Abstract
Hepatic lipid synthesis is known to be regulated by food consumption. In rodents fasting decreases the synthesis of cholesterol as well as fatty acids. Refeeding a high carbohydrate/low fat diet enhances fatty acid synthesis by 5- to 20-fold above the fed state, whereas cholesterol synthesis returns only to the prefasted level. Sterol regulatory element binding proteins (SREBPs) are transcription factors that regulate genes involved in cholesterol and fatty acid synthesis. Here, we show that fasting markedly reduces the amounts of SREBP-1 and -2 in mouse liver nuclei, with corresponding decreases in the mRNAs for SREBP-activated target genes. Refeeding a high carbohydrate/low fat diet resulted in a 4- to 5-fold increase of nuclear SREBP-1 above nonfasted levels, whereas nuclear SREBP-2 protein returned only to the nonfasted level. The hepatic mRNAs for fatty acid biosynthetic enzymes increased 5- to 10-fold above nonfasted levels, a pattern that paralleled the changes in nuclear SREBP-1. The hepatic mRNAs for enzymes involved in cholesterol synthesis returned to the nonfasted level, closely following the pattern of nuclear SREBP-2 regulation. Transgenic mice that overproduce nuclear SREBP-1c failed to show the normal decrease in hepatic mRNA levels for cholesterol and fatty acid synthetic enzymes upon fasting. We conclude that SREBPs are regulated by food consumption in the mouse liver and that the decline in nuclear SREBP-1c upon fasting may explain in part the decrease in mRNAs encoding enzymes of the fatty acid biosynthetic pathway.
Keywords: cholesterol biosynthesis, fatty acid biosynthesis, transgenic mice, transcriptional regulation
Sterol regulatory element binding proteins (SREBPs) are membrane-bound transcription factors of the basic-helix–loop–helix–leucine zipper (bHLH-Zip) family that have been shown to regulate enzymes responsible for the synthesis of cholesterol, fatty acids, and the low density lipoprotein (LDL) receptor (see ref. 1 for review). Three members of the SREBP family have been identified and characterized (1). SREBP-1a and -1c are derived from a single gene through the use of alternative transcription start sites that produce alternate forms of exon 1 (2). The rat homologue of SREBP-1c (adipocyte determination differentiation-dependent factor 1; ADD1) was independently cloned by Tontonoz, et al. (3) as a transcription factor that bound to E-box sequences and may be involved in adipocyte differentiation. The third protein, SREBP-2, is derived from a separate gene (4).
All SREBPs are synthesized as ≈1150-amino acid precursors bound to the endoplasmic reticulum and nuclear envelope (1). The NH2-terminal segment of ≈500 amino acids contains all of the sequences necessary to activate transcription. To be active, the membrane-bound SREBP must be proteolytically cleaved to release the NH2-terminal segment so that it can enter the nucleus. The cleaved form of SREBPs, designated the nuclear form, binds to 10-bp sterol regulatory elements in the enhancer regions of target genes to activate transcription. The target genes involved in cholesterol metabolism include the LDL receptor, 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) synthase, HMG-CoA reductase, farnesyl-diphosphate (FPP) synthase, squalene synthase, and SREBP-2 (1, 5–8). Genes involved in fatty acid and triglyceride synthesis that are regulated by SREBPs include acetyl-CoA carboxylase (ACC), fatty acid synthase (FAS), and glycerol-3-phosphate acyltransferase (9–12).
In cultured cells, the cleavage of SREBP-1 and -2 is coordinately regulated (1). When cells are overloaded with sterols, the proteolytic cleavage is suppressed, thereby decreasing the active nuclear form of all SREBPs with a consequent decline in transcription of all target genes. In vivo experiments using hamsters fed a cholesterol-rich diet also demonstrated decreased nuclear forms of SREBP-1 and -2 in liver (13). When cultured cells are deprived of sterols by growth in lipoprotein-deficient serum and an HMG-CoA reductase inhibitor (compactin), SREBP cleavage is induced and the active nuclear forms of SREBP-1 and -2 increase (1). Cholesterol depletion had a different effect in liver. When hamsters were fed a cholesterol-depleting diet (lovastatin and colestipol), the nuclear form of SREBP-2 was markedly increased, but the nuclear form of SREBP-1 was decreased (14). A rationale for the discrepancy between cultured cells and liver was provided by the discovery that the predominant form of SREBP-1 in hamster, mouse, and human liver is SREBP-1c (13, 15). All actively growing cell culture lines studied to date have predominantly the SREBP-1a and -2 isoforms (15). We have previously shown that SREBP-1c is a much weaker activator of transcription than is SREBP-1a, presumably because the former has a much shorter transcriptional activation domain (16). Therefore, when animals are fed cholesterol-depleting diets, a weak activator of transcription (SREBP-1c) is replaced by a much stronger activator of transcription (SREBP-2) (1), and this likely explains the increase in mRNAs for cholesterol biosynthetic enzymes.
In studies with transgenic mice overexpressing the nuclear form of SREBP-1c in the liver, we observed 2- to 4-fold increases in mRNAs for genes involved in fatty acid synthesis, including ACC, FAS, and stearoyl-CoA desaturase 1 (SCD-1). In contrast, there were no significant changes in any mRNAs for genes involved in cholesterol synthesis (16).
Physiological regulation of hepatic lipogenic genes is most dramatic in animal studies using fasting/refeeding protocols. Fasting markedly reduces the substrate flux through the fatty acid synthesis pathway (see ref. 17 for review). Similar changes occur in cholesterol synthesis (18). Refeeding a fat-free, high carbohydrate diet induces the synthesis of fatty acids to levels significantly higher than the normal fed state (often referred to as “overshoot”), whereas the synthesis of cholesterol returns only to the prefasted level (17). In white fat, Kim et al. (19) have shown that the mRNA for SREBP-1c/ADD1 is markedly reduced by fasting and elevated upon refeeding. They also showed that SREBP-1c/ADD1 binds to the previously defined insulin response element in the promoter of FAS, suggesting that SREBP-1c/ADD1 could be responsible for feeding-induced changes observed in genes that regulate fatty acid synthesis in adipose tissue.
In the current studies, we employ the fasting/refeeding protocol in wild-type mice to determine if nuclear SREBP-1a, -1c, and -2 are regulated in liver under these metabolic conditions. To further study the effects of SREBP-1c on potential target genes, we take advantage of the rat phosphoenolpyruvate carboxykinase (PEPCK) promoter used to overexpress the dominant-positive form of human SREBP-1c in transgenic mice (16). Fasted SREBP-1c transgenic mice maintain high levels of hepatic SREBP-1c expression, thus allowing the examination of its effects on potential target genes in the fasted liver.
MATERIALS AND METHODS
RNA Analysis.
Northern blots (11) and RNase protection assays (15) of total RNA were carried out as described in the indicated references. A 32P-labeled 25-bp oligonucleotide probe for the 28S ribosomal RNA was used as a loading control (20).
Immunoblotting.
Immunoblot analysis were carried out with the Enhanced Chemiluminesence Western Blotting Detection System kit (Amersham) as described (11). The primary antibodies were as previously described (11, 21).
Wild-Type Mice.
All mice were housed in colony cages with a 14-h light/10-h dark cycle. Six-week-old SJL male mice were purchased from The Jackson Laboratory and adapted to the environment for 2 wk prior to study. For the fasting and refeeding time course experiments, three male mice were included in each treatment group. The treatment groups in the fasting time course consisted of mice fed ad libitum with Teklad 4% (wt/wt) Mouse/Rat Diet 7001 from Harlan Teklad Premier Laboratory Diets (Madison, WI) or mice fasted for 1, 3, 6, 9, 12, or 24 h before sacrifice. For the refeeding time course, mice were fed ad libitum; fasted for 24 h; or fasted for 24 h then refed a high carbohydrate/low fat diet (catalog no. TD 88122; Harlan Teklad Diets) for 1, 3, 6, 9, 12, or 24 h before sacrifice. Pooled liver membranes and nuclear extracts from each group were prepared for immunoblotting as described (11). The starting times for the experiments were staggered so that all mice were sacrificed at the same time, which was 10 h into the light cycle.
For all other fasting/refeeding experiments, 8-wk-old SJL male mice were divided into three treatment groups. Five mice were maintained on Teklad 4% laboratory chow, five were fasted for 24 h, and five were fasted for 24 h then refed the high carbohydrate/low fat diet for 12 h prior to sacrifice as described above. Pooled liver membranes and nuclear extracts were prepared as described above. The characteristics of the mice in this study are shown in Table 1.
Table 1.
Effects of fasting and refeeding on physiologic parameters of wild-type SJL mice
| Parameter | Nonfasted | Fasted | Refed |
|---|---|---|---|
| No. and sex | 5 ♂ | 5 ♂ | 5 ♂ |
| Body weight, g | 21 ± 0.30 | 17 ± 0.17 | 19 ± 0.30 |
| Liver weight, g | 1.2 ± 0.02 | 0.80 ± 0.02 | 1.5 ± 0.08 |
| Liver weight/body weight | 0.058 ± 0.001 | 0.047 ± 0.001 | 0.079 ± 0.003 |
| Liver cholesterol content, mg/g | 2.2 ± 0.07 | 2.5 ± 0.04 | 1.5 ± 0.08 |
| Liver triglyceride content, mg/g | 9.5 ± 1.3 | 16 ± 2.5 | 14 ± 1.9 |
| Total plasma cholesterol, mg/dl | 100 ± 6.4 | 115 ± 2.3 | 78 ± 4.7 |
| Total plasma triglycerides, mg/dl | 159 ± 16 | 128 ± 5.4 | 369 ± 34 |
Each value represents the mean ± SEM. All of the animals were 8 wk old at the time of sacrifice and were fed chow ad libitum, fasted for 24 h, or fasted for 24 h and refed a high carbohydrate/low fat diet for 12 h.
SREBP-1c Transgenic Mice.
A transgene construct encoding amino acids 1–436 of human SREBP-1c under control of the rat PEPCK promoter was made as previously described and used to generate mice overexpressing the nuclear form of SREBP-1c (16). The line used in all transgenic animal studies was TgSREBP-1c436, and the mice were sacrificed at 12 wk of age. Two weeks before sacrifice, six transgenic and six littermate controls were placed on a low carbohydrate/high protein diet (no. 5789C-3) from Purina Mills (St. Louis) containing 71% (wt/wt) casein and 4.25% (wt/wt) sucrose. This diet was used to induce maximal expression of the transgene (16). Prior to sacrifice, three TgSREBP-1c436 and three littermate control mice were fasted for 6 h to further induce the transgene, while the remaining three TgSREBP-1c436 and three control mice were maintained on the low carbohydrate/high protein diet. The phenotypic characteristics of the mice used for this experiment are presented in Table 2.
Table 2.
Phenotypic comparison of wild-type and TgSREBP-1c436 mice in the fed and fasted state
| Parameter | Wild-type
|
TgSREBP-1c436
|
||
|---|---|---|---|---|
| Fed | Fasted | Fed | Fasted | |
| No. and sex | 2 ♂, 1 ♀ | 1 ♂, 2 ♀ | 2 ♂, 1 ♀ | 2 ♂, 1 ♀ |
| Body weight, g | 28.0 ± 0.5 | 25.4 ± 2.8 | 25.0 ± 3.1 | 25.4 ± 2.1 |
| Liver weight, g | 1.47 ± 0.13 | 1.29 ± 0.14 | 1.44 ± 0.24 | 1.39 ± 0.19 |
| Liver weight/body weight | 0.05 ± 0.004 | 0.05 ± 0.003 | 0.06 ± 0.003 | 0.05 ± 0.003 |
| Liver cholesterol content, mg/g | 2.21 ± 0.12 | 2.45 ± 0.04 | 2.41 ± 0.14 | 2.84 ± 0.21 |
| Liver triglyceride content, mg/g | 6.3 ± 2.5 | 12 ± 4.9 | 24 ± 2.9 | 21 ± 4.0 |
| Total plasma cholesterol, mg/dl | 93 ± 19 | 86 ± 18 | 99 ± 14 | 111 ± 12 |
| Total plasma triglycerides, mg/dl | 130 ± 30 | 93 ± 7.2 | 100 ± 14 | 107 ± 14 |
Each value represents the mean ± SEM. All of the mice were 12 wk old at the time of sacrifice and were fed the high protein/low carbohydrate diet for 2 wk before study. Food was removed from fasted mice 6 h before sacrifice.
RESULTS
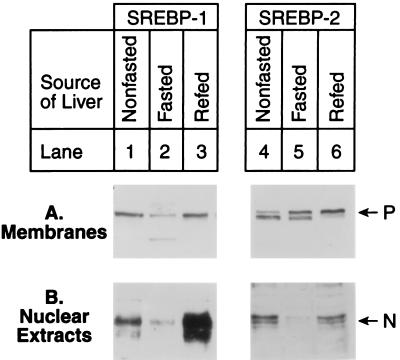
Fig. 1 shows an experiment in which mice were fasted for 24 h and refed a high carbohydrate/low fat diet for 12 h, after which liver extracts were assayed by immunoblotting for the membrane-bound precursor forms and mature nuclear forms of SREBP-1 and -2. Inasmuch as our antibody does not distinguish between SREBP-1a and -1c, we use the noncommittal term SREBP-1. The phenotypic characteristics of the mice used in this study are shown in Table 1. Nonfasted mice showed the precursor forms of both SREBPs in membranes and the cleaved forms in nuclear extracts (Fig. 1, lanes 1 and 4). In this experiment and others, we noted that the SREBP bands often appeared as closely spaced doublets and that the migration of upper and lower bands varied between preparations with no consistent relation to the physiological state of the animal. For purposes of the present study, we consider both bands together to represent either the precursor or nuclear forms of SREBPs.
Figure 1.
Immunoblot analysis of SREBP-1 (lanes 1–3) and SREBP-2 (lanes 4–6) in membranes (A) and nuclear extracts (B) from livers of mice in a nonfasted state (lanes 1 and 4), fasted for 24 h (lanes 2 and 5), or fasted for 24 h and refed a high carbohydrate/low fat diet for 12 h (lanes 3 and 6). For each group, livers from five mice shown in Table 1 were pooled, and aliquots (30 μg) of membranes and nuclear extracts were subjected to SDS/PAGE and electrophoretically transferred to a nitrocellulose filter. Immunoblot analysis was carried out with 5 μg/ml rabbit anti-mouse SREBP-1 IgG (lanes 1–3) or SREBP-2 IgG (lanes 4–6). Bound antibodies were visualized as described in the text. Filters were exposed to film for 60 s for SREBP-1 (lanes 1–3) and 90 s for SREBP-2 (lanes 4–6). P and N denote the precursor and cleaved nuclear forms of SREBP-1 or SREBP-2, respectively.
When the mice were fasted for 24 h, there was a marked decline in the nuclear forms of both SREBPs (Fig. 1B, lanes 2 and 5). The precursor form of SREBP-1 also declined (lane 2), but the precursor form of SREBP-2 did not change (lane 5). When the mice were refed for 12 h, the amount of the precursor form of SREBP-1 returned to normal, and there was a marked increase in the nuclear form, which reached a level that was approximately 4- to 5-fold higher than that seen in nonfasted animals (compare lanes 3 and 1). Refeeding caused no change in the amount of SREBP-2 precursor, and it restored the mature nuclear SREBP-2 to the same level measured in nonfasted animals (compare lanes 6 and 4).
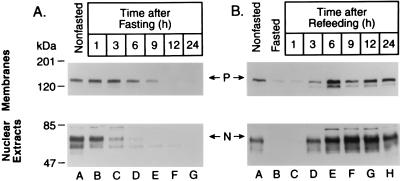
To study the fasting-induced decrease and the refeeding-induced overshoot of nuclear SREBP-1 in more detail, we performed a time-course experiment (Fig. 2). The nuclear form of SREBP-1 declined perceptibly after a 3-h fast, and it was barely detectable after 6 h. At this time point, the precursor form also declined. When the animals had been fasted for 24 h and then refed, the amount of nuclear SREBP-1 reached prefasting levels at 3 h and exceeded this level at 6–24 h.
Figure 2.
Changes in SREBP-1 protein in the livers of mice at various times after fasting (A) and refeeding a high carbohydrate/low fat diet after 24-h fast (B). Livers from three 8-wk-old SJL male mice were pooled for each treatment group, and aliquots (30 μg) of membranes (upper gels) and nuclear extracts (lower gels) were subjected to SDS/PAGE and electrophoretically transferred to a nitrocellulose filter. Immunoblot analysis was carried out with 5 μg/ml rabbit anti-mouse SREBP-1 IgG. Bound antibodies were visualized as described in the text. Filters were exposed to film for 60 s. P and N denote the precursor and nuclear cleaved forms of SREBP-1, respectively.
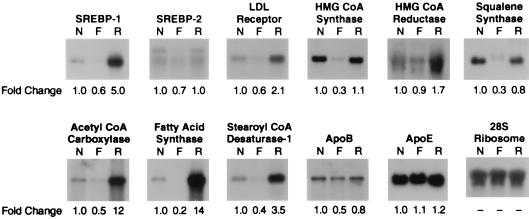
Fig. 3 shows the levels of various lipid-related mRNAs in livers of nonfasted mice, mice fasted for 24 h, and mice fasted for 24 h and refed for 12 h. The mRNAs were assessed by Northern blotting. Fasting reduced the levels of mRNA for SREBP-1 and -2 by 40% and 30%, respectively. Refeeding led to a 5-fold overshoot in the mRNA for SREBP-1, but not SREBP-2. These results paralleled those seen at the nuclear protein level. The mRNAs for some of the cholesterol-supply genes (LDL receptor, HMG-CoA synthase, and squalene synthase) declined by 40–70% upon fasting and returned either to basal levels or to slightly elevated levels after refeeding. The mRNAs for the fatty acid synthetic enzymes (ACC, FAS, and SCD-1) also declined by 60–80% after fasting, and they rose markedly by 3.4- to 14-fold after refeeding. HMG-CoA reductase mRNAs showed little change on fasting but increased on refeeding. The mRNAs for apoproteins B and E showed only minor changes after fasting and refeeding. The hepatic mRNA changes in fasted and refed mice were similar in three additional independent studies (data not shown).
Figure 3.
Amounts of various mRNAs in livers from nonfasted mice (N), mice fasted for 24 h (F), or mice fasted for 24 h and refed a high carbohydrate/low fat diet for 12 h (R) as measured by Northern blot hybridization. The mice are described in Table 1. Total RNA was isolated from five mice in each treatment group, pooled, and subjected to electrophoresis and blot hybridization with the indicated 32P-labeled probe. The amount of radioactivity in each band was quantified by exposure of the filters to a Bio-Imaging Analyzer with BAS1000 MacBus software (Fuji Medical Systems). The fold change in each mRNA relative to that of nonfasted mice was calculated after correction for loading differences by using the 28S ribosomal RNA as a loading control. These values are shown below each blot.
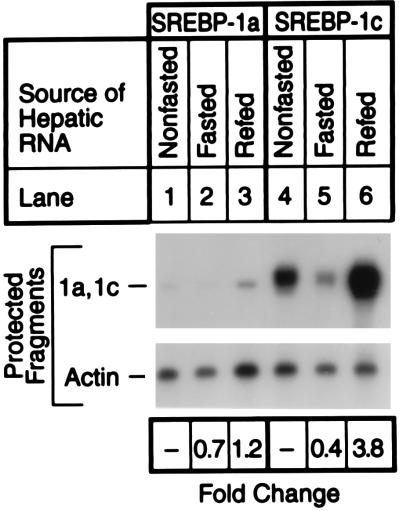
The SREBP-1 gene uses two different promoters to produce two different transcripts, -1a and -1c, that differ only in the first exon (15, 22). To determine whether the fasting–refeeding protocol affected the amounts of one or both transcripts, we performed an RNase protection experiment (Fig. 4). As noted previously, in nonfasted mice the SREBP-1c transcript in the liver was considerably more abundant than the SREBP-1a transcript (15). Fasting reduced the amount of the SREBP-1c transcript by 60%, and refeeding raised the level by 3.8-fold. In contrast, the fasting–refeeding protocol produced only minor changes in the amount of the SREBP-1a transcript. Under all conditions, the SREBP-1c transcript was more abundant than the SREBP-1a transcript, suggesting that the -1c isoform accounted for the bulk of SREBP-1 observed on the immunoblots.
Figure 4.
RNase protection assay for SREBP-1a and SREBP-1c mRNAs from livers of nonfasted mice (lanes 1 and 4), mice fasted for 24 h (lanes 2 and 5), or mice fasted for 24 h and refed a high carbohydrate/low fat diet for 12 h (lanes 3 and 6). The animals are described in Table 1. Pooled total RNA was isolated from five mice in each treatment group, and a 15-μg aliquot was hybridized to 32P-labeled cRNA probes for mouse SREBP-1a, SREBP-1c, and β-actin for 10 min at 68°C. After RNase digestion, the protected fragments were separated by gel electrophoresis and exposed to film with an intensifying screen for 8 h at −80°C. The gels were also quantified in a PhosphorImager as described in the text. The fold change in each mRNA relative to that of the nonfasted mice was calculated after correction for loading differences, using β-actin mRNA as a loading control.
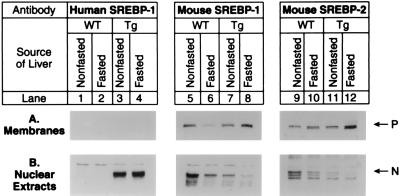
To determine the effects of SREBP-1c expression in the fasted state, we used the previously described transgenic mice that overexpress the nuclear form of human SREBP-1c (16). The transgene was constructed by fusing the PEPCK promoter to a cDNA encoding amino acids 1–436 of human SREBP-1c. Inasmuch as the PEPCK promoter is more active in the fasted state than in the fed state, expression of nuclear SREBP-1c was maintained in livers of fasted TgSREBP-1c436 mice. Table 2 compares quantitative parameters of wild-type and TgSREBP-1c436 littermate mice sacrificed in the fed state or after a 6-h fast. Fig. 5 shows immunoblots of the membrane-bound precursor and nuclear forms of human SREBP-1c (transgene) and endogenous mouse SREBP-1 and -2 in pooled samples from the same livers. Expression of the human SREBP-1c transgene protein is similar in the fasted and nonfasted states (lanes 3 and 4). The nuclear forms of endogenous SREBP-1 and -2 were again decreased in livers from fasted wild-type mice compared with nonfasted mice (lanes 5 vs. 6 and lanes 9 vs. 10). The nuclear forms of endogenous SREBP-1 and -2 in the SREBP-1c transgenic mice were essentially suppressed in both the fasted and nonfasted states (lanes 7 vs. 8 and lanes 11 vs. 12).
Figure 5.
Immunoblot analysis of transgenic human SREBP-1c436 (lanes 1–4), endogenous mouse SREBP-1 (lanes 5–8), and endogenous mouse SREBP-2 (lanes 9–12) in membranes (A) and nuclear extracts (B) from livers of wild-type (WT) and TgSREBP-1c436 (Tg) mice in the nonfasted state or after fasting for 6 h as indicated. For each group, livers from mice shown in Table 2 were pooled, and aliquots (30 μg) of membranes and nuclear extracts were subjected to SDS/PAGE and electrophoretically transferred to a nitrocellulose filter. Immunoblot analysis was carried out using 5 μg/ml rabbit anti-human SREBP-1 IgG (lanes 1–4), anti-mouse SREBP-1 IgG (lanes 5–8), or anti-mouse SREBP-2 IgG (lanes 9–12) as the primary antibody. The anti-mouse SREBP-1 IgG antibody is specific for mouse SREBP-1 and does not cross-react with the human SREBP-1 transgene product. Bound antibodies were visualized as described in the text. Filters were exposed to film for 15 s for the truncated human SREBP-1, 25 s for mouse SREBP-1, and 90 s for mouse SREBP-2.
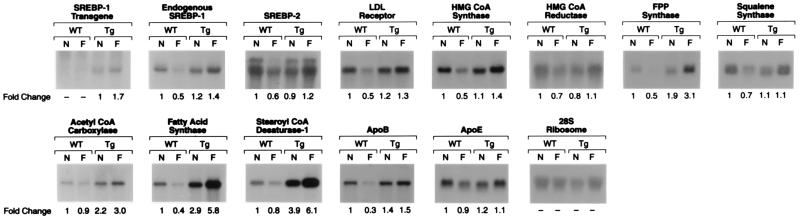
Fig. 6 shows Northern blots of various mRNAs involved in cholesterol and fatty acid metabolism in livers from wild-type and TgSREBP-1c436 mice that were either fed ad libitum (nonfasted) or fasted for 6 h. The mRNAs involved in cholesterol synthesis and regulation (SREBP-1, SREBP-2, LDL receptor, HMG-CoA synthase, HMG-CoA reductase, FPP synthase, and squalene synthase) were decreased by 30–50% in the livers of fasted wild-type mice. However, in livers from fasted TgSREBP-1c436 mice, the cholesterologenic mRNAs did not decrease but either were maintained or were slightly greater than the levels measured in livers from nonfasted TgSREBP-1c436 mice. The hepatic mRNAs for the fatty acid synthetic enzymes (ACC, FAS, and SCD-1) were decreased in the fasted wild-type mice. The fold decrease was less dramatic than the fold decreases shown in Fig. 3, possibly owing to the shorter fasting period. mRNAs encoding fatty acid synthetic enzymes were 2- to 4-fold higher in nonfasted TgSREBP-1c436 than in nonfasted wild-type mice. These changes are consistent with previously published results (16). In the livers of fasted TgSREBP-1c436 mice, the fatty acid synthetic enzyme mRNAs were further increased by 1.4- to 2-fold above nonfasted levels. The 28S ribosome RNA and mRNAs for apoprotein E were essentially unchanged in all groups. The hepatic mRNA changes for wild-type and TgSREBP-1c436 were similar in two additional independent studies (data not shown).
Figure 6.
Amounts of various mRNAs in livers from wild-type (WT) and TgSREBP-1c436 (Tg) mice in the nonfasted state (N) or fasted (F) for 6 h as measured by blot hybridization. The mice used in this experiment are described in Table 2. Total RNA was isolated, pooled, and subjected to electrophoresis and blot hybridization with the indicated 32P-labeled probe. The amount of radioactivity in each band was quantified as described in the text. The fold change in each mRNA relative to that of wild-type nonfasted mice was calculated after correction for loading differences by using the 28S ribosomal RNA as a loading control. These values are shown below each blot.
DISCUSSION
In the current experiments with SJL mice, the amount of hepatic nuclear SREBP-1 declined after fasting, and it increased approximately 4-fold above nonfasted levels when the mice were refed a high carbohydrate/low fat diet (Fig. 1). The changes in SREBP-1 protein were accompanied by a corresponding change in the amount of SREBP-1c mRNA, suggesting that the fasting–refeeding protocol changed the synthesis rate of the full-length SREBP-1c precursor (Figs. 3 and 4). Because the amount of SREBP-1c precursor protein in liver membranes from refed animals was not increased compared with nonfasted animals, the finding of an increase in the nuclear form of SREBP-1c after refeeding suggests that refeeding also increased the cleavage of the SREBP-1c precursor. However, it is also possible there is a concomitant reduction in the rate of SREBP-1c degradation.
The nuclear form of SREBP-2 decreased after fasting, but it returned only to basal levels after refeeding. The changes in nuclear SREBP-2 coincided with changes in its mRNA level. Despite the fall in mRNA during fasting, the amount of precursor in the fasted state did not fall. This result suggests that fasting reduces both the synthesis and the cleavage of SREBP-2.
Considered together, the data suggest that the changes observed in nuclear levels of SREBP-1 and -2 with fasting and refeeding are a result of both transcriptional and post-transcriptional mechanisms. We have also observed changes in the nuclear forms of SREBP-1 and -2 in epididymal fat pads from fasted and refed mice identical to those observed in the liver (unpublished observations). The changes that we observed in nuclear SREBP-1 in white fat are similar to the changes in SREBP-1c/ADD1 mRNA that were recently reported by Kim et al. (19).
The fall in nuclear SREBP-1 and -2 during fasting correlated with reductions in the levels of mRNAs encoding cholesterol and fatty acid biosynthetic enzymes as well as the LDL receptor. Upon refeeding the large overshoot in nuclear SREBP-1 paralleled the overshoot in the levels of mRNA for the lipogenic enzymes, such as ACC and FAS (Figs. 1 and 3). These mRNAs increased to levels more than 10-fold above nonfasted levels after refeeding. In contrast, the mRNAs for cholesterologenic enzymes (HMG-CoA synthase, HMG-CoA reductase, and squalene synthase) and the LDL receptor rose only to basal levels during refeeding. This correlated with the changes in nuclear SREBP-2. These data are consistent with other accumulating data suggesting that SREBP-2 may be more specific than SREBP-1c in regulating cholesterologenic genes.
To test the hypothesis that the fall in SREBP-1 is responsible for the fall in fatty acid synthesis in fasted mice, we studied this regulation in transgenic mice that overproduce SREBP-1c in a nonsuppressible fashion. In SREBP-1c transgenic mice the level of mRNAs for cholesterologenic enzymes, fatty acid synthase and the LDL receptor, failed to decline during fasting. This result suggests that SREBP-1c, though a weaker activator of transcription, can support basal mRNA levels of target enzymes in both the fatty acid and cholesterol synthesis pathways during fasting.
The conclusion that SREBP-1c is important in maintaining the basal level of transcription of ACC, FAS, and SCD-1 is supported by recently published data obtained from the SREBP-1 knockout mouse (21). Mice homozygous for the disrupted SREBP-1 allele had decreased basal hepatic mRNA levels of ACC, FAS, and SCD-1 and had significantly reduced rates of hepatic fatty acid synthesis as measured by [3H]water incorporation (21).
The lipogenic response to refeeding in liver is generally thought to reflect the reciprocal effects of insulin and glucagon which rise and fall, respectively, after refeeding a high carbohydrate diet (23). The molecular mechanisms included in the refeeding response are complex, but reportedly involve transcriptional activation, mRNA stability, and catalytic activation of several key lipogenic enzymes (24). The transcriptional activation of these lipogenic genes occurs through promoter elements that are often referred to as carbohydrate or insulin response elements. Insulin response elements have been localized in the promoters of several genes involved in fatty acid and triglyceride synthesis (see ref. 23 for review), although no clear consensus sequence has been identified. The specific transcription factors involved in the insulin response have been difficult to identify and characterize.
The insulin response element in the promoter of the FAS gene has been intensively studied. Recent work has localized this element to an E-box sequence at position −64 to −59 (19, 25). This region has also been shown to be critical for the transcriptional activation of FAS by SREBPs (10, 19). Magaña and Osborne (10) reported SREBP-1a and -2 bind to two separate sites, each of which contains one half of the E-box at −64. However, Kim et al. (19) have shown that SREBP-1c/ADD1 can bind to E-box promoter sequences (CANNTG) as well as sterol regulatory elements (ATCACCCCAC) associated with genes involved in cholesterol metabolism (26, 27). They conclude that SREBP-1c/ADD1 binds to the E-box at −64 in the FAS promoter and that SREBP-1c/ADD1 is the insulin response factor responsible for the changes in FAS mRNA observed in adipose tissue from fasted and refed rats. On the other hand, Wang and Sul (25) have published evidence that upstream stimulatory factors (USF)-1 and -2 bind to the same E-box at −64 in the FAS promoter and mediate the insulin response independent of SREBPs. The current data with the SREBP-1c transgenic mice support the idea that forced expression of nuclear SREBP-1c raises the mRNAs for lipogenic enzymes even during fasting. These data indicate that SREBP-1c is sufficient to drive transcription of these genes, but they do not address the question of whether the USFs would also be sufficient under the same circumstances. More detailed promoter studies will be required to determine the relative contributions of SREBP-1c and/or USFs to the insulin response for each particular gene known to have such an element.
Acknowledgments
We thank Drs. Michael S. Brown and Joseph L. Goldstein for their continued encouragement and critical reading of the manuscript. We also thank Richard Gibson for invaluable help with the animals and Scott Clark and Robin Craddock for excellent technical assistance. This work was supported by research funds from the National Institutes of Health (HL20948), the Moss Heart Foundation, and the Perot Family Foundation. J.D.H. is the recipient of a Postdoctoral Fellowship for Physicians from the Howard Hughes Medical Institute. I.S. is the recipient of a postdoctoral fellowship from the Manpei Suzuki Diabetes Foundation of Tokyo, Japan.
Footnotes
Abbreviations: ACC, acetyl-CoA carboxylase; ADD, adipocyte determination differentiation-dependent factor; FAS, fatty acid synthase; FPP, farnesyl diphosphate; HMG-CoA, 3-hydroxy-3-methylglutaryl-CoA; LDL, low density lipoprotein; PEPCK, phosphoenolpyruvate carboxykinase; SCD-1, stearoyl-CoA desaturase 1; SREBP, sterol regulatory element binding protein.
References
- 1.Brown M S, Goldstein J L. Cell. 1997;89:331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 2.Yokoyama C, Wang X, Briggs M R, Admon A, Wu J, Hua X, Goldstein J L, Brown M S. Cell. 1993;75:187–197. [PubMed] [Google Scholar]
- 3.Tontonoz P, Kim J B, Graves R A, Spiegelman B M. Mol Cell Biol. 1993;13:4753–4759. doi: 10.1128/mcb.13.8.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hua X, Yokoyama C, Wu J, Briggs M R, Brown M S, Goldstein J L, Wang X. Proc Natl Acad Sci USA. 1993;90:11603–11607. doi: 10.1073/pnas.90.24.11603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vallett S M, Sanchez H B, Rosenfeld J M, Osborne T F. J Biol Chem. 1996;271:12247–12253. doi: 10.1074/jbc.271.21.12247. [DOI] [PubMed] [Google Scholar]
- 6.Ericsson J, Jackson S M, Lee B C, Edwards P A. Proc Natl Acad Sci USA. 1996;93:945–950. doi: 10.1073/pnas.93.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan G, Dai P-H, Osborne T F, Kim J B, Shechter I. J Biol Chem. 1997;272:10295–10302. doi: 10.1074/jbc.272.15.10295. [DOI] [PubMed] [Google Scholar]
- 8.Sato R, Inoue J, Kawabe Y, Kodama T, Takano T, Maeda M. J Biol Chem. 1996;271:26461–26464. doi: 10.1074/jbc.271.43.26461. [DOI] [PubMed] [Google Scholar]
- 9.Magaña M M, Lin S S, Dooley K A, Osborne T F. J Lipid Res. 1997;38:1630–1638. [PubMed] [Google Scholar]
- 10.Magaña M M, Osborne T F. J Biol Chem. 1996;271:32689–32694. doi: 10.1074/jbc.271.51.32689. [DOI] [PubMed] [Google Scholar]
- 11.Shimano H, Horton J D, Hammer R E, Shimomura I, Brown M S, Goldstein J L. J Clin Invest. 1996;98:1575–1584. doi: 10.1172/JCI118951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ericsson J, Jackson S M, Kim J B, Spiegelman B M, Edwards P A. J Biol Chem. 1997;272:7298–7305. doi: 10.1074/jbc.272.11.7298. [DOI] [PubMed] [Google Scholar]
- 13.Shimomura I, Bashmakov Y, Shimano H, Horton J D, Goldstein J L, Brown M S. Proc Natl Acad Sci USA. 1997;94:12354–12359. doi: 10.1073/pnas.94.23.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheng Z, Otani H, Brown M S, Goldstein J L. Proc Natl Acad Sci USA. 1995;92:935–938. doi: 10.1073/pnas.92.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shimomura I, Shimano H, Horton J D, Goldstein J L, Brown M S. J Clin Invest. 1997;99:838–845. doi: 10.1172/JCI119247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimano H, Horton J D, Shimomura I, Hammer R E, Brown M S, Goldstein J L. J Clin Invest. 1997;99:846–854. doi: 10.1172/JCI119248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hillgartner F B, Salati L M, Goodridge A G. Physiol Rev. 1995;75:47–76. doi: 10.1152/physrev.1995.75.1.47. [DOI] [PubMed] [Google Scholar]
- 18.Tomkins G M, Chaikoff I L. J Biol Chem. 1952;192:569–573. [PubMed] [Google Scholar]
- 19.Kim J B, Sarraf P, Wright M, Yao K M, Mueller E, Solanes G, Lowell B B, Spiegelman B M. J Clin Invest. 1998;101:1–9. doi: 10.1172/JCI1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hassouna N, Michot B, Bachellerie J-P. Nucleic Acids Res. 1984;12:3563–3583. doi: 10.1093/nar/12.8.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimano H, Shimomura I, Hammer R E, Herz J, Goldstein J L, Brown M S, Horton J D. J Clin Invest. 1997;100:2115–2124. doi: 10.1172/JCI119746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hua X, Wu J, Goldstein J L, Brown M S, Hobbs H H. Genomics. 1995;25:667–673. doi: 10.1016/0888-7543(95)80009-b. [DOI] [PubMed] [Google Scholar]
- 23.Towle H C. J Biol Chem. 1995;270:23235–23238. doi: 10.1074/jbc.270.40.23235. [DOI] [PubMed] [Google Scholar]
- 24.Iritani N. Eur J Biochem. 1992;205:433–442. doi: 10.1111/j.1432-1033.1992.tb16797.x. [DOI] [PubMed] [Google Scholar]
- 25.Wang D, Sul H S. J Biol Chem. 1997;272:26367–26374. doi: 10.1074/jbc.272.42.26367. [DOI] [PubMed] [Google Scholar]
- 26.Briggs M R, Yokoyama C, Wang X, Brown M S, Goldstein J L. J Biol Chem. 1993;268:14490–14496. [PubMed] [Google Scholar]
- 27.Wang X, Briggs M R, Hua X, Yokoyama C, Goldstein J L, Brown M S. J Biol Chem. 1993;268:14497–14504. [PubMed] [Google Scholar]