Abstract
Roles of hydrogen bonding interaction between Ser186 of the actuator (A) domain and Glu439 of nucleotide binding (N) domain seen in the structures of ADP-insensitive phosphorylated intermediate (E2P) of sarco(endo)plasmic reticulum Ca2+-ATPase were explored by their double alanine substitution S186A/E439A, swap substitution S186E/E439S, and each of these single substitutions. All the mutants except the swap mutant S186E/E439S showed markedly reduced Ca2+-ATPase activity, and S186E/E439S restored completely the wild-type activity. In all the mutants except S186E/E439S, the isomerization of ADP-sensitive phosphorylated intermediate (E1P) to E2P was markedly retarded, and the E2P hydrolysis was largely accelerated, whereas S186E/E439S restored almost the wild-type rates. Results showed that the Ser186-Glu439 hydrogen bond stabilizes the E2P ground state structure. The modulatory ATP binding at sub-mm∼mm range largely accelerated the EP isomerization in all the alanine mutants and E439S. In S186E, this acceleration as well as the acceleration of the ATPase activity was almost completely abolished, whereas the swap mutation S186E/E439S restored the modulatory ATP acceleration with a much higher ATP affinity than the wild type. Results indicated that Ser186 and Glu439 are closely located to the modulatory ATP binding site for the EP isomerization, and that their hydrogen bond fixes their side chain configurations thereby adjusts properly the modulatory ATP affinity to respond to the cellular ATP level.
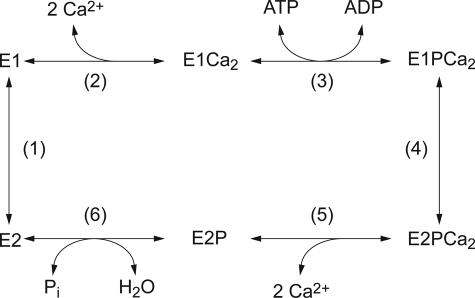
Sarcoplasmic reticulum Ca2+-ATPase (SERCA1a)2 is a representative member of P-type ion-transporting ATPases and catalyzes Ca2+ transport coupled with ATP hydrolysis (Fig. 1) (1–9). In the catalytic cycle, the enzyme is activated by binding of two Ca2+ ions at the transport sites (E2 to E1Ca2, steps 1–2) and then autophosphorylated at Asp351 with MgATP to form ADP-sensitive phosphoenzyme (E1P, step 3), which can react with ADP to regenerate ATP. Upon formation of this EP, the bound Ca2+ ions are occluded in the transport sites (E1PCa2). The subsequent isomeric transition to ADP-insensitive form (E2P) results in a change in the orientation of the Ca2+ binding sites and reduction of their affinity, and thus Ca2+ release into lumen (steps 4 and 5). Finally, the hydrolysis takes place and returns the enzyme into an unphosphorylated and Ca2+-unbound form (E2, step 6). E2P can also be formed from Pi in the presence of Mg2+ and the absence of Ca2+ by reversal of its hydrolysis.
FIGURE 1.
Reaction cycle of sarco(endo)plasmic reticulum Ca2+-ATPase.
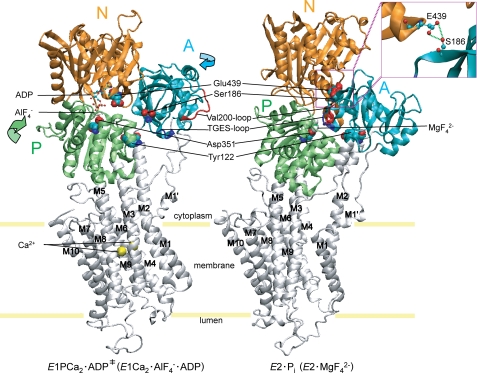
The cytoplasmic three domains N, A, and P largely move and change their organization states during the Ca2+ transport cycle (10–22). These changes are linked with the rearrangements in the transmembrane helices. In the EP isomerization (loss of ADP sensitivity) and Ca2+ release, the A domain largely rotates (by ∼110° parallel to membrane plane), intrudes into the space between the N and P domains, and the P domain largely inclines toward the A domain. Thus in E2P, these domains produce the most compactly organized state (see Fig. 2 for the change E1Ca2·AlF4−·ADP →E2·MgF42− as the model for the overall process E1PCa2·ADP‡ → E2·Pi).
FIGURE 2.
Structure of SERCA1a and formation of Ser186-Glu439 hydrogen bond between the A and N domains. The coordinates for the structures E1Ca2·AlF4−·ADP, (the analog for the transition state of the phosphoryl transfer E1PCa2·ADP‡, left panel) and E2·MgF42− (E2·Pi analog (21), right panel) of Ca2+-ATPase were obtained from the Protein Data Bank (PDB accession code 1T5T and 1WPG, respectively (12, 14)). The arrows indicate approximate movements of the A and P domains in the change from E1Ca2·AlF4− ·ADP to E2·MgF42−. Ser186 and Glu439 are depicted as van der Waals spheres. These two residues form a hydrogen bond in E2·MgF42− (see inset). The phosphorylation site Asp351, two Ca2+ at the transport sites and ADP with AlF4− at the catalytic site in E1Ca2·AlF4−·ADP, MgF42− bound at the catalytic site in E2·MgF42− are depicted. The TGES184 loop and Val200 loop of the A domain and Tyr122 on the top part of M2 are shown. These elements produce three interaction networks between A and P domains and M2 (Tyr122) in E2·MgF42− (23–26). M1′ and M1-M10 are also indicated.
We have found that the interactions between the A and P domains at the Val200-loop (Asp196-Asp203) with the residues of the P domain (Arg678/Glu680/Arg656/Asp660) (23) and at the Tyr122 hydrophobic cluster (24–26) (see Fig. 2) play critical roles for Ca2+ deocclusion/release in E2PCa2 → E2P + 2Ca2+ after the loss of ADP sensitivity (E1PCa2 to E2PCa2 isomerization). The proper length of the A/M1′ linker is critical for inducing the inclining motion of the A and P domains for the Ca2+ deocclusion and release from E2PCa2 (27, 28). The importance of the interdomain interaction between Arg678 (P) and Asp203 (A) in stabilizing the E2P and E2 intermediates and its influence on modulatory ATP activation were pointed out by the mutation R678A (29). Regarding the N domain, the importance of Glu439 in the EP isomerization and E2P hydrolysis was previously noted by its alanine substitution, and possible importance of its interaction with Ser186 on the A domain has been suggested since Glu439 forms a hydrogen bond with Ser186 in the E2P analog structures (29) (see Fig. 2). The Darier disease-causing mutations of Ser186 of SERCA2b, S186P and S186F also alter the kinetics of the EP processing and its importance as the residue in the immediate vicinity of TGES184 has been pointed out (30, 31). Notably also, Glu439 is situated near the adenine binding pocket and its importance in the ATP binding and ATP-induced structural change have been shown (32, 33). In the structure E2(TG)AMPPCP (E2·ATP), Glu439 interacts with the modulatory ATP binding via Mg2+, and is involved in the acceleration of the Ca2+-ATPase cycle (16).
Considering these critical findings on each of Glu439 and Ser186, it is crucial to reveal the role of the Ser186-Glu439 hydrogen-bonding interaction between the A and N domains in the EP processing and its ATP modulation (i.e. regulatory ATP-induced acceleration). We therefore made a series of mutants on both Ser186 and Glu439 including the swap substitution mutant, S186A, E439A, S186A/E439A, S186E, E439S, S186E/E439S, and explored their kinetic properties. Results showed that the Ser186-Glu439 hydrogen bond is critical for the stabilization of the E2P ground state structure, and possibly functioning as to make the E2P resident time long enough for Ca2+ release (E2PCa2 → E2P + 2Ca2+) thus to avoid its hydrolysis without Ca2+ release. Results also revealed that the side-chain configurations of Ser186 and Glu439 are fixed by their hydrogen bond, thereby conferring the proper modulatory ATP binding to occur at the cellular ATP level to accelerate the rate-limiting EP isomerization.
EXPERIMENTAL PROCEDURES
Mutagenesis and Expression
The Stratagene QuikChangeTM site-directed mutagenesis method (Stratagene, La Jolla, CA) was utilized for the substitution in the rabbit SERCA1a cDNA. The ApaI-KpnI or KpnI-SalI restriction fragments with the desired mutation were excised from the plasmid and ligated back into the corresponding region in the full-length SERCA1a cDNA in the pMT2 expression vector (34). The pMT2 DNA was transfected into COS-1 cells by the liposome-mediated transfection method. Microsomes were prepared from the cells as described (35). “Control microsomes” were prepared from COS-1 cells transfected with the pMT2 vector containing no SERCA1a cDNA. The amount of expressed SERCA1a was quantified by immunosorbent assay (36). Expression levels of wild-type SERCA1a and the mutants were 2–3% of total microsomal proteins.
Ca2+-ATPase Activity
The rate of ATP hydrolysis was determined at 25 °C with 20 μg/ml microsomal protein in various concentrations of ATP, 1 μm A23187, 0.1 m KCl, 7 mm MgCl2, 0.05 mm CaCl2, or 5 mm EGTA, and 50 mm MOPS/Tris (pH 7.0), otherwise as noted in the legends for figures. The Ca2+-ATPase activity of the expressed SERCA1a of the microsomes was obtained by subtracting the Ca2+-ATPase activity of the control microsome.
Formation and Hydrolysis of EP
Phosphorylation of SERCA1a in microsomes with [γ-32P]ATP or 32Pi, and dephosphorylation of 32P-labeled SERCA1a were performed as described in the legends to figures. The reactions were quenched with ice-cold trichloroacetic acid containing Pi. Rapid kinetics measurements were performed with a handmade rapid mixing apparatus (37). The precipitated proteins were separated by 5% SDS-PAGE at pH 6.0 according to Weber and Osborn (38). The radioactivity associated with the separated Ca2+-ATPase was quantitated by digital autoradiography (39). The amount of EP formed with the expressed SERCA1a was obtained by subtracting the background radioactivity with the control microsomes, which was less than 5% of the radioactivity of EP of the expressed wild-type SERCA1a.
Miscellaneous
Protein concentrations were determined by the method of Lowry et al. (40) with bovine serum albumin as a standard. Data were analyzed by nonlinear regression using the program Origin (Microcal Software, Inc., Northampton, MA). The concentrations of free Ca2+, Mg2+, ATP, and MgATP were calculated by Calcon program. Three-dimensional models of the enzyme were reproduced by the program VMD (41).
RESULTS
Ca2+-ATPase Activity
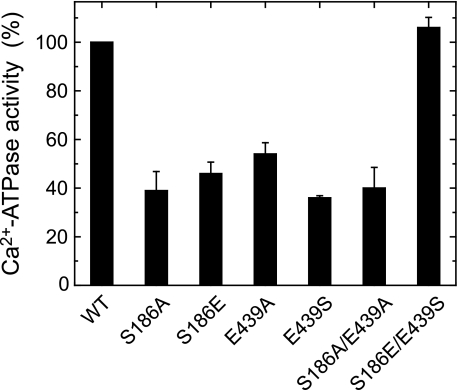
All the mutants S186A, S186E, E439A, E439S, S186A/E439A, and S186E/E439S were expressed in COS-1 cells at the levels comparable to the wild type. The amounts of EP formed with ATP at a saturating 50 μm Ca2+ in the mutants were comparable to the wild type (see the maximum levels in supplemental Fig. S1). In Fig. 3, the Ca2+-ATPase activities of the mutants and wild type in the microsomes were determined at 0.1 mm MgATP, and the turnover rate was calculated by dividing the activity with the amount of maximum EP. The mutations S186A, S186E, E439A, E439S, and S186A/E439A caused significant reduction of the activity, whereas the swap mutation S186E/E439S completely restored the wild-type activity. The result for E439A agrees with the previous finding by Clausen et al. (29).
FIGURE 3.
Ca2+-ATPase activities of expressed SERCA1a. The Ca2+-ATPase activity of microsomes expressing the wild-type or mutant SERCA1a was determined with 0.1 mm [γ-32P]ATP as described under “Experimental Procedures.” The activities are divided by the amount of EP formed at steady state (see supplemental Fig. S1), and the turnover rates thus obtained are shown as percentage of that of the wild type (7.29 ± 0.42 s−1 (n = 5)). The values of the mutants presented are the mean ± S.D. (n = 3–5).
Ca2+ Affinity and E2 to E1Ca2 Transition
The Ca2+ affinities at the transport sites estimated by the activation of EP formation were nearly the same in all the mutants as in the wild type (Table 1 and supplemental Fig. S2). The rates of the E2 to E1Ca2 transition were then determined at pH 6 where the equilibrium between E1 and E2 in the absence of Ca2+ is most shifted to E2 (42). In this experiment, the enzyme was first preincubated without Ca2+ and then phosphorylated by simultaneous addition of saturating Ca2+ and ATP. The time courses of EP formation were well described by first-order kinetics (supplemental Fig. S1), and the rates obtained are listed in Table 1. When ATP was added to the enzyme preincubated with Ca2+ (E1Ca2) otherwise as above, the EP formation was much faster and completed within ∼1 s; therefore, the rates obtained above reflect the rate-limiting E2 to E1Ca2 transition. The substitutions of Ser186 and Glu439 did not give much changes, although S186E/E439S somewhat increased the rate.
TABLE 1.
Kinetic parameters determined for partial reaction steps
The affinity of the transport sites for Ca2+ was estimated in supplemental Fig. S2 as the Ca2+-induced activation of EP formation, and the K0.5 value and Hill coefficient (nH) are shown. The rate of the E2 to E1Ca2 transition in steps 1–2 were determined by the E1PCa2 formation from the E2 state (i.e. the rate-limiting E2 to E1Ca2 transition in steps 1–2) upon the simultaneous addition of saturating 100 μm Ca2+ and ATP in supplemental Fig. S1. The rates in the other steps were obtained in Fig. 5 (loss of ADP sensitivity in steps 4–5 in the absence of K+), Fig. 6 (decay of E1PCa2 formed from ATP in the presence of Ca2+ (EPATP), i.e. rate-limiting E1PCa2 to E2P isomerization in steps 4–5 in 0.1 m K+), and Fig. 7 (hydrolysis of E2P formed from Pi in the absence of Ca2+ (E2PPi) in step 6). The concentrations of BeF3− and AlF4− (K0.5) giving the half-maximum formation of E2·BeF3− and E2·AlF4− from the E2 state were obtained in supplemental Fig. S3.
| Affinity for Ca2+ |
E2 to E1Ca2 | Loss of ADP sensitivity |
Decay of EPATPa |
Hydrolysis of E2PPi |
K0.5 in formation of E2P analog |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K0.5 | nH | (−)K+ | (+)K+ | (+)K+ | BeF3− | AlF4− | ||||||||
| μm | s−1 | (%) | s−1 | (%) | s−1 | (%) | s−1 | (%) | μm (%) | |||||
| Wild type | 0.149 | 2.26 | 0.184 | (100) | 0.270 | (100) | 0.0250 | (100) | 0.144 | (100) | 3.2 | (100) | 6.7 | (100) |
| S186A | 0.181 | 1.76 | 0.194 | (106) | —b | 0.0034 | (14) | 0.590 | (410) | 5.2 | (162) | 8.3 | (124) | |
| S186E | 0.127 | 1.84 | 0.254 | (139) | 0.184 | (68) | 0.0159 | (64) | 0.119 | (83) | 2.2 | (68) | 4.3 | (64) |
| E439A | 0.172 | 2.00 | 0.174 | (95) | —b | 0.0067 | (27) | 0.452 | (314) | 6.7 | (217) | 6.2 | (92) | |
| E439S | 0.142 | 1.86 | 0.250 | (136) | —b | 0.0121 | (49) | 0.306 | (213) | 8.7 | (273) | 13.4 | (200) | |
| S186A/E439A | 0.154 | 1.78 | 0.116 | (63) | —b | 0.0052 | (21) | 0.491 | (341) | 5.4 | (169) | 6.6 | (99) | |
| S186E/E439S | 0.146 | 1.64 | 0.363 | (198) | 0.185 | (68) | 0.0221 | (89) | 0.261 | (181) | 3.5 | (110) | 5.9 | (88) |
a The rate most likely reflects the rate-limiting E1PCa2 to E2P transition in step 4 in the presence of 0.1 m K±.
b Not determined because E2P was not accumulated.
E1PCa2 to E2P Isomerization and Decay of E1PCa2 Formed from ATP
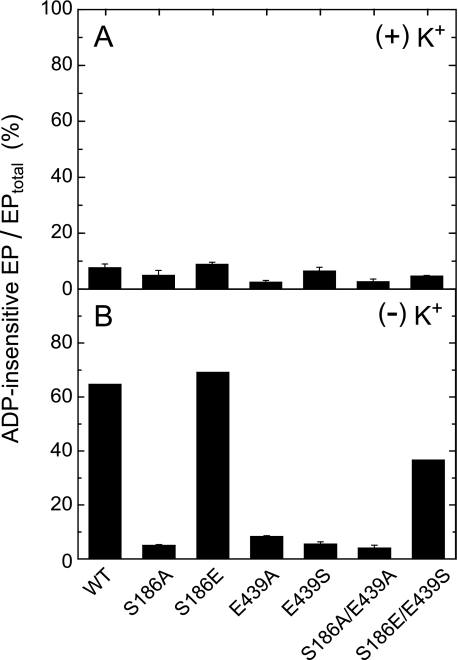
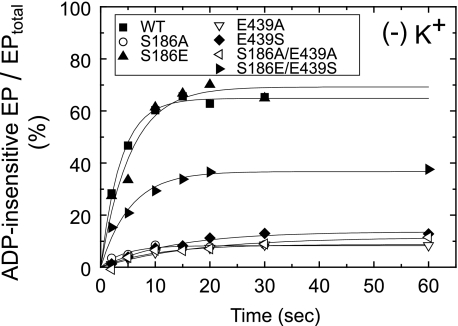
In Fig. 4, the accumulation of ADP-insensitive EP (E2P) in the presence and absence of K+ was determined at 0 °C and at 15 s (nearly the steady state) after addition of 10 μm ATP. In the presence of K+, which strongly accelerates the hydrolysis of E2P and thus suppresses its accumulation in the wild type (43, 44), the fraction of accumulated E2P in all the mutants was very low as in the wild type. In the absence of K+, the E2P accumulation largely increased in the wild type, S186E, and S186E/E439S. By contrast, the E2P accumulation in S186A, E439A, E439S, and S186A/E439A was very low even in the absence of K+, therefore the E1PCa2 to E2P isomerization was likely retarded and/or the E2P hydrolysis was accelerated in these mutants.
FIGURE 4.
Accumulation of ADP-insensitive EP in the presence (A) and absence (B) of K+. Microsomes expressing the wild type or mutant were phosphorylated with 10 μm [γ-32P]ATP at 0 °C for 15 s in 50 μl of a mixture containing 2.5 μg of microsomal protein, 1 μm A23187, 7 mm MgCl2, 0.05 mm CaCl2, 50 mm MOPS/Tris (pH 7.0), and 0.1 m KCl (A) or 0.1 m LiCl without KCl (B). The total amount of EP formed was determined by the acid-quenching. For determination of ADP-insensitive EP (E2P), an equal volume of a mixture containing 10 mm ADP, 7 mm MgCl2, 10 mm EGTA, 50 mm MOPS/Tris (pH 7.0), and 0.1 m KCl (A) or LiCl without KCl (B) was added to the above phosphorylation mixture, and the reaction was quenched at 1 s after the ADP addition. ADP-sensitive EP (E1PCa2) disappeared entirely within 1 s after the ADP addition. The amount of E2P is shown as percentage of the total amount of EP.
In Fig. 5, the time course of E2P accumulation upon the addition of ATP to E1Ca2 was determined in the absence of K+. The total amount of EP reached its maximum level very rapidly (within ∼1 s) and remained unchanged during the period of observation and therefore the time course actually reflects the accumulation of E2P from E1PCa2. The E2P accumulation proceeded with first-order kinetics, and the rates obtained are listed in Table 1. The rates in the mutants S186E and S186E/E439S were only slightly slowed. In S186A, E439A, S186A/E439A, and E439S, E2P was not accumulated.
FIGURE 5.
Time course of accumulation of ADP-insensitive EP from E1Ca2 and ATP. Microsomes expressing the wild type or mutant were phosphorylated with [γ-32P]ATP at 0 °C for various periods in the presence of 0.1 m LiCl without KCl otherwise as in Fig. 4B. The total amount of EP and the amount of ADP-insensitive EP (E2P) were determined at the indicated time without and with the ADP addition in 0.1 m LiCl (no KCl), otherwise as in Fig. 4B. Solid lines show the least squares fit to a single exponential, and the apparent rates to reach the steady-state E2P level are given in Table 1.
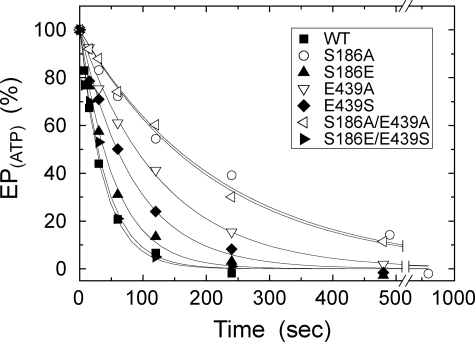
For the analysis of the E1PCa2 to E2P isomerization in the presence of K+, E1PCa2 was first accumulated with ATP and its decay time course was determined in Fig. 6. This is because, as well known with the wild type (44), the E1PCa2 decay reflects the rate-limiting E1PCa2 to E2P isomerization followed by the rapid E2P hydrolysis. In fact, almost all of EP present at each time point was E1PCa2 in the mutants as well as in the wild type (data not shown, but see Fig. 4A). The decay time courses were well fitted with a single exponential, and the rates obtained are listed in Table 1. In S186A, E439A, and S186A/E439A, the decay rate was markedly reduced to 14∼27% of the wild type. The rate was also reduced significantly in E439S and to some extent in S186E. In the swap mutant S186E/E439S, the wild-type rate was almost restored.
FIGURE 6.
Decay of E1PCa2 formed from ATP. Microsomes expressing the wild type or mutant were phosphorylated in 0.1 m KCl at 0 °C for 15 s as in Fig. 4A. Phosphorylation was terminated by addition of an equal volume of a buffer containing 8 mm EGTA, 0.1 m KCl, 7 mm MgCl2, and 50 mm MOPS/Tris (pH 7.0) at 0 °C. The total amount of EP remaining after the EGTA addition was determined at the indicated time. The total amounts of EP obtained at zero time (i.e. immediately before the EGTA addition) are normalized to 100%. Solid lines show the least squares fit to a single exponential, and the decay rates obtained are given in Table 1.
Hydrolysis of E2P Formed from Pi
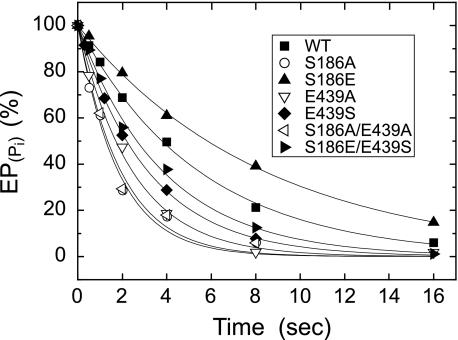
The E2P hydrolysis was examined by first phosphorylating the enzyme with 32Pi in the absence of Ca2+ and K+ and presence of 35% (v/v) Me2SO, which extremely favors E2P formation (45), and then by diluting the phosphorylated samples at 0 °C with a large volume of solution containing non-radioactive Pi and K+ without Ca2+ (Fig. 7). The conditions were thus made otherwise the same as those for the decay of E1PCa2 formed from ATP (E1PCa2 to E2P isomerization) in Fig. 6. Hydrolysis of 32P-labeled E2P proceeded with first-order kinetics, and the rates obtained are listed in Table 1. As compared with the wild type, the rate was markedly increased in the alanine mutants S186A, E439A, S186A/E439A, and also in E439S at a lower extent. The result of E439A is consistent with the previous observation by Clausen et al. (29). The rate was slightly decreased in S186E, in contrast to the marked increase in S186A and the Darier disease mutant S186F of SERCA2b (30). The swap mutant S186E/E439S exhibited an intermediate rate between those of E439S and S186E, and thus brought the markedly increased rate of E439S toward the wild-type one.
FIGURE 7.
Hydrolysis of E2P formed from Pi without Ca2+. Microsomes expressing the wild type or mutant were phosphorylated with 0.1 mm 32Pi at 25 °C for 10 min in 5 μl of a mixture containing 2 μg of microsomal protein, 20 μm A23187, 1 mm EGTA, 7 mm MgCl2, 50 mm MOPS/Tris (pH 7.0), and 35% (v/v) Me2SO. The mixture was then cooled and diluted at 0 °C by 100 μl of a mixture containing 1.05 mm non-radioactive Pi, 105 mm KCl, 15.8 mm EGTA, and 50 mm MOPS/Tris (pH 7.0). At different times after the dilution, the E2P hydrolysis was quenched by acid. The amounts of E2P formed with 32Pi at zero time are normalized to 100%. Solid lines show the least squares fit to a single exponential, and the rates obtained are given in Table 1.
Note that the overall Ca2+-ATPase activity was significantly inhibited in all the alanine mutants, S186E, and E439S, despite the markedly accelerated E2P hydrolysis (or unretarded hydrolysis in S186E). Therefore, the inhibition of the Ca2+-ATPase activity in the mutants is ascribed to the retardation of the rate-limiting E1PCa2 to E2P isomerization.
BeF3− and AlF4− Affinities in Formation of E2·BeF3− and E2·AlF4−
The E2 state Ca2+-ATPase in the absence of Ca2+ forms the complexes E2·BeF3− and E2·AlF4−, which are analogs of the E2P ground state and of the transition state of the E2P hydrolysis, respectively (22). In supplemental Fig. S3 and Table 1, the effects of the mutations on the affinities for BeF3− and AlF4− were determined by changing the beryllium and aluminum concentrations in the presence of excess 2 mm fluoride and by determining the inhibition of EP formation from ATP. In the E2·BeF3− formation, the mutations S186A, E439A, S186A/E439A, and E439S significantly decreased the BeF3− affinity, S186E increased slightly, and the swap mutation S186E/E439S restored the wild-type affinity. In the E2·AlF4− formation, the mutation effects were much less pronounced or not exhibited. The results agree with the mutation effects on the E2P hydrolysis (Fig. 7) that the alanine mutations and E439S markedly enhance the hydrolysis rate, S186E reduces slightly, and the swap mutation S186E/E439A restores the wild-type rate. The results indicated that the hydrogen-bonding interaction between Ser186 and Glu439 functions to stabilize the E2P ground state.
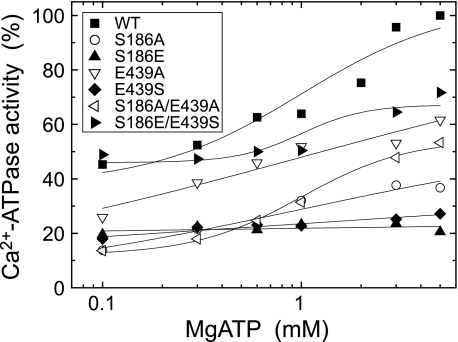
Modulatory MgATP-induced Acceleration of Ca2+-ATPase Activity
As known for a long time (46), the Ca2+-ATPase activity of the wild type is markedly increased by MgATP at sub-mm ∼ mm range. This modulatory MgATP effect was examined at various MgATP concentrations in Fig. 8. The mutants S186A, E439A, and S186A/E439A exhibited the marked increase with increasing MgATP concentration despite their significantly reduced activity at the low ATP concentrations (see Fig. 3). The result on E439A is consistent with the previous observation by Clausen et al. (29). The activity was increased only slightly in E439S with MgATP and not increased at all in S186E. In the swap mutant S186E/E439S, the activity was increased with MgATP, and thus the MgATP modulatory effect as well as the activity was almost restored. In Figs. 9–11, the modulation by MgATP and free ATP was further explored in each of the steps; the E1PCa2 to E2P isomerization and the E2P hydrolysis.
FIGURE 8.
MgATP dependence of Ca2+-ATPase activity. The Ca2+-ATPase activity of microsomes expressing the wild type or mutant was determined at various concentrations of [γ-32P]ATP otherwise as in Fig. 3. Almost all of ATP (more than 97% of total ATP) is in MgATP. The activities of the mutants are presented as a percentage of that of the wild type at 5 mm MgATP.
FIGURE 9.
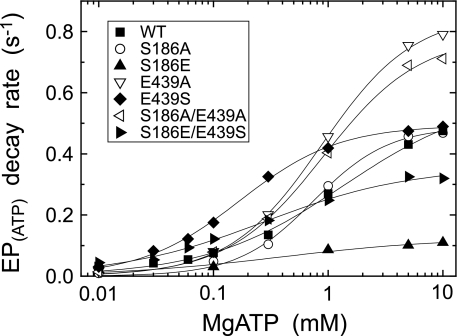
MgATP dependence of the decay rate of E1PCa2 formed from ATP. E1PCa2 was first formed in 50 μl of a microsomes suspension in 10 μm [γ-32P]ATP, 10 μm Ca2+ (0.98 mm CaCl2 with 1 mm EGTA), and 0.1 m KCl, otherwise as in Fig. 4A. Phosphorylation was terminated by 100 μl of a buffer containing 8 mm EGTA, 1 μm A23187, 0.1 m KCl, 50 mm MOPS/Tris (pH 7.0), and various concentrations of ATP and MgCl2 (producing MgATP (more than 97% of the total ATP) with 6.2 mm free Mg2+). At different times after this MgATP addition, the decay reaction of E1PCa2 at 0 °C was quenched by acid. The rate of the single exponential decay of E1PCa2 obtained was plotted versus the MgATP concentration. Solid lines show the least squares fit to the Hill equation, and the parameters V0 (the rate at the lowest 10 μm MgATP), Vmax (the maximum rate), and K0.5 (MgATP concentration giving the half-maximal change) are given in Table 2.
FIGURE 10.
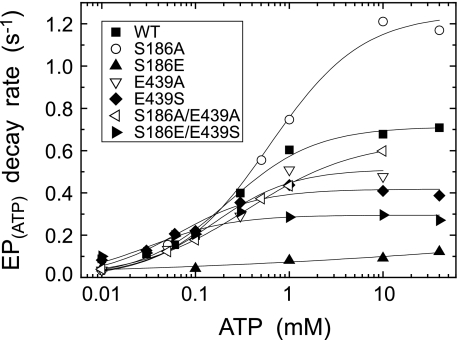
ATP dependence of the decay rate of E1PCa2 formed from ATP. The E1PCa2 decay was followed after addition of various concentrations of ATP and 30 mm EDTA without MgCl2 (in place of 8 mm EGTA) otherwise as in Fig. 9. The single exponential decay rate of E1PCa2 obtained was plotted versus the metal-free ATP concentration, and the parameters V0, Vmax, and K0.5 estimated as in Fig. 9 are given in Table 2.
FIGURE 11.
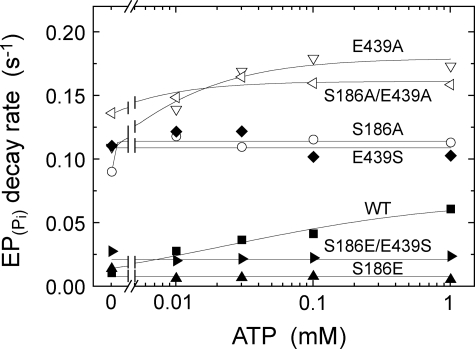
ATP dependence of the rate of E2P hydrolysis. Microsomes expressing the wild type or mutant were phosphorylated with 32Pi at 25 °C for 10 min in 4 μl of a mixture containing 1.6 μg of microsomal protein, 0.1 mm 32Pi, 20 μm A23187, 1 mm EGTA, 7 mm MgCl2, 50 mm MOPS/Tris (pH 7.0), and 35% (v/v) Me2SO. The mixture was then diluted at 0 °C by addition of 196 μl of a mixture containing 1.02 mm non-radioactive Pi, 50 mm MOPS/Tris (pH 7.0), 15.3 mm EDTA, and various concentration of ATP. At different times after the dilution, the E2P hydrolysis was quenched by acid. The rate of the single exponential E2P hydrolysis was plotted versus the ATP concentration, and the parameters V0 (the rate without ATP), Vmax (the rate at the highest ATP concentration, 1 mm), and K0.5 (ATP concentration giving the half-maximal change) are given in Table 2. It should be noted that Mg2+ bound at the catalytic site of E2P is occluded (53), and therefore the E2P hydrolysis takes place even after removal of free Mg2+.
Modulatory MgATP- and ATP-induced Acceleration of E1PCa2 to E2P Isomerization
E1PCa2 was first formed with 10 μm MgATP in the presence of K+ under conditions in which E1PCa2 accumulates dominantly (see Fig. 4A). Then, the MgATP modulation of the E1PCa2 decay (the rate-limiting E1PCa2 to E2P isomerization) was examined by the subsequent addition of various concentrations of MgATP together with an excess EGTA to remove free Ca2+. The decay time courses were fitted well with a single exponential (data not shown). In Fig. 9, the rates thus determined are plotted versus the MgATP concentration. The values V0 (the rate at 10 μm MgATP), Vmax (the maximum rate at a saturating MgATP concentration), and K0.5 (giving the half-maximum rate) are obtained by fitting the curves to the Hill equation and shown in Table 2. In the wild-type, MgATP enhanced the EP isomerization rate by 18-fold (Vmax/V0) with K0.5 of 1.35 mm. In the alanine mutants S186A, E439A, and S186A/E439A, the magnitudes of MgATP-induced acceleration were much larger (54-, 50-, and 56-fold, respectively) with slightly higher MgATP affinities than the wild type. In E439S and the swap mutant S186E/E439S, the magnitudes of MgATP-induced acceleration were similar to or somewhat smaller than the wild type (16.5- and 8.3-fold, respectively) and their MgATP affinities were markedly higher than the wild type. In S186E, the MgATP-induced acceleration was least (5.5-fold), and consequently, the rate in this mutant at the high MgATP concentrations, e.g. at 10 mm became much lower than the wild type. Note that this markedly reduced rate in S186E was increased by the swap mutation S186E/E439S to the significant level close to the wild type.
TABLE 2.
Parameters determined for the MgATP/ATP-induced acceleration of partial reaction steps
The modulatory MgATP- and metal-free ATP-induced acceleration of the decay rate of EPATP formed from ATP and Ca2+ (i.e. the rate-limiting E1PCa2 to E2P isomerization) and that of the hydrolysis of E2PPi formed from Pi in the absence of Ca2+ were determined as described in Figs. 9–11. The kinetic parameters in the nucleotide dependence curves were obtained by the fitting to the Hill equation in Figs. 9 and 10, or roughly by the eye inspection in Fig. 11; K0.5 (the MgATP or ATP concentration giving the half-maximum acceleration), V0 (the rate at 10 μm MgATP or ATP in Figs. 9 and 10, and no ATP in Fig. 11), and Vmax (the maximum rate in Figs. 9 and 10, or the rate at the highest 1 mm ATP in Fig. 11).
| MgATP dependence of EPATP decay |
ATP dependence of EPATP decay |
ATP dependence of E2PPi hydrolysis |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| (+)Mg2+ |
(−)Mg2+ |
(−)Mg2+ |
|||||||
| K0.5 | V0 | Vmax | K0.5 | V0 | Vmax | K0.5 | V0 (no ATP) | Vmax (1 mm ATP) | |
| mm | s−1 | s−1 | mm | s−1 | s−1 | μm | s−1 | s−1 | |
| Wild type | 1.351 | 0.033 | 0.591 | 0.224 | 0.088 | 0.714 | 60 | 0.016 | 0.066 |
| S186A | 0.738 | 0.009 | 0.489 | 0.612 | 0.033 | 1.248 | —a | 0.095 | 0.118 |
| S186E | —a | 0.020 | 0.110b | —a | 0.045 | 0.121c | —a | 0.019 | 0.010 |
| E439A | 0.874 | 0.017 | 0.857 | 0.155 | 0.035 | 0.513 | 10 | 0.115 | 0.178 |
| E439S | 0.170 | 0.030 | 0.494 | 0.065 | 0.082 | 0.418 | —a | 0.116 | 0.108 |
| S186A/E439A | 0.913 | 0.014 | 0.786 | 0.360 | 0.039 | 0.647 | 12 | 0.141 | 0.164 |
| S186E/E439S | 0.281 | 0.043 | 0.357 | 0.032 | 0.010 | 0.295 | —a | 0.033 | 0.029 |
a Not determined.
b The value at the highest (10 mm) ATP.
c The value at the highest (40 mm) ATP.
The modulatory effect of metal-free ATP on the EP isomerization was also explored, in this case, by adding various concentrations of ATP together with an excess EDTA to remove free Ca2+ and Mg2+ (Fig. 10). The E1PCa2 decay time courses of the wild type and mutants were apparently fitted to a single exponential kinetics but not strictly (data not shown). This complicated kinetics may be because Mg2+ bound at the catalytic site was likely removed by the added EDTA in some E1PCa2 fraction as previously also noted (29). Nevertheless, for simplicity, the single exponential rates estimated were plotted in Fig. 10. The parameters K0.5, V0 (at 10 μm ATP), Vmax in the ATP dependence curve are listed in Table 2.
In the wild type, the affinity of metal-free ATP for the modulation was significantly higher than that of MgATP (29); thus Mg2+ in MgATP brings its modulatory binding to correspond to cellular level of ATP (mostly MgATP complex). In the alanine mutants S186A, E439A, and S186A/E439A (most profoundly in S186A), the increase in free ATP concentration from 0.01 to 40 mm exhibited the marked acceleration of the E1PCa2 decay by 38-, 15-, and 17-fold, respectively, which are even much more than that of the wild type (8-fold). The free ATP affinities in the alanine mutants were similar to and not higher than the wild type. The results of E439A are in agreement with the previous study by Clausen et al. (29).
In E439S, the extent of the acceleration was 5.1-fold and slightly smaller than that of the wild type with somewhat increased ATP affinity. In S186E, the ATP-induced acceleration of EP isomerization was very small (2.7-fold), and actually at the highest 40 mm ATP, the isomerization rate was only 17% of the wild-type rate. However, in the swap mutation S186E/E439S, the marked ATP-induced acceleration (by 30-fold) was restored with the ATP affinity much higher than the wild type.
ATP Modulation of E2P Hydrolysis
The modulation of the E2P hydrolysis was examined only with metal-free ATP, because metal-free ATP but not MgATP is able to bind to E2P for the modulation with a reasonable affinity (47, 48). E2P was first formed by 32Pi in the absence of Ca2+ and K+ and presence of Me2SO as in Fig. 7, then the phosphorylated sample was largely diluted with a buffer containing excess EDTA and various concentrations of ATP, and the E2P hydrolysis was followed in the absence of K+ (in the presence of K+, the hydrolysis especially at high ATP was too fast to be followed). In Fig. 11, the single exponential rates of E2P hydrolysis were plotted versus the ATP concentration. In the absence of K+ without ATP, the hydrolysis rate (V0 in Table 2) was significantly faster in the mutants S186A, E439A, S186A/E439A, and E439S than in the wild type, as was found in the presence of K+ without ATP (Fig. 7 and Table 1). The V0 of S186E was similar to that of the wild type, and the swap mutant S186E/E439S exhibited the intermediate V0 value between S186E and E439S; thus, restored almost the wild-type rate from the markedly enhanced one in E439S.
With increasing ATP to 1 mm, the hydrolysis rate in the wild type increased by 4.1-fold. In S186E and in S186E/E439S, the rate was not increased or rather decreased slightly by 1 mm ATP; therefore, the swap mutation did not restore the ATP modulation. No ATP acceleration was found with E439S, although its rate was markedly elevated as compared with the wild type. In E439A, S186A, and S186A/E439A, the markedly elevated rates without ATP were slightly increased with increasing ATP by 1.2∼1.5-fold.
DISCUSSION
Roles of Ser186-Glu439 Interactions in E1PCa2 to E2P Isomerization and E2P Hydrolysis in the Absence of Modulatory ATP
In the absence of modulatory ATP, we observed here that the substitutions of Ser186 and Glu439, especially by alanine(s) S186A, E439A, and S186A/E439A result in the markedly retarded E1PCa2 to E2P isomerization and markedly accelerated E2P hydrolysis. Such changes were also found in E439S and S186E with a somewhat less extent (in S186E the change was only the retardation of the EP isomerization). Most importantly, these changes as well as the inhibition of the overall Ca2+-ATPase activity were almost relieved by the swap mutation S186E/E439S, and thus the wild-type properties were restored. The results demonstrated that the Ser186-Glu439 hydrogen bond between the A and N domains functions to stabilize the E2P structure, consistent with the prediction made with the mutation E439A (29). The loss of the Ser186-Glu439 interdomain interaction destabilized the E2P structure. In theory, the transition state structure is close to the product state (e.g. see the textbook by Fersht, Ref. 49); therefore, the transition state of the E1PCa2 to E2P isomerization is probably also destabilized by the loss of the interaction causing the retardation of EP isomerization.
Note in Table 1 that the disruption of the Ser186-Glu439 hydrogen bond by the mutations caused the reduction of the BeF3− affinity for the E2·BeF3− formation concomitantly with the marked acceleration of the E2P hydrolysis, and that the swap mutation S186E/E439S restored the wild type properties. The results obviously show that the E2P ground state is stabilized by the Ser186-Glu439 hydrogen bond. This interaction therefore functions to retard the catalytic structural events, which involve rearrangement of the catalytic site for the attack of the TGES184-coordinated specific water on the Asp351-acylphosphate. The E2P stabilization may be important to avoid the too (un-physiologically) rapid E2P hydrolysis, and thereby making the resident time of E2P long enough for Ca2+-release (E2PCa2 → E2P + 2Ca2+) and preventing a possible E2PCa2 hydrolysis without Ca2+-release for the energy coupling.
Regarding the positioning of the TGES184 loop, the A domain largely rotates for the E1PCa2 to E2P isomerization and its outermost TGES184 comes above the Asp351 region of the P domain; therefore blocking the access of the ADP β-phosphate to Asp351-acylphosphate. For the subsequent acylphosphate hydrolysis, the rearrangement in the catalytic site takes place so as to produce the appropriate positioning of the TGES184 loop with the coordinated attacking water molecule. Because Ser186 is situated in the immediate C terminus of TGES184, it is likely that the Ser186-Glu439 hydrogen bond in E2P may be stabilizing the TGES184 loop at the position after the loss of the ADP sensitivity but before gaining the catalytic activity for the Asp351-acylphosphate hydrolysis. Then, during the rearrangement of the catalytic site structure via the transition state for the hydrolysis, E2P + H2O → E2 + Pi, the Ser186-Glu439 hydrogen bond would be lost.
In the crystal structures of E2·BeF3− (E2P ground state), Ser186 and Glu439 are actually closely located (2.5–2.8 Å) with their hydrogen bond, whereas in E2·AlF4− (transition state of E2P hydrolysis) they are more separated (2.9–3.4 Å) (15, 18, 19). In the E2(TG) structures, the two residues are far more separated (∼10 Å). The loss of the Ser186-Glu439 interaction is of course associated with the motions and separation of the cytoplasmic domains A, N, and P. Such rearrangement is actually reflected by the change in the catalytic site from the strongly hydrophobic closed structure in the E2P ground state to the hydrophilic opened one in the transition state and product E2·Pi state as well as in the E2 state (22).
We examined the structure of the mutants S186E/E439S, S186E, and E439S with the crystal structures E2·BeF3−, 2ZBE (18) and 3B9B (19) by the program Swiss-PdbViewer (50). The structural modeling of the mutants actually agreed with their kinetic consequences and with the predicted roles of the Ser186-Glu439 hydrogen-bonding interaction in the EP processing (i.e. marked effects by the alanine mutations, smaller effects in E439S, and further smaller effects in S186E, and the restoration in S186E/E439S). Namely, in S186E/E439S, the two introduced residues are able to produce their hydrogen bond as in the wild type. In S186E, the introduced glutamate is still potentially able to produce a hydrogen bond with the partner residue Glu439. In this case, pKa of the introduced glutamate was estimated by ProPKa (51) to become extremely high, 7.61 (2ZBE) or 9.20 (3B9B); therefore, it is protonated thus avoiding repulsion with Glu439. In E439S, the introduced small serine may not reach the partner residue (Ser186) but possibly produces a polar interaction. In the alanine mutants, the introduced non-polar small alanine(s) at the position(s) of Ser186 and Glu439 is obviously not able to produce a hydrogen bond.
Ser186-Glu439 Interaction in ATP Modulation of EP Processing
The retarded E1PCa2 to E2P isomerization in the alanine mutants S186A, E439A, and S186A/E439A was markedly accelerated with increasing ATP/MgATP, and the isomerization became even faster in some mutants than in the wild type at high ATP/MgATP concentrations. Thus the modulatory ATP/MgATP binding overcame the mutation-induced destabilization of E2P structure. Results indicate that Ser186 and Glu439 are not involved directly in the modulatory ATP/MgATP binding for the E1PCa2 to E2P isomerization. Results also show that formation of both the Ser186-Glu439 interdomain hydrogen bond and the structure needed for the modulatory ATP/MgATP binding occurs upon the E1PCa2 to E2P isomerization, in which the A, N, and P domains gather to form the most compactly organized state. The necessity for a close interaction between the A and N domains was recently supported by the observation (52) that the residues of the A domain Arg174, Ile188, and Lys205 are involved in the binding of modulatory ATP/MgATP in the E2P state. Our mutations further showed that S186E disrupts seriously the modulatory ATP/MgATP acceleration of the E1PCa2 to E2P isomerization, and that the swap mutation S186E/E439S restores almost the wild-type acceleration. The introduced long and negatively charged glutamate in S186E likely caused a steric collision (repulsion) with the modulatory ATP/MgATP thus inhibiting the modulation. In S186E/E439S, the hydrogen bond between the introduced two residues probably fixed the glutamate configuration of S186E; thereby avoided its inhibitory effect and restored the ATP/MgATP modulation of the EP isomerization. Notably, the affinities of S186E/E439S and E439S for the modulatory ATP and MgATP in the EP isomerization were significantly higher than those of the wild type (see Table 2), indicating the importance of the Ser186-Glu439 hydrogen bond in the affinity of the modulatory MgATP (ATP) suitable to its cellular level.
In S186E, the ATP modulation of the E2P hydrolysis was also lost. The swap mutation S186E/E439S did not restore it, in contrast to the restored modulation of the E1PCa2 to E2P isomerization. Results show that the structure of the modulatory ATP binding site and/or configuration of ATP for the E1PCa2 to E2P isomerization are distinct from those for the E2P hydrolysis; therefore altered for (during) the E2P hydrolysis. This agrees with the fact that the E2P hydrolysis involves separation of the N, A, and P domains to the more loosely organized state in E2. Actually, in E2(TG)AMPPCP structure, Ser186 is far from Glu439 and ATP (10∼13 Å), and Glu439 participates in binding of the ATP phosphate moiety via Mg2+ (16). In E2·AlF4− with bound AMPPCP, Ser186 is still close to Glu439 and the bound modulatory ATP (AMPPCP α-phosphate). The modulatory ATP likely accelerates the release of the A domain (Ser186) from the N domain (Glu439). As Glu439 becomes involved in the modulatory ATP binding during the E2P hydrolysis to E2, the swap mutation S186E/E439S did not restore the ATP modulation of the E2P hydrolysis that is disrupted in S186E.
Supplementary Material
Acknowledgments
We thank Dr. David H. MacLennan, University of Toronto, for the generous gift of SERCA1a cDNA and Dr. Randal J. Kaufman, Genetics Institute, Cambridge, MA, for the generous gift of the expression vector pMT2. We are also grateful to Dr. Chikashi Toyoshima, University of Tokyo, for helpful discussions.
This work was supported by a grant-in-aid for scientific research (B) (to H. S.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
- SERCA1a
- adult fast-twitch skeletal muscle sarcoplasmic reticulum Ca2+-ATPase
- EP
- phosphoenzyme
- E1P
- ADP-sensitive phosphoenzyme
- E2P
- ADP-insensitive phosphoenzyme
- MOPS
- 3-(N-morpholino)propanesulfonic acid
- TG
- thapsigargin
- PDB
- Protein Data Bank
- A
- actuator domain
- N
- nucleotide binding domain
- P
- phosphorylation domain.
REFERENCES
- 1.Hasselbach W., Makinose M. (1961) Biochem. Z. 333, 518–528 [PubMed] [Google Scholar]
- 2.Ebashi S., Lipmann F. (1962) J. Cell Biol. 14, 389–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inesi G., Sumbilla C., Kirtley M. E. (1990) Physiol. Rev. 70, 749–760 [DOI] [PubMed] [Google Scholar]
- 4.Møller J. V., Juul B., le Maire M. (1996) Biochim. Biophys. Acta 1286, 1–51 [DOI] [PubMed] [Google Scholar]
- 5.MacLennan D. H., Rice W. J., Green N. M. (1997) J. Biol. Chem. 272, 28815–28818 [DOI] [PubMed] [Google Scholar]
- 6.McIntosh D. B. (1998) Adv. Mol. Cell. Biol. 23, 33–99 [Google Scholar]
- 7.Toyoshima C., Inesi G. (2004) Annu. Rev. Biochem. 73, 269–292 [DOI] [PubMed] [Google Scholar]
- 8.Toyoshima C. (2008) Arch. Biochem. Biophys. 476, 3–11 [DOI] [PubMed] [Google Scholar]
- 9.Toyoshima C. (2009) Biochim. Biophys. Acta 1793, 941–946 [DOI] [PubMed] [Google Scholar]
- 10.Toyoshima C., Nakasako M., Nomura H., Ogawa H. (2000) Nature 405, 647–655 [DOI] [PubMed] [Google Scholar]
- 11.Toyoshima C., Nomura H. (2002) Nature 418, 605–611 [DOI] [PubMed] [Google Scholar]
- 12.Sørensen T. L.-M., Møller J. V., Nissen P. (2004) Science 304, 1672–1675 [DOI] [PubMed] [Google Scholar]
- 13.Toyoshima C., Mizutani T. (2004) Nature 430, 529–535 [DOI] [PubMed] [Google Scholar]
- 14.Toyoshima C., Nomura H., Tsuda T. (2004) Nature 432, 361–368 [DOI] [PubMed] [Google Scholar]
- 15.Olesen C., Sørensen T. L., Nielsen R. C., Møller J. V., Nissen P. (2004) Science 306, 2251–2255 [DOI] [PubMed] [Google Scholar]
- 16.Jensen A. M., Sørensen T. L., Olesen C., Møller J. V., Nissen P. (2006) EMBO J. 25, 2305–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi M., Kondou Y., Toyoshima C. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 5800–5805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toyoshima C., Norimatsu Y., Iwasawa S., Tsuda T., Ogawa H. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 19831–19836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olesen C., Picard M., Winther A. M., Gyrup C., Morth J. P., Oxvig C., Møller J. V., Nissen P. (2007) Nature 450, 1036–1042 [DOI] [PubMed] [Google Scholar]
- 20.Danko S., Daiho T., Yamasaki K., Kamidochi M., Suzuki H., Toyoshima C. (2001) FEBS Lett. 489, 277–282 [DOI] [PubMed] [Google Scholar]
- 21.Danko S., Yamasaki K., Daiho T., Suzuki H., Toyoshima C. (2001) FEBS Lett. 505, 129–135 [DOI] [PubMed] [Google Scholar]
- 22.Danko S., Yamasaki K., Daiho T., Suzuki H. (2004) J. Biol. Chem. 279, 14991–14998 [DOI] [PubMed] [Google Scholar]
- 23.Kato S., Kamidochi M., Daiho T., Yamasaki K., Gouli W., Suzuki H. (2003) J. Biol. Chem. 278, 9624–9629 [DOI] [PubMed] [Google Scholar]
- 24.Yamasaki K., Daiho T., Danko S., Suzuki H. (2004) J. Biol. Chem. 279, 2202–2210 [DOI] [PubMed] [Google Scholar]
- 25.Wang G., Yamasaki K., Daiho T., Suzuki H. (2005) J. Biol. Chem. 280, 26508–26516 [DOI] [PubMed] [Google Scholar]
- 26.Yamasaki K., Wang G., Daiho T., Danko S., Suzuki H. (2008) J. Biol. Chem. 283, 29144–29155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daiho T., Yamasaki K., Wang G., Danko S., Iizuka H., Suzuki H. (2003) J. Biol. Chem. 278, 39197–39204 [DOI] [PubMed] [Google Scholar]
- 28.Daiho T., Yamasaki K., Danko S., Suzuki H. (2007) J. Biol. Chem. 282, 34429–34447 [DOI] [PubMed] [Google Scholar]
- 29.Clausen J. D., McIntosh D. B., Anthonisen A. N., Woolley D. G., Vilsen B., Andersen J. P. (2007) J. Biol. Chem. 282, 20686–20697 [DOI] [PubMed] [Google Scholar]
- 30.Dode L., Andersen J. P., Leslie N., Dhitavat J., Vilsen B., Hovnanian A. (2003) J. Biol. Chem. 278, 47877–47889 [DOI] [PubMed] [Google Scholar]
- 31.Miyauchi Y., Daiho T., Yamasaki K., Takahashi H., Ishida-Yamamoto A., Danko S., Suzuki H., Iizuka H. (2006) J. Biol. Chem. 281, 22882–22895 [DOI] [PubMed] [Google Scholar]
- 32.Ma H., Inesi G., Toyoshima C. (2003) J. Biol. Chem. 278, 28938–28943 [DOI] [PubMed] [Google Scholar]
- 33.Inesi G., Ma H., Lewis D., Xu C. (2004) J. Biol. Chem. 279, 31629–31637 [DOI] [PubMed] [Google Scholar]
- 34.Kaufman R. J., Davies M. V., Pathak V. K., Hershey J. W. B. (1989) Mol. Cell. Biol. 9, 946–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maruyama K., MacLennan D. H. (1988) Proc. Natl. Acad. Sci. U.S.A. 85, 3314–3318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daiho T., Yamasaki K., Suzuki H., Saino T., Kanazawa T. (1999) J. Biol. Chem. 274, 23910–23915 [DOI] [PubMed] [Google Scholar]
- 37.Kanazawa T., Saito M., Tonomura Y. (1970) J. Biochem. 67, 693–711 [DOI] [PubMed] [Google Scholar]
- 38.Weber K., Osborn M. (1969) J. Biol. Chem. 244, 4406–4412 [PubMed] [Google Scholar]
- 39.Daiho T., Suzuki H., Yamasaki K., Saino T., Kanazawa T. (1999) FEBS Lett. 444, 54–58 [DOI] [PubMed] [Google Scholar]
- 40.Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. (1951) J. Biol. Chem. 193, 265–275 [PubMed] [Google Scholar]
- 41.Humphrey W., Dalke A., Schulten K. (1996) J. Mol. Graph. 14, 33–38, 27–28 [DOI] [PubMed] [Google Scholar]
- 42.Pick U., Karlish S. J. D. (1982) J. Biol. Chem. 257, 6120–6126 [PubMed] [Google Scholar]
- 43.Shigekawa M., Pearl L. J. (1976) J. Biol. Chem. 251, 6947–6952 [PubMed] [Google Scholar]
- 44.Shigekawa M., Dougherty J. P. (1978) J. Biol. Chem. 253, 1451–1457 [PubMed] [Google Scholar]
- 45.de Meis L., Inesi G. (1982) J. Biol. Chem. 257, 1289–1294 [PubMed] [Google Scholar]
- 46.Yamamoto T., Tonomura Y. (1967) J. Biochem. 62, 558–575 [DOI] [PubMed] [Google Scholar]
- 47.Champeil P., Riollet S., Orlowski S., Guillain F., Seebregts C. J., McIntosh D. B. (1988) J. Biol. Chem. 263, 12288–12294 [PubMed] [Google Scholar]
- 48.Andersen J. P., Møller J. V. (1985) Biochim. Biophys. Acta 815, 9–15 [DOI] [PubMed] [Google Scholar]
- 49.Fersht A. R. (1999) Structure and Mechanism in Protein Science: A Guide to Enzyme Catalysis and Protein Folding, pp. 132–168, W. H. Freeman and Co., New York [Google Scholar]
- 50.Guex N., Peitsch M.C. (1997) Electrophoresis 18, 2714–2723 [DOI] [PubMed] [Google Scholar]
- 51.Li H., Robertson A. D., Jensen J. H. (2005) Proteins 61, 704–721 [DOI] [PubMed] [Google Scholar]
- 52.Clausen J. D., McIntosh D. B., Woolley D. G., Andersen J. P. (2008) J. Biol. Chem. 283, 35703–35714 [DOI] [PubMed] [Google Scholar]
- 53.Ogurusu T., Wakabayashi S., Shigekawa M. (1991) J. Biochem. 109, 472–476 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.