Abstract
Fredericamycin (FDM) A, a highly oxidized aromatic pentadecaketide natural product, exhibits potent cytotoxicity and has been studied as a new anticancer drug lead. The FDM biosynthetic gene cluster has been previously cloned from Streptomyces griseus ATCC 49344 and successfully expressed in the heterologous host Streptomyces albus J1074. The fdmM and fdmM1 genes code for two proteins with high sequence homology to each other but unknown function. In-frame deletion of each of the genes from the fdm cluster was accomplished in the S. albus host. Each mutant failed to produce FDM A and the key biosynthetic intermediate FDM E but produced various new metabolites, the titers of which were dramatically increased via overexpression of an fdm pathway-specific activator fdmR1. The ΔfdmM mutant strain accumulated three new compounds FDM M-1, FDM M-2, and FDM M-3, whereas the ΔfdmM1 mutant strain produced one new compound FDM M1-1. Isolation and structural characterization of these compounds enable us to propose that FdmM and FdmM1 catalyze the C-6 and C-8 hydroxylations for FDM biosynthesis, respectively. Homologs of FdmM and FdmM1 can be found in biosynthetic gene clusters of many other aromatic polyketides, ranging from dodecaketides to pentadecaketides, but to date all of them were annotated as proteins of unknown function. Based on the findings reported here for FdmM and FdmM1, we now propose similar functions for those proteins, and FdmM and FdmM1 therefore represent an emerging family of novel oxygenases responsible for hydroxylation of aromatic polyketide natural products.
Studies on natural product biosynthetic pathways have uncovered a number of intriguing oxygenases. For instance, the first reported metal- and cofactor-free monooxygenase TcmH is responsible for quinone moiety formation in tetracenomycin D3 and represents a rapidly growing family of antibiotic biosynthetic monooxygenases (1, 2). Another example is DpgC, which is involved in the biosynthesis of vancomycin building block 3, 5-dihydroxyphenylglycine. The crystal structure of DpgC has been used to understand the reaction mechanism of metal- and cofactor-free dioxygenases (3). MomA, involved in mompain biosynthesis (4) is a metal ion-dependent monooxygenase belonging to the cupin superfamily of oxidases bearing no prosthetic group. The discovery of enzymes such as TcmH, DpgC, and MomA continually encourage the community to search for and characterize new oxygenases from proteins of unknown functions involved in natural products biosynthesis.
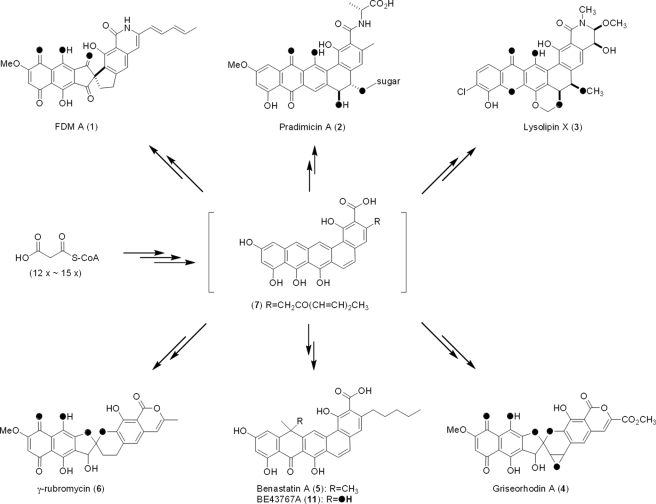
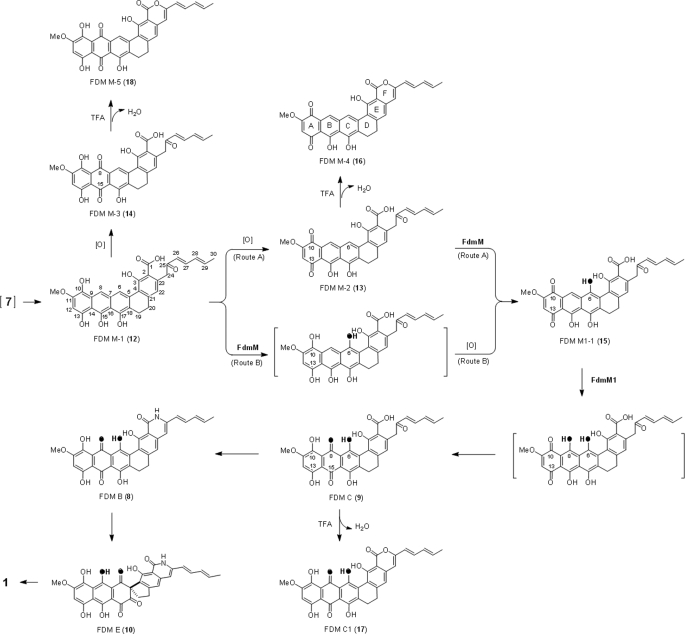
Whereas octaketides (C-16), nonaketides (C-18), and decaketides (C-20) are the most common aromatic polyketide natural products whose biosyntheses have been extensively investigated, aromatic polyketides ranging from dodecaketides (C-24) to pentadecaketides (C-30) are also known including biologically active compounds such as fredericamycin (FDM)2 A (1) (5, 6), pradimicin A (2) (7), lysolipin X (3) (8), griseorhodin A (4) (9), benastatin A (5) (10), and γ-rubromycin (6) (Fig. 1 and Ref. 11). The highly oxidized nature of these molecules suggests their biosynthetic pathways as potential resources for the discovery of new oxygenases. We have recently cloned and sequenced the biosynthetic gene cluster of 1 from Streptomyces griseus ATCC 49344, proposing that 1 is derived from a pentadecaketide core structure (7) (Fig. 1). The complete fdm cluster was successfully expressed in the heterologous host Streptomyces albus J1074, providing another amenable system with which to perform in vivo studies (12). Based on the structures of FDM A and its biosynthetic intermediates, FDM B (8), FDM C (9) (13), and FDM E (10) (14), 1 was proposed to be generated from 8 through a benzylic acid rearrangement (Fig. 2). The biosynthetic production of 1 from 7 was proposed to include at least seven oxidative steps (14). Upon bioinformatics analysis of the fdm cluster, however, only three genes, fdmJ, fdmP, and fdmQ, were identified as candidates to encode oxygenases, and five additional genes, fdmK, fdmL, fdmM, fdmM1, and fdmU, were annotated to encode proteins of unknown function. The latter implies the existence of novel oxygenases responsible for the some of the oxidative steps in FDM biosynthesis (12).
FIGURE 1.
Structures of selected aromatic dodecaketide (2, 3), tridecaketide (4, 6), tetradecaketide (5, 11), and pentadecaketide (1) natural products whose biosynthetic gene clusters have been cloned. Oxygen atoms predicted or confirmed to be derived from O2 are shown in black.
FIGURE 2.
FDM biosynthetic intermediates, metabolites accumulated in the recombinant ΔfdmM and ΔfdmM1 mutant strains and related compounds. FdmM-catalyzed C-6 and FdmM1-catalyzed C-8 hydroxylations are shown in black.
FdmM and FdmM1, showing 30.8% identity to each other, are two of five proteins of unknown function involved in biosynthesis of 1 on the basis of bioinformatics analysis. Homologs of FdmM and FdmM1 can be found in biosynthetic gene clusters of many other aromatic polyketides ranging from dodecaketides to pentadecaketides. However, all of them to date were annotated as proteins of unknown functions. These include Prm-Orf5 (29.5% identity to FdmM, 42.3% identity to FdmM1) in the pradimicin A (2) biosynthetic pathway (7), LlpB (30.1% identity to FdmM, 50% identity to FdmM1), and LlpQ (41.2% identity to FdmM, 36.1% identity to FdmM1) in the lysolipin X (3) biosynthetic pathway (8), GrhM (42.2% identity to FdmM, 31% identity to FdmM1) in the griseorhodin A (4) biosynthetic pathway (9), BenG (28.6% identity to FdmM, 26.5% identity to FdmM1) in the benastatin A (5) biosynthetic pathway (10), and RubQ (43.8% identity to FdmM, 31.6% identity to FdmM1) in the γ-rubromycin (6) biosynthetic pathway (supplemental Fig. S1 and Ref. 11). Like 1, inspection of those aromatic polyketides reveals the crucial role of oxygenation in their biosynthesis (Fig. 1). It should be noted that although no oxygenation is needed in the biosynthesis of benastatin (5), its biosynthetic congener BE43767A (11) contains a C-8 hydroxyl group (15).
We report here in vivo investigations of the roles of FdmM and FdmM1 in the biosynthesis of 1 by (i) construction of ΔfdmM and ΔfdmM1 mutants, respectively and (ii) structural characterization of the accumulated metabolites for each mutant. These data provide new insights into the biosynthesis of 1 and unveil FdmM and FdmM1 as members of an emerging family of novel oxygenases involved in aromatic polyketide biosynthesis.
EXPERIMENTAL PROCEDURES
General
IR spectra were measured on a Bruker EQUINOX 55/S FT-IR/NIR spectrophotometer (Ettlingen). Electrospray ionization mass spectroscopy (ESI-MS) or high-resolution (HR) ESI-MS was performed on an Agilent 1100 HPLC-MSDSL ion trap mass spectrometer (Santa Clara, CA). Atmospheric pressure chemical ionization mass spectroscopy (APCI-MS) was performed on an Agilent 1100 VL APCI mass spectrometer (Santa Clara, CA). HR Matrix-Assisted Laser Desorption Ionization-Fourier Transform (MALDI-FT)-MS was measured on an IonSpec HiResMALDI FT mass spectrometer (Lake Forest, CA). NMR data were obtained using a Varian Unity Inova 500 MHz NMR spectrometer (Palo Alto, CA). Analytical or semi-preparative HPLC was carried on a Varian HPLC system with in-line Prostar 330 PDA detector (Woburn, MA). The cytotoxity assay of FDM compounds was performed as described previously (14).
Bacterial Strains and Culture Conditions
Streptomyces albus J1074 was used as heterologous expression host (12). For protoplast preparation, S. albus was grown in YEME medium (16). Escherichia coli ET12567/pUZ8002 (17) and BW25113 (18) were used for intergeneric conjugation and λ RED-mediated PCR-targeting mutagenesis, respectively. S. albus recombinant strains were cultured in R2YE medium to prepare seed cultures, and then inoculated to production medium and cultured at 28 °C, 250 rpm for FDM production as described previously (12). The production medium consisted of 8 g of yeast extract, 20 g of malt extract, 2 g of NaCl, 10 g of MOPS sodium salt in 500 ml of tap water, and 380 ml of Milli-Q® water (Millipore Corp, Bedford, MA). After autoclaving, 1 ml of 10% MgSO4, 1 ml of 1% FeSO4, 0.1 ml of 10% ZnSO4, and 120 ml of 50% glucose were added. The concentrations of antibiotics used for selection were ampicillin (100 μg/ml), apramycin (50 μg/ml), chloramphenicol (25 μg/ml), and thiostrepton (10 μg/ml) (16).
Plasmids and DNA Manipulation
General DNA manipulations were performed as described (17). MYME was used for genomic DNA isolation (16). Protoplast transformation and intergeneric conjugation were performed as previously described (19). PCR reactions were performed with the GoTaq DNA polymerase kit (Promega, Madison, WI) or Platinum Pfx DNA polymerase kit (Invitrogen, Carlsbad, CA). λ RED-mediated recombination was carried out according to the standard procedure (18). Plasmids pSET152, pWHM1250, pKC1218, and pBS4028 were described previously (19).
Construction of the ΔfdmM and ΔfdmM1 In-frame Deletion Mutant Strains
The inactivation of fdmM and fdmM1 took advantage of the successful heterologous expression of the fdm cluster carried by pBS4028 in S. albus (12). The fdmM gene in pBS4028 was disrupted by replacing a 0.4-kb fragment internal fdmM with the 1.4-kb aac(3)IV+oriT cassette through λ RED-mediated recombination; the primers fdmMf and fdmMr (supplemental Table S1) were used for cloning the aac(3)IV+oriT cassette used in fdmM inactivation. The ΔfdmM in-frame-deleted fdm heterologous expression plasmid pBS4052 was obtained by excising the aac(3)IV+oriT cassette with XbaI and self-ligation. Correct construction of pBS4052 was confirmed by DNA sequencing and PCR using primers fdmMM1a and fdmMM1b (supplemental Table S1 and Fig. S2). The ΔfdmM recombinant mutant strain SB4021 was afforded by transforming pBS4052 into S. albus J1074.
The ΔfdmM1 in-frame-deleted fdm heterologous plasmid pBS4053 was constructed from pBS4028 in a similar way as pBS4052 using the primers fdmM1f and fdmM1r (supplemental Table S1). Following confirmation by sequencing and PCR using fdmMM1a and fdmMM1b as primers (supplemental Table S1), pBS4053 was transformed to S. albus to afford the ΔfdmM1 recombinant mutant strain SB4022 (supplemental Fig. S3).
Complementation of SB4021 and SB4022
The 2.5-kb BamHI/NcoI fragment containing fdmKLM was excised and inserted into the same sites of pET28a. The resultant plasmid was cut off a 0.7-kb ApaLI fragment and self-ligated. Then, the entire fdmM gene together with its native promoter was removed as a 1.9-kb EcoRI/XbaI fragment and inserted into the same sites of pKC1218 to generate pBS4054. Plasmid pBS4054 was introduced into SB4021 by E. coli-Streptomyces conjugation, affording the ΔfdmM-complemented strain SB4023.
The 1.0-kb EcoRV/KpnI fragment, containing fdmM1 and its native promoter, was inserted into the same sites of pET29a. The same locus was then removed as a 1.1-kb EcoRI/XbaI fragment and inserted into the same sites of pKC1218 to yield pBS4055. Introduction of pBS4055 into SB4021 finally afforded the ΔfdmM1 complemented strain SB4024.
Overexpression of fdmR1 in SB4021 and SB4022
Plasmid pBS4045 (19) was cut by HindIII and blunted by DNA polymerase I large (Klenow) fragment (Promega). A 2.5-kb fragment containing fdmR1 downstream of ErmE* promoter was excised by EcoRI and inserted into the EcoRI/EcoRV sites of pSET152 to generate pBS4056. Introducing pBS4056 into SB4021 and SB4022 by E. coli-Streptomyces conjugation afforded SB4025 and SB4026, respectively.
Production and Isolation of 12, 13, and 14
Growth of S. albus SB4025 to produce 12, 13, and 14 was done in a fashion similar to that for FDM production in S. griseus (14). Briefly, the seed cultures were prepared by inoculating spores into R2YE medium and growing at 28 °C, 250 rpm for 1 day, added to the production medium (2%, v/v), and the resulting cultures were grown for 8 days under the same conditions. For preparation of compound 12 cultures were grown for 5 days instead of the usual 8 days. Cultures were acidified to pH 2.0 with 2 m HCl and centrifuged. The pellet (mycelia and any other precipitate) was harvested and extracted three times with acetone. Following solvent removal in vacuo, the resulting residue was extracted with EtOAc three times. Solvent removal in vacuo was followed by chromatography over a polyamide 6 column (Fluka, Steinheim, Germany) using CHCl3-MeOH-HCO2H (500:0:0, 230:20:0, 200:50:0, 200:50:0.1, and 400:100:1.0). Compound 14 was eluted by CHCl3-MeOH-HCO2H 200:50:0.1. However, 14 could not be cleanly separated from 13. Compounds 12 and 13 were co-eluted by CHCl3-MeOH-HCO2H 400:100:1.0. Fractions containing any one of the three compounds were concentrated and subjected to silica gel chromatography (230–400 mesh, Natland Inc., Morrisville, NC) eluted with CHCl3-MeOH-AcOH (250:0:0, 240:10:0, 230:20:0, 210:40:0, 200:50:0, and 200:50:0.5). Compound 14 was eluted by CHCl3-MeOH-AcOH 210:40:0, 12 was eluted by CHCl3-MeOH-AcOH 200:50:0, and 13 was eluted by CHCl3-MeOH-AcOH 200:50:0.5. Fractions were concentrated and resolved by semi-preparative HPLC on an Alltima C18 column (5 μm, 250 × 10 mm, Alltech, Deerfield, IL), eluted as described previously (14). Solvents tested for NMR data collection of 13 included acetone, MeOD, CDCl3, MeOD-CDCl3 in different ratios, DMSO, DMF, pyridine, and trifluoroacetic acid.
Production and Isolation of 15
Compound 15 was produced by SB4026 using the same culture procedures described for SB4025. Isolation of 15 was executed using the same procedure applied to 13 and 14, except that 15 was eluted from the polyamide 6 column with a solvent of 200:50:1.0 (CHCl3-MeOH-HCO2H). When subjected to silica gel chromatography, 15 was eluted by CHCl3-MeOH-AcOH 140:60:0.4 and was completely eluted by a solvent ratio of 140:60:1.0. Partially purified 15 was further refined by semi-preparative HPLC affording pure 15 for NMR spectroscopy.
FDM M-1 (12)
12, isolated as a yellow solid, afforded IR νmax (CHCl3) at 3409, 2933, 2851, 1617, 1594, 1437, 1377, 1354, 1259, 1191, 1143, 1073, 1001, 951, 808, 767, 704, 674 cm−1. APCI-MS yielded [M-H2O+H]+ and [M-H]− ions at m/z 525 and 541, respectively, and HR-ESI-MS yielded an [M-H]− ion at m/z 541.1479 (541.1504 calculated for the [M-H]− ion of molecular formula C31H25O9). For NMR data, see Table 1.
TABLE 1.
Summary of 1H and 13C NMR data for 12, 13, 14, 15, 16, and 18
| Position | 12 in DMSO |
13 in DMSO |
16 in TFA:CDCl3 (1:9) |
14 in DMSO |
18 in TFA:CDCl3 (1:9) |
15 in DMSO |
|||
|---|---|---|---|---|---|---|---|---|---|
| δH | δH | δH | δC | δH | δH | δC | δH | δC | |
| J in Hz | J in Hz | J in Hz | ppm | J in Hz | J in Hz | ppm | J in Hz | ppm | |
| 1 (COOH-1) | –a | –a | 168.4 | –a | 167.7 | –a | 171.8e | ||
| 2 | 104.5 | 104.5 | 115.5 | ||||||
| 3 | 160.0 | 158.8 | 162.5 | ||||||
| 4 | 119.4 | 119.4 | 121.4 | ||||||
| 5 | 137.4 | 139.0 | 127.5 | ||||||
| 6 | 8.29 (1H, s) | 8.65 (1H, s) | 8.51 (1H, s) | 124.6 | 9.16 (1H, s) | 8.81 (1H, s) | 120.4 | 150.0e | |
| 7 | 134.8 | 128.5 | 126.2 | ||||||
| 8 | 8.09 (1H, s) | 7.95 (1H, s) | 8.14 (1H, s) | 129.1 | 187.2 | 8.49 (1H, s) | 121.2 | ||
| 9 | 125.6 | 112.8 | 125.1 | ||||||
| 10 | 180.0 | 149.7 | 175.9 | ||||||
| 11 | 162.8 | 157.7 | 163.0 | ||||||
| OCH3-11 | 3.93 (3H, s) | 3.87 (3H, s) | 3.97 (3H, s) | 57.3 | 4.00 (3H, s) | 4.03 (3H, s) | 57.0 | 3.93 (3H, s) | 57.7 |
| 12 | 6.49 (1H, s) | 6.43 (1H, s) | 6.43 (1H, s) | 110.6 | 6.53 (1H, s) | 6.77 (1H, s) | 106.9 | 6.49 (1H, s) | 109.7 |
| 13 | 188.0 | 159.9 | 185.5 | ||||||
| 14 | 106.0 | 106.2 | 106.6 | ||||||
| 15 | 165.5 | 188.1 | 167.7 | ||||||
| 16 | 113.9 | 114.2 | 114.0 | ||||||
| 17 | 154.2 | 151.1 | 148.7 | ||||||
| 18 | 127.6 | 133.8 | 129.8 | ||||||
| 19 | –b | 2.84 (2H, br) | 2.99 (2H, br) | 20.7 | 2.88 (2H, br) | 2.96 (2H, br) | 20.2 | 2.84 (2H, br) | 22.3 |
| 20 | –b | 2.70 (2H, br) | 2.91 (2H)d | 30.4 | 2.75 (2H, br) | 2.92 (2H)d | 29.8 | 2.68 (2H, br) | 30.1 |
| 21 | 151.4 | 150.8 | 145.6 | ||||||
| 22 | 6.57 (1H, s) | 6.66 (1H, s) | 6.83 (1H, s) | 116.4 | 7.01 (1H, s) | 6.85 (1H, s) | 116.3 | 6.66 (1H, s) | 122.6 |
| 23 | 138.9 | 139.3 | 140.2 | ||||||
| 24 | 4.22 (2H, s) | 4.21 (2H, s) | 6.35 (1H, s) | 105.9 | 4.30 (2H, s) | 6.36 (1H, s) | 105.6 | 4.28 (2H, s) | 48.1 |
| 25 | 153.7 | 153.7 | 197.5 | ||||||
| 26 | 6.11 (1H, d, 15.5) | 6.09 (1H, d, 15.0) | 5.99 (1H, d, 15.0) | 119.2 | 6.16 (1H, d, 15.0) | 5.99 (1H, d, 15.0) | 118.8 | 6.17 (1H, d, 15.5) | 128.5 |
| 27 | 7.11 (1H, dd, 15.0, 9.5) | 7.18 (1H, m) | 6.98 (1H, dd, 15.5, 11.0) | 135.9 | 7.20 (1H, m) | 6.96 (1H, dd, 15.5, 11.0) | 135.8 | 7.21 (1H,dd,15.0, 9.5) | 142.7 |
| 28 | 6.19 (1H, m) | 6.25 (1H, m) | 6.19 (1H, m) | 130.7 | 6.31 (1H, m) | 6.17 (1H, m) | 130.8 | 6.32 (1H, m) | 131.1 |
| 29 | 6.18 (1H, m) | 6.24 (1H, m) | 6.09 (1H, dq, 13.0, 6.5) | 137.5 | 6.30 (1H, m) | 6.08 (1H, dq, 15.0, 6.5) | 137.4 | 6.30 (1H, m) | 140.5 |
| 30 | 1.78 (3H, d, 5.0) | 1.78 (3H, d, 5.0) | 1.88 (3H, d, 6.5) | 18.8 | 1.85 (3H, d, 4.5) | 1.89 (3H, d, 6.5) | 19.0 | 1.85 (3H, d, 4.5) | 19.3 |
| OH-3 | –c | –a | –a | 13.54 (1H, s)c | –a | –a | |||
| OH-6 | –a | ||||||||
| OH-10 | 12.67 (1H, s)c | 12.72 (1H, s)c | –a | ||||||
| OH-13 | 12.33 (1H, s)c | 12.67 (1H, s)c | –a | ||||||
| OH-15 | 12.31 (1H, s)c | –a | –a | –a | |||||
| OH-17 | 12.71 (1H, s)c | –a | –a | –c | –a | –a | |||
a Not observed.
b Overlapped HOD signal.
c Interchangeable.
d Overlap with DMSO signal, determined by HSQC.
e Determined by gHMBC.
FDM M-2 (13)
13, isolated as a purple solid, afforded IR νmax (CHCl3) at 3431, 2906, 2832, 1772, 1684, 1593, 1489, 1437, 1376, 1355, 1276, 1236, 1206, 1141, 1073, 1025, 845, 803, 760, 725, 674 cm−1. APCI-MS yielded [M+H]+ and [M-H]− ions at m/z 541 and 539, respectively, and HR-ESI-MS yielded an [M-H]− ion at m/z 539.1321 (539.1348 calculated for the [M-H]− ion of molecular formula C31H23O9). For NMR data, see Table 1.
FDM M-3 (14)
14, isolated as a red solid, afforded IR νmax (CHCl3) at 3177, 3021, 2939, 2845, 1869, 1771, 1718, 1676, 1660, 1592, 1475, 1435, 1402, 1378, 1366, 1313, 1260, 1205, 1189, 1166, 1129, 1098, 1070, 998, 986, 952, 892, 823, 786, 763, 745, 707, 687 cm−1. APCI-MS yielded [M+H]+ and [M-H]− ions at m/z 557 and 555, respectively, and HR-ESI-MS yielded an [M-H]− ion at m/z 555.1273 (557.1297 calculated for the [M-H]− ion of molecular formula C31H23O10). For NMR data, see Table 1.
FDM M1–1 (15)
15, isolated as a dark blue solid, afforded IR νmax (CHCl3) at 3385, 3223, 3016, 2941, 2843, 1664, 1599, 1437, 1387, 1254, 1234, 1197, 1115, 1069, 1023, 1000, 952, 852, 791, 755, 680, 665 cm−1. APCI-MS yielded an [M+H]+ ion at m/z 557, and HR-ESI-MS yielded [M-H]− ion at m/z 555.1269 (557.1297 calculated for the [M-H]− ion of molecular formula C31H23O10). For NMR data, see Table 1 and supplemental Table S1.
FDM M-4 (16)
16, isolated as a brown solid, afforded IR νmax (CHCl3) at 3384, 3015, 2880, 2837, 1684, 1618, 1558, 1490, 1437, 1320, 1204, 1146, 1025, 954, 801, 765, 722, 708, 688 cm−1. APCI-MS yielded [M+H]+ and [M-H]− ions at m/z 523 and 521, respectively, and HR-MALDI-FT-MS yielded an [M+H]+ ion at m/z 523.1396 (523.1387 calculated for the [M+H]+ ion of molecular formula C31H23O8). For NMR data, see Table 1.
FDM M-5 (18)
18, isolated as a dark red solid, afforded IR νmax (CHCl3) at 3429, 2983, 2905, 2842, 1869, 1783, 1684, 1618, 1559, 1542, 1521, 1507, 1474, 1458, 1437, 1407, 1376, 1314, 1260, 1208, 1165, 1145, 1016, 988, 962, 844, 804, 760, 725, 674 cm−1. APCI-MS yielded an [M+H]+ ion at m/z 539, and HR-MALDI-FT-MS yielded [M+H]+ and [M+Na]+ ions at m/z 539.1325 and 561.1148, respectively, (539.1337 and 561.1156 calculated for the [M+H]+ and [M+Na]+ ions, respectively, of molecular formula C31H23O9). For NMR data, see Table 1.
RESULTS
Construction and Evaluation of the ΔfdmM and ΔfdmM1 In-frame Deletion Mutants
Inactivation of both fdmM and fdmM1 was carried out on the basis of successful heterologous expression of the fdm cluster in S. albus (12). For fdmM, a 0.4-kb internal fragment of fdmM was replaced by the aac(3)IV+oriT cassette through λ RED-mediated PCR-targeting protocol (18) in plasmid pBS4028 (19). The cassette was then removed by XbaI leaving an 81-bp scar in the resultant construct pBS4052. The ΔfdmM in-frame deletion recombinant mutant strain SB4021 was generated by transforming pBS4052 into S. albus. When cultured in production medium, SB4021 failed to produce 1 and 10 but instead yielded three new compounds FDM M-1 (12), FDM M-2 (13), and FDM M-3 (14) in very low titer. SB4021 was complemented by in trans expression of fdmM with its native promoter to afford SB4023, in which production of 1 and 10 was restored with the concomitant disappearance of 12, 13, and 14. Titers of 12, 13, and 14 were dramatically increased (>12-fold) by overexpression of an fdm pathway specific activator fdmR1 (19) in SB4021 to afford SB4025 (Fig. 3a). The titer enhancement in SB4025 vastly hastened isolation and characterization of the three accumulated compounds.
FIGURE 3.
HPLC traces of metabolite profiles from selected recombinant S. albus strains. a, SB4006, S. albus strain harboring the intact fdm cluster; SB4021, S. albus harboring the mutated fdm cluster with the ΔfdmM in-frame deletion; SB4025, SB4021 overexpressing fdmR1; SB4023, SB4021 complemented by expressing fdmM in trans. b, SB4006; SB4022, S. albus harboring the mutated fdm cluster with the ΔfdmM1 in-frame deletion; SB4026, SB4022 with overexpressing fdmR1; SB4024, SB4022 complemented by expressing fdmM1 in trans. (▾) FDM A (1); (▿) FDM E (10); (•) FDM M-1 (12); (○) FDM M-2 (13); (♦) FDM M-3 (14); (♢) FDM M1-1 (15). Different wavelengths were used in panels a and b to maximize the detection sensitivity to the compounds of interest.
The ΔfdmM1 in-frame deletion expression plasmid pBS4053 was constructed from pBS4028 in a fashion similar to that used to make pBS4052, affording the mutated fdm cluster with a 0.4-kb internal fragment of fdmM1 being substituted with an 81-bp scar. Transformation of pBS4053 into S. albus afforded the ΔfdmM1 in-frame deletion recombinant mutant strain SB4022, in which production of 1 and 10 was abolished. HPLC analysis of the SB4022 metabolite profile revealed two minor peaks at 12.8 and 15.4 min. When the activator FdmR1 was overproduced in SB4022 to afford SB4026, the titer of FDM M1-1 (15), with retention time at 12.8 min, was increased by ∼25-fold. In contrast, there was almost no change for the compound eluting at 15.4 min in SB4022 and SB4026, implying that this compound has no relationship to the fdm biosynthetic pathway. Complementation of the ΔfdmM1 mutation was achieved by introducing a plasmid, pBS4055 carrying fdmM1 with its native promoter into SB4022, restoring the production of both 1 and 10 (Fig. 3b).
Isolation and Characterization of 12, 13, and 14
Compounds 12, 13, and 14 were extracted from the pellet of acidified SB4025 culture with acetone. Following re-extraction with ethyl acetate, the concentrated extract was subjected to several rounds of chromatography. The three compounds were separated by silica gel chromatography and refined using semi-preparative reverse phase HPLC. Compounds 12, 13, and 14 were isolated as yellow, purple, and red solids, respectively. Compound 12 was found to be very unstable; compounds 13 and 14 showed extremely poor solubility in almost all common solvents.
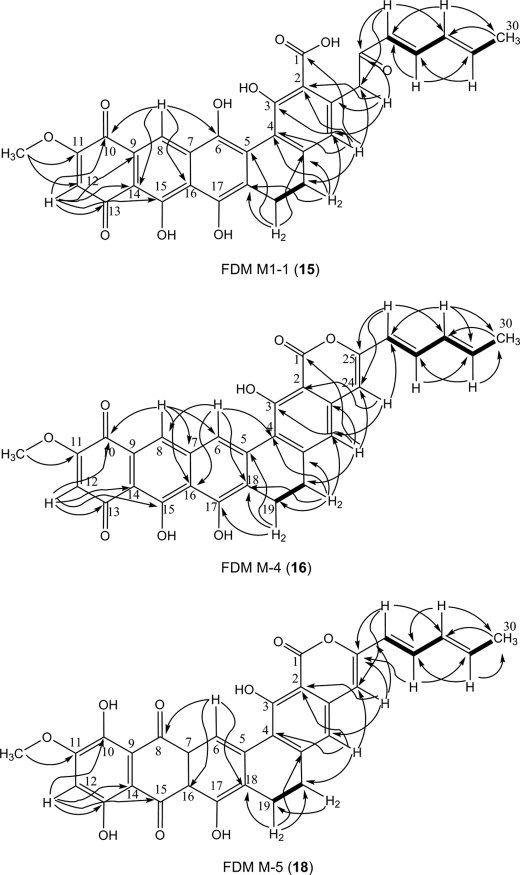
HR ESI-MS analysis of analytically pure 13 afforded an [M-H]− ion at m/z 539.1321 consistent with the molecular formula C31H23O9 (calculated 539.1348 for the [M-H]− ion). The limited solubility of 13 dictated that only 1H NMR data of this molecule could be obtained (Table 1). After exhaustive testing, 13 was found to be soluble in trifluoroacetic acid. However, the use of trifluoroacetic acid rapidly and quantitatively converted 13 to the dehydration product FDM M-4 (16) (Fig. 2). The mass of 16 obtained by HR MALDI-FT-MS was determined to be m/z 523.1396 [M+H]+, in accordance with the formula C31H23O8 (calculated 523.1387) for the [M+H]+ ion. Inspection of the 1H, 13C, and two-dimensional (gCOSY, HSQC, and gHMBC) NMR spectra of 16 enabled full correlations of all C and H signals (Table 1 and Fig. 4). The right half of 16 (C-1 to C-4 and C-19 to C-30) showed NMR data almost identical to that of previously reported FDM C1 (17) (13), strongly supporting the notion that 16 and 17 possess the same ring D, E, F, and diene side chain structures. Ring A of 16 was assigned to be in the quinone oxidation state based on gHMBC correlations from H-12 (6.43 ppm) to C-10 (180.0 ppm) and C-13 (188.0 ppm). Comparison of the 1H NMR spectra of 16 and 17 revealed that 16 possessed two extra proton signals at 8.14 ppm and 8.51 ppm. One proton was assigned as H-8 (8.14 ppm) in ring B based on its HSQC correlation to C-8 (129.1 ppm) and gHMBC correlations to C-10 and C-14 (106 ppm); the other was determined as H-6 (8.51 ppm) by its HSQC correlation to C-6 (124.6 ppm) and gHMBC correlations to C-4 (119.4 ppm), C-8, and C-18 (127.6). The structures of ring B and C of 16 were confirmed by gHMBC correlations originating from H-6, H-8, and H-12 (Fig. 4). The angular ring structure of 16, supported by NMR data, was consistent with the proposed FDM biosynthetic pathway. Comparison of the molecular weight of 13 and 16 suggested that 16 was generated from 13 via dehydrative cyclization (Fig. 2). This was confirmed by analysis of the 1H NMR spectra of 13 and 16. The two α-proton signals at C-24 (4.21 ppm) of 13 were shifted and replaced to a single proton signal at C-24 (6.35 ppm) of 16, supporting the formation of lactone ring F in going from 13 to 16. Further evidence supporting this transformation was that 16 lacked the peak at 1593 cm−1 present in the IR spectrum of 13; the C-25 keto group of 13 is absent in 16.
FIGURE 4.
Selected gCOSY (bold lines) and gHMBC (arrows) correlations of FDM M1-1 (15), FDM M-4 (16), and FDM M-5 (18).
HR ESI-MS analysis of 14 revealed an [M-H]− ion at m/z 555.1273, consistent with formula C31H23O10 (calculated 555.1297 for the [M-H]− ion). Only the 1H NMR data of 14 was obtained due to the poor solubility of this compound (Table 1). When dissolved in anhydrous trifluoroacetic acid, 14 was converted to FDM M-5 (18) with m/z 539.1325 for the [M+H]+ ion as detected by HR MALDI-FT-MS (Fig. 2). The mass detected for 18 supported its formula as C31H23O9 (calculated 539.1337 for the [M+H]+ ion). Analyses of the 1H, 13C, and two-dimensional (gCOSY, HSQC, and gHMBC) NMR spectra of 18 enabled us to propose its structure (Table 1 and Fig. 4). The only difference between 17 and 18 is the C-6 hydroxyl group of 17, which is absent in 18. This was consistent with the almost identical nature of 1H and 13C NMR data for 17 and 18 except for data relating to ring C. The 1H NMR signal at 8.81 ppm, HSQC correlation to C-6 (120.4 ppm) and gHMBC correlations from H-6 to C-8 (187.2 ppm) and C-18 (133.8 ppm) support the C-6 phenyl proton of 18. Compound 18 was proposed to be the dehydrated derivative of 14 according to their mass difference. By analogy to the conversion of 13 to 16, dehydrative cyclization of 14 affords the lactone ring F of 18. As with 16, this is substantiated by the absence of the carbonyl IR stretch at 1592 cm−1 for 18, which is present in 14.
Compound 12 was found to be very unstable and, consistent with its predicted reactivity, converts to oxidized species 13 and 14 during culture and isolation. Accordingly, SB4025 was cultured in production medium for 5 days, instead of 8 days, to optimize the chance of isolating 12. All manipulations were carried out under 20 °C to minimize loss of 12. Subsequent HR ESI-MS analysis of purified 12 revealed an [M-H]− ion at m/z 541.1479, consistent with formula C31H25O9 (calculated 541.1504 for the [M-H]− ion). The tentative formula for 12 bore two protons more than 13. 1H NMR of 12 (Table 1) revealed a spectrum almost identical to that of 13, with the notable exception that 12 was determined to be in the hydroquinone oxidation state. This structure assignment was supported by the fact that 12 can be converted to 13 and 14 spontaneously in air. Furthermore, IR spectra revealed that 12 lacks IR bands typically associated with the presence of quinones (1684 cm−1 in 13 and 1676 cm−1 in 14).
Conversion of 12 to 13 and 14
During the growth of SB4025 in production medium it was noted that 12 is produced prior to 13 and 14. It was also observed that 12 was very unstable and was primarily converted to 13 during the course of isolations at room temperature. When 12 was dissolved in acetone (922 μm) under aerobic conditions at 30 °C for 6 h, it was almost completely converted to 13 and 14 in a ratio of 1:3 (supplemental Fig. S4a). Analogous conditions devoid of O2 led to significantly reduced production of 13 and 14 from 12 (supplemental Fig. S4b). To exclude the possibility that 12 was converted to 14 via 13 as an intermediate, a 370 μm solution of 13 in acetone was exposed to air at 30 °C for 6 h, followed by HPLC analysis of the mixture. No loss of 13 was observed nor was any new product detected.
Isolation and Characterization of 15
Compound 15, a dark blue solid, was isolated from SB4026 culture in a process similar to that used for 13 and 14. It was proposed as an isomer of 14, in that its molecular formula was also determined to be C31H23O10 based on high-resolution ESI-MS (calculated 555.1297 for [M-H]− ion), which compared favorably to the acquired [M-H]− ion of m/z 555.1269 for 15. Analyses of the 1H, 13C, and two-dimensional (gCOSY, HSQC, and gHMBC) NMR spectra for 15 in DMSO enabled structural assignment (Table 1 and Fig. 4). This molecule has the same right hemisphere as 9 (rings C, D, and E and the diene moiety) as supported by almost identical 1H and 13C NMR resonances for C-1 to C-4 and C-19 to C-30. The ring A of 15 was assigned to be in the quinone oxidation state based on gHMBC correlations originating from H-12 (6.49 ppm) to C-10 (175.9 ppm) and C-13 (185.5 ppm). This assignment was confirmed by the similarity of 1H and 13C NMR data to that of the ring A of 16. H-8 was assigned based on the chemical shift at 8.49 ppm in 1H NMR, HSQC correlation to C-8 (121.2 ppm) and the gHMBC correlations from H-8 to C-6 (150 ppm) and C-10. The structure of ring B was determined by the gHMBC correlations from H-8 and H-12 while the structure of ring C was elucidated according to the gHMBC correlations originating from H-8 to C-6 and C-16 (114 ppm) and from H-19 (2.84 ppm) to C-5 (127.5 ppm) and C-18 (129.8 ppm). The structure of 15 as elucidated on the basis of NMR data acquired in d6-DMSO was confirmed by NMR experiments conducted in CDCl3-MeOD (supplemental Table S2).
DISCUSSION
The FDMs are aromatic polyketides originating from a pentadecaketide (C-30) precursor, representing aromatic polyketides derived from the longest polyketide chain whose biosynthetic machinery has been characterized. With its unique carbaspirocycle, 1 exhibits potent cytotoxicities against selected cancer cell lines, exerts its activities via several novel mode of actions, and has been studied as a new anticancer drug lead (14). Biosynthesis of 1 includes several oxidative reactions, which cannot be assigned to specific enzymes on the basis of bioinformatics analysis of the gene cluster alone (12). These features of 1 heighten the interest in investigations of the biosynthetic pathway for this unique natural product. Feeding experiments (20) and cloning of its biosynthetic gene cluster confirm the polyketide origin of 1 (12). Characterization of intermediates 8, 9, and 10 unveils the final steps en route to 1 and suggests that its carbaspirocycle moiety is formed through a benzilic acid rearrangement process from 10 to 1 (Fig. 2 and Ref. 14). The pathway from 7 to 9, previously unaccounted for, is now significantly illuminated by this work.
Inactivation of two functionally unknown genes, fdmM and fdmM1, was facilitated by successful expression of the fdm cluster in S. albus, a model heterologous host with expedient enabling tools for in vivo genetic pathway manipulations (12). Abolishment of 1 and 10 production in each of the mutant strains could be readily complemented confirming that both genes are essential to FDM biosynthesis. The titers of compounds accumulated in both ΔfdmM and ΔfdmM1 in-frame deletion mutant strains were dramatically improved by overexpression of the well studied activator fdmR1 (17). Compound 15 was isolated from SB4026, the ΔfdmM1 mutant overexpressing FdmR1. Structural comparison of 9 and 15 suggests that FdmM1 catalyzes C-8 hydroxylation of 15 followed by tautomerization to afford 9 (Fig. 2). Compounds 12, 13, and 14 were produced by SB4025, the ΔfdmM in-frame deletion recombinant mutant strain in which FdmR1 was overproduced. Compound 12 was unstable, rapidly oxidizing to both 13 and 14, under aerobic conditions. The enrichment of 12 prior to 13 and 14 observed during culture, suggests that 12 is the intermediate accumulated in SB4025 whereas 13 and 14 are shunt products derived from 12 by spontaneous oxidization. There are two proposed pathways from 12 to 15 (Fig. 2). The first invokes that 12 is either spontaneously or enzymatically oxidized to 13. Subsequent C-6 hydroxylation of 13 by FdmM would afford 15 (Route A). A second route (Route B) that cannot be excluded, invokes C-6 hydroxylation of 12 by FdmM prior to formation of the quinone-type ring A. Accumulation of 12 in SB4025 clearly demonstrates that the modification steps, including ring D reduction, O-11 methylation, and C-10 hydroxylation, occur prior to C-6 and C-8 hydroxylation.
Investigation of aromatic polyketide biosyntheses revealed that their hydroxylation steps could be accomplished by a diverse set of monooxygenases, including the typical phenol hydroxylase-like FAD-dependent monooxygenase (e.g. RdmE catalyzing aklavinone-11-hydroxylation in Streptomyces purpurascens) (21) and some novel oxygenases. Aclacinomycin 10-hydroxylase involved in rhodomycin biosynthesis in S. purpurascens was reported as the first unveiled hydroxylase using S-adenosyl-l-methionine as a cofactor (22). SnoaL2 and AclR are proposed to be hydroxylases responsible for the conversion from aklavinone to 1-hydroxyaklavinone in nogalamycin and cinerubin biosynthetic pathways, respectively. These hydroxylases are postulated to be evolutionarily related to cyclases based on their primary and tertiary structures (23). However, little is known about the hydroxylation of aromatic polyketide natural products derived from polyketide chains longer than 18 carbons prior to this work. Bioinformatics analysis of the biosynthetic gene clusters for these aromatic polyketide natural products fell short of identifying candidates to account for all of the proposed oxidative steps. Instead, these clusters are often characterized with several genes whose deduced products show no sequence homology to proteins of known functions, as exemplified by FdmM and FdmM1.
Characterization of the compounds accumulated from the ΔfdmM and ΔfdmM1 mutant strains has enabled us to assign FdmM and FdmM1 as hydroxylases involved in the biosynthesis of 9. Homologs of FdmM and FdmM1 are known, such as BenG (7), LlpB (8), LlpQ (8), Prm-Orf5 (9), GrhM (10), and RubQ (11) (supplemental Fig. S1) involved in the biosynthesis of other aromatic polyketide natural products ranging from dodecaketides to pentadecaketides, and, like FdmM and FdmM1; however, they were all annotated previously as proteins of unknown function. Based on the findings reported here for FdmM and FdmM1, we now propose similar functions for these proteins, catalyzing region-specific hydroxylations in their respective biosynthetic pathways.
Alignment of FdmM and FdmM1 and their homologs reveals that a conserved motif (G/AXGXXAG) embedded within the N-terminal domain of most of these proteins (supplemental Fig. S3), which is reminiscent of the classical N-terminal Rossman fold characteristic of FAD-dependent oxygenases (24). However, the small size of FdmM (149 amino acids) and FdmM1 (154 amino acids) distinguishes these enzymes from the FAD-dependent oxygenases (e.g. p-hydroxybenzoate hydroxylase from E. coli, 394 amino acids) (25). Very few of the known oxygenases are as small as FdmM and FdmM1, with the exception of the aforementioned TcmH family of metal- and cofactor-free monooxygenases (2). Future efforts are clearly warranted to determine the cofactor dependence of FdmM and FdmM1 and to ensure a complete mechanistic understanding of this family of novel oxygenases.
Previous cytotoxity assays of the carbaspirocyclic FDM analogs 1 and 10 revealed that they exhibited comparable bioactivities against the selected cancer cell lines (14). Bioassays of newly isolated planar FDM intermediates 13, 14, and 15, as well as 9, showed their cytotoxicities to be compromised (ranging from one to three orders of magnitude less relative to 1). The significantly reduced potency observed for 9 and 13-15 relative to 1 supports previous assertions that the carbaspirocyclic center of the FDMs is crucial to their bioactivity (14).
Taken together, our ability to correlate specific gene inactivation events, as exemplified here with FdmM and FdmM1, with specific structural modifications to FDM A, supports the significance of S. albus as a heterologous host for combinatorial biosynthesis efforts to produce new FDM analogs. More importantly, the data resulting from this work highlight FdmM and FdmM1 as novel oxygenases previously assigned simply as proteins of unknown function. FdmM and FdmM1 catalyze hydroxylations at the C-6 and C-8 positions of FDM A and, in the heterologous host S. albus, are compatible to genetic inactivations during overexpression of the pathway specific activator FdmR1. The coordination of genetic inactivations and tandem FdmR1 overexpression within a heterologous host highlights the plasticity of the fdm biosynthetic gene cluster establishing an excellent stage for future investigations of FDM A biosynthesis and engineering.
Supplementary Material
Acknowledgments
We thank Dr. José A. Salas, University of Oviedo, Spain for the S. albus J1074 strain, the Analytical Instrumentation Center of the School of Pharmacy, UW-Madison and the Biotechnology Center, UW-Madison for support in obtaining MS and NMR data, the UW Paul P. Carbone Comprehensive Cancer Center SMSF for acquisition of cytotoxicity data, and the John Innes Center, Norwich, UK, for providing the λ RED-mediated PCR-targeting mutagenesis kit.
This work was supported, in whole or in part, by National Institutes of Health Grant CA113297.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables S1 and S2 and Figs. S1–S4.
- FDM
- fredericamycin
- ACPI-MS
- atmospheric pressure chemical ionization MS
- gCOSY
- 1H-1H correlation spectroscopy
- gHMBC
- heteronuclear multiple bond correlation
- HR-ESI-MS
- high resolution electrospray ionization mass spectroscopy
- HSQC
- heteronuclear single quantum coherence
- MALDI-FT-MS
- matrix-assisted laser desorption ionization-Fourier transform-MS
- MOPS
- 4-morpholinepropanesulfonic acid.
REFERENCES
- 1.Shen B., Hutchinson C. R. (1993) Biochemistry 32, 6656–6663 [DOI] [PubMed] [Google Scholar]
- 2.Fetzner S. (2002) Appl. Microbiol. Biotechnol. 60, 243–257 [DOI] [PubMed] [Google Scholar]
- 3.Widboom P. F., Fielding E. N., Liu Y., Bruner S. D. (2007) Nature 447, 342–345 [DOI] [PubMed] [Google Scholar]
- 4.Funa N., Funabashi M., Yoshimura E., Horinouchi S. (2005) J. Biol. Chem. 280, 14514–14523 [DOI] [PubMed] [Google Scholar]
- 5.Pandey R. C., Toussaint M. W., Stroshane R. M., Kalita C. C., Aszalos A. A., Garretson A. L., Wei T. T., Byrne K. M., Geoghegan R. F., Jr., White R. J. (1981) J. Antibiot. 34, 1389–1401 [DOI] [PubMed] [Google Scholar]
- 6.Misra R., Pandey R. C. (1982) J. Am. Chem. Soc. 104, 4478–4479 [Google Scholar]
- 7.Kim B. C., Lee J. M., Ahn J. S., Kim B. S. (2007) J. Microbiol. Biotechnol. 17, 830–839 [PubMed] [Google Scholar]
- 8.Bockholt H., Udvarnoki G., Rohr J., Mocek U., Beale J. M., Floss H. G. (1994) J. Org. Chem. 59, 2064–2069 [Google Scholar]
- 9.Li A., Piel J. (2002) Chem. Biol. 9, 1017–1026 [DOI] [PubMed] [Google Scholar]
- 10.Xu Z., Schenk A., Hertweck C. (2007) J. Am. Chem. Soc. 129, 6022–6030 [DOI] [PubMed] [Google Scholar]
- 11.Martin R., Sterner O., Alvarez M. A., de Clercq E., Bailey J. E., Minas W. (2001) J. Antibiot. 54, 239–249 [DOI] [PubMed] [Google Scholar]
- 12.Wendt-Pienkowski E., Huang Y., Zhang J., Li B., Jiang H., Kwon H., Hutchinson C. R., Shen B. (2005) J. Am. Chem. Soc. 127, 16442–16452 [DOI] [PubMed] [Google Scholar]
- 13.Sontag B., Müller J. G., Hansske F. G. (2004) J. Antibiot. 57, 823–828 [DOI] [PubMed] [Google Scholar]
- 14.Chen Y., Luo Y., Ju J., Wendt-Pienkowski E., Rajski S. R., Shen B. (2008) J. Nat. Prod. 71, 431–437 [DOI] [PubMed] [Google Scholar]
- 15.Schenk A., Xu Z., Pfeiffer C., Steinbeck C., Hertweck C. (2007) Angew. Chem. Int. Ed. Engl. 46, 7035–7038 [DOI] [PubMed] [Google Scholar]
- 16.Kieser T., Bibb M. J., Buttner M. J., Chater K. F., Hopwood D. A. (2000) Practical Streptomyces Genetics, The John Innes Foundation, Norwich, UK [Google Scholar]
- 17.Sambrook J., Fritsch E. F., Maniatis T. (2000) Molecular Cloning: A Laboratory Manual, 3rd Ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 18.Gust B., Challis G. L., Fowler K., Kieser T., Chater K. F. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 1541–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y., Wendt-Pienkowski E., Shen B. (2008) J. Bacteriol. 190, 5587–5596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byrne K. M., Hilton B. D., White R. J., Misra R., Pandey R. C. (1985) Biochemistry 24, 478–486 [DOI] [PubMed] [Google Scholar]
- 21.Niemi J., Wang Y., Airas K., Ylihonko K., Hakala J., Mäntsälä P. (1999) Biochem. Biophys. Acta 1430, 57–64 [DOI] [PubMed] [Google Scholar]
- 22.Jansson A., Koskiniemi H., Erola A., Wang J., Mäntsälä P., Schneider G., Niemi J. (2005) J. Biol. Chem. 280, 3636–3644 [DOI] [PubMed] [Google Scholar]
- 23.Beinker P., Lohkamp B., Peltonen T., Niemi J., Mäntsälä P., Schneider G. (2006) J. Mol. Biol. 359, 728–740 [DOI] [PubMed] [Google Scholar]
- 24.Kleiger G., Eisenberg D. (2002) J. Mol. Biol. 323, 69–76 [DOI] [PubMed] [Google Scholar]
- 25.Gatti D. L., Entsch B., Ballou D. P., Ludwig M. L. (1996) Biochemistry 35, 567–578 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.