Abstract
The Mycobacterium tuberculosis P450 enzymes are of interest for their pharmacological development potential, as evidenced by their susceptibility to inhibition by antifungal azole drugs that normally target sterol 14α-demethylase (CYP51). Although antifungal azoles show promise, direct screening of compounds against M. tuberculosis P450 enzymes may identify novel, more potent, and selective inhibitory scaffolds. Here we report that CYP130 from M. tuberculosis has a natural propensity to bind primary arylamines with particular chemical architectures. These compounds were identified via a high throughput screen of CYP130 with a library of synthetic organic molecules. As revealed by subsequent x-ray structure analysis, selected compounds bind in the active site by Fe-coordination and hydrogen bonding of the arylamine group to the carbonyl oxygen of Gly243. As evidenced by the binding of structural analogs, the primary arylamine group is indispensable, but synergism due to hydrophobic contacts between the rest of the molecule and protein amino acid residues is responsible for a binding affinity comparable with that of the antifungal azole drugs. The topology of the CYP130 active site favors angular coordination of the arylamine group over the orthogonal coordination of azoles. Upon substitution of Gly243 by an alanine, the binding mode of azoles and some arylamines reverted from type II to type I because of hydrophobic and steric interactions with the alanine side chain. We suggest a role for the conserved Ala(Gly)243-Gly244 motif in the I-helix in modulating both the binding affinity of the axial water ligand and the ligand selectivity of cytochrome P450 enzymes.
CYP130 is one of the 20 Mycobacterium tuberculosis cytochrome P450 (P450, CYP)2 enzymes and is one of three (CYP51, CYP121, and CYP130) that have been studied as individually expressed proteins at the structural level. Evidence has accumulated for the importance of M. tuberculosis P450 enzymes in virulence (CYP132) (1), host infection (CYP125) (2), and pathogen viability (CYP128, CYP121) (3, 4), although neither their exact biological functions nor any of the endogenous substrates upon which these enzymes operate have yet been established. However, it has recently been shown in vitro that CYP121 catalyzes a C–C coupling reaction between two tyrosine groups (5). CYP130 is absent from the genome of Mycobacterium bovis, suggesting that it might play specific role(s) in the infection of the human host and thus constitute a potential therapeutic target.
The potential of M. tuberculosis P450 enzymes for pharmacologic development was initially suggested by their susceptibility to inhibition by antifungal azole drugs such as fluconazole, econazole, and clotrimazole. These drugs block sterol 14α-demethylase CYP51 in fungi (6), tightly bind to M. tuberculosis P450 proteins (7, 8), and display inhibitory potential against latent and multidrug-resistant forms of tuberculosis both in vitro and in tuberculosis-infected mice (9–14).
The substantial differences between fungal CYP51 and the potential P450 targets in microbial pathogens, including M. tuberculosis, suggest that the direct screening of compounds against M. tuberculosis CYP enzymes could identify novel inhibitory scaffolds that are more potent and selective than antifungal drugs. Structurally characterized screening targets are advantageous, as the already defined purification and crystallization protocols can be applied to obtain co-crystal structures and to elucidate the binding modes of screening hits. This approach has been successfully applied to CYP51, resulting in identification of novel inhibitory scaffolds for CYP51 therapeutic targets (15, 16).
Toward this goal, the property of P450 enzymes to shift the ferric heme iron Soret band on ligand binding (17) provides an experimental platform for high throughput screening of compound libraries to select chemotypes with high binding affinities for the target. Expulsion of the heme iron axial water ligand from the Fe-coordination sphere by the incoming substrate followed by transition of the ferric heme from the low-spin hexacoordinated to the high-spin pentacoordinated state characterize type I spectral shifts and are a prerequisite for P450 catalytic activity. Replacement of a weak axial ligand, the water molecule, with a stronger one possessing a nitrogen-containing aliphatic or aromatic group coordinating to the heme iron characterizes type II spectral shifts.
To find new high affinity ligands of CYP130, a commercial library of 20,000 small organic molecules comprising a large selection of molecular scaffolds was screened against the enzyme. In contrast to the results with CYP51, no type I binding hits were identified. Screening produced about a dozen structurally diverse type II hits that were unexpectedly devoid of the usual aromatic nitrogen atoms readily accessible for axial coordination of the heme iron, suggesting an alternative coordination mode. High resolution x-ray structure analysis determined that two compounds coordinated to the heme iron via a primary arylamine group, providing the first structural evidence on P450-heterocyclic arylamine interactions.
EXPERIMENTAL PROCEDURES
Materials and Reagents
The CYP130G243A mutant is a new protein construct. Amino acid substitution was carried out using the QuikChange site-directed mutagenesis kit (Stratagene) following the recommended protocol. The cloned M. tuberculosis cyp130 gene was used as a template with the complementary oligonucleotide primers 5′-TTCACCATGGTCACCGCCGGCAACGACACCGTC-3′ and 5′-GACGGTGTCGTTGCCGGCGGTGACCATGGTGAA-3′. The correctness of the DNA construct was confirmed by sequencing. Wild-type recombinant CYP130 and its G243A mutant were purified as described previously (8). CYP142 was prepared similarly to CYP130.
Both the library and most of the separately purchased compounds, including compound 2, (5-amino-2-{4-[(4-aminophenyl)sulfanyl]phenyl}-1H-isoindole-1,3(2H)-dione), and compound 4, (1-(3-methylphenyl)-1H-benzimidazole-5-amine), were from ChemDiv (San Diego). Compound 5 (4-(2,3-bis(3,4,5,6-tetrahydropyridin-2-yl)quinoxalin-6-yloxy)aniline was from ChemBridge (San Diego). The nitro analog of compound 2, 2-(4-(4-nitrophenylthio)phenyl)isoindole-1,3-dione was from Asinex USA (Winston-Salem, NC). Aniline, benzylamine, econazole, glucose, Aspergillus niger glucose oxidase, bovine liver catalase, spinach ferredoxin, spinach NADP+-ferredoxin reductase, and NADPH were from Sigma-Aldrich.
Automated Library Screening and Data Analysis
Screening of the FMP-20 compound library (ChemDiv) containing 20,000 small organic molecules was performed as described elsewhere (15). Briefly, library compounds were solubilized at a stock concentration of 10 mm in Me2SO and distributed in 0.4-μl aliquots in 384-well microtiter plates that were stored sealed with aluminum foil at −20 °C. Prior to use, 40 μl of 50 mm Tris-HCl, pH 7.5, containing 10% glycerol was added to each well to achieve a concentration of 100 μm and to reduce the Me2SO concentration to 1%. The plates were subsequently incubated at 37 °C for 15 min followed by 5 min of sonication using an ultrasonic water bath (Transsonic 460, Elma) to allow the suspensions of the most hydrophobic compounds to solubilize. Wells were not inspected visually for precipitates or cloudiness upon dilution. To account for the optical properties of the test compounds, compound-specific changes of absorption spectra (310–450 nm) were recorded before and after 10 μl of 4 μm wild-type CYP130 was added to each well containing either test or reference compounds in the same buffer. Econazole at two different concentrations, 10 and 50 μm, was used as reference for the type II binding mode. No reference compounds for type I binding were available for CYP130.
Absorption data were exported to an EXCEL spreadsheet, and background spectra obtained for compounds in the absence of protein, as well as protein in the absence of compound, were subtracted. Absorption values were plotted against wavelength. Curves obtained for the reference were superimposed on the curves obtained for the test compounds. Novel ligands were identified by curve similarity.
Hit validation in the automated regime was performed at 10-fold increased CYP130 target concentration (from 1 to 10 μm) to achieve better signal-to-noise ratio, and 10-fold reduced (from 100 to 10 μm) concentration of the hit compounds. Hits that demonstrated absorption difference spectra compatible with the econazole reference even at the lowest concentration passed the validation test.
Spectroscopic Binding Assays
All ligand binding assays were performed by spectrophotometric titration in 50 mm potassium phosphate buffer, pH 7.4, containing 0.1 mm EDTA using a Cary UV-visible scanning spectrophotometer (Varian). Stock solutions of the ligands were prepared in Me2SO. To account for the absorbance of the tested compounds, 1 ml of protein (2.5 μm) in buffer was placed in the first chamber of two split cuvettes, and 1 ml of buffer was placed in the second chamber. After background scanning, equal volumes (0.25–1.0 μl) of ligand solution were titrated into both the second chamber of the reference cuvette containing only buffer and the first chamber of the sample cuvette containing the protein. The same volume of Me2SO was added into the alternate chambers to correct for organic solvent effects. Difference spectra were recorded from 300 to 500 nm. To determine the KD values, titration data points were fitted to either the rectangular hyperbola (Equation 1), the quadratic hyperbola (Equation 2), or the Hill equation (Equation 3) using the Kaleidagraph software (Synergy). In all equations, Aobs is the absorption shift determined at any ligand concentration; Amax is the maximal absorption shift obtained at saturation; KD is the apparent dissociation constant for the inhibitor-enzyme complex; Et is the total enzyme concentration used; S is the ligand concentration; and n is a Hill coefficient, a measure of cooperativity.
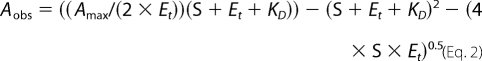 |
Anaerobic Conditions
To reproducibly maintain satisfactorily strict anaerobic conditions, when required, an enzymatic O2-scavenging system consisting of 2 units/ml glucose oxidase, 10 mm glucose, and 130 units/ml catalase was added to protein samples.
Crystallization, Data Collection, and Structure Determination
Compounds 2–5 were used to screen crystallization conditions using commercial high throughput screening kits available in deep-well format (Hampton Research), a nanoliter drop-setting Mosquito robot (TTP LabTech) operating with 96-well plates, and a hanging drop crystallization protocol. Optimization of conditions was carried out manually in 24-well plates. The protein was from 0.85 mm frozen stock in 20 mm Tris-HCl, pH 7.5, 200 mm NaCl, and 0.5 mm EDTA. Prior to crystallization, the protein was diluted to 0.1–0.2 mm by mixing with the compound dissolved at 2 mm in 10 mm Tris-HCl, pH 7.5.
Crystals of the CYP130-compound 2 complex grew in 12–16% polyethylene glycol 4000, 0.1 m sodium acetate, pH 5.0, 200 mm ammonium sulfate, and 1% isopropyl alcohol. Crystals of the CYP130-compound 4 complex grew in 18–22% polyethylene glycol 4000, 0.1 m sodium acetate, pH 5.5, 200 mm ammonium sulfate, and 2% isopropyl alcohol. Crystals of the CYP130G243A mutant were obtained from 1.5 m ammonium sulfate, 0.1 m citric acid, pH 5.2, and 3% isopropyl alcohol. Prior to data collection, the crystals were cryoprotected by plunging them into a drop of reservoir solution supplemented with 18% ethylene glycol and flash-frozen in liquid nitrogen. Diffraction data were collected at 100–110 K at Beamline 8.3.1, Advanced Light Source, Lawrence Berkeley National Laboratory. Data indexing, integration, and scaling were conducted using MOSFLM (18) and the HKL2000 software suite (19). Crystal structures were determined by molecular replacement using the atomic coordinates of the non-ligand-bound CYP130 (PDB ID code: 2UUQ) as a search model (Table 1).
TABLE 1.
Crystallographic data and statistics
Numbers in parentheses correspond to the highest resolution shell. r.m.s., root mean square; NA, not applicable.
| CYP130-compound 2 | CYP130-compound 4 | CYP130G243A ligand-free | |
|---|---|---|---|
| PDB ID code | 2WH8 | 2WHF | 2WGY |
| Data collection | |||
| Wavelength (Å) | 1.11587 | 1.11587 | 1.11587 |
| Resolution (Å) | 1.70 | 1.58 | 1.50 |
| Unique reflections | 164,200 | 51,244 | 59,681 |
| Redundancy | 1.8 (1.7)a | 3.6 (3.4) | 3.5 (2.5) |
| Completeness (%) | 96.0 (93.1) | 100.0 (99.6) | 97.0 (78.2) |
| Space group | P1 | C2 | C2 |
| Cell dimensions | |||
| a, b, c (Å) | 53.7, 84.9, 90.2 | 160.9, 53.9, 44.0 | 160.2, 54.1, 43.3 |
| α, β, γ (°) | 96.6, 90.0, 108.4 | 90.0, 96.7, 90.0 | 90.0, 96.2, 90 |
| Molecules in asymmetric unit | 4 | 1 | 1 |
| Solvent content (%) | 41.2 | 39.3 | 41.2 |
| Rsyma (%) | 3.4 (33.1) | 3.7 (44.7) | 3.4 (12.0) |
| I/σ | 19.9 (2.1) | 39.7 (3.1) | 45.3 (9.5) |
| Refinement | |||
| Reflections used in refinement | 142,540 | 54,902 | 54,979 |
| Rcryst (Rfree)b (%) | 19.9/25.9 | 19.6/23.9 | 18.3/21.3 |
| No. of atoms | 13,226 | 3,319 | 3,586 |
| Protein | 12,098 | 3,078 | 3,180 |
| Heme | 172 | 43 | 43 |
| Ligand | 104 | 34 | NA |
| Water/solvent | 851/0 | 164/0 | 323/40 |
| Wilson plot B-values (Å2) | 21.2 | 23.9 | 16.1 |
| Mean B-factor (Å2) | 22.2 | 25.1 | 16.6 |
| Protein (Å2) | 22.5d | 24.8 | 15.7 |
| Heme (Å2) | 11.9d | 15.7 | 10.1 |
| Ligand (Å2) | 32.5d | 4-1: 38.0; 4-2: 43.7 | NA |
| Water (Å2) | 28.4 | 30.0 | 23.9 |
| r.m.s. deviations | |||
| Bond length (Å) | 0.019 | 0.015 | 0.009 |
| Bond angles (°) | 1.8 | 1.7 | 1.2 |
| Ramachandranc (%) | 89.4/10.0/0.2/0.3d | 89.2/10.5/0.3/0.0 | 90.7/9.0/0.3/0.0 |
aRsym = Σ|Ii − 〈I〉|/ΣIi, where Ii is the intensity of the ith observation and 〈I〉 is the mean intensity of reflection.
b Rcryst = Σ‖Fo| − |Fc‖/Σ|Fo|, calculated with the working reflection set. Rfree is the same as Rcryst but calculated with the reserved reflection set.
c PROCHECK program (32), portions of the protein residues in most favored/additional allowed/generously allowed/disallowed regions.
d Averaged for chain A only.
RESULTS
Identification of Binding Hits
Automated screening of the library of organic molecules against the M. tuberculosis CYP130 target resulted in about a dozen type II, but no type I, binding hits (supplemental Fig. S1A). The absence of type I hits is consistent with our previous observation that none of the compounds tested thus far could shift the CYP130 wild-type form into the pentacoordinated high-spin state that is a prerequisite for catalytic activity. Thus, no catalytic activity of any kind has so far been observed for CYP130. Most type II screen hits identified in these studies were heterocyclic arylamines with an overall chemical architecture that differs from that of the antifungal azoles. Remarkably, none of the top compounds contained a nitrogen atom within an aromatic heterocyclic ring in a position readily accessible for axial coordination to the heme iron.
Spectrophotometric Characterization of the Complexes
The electronic absorption spectra of ferric CYP130 in complex with compound 2 (Fig. 1 and supplemental Table S1) and compounds 4 and 5 (supplemental Fig. S2 and Table S1) exhibit red-shifted Soret bands, confirming the type II nature of these ligands. Because of the previously observed instability of the ferrous ligand-free form (20), it was impossible to monitor direct addition of the ligands to ferrous CYP130. Instead, we reduced the ferric complexes anaerobically using sodium dithionite. Of the three compounds tested, only compound 2 appeared to form a stable ferrous hexacoordinate complex. Indeed, a new species with very distinct features at 445.0, 527.8, and 557.6 nm was slowly formed (Fig. 1). Bubbling of CO into the solution after 60 min resulted in very slow conversion to the hallmark ferrous CO-bound form, supporting the assumption that the new species is a ferrous compound 2 complex in the native state. On the other hand, reduction of the ferric complexes of econazole and compounds 4 and 5 resulted in the formation of a completely denaturated, partially denatured, and native ferrous ligand-free species, respectively (supplemental Fig. S2 and Table S1). The spectral identification is based on a previous stopped-flow study of the reduction of the ferric ligand-free CYP130 by sodium dithionite (20) and is further supported by their different ferrous CO species (supplemental Fig. S2 and Table S1). Also of particular interest is the failure to reduce the ferric CYP130-compound 2 complex using spinach ferredoxin and NADP+-ferredoxin reductase, indicating that the binding of this type II ligand to CYP130 decreases the redox potential of the heme.
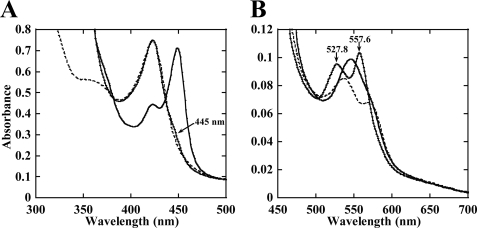
FIGURE 1.
Electronic absorption spectra of the CYP130-compound 2 complex. The Soret (A) and enlarged visible (B) regions are shown. The ferric complex (dashed trace) was reduced to a stable ferrous form (thin trace). Once reduction was complete (∼60 min), CO was bubbled for 30 s, and spectra were collected until equilibrium was reached (thick trace). The position of band maxima and their respective extinction coefficient values are summarized in the supporting material (supplemental Table S1). The spectra were recorded at the protein concentration of 5 μm in 100 mm potassium phosphate buffer, pH 7.4, containing 0.1 mm EDTA, and compound 2 was added at a saturating concentration of 25 μm.
Binding Affinity of the Arylamine Compounds
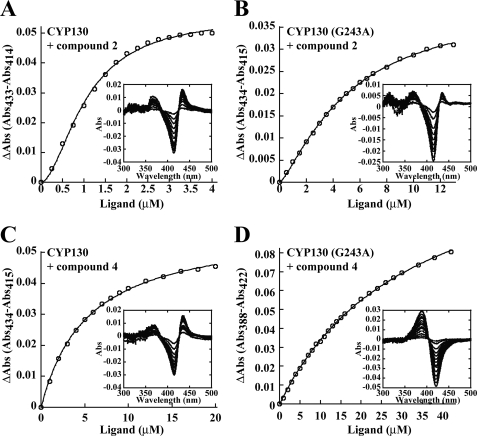
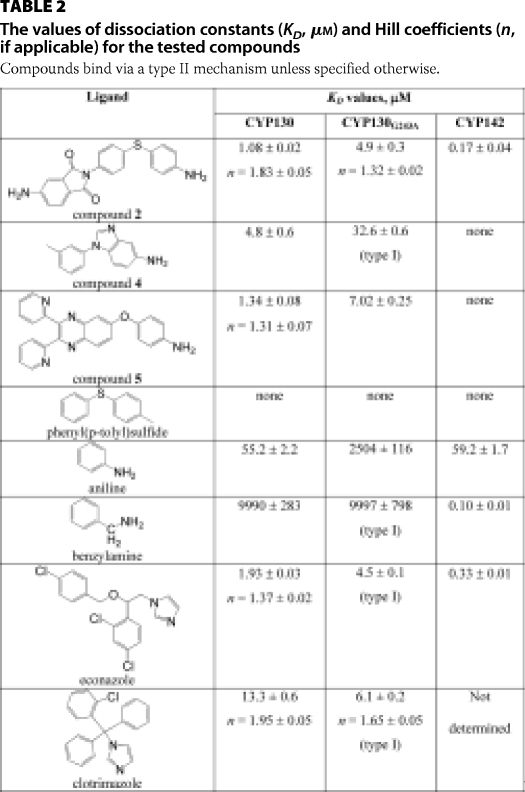
The KD values for the ligands were obtained from the spectral titration curves (Fig. 2) and are summarized in Table 2. For comparison, the KD values for econazole and clotrimazole obtained previously (8) are also listed. The sigmoid titration plots obtained for compound 2 (Fig. 2A) and compound 5 (not shown) were best-fitted to the Hill equation (Equation 3) with coefficients of 1.83 and 1.31, respectively, indicating binding cooperativity, as reported previously for the binding of econazole and clotrimazole (8). In contrast, the titration curve for compound 4 (Fig. 2B) was fitted with a rectangular hyperbola (Equation 1), indicating the absence of cooperativity, even though the active site can accommodate two molecules of this ligand (see below). Collectively, the binding affinities of all the tested arylamines were in the low micromolar range and comparable with those of the antifungal azole drugs (Table 2).
FIGURE 2.
Binding of heterocyclic arylamines to M. tuberculosis CYP130 and CYP130G243A mutant. The concentration dependence of compound 2 (A and B) and compound 4 (C and D) binding deduced from the difference absorption changes obtained from the titration of protein (2.5 μm) with increasing concentrations of the inhibitor. Representative sets of difference spectra that were recorded are shown in the insets. Spectrophotometric titrations were carried out as described under “Experimental Procedures.”
TABLE 2.
The values of dissociation constants (KD, μm) and Hill coefficients (n, if applicable) for the tested compounds
Compounds bind via a type II mechanism unless specified otherwise.
The binding of heterocyclic arylamines to CYP130 was specific. Among all of the compounds tested only compound 2 bound to CYP142 with a higher affinity than to CYP130 (Table 2). Co-crystallization of CYP142 with compound 2 or other compounds has so far been unsuccessful. No significant binding of the arylamine compounds to two other characterized M. tuberculosis P450 enzymes, CYP125 and CYP51, was detected.
CYP130 in Complex with Heterocyclic Amines
Co-crystal structures were determined for compounds 2 and 4 by x-ray crystallography (Fig. 3), but co-crystals for compounds 3 and 5 were unsuitable for data collection. No significant conformational differences in the polypeptide chain associated with the binding of the heterocyclic amines were observed. Overall root mean square deviations compared with the ligand-free protein were within 0.47 Å for the CYP130-compound 2 complex and 0.26 Å for the CYP130-compound 4 complex. Some conformational differences were evident at the C terminus of the F-helix and in the BC-loop, which are among the inherently flexible and mobile structural elements in the P450 protein fold.
FIGURE 3.
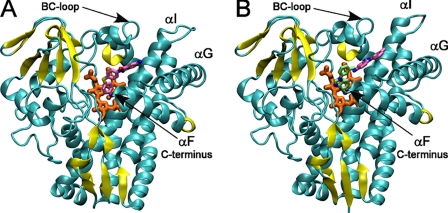
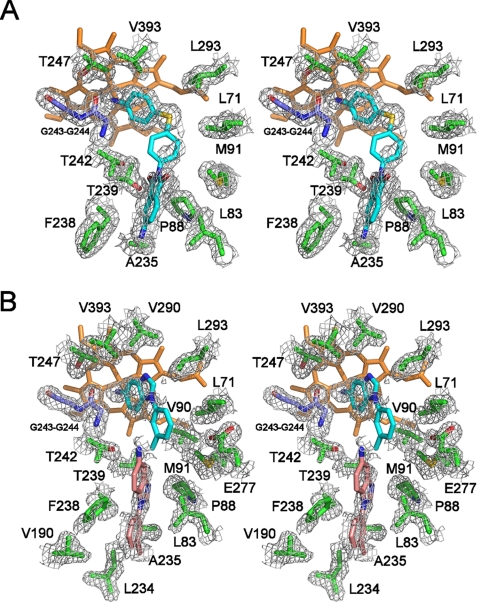
Overall view of CYP130 with ligands bound in the active site. A, compound 2 is highlighted in pink. B, two molecules of compound 4 are highlighted in lime and pink. Protein is shown as a ribbon with the β-sheets highlighted in yellow, and heme is in orange. Images were generated using the Visual Molecular Dynamics (VMD) program (28).
Although two amino groups are present in the chemical structure of compound 2 (Table 2), this ligand binds in a single preferred orientation that places the aminophenylthio group in the Fe-coordinating position. In all four molecules in the asymmetric unit, electron density was well defined for the ligating aminophenylthio moiety but scattered for the non-ligating phenyl isoindole moiety (Fig. 4A). An angular geometry of compound 2 was clearly indicated by the curvature of the electron density map bending at the thio group connecting the aminophenyl ring to the phenyl isoindole-1,3-dione moiety. Bending served to avoid a clash with the active site residues Leu71, Val90, and Leu293 as compound 2 spiraled around the I-helix with the isoindole ring nearly co-planar to the Phe238-Thr239 fragment of the I-helix backbone and the Phe238 side chain. Other hydrophobic contacts within 4 Å of the isoindole ring included Pro88 and Met91 in the BC-loop.
FIGURE 4.
Stereo view of compounds 2 (A) and 4 (B) in the active site of CYP130. A, the electron densities (gray mesh) of compound 2 (cyan) and the active site residues (green) located within 5 Å of the ligand are represented by a fragment of a 2Fo − Fc composite omit map, where Fo is the observed structure factor and Fc is the calculated structure factor contoured at 1.0 σ. B, the first molecule of compound 4 is cyan, and the second molecule is pink. The fragment of the I-helix comprising consecutive glycine residues 243–244 is blue. Fragments of the electron density are shown in gray mesh. Images were generated using PYMOL (29).
Compound 4 binds via a single benzoimidazole amine group. Again, electron density around the heme clearly indicated the direction of Fe-coordination but is progressively less defined toward the non-ligating tolyl end of the molecule (Fig. 4B, molecule 4-1, cyan). A second molecule of compound 4 (Fig. 4B, molecule 4-2, pink) was fitted into the scattered electron density observed within the binding space delineated by compound 2. This molecule binds through hydrophobic interactions.
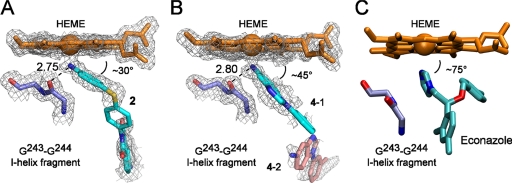
In both structurally characterized complexes the ligands bound to the heme iron via the nitrogen lone pair of electrons in a sp3 atomic orbital of the primary arylamine. The coordination bond length for compounds 2 and 4 varied from 2.09 to 2.15 Å. The tetrahedral orientations of the sp3 orbitals allowed the arylamine group to approach the heme macrocycle at an angle of ∼30 to 45° (Fig. 5, A and B), in contrast to the aromatic nitrogen heterocyclic compounds, including azoles, that bind orthogonally via an sp2-hybridized aromatic nitrogen atom (Fig. 5C). Furthermore, the binding of arylamines is facilitated by hydrogen bonding of one of the amine protons to the Gly243 carbonyl group (H-bond length varied between 2.73 and 2.88 Å) (Fig. 5), an interaction that alleviates the accumulation of positive charge on the nitrogen coordinated to the iron atom.
FIGURE 5.
Coordination of heme iron by the ligands. Compound 2 (cyan) (A), two molecules of compound 4 (cyan (4-1) and pink (4-2) (B), and econazole (cyan) (C) are shown bound in the active site of CYP130. Econazole is shown as bound in the active site of CYP130 (PDB ID code: 2UVN). Fragment Gly243-Gly244 of the I-helix is shown as blue sticks. The electron densities are represented by a fragment of a 2Fo − Fc composite omit map (gray mesh) contoured at 1.0 σ. Heme is orange. H-bonds are shown as dashed lines with the distances given in angstroms.
Structural Basis for Binding Cooperativity
As only one molecule of compound 2 was found in the active site in the co-crystal structure, we attribute binding cooperativity to intermolecular interactions. A similar phenomenon was described previously for the CYP130-econazole complex, where binding cooperativity resulted from CYP130 dimerization upon econazole binding manifested in the repacking of protein molecules in the crystal lattice (8). Although the small amplitude of the conformational changes observed in the CYP130-compound 2 complex is in notable contrast with those that occur on econazole binding, compound 2 also affected CYP130 packing in the crystal resulting in loss of crystallographic symmetry and changes in the space group, unit cell dimensions, and number of molecules per asymmetric unit (Table 1). These changes in the intermolecular contacts may impact the binding affinity of the ligand resulting in binding cooperativity. It is worth mentioning that no changes were observed in the crystal parameters for the CYP130-compound 4 complex compared with the ligand-free CYP130 (8).
Ligand Binding to CYP130G243A
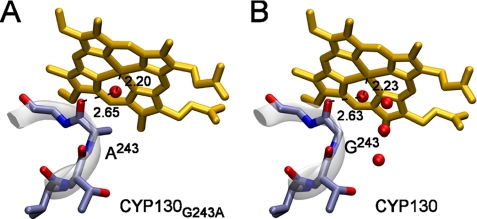
The Gly243-Gly244 motif in the middle of the I-helix is not rare among P450 enzymes, although the Ala-Gly pair is more common. Three M. tuberculosis P450 enzymes, specifically CYP130, CYP141, and CYP142, possess the Gly-Gly motif (Fig. 6), which appears to sterically favor angular coordination of the arylamines in CYP130. To investigate the role of an alanine versus glycine at this site, we replaced Gly243 with an alanine in CYP130 via site-directed mutagenesis. The electronic absorption spectrum of the CYP130G243A mutant in the ferric resting state showed a slight red shift of the Soret band relative to the wild-type protein with a higher molar extinction coefficient (supplemental Fig. S3 and Table S2), suggesting an enhanced low-spin character for the CYP130G243A mutant in the ferric resting state. The Fe–O coordination bond and H-bond distances are consistent with this trend with the Fe–O bond being slightly shorter in the CYP130G243A crystals (2.20 versus 2.23 Å in the wild type) and the H-bond to the carbonyl oxygen of Ala243 slightly longer (2.65 versus 2.62 Å) (Fig. 7). However, the absolute differences do not exceed 0.03 Å, which is only three times the bond length root-mean-square deviation of 0.009 Å (Table 1 and Ref 8), and therefore are at the borderline of credibility. The overall geometry of binding and temperature factors (B-factors) of the axial water ligand were unaffected by the G243A substitution.
FIGURE 6.
Alignments of the I-helix in M. tuberculosis P450. Fragments of the multiple sequence alignments of the 20 P450 proteins in M. tuberculosis representing the I-helix are shown. The secondary structure annotation and residue numbering at the top correspond to CYP130. The spiral represents the α-helix with the gap indicating disruption of the α-helix hydrogen bonding pattern at the conserved Ala(Gly)243-Gly244 motif (highlighted in bold). Accession numbers of the proteins in the Swiss-Prot/TrEMBL (us.expasy.org/sprot) or NCBI (www.ncbi.nlm.nih.gov) data bases are given next to the name of the protein. Alignments were performed using the MAP algorithm as implemented in the BCM Search Launcher (30). The figure was generated using ESPript (31).
FIGURE 7.
Binding of the water molecules in the active site of CYP130 and CYP130G243A. A more hydrophobic environment due to the side chain of Ala243 (A) results in the loss of three water molecules from the active site, which are present in the wild-type CYP130 (PDB ID code: 2UUQ) (B). The heme is in yellow, the water molecules are shown as red spheres, the 243–344 fragment of the I-helix is shown as a stick representation (blue), and the ribbon in gray. Distances between the atoms are given in angstroms.
The G243A substitution affects the binding of both the arylamine and azole compounds (Table 2). The binding affinities of compounds 2 and 5 were reduced by factors of 4.5 and 5.2, respectively. The titration plot obtained for these two compounds revealed that the cooperativity effect is also significantly reduced for compound 2 (Fig. 2B), as indicated by a smaller Hill coefficient value of 1.32, and is totally abolished for compound 5 (data not shown). The binding affinity of compound 4 was not only 6.8-fold reduced, but its binding mode reversed from type II to type I, as were the binding modes of econazole and clotrimazole, both with surprisingly small losses in binding affinity. Given our multiple unsuccessful attempts to obtain the pentacoordinated high-spin state of CYP130, the ease of this transition in CYP130G243A was surprising and must be due to the hydrophobic effect of the alanine side chain. Consistent with increased hydrophobicity, three water molecules (HOH-2187, HOH-2292, and HOH-2293) in the vicinity of Gly243 in the CYP130 wild-type (PDB ID code: 2UUG) were lost from CYP130G243A (Fig. 7).
Binding of Aniline and Analogs
The contribution of the arylamine group to the binding affinity of compound 2 was estimated by examining the binding of the prototypic arylamine aniline and related compounds to CYP130 and the CYP130G243A mutant (Table 2). The binding affinity of aniline to CYP130 was ∼55 μm, which is 10–20 times stronger than reported for the hepatic microsomal fraction of P450 enzymes in earlier studies (21). The affinity of aniline dropped to 2.5 mm (∼50-fold) in CYP130G243A, which may be due to the steric effect of the alanine side chain that interferes with both the coordination bond to the heme iron and the H-bond to the carbonyl oxygen.
Insertion of a methylene unit between the aromatic ring and the amine group resulted in a 200-fold drop of benzylamine affinity toward both CYP130 and CYP130G243A compared with that of aniline, together with a reversal of the benzylamine binding mode from type II to type I in CYP130G243A. According to the crystal structure, steric constraints imposed by the residues Leu71, Val90, and Leu293 contribute to the drop in the binding affinity for benzylamine versus aniline. Also, insertion of the methylene unit increases the pKa of the amine group from 4.6 in aniline to 9.4 in benzylamine, resulting in protonation of the latter at neutral pH in aqueous solutions. Although the general acid-base theory does not hold in the dielectric environment of the proteins, where the pKa of functional groups is determined largely by interactions with the surrounding groups, the apparent concentration of the active unionized form of benzylamine in solution will be strongly reduced by the protonation.
Unlike aniline, phenyl p-tolyl sulfide, having the angular architecture of compound 2 but lacking both the amine group and isoindole-1,3-dione moiety, did not cause any perturbation in the Soret region of the optical spectra. Although this molecule cannot bind via a type II mechanism, one could expect type I perturbations in the optical spectra if the axial water ligand were displaced.
Interaction of CYP130 with the Nitro Analog of Compound 2
The nitro analog of compound 2, 2-(4-(4-nitrophenylthio)phenyl)isoindole-1,3-dione, in which the amino group coordinating to the heme iron is substituted by a non-coordinating nitro group (supplemental Fig. S1B), also did not perturb the Soret band nor did it compete with the weak ligand imidazole for binding in the CYP130 active site (not shown). Upon enzymatic reduction of the CYP130-nitro analog mixture by the spinach ferredoxin/NADP+-ferredoxin reductase system under strict anaerobic conditions, a slow transition from the resting ferric ligand-free form to a ferric arylamine-bound form was observed (supplemental Fig. S4). In contrast to the liver microsome P450 preparations under similar anaerobic conditions (22–24), no formation of the nitrosoarene intermediate with a spectral feature at 454 nm has been detected. A control experiment, in which the nitro compound was preincubated with spinach ferredoxin/NADP+-ferredoxin reductase and NADPH for 60 min prior to the addition of ferric CYP130, confirmed that the formation of the amine form was due to the action of CYP130. Lack of nitrosoarene intermediate detection may suggest either low affinity of the latter to CYP130 or its fast conversion to the hydroxylamine and the amine forms.
DISCUSSION
The pharmacological potential of M. tuberculosis P450 enzymes motivated us to further explore the topology of the CYP130 active site by screening a library of synthetic organic molecules against this protein. In this screen, we identified inhibitors capable of specific binding to CYP130 via a type II mechanism that involves replacing the axial water ligand by a primary arylamine group. Coordination via the arylamine allowed compounds to approach the heme macrocycle at a sharp angle, avoiding steric constrains imposed by the I-helix that lies close to the heme plane, in contrast to the orthogonal binding of the aromatic amines (Fig. 5).
CYP130, as well as two other M. tuberculosis P450 enzymes, CYP141 and CYP142, has two consecutive glycine residues, Gly243-Gly244, on the heme-facing surface of the I-helix (Fig. 6), which favors close contacts between the I-helix and the heme macrocycle. In the ferric resting state, the carbonyl oxygen of Gly243 hydrogen bonds to the iron axial water ligand (8). This interaction is conserved across the P450 protein family, as judged from the conservation of the Ala(Gly)-Gly motif and the crystal structure analysis of multiple representatives of the P450 protein family.
Spectroscopic binding studies conducted on the CYP130G243A mutant in this work revealed that the alanine side chain substituted for Gly243 interfered with the coordination of azoles and some arylamines, reversing their binding modes from type II to type I (Table 2). Facilitated release of the axial water ligand in CYP130G243A is attributed to the increased hydrophobicity of the oxygen-scission site due to the added methyl group. This difference suggests a role for the conserved Ala(Gly)-Gly motif in modulating the binding of the axial water. The wild-type CYP130 has a more hydrophilic Gly-Gly motif and tends to retain the axial water ligand, whereas the Ala-Gly variant, CYP130G243A, more readily releases the axial water on binding of hydrophobic ligands.
Remarkably, the binding affinities of econazole and clotrimazole are virtually independent of the differential binding mode in the wild type and mutant, suggesting that the coordination bond between the azole and the heme iron was already impaired in the wild type and was lost entirely in the CYP130G243A mutant. This assumption is consistent with a previous report on steric hindrance, in which the azole drug fluconazole was forced to adopt alternative binding modes in the CYP121-fluconazole complex (25). In wild-type CYP130, econazole binds with distorted geometry (Fig. 5) by pushing Gly243 away to create an additional space (8). In CYP130G243A, the steric bulk of the alanine side chain created even more steric constraints, which interfered with the orthogonal coordination of the azoles and attenuate the affinity of heterocyclic amines.
Arylamines and heterocyclic arylamines are widespread in the environment as industrial chemicals, food additives, and drugs. They are notorious because N-oxidation by human liver cytochrome P450 enzymes converts them into mutagenic and carcinogenic products. Based on a wealth of experimental data accumulated for the mammalian P450 enzymes, structurally diverse primary alkyl- and arylamines show the entire spectrum of binding modes, ranging from type I to reverse type I to type II, with binding affinities for type II ligands (from 5 μm to 7 mm) on average being considerably lower than for type I (from 0.1 to 500 μm) (26). Type I binding is imperative for catalytic conversion by P450. The diversity of binding modes suggests that multiple factors govern access, binding, and orientation of primary amines within the P450 active site. The characterization of heterocyclic arylamine binding reported here shows that the synergism of Fe-coordination, hydrogen bonding, hydrophobic interactions, and differential active site geometry all single out a compound for strong inhibitory potential toward the P450 target.
Our data suggest that compounds angularly coordinating to the heme iron have potential utility as selective inhibitors of some members of the subgroup of P450 enzymes with a Gly-Gly motif in the I-helix and should be investigated further for their antimicrobial potential, with the proviso that the arylamine functionality must be used with caution because of the incidence of idiosyncratic toxicities associated with older drugs of this category (27).
Supplementary Material
Acknowledgments
We thank Potter Wickware for critical reading of the manuscript; Petrea Kells for excellent technical support; Chris Waddling for assistance with software and instrumentation; and Chiung-Kuang Chen and the staff members of Beamline 8.3.1, James Holton, George Meigs, and Jane Tanamachi (Advanced Light Source at Lawrence Berkeley National Laboratory) for assistance with data collection. The Advanced Light Source is supported by the Director, Office of Science, Office of Basic Energy Sciences of the U. S. Department of Energy under Contract DE-AC02-05CH11231.
This work was supported, in whole or in part, by National Institutes of Health RO1 Grants AI07824 and GM25515 (to P. O. d. M.) and GM078553 (to L. M. P.). This work was also supported by Grants BIO/0312992A (to J. P. K.) from the X-Mtb Consortium Bundesministerium fuer Bildung und Forschung/Projekttraeger Juelich (BMBF/PTJ).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4 and Tables S1 and S2.
The atomic coordinates and structure factors (codes 2WH8, 2WGY, and 2WHF) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
- CYP or P450
- cytochrome P450
- PDB
- Protein Data Bank.
REFERENCES
- 1.Recchi C., Sclavi B., Rauzier J., Gicquel B., Reyrat J. M. (2003) J. Biol. Chem. 278, 33763–33773 [DOI] [PubMed] [Google Scholar]
- 2.Chang J. C., Harik N. S., Liao R. P., Sherman D. R. (2007) J. Infect. Dis. 196, 788–795 [DOI] [PubMed] [Google Scholar]
- 3.Sassetti C. M., Rubin E. J. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 12989–12994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McLean K. J., Carroll P., Lewis D. G., Dunford A. J., Seward H. E., Neeli R., Cheesman M. R., Marsollier L., Douglas P., Smith W. E., Rosenkrands I., Cole S. T., Leys D., Parish T., Munro A. W. (2008) J. Biol. Chem. 283, 33406–33416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belin P., Le Du M. H., Fielding A., Lequin O., Jacquet M., Charbonnier J. B., Lecoq A., Thai R., Courçcon M., Masson C., Dugave C., Genet R., Pernodet J. L., Gondry M. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 7426–7431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheehan D. J., Hitchcock C. A., Sibley C. M. (1999) Clin. Microbiol. Rev. 12, 40–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McLean K. J., Cheesman M. R., Rivers S. L., Richmond A., Leys D., Chapman S. K., Reid G. A., Price N. C., Kelly S. M., Clarkson J., Smith W. E., Munro A. W. (2002) J. Inorg. Biochem. 91, 527–541 [DOI] [PubMed] [Google Scholar]
- 8.Ouellet H., Podust L. M., de Montellano P. R. (2008) J. Biol. Chem. 283, 5069–5080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmad Z., Sharma S., Khuller G. K. (2005) FEMS Microbiol. Lett. 251, 19–22 [DOI] [PubMed] [Google Scholar]
- 10.Ahmad Z., Sharma S., Khuller G. K., Singh P., Faujdar J., Katoch V. M. (2006) Int. J. Antimicrob. Agents 28, 543–544 [DOI] [PubMed] [Google Scholar]
- 11.Ahmad Z., Sharma S., Khuller G. K. (2006) FEMS Microbiol. Lett. 261, 181–186 [DOI] [PubMed] [Google Scholar]
- 12.Ahmad Z., Sharma S., Khuller G. K. (2006) FEMS Microbiol. Lett. 258, 200–203 [DOI] [PubMed] [Google Scholar]
- 13.Banfi E., Scialino G., Zampieri D., Mamolo M. G., Vio L., Ferrone M., Fermeglia M., Paneni M. S., Pricl S. (2006) J. Antimicrob. Chemother. 58, 76–84 [DOI] [PubMed] [Google Scholar]
- 14.Byrne S. T., Denkin S. M., Gu P., Nuermberger E., Zhang Y. (2007) J. Med. Microbiol. 56, 1047–1051 [DOI] [PubMed] [Google Scholar]
- 15.Podust L. M., von Kries J. P., Nasser Eddine A., Kim Y., Yermalitskaya L. V., Kuehne R., Ouellet H., Warrier T., Alteköster M., Lee J. S., Rademann J., Oschkinat H., Kaufmann S. H., Waterman M. R. (2007) Antimicrob. Agents Chemother. 51, 3915–3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen C. K., Doyle P. S., Yermalitskaya L. V., Mackey Z. B., Ang K. K., McKerrow J. H., Podust L. M. (2009) PLoS Negl. Trop. Dis. 3, e372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schenkman J. B., Remmer H., Estabrook R. W. (1967) Mol. Pharmacol. 3, 113–123 [PubMed] [Google Scholar]
- 18.Leslie A. G. W. (1992) Joint CCP4-ESF-EAMCB Newsletter on Protein Crystallography, Vol. 26, Daresbury Laboratory, Warrington, UK [Google Scholar]
- 19.Otwinowski Z., Minor W. (1997) Methods Enzymol. 276, 307–326 [DOI] [PubMed] [Google Scholar]
- 20.Ouellet H., Lang J., Couture M., Ortiz de Montellano P. R. (2009) Biochemistry 48, 863–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Remmer H., Schenkman J., Estabrook R. W., Sasame H., Gillette J., Narasimhulu S., Cooper D. Y., Rosenthal O. (1966) Mol. Pharmacol. 2, 187–190 [PubMed] [Google Scholar]
- 22.Keilin D., Hartree E. F. (1943) Nature 151, 390–391 [Google Scholar]
- 23.Franklin M. R. (1976) Chem. Biol. Interact. 14, 337–346 [DOI] [PubMed] [Google Scholar]
- 24.Mansuy D., Beaune P., Cresteil T., Bacot C., Chottard J. C., Gans P. (1978) Eur. J. Biochem. 86, 573–579 [DOI] [PubMed] [Google Scholar]
- 25.Seward H. E., Roujeinikova A., McLean K. J., Munro A. W., Leys D. (2006) J. Biol. Chem. 281, 39437–39443 [DOI] [PubMed] [Google Scholar]
- 26.Hlavica P. (2006) Biochim. Biophys. Acta 1764, 645–670 [DOI] [PubMed] [Google Scholar]
- 27.Kim D., Guengerich F. P. (2005) Annu. Rev. Pharmacol. Toxicol. 45, 27–49 [DOI] [PubMed] [Google Scholar]
- 28.Humphrey W., Dalke A., Schulten K. (1996) J. Mol. Graph. 14, 33–38 [DOI] [PubMed] [Google Scholar]
- 29.DeLano W. L. (2002) The PyMOL Molecular Graphics System, DeLano Scientific, San Carlos, CA [Google Scholar]
- 30.Smith R. F., Wiese B. A., Wojzynski M. K., Davison D. B., Worley K. C. (1996) Genome Res. 6, 454–462 [DOI] [PubMed] [Google Scholar]
- 31.Gouet P., Courcelle E., Stuart D. I., Métoz F. (1999) Bioinformatics 15, 305–308 [DOI] [PubMed] [Google Scholar]
- 32.Laskowski R. A., MacArthur M. W., Moss D. S., Thornton J. M. (1993) J. Appl. Crystallogr. 26, 283–291 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.