al., 1994 [11]; Bonkhoff et al., 1993 [19]; Nagle et al., 1995 [20] |
| αIIb (truncated) | ↑ | unknown | Trikha et al, 1998 [33] |
| β1 | ↑ | unknown | Murant et al., 1997 [10]; Knox et al., 1994 [11]; Goel et al., 2007 [22] |
| β3 | ↑ | ↑ | Zheng et al., 1999 [30] |
| β6 | ↑ | ↑ | Li and Languino, 2007 [31] |
| Down-Regulated | |||
|---|---|---|---|
| α3, α4 α5 | ↓ | unknown | Nagle et al., 1994 [18] |
| α7 | ↓ | unknown | Ren et al., 2007 [17] |
| β1C | ↓ | unknown | Fornaro et al., 1996, 1998, 1999 [25–27]; Perlino et al., 2000 [28] |
| β4 | ↓ | unknown | Nagle et al., 1995 [20]; Davis et al., 2001 [23]; Allen et al., 1998 [24] |
This table shows the expression of integrin subunits found to be deregulated in human primary and metastatic prostate cancer.
Integrin deregulation in prostate cancer
Integrins are heterodimers consisting of α and β subunits. At this time, 24 heterodimers of the integrin family, consisting of 18 α and 8 β subunits, have been described [12, 13], and their ability to activate specific signaling path-ways has been investigated [13]. Integrin signaling plays a key role in the alteration of cellular growth and tumor progression through the regulation of gene expression, apoptosis, cell adhesion, proliferation, migration and angiogenesis [14, 15], as well as proteinase expression [16]. Most α and β subunits have been shown to be downregulated in prostate cancer, whereas only α6, β1, β3 and β6 are upregulated [6]. Among the α subunits, several reports show that α3, α4, α5 and α7 are downregulated [17, 18]; α2 and α6 are aberrantly expressed, whereas there are no reports on the remaining subunits [6]. A unique expression pattern has been shown for α2, which is downregulated in prostate cancer, but upregulated in lymph node metastases as compared to primary lesions [18, 19]. An extensive analysis of α6 expression in prostate cancer shows that α6 expression is either maintained or overexpressed in prostate cancer, and increases in lymph node metastases [11, 19–21].
Among the β subunits, β1, β3, and β6 are upregulated, while β1C and β4 are downregulated in human prostate cancer [6, 20, 22–24]. No reports are available for β5, β7, and β8. Five β1 variant subunits, β1A, β1B, β1C, β1C-2, and β1D, generated by alternative splicing, have been described. Two variants, β1C and β1A, are shown to be expressed in benign prostatic epithelium. β1C is expressed at both protein and mRNA levels in benign prostatic epithelial cells, but is markedly downregulated in adenocarcinoma [25–28]. Fornaro et al. show that the expression of β1C integrin increases p27kip1 levels, a cell cycle inhibitor, as well as p27kip1 association with cyclin A [26]. In contrast, the findings that the expression of the β1A integrin variant is upregulated and is necessary for the cell's ability to grow in an anchorage-independent manner [29], point to the important role that the β1A integrin may have during prostate cancer progression and will be helpful in formulating new therapeutic strategies.
Upregulation of αvβ3, αvβ6 and the truncated αIIb integrin variant has been described [6]. Zheng et al., using human prostate cancer cells isolated from 16 surgical specimens, show that these cells express αvβ3, whereas normal prostate epithelial cells do not [30]. Similarly, αvβ6 [31, 32] and the truncated αIIb integrin variant [33] are found to be expressed in adenocarcinoma.
The β1 and β3 integrin subunits are known to localize in focal contacts and to mediate spreading and cytoskeletal rearrangement in normal cells [12, 13]. However, when we either downregulated or upregulated these sub-units by siRNA or ectopic expression analysis, we show that cancer cell spreading is not affected [29, 34]. These results demonstrate that the ability of the β1 and β3 subunits to promote cancer progression is independent of cell spreading.
Overall, these findings indicate that the expression of selective integrin subunits is deregulated during prostate cancer progression, and that these subunits are potential diagnostic markers in prostate cancer.
Activation of unique signaling pathways by integrins
The expression of the β1 and β3 subunits activates specific signaling pathways and supports distinct cancer cell functions [34, 35]. Analysis of the mechanism by which β1 may promote tumor growth in vivo, shows that β1 is uniquely required in cancer cells for the localization, expression and function of insulin-like growth factor type 1 receptor (IGF-IR), which is known to support cancer cell proliferation and survival [29, 35]. The mechanism proposed for β1 integrins' control of IGF-IR activity involves β1 recruiting specific adaptors to the plasma membrane, thus increasing the concentration of specific adaptors proximal to the growth factor receptor [35]. This study provides evidence that the β1 cytodomain plays an important role in mediating β1 integrin association with either insulin receptor substrate-1 (IRS-1) or Grb2-associated binder1 (Gab1)/SH2-containing protein-tyrosine phosphate 2 (Shp2), downstream effectors of IGF-IR. Specifically, β1A associates with IRS-1 and β1C with Gab1/Shp2 [29, 35, 36].
In parallel studies, we have discovered that β3 is uniquely required in cancer cells for increasing cdc2 levels, as well as cdc2 kinase activity. While β1 integrin expression does not increase cancer cell motility or cdc2 levels, and appears to predominantly modulate cell proliferation and survival, these effects are specific for β3. Higher levels of cdc2 result in increased cell migration mediated by the specific association of cdc2 with cyclin B2 and the phosphorylation of caldesmon, a substrate of cdc2. These results show that cdc2 acts as a downstream mediator of the αvβ3 integrin and promotes cancer cell migration [34]. In conclusion, the β1 and β3 integrins promote activation of selective signaling pathways that support prostate cancer progression.
Integrin downstream effectors
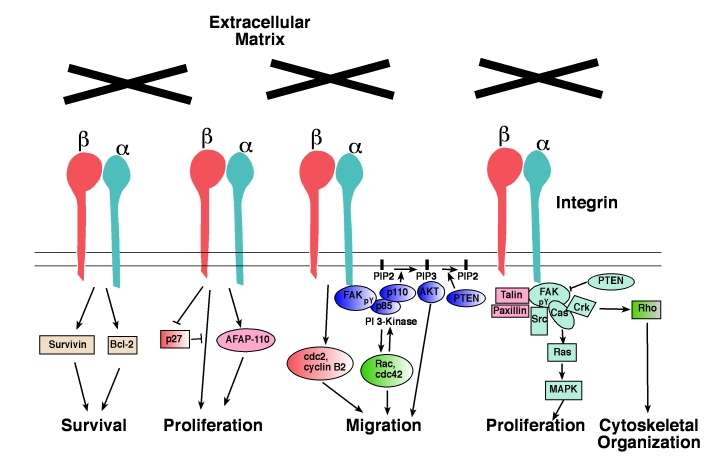
Since integrins lack catalytic activity, they depend on intracellular effector proteins to transduce signals [37, 38]. In this section, we discuss the major signaling effectors that are likely to contribute to prostate cancer progression (Figure 1 and Table 2).
Figure 1.
Integrin-dependent signaling pathways. Schematic drawing showing the signal transduction path-ways regulated by integrins that control prostate cancer cell survival, proliferation, adhesion, migration, and cytoskeletal organization. For a detailed description of integrin downstream effectors like Rac, cdc42, Src, Cas, Rho and Crk, or cytoskeletal proteins like AFAP-110, talin and paxillin, readers should refer to previous articles [38, 86].
Table 2.
Aberrant Integrin-Dependent Pathways in Prostate Cancer
| Downstream Effectors | Expression/Activity | Prostate cancer stage | References |
|---|---|---|---|
| FAK | upregulated expression | invasive cancer and metastasis | Rovin et al, 2002 [41]; Tremblay et al, 1996 [42] |
| MAP kinase | increased kinase activity | androgen-independent state | Bakin et al, 2003 [53] |
| PTEN AKT | downregulated expression increased kinase activity | cancer and metastasis cancer with high Gleason score | Schmitz et al, 2007 [61] Sun et al, 2001 [67]; Malik et al, 2002 [68] |
| Survivin | upregulated expression | PIN, primary tumors and metastasis | Shariat et al, 2004 [71]; Krajewska et al, 2003 [72]; Kishi et al, 2004 [73] |
| Bcl-2 | upregulated expression | PIN, primary tumors and metastasis from recurrent cancer | Colombel et al, 1993 [76]; Zellweger et al, 2005 [77]; Krajewska et al, 1996 [78] |
Signaling proteins and inhibitors of apoptosis known to be regulated by integrins and to affect prostate cancer progression are shown. FAK, Focal adhesion kinase; PTEN, phosphatase and tensin homolog; MAP kinase, mitogen-activated protein kinase.
Focal adhesion kinase (FAK)
FAK is a non-receptor tyrosine kinase, which becomes activated upon integrin-extracellular matrix (ECM) interactions and integrin clustering [39, 40]. Upon phosphorylation, FAK interacts with several signaling proteins, including Src kinases, Cas, paxillin and Phosphoinositide 3-Kinase (PI 3-Kinase) [39, 40]. FAK signaling is altered in prostate cancer. In normal prostate, FAK expression is absent or weak in secretory epithelium and is expressed predominantly in the basal layers. Prostate carcinoma shows a greater expression of FAK compared to the secretory layer of normal prostate. FAK expression is further increased in invasive prostate cancer [41, 42].
A well established role for FAK is its ability to regulate cancer cell motility [43]. The expression of dominant negative FAK inhibits the migration of prostate carcinoma cells [44]. In our previous study, we show that the β3 integrin induces cell migration on vitronectin, which is mediated by FAK [30]. Recently, the role of FAK in cell migration has been confirmed by using an inhibitor of FAK phosphorylation, PF-573,228. This inhibitor fails to inhibit cell growth or to induce apoptosis. In contrast, treatment with PF-573,228 inhibits both chemotactic and haptotactic migration concomitant with the inhibition of focal adhesion turnover [45]. In addition, Dasatinib, an inhibitor of Src family kinases/Abl, blocks FAK and Cas signaling in human prostate cancer cells, resulting in the suppression of invasion, migration and adhesion of prostate cancer cells [46].
Bombesin is shown to stimulate PC-3 cell migration and tyrosine phosphorylation of FAK. In addition, bombesin also increases the association between FAK and the β1, β3 and β5 integrins [47]. Bombesin induces relocalization of FAK in focal contacts, followed by its tyrosine phosphorylation and the formation of actin lamellipodia. FAK inhibitors cause reduced cell motility upon bombesin treatment [48]. FAK is also required for bombesin stimulated activation of RhoA, a GTPase required for cell migration [49]. Another example of the role that FAK plays in cell migration is provided by Sumitomo et al., who use Neutral endopeptidase 24.11 (NEP) [50]. NEP is an enzyme which cleaves neuropeptides such as bombesin and endothelin-1. NEP treatment blocks bombesin and endothelin-stimulated cell migration and FAK phosphorylation. This study suggests that NEP expression results in the formation of a complex containing NEP, Lyn and PI 3-Kinase and this complex competitively blocks FAK/PI 3-Kinase interactions [50]. The FAK/PI 3-Kinase interactions are also shown to promote prostate cancer cell invasion: α5β1 interacts with the PHSRN sequence of fibronectin (FN), which induces FAK phosphorylation and FAK association with PI 3-Kinase, resulting in prostate cancer cell invasion [51]. FAK siRNA, or specific PI 3-Kinase inhibitors, block PHSRN-mediated invasion [51]. Overall, these studies highlight a crucial role for the FAK in prostate cancer cell invasion mediated by integrins.
Ras/Raf/MAP kinase
Mitogen-activated protein (MAP) kinases, the principal effectors of Ras and known down-stream effectors of integrins, are major regulators of cell proliferation and cell differentiation [52]. Although Ras and Raf mutations are not common in prostate cancer, it is known that the activation of the Ras/MAP kinase pathway might be sufficient for progression towards the androgen-independent state [53, 54]. A high ERK/p38 activity ratio favors prostate tumor growth and activation of α5β1 integrin is proposed as a determinant of the in vivo growth promoting activity of a high ERK/p38 ratio [55]. Furthermore, inhibition of MAP kinase, using U0126, decreases α6 integrin mRNA levels in androgen-independent prostate cancer cells [56]. Thus, blocking MAP kinase activation provides an important tool to regulate integrin signaling during prostate cancer progression.
PTEN
PTEN, a dual specificity phosphatase, has the ability to dephosphorylate inositol phospholipids such as phosphatidylinositol-3,4,5-triphosphate (PIP3) and, as a consequence, negatively regulates AKT activation. By virtue of its ability to inhibit the AKT pathway, PTEN acts as a tumor suppressor [57]. The Pten gene is frequently deleted or mutated in human cancers and is shown to be involved in the regulation of cell migration on integrin substrates [58]. In 1997, PTEN was cloned from the 10q23 region, a region frequently targeted by loss of heterozygosity in advanced cancer [59, 60]. PTEN alterations are common in prostate cancer. Recently, Schmitz et al. have shown that 23% of patients with first time diagnoses lost PTEN expression, and 59% of patients with lymph node metastasis no longer express PTEN. These findings suggest that loss of PTEN expression is a possible early prognostic marker for prostate cancer metastasis [61].
Overexpression of PTEN inhibits cell migration, whereas antisense to PTEN enhances cell migration. These effects are suggested to be mediated by FAK regulation, since overexpression of FAK partially antagonizes the effects of PTEN. Thus, PTEN phosphatase may function as a tumor suppressor by negatively regulating cell interactions with the ECM, mediated by integrins [58]. PTEN is shown to regulate the adhesion and proliferation of LNCaP-C4-2 prostate cancer cells stimulated by vascular endothelial growth factor [62]. PTEN expression inhibits LNCaP-C4-2 cell migration toward calvaria-conditioned medium, but has no effect on migration toward lung-conditioned medium, and this inhibitory effect is dependent on PTEN lipid phosphatase activity [63]. All these studies suggest that PTEN downregulation contributes to integrin activation of signaling pathways that mediate cancer progression, although the mechanisms underlying this cross-talk remain to be investigated.
PI 3-Kinase/AKT pathway
PI 3-Kinase is a major downstream component of the integrin and growth factor signaling pathways [64, 65]. PI 3-Kinase catalyzes the production of the lipid secondary messenger PIP3 at the cell membrane. PIP3, in turn, contributes to the recruitment and activation of a wide range of downstream targets, including the serine-threonine protein kinase AKT [64]. Several studies show that integrin-mediated activation of PI 3-Kinase plays a crucial role in cancer cell survival, preventing anoikis and promoting cell migration (for review, [37, 66]). AKT1 kinase activity is significantly increased in primary carcinomas of the prostate [67]. AKT activation, assessed by immunohisto-chemical staining of human prostate cancer biopsies, shows greater intensity in high Gleason grade compared to PIN and all other grades of prostate cancer [68]. Similarly, using protein microarrays, it is shown that prostate cancer progression is associated with increased phosphorylation of AKT [69]. Although AKT promotes several integrin-mediated functions, our studies indicate a predominant role for the PI 3-Kinase/AKT pathway in prostate cancer cell migration [70].
Survivin/Bcl-2
Survivin, an important member of the inhibitor of apoptosis family, is a dual regulator of cell proliferation and cell viability. Survivin is expressed in embryonic and fetal organs, but is undetectable in most differentiated tissues. Survivin is shown to be upregulated in prostate cancer, especially in aggressive forms, such as high grade carcinoma and metastasis [71–73]. We demonstrate that β1 integrin engagement by FN upregulates the expression of survivin, and increases protection from apoptosis induced by the TNF-α in aggressive prostate cancer cells. The expression of dominant negative survivin counteracts the ability of FN to protect cells from undergoing apoptosis. We also show that the regulation of survivin levels by integrins is mediated by the AKT pathway [74]. It should be noted that in addition to integrin-ECM interactions, IGF/mTOR signaling and anti-androgen therapy are associated with the modulation of survivin levels in prostate cancer [75].
Bcl-2 is another important regulator of cell survival. Bcl-2 expression is restricted to the basal cells in normal and hypertrophic prostate glands, but all epithelial cells in areas of PIN express Bcl-2 [76]. All primary prostatic carcinomas and metastases obtained from hormone-refractory tumors are shown to express Bcl-2 [76–78]. This suggests that they may protect tumor cells from apoptosis induced in response to radiotherapy or chemotherapy. Integrin ligation, specifically by α5β1 and αvβ3, but not αvβ1, stimulates Bcl-2 expression via the FAK and PI 3-Kinase pathways [79, 80]. This integrin-mediated regulation of Bcl-2 is also controlled by the activation of Ca2+/calmodulin-dependent protein kinase IV, NF-kappaB and CREB transcription factors [79, 80]. Bcl-2 is also known to suppress anoikis induced by quinazoline based α1-adrenoceptor antagonists in prostate cancer cells [81].
All these recent studies highlight a crucial role for survivin and Bcl-2 in prostate cancer cell survival mediated by integrins.
Conclusions and future studies
The studies reviewed here indicate that designing new diagnostic and therapeutic approaches for prostate cancer, based on inhibitors of integrin functions or of integrin down-stream signaling, will prove to be a successful strategy. However, the molecular pathways by which integrins contribute to prostate cancer progression, and in general, the molecular mechanisms that promote this disease remain to be fully investigated. Several areas of research appear under-investigated. Among others, a major effort is needed to study the mechanisms by which integrins are deregulated in prostate cancer and to characterize integrin-mediated pathways which support survival of prostate cancer stem cells. Furthermore, new preclinical studies to test the efficacy of integrin inhibitors in prostate cancer are necessary. For this purpose, prostate cancer mouse models, such as the TRAMP mouse or the mouse which carries a conditional Pten deletion in the prostate are useful tools. Future studies will also take advantage of the use of recently developed novel small animal molecular imaging approaches, such as bioluminescence imaging (BLI) [82, 83]. A very innovative study, using BLI in mice that ubiquitously express luciferase (FLASH, firefly luciferase activated systemically in homozygotes), proves that we can increase our ability to detect tumor response to therapeutic agents like siR-NAs [84, 85].
In conclusion, studies aimed at elucidating the mechanisms by which deregulation of integrin-mediated signaling pathways occurs in prostate cancer will provide novel therapeutic approaches for this disease.
Acknowledgments
This study was supported by NIH grant RO1 CA-89720 and RO1 CA-109874); DOD (PCRP DAMD PC040221) to LRL and from American Cancer Society (Institutional Research Grant-93-033) to HLG. We would like to thank Heather Ehlers for help during the preparation of this article.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Scher HI, Heller G. Clinical states in prostate cancer: toward a dynamic model of disease progression. Urology. 2000;55:323–327. doi: 10.1016/s0090-4295(99)00471-9. [DOI] [PubMed] [Google Scholar]
- 3.De Marzo AM, Meeker AK, Zha S, Luo J, Na-kayama M, Platz EA, Isaacs WB, Nelson WG. Human prostate cancer precursors and patho-biology. Urology. 2003;62:55–62. doi: 10.1016/j.urology.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 4.Fornaro M, Manes T, Languino LR. Integrins and prostate cancer metastases. Cancer Metastasis Rev. 2001;20:321–331. doi: 10.1023/a:1015547830323. [DOI] [PubMed] [Google Scholar]
- 5.Demetriou MC, Cress AE. Integrin clipping: a novel adhesion switch? J Cell Biochem. 2004;91:26–35. doi: 10.1002/jcb.10675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goel HL, Li J, Kogan S, Languino LR. Integrins in prostate cancer progression. Endocr Relat Cancer. 2008;3:657–664. doi: 10.1677/ERC-08-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boudreau N, Bissell MJ. Extracellular matrix signaling: integration of form and function in normal and malignant cells. Curr Opin Cell Biol. 1998;10:640–646. doi: 10.1016/s0955-0674(98)80040-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knudsen BS, Miranti CK. The impact of cell adhesion changes on proliferation and survival during prostate cancer development and pro-gression. J Cell Biochem. 2006;99:345–361. doi: 10.1002/jcb.20934. [DOI] [PubMed] [Google Scholar]
- 9.Edlund M, Sung SY, Chung LW. Modulation of prostate cancer growth in bone microenviron-ments. J Cell Biochem. 2004;91:686–705. doi: 10.1002/jcb.10702. [DOI] [PubMed] [Google Scholar]
- 10.Murant SJ, Handley J, Stower M, Reid N, Cus-senot O, Maitland NJ. Co-ordinated changes in expression of cell adhesion molecules in prostate cancer. Eur J Cancer. 1997;33:263–271. doi: 10.1016/s0959-8049(96)00418-2. [DOI] [PubMed] [Google Scholar]
- 11.Knox JD, Cress AE, Clark V, Manriquez L, Affini-to KS, Dalkin BL, Nagle RB. Differential expression of extracellular matrix molecules and the α6-integrins in the normal and neoplastic prostate. Am J Pathol. 1994;145:167–174. [PMC free article] [PubMed] [Google Scholar]
- 12.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 13.Alam N, Goel HL, Zarif MJ, Butterfield JE, Perkins HM, Sansoucy BG, Sawyer TK, Languino LR. The integrin-growth factor receptor duet. J Cell Physiol. 2007;213:649–653. doi: 10.1002/jcp.21278. [DOI] [PubMed] [Google Scholar]
- 14.Akalu A, Cretu A, Brooks PC. Targeting integrins for the control of tumour angiogenesis. Expert Opin Investig Drugs. 2005;14:1475–1486. doi: 10.1517/13543784.14.12.1475. [DOI] [PubMed] [Google Scholar]
- 15.Felding-Habermann B. Integrin adhesion receptors in tumor metastasis. Clin Exp Metastasis. 2003;20:203–213. doi: 10.1023/a:1022983000355. [DOI] [PubMed] [Google Scholar]
- 16.Munshi HG, Stack MS. Reciprocal interactions between adhesion receptor signaling and MMP regulation. Cancer Metastasis Rev. 2006;25:45–56. doi: 10.1007/s10555-006-7888-7. [DOI] [PubMed] [Google Scholar]
- 17.Ren B, Yu YP, Tseng GC, Wu C, Chen K, Rao UN, Nelson J, Michalopoulos GK, Luo JH. Analysis of integrin α7 mutations in prostate cancer, liver cancer, glioblastoma multiforme, and leiomyosarcoma. J Natl Cancer Inst. 2007;99:868–880. doi: 10.1093/jnci/djk199. [DOI] [PubMed] [Google Scholar]
- 18.Nagle RB, Knox JD, Wolf C, Bowden GT, Cress AE. Adhesion molecules, extracellular matrix, and proteases in prostate carcinoma. J Cell Bi-ochem Suppl. 1994;19:232–237. [PubMed] [Google Scholar]
- 19.Bonkhoff H, Stein U, Remberger K. Differential expression of α6 and α2 very late antigen integrins in the normal, hyperplastic, and neoplastic prostate: simultaneous demonstration of cell surface receptors and their extracellular ligands. Hum Pathol. 1993;24:243–248. doi: 10.1016/0046-8177(93)90033-d. [DOI] [PubMed] [Google Scholar]
- 20.Nagle RB, Hao J, Knox JD, Dalkin BL, Clark V, Cress AE. Expression of hemidesmosomal and extracellular matrix proteins by normal and malignant human prostate tissue. Am J Pathol. 1995;146:1498–1507. [PMC free article] [PubMed] [Google Scholar]
- 21.Pontes-Junior J, Reis ST, Dall'oglio M, Neves de Oliveira LC, Cury J, Carvalho PA, Ribeiro-Filho LA, Moreira Leite KR, Srougi M. Evaluation of the expression of integrins and cell adhesion molecules through tissue microarray in lymph node metastases of prostate cancer. J Carcinog. 2009;8:3. doi: 10.4103/1477-3163.48453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goel HL, Zarif MJ, Saluja V, Breen M, Garlick DS, Jiang Z, Wu CL, Davis RJ, FitzGerald TJ, Languino LR. Down-regulation of β1 integrin in vivo delays prostate cancer progression and increases radiosensitivity. IMPaCT Meeting; September 5–8, 2007; Atlanta, GA. 2007. [Google Scholar]
- 23.Davis TL, Cress AE, Dalkin BL, Nagle RB. Unique expression pattern of the α6β4 integrin and laminin-5 in human prostate carcinoma. Prostate. 2001;46:240–248. doi: 10.1002/1097-0045(20010215)46:3<240::aid-pros1029>3.0.co;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen MV, Smith GJ, Juliano R, Maygarden SJ, Mohler JL. Downregulation of the β4 integrin subunit in prostatic carcinoma and prostatic intraepithelial neoplasia. Hum Pathol. 1998;29:311–318. doi: 10.1016/s0046-8177(98)90109-5. [DOI] [PubMed] [Google Scholar]
- 25.Fornaro M, Tallini G, Bofetiado CJ, Bosari S, Languino LR. Down-regulation of β1C integrin, an inhibitor of cell proliferation, in prostate carcinoma. Am J Pathol. 1996;149:765–773. [PMC free article] [PubMed] [Google Scholar]
- 26.Fornaro M, Tallini G, Zheng DQ, Flanagan WM, Manzotti M, Languino LR. p27(kip1) acts as a downstream effector of and is coexpressed with the β1C integrin in prostatic adenocarcinoma. J Clin Invest. 1999;103:321–329. doi: 10.1172/JCI4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fornaro M, Manzotti M, Tallini G, Slear AE, Bo-sari S, Ruoslahti E, Languino LR. β1C integrin in epithelial cells correlates with a nonproliferative phenotype: forced expression of β1C inhibits prostate epithelial cell proliferation. Am J Pathol. 1998;153:1079–1087. doi: 10.1016/s0002-9440(10)65652-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perlino E, Lovecchio M, Vacca RA, Fornaro M, Moro L, Ditonno P, Battaglia M, Selvaggi FP, Mastropasqua MG, Bufo P, Languino LR. Regulation of mRNA and protein levels of β1 integrin variants in human prostate carcinoma. Am J Pathol. 2000;157:1727–1734. doi: 10.1016/s0002-9440(10)64809-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goel HL, Breen M, Zhang J, Das I, Aznavoorian-Cheshire S, Greenberg NM, Elgavish A, Languino LR. β1A integrin expression is required for type 1 insulin-like growth factor receptor mito-genic and transforming activities and localization to focal contacts. Cancer Res. 2005;65:6692–6700. doi: 10.1158/0008-5472.CAN-04-4315. [DOI] [PubMed] [Google Scholar]
- 30.Zheng DQ, Woodard AS, Fornaro M, Tallini G, Languino LR. Prostatic carcinoma cell migration via αvβ3 integrin is modulated by a focal adhesion kinase pathway. Cancer Res. 1999;59:1655–1664. [PubMed] [Google Scholar]
- 31.Li J, Wang T, Goel HL, Jiang Z, Cai Y, Crockett KA, Zhang JZ, Jain D, Coonradt M, Manes T, Violette MS, Weinreb H, Dolinski B, FitzGerald TJ, Languino LR. A Novel Mechanism of Prostate Cancer Growth Mediated by αvβ6 Integrin and Androgen Receptor. IMPaCT Meeting; September 5–8, 2007; Atlanta, GA. 2007. [Google Scholar]
- 32.Azare J, Leslie K, Al-Ahmadie H, Gerald W, Weinreb PH, Violette SM, Bromberg J. Constitutively activated Stat3 induces tumorigenesis and enhances cell motility of prostate epithelial cells through integrin β6. Mol Cell Biol. 2007;27:4444–4453. doi: 10.1128/MCB.02404-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trikha M, Cai Y, Grignon D, Honn KV. Identification of a novel truncated αIIb integrin. Cancer Res. 1998;58:4771–4775. [PubMed] [Google Scholar]
- 34.Manes T, Zheng DQ, Tognin S, Woodard AS, Marchisio PC, Languino LR. αvβ3 integrin expression up-regulates cdc2, which modulates cell migration. J Cell Biol. 2003;161:817–826. doi: 10.1083/jcb.200212172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goel HL, Fornaro M, Moro L, Teider N, Rhim JS, King M, Languino LR. Selective modulation of type 1 insulin-like growth factor receptor signaling and functions by β1 integrins. J Cell Biol. 2004;166:407–418. doi: 10.1083/jcb.200403003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goel HL, Moro L, King M, Teider N, Centrella M, McCarthy TL, Holgado-Madruga M, Wong AJ, Marra E, Languino LR. β1 integrins modulate cell adhesion by regulating insulin-like growth factor-II levels in the microenvironment. Cancer Res. 2006;66:331–342. doi: 10.1158/0008-5472.CAN-05-2588. [DOI] [PubMed] [Google Scholar]
- 37.Reddig PJ, Juliano RL. Clinging to life: cell to matrix adhesion and cell survival. Cancer Me-tastasis Rev. 2005;24:425–439. doi: 10.1007/s10555-005-5134-3. [DOI] [PubMed] [Google Scholar]
- 38.Goel HL, Languino LR. Integrin signaling in cancer. In: Kumar R, editor. Molecular targeting and signal transduction. Norwell: Kluwer Academic Publishers; 2004. pp. 15–31. [Google Scholar]
- 39.Tilghman RW, Parsons JT. Focal adhesion kinase as a regulator of cell tension in the progression of cancer. Semin Cancer Biol. 2007;18:45–52. doi: 10.1016/j.semcancer.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006;18:516–523. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 41.Rovin JD, Frierson HF, Jr, Ledinh W, Parsons JT, Adams RB. Expression of focal adhesion kinase in normal and pathologic human prostate tissues. Prostate. 2002;53:124–132. doi: 10.1002/pros.10114. [DOI] [PubMed] [Google Scholar]
- 42.Tremblay L, Hauck W, Aprikian AG, Begin LR, Chapdelaine A, Chevalier S. Focal adhesion kinase pp125FAK expression, activation and association with paxillin and p50CSK in human metastatic prostate carcinoma. Int J Cancer. 1996;68:164–171. doi: 10.1002/(sici)1097-0215(19961009)68:2<169::aid-ijc4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 43.Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T, Alzawa S. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–543. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 44.Slack JK, Adams RB, Rovin JD, Bissonette EA, Stoker CE, Parsons JT. Alterations in the focal adhesion kinase/Src signal transduction path-way correlate with increased migratory capacity of prostate carcinoma cells. Oncogene. 2001;20:1152–1163. doi: 10.1038/sj.onc.1204208. [DOI] [PubMed] [Google Scholar]
- 45.Slack-Davis JK, Martin KH, Tilghman RW, Iwanicki M, Ung EJ, Autry C, Luzzio MJ, Cooper B, Kath JC, Roberts WG, Parsons JT. Cellular characterization of a novel focal adhesion kinase inhibitor. J Biol Chem. 2007;282:14845–14852. doi: 10.1074/jbc.M606695200. [DOI] [PubMed] [Google Scholar]
- 46.Nam S, Kim D, Cheng JQ, Zhang S, Lee JH, Buettner R, Mirosevich J, Lee FY, Jove R. Action of the Src family kinase inhibitor, dasatinib (BMS-354825), on human prostate cancer cells. Cancer Res. 2005;65:9185–9189. doi: 10.1158/0008-5472.CAN-05-1731. [DOI] [PubMed] [Google Scholar]
- 47.Aprikian AG, Tremblay L, Han K, Chevalier S. Bombesin stimulates the motility of human prostate-carcinoma cells through tyrosine phosphorylation of focal adhesion kinase and of integrin-associated proteins. Int J Cancer. 1997;72:498–504. doi: 10.1002/(sici)1097-0215(19970729)72:3<498::aid-ijc19>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 48.Lacoste J, Aprikian AG, Chevalier S. Focal adhesion kinase is required for bombesin-induced prostate cancer cell motility. Mol Cell Endocrinol. 2005;235:51–61. doi: 10.1016/j.mce.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 49.Zheng R, Iwase A, Shen R, Goodman OB, Jr, Sugimoto N, Takuwa Y, Lerner DJ, Nanus DM. Neuropeptide-stimulated cell migration in prostate cancer cells is mediated by RhoA kinase signaling and inhibited by neutral endopeptidase. Oncogene. 2006;25:5942–5952. doi: 10.1038/sj.onc.1209586. [DOI] [PubMed] [Google Scholar]
- 50.Sumitomo M, Shen R, Walburg M, Dai J, Geng Y, Navarro D, Boileau G, Papandreou CN, Giancotti FG, Knudsen B, Nanus DM. Neutral endopeptidase inhibits prostate cancer cell migration by blocking focal adhesion kinase signaling. J Clin Invest. 2000;106:1399–1407. doi: 10.1172/JCI10536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeng ZZ, Jia Y, Hahn NJ, Markwart SM, Rock-wood KF, Livant DL. Role of focal adhesion kinase and phosphatidylinositol 3′-kinase in integrin fibronectin receptor-mediated, matrix metalloproteinase-1-dependent invasion by metastatic prostate cancer cells. Cancer Res. 2006;66:8091–8099. doi: 10.1158/0008-5472.CAN-05-4400. [DOI] [PubMed] [Google Scholar]
- 52.Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 53.Bakin RE, Gioeli D, Bissonette EA, Weber MJ. Attenuation of Ras signaling restores androgen sensitivity to hormone-refractory C4–2 prostate cancer cells. Cancer Res. 2003;63:1975–1980. [PubMed] [Google Scholar]
- 54.Weber MJ, Gioeli D. Ras signaling in prostate cancer progression. J Cell Biochem. 2004;91:13–25. doi: 10.1002/jcb.10683. [DOI] [PubMed] [Google Scholar]
- 55.Aguirre-Ghiso JA, Estrada Y, Liu D, Ossowski L. ERK(MAPK) activity as a determinant of tumor growth and dormancy; regulation by p38(SAPK) Cancer Res. 2003;63:1684–1695. [PubMed] [Google Scholar]
- 56.Onishi T, Yamakawa K, Franco OE, Kawamura J, Watanabe M, Shiraishi T, Kitazawa S. Mitogen-activated protein kinase pathway is involved in α6 integrin gene expression in androgen-independent prostate cancer cells: role of proximal Sp1 consensus sequence. Biochim Biophys Acta. 2001;1538:218–227. doi: 10.1016/s0167-4889(01)00068-4. [DOI] [PubMed] [Google Scholar]
- 57.Majumder PK, Sellers WR. Akt-regulated path-ways in prostate cancer. Oncogene. 2005;24:7465–7474. doi: 10.1038/sj.onc.1209096. [DOI] [PubMed] [Google Scholar]
- 58.Tamura M, Gu J, Matsumoto K, Aota S, Parsons R, Yamada KM. Inhibition of cell migration, spreading and focal adhesions by tumor sup-pressor PTEN. Science. 1998;280:1614–1617. doi: 10.1126/science.280.5369.1614. [DOI] [PubMed] [Google Scholar]
- 59.Steck PA, Pershouse MA, Jasser SA, Yung WK, Lin H, Ligon AH, Langford LA, Baumgard ML, Hattier T, Davis T, Frye C, Hu R, Swedlund B, Teng DH, Tavtigian SV. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat Genet. 1997;15:356–362. doi: 10.1038/ng0497-356. [DOI] [PubMed] [Google Scholar]
- 60.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, Bigner SH, Giovanella BC, Itt-mann M, Tycko B, Hibshoosh H, Wigler MH, Parsons R. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 61.Schmitz M, Grignard G, Margue C, Dippel W, Capesius C, Mossong J, Nathan M, Giacchi S, Scheiden R, Kieffer N. Complete loss of PTEN expression as a possible early prognostic marker for prostate cancer metastasis. Int J Cancer. 2007;120:1284–1292. doi: 10.1002/ijc.22359. [DOI] [PubMed] [Google Scholar]
- 62.Chen J, De S, Brainard J, Byzova TV. Metastatic properties of prostate cancer cells are controlled by VEGF. Cell Commun Adhes. 2004;11:1–11. doi: 10.1080/15419060490471739. [DOI] [PubMed] [Google Scholar]
- 63.Wu Z, McRoberts KS, Theodorescu D. The role of PTEN in prostate cancer cell tropism to the bone micro-environment. Carcinogenesis. 2007;28:1393–1400. doi: 10.1093/carcin/bgm050. [DOI] [PubMed] [Google Scholar]
- 64.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 65.Khwaja A, Rodriguez-Viciana P, Wennstrom S, Warne PH, Downward J. Matrix adhesion and Ras transformation both activate a phosphoi-nositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lipscomb EA, Mercurio AM. Mobilization and activation of a signaling competent α6β4 integrin underlies its contribution to carcinoma progression. Cancer Metastasis Rev. 2005;24:413–423. doi: 10.1007/s10555-005-5133-4. [DOI] [PubMed] [Google Scholar]
- 67.Sun M, Wang G, Paciga JE, Feldman RI, Yuan ZQ, Ma XL, Shelley SA, Jove R, Tsichlis PN, Nicosia SV, Cheng JQ. AKT1/PKBα kinase is frequently elevated in human cancers and its constitutive activation is required for oncogenic transformation in NIH3T3 cells. Am J Pathol. 2001;159:431–437. doi: 10.1016/s0002-9440(10)61714-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Malik SN, Brattain M, Ghosh PM, Troyer DA, Prihoda T, Bedolla R, Kreisberg JI. Immunohistochemical demonstration of phospho-Akt in high Gleason grade prostate cancer. Clin Cancer Res. 2002;8:1168–1171. [PubMed] [Google Scholar]
- 69.Paweletz CP, Charboneau L, Bichsel VE, Simone NL, Chen T, Gillespie JW, Emmert-Buck MR, Roth MJ, Petricoin IE, Liotta LA. Reverse phase protein microarrays which capture disease progression show activation of pro-survival pathways at the cancer invasion front. Oncogene. 2001;20:1981–1989. doi: 10.1038/sj.onc.1204265. [DOI] [PubMed] [Google Scholar]
- 70.Zheng DQ, Woodard AS, Tallini G, Languino LR. Substrate specificity of αvβ3 integrin-mediated cell migration and phosphatidylinositol 3-kinase/AKT pathway activation. J Biol Chem. 2000;275:24565–24574. doi: 10.1074/jbc.M002646200. [DOI] [PubMed] [Google Scholar]
- 71.Shariat SF, Lotan Y, Saboorian H, Khoddami SM, Roehrborn CG, Slawin KM, Ashfaq R. Survivin expression is associated with features of biologically aggressive prostate carcinoma. Cancer. 2004;100:751–757. doi: 10.1002/cncr.20039. [DOI] [PubMed] [Google Scholar]
- 72.Krajewska M, Krajewski S, Banares S, Huang X, Turner B, Bubendorf L, Kallioniemi OP, Shabaik A, Vitiello A, Peehl D, Gao GJ, Reed JC. Elevated expression of inhibitor of apoptosis proteins in prostate cancer. Clin Cancer Res. 2003;9:4914–4925. [PubMed] [Google Scholar]
- 73.Kishi H, Igawa M, Kikuno N, Yoshino T, Urakami S, Shiina H. Expression of the survivin gene in prostate cancer: correlation with clinicopatho-logical characteristics, proliferative activity and apoptosis. J Urol. 2004;171:1855–1860. doi: 10.1097/01.ju.0000120317.88372.03. [DOI] [PubMed] [Google Scholar]
- 74.Fornaro M, Plescia J, Chheang S, Tallini G, Zhu YM, King M, Altieri DC, Languino LR. Fibronectin protects prostate cancer cells from tumor necrosis factor α-induced apoptosis via the AKT/Survivin pathway. J Biol Chem. 2003;278:50402–50411. doi: 10.1074/jbc.M307627200. [DOI] [PubMed] [Google Scholar]
- 75.Vaira V, Lee CW, Goel HL, Bosari S, Languino LR, Altieri DC. Regulation of survivin expression by IGF-1/mTOR signaling. Oncogene. 2007;26:2678–2684. doi: 10.1038/sj.onc.1210094. [DOI] [PubMed] [Google Scholar]
- 76.Colombel M, Symmans F, Gil S, O'Toole KM, Chopin D, Benson M, Olsson CA, Korsmeyer S, Buttyan R. Detection of the apoptosis-suppressing oncoprotein bcl-2 in hormone- refractory human prostate cancers. Am J Pathol. 1993;143:390–400. [PMC free article] [PubMed] [Google Scholar]
- 77.Zellweger T, Ninck C, Bloch M, Mirlacher M, Koivisto PA, Helin HJ, Mihatsch MJ, Gasser TC, Bubendorf L. Expression patterns of potential therapeutic targets in prostate cancer. Int J Cancer. 2005;113:619–628. doi: 10.1002/ijc.20615. [DOI] [PubMed] [Google Scholar]
- 78.Krajewska M, Krajewski S, Epstein JI, Shabaik A, Sauvageot J, Song K, Kitada S, Reed JC. Immunohistochemical analysis of bcl-2, bax, bcl-X, and mcl-1 expression in prostate cancers. Am J Pathol. 1996;148:1567–1576. [PMC free article] [PubMed] [Google Scholar]
- 79.Matter ML, Ruoslahti E. A signaling pathway from the α5β1 and αvβ3 integrins that elevates bcl-2 transcription. J Biol Chem. 2001;276:27757–27763. doi: 10.1074/jbc.M102014200. [DOI] [PubMed] [Google Scholar]
- 80.Lee BH, Ruoslahti E. α5β1 integrin stimulates Bcl-2 expression and cell survival through Akt, focal adhesion kinase, and Ca2+/calmodulin-dependent protein kinase IV. J Cell Biochem. 2005;95:1214–1223. doi: 10.1002/jcb.20488. [DOI] [PubMed] [Google Scholar]
- 81.Keledjian K, Kyprianou N. Anoikis induction by quinazoline based α1-adrenoceptor antagonists in prostate cancer cells: antagonistic effect of bcl-2. J Urol. 2003;169:150–156. doi: 10.1097/01.ju.0000042453.12079.77. [DOI] [PubMed] [Google Scholar]
- 82.Drake JM, Gabriel CL, Henry MD. Assessing tumor growth and distribution in a model of prostate cancer metastasis using biolumines-cence imaging. Clin Exp Metastasis. 2005;22:674–684. doi: 10.1007/s10585-006-9011-4. [DOI] [PubMed] [Google Scholar]
- 83.Liao CP, Zhong C, Saribekyan G, Bading J, Park R, Conti PS, Moats R, Berns A, Shi W, Zhou Z, Nikitin AY, Roy-Burman P. Mouse models of prostate adenocarcinoma with the capacity to monitor spontaneous carcinogenesis by bioluminescence or fluorescence. Cancer Res. 2007;67:7525–7533. doi: 10.1158/0008-5472.CAN-07-0668. [DOI] [PubMed] [Google Scholar]
- 84.Svensson RU, Barnes JM, Rokhlin OW, Cohen MB, Henry MD. Chemotherapeutic agents up-regulate the cytomegalovirus promoter: implications for bioluminescence imaging of tumor response to therapy. Cancer Res. 2007;67:10445–10454. doi: 10.1158/0008-5472.CAN-07-1955. [DOI] [PubMed] [Google Scholar]
- 85.Svensson RU, Shey MR, Ballas ZK, Dorkin JR, Goldberg M, Akinc A, Langer R, Anderson DG, Bumcrot D, Henry MD. Assessing siRNA pharmacodynamics in a luciferase-expressing mouse. Mol Ther. 2008;16:1995–2001. doi: 10.1038/mt.2008.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang J, Park SI, Artime MC, Summy JM, Shah AN, Bomser JA, Dorfleutner A, Flynn DC, Gallick GE. AFAP-110 is overexpressed in prostate cancer and contributes to tumorigenic growth by regulating focal contacts. J Clin Invest. 2007;117:2962–2973. doi: 10.1172/JCI30710. [DOI] [PMC free article] [PubMed] [Google Scholar]