Abstract
The alpha 6 beta 4 integrin is structurally distinct from all the other known integrins because the cytoplasmic domain of beta 4 is unusually large and contains four type III fibronectin-like modules toward its C-terminus. To examine the function of the beta 4 cytoplasmic tail, we have expressed full-length and truncated human beta 4 cDNAs in rat bladder epithelial 804G cells, which form hemidesmosome-like adhesions in vitro. The cDNA encoded wild-type beta 4 subunit associated with endogenous alpha 6 and was recruited at the cell surface within hemidesmosome-like adhesions. A recombinant form of beta 4, lacking almost the entire cytoplasmic domain associated with alpha 6, reached the cell surface but remained diffusely distributed. A beta 4 molecule lacking almost the entire extracellular portion did not associate with alpha 6 but was correctly targeted to the hemidesmosome-like adhesions. Thus, the cytoplasmic portion of beta 4 contains sequences that are required and may be sufficient for the assembly of the alpha 6 beta 4 integrin into hemidesmosomes. To localize these sequences we examined the properties of additional mutant forms of beta 4. A truncated beta 4 subunit, lacking the most C-terminal pair of type III fibronectin homology domains, was incorporated into hemidesmosome-like adhesions, but another recombinant beta 4 molecule, lacking both pairs of type III fibronectin repeats, was not. Finally a recombinant beta 4 molecule, which was created by adjoining the region of the cytoplasmic domain including all type III repeats to the transmembrane segment, was efficiently recruited in hemidesmosome-like adhesions. Taken together these results suggest that the assembly of the alpha 6 beta 4 integrin into hemidesmosomes is mediated by a 303-amino acid region of beta 4 tail that comprises the first pair of type III fibronectin repeats and the segment between the second and third repeats. These data imply a function of a specific segment of the beta 4 cytoplasmic domain in interaction with cytoskeletal components of hemidesmosomes.
Full text
PDF


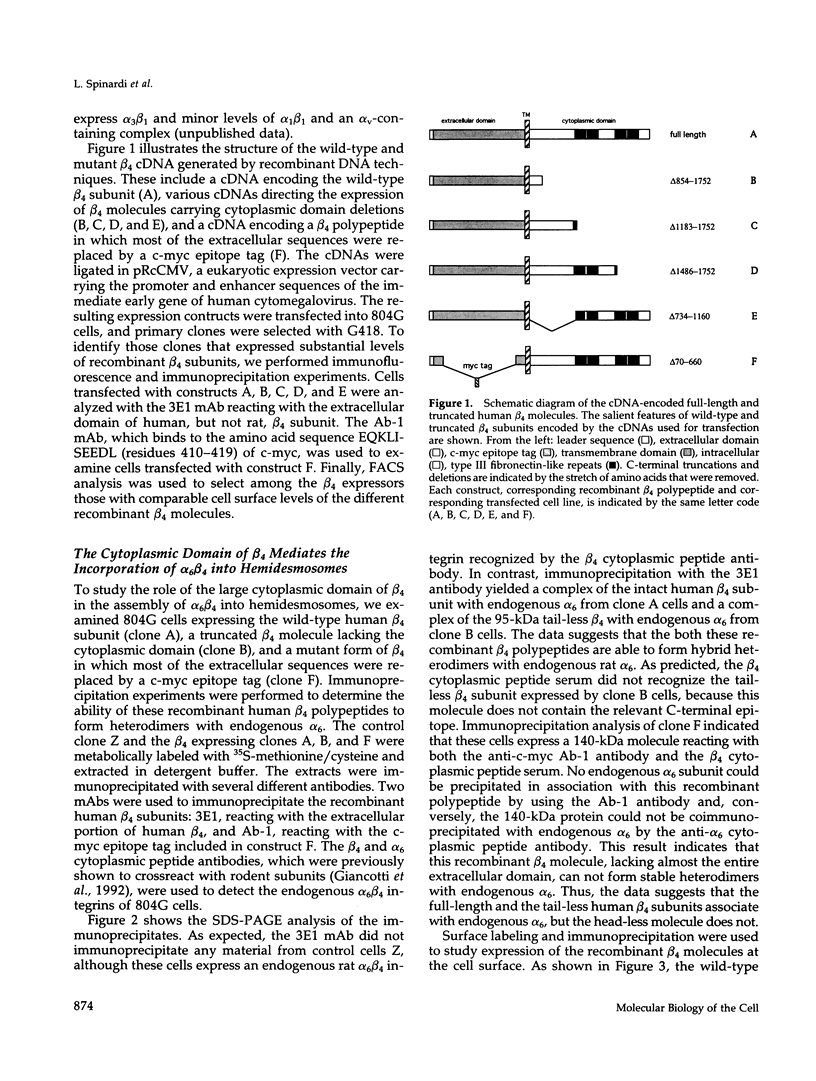
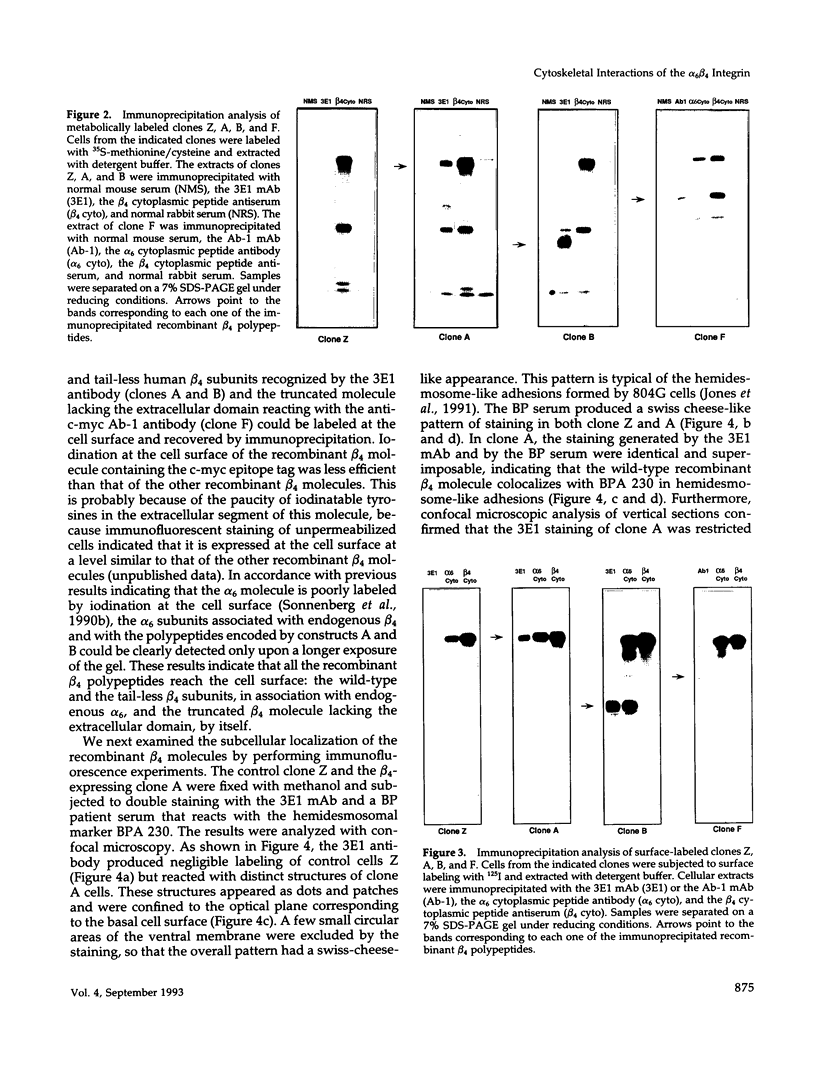
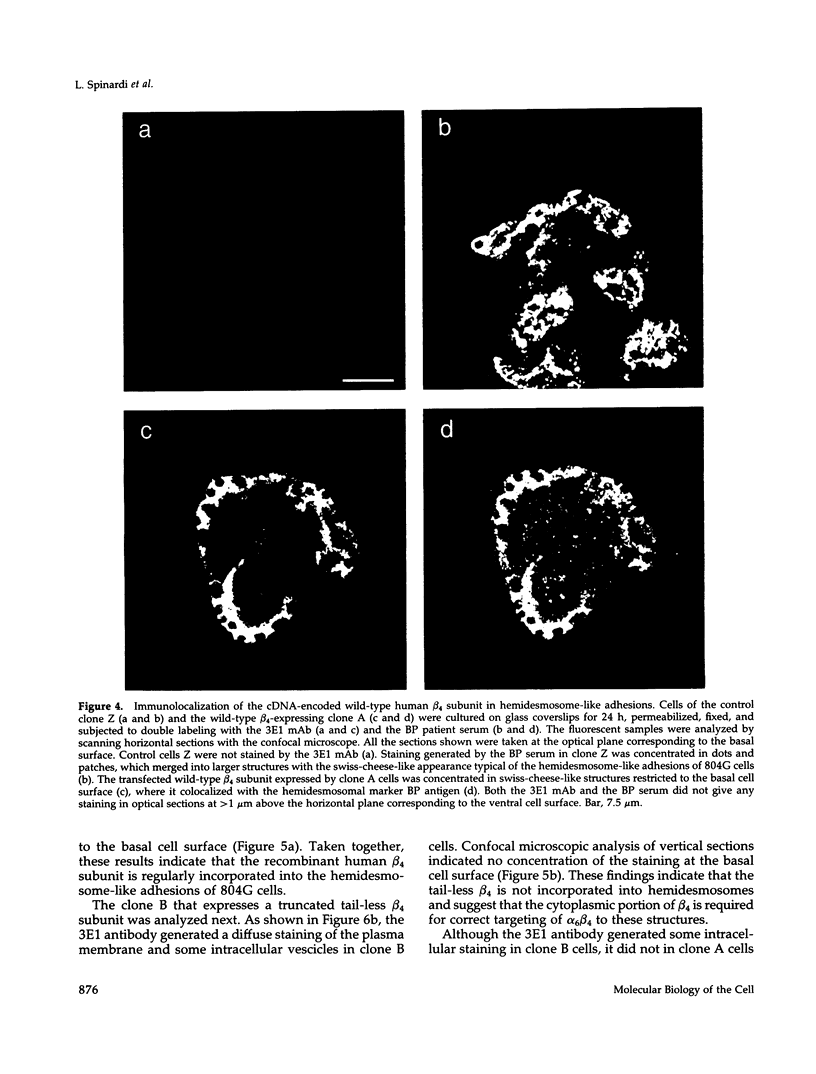



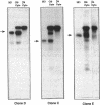
Images in this article
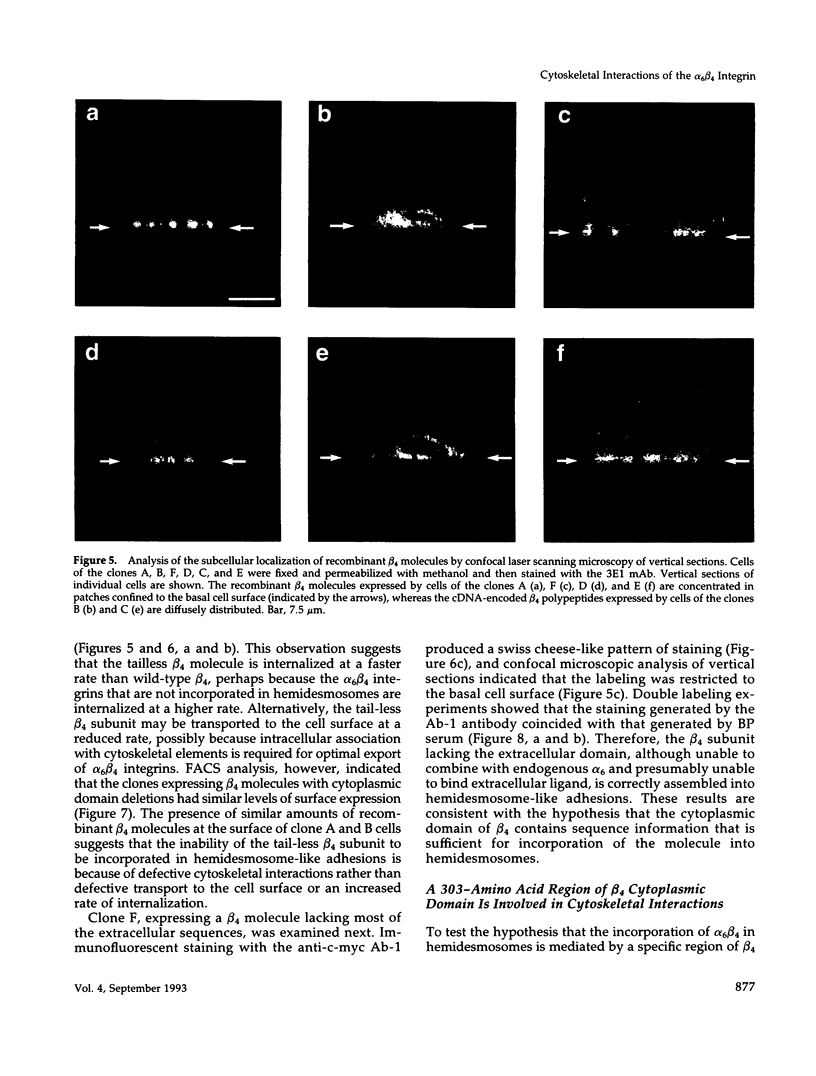
Selected References
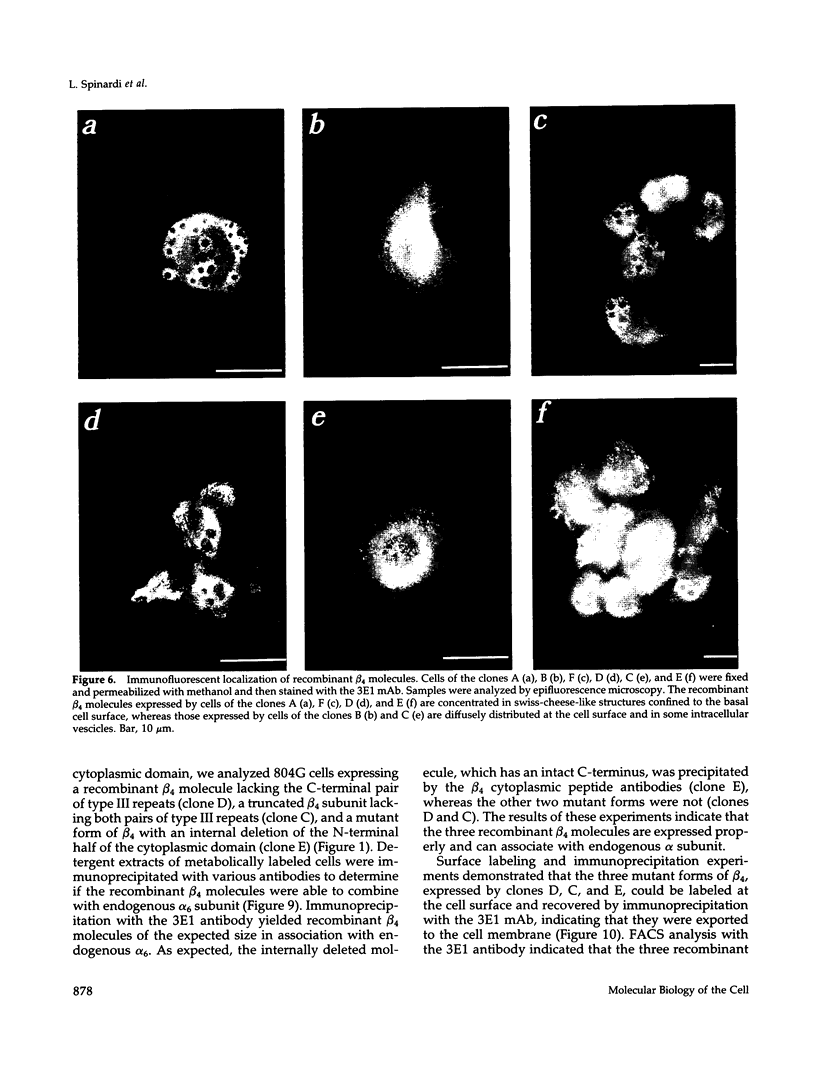
These references are in PubMed. This may not be the complete list of references from this article.
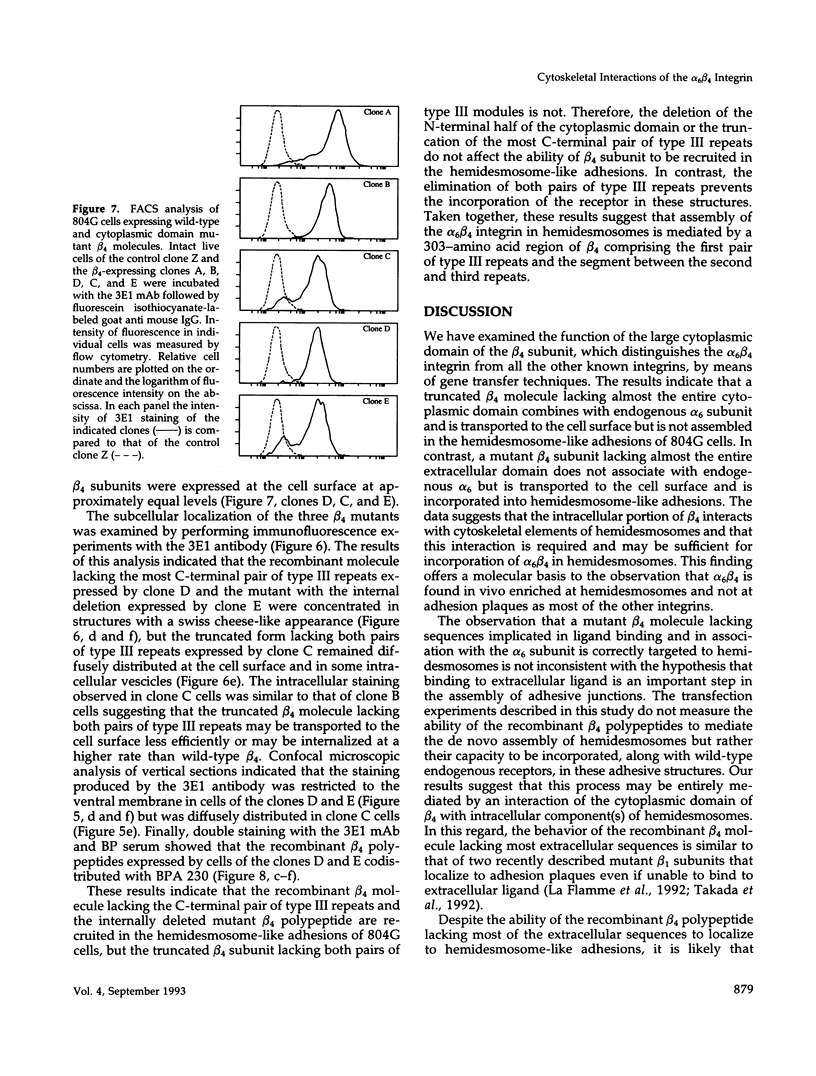
- Benian G. M., Kiff J. E., Neckelmann N., Moerman D. G., Waterston R. H. Sequence of an unusually large protein implicated in regulation of myosin activity in C. elegans. Nature. 1989 Nov 2;342(6245):45–50. doi: 10.1038/342045a0. [DOI] [PubMed] [Google Scholar]
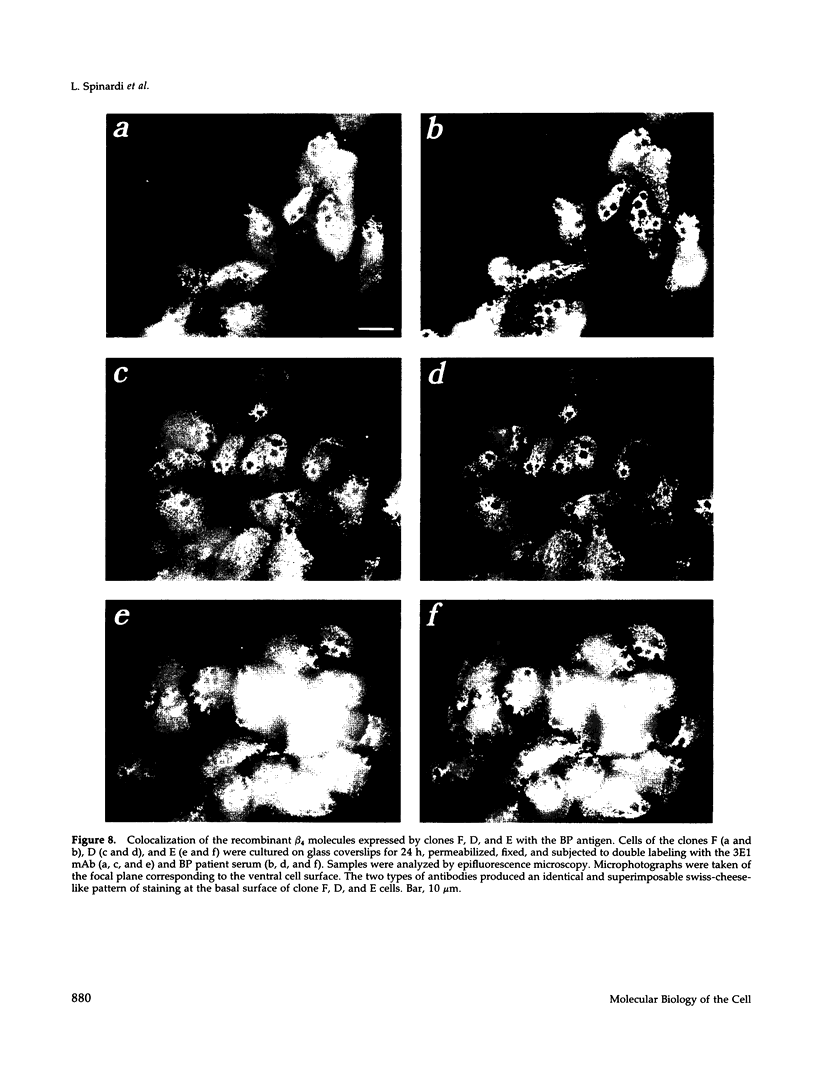
- Buck C. A., Horwitz A. F. Cell surface receptors for extracellular matrix molecules. Annu Rev Cell Biol. 1987;3:179–205. doi: 10.1146/annurev.cb.03.110187.001143. [DOI] [PubMed] [Google Scholar]
- Burn P., Kupfer A., Singer S. J. Dynamic membrane-cytoskeletal interactions: specific association of integrin and talin arises in vivo after phorbol ester treatment of peripheral blood lymphocytes. Proc Natl Acad Sci U S A. 1988 Jan;85(2):497–501. doi: 10.1073/pnas.85.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K., Fath K., Kelly T., Nuckolls G., Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Burridge K., Petch L. A., Romer L. H. Signals from focal adhesions. Curr Biol. 1992 Oct;2(10):537–539. doi: 10.1016/0960-9822(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Carter W. G., Kaur P., Gil S. G., Gahr P. J., Wayner E. A. Distinct functions for integrins alpha 3 beta 1 in focal adhesions and alpha 6 beta 4/bullous pemphigoid antigen in a new stable anchoring contact (SAC) of keratinocytes: relation to hemidesmosomes. J Cell Biol. 1990 Dec;111(6 Pt 2):3141–3154. doi: 10.1083/jcb.111.6.3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter W. G., Wayner E. A., Bouchard T. S., Kaur P. The role of integrins alpha 2 beta 1 and alpha 3 beta 1 in cell-cell and cell-substrate adhesion of human epidermal cells. J Cell Biol. 1990 Apr;110(4):1387–1404. doi: 10.1083/jcb.110.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan B. M., Kassner P. D., Schiro J. A., Byers H. R., Kupper T. S., Hemler M. E. Distinct cellular functions mediated by different VLA integrin alpha subunit cytoplasmic domains. Cell. 1992 Mar 20;68(6):1051–1060. doi: 10.1016/0092-8674(92)90077-p. [DOI] [PubMed] [Google Scholar]
- Chen W. T., Hasegawa E., Hasegawa T., Weinstock C., Yamada K. M. Development of cell surface linkage complexes in cultured fibroblasts. J Cell Biol. 1985 Apr;100(4):1103–1114. doi: 10.1083/jcb.100.4.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsky C. H., Knudsen K. A., Bradley D., Buck C. A., Horwitz A. F. Distribution of the cell substratum attachment (CSAT) antigen on myogenic and fibroblastic cells in culture. J Cell Biol. 1985 May;100(5):1528–1539. doi: 10.1083/jcb.100.5.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca M., Tamura R. N., Kajiji S., Bondanza S., Rossino P., Cancedda R., Marchisio P. C., Quaranta V. Polarized integrin mediates human keratinocyte adhesion to basal lamina. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6888–6892. doi: 10.1073/pnas.87.17.6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana E., Colella S., Conforti G., Abbadini M., Gaboli M., Marchisio P. C. Fibronectin and vitronectin regulate the organization of their respective Arg-Gly-Asp adhesion receptors in cultured human endothelial cells. J Cell Biol. 1988 Sep;107(3):1215–1223. doi: 10.1083/jcb.107.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einheber S., Fischman D. A. Isolation and characterization of a cDNA clone encoding avian skeletal muscle C-protein: an intracellular member of the immunoglobulin superfamily. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2157–2161. doi: 10.1073/pnas.87.6.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancotti F. G., Comoglio P. M., Tarone G. A 135,000 molecular weight plasma membrane glycoprotein involved in fibronectin-mediated cell adhesion. Immunofluorescence localization in normal and RSV-transformed fibroblasts. Exp Cell Res. 1986 Mar;163(1):47–62. doi: 10.1016/0014-4827(86)90557-4. [DOI] [PubMed] [Google Scholar]
- Giancotti F. G., Ruoslahti E. Elevated levels of the alpha 5 beta 1 fibronectin receptor suppress the transformed phenotype of Chinese hamster ovary cells. Cell. 1990 Mar 9;60(5):849–859. doi: 10.1016/0092-8674(90)90098-y. [DOI] [PubMed] [Google Scholar]
- Giancotti F. G., Stepp M. A., Suzuki S., Engvall E., Ruoslahti E. Proteolytic processing of endogenous and recombinant beta 4 integrin subunit. J Cell Biol. 1992 Aug;118(4):951–959. doi: 10.1083/jcb.118.4.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg M. H., Loftus J. C., Plow E. F. Cytoadhesins, integrins, and platelets. Thromb Haemost. 1988 Feb 25;59(1):1–6. [PubMed] [Google Scholar]
- Giudice G. J., Emery D. J., Diaz L. A. Cloning and primary structural analysis of the bullous pemphigoid autoantigen BP180. J Invest Dermatol. 1992 Sep;99(3):243–250. doi: 10.1111/1523-1747.ep12616580. [DOI] [PubMed] [Google Scholar]
- Hayashi Y., Haimovich B., Reszka A., Boettiger D., Horwitz A. Expression and function of chicken integrin beta 1 subunit and its cytoplasmic domain mutants in mouse NIH 3T3 cells. J Cell Biol. 1990 Jan;110(1):175–184. doi: 10.1083/jcb.110.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemler M. E. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Annu Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- Hibbs M. L., Xu H., Stacker S. A., Springer T. A. Regulation of adhesion of ICAM-1 by the cytoplasmic domain of LFA-1 integrin beta subunit. Science. 1991 Mar 29;251(5001):1611–1613. doi: 10.1126/science.1672776. [DOI] [PubMed] [Google Scholar]
- Hieda Y., Nishizawa Y., Uematsu J., Owaribe K. Identification of a new hemidesmosomal protein, HD1: a major, high molecular mass component of isolated hemidesmosomes. J Cell Biol. 1992 Mar;116(6):1497–1506. doi: 10.1083/jcb.116.6.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogervorst F., Kuikman I., von dem Borne A. E., Sonnenberg A. Cloning and sequence analysis of beta-4 cDNA: an integrin subunit that contains a unique 118 kd cytoplasmic domain. EMBO J. 1990 Mar;9(3):765–770. doi: 10.1002/j.1460-2075.1990.tb08171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz A., Duggan K., Buck C., Beckerle M. C., Burridge K. Interaction of plasma membrane fibronectin receptor with talin--a transmembrane linkage. Nature. 1986 Apr 10;320(6062):531–533. doi: 10.1038/320531a0. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Izumi K., Hirao Y., Hopp L., Oyasu R. In vitro induction of ornithine decarboxylase in urinary bladder carcinoma cells. Cancer Res. 1981 Feb;41(2):405–409. [PubMed] [Google Scholar]
- Jones J. C., Kurpakus M. A., Cooper H. M., Quaranta V. A function for the integrin alpha 6 beta 4 in the hemidesmosome. Cell Regul. 1991 Jun;2(6):427–438. doi: 10.1091/mbc.2.6.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiji S., Tamura R. N., Quaranta V. A novel integrin (alpha E beta 4) from human epithelial cells suggests a fourth family of integrin adhesion receptors. EMBO J. 1989 Mar;8(3):673–680. doi: 10.1002/j.1460-2075.1989.tb03425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatte D. H., Kurpakus M. A., Grelling K. A., Jones J. C. Immunochemical characterization of three components of the hemidesmosome and their expression in cultured epithelial cells. J Cell Biol. 1989 Dec;109(6 Pt 2):3377–3390. doi: 10.1083/jcb.109.6.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurpakus M. A., Jones J. C. A novel hemidesmosomal plaque component: tissue distribution and incorporation into assembling hemidesmosomes in an in vitro model. Exp Cell Res. 1991 May;194(1):139–146. doi: 10.1016/0014-4827(91)90143-i. [DOI] [PubMed] [Google Scholar]
- Kurpakus M. A., Quaranta V., Jones J. C. Surface relocation of alpha 6 beta 4 integrins and assembly of hemidesmosomes in an in vitro model of wound healing. J Cell Biol. 1991 Dec;115(6):1737–1750. doi: 10.1083/jcb.115.6.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFlamme S. E., Akiyama S. K., Yamada K. M. Regulation of fibronectin receptor distribution. J Cell Biol. 1992 Apr;117(2):437–447. doi: 10.1083/jcb.117.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labeit S., Barlow D. P., Gautel M., Gibson T., Holt J., Hsieh C. L., Francke U., Leonard K., Wardale J., Whiting A. A regular pattern of two types of 100-residue motif in the sequence of titin. Nature. 1990 May 17;345(6272):273–276. doi: 10.1038/345273a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee E. C., Lotz M. M., Steele G. D., Jr, Mercurio A. M. The integrin alpha 6 beta 4 is a laminin receptor. J Cell Biol. 1992 May;117(3):671–678. doi: 10.1083/jcb.117.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legan P. K., Collins J. E., Garrod D. R. The molecular biology of desmosomes and hemidesmosomes: "what's in a name"? Bioessays. 1992 Jun;14(6):385–393. doi: 10.1002/bies.950140608. [DOI] [PubMed] [Google Scholar]
- Marcantonio E. E., Guan J. L., Trevithick J. E., Hynes R. O. Mapping of the functional determinants of the integrin beta 1 cytoplasmic domain by site-directed mutagenesis. Cell Regul. 1990 Jul;1(8):597–604. doi: 10.1091/mbc.1.8.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morla A., Ruoslahti E. A fibronectin self-assembly site involved in fibronectin matrix assembly: reconstruction in a synthetic peptide. J Cell Biol. 1992 Jul;118(2):421–429. doi: 10.1083/jcb.118.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson N. J., Pearson R. B., Needleman D. S., Hurwitz M. Y., Kemp B. E., Means A. R. Regulatory and structural motifs of chicken gizzard myosin light chain kinase. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2284–2288. doi: 10.1073/pnas.87.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otey C. A., Pavalko F. M., Burridge K. An interaction between alpha-actinin and the beta 1 integrin subunit in vitro. J Cell Biol. 1990 Aug;111(2):721–729. doi: 10.1083/jcb.111.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patthy L. Homology of a domain of the growth hormone/prolactin receptor family with type III modules of fibronectin. Cell. 1990 Apr 6;61(1):13–14. doi: 10.1016/0092-8674(90)90208-v. [DOI] [PubMed] [Google Scholar]
- Prendergast G. C., Lawe D., Ziff E. B. Association of Myn, the murine homolog of max, with c-Myc stimulates methylation-sensitive DNA binding and ras cotransformation. Cell. 1991 May 3;65(3):395–407. doi: 10.1016/0092-8674(91)90457-a. [DOI] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ruoslahti E. Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell. 1985 Jan;40(1):191–198. doi: 10.1016/0092-8674(85)90322-8. [DOI] [PubMed] [Google Scholar]
- Reszka A. A., Hayashi Y., Horwitz A. F. Identification of amino acid sequences in the integrin beta 1 cytoplasmic domain implicated in cytoskeletal association. J Cell Biol. 1992 Jun;117(6):1321–1330. doi: 10.1083/jcb.117.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddelle K. S., Green K. J., Jones J. C. Formation of hemidesmosomes in vitro by a transformed rat bladder cell line. J Cell Biol. 1991 Jan;112(1):159–168. doi: 10.1083/jcb.112.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Sawamura D., Li K., Chu M. L., Uitto J. Human bullous pemphigoid antigen (BPAG1). Amino acid sequences deduced from cloned cDNAs predict biologically important peptide segments and protein domains. J Biol Chem. 1991 Sep 25;266(27):17784–17790. [PubMed] [Google Scholar]
- Schwarz M. A., Owaribe K., Kartenbeck J., Franke W. W. Desmosomes and hemidesmosomes: constitutive molecular components. Annu Rev Cell Biol. 1990;6:461–491. doi: 10.1146/annurev.cb.06.110190.002333. [DOI] [PubMed] [Google Scholar]
- Singer I. I., Scott S., Kawka D. W., Kazazis D. M., Gailit J., Ruoslahti E. Cell surface distribution of fibronectin and vitronectin receptors depends on substrate composition and extracellular matrix accumulation. J Cell Biol. 1988 Jun;106(6):2171–2182. doi: 10.1083/jcb.106.6.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solowska J., Edelman J. M., Albelda S. M., Buck C. A. Cytoplasmic and transmembrane domains of integrin beta 1 and beta 3 subunits are functionally interchangeable. J Cell Biol. 1991 Sep;114(5):1079–1088. doi: 10.1083/jcb.114.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solowska J., Guan J. L., Marcantonio E. E., Trevithick J. E., Buck C. A., Hynes R. O. Expression of normal and mutant avian integrin subunits in rodent cells. J Cell Biol. 1989 Aug;109(2):853–861. doi: 10.1083/jcb.109.2.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg A., Calafat J., Janssen H., Daams H., van der Raaij-Helmer L. M., Falcioni R., Kennel S. J., Aplin J. D., Baker J., Loizidou M. Integrin alpha 6/beta 4 complex is located in hemidesmosomes, suggesting a major role in epidermal cell-basement membrane adhesion. J Cell Biol. 1991 May;113(4):907–917. doi: 10.1083/jcb.113.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenberg A., Linders C. J., Daams J. H., Kennel S. J. The alpha 6 beta 1 (VLA-6) and alpha 6 beta 4 protein complexes: tissue distribution and biochemical properties. J Cell Sci. 1990 Jun;96(Pt 2):207–217. doi: 10.1242/jcs.96.2.207. [DOI] [PubMed] [Google Scholar]
- Sonnenberg A., Linders C. J., Modderman P. W., Damsky C. H., Aumailley M., Timpl R. Integrin recognition of different cell-binding fragments of laminin (P1, E3, E8) and evidence that alpha 6 beta 1 but not alpha 6 beta 4 functions as a major receptor for fragment E8. J Cell Biol. 1990 Jun;110(6):2145–2155. doi: 10.1083/jcb.110.6.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Stepp M. A., Spurr-Michaud S., Tisdale A., Elwell J., Gipson I. K. Alpha 6 beta 4 integrin heterodimer is a component of hemidesmosomes. Proc Natl Acad Sci U S A. 1990 Nov;87(22):8970–8974. doi: 10.1073/pnas.87.22.8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S., Naitoh Y. Amino acid sequence of a novel integrin beta 4 subunit and primary expression of the mRNA in epithelial cells. EMBO J. 1990 Mar;9(3):757–763. doi: 10.1002/j.1460-2075.1990.tb08170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura R. N., Rozzo C., Starr L., Chambers J., Reichardt L. F., Cooper H. M., Quaranta V. Epithelial integrin alpha 6 beta 4: complete primary structure of alpha 6 and variant forms of beta 4. J Cell Biol. 1990 Oct;111(4):1593–1604. doi: 10.1083/jcb.111.4.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Parry D. A., Klaus-Kovtun V., Steinert P. M., Stanley J. R. Comparison of molecularly cloned bullous pemphigoid antigen to desmoplakin I confirms that they define a new family of cell adhesion junction plaque proteins. J Biol Chem. 1991 Jul 5;266(19):12555–12559. [PubMed] [Google Scholar]
- Westgate G. E., Weaver A. C., Couchman J. R. Bullous pemphigoid antigen localization suggests an intracellular association with hemidesmosomes. J Invest Dermatol. 1985 Mar;84(3):218–224. doi: 10.1111/1523-1747.ep12265229. [DOI] [PubMed] [Google Scholar]
- Woods A., Couchman J. R. Protein kinase C involvement in focal adhesion formation. J Cell Sci. 1992 Feb;101(Pt 2):277–290. doi: 10.1242/jcs.101.2.277. [DOI] [PubMed] [Google Scholar]