Abstract
We have identified, purified, and characterized three subcomplexes of PA700, the 19 S regulatory complex of the 26 S proteasome. These subcomplexes (denoted PS-1, PS-2, and PS-3) collectively account for all subunits present in purified PA700 but contain no overlapping components or significant levels of non-PA700 proteins. Each subcomplex contained two of the six AAA subunits (Rpt1–6) that form the binding interface of PA700 with the 20 S proteasome, the protease component of the 26 S proteasome. Unlike intact PA700, no individual PA700 subcomplex displayed ATPase activity or proteasome activating activity. However, both activities were manifested by ATP-dependent in vitro reconstitution of PA700 from the subcomplexes. We exploited functional reconstitution to define and distinguish roles of different PA700 subunits in PA700 function by selective alteration of subunits within individual subcomplexes prior to reconstitution. Carboxypeptidase treatment of either PS-2 or PS-3, subcomplexes containing specific Rpt subunits previously shown to have important roles in 26 S proteasome assembly and activation, inhibited these processes but did not affect PA700 reconstitution or ATPase activity. Thus, the intact C termini of both subunits are required for 26 S proteasome assembly and activation but not for PA700 reconstitution. Surprisingly, carboxypeptidase treatment of PS-1 also inhibited 26 S proteasome assembly and activation upon reconstitution with untreated PS-2 and PS-3. These results suggest a previously unidentified role for other PA700 subunits in 26 S proteasome assembly and activation. Our results reveal relative structural and functional relationships among the AAA subunits of PA700 and new insights about mechanisms of 26 S proteasome assembly and activation.
The 26 S proteasome is a 2,500,000-Da protease complex that degrades polyubiquitylated proteins by an ATP-dependent mechanism (1, 2). The biochemical processes required for this function are divided between two subcomplexes that compose the holoenzyme (3, 4). The first, called 20 S proteasome or core particle, is a 700,000-Da complex that catalyzes peptide bond hydrolysis (5). The second, called PA700 or 19 S regulatory particle, is a 700,000-Da complex that mediates multiple aspects of proteasome function related to initial binding and subsequent delivery of substrates to the catalytic sites of the 20 S proteasome (6). The 20 S proteasome is composed of 28 subunits representing the products of 14 genes arranged in four axially stacked heteroheptameric rings (7, 8). Each of the two center β rings contains three different protease subunits that utilize N-terminal threonine residues as catalytic nucleophiles (5, 8, 9). These residues line an interior lumen formed by the stacked rings and thus are sequestered from interaction with substrates by a shell of 20 S proteasome subunits.
PA700 is composed of 20 different subunits. Six of these subunits, termed Rpt1–6, are AAA2 (ATPases Associated with various cellular Activities) family members that confer ATPase activity to the complex and mediate energy-dependent proteolysis by the 26 S proteasome (2, 10). 26 S proteasome assembly from PA700 and 20 S proteasome requires ATP binding to Rpt subunits (11–15). Binding of PA700 to the 20 S proteasome occurs at an axial interface between a heterohexameric ring of the PA700 Rpt subunits and the heteroheptameric outer ring of α-type 20 S proteasome subunits (16). Substrates enter the proteasome through a pore in the center of the α subunit ring that is reversibly gated by conformationally variable N-terminal residues of certain α subunits in response to PA700 binding (12, 17–19). Although the degradation of polyubiquitylated proteins requires additional ATP hydrolysis-dependent actions by PA700, the assembled 26 S proteasome displays greatly increased rates of energy-independent degradation of short peptides by virtue of their increased access to catalytic sites via diffusion through the open pore (15, 18, 20).
Recently, specific interactions between Rpt and α subunits that determine PA700-20 S proteasome binding and gate opening have been defined. These findings established nonequivalent roles among the six different Rpt subunits for these processes (12, 19). For example, carboxypeptidase A treatment of PA700 selectively cleaves the C termini of two Rpt subunits (Rpt2 and Rpt5) and renders PA700 incompetent for proteasome binding and activation (19). Remarkably, short peptides corresponding to the C terminus of either Rpt2 or Rpt5, but none of the other Rpt subunits, were sufficient to bind to the 20 S proteasome and activate peptide substrate hydrolysis by inducing gate opening (12, 15, 18). The C-terminal peptides of Rpt2 and Rpt5 appear to bind to different and distinct sites on the proteasome and produce additive effects on rates of peptide substrate hydrolysis, suggesting that pore size or another feature of gating can be variably modulated (19). These various results, however, do not specify whether the action of one or the other or both C-terminal peptides is essential for function of intact PA700.
In addition to its role in activation, PA700 plays other essential roles in 26 S proteasome function related to substrate selection and processing. For example, PA700 captures polyubiquitylated proteins via multiple subunits that bind polyubiquitin chains (21–23). Moreover, to ensure translocation of the bound ubiquitylated protein through the narrow opened substrate access pore for proteolysis, PA700 destabilizes the tertiary structure of the protein via chaperone-like activity and removes polyubiquitin chains via deubiquitylating activities of several different subunits (24–30). These various functions appear to be highly coordinated and may be mechanistically linked to one another and to the hydrolysis of ATP by Rpt subunits during substrate processing.
Despite support for this general model of PA700 action, there is a lack of detailed knowledge about how PA700 subunits are structurally organized and functionally linked. Previously, we identified and characterized a subcomplex of PA700 called “modulator” that contained two ATPase subunits, Rpt4 and Rpt5, and one non-ATPase subunit, p27 (31). Although this protein was identified by an assay that measured increased PA700-dependent proteasome activation, the mechanistic basis of this effect was not clear. Moreover, the modulator lacked detectable ATPase activity and proteasome activating activity. The latter feature is surprising in retrospect because of the newly identified capacity of Rpt5 to activate the proteasome directly (12, 19). This disparity suggests that specific interactions among multiple PA700 subunits determine the manifestation and regulation of various activities.
This study extends our recent findings regarding relative roles of Rpt subunits in the regulation of proteasome function. It also provides new insights and significance to older work that identified and characterized the modulator as a subcomplex of PA700. Our findings unite two different lines of investigation to offer new information about the structure, function, and regulation of 26 S proteasome. They also offer insights about alternative models for assembly of PA700 and 26 S proteasome in intact cells.
EXPERIMENTAL PROCEDURES
Protein Purification
20 S proteasome and PA700 were purified from bovine red blood cells as described previously (11, 32, 33). PA700 subcomplexes PS-1, PS-2, and PS-3 were purified by variations of the PA700 purification, as described in detail below.
Purification of PS-1
Soluble lysates of bovine red blood cells were prepared as for purification of PA700 and subjected to gel filtration column chromatography on Sephacryl S-300, as described previously (32). Column fractions containing peak activity of PA700 were pooled and subjected to ion-exchange chromatography on DEAE-Fractogel (2.5 × 9-cm column) equilibrated with 20 mm Tris-HCl, pH 7.6, 5 mm β-mercaptoethanol, and 20% glycerol and eluted with the same buffer with a linear gradient of NaCl (100–400 mm). PA700-containing fractions were identified by functional assays for the activation of 20 S proteasome-catalyzed hydrolysis of Suc-Leu-Leu-Val-Tyr-AMC, as described below, and by identification of the characteristic component subunits by Coomassie Blue staining of SDS-polyacrylamide gels. PA700 eluted at a NaCl concentration of ∼300 mm. PS-1 was identified as a subset of PA700 proteins with coincident distribution profiles that eluted at a NaCl concentration of ∼200–250 mm but were devoid of proteasome activating activity. These fractions were pooled, concentrated, and re-chromatographed on the Sephacryl S-300 column (120 × 5 cm) in buffer consisting of 20 mm Tris-HCl, pH 7.6, 100 mm NaCl, 5 mm β-mercaptoethanol, and 20% glycerol. PS-1 was identified by Western blotting against select PA700 subunits and by Coomassie Blue staining after SDS-PAGE of column fractions. PS-1-containing fractions were pooled, dialyzed against 5 mm potassium phosphate buffer, pH 7.6, containing 5 mm β-mercaptoethanol, and 10% glycerol, applied to a column of hydroxylapatite (7 × 2.5 cm), and eluted with a linear gradient (5–200 mm) of phosphate buffer. Fractions containing characteristic PA700 subunits, as detected by Coomassie Blue staining and Western blotting against select PA700 subunits, were pooled and concentrated. If additional purification was required, PS-1 was subjected to glycerol density gradient centrifugation (12.5–37.5% glycerol) as described previously (15). Purified samples of PS-1 were concentrated to greater than 1 mg/ml, dialyzed extensively against 20 mm Tris-HCl, pH 7.6, 20 mm NaCl, 5 mm β-mercaptoethanol, and 10% glycerol, and stored at −80 °C until use.
Purification of PS-2
PS-2 was purified from the extract of bovine red blood cells that remained soluble after treatment with 40% saturated ammonium sulfate. Additional solid ammonium sulfate was added to this fraction to achieve 80% saturation. The precipitated proteins were collected by centrifugation, redissolved in 20 mm Tris-HCl, pH 7.6, 100 mm NaCl, 5 mm β-mercaptoethanol, and 10% glycerol, dialyzed extensively against the same buffer, concentrated, and subjected to gel filtration chromatography on Sephacryl S-300. The column was equilibrated and eluted with 20 mm Tris-HCl, pH 7.6, 100 mm NaCl, 5 mm β-mercaptoethanol, and 10% glycerol. Column fractions were assayed for PA700 activity and subjected to Western blotting for Rpt1, Rpt2, and other selected PA700 subunits. Fractions containing Rpt1 and Rpt2, but no PA700 activity, were pooled, applied to a DEAE-Fractogel column equilibrated with 20 mm Tris-HCl, pH 7.6, 100 mm NaCl, 5 mm β-mercaptoethanol, and 10% glycerol, and eluted with a linear gradient of 100–450 mm NaCl in the same buffer. Fractions containing Rpt1 and Rpt2 as detected by Western blotting, were pooled, dialyzed against 20 mm Tris-HCl, pH 7.6, 100 mm NaCl, 5 mm β-mercaptoethanol, and 10% glycerol, and subjected to a second round of ion-exchange chromatography on DEAE-Fractogel using an elution gradient of 100–350 mm NaCl. Column fractions containing PS-2 were identified by Coomassie Blue staining and by Western blotting for component proteins. If additional purification was required, PS-2-containing fractions were pooled, concentrated, and subjected to glycerol density gradient centrifugation as described above for PS-1. Purified samples of PS-2 were concentrated to greater than 1 mg/ml, dialyzed extensively against 20 mm Tris-HCl, pH 7.6, 20 mm NaCl, 5 mm β-mercaptoethanol, and 10% glycerol, and stored at −80 °C until use.
Purification of PS-3
PS-3, formerly termed modulator, was purified from bovine red blood cells, as described previously (31).
Proteasome Activity
Proteasome activity was measured by determining rates of enzymatic cleavage of 7-amino-4-methylcoumarin (AMC) from peptide substrates Suc-Leu-Leu-Val-Tyr-AMC, Suc-Leu-Leu-Glu-AMC, and carbobenzoxy-Val-Leu-Arg-AMC, as described previously (11, 19, 33). Standard assay conditions included 50 mm Tris-HCl, pH 7.8, and 5 mm β-mercaptoethanol, 0.2–1.0 μg of latent 20 S proteasome, and 50 μm substrate in a volume of 200 μl. Incubations were carried out at 37 °C for 10 or 20 min (depending on the experiment) in a Biotek FL600 fluorescence plate reader with filters at 380 nm excitation/460 nm emission. AMC fluorescence was monitored once per min during the assay and analyzed with kinetic software. Activity is expressed as arbitrary fluorescent units (AFU) produced per min. Routine control assays included reactions without proteasome. Other details of individual experiments are provided in the appropriate figure legends. In some experiments semi-quantitative measures of proteasome activity were obtained by overlay of peptide substrates in situ on proteins separated in native polyacrylamide gels, as described previously (34). After incubation at 37 °C for 10–30 min, AMC at the position of the protease responsible for its production was visualized by UV light.
Proteasome Activating Activity
Activation of 20 S proteasome activity by PA700 or PA700 subcomplexes was measured by a variation of the proteasome activity assays described above. Activating proteins were preincubated with 20 S proteasome for 30 min at 37 °C in buffer consisting of 50 mm Tris-HCl, pH 7.8, 5 mm β-mercaptoethanol, 1 mm ATP, 5 mm MgCl2 in a final volume of 50 μl. Peptide substrate (150 μl) was added, and proteasome activity was measured as described above. Routine controls included incubations in the absence of ATP and incubations lacking either 20 S proteasome or PA700.
PA700 Reconstitution from PA700 Subcomplexes
To reconstitute PA700 from PA700 subcomplexes, proteins were preincubated for 15–30 min at 25 °C in 50 mm Tris-HCl, pH 7.8, and 5 mm β-mercaptoethanol, 1 mm ATP, 5 mm MgCl2 in a final volume of 50 μl. Samples were then subjected to analysis as described in the appropriate figure or table legends of the specific experiments. ATPase and proteasome stimulating activities of reconstituted subcomplexes are not reported by specific activities on a molar basis because of incomplete reconstitution.
ATPase Activity
ATPase activity was determined by two different assays, including production of [32P]phosphate from [γ-32P]ATP (35) and colorimetric detection of phosphate using Malachite green (36).
Carboxypeptidase Treatment of PA700
Carboxypeptidase A (CbpA) was purchased from Sigma. CbpA was incubated with PA700 or PA700 subcomplexes (usually 0.5 milliunits of CbpA/mg of PA700 protein) at 25 °C as described previously and in figure legends for specific experiments (19). Control experiments documented that CbpA had no direct effect on the activity of the 20 S proteasome. CbpA was inhibited by carboxypeptidase inhibitor from the potato tuber (C0279, Sigma).
Glycerol Density Gradient Centrifugation
Glycerol density gradient centrifugation was conducted as described in detail previously (30).
Native PAGE
Native PAGE was conducted as described previously using 4% polyacrylamide gels (15).
Mass Spectrometry
Protein identification by mass spectrometry was conducted by the Protein Chemistry Core Research Facility at the University of Texas Southwestern Medical Center. Details of procedures for specific analysis are provided in the appropriate figure and table legends.
RESULTS
Identification of PS-1, a Novel Subcomplex of PA700
We previously established a procedure for the purification of PA700 from bovine red blood cells based on a functional assay that monitors ATP-dependent activation of 20 S proteasome-catalyzed hydrolysis of peptide substrates (11). This procedure initially isolates PA700 as a homogeneous peak of activity upon Sephacryl S-300 gel filtration chromatography of an ammonium sulfate-precipitated fraction of a soluble cell extract (supplemental Fig. 1). Subsequent ion-exchange chromatography of fractions containing this activity also produces an activity peak that is coincident with a complement of proteins characteristic of PA700 subunits, as shown by Coomassie Blue staining of SDS-polyacrylamide gels (Fig. 1A). Inspection of the gel, however, revealed that some, but not all, of the PA700 subunits also eluted coincidently as a separate peak earlier than PA700 in the salt gradient. These fractions had no detectable proteasome activating activity and also contained proteins not characteristic of PA700. The same elution pattern of stained proteins was observed in multiple independent PA700 preparations, indicating that it was a reproducible feature of the method. Western blotting of selected PA700 subunits confirmed that some PA700 subunits (e.g. Rpt6) had bimodal distributions corresponding to the staining pattern described above, whereas others (e.g. Rpt2) were specific for functional PA700 (Fig. 1A). These results, as well as similar distinctions in distribution patterns of other PA700 subunits (data not shown), suggested that bovine red blood cell extracts contain a subcomplex of PA700 that lacks proteasome activating activity. We termed this putative complex PA700 Subcomplex-1 (PS-1) and sought to purify it and characterize its structure and function.
FIGURE 1.
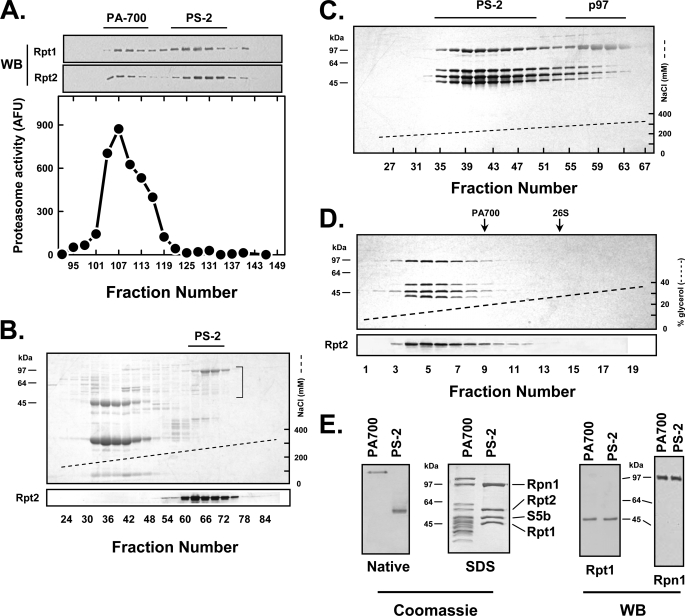
Identification and purification of PS-1. PS-1 was identified and purified as described under “Experimental Procedures.” A, DEAE-Fractogel ion-exchange chromatography. PA700 isolated by gel filtration chromatography (supplemental Fig. 1) was subjected to ion-exchange chromatography. Column fractions were assayed for PA700 activity (upper panel) and stained with Coomassie Blue and Western-blotted for Rpt6 and Rpt2 subunits after SDS-PAGE (lower panel). PA700 activity assays contained purified 20 S proteasome with 1.9 AFU of activity per assay. Column fractions contained negligible endogenous proteasome activity (data not shown). Distributions of PA700 and PS-1 are indicated by respective brackets. B, Sephacryl S-300 gel filtration chromatography. PS-1-containing fractions from A were pooled and chromatographed. Fractions were stained by Coomassie Blue after SDS-PAGE. Normal elution positions of 26 S proteasome and PA700 are noted with arrows. C, hydroxylapatite chromatography. PS-1 containing fractions from B were pooled and chromatographed. Fractions were stained with Coomassie Blue and Western-blotted for Rpn10, Rpt6, and Rpt2. D, glycerol density gradient centrifugation of PS-1. PS-1 from the hydroxylapatite column was subjected to glycerol density gradient centrifugation (12.5–37.5% glycerol). Fractions were stained with silver after SDS-PAGE. Normal sedimentation positions of purified PA700 and 26 S proteasome are indicated by arrows. E, equimolar amounts of purified PS-1 and PA700 were subjected to native PAGE and SDS-PAGE and stained with Coomassie Blue. F, equimolar amounts of purified PA700 and PS-1 were subjected to Western blotting for the indicated proteins.
Purification of PS-1
To purify PS-1, fractions containing PS-1 were pooled and subjected to a second round of gel filtration chromatography on Sephacryl S-300. SDS-PAGE of eluted fractions revealed that the same set of PA700 subunits identified by ion-exchange chromatography co-eluted with an apparent molecular weight of ∼450,000, a value distinctly smaller than that of intact PA700 (Fig. 1B). Because the PS-1-containing fractions overlapped with proteins featuring distinctly different distribution profiles, we subjected PS-1 to further purification on hydroxylapatite chromatography. This procedure isolated a set of ∼11 proteins with a homogeneous distribution profile (Fig. 1C). The composition and co-distribution of this set of proteins were not altered by additional purification procedures such as gel filtration chromatography on Superose 6, glycerol density gradient centrifugation, or ion-exchange chromatography on DEAE-Affi-Gel Blue (Fig. 1D and data not shown). Like PA700, these proteins migrated as a single band upon native PAGE (Fig. 1E). Thus, these proteins appear to form a single, structurally stable complex.
To identify the component subunits of PS-1 and compare them to those of PA700, we subjected the complex to mass spectrometry and to Western blot analysis with antibodies against representative PA700 subunits. Mass spectrometry of PS-1 identified Rpt3, Rpt6, Rpn2, Rpn3, Rpn5, Rpn6, Rpn7, Rpn8, Rpn9, Rpn10, Rpn11, Rpn12, and Uch37 but no detectable Rpn1, Rpt1, Rpt2, Rpt4, and Rpt5 (supplemental Table 1). Peptides providing appreciable coverage of PAAF-1 (Rpn14 in yeast), a protein previously described alternatively as a PA700 subunit or a PA700-associated protein (37, 38), were also identified in the PS-1 samples (supplemental Table 1). Western blotting of selected subunits confirmed the presence of Rpt3, Rpt6, Rpn8, Rpn10, Rpn11, and Rpn12 and the absence of Rpt1, Rpt2, Rpt4, and Rpt5 in PS-1 (Fig. 1F). The PA700 subunit Rpn13, which was not identified in PS-1 by mass spectrometry, was detected by Western blotting (Fig. 1F). We were unable to corroborate the mass spectrometry identification of PAAF-1 by Western blotting due to lack of an appropriate antibody. Nevertheless, all proteins detected by Western blotting were present in PS-1 and PA700 at similar molar ratios, indicating that PS-1 represents the subcomplex of PA700 with equimolar levels of component subunits. Thus, PS-1 appears to be a novel subcomplex of PA700.
Identification and Purification of PS-2 and PS-3, Distinct Subcomplexes of PA700
Identification of PS-1 as a subcomplex of PA700 suggested that PA700 subunits absent from it might exist in one or more complementary subcomplexes. Previously, we identified a complex we called modulator that contained two PA700 ATPase subunits (Rpt4 and Rpt5) and one non-ATPase subunit, p27 (Nas2 in yeast) (31, 39, 40). Consistent with those results and with similar results subsequently reported by others (41, 42), we employed Western blotting for Rpt4 and Rpt5 to identify modulator in fractions that eluted after PA700 during the initial Sephacryl S-300 gel filtration chromatography (supplemental Fig. 1 and data not shown). Using our previously published procedure, we purified modulator from these fractions (supplemental Fig. 1B). Thus, Rpt4, Rpt5, and p27, three PA700 subunits absent from PS-1, are present in a distinct subcomplex.
To determine whether the remaining PA700 subunits absent from PS-1 and modulator could be identified apart from intact PA700, we screened cell extracts with antibodies against several of those subunits. We noted a differential distribution of two of these proteins, the ATPase subunits Rpt1 and Rpt2, when compared with some other PA700 subunits. Thus, unlike Rpt3, Rpt4, Rpt5, and Rpt6 that were enriched in the extract fraction precipitated between 0 and 40% ammonium sulfate saturation, Rpt1 and Rpt2 were about equally distributed between extract fractions that precipitated between 0 and 40% and 40 and 80% saturation of ammonium sulfate (data not shown). Therefore, we used Western blotting to monitor Rpt1 and Rpt2 during fractionation of proteins that precipitated between 40 and 80% saturated ammonium sulfate. Gel filtration chromatography separated two Rpt1/Rpt2 peaks with coincident distribution profiles (Fig. 2A). The first peak eluted at a position similar to that of PA700 and probably represents residual PA700 in this ammonium sulfate fraction. ATP-dependent stimulation of 20 S proteasome activity and Western blotting of other PA700 subunits confirmed this identification (Fig. 2A and data not shown). The later eluting second peak containing most of the Rpt1 and Rpt2 protein was subjected to ion-exchange chromatography on DEAE-Fractogel. Rpt1 and Rpt2 eluted from this column coincidently and shared a distribution profile with two other proteins with molecular weights of 97,000 and 55,000 (Fig. 2B). These fractions were pooled and subjected to a repeated round of ion-exchange chromatography that separated these four coincidently eluting proteins from all others, including a major contaminant with the same molecular weight (97,000) as a component subunit (Fig. 2C). The contaminant was subsequently identified as p97 (also known as VCP, data not shown) (43–45). The co-eluting proteins behaved as a single structurally stable complex during additional purification procedures, including gel filtration chromatography, glycerol density gradient centrifugation, ion-exchange chromatography (Fig. 2D and data not shown), and native PAGE (Fig. 2E). Mass spectrometry and Western blotting confirmed the identity of two monitored components as Rpt1 and Rpt2 and identified the others as Rpn1 and S5b (Fig. 2E and supplemental Table 2). Thus, PS-1, PS-2, and modulator (hereafter termed PS-3) appear to contain most, if not all, subunits found in intact PA700 but have no common, overlapping components or appreciable levels of nonconstituent PA700 proteins. These results suggest that PS-1, PS-2, and PS-3 represent complementary subcomplexes of PA700. Remarkably, each subcomplex contains two different AAA subunits of six found in intact PA700.
FIGURE 2.
Identification and purification of PS-2. PS-2 was identified and purified as described under “Experimental Procedures.” A, bovine red blood cell extract precipitated between 40 and 80% saturated ammonium sulfate was chromatographed on Sephacryl S-300. Fractions were assayed for PA700 activity (upper panel) and Western-blotted (WB) for Rpt1 and Rpt2 after SDS-PAGE. PA700 activity assays contained purified 20 S proteasome with 3.5 AFU of activity per assay. Pooled fractions for PS-2 are indicated by a bar. B, DEAE-Fractogel ion-exchange column chromatography of PS-2 from A. Fractions were stained with Coomassie Blue and subjected to Western blotting (Rpt2 shown and other subunits not shown). Fractions indicated by bar were pooled. C, hydroxylapatite column chromatography of PS-2-containing fractions from B. Fractions were stained with Coomassie Blue. D, glycerol density gradient centrifugation of PS-2 from C. Fractions were stained with silver. Normal sedimentation positions of purified PA700 and 26 S proteasome are indicated by arrows. E, native- and SDS-PAGE of purified PA700 and purified PS-2. Equimolar amounts of PA700 and PS-2 were subjected to native- and SDS-PAGE and stained with Coomassie Blue. Equimolar amounts of PA700 and PS-2 were also Western-blotted for the indicated proteins after SDS-PAGE. Individual bands of PS-2 were excised and subjected to protein identification by mass spectrometry as described in supplemental Table 2.
Functional Characterization of Purified PS-1, PS-2, and PS-3
PA700 displays several biochemical activities, including ATPase activity (35, 46), ATP-dependent binding to the 20 S proteasome (47), and activation of 20 S proteasome-catalyzed peptidase activity (48, 49). To determine whether isolated PS-1, PS-2, or PS-3 displayed any of these features, we subjected them to standard assays for these activities. Despite the presence of two different AAA subunits in each of the three subcomplexes, no subcomplex hydrolyzed ATP at detectable rates in either of two sensitive ATPase assays (Table 1 and data not shown). Neither PS-2 nor PS-3 had detectable binding to 20 S proteasome in the presence or absence of ATP as determined by glycerol density gradient centrifugation (data not shown). These results are surprising because PS-2 and PS-3 contain subunits (Rpt2 and Rpt5, respectively) whose C termini are independently capable of binding to the proteasome (12, 19). In contrast, PS-1 displayed detectable binding to the 20 S proteasome, but to a much lower extent than that achieved by comparable levels of intact PA700 and by a mechanism that was independent of ATP (supplemental Fig. 2). This finding is also surprising because C-terminal peptides of neither Rpt subunit of PS-1 can activate the proteasome (12, 19). Finally, none of the PA700 subcomplexes activated 20 S proteasome peptidase activity to appreciable extents in the presence or absence of ATP (Table 2). As with the proteasome binding results, these negative effects on proteasome activation are surprising for PS-2 and PS-3 because the isolated C termini of Rpt2 and Rpt5 are each sufficient for proteasome activation. Mass spectrometry revealed that the Rpt2 and Rpt5 in these subcomplexes had intact C termini (supplemental Table 2 and data not shown). Collectively, these various results demonstrate that isolated PS-1, PS-2, and PS-3 lack important functions of intact PA700 and suggest that these functions require specific subunit interactions present in the intact complex.
TABLE 1.
ATPase activities of PA700 and PA700 subcomplexes
PA700 and PA700 subcomplexes were purified as described in the text and assayed for ATPase activity by the Malachite green method. PA700 (2 or 6 μg/assay, as indicated) or PA700 subcomplexes (4 μg of an individual subcomplex or 4 μg of each subcomplex for combinations) were assayed for ATPase activity in the presence (+) or absence (−) of 15 μg of 20 S proteasome after preincubation reaction for reconstitution, as described under “Experimental Procedures.” Data represent mean values of triplicate assays (±S.E.). Similar results were obtained in at least three independent experiments for each condition. Similar results also were obtained with an independent experiment using a radiometric assay of ATPase activity.
| Protein | ATPase |
|
|---|---|---|
| −20 S | +20 S | |
| pmol Pi/min/μg protein | ||
| PA700 (2 μg) | 26 ± 3.7 | 32 ± 4.4 |
| PA700 (6 μg) | 25 ± 1.8 | 29 ± 2.1 |
| PS-1 | 0 | 0 |
| PS-2 | 0 | 0 |
| PS-3 | 0 | 0 |
| PS-1/PS-2 | 0 | 0 |
| PS-2/PS-3 | 0 | 0 |
| PS-1/PS-2/PS-3 | 11.5 ± 1.6 | 13.3 ± 2.3 |
TABLE 2.
Proteasome activating activity of PA700 and PA700 subcomplexes
The indicated purified protein complexes were assayed for 20 S proteasome activating activity as described under “Experimental Procedures” after preincubation under reconstitution conditions for 30 min. Control assays (−20 S proteasome) were conducted to confirm the lack of endogenous proteasome activity in protein samples. Experimental samples (+20 S proteasome) contained 20 S proteasome (0.4 μg/assay) and PA700 (2 μg/assay) or PA700 subcomplexes (2 μg of each subcomplex per assay). The presence (+) or absence (−) of ATP indicates ATP status during both the subcomplex reconstitution and proteasome activation phases of the experiment. Proteasome activity is expressed as arbitrary fluorescent units (AFU) of AMC produced per min from hydrolysis of Suc-Leu-Leu-Val-Tyr-AMC. Data represent mean values of triplicate assays ± S.E. Similar results were obtained in at least three independent experiments for each condition. Similar results also were obtained using Suc-Leu-Leu-Glu-AMC and carbobenzoxy-Val-Leu-Arg-AMC substrates in two additional experiments.
| Addition | −20 S proteasome |
+20 S proteasome |
|||
|---|---|---|---|---|---|
| −ATP | +ATP | −ATP | +ATP | +ATPγS | |
| proteasome activity (AFU/min) | |||||
| No addition | 0 | 0 | 15.3 ± 2.6 | 13.4 ± 1.3 | 12.1 ± 1.5 |
| PA700 | 0 | 0 | 16.7 ± 2.0 | 287 ± 20 | 254 ± 34 |
| PS-1 | 0 | 0.5 | 12.4 ± 3.5 | 12.0 ± 3.3 | 17.5 ± 3.8 |
| PS-2 | 0 | 0 | 12.7 ± 1.9 | 11.9 ± 1.1 | 16.6 ± 2.3 |
| PS-3 | 0.2 | 0 | 15.9 ± 3.1 | 18.0 ± 2.7 | 13.0 ± 2.7 |
| PS-1/PS-2/PS-3 | 0.2 | 0.3 | 13.0 ± 2.5 | 198 ± 16 | 158 ± 28 |
Reconstitution of PA700 Functions from PS-1, PS-2, and PS-3
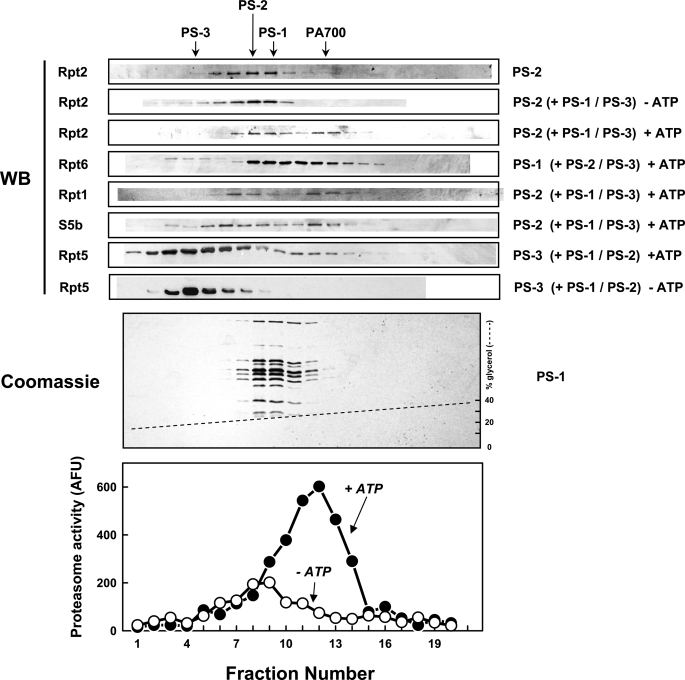
To further evaluate the significance of the lack of PA700-like functions in individual PS-1, PS-2, and PS-3 subcomplexes and to test the hypothesis that the lack of these functions resulted from the absence of otherwise required interactions among subunits, we determined whether functional PA700 could be reconstituted from the subcomplexes. To this end, we repeated the assays described above after preincubating PS-1, PS-2, and PS-3 in the presence or absence of ATP. Remarkably, both ATPase activity (Table 1) and activated 20 S proteasome peptidase activity (Table 2) were manifested by this procedure. As with intact PA700, the latter effect required ATP but was supported by ATPγS (Table 2). Neither ATPase activity nor proteasome activation was manifested by any two subcomplexes in the absence of the third (Table 1 and Fig. 3). ATPase activity was reconstituted equally well in the presence or absence of the 20 S proteasome, indicating that functional reconstitution of the subcomplexes did not require the proteasome (Table 1). Likewise, similar degrees of proteasome activation were achieved when initial reconstitution was conducted in the presence or absence of the 20 S proteasome (data not shown). To determine directly whether the restoration of PA700-like functions resulted from physical assembly of the subcomplexes into PA700 or a PA700-like complex, we subjected the preincubated subcomplexes to native PAGE. In the presence of 20 S proteasome, a proteolytically active complex was formed featuring an electrophoretic mobility indistinguishable from that of purified 26 S proteasome or 26 S proteasome assembled by ATP-dependent binding of 20 S proteasome and intact PA700 (Fig. 4, lanes 9 and 10, and data not shown). Formation of this activated complex required all three subcomplexes and ATP or ATPγS (Fig. 4 and data not shown). In the absence of 20 S proteasome, a reconstituted complex with mobility similar to intact PA700 was difficult to detect unambiguously because of the similar migration positions of PA700 and PS-1 on native PAGE and because the subcomplexes were not reconstituted quantitatively. The latter effect probably is a consequence of uncertainty about the conditions required for optimal reconstitution, including absolute and relative concentrations of the subcomplexes and other factors (see under “Discussion”). Nevertheless, clear evidence for ATP-dependent reconstitution of a PA700-like complex from the subcomplexes in the absence of 20 S proteasome was detected by glycerol density gradient centrifugation. We preincubated the subcomplexes in the presence or absence of ATP prior to centrifugation. Gradient fractions then were subjected to Western blotting for various subunits and assayed for proteasome activating activity. Samples preincubated in the absence of ATP displayed subunit distribution patterns characteristic of those expected for individual subcomplexes, indicating that no detectable reconstitution occurred under these conditions (Fig. 5). In contrast, subcomplexes preincubated with ATP showed a redistribution of representative subunits of each subcomplex to a faster sedimenting position characteristic of intact PA700 (Fig. 5). Both the redistribution and the co-sedimentation of subunits from each subcomplex at this new position indicate that the subcomplexes were reconstituted into a PA700-like complex. Moreover, the position of the redistributed subunits coincided with a peak of proteasome activating activity absent from corresponding fractions of the subcomplex sample preincubated without ATP. A broad peak of low level proteasome activating activity was observed in slower sedimenting gradient fractions from samples preincubated both with and without ATP. This probably reflects ATP-dependent reconstitution of PA700 from the subcomplexes during the proteasome activation assay (which by necessity contains ATP) because of their overlapping distribution profiles and thus their co-presence in the assays (Fig. 5). These results support the conclusion that PA700 can be structurally reconstituted from PS-1, PS-2, and PS-3 in a manner that requires ATP but not the 20 S proteasome. In sum, these various results indicate that PS-1, PS-2, and PS-3 assemble into a PA700-like complex in an ATP-dependent fashion and that the resulting complex is competent for ATP hydrolysis and proteasome binding and activation.
FIGURE 3.
Reconstitution of PA700 activity from PS-1, PS-2, and PS-3 subcomplexes. Purified PS-1, PS-2, and PS-3 were subjected to reconstitution assays for proteasome activating activity as described under “Experimental Procedures.” The indicated amounts of PS-1 (left panel), PS-2 (center panel), or PS-3 (right panel) were incubated in the absence (−) or presence (+) of fixed amounts (4 μg each) of the corresponding other two PA700 subcomplexes in the presence of 1 mm ATP, 5 mm MgCl2 for 15 min at 25 °C. 20 S proteasome (0.4 μg) was added, and the incubation was continued for an additional 30 min prior to addition of Suc-Leu-Leu-Val-Tyr-AMC and measurement of rates of substrate hydrolysis. AMC production was measured for 20 min after addition of substrate. Proteasome activity is expressed as AFU/min.
FIGURE 4.
ATP-dependent reconstitution of 26 S proteasome from PS-1, PS-2, PS-3, and 20 S proteasome. PS-1 (4 μg), PS-2 (4 μg), PS-3 (4 μg), and PA700 (4 μg) were preincubated for 30 min at 25 °C in the presence (+) or absence (−) of ATP (1 mm) and 20 S proteasome (40 μg), as indicated (lanes 6–10). Samples were subjected to native PAGE and either stained with Coomassie Blue (upper panel) or overlaid with Suc-Leu-Leu-Val-Tyr-AMC for visualization of proteasome activity (lower panel). Indicated individual proteins in corresponding amounts also were electrophoresed (lanes 1–5). Arrows indicate positions of active proteasome complexes equivalent to positions of 26 S proteasome.
FIGURE 5.
ATP-dependent reconstitution of PA700 from PS-1, PS-2, and PS-3. Limiting concentrations of PS-1, PS-2, and PS-3 (10 μg) were preincubated at 25 °C for 30 min with excess concentrations (60 μg each) of the corresponding other two subcomplexes (shown in parentheses) in the presence (+) or absence (−) of 1 mm ATP and then subjected to glycerol density gradient centrifugation (12.5–37.5% glycerol, no ATP in gradient buffers). Gradient fractions were subjected to Western blotting for the indicated subunits of the limiting subcomplex (upper panels, WB), stained with Coomassie Blue (middle panel), or assayed for 20 S proteasome activating activity (lower panel). The relative sedimentation positions of isolated PS-1, PS-2, PS-3, and intact PA700 were determined by independent experiments and are shown with arrows. Data for Rpt2 Western blotting of isolated PS-2 (upper panel) and Coomassie Blue staining of isolated PS-1 (middle panel) are shown. Gradient fractions were also assayed for proteasome activating activity (lower panel). Assays contained the indicated column fractions, 0.4 μg of 20 S proteasome and 1 mm ATP. “+ATP” and “−ATP” refer to the presence or absence, respectively, of ATP in the preincubation reactions prior to sample application to the gradient. The figure shows results for the condition of 10 μg of PS-1 and 60 μg each of PS-2 and PS-3. Qualitatively similar results were obtained with other conditions shown in the upper panel. No proteasome activating activity was observed in gradient fractions from PS-1, PS-2, and PS-3 samples preincubated in the absence of ATP and assayed in the absence of ATP (data not shown).
Relative Roles of PS-1, PS-2, and PS-3 for PA700 Functions
The ability to reconstitute PA700 function from isolated subcomplexes provides a unique opportunity to determine the relative roles of individual PA700 subunits for both PA700 and 26 S proteasome function. In particular, we sought to exploit this result to clarify previous findings about the relative roles of Rpt2 and Rpt5 in proteasome binding and activation. For example, although isolated C-terminal peptides of either subunit can bind to and activate the proteasome, CbpA treatment of intact PA700 sufficient to abolish proteasome activating activity removed the responsible C-terminal residues from both of these subunits (but not from other Rpt subunits) (19). Thus, these results left unresolved whether loss of PA700 function was caused by modification of one or the other or both of these Rpt subunits. To resolve this issue, we used CbpA to selectively alter subunits of individual PA700 subcomplexes prior to reconstitution with the two remaining untreated subcomplexes. We first established that CbpA did not affect the overall quaternary structure of any PA700 subcomplex. Indeed, CbpA treatment of each subcomplex had no effect on its sedimentation position or subunit pattern during glycerol density gradient centrifugation or on its migration as a single band during native PAGE. Second, we verified that CbpA treatment of the reconstituted subcomplexes, like treatment of intact PA700, inhibited proteasome activating activity but not ATPase activity (Table 3). CbpA treatment of the subcomplexes prior to reconstitution produced similar differential results on these activities (Table 3). These results demonstrate that CbpA affected subcomplex subunits in a manner that inhibited proteasome activation but not subcomplex reconstitution. Thus, inhibition of proteasome activating activity does not result indirectly from failure of the treated subcomplexes to assemble into a PA700-like complex.
TABLE 3.
Effect of carboxypeptidase treatment on activities of PA700 and PA700 subcomplexes
The indicated purified protein complexes were assayed for ATPase activity or 20 S proteasome activating activity after preincubation in the absence (−) or presence (+) of CbpA. CbpA treatment was conducted either after (row 2) or before (row 3) preincubation of PS-1, PS-2, and PS-3 (4 μg each) for reconstitution. ATPase activity was determined by the Malachite green method. Proteasome activating activity represents the relative increase of 20 S proteasome activity (measured by the rate of hydrolysis of Suc-Leu-Leu-Val-Tyr-AMC) by the indicated proteins. The activity of 20 S proteasome (0.4 μg/assay) in the absence of other proteins is set to a relative value of 1.0. Data represent mean values of triplicate assays ± S.E. Similar results were observed in at least three independent experiments for each condition and in one experiment using Suc-Leu-Leu-Glu-AMC as substrate.
| Protein | ATPase activity |
Proteasome activating activity (relative activity) |
||
|---|---|---|---|---|
| −CbpA | +CbpA | −CbpA | +CbpA | |
| pmol Pi/min/μg protein | ||||
| PA700 | 28.8 ± 3.6 | 26.9 ± 3.4 | 22.8 + 0.7 | 1.2 ± 0.5 |
| (PS-1/PS-2/PS-3) ± CbpA | 18.9 ± 2.8 | 15.3 ± 3.9 | 17.2 ± 1.6 | 1.3 ± 0.8 |
| CbpA ± (PS-1/PS-2/PS-3) | 20.5 ± 2.0 | 18.6 ± 1.5 | 16.0 ± 3.2 | 1.3 ± 0.2 |
To determine the roles of Rpt subunits in individual subcomplexes in proteasome binding and activation, we treated each subcomplex with CbpA and then performed reconstitution with the two other untreated subcomplexes. After treatment, carboxypeptidase activity was inhibited during the reconstitution reaction by a peptide inhibitor of CbpA (50). The inhibitor completely blocked the normal effect of CbpA on both intact PA700 and PA700 reconstituted from all subcomplexes when added to the proteins prior to CbpA treatment (supplemental Tables 3 and 4). Additional control experiments established that the inhibitor had no nonspecific effects on assays for proteasome activity or ATPase activity (supplemental Tables 3 and 4 and data not shown). CbpA treatment of either PS-2 or PS-3 inhibited proteasome activation when these subcomplexes were reconstituted with untreated PS-1 and PS-3 or PS-1 and PS-2, respectively (Table 4). These effects on activity were mirrored by corresponding inhibition of assembly of active 26 S proteasome, as revealed by native PAGE (data not shown). None of these various treatments had significant effects on the reconstitution of ATPase activity in the presence or absence of 20 S proteasome (Table 4). These results indicate that modification of the C terminus of either previously identified target of CbpA (Rpt2 in PS-2 or Rpt5 in PS-3) is sufficient to inhibit assembly and activation of 26 S proteasome. Surprisingly, however, CbpA treatment of PS-1 also inhibited subsequent proteasome activation by the complex reconstituted with untreated PS-2 and PS-3 (Table 4). This effect, which was blocked by the carboxypeptidase inhibitor, was unexpected because PS-1 contains Rpt subunits (Rpt3 and Rpt6) whose C termini do not activate the proteasome directly and are not processed by CbpA treatment of intact PA700 (19). To determine the basis for the loss of reconstituted PA700 functions after CbpA treatment of PS-1, we attempted to determine whole mass values of Rpt3 and Rpt6, as previously accomplished for all Rpt subunits of PA700 (19). Each subunit from treated PS-1 eluted from the high pressure liquid chromatography column utilized for its isolation in a position similar to that of its counterpart in both untreated PS-1 and PA700 and was indistinguishable from these respective proteins after Western blotting. However, we were unable to obtain unambiguous whole mass values for these CbpA-treated proteins. Although the basis for this result is unclear, it may reflect the production of multiple forms of CbpA-processed Rpt proteins from PS-1. Nevertheless, these indirect results suggest that one or more carboxypeptidase-sensitive PS-1 subunits are important for 26 S proteasome assembly and activation.
TABLE 4.
Effect of carboxypeptidase treatment on activities of PA700 and PA700 subcomplexes
PA700 or the indicated PA700 subcomplexes were treated with CbpA. After treatment, carboxypeptidase inhibitor was added to ensure complete inhibition of CbpA. The treated protein (2 μg of PA700 or 4 μg each of PS-1, PS-2, and PS-3) was then mixed with the indicated untreated proteins and preincubated under conditions for reconstitution of PA700 activities from PS-1, PS-2, and PS-3. Proteins were then assayed for ATPase activity or proteasome activating activity. ATPase activity was determined by the Malachite green method. Proteasome activating activity represents the relative increase of 20 S proteasome activity (measured by the rate of hydrolysis of Suc-Leu-Leu-Val-Tyr-AMC) by the indicated proteins. The activity of 20 S proteasome (0.4 μg/assay) in the absence of other proteins is set to a relative value of 1.0. Data represent mean values of triplicate assays ± S.E. Similar results were observed in at least three independent experiments.
| CbpA-treated protein | Untreated protein | ATPase activity | Proteasome activating activity (relative activity) |
|---|---|---|---|
| pmol Pi/min/μg protein | |||
| PA700 | 20.5 ± 3.9 | 18.1 ± 2.5 | |
| PA700 | 23.2 ± 2.6 | 0.9 ± 0.6 | |
| PS-1 + PS-2 + PS-3 | 16.4 ± 2.1 | 14.4 ± 1.5 | |
| PS-1 + PS-2 + PS-3 | 14.9 ± 2.7 | 1.4 ± 0.2 | |
| PS-1 | PS-2 + PS-3 | 17.9 ± 1.4 | 1.8 ± 0.5 |
| PS-2 | PS-1 + PS-3 | 20.6 ± 1.2 | 1.5 ± 0.4 |
| PS-3 | PS-1 + PS-2 | 15.1 ± 3.6 | 0.8 ± 0.3 |
To determine whether inhibition of proteasome activation by CbpA treatment of PS-1 could be explained by consequential loss of the PS-1 binding to the proteasome, we compared proteasome binding of CbpA-treated and untreated PS-1. Surprising, CbpA treatment did not affect the apparent binding of PS-1 to the proteasome (supplemental Fig. 2). These results suggest that CbpA-sensitive PS-1 functions other than proteasome binding may be responsible for the PS-1 contribution to proteasome activation after subcomplex reconstitution. However, additional work will be required to determine the precise basis for this effect (see “Discussion”).
DISCUSSION
Relative Roles and Functions of Rpt Subunits of PS-1, PS-2, and PS-3
The results presented here reveal new insights about the structural and functional relationships among subunits of PA700, the 19 S ATPase regulatory complex of the 26 S proteasome. We have identified, purified, and characterized three subcomplexes of PA700 that individually lack critical functions of the intact complex but can be reconstituted in an ATP-dependent fashion to manifest these functions. Notably, each subcomplex contains two of the six different Rpt subunits that form the binding surface of PA700 to the 20 S proteasome. Two of these subunits, Rpt2 and Rpt5 of the PS-2 and PS-3 subcomplexes, respectively, were shown previously to be the only Rpt subunits capable of proteasome activation via binding of their C termini to cognate binding sites on the 20 S proteasome (12, 19). Unlike intact PA700, which binds to the 20 S proteasome in an ATP-dependent fashion, neither PS-2 nor PS-3 bound to the proteasome in the presence or absence of ATP. These results suggest that the ATP binding-induced conformation of PA700 that promotes binding of the C termini of Rpt2 and Rpt5 to the proteasome is not mimicked in these subcomplexes. Nevertheless, the ATP dependence of PA700 reconstitution from the subcomplexes indicates that one or more of them is conformationally sensitive to ATP binding. Despite the ATP dependence of reconstitution, intact PA700 is relatively stable in the absence of ATP; our PA700 purification, which normally is conducted with ATP-free buffers, is not improved when ATP is present.
In contrast to PS-2 and PS-3, PS-1 contains no Rpt subunits with C termini that can independently activate the proteasome (12, 19). Therefore, several results regarding functional features of isolated PS-1 and its contribution to functions of reconstituted subcomplexes were surprising, contradictory, and remain unexplained. Assuming that the observed binding of PS-1 to the proteasome resulted from a specific interaction, contradictory results were obtained for the basis and functional significance of this effect. For example, CbpA-treated PS-1 failed to support proteasome activation after it was reconstituted with untreated PS-2 and PS-3 but retained its proteasome binding capacity. Thus, PS-1 may contain CbpA-sensitive sites that affect proteasome activation by mechanisms independent of a contribution to binding. Our previous work showed that neither Rpt3 nor Rpt6, whose C termini represent likely proteasome binding elements of this subcomplex, were not modified by CbpA treatment of intact PA700. Unfortunately, we were unable to determine unambiguous masses of CbpA-treated Rpt3 and Rpt6, although our data suggest that these subunits were processed to multiple forms. If the latter speculation is confirmed, these results indicate the presence of one or more cryptic CbpA-sensitive subunits in intact PA700 that become exposed in isolated PS-1. PS-1 also contains Rpn2, a non-ATPase component of PA700, which a recent report implicated in proteasome binding and activation (51). The large mass of Rpn2 (112,000 Da) also prevented an unambiguous determination of whether this subunit was modified by CbpA, and additional work will be required to determine its possible role in both PS-1 and PA700 binding to the proteasome. In sum, our results indicate that subunits in each of the three subcomplexes contribute to 26 S proteasome assembly and activation and that interference with the contribution of any one of these is sufficient to inhibit these processes. The roles of Rpt2 of PS-2 and Rpt5 of PS-3 are consistent with previous results, but additional work will be required to define the role of PS-1 subunits in proteasome binding and activation.
Structure-Function Relationships for Rpt Subunits
The current data provide new insights about the structural organization of PA700 subunits. Although there is no x-ray structure of PA700, data from biochemical, genetic, and imaging experiments have provided a general model of PA700 architecture and an interaction map for component subunits (13, 16, 52–54). These various results have been interpreted as a model featuring a fixed arrangement of the AAA subunits within a heterohexameric ring, Rpt1-Rpt2-Rpt6-Rpt4-Rpt5-Rpt3 (52, 54). Thus, the adjacent positions of Rpt1 and Rpt2 and of Rpt4 and Rpt5 are consistent with their presence in the PS-2 and PS-3 subcomplexes, respectively. In contrast, this model places Rpt3 and Rpt6, the ATPase components of PS-1, ∼180° opposite one another in the hexameric ring, suggesting either that they are linked indirectly by other PS-1 subunits or that the structural model indicated above is incorrect. Previous work showing formation of a tetrameric complex of Rpt1, Rpt2, Rpt3, and Rpt6 upon in vitro co-expression of these proteins in a transcription-translation linked expression system is compatible with either interpretation (55). Therefore, additional work will be required to establish whether Rpt3 and Rpt6 occupy adjacent or separated positions within the heterohexameric ring of PA700.
Origin and Significance of PA700 Subcomplexes
The origin and significance of PA700 subcomplexes in cell extracts are unclear. Although these subcomplexes could represent artifacts of the purification procedure generated by dissociation of intact PA700, we instead speculate that they represent physiologic intermediates of PA700 and/or 26 S proteasome assembly. In contrast to the cellular mechanisms of 20 S proteasome assembly, relatively little is known about corresponding processes for PA700 and 26 S proteasome (56). Thus, it remains unclear whether the 26 S proteasome is formed in cells by binding of fully assembled PA700 to 20 S proteasome or by sequential binding of subcomplexes of PA700 to the 20 S proteasome. Although our in vitro data indicate that intact PA700 is competent to bind to the 20 S proteasome directly in the presence of ATP and that PA700 can be reconstituted from PS-1, PS-2, and PS-3 subcomplexes in the absence of 20 S proteasome, they do not exclude the possibility that in cells PA700 is assembled sequentially from subcomplexes using the 20 S proteasome as a template. In the latter case, it will be important to establish the order and determinants of assembly.
An obvious potential determinant of cellular assembly of PA700 by either mechanism described above involves chaperones or other assembly factors. Several such factors are known to be involved in the assembly of the 20 S proteasome, and it is reasonable to assume their existence for PA700 (57–60). Indeed, immediately prior to submission of our manuscript a series of papers was published identifying Hms3, Nas2, Rpn14, and Nas6 and their respective mammalian orthologs, S5b, p27, PAAF-1, and p28 (gankyrin), as chaperones for PA700 (19 S regulatory particle) assembly in yeast and mammalian cells (61–66). Each of these proteins has been designated previously as either a PA700 subunit or a PA700-associated protein, and the former three have been identified as components of the subcomplexes reported here. Despite some inconsistencies, these new reports contain largely concordant data with regard to the role of the PA700 assembly of these proteins. Likewise, they feature both consistent and apparently discordant data with those reported here. For example, PA700 subcomplexes identical to PS-2 and/or PS-3 were also identified in the new reports. Moreover, the precise pairing of Rpt subunits among the three different subcomplexes described by our work (i.e. Rpt1-Rpt2, Rpt3-Rpt6, and Rpt4-Rpt5) as well as their association with the proteins described above (i.e. Rpt1-Rpt2 with Hms3/S5b, Rpt3-Rpt6 with Rpn14/PAAF-1, and Rpt4-Rpt5 with Nas2/p27) were also established by three other studies (61, 62, 66). In contrast, there are several major apparent differences between our results and some of those contained in the new reports. First, none of the other identified Rpt subunit-containing subcomplexes was associated with significant content of other PA700 subunits, especially those of the “lid” (see below; a possible exception was noted in the study of mammalian cells, cf. Ref. 62). Specifically, the complexes containing Rpt3 and Rpt6 were structurally far simpler than PS-1, featuring only Rpn2, Nas6, and Rpn14 as additional proteins. In fact, these papers concluded that a complete ring of Rpt subunits was assembled prior to addition of most other PA700 subunits. In contrast, PS-1 contained nearly all established lid proteins. Second, each of the chaperones identified in the papers was excluded from the final assembled complex. Because of a lack of suitable reagents for p27 and PAAF-1 and the absence of p28/gankyrin/Nas6 from any of our proteins, we examined only S5b in this context. In contrast to Hsm3, our work identified S5b, a component of PS-2, in both purified PA700 and 26 S proteasome (supplemental Fig. 3). Moreover, S5b, which was originally identified in purified human 26 S proteasome (67) and was considered one of the few 26 S proteasome subunits not shared between yeast and most other eukaryotic species, was incorporated into PA700 reconstituted from subcomplexes and into the 26 S proteasome formed by in vitro assembly from PA700 and 20 S proteasome.3 Despite these apparent discrepancies, our experiments do not specifically address the functional role of S5b or the other proteins in assembly of either PA700 or 26 S proteasome and additional work will be required to resolve this disparity. Finally, regardless of the roles of chaperones, our results demonstrate that reconstitution of PA700 from subcomplexes neither required nor was improved by the 20 S proteasome. Although these results suggest that the 20 S proteasome is not an obligatory template for PA700 assembly, as indicated by other studies, such a 20 S proteasome-dependent pathway may be favored in intact cells. In this regard, we note that the in vitro assembly of neither PA700 from PS-1, PS-2, and PS-3 nor 26 S proteasome from purified 20 S proteasome and PA700 components was completely efficient. Thus, many factors, both identified and unidentified, may be required for efficient PA700 assembly in vitro and in cells. Additional work will be required to define such factors and their roles.
The PS-1, PS-2, and PS-3 subcomplexes described here, and the corresponding complexes in the aforementioned recent papers differ from other subcomplexes of PA700 described previously. The best characterized of these are the “base” and lid, originally identified and most extensively characterized in Saccharomyces cerevisiae (13). Like PS-1, PS-2, and PS-3, the base and lid are complementary subcomplexes of intact PA700. In contrast, however, the base contains all Rpt subunits as well as Rpn1, Rpn2, and Rpn13. Moreover, the base mimics the ability of intact PA700 to bind to and activate the proteasome in an ATP-dependent fashion, presumably because it has an intact ring of Rpt subunits. The lid contains all remaining subunits and appears normally to be physically linked to the base via Rpn10. We have not identified base and lid subcomplexes at appreciable levels in our mammalian cell preparations, nor have we been able to generate them from intact PA700 or 26 S proteasome by treatment of the purified complexes with high concentrations of salts or mild denaturing conditions, as has been described in yeast (68).4 The significance of these distinctions is unclear. We note, however, that subcomplexes similar to those described in this study were reported previously (41, 42, 69). For example, PS-3, originally termed modulator by us, was independently identified and isolated from extracts of other mammalian cells (41, 42). Moreover, Rpt1, Rpt2, Rpn1, and S5b, the four components of PS-2, originally were shown to form a complex when co-expressed in an in vitro transcription-translation linked system (69). Such studies highlight the specificity of interactions among these proteins, suggesting that they also assemble preferentially in cells.
An alternative explanation for the existence of subcomplexes of PA700 is that they have functions exclusive of the proteasome regulation. AAA proteins play many cellular roles, and it is possible that these subcomplexes, either directly or in combination with other proteins to which they bind, have nonproteolytic actions. Nonproteolytic roles of Rpt subunits of PA700 have been described previously in transcription and in other processes (70–74). Although we failed to identify non-PA700 proteins at appreciable levels in any of the subcomplexes, it is possible that subpopulations of these or other PA700 subcomplexes associate with other proteins for specific cellular roles.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grant R01 DK46181. This work was also supported by The Welch Foundation Grant I-500 (to G. N. D.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables 1–4, Figs. 1–3, and additional references.
D. Thompson and G. N. DeMartino, unpublished observations.
K. Hakala, D. Thompson, and G. N. DeMartino, unpublished observations.
- AAA
- ATPases associated with a variety of cellular activities
- AMC
- 7-amino-4-methylcoumarin
- CbpA
- carboxypeptidase A
- AFU
- arbitrary fluorescent units
- ATPγS
- adenosine 5′-O-(thiotriphosphate)
- Suc
- succinyl.
REFERENCES
- 1.Voges D., Zwickl P., Baumeister W. (1999) Annu. Rev. Biochem. 68, 1015–1068 [DOI] [PubMed] [Google Scholar]
- 2.Smith D. M., Benaroudj N., Goldberg A. (2006) J. Struct. Biol. 156, 72–83 [DOI] [PubMed] [Google Scholar]
- 3.Demartino G. N., Gillette T. G. (2007) Cell 129, 659–662 [DOI] [PubMed] [Google Scholar]
- 4.Coux O., Tanaka K., Goldberg A. L. (1996) Annu. Rev. Biochem. 65, 801–847 [DOI] [PubMed] [Google Scholar]
- 5.Baumeister W., Walz J., Zühl F., Seemüller E. (1998) Cell 92, 367–380 [DOI] [PubMed] [Google Scholar]
- 6.Finley D. (2009) Annu. Rev. Biochem. 78, 477–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bochtler M., Ditzel L., Groll M., Hartmann C., Huber R. (1999) Annu. Rev. Biophys. Biomol. Struct. 28, 295–317 [DOI] [PubMed] [Google Scholar]
- 8.Groll M., Bochtler M., Brandstetter H., Clausen T., Huber R. (2005) ChemBioChem. 6, 222–256 [DOI] [PubMed] [Google Scholar]
- 9.Groll M., Ditzel L., Löwe J., Stock D., Bochtler M., Bartunik H. D., Huber R. (1997) Nature 386, 463–471 [DOI] [PubMed] [Google Scholar]
- 10.Striebel F., Kress W., Weber-Ban E. (2009) Curr. Opin. Struct. Biol. 19, 209–217 [DOI] [PubMed] [Google Scholar]
- 11.Ma C.-P., Vu J. H., Proske R. J., Slaughter C. A., DeMartino G. N. (1994) J. Biol. Chem. 269, 3539–3547 [PubMed] [Google Scholar]
- 12.Smith D. M., Chang S. C., Park S., Finley D., Cheng Y., Goldberg A. L. (2007) Mol. Cell 27, 731–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glickman M. H., Rubin D. M., Coux O., Wefes I., Pfeifer G., Cjeka Z., Baumeister W., Fried V. A., Finley D. (1998) Cell 94, 615–623 [DOI] [PubMed] [Google Scholar]
- 14.Köhler A., Cascio P., Leggett D. S., Woo K. M., Goldberg A. L., Finley D. (2001) Mol. Cell 7, 1143–1152 [DOI] [PubMed] [Google Scholar]
- 15.Liu C. W., Li X., Thompson D., Wooding K., Chang T. L., Tang Z., Yu H., Thomas P. J., DeMartino G. N. (2006) Mol. Cell 24, 39–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.da Fonseca P. C., Morris E. P. (2008) J. Biol. Chem. 283, 23305–23314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groll M., Bajorek M., Köhler A., Moroder L., Rubin D. M., Huber R., Glickman M. H., Finley D. (2000) Nat. Struct. Biol. 7, 1062–1067 [DOI] [PubMed] [Google Scholar]
- 18.Smith D. M., Kafri G., Cheng Y., Ng D., Walz T., Goldberg A. L. (2005) Mol. Cell 20, 687–698 [DOI] [PubMed] [Google Scholar]
- 19.Gillette T. G., Kumar B., Thompson D., Slaughter C. A., DeMartino G. N. (2008) J. Biol. Chem. 283, 31813–31822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rabl J., Smith D. M., Yu Y., Chang S. C., Goldberg A. L., Cheng Y. (2008) Mol. Cell 30, 360–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deveraux Q., Ustrell V., Pickart C., Rechsteiner M. (1994) J. Biol. Chem. 269, 7059–7061 [PubMed] [Google Scholar]
- 22.Husnjak K., Elsasser S., Zhang N., Chen X., Randles L., Shi Y., Hofmann K., Walters K. J., Finley D., Dikic I. (2008) Nature 453, 481–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lam Y. A., Lawson T. G., Velayutham M., Zweier J. L., Pickart C. M. (2002) Nature 416, 763–767 [DOI] [PubMed] [Google Scholar]
- 24.Liu C. W., Millen L., Roman T. B., Xiong H., Gilbert H. F., Noiva R., DeMartino G. N., Thomas P. J. (2002) J. Biol. Chem. 277, 26815–26820 [DOI] [PubMed] [Google Scholar]
- 25.Strickland E., Hakala K., Thomas P. J., DeMartino G. N. (2000) J. Biol. Chem. 275, 5565–5572 [DOI] [PubMed] [Google Scholar]
- 26.Braun B. C., Glickman M., Kraft R., Dahlmann B., Kloetzel P. M., Finley D., Schmidt M. (1999) Nat. Cell Biol. 1, 221–226 [DOI] [PubMed] [Google Scholar]
- 27.Lam Y. A., Xu W., DeMartino G. N., Cohen R. E. (1997) Nature 385, 737–740 [DOI] [PubMed] [Google Scholar]
- 28.Yao T., Cohen R. E. (2002) Nature 419, 403–407 [DOI] [PubMed] [Google Scholar]
- 29.Verma R., Aravind L., Oania R., McDonald W. H., Yates J. R., 3rd, Koonin E. V., Deshaies R. J. (2002) Science 298, 611–615 [DOI] [PubMed] [Google Scholar]
- 30.Koulich E., Li X., DeMartino G. N. (2008) Mol. Biol. Cell 19, 1072–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeMartino G. N., Proske R. J., Moomaw C. R., Strong A. A., Song X., Hisamatsu H., Tanaka K., Slaughter C. A. (1996) J. Biol. Chem. 271, 3112–3118 [DOI] [PubMed] [Google Scholar]
- 32.DeMartino G. N. (2005) Methods Enzymol. 398, 295–306 [DOI] [PubMed] [Google Scholar]
- 33.McGuire M. J., DeMartino G. N. (1986) Biochim. Biophys. Acta 873, 279–289 [DOI] [PubMed] [Google Scholar]
- 34.Elsasser S., Schmidt M., Finley D. (2005) Methods Enzymol. 398, 353–363 [DOI] [PubMed] [Google Scholar]
- 35.DeMartino G. N., Moomaw C. R., Zagnitko O. P., Proske R. J., Ma C.-P., Afendis S. J., Swaffield J. C., Slaughter C. A. (1994) J. Biol. Chem. 269, 20878–20884 [PubMed] [Google Scholar]
- 36.Van Veldhoven P. P., Mannaerts G. P. (1987) Anal. Biochem. 161, 45–48 [DOI] [PubMed] [Google Scholar]
- 37.Park Y., Hwang Y. P., Lee J. S., Seo S. H., Yoon S. K., Yoon J. B. (2005) Mol. Cell. Biol. 25, 3842–3853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seong K. M., Baek J. H., Yu M. H., Kim J. (2007) FEBS Lett. 581, 2567–2573 [DOI] [PubMed] [Google Scholar]
- 39.Watanabe T. K., Saito A., Suzuki M., Fujiwara T., Takahashi E., Slaughter C. A., DeMartino G. N., Hendil K. B., Chung C. H., Tanahashi N., Tanaka K. (1998) Genomics 50, 241–250 [DOI] [PubMed] [Google Scholar]
- 40.Russell S. J., Steger K. A., Johnston S. A. (1999) J. Biol. Chem. 274, 21943–21952 [DOI] [PubMed] [Google Scholar]
- 41.Hastings R., Walker G., Eyheralde I., Dawson S., Billett M., Mayer R. J. (1999) Mol. Biol. Rep. 26, 35–38 [DOI] [PubMed] [Google Scholar]
- 42.Hastings R. A., Eyheralde I., Dawson S. P., Walker G., Reynolds S. E., Billett M. A., Mayer R. J. (1999) J. Biol. Chem. 274, 25691–25700 [DOI] [PubMed] [Google Scholar]
- 43.Wójcik C., Yano M., DeMartino G. N. (2004) J. Cell Sci. 117, 281–292 [DOI] [PubMed] [Google Scholar]
- 44.Wójcik C., Rowicka M., Kudlicki A., Nowis D. M., Kujawa M., DeMartino G. N. (2006) Mol. Biol. Cell 17, 4606–4618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jentsch S., Rumpf S. (2007) Trends Biochem. Sci. 32, 6–11 [DOI] [PubMed] [Google Scholar]
- 46.Hoffman L., Rechsteiner M. (1996) J. Biol. Chem. 271, 32538–32545 [DOI] [PubMed] [Google Scholar]
- 47.Adams G. M., Crotchett B., Slaughter C. A., DeMartino G. N., Gogol E. P. (1998) Biochemistry 37, 12927–12932 [DOI] [PubMed] [Google Scholar]
- 48.Ma C.-P., Slaughter C. A., DeMartino G. N. (1992) Biochim. Biophys. Acta 1119, 303–311 [DOI] [PubMed] [Google Scholar]
- 49.Hoffman L., Pratt G., Rechsteiner M. (1992) J. Biol. Chem. 267, 22362–22368 [PubMed] [Google Scholar]
- 50.Ryan C. A., Hass G. M., Kuhn R. W. (1974) J. Biol. Chem. 249, 5495–5499 [PubMed] [Google Scholar]
- 51.Rosenzweig R., Osmulski P. A., Gaczynska M., Glickman M. H. (2008) Nat. Struct. Mol. Biol. 15, 573–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ferrell K., Wilkinson C. R., Dubiel W., Gordon C. (2000) Trends Biochem. Sci. 25, 83–88 [DOI] [PubMed] [Google Scholar]
- 53.Hartmann-Petersen R., Tanaka K., Hendil K. B. (2001) Arch. Biochem. Biophys. 386, 89–94 [DOI] [PubMed] [Google Scholar]
- 54.Chen C., Huang C., Chen S., Liang J., Lin W., Ke G., Zhang H., Wang B., Huang J., Han Z., Ma L., Huo K., Yang X., Yang P., He F., Tao T. (2008) Proteomics 8, 508–520 [DOI] [PubMed] [Google Scholar]
- 55.Richmond C., Gorbea C., Rechsteiner M. (1997) J. Biol. Chem. 272, 13403–13411 [DOI] [PubMed] [Google Scholar]
- 56.Isono E., Nishihara K., Saeki Y., Yashiroda H., Kamata N., Ge L., Ueda T., Kikuchi Y., Tanaka K., Nakano A., Toh-e A. (2007) Mol. Biol. Cell 18, 569–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kusmierczyk A. R., Hochstrasser M. (2008) Biol. Chem. 389, 1143–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kusmierczyk A. R., Kunjappu M. J., Funakoshi M., Hochstrasser M. (2008) Nat. Struct. Mol. Biol. 15, 237–244 [DOI] [PubMed] [Google Scholar]
- 59.Murata S., Yashiroda H., Tanaka K. (2009) Nat. Rev. Mol. Cell. Biol. 10, 104–115 [DOI] [PubMed] [Google Scholar]
- 60.Ramos P. C., Dohmen R. J. (2008) Structure 16, 1296–1304 [DOI] [PubMed] [Google Scholar]
- 61.Funakoshi M., Tomko R. J., Jr., Kobayashi H., Hochstrasser M. (2009) Cell 137, 887–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaneko T., Hamazaki J., Iemura S., Sasaki K., Furuyama K., Natsume T., Tanaka K., Murata S. (2009) Cell 137, 914–925 [DOI] [PubMed] [Google Scholar]
- 63.Park S., Roelofs J., Kim W., Robert J., Schmidt M., Gygi S. P., Finley D. (2009) Nature 459, 866–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roelofs J., Park S., Haas W., Tian G., McAllister F. E., Huo Y., Lee B. H., Zhang F., Shi Y., Gygi S. P., Finley D. (2009) Nature 459, 861–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Le Tallec B., Barrault M. B., Guérois R., Carré T., Peyroche A. (2009) Mol. Cell 33, 389–399 [DOI] [PubMed] [Google Scholar]
- 66.Saeki Y., Toh-E A., Kudo T., Kawamura H., Tanaka K. (2009) Cell 137, 900–913 [DOI] [PubMed] [Google Scholar]
- 67.Deveraux Q., Jensen C., Rechsteiner M. (1995) J. Biol. Chem. 270, 23726–23729 [DOI] [PubMed] [Google Scholar]
- 68.Saeki Y., Toh-e A., Yokosawa H. (2000) Biochem. Biophys. Res. Commun. 273, 509–515 [DOI] [PubMed] [Google Scholar]
- 69.Gorbea C., Taillandier D., Rechsteiner M. (2000) J. Biol. Chem. 275, 875–882 [DOI] [PubMed] [Google Scholar]
- 70.Ferdous A., Sikder D., Gillette T., Nalley K., Kodadek T., Johnston S. A. (2007) Genes Dev. 21, 112–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gonzalez F., Delahodde A., Kodadek T., Johnston S. A. (2002) Science 296, 548–550 [DOI] [PubMed] [Google Scholar]
- 72.Collins G. A., Tansey W. P. (2006) Curr. Opin. Genet. Dev. 16, 197–202 [DOI] [PubMed] [Google Scholar]
- 73.Ezhkova E., Tansey W. P. (2004) Mol. Cell 13, 435–442 [DOI] [PubMed] [Google Scholar]
- 74.Lee D., Ezhkova E., Li B., Pattenden S. G., Tansey W. P., Workman J. L. (2005) Cell 123, 423–436 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.