Abstract
Plant and algal prolyl 4-hydroxylases (P4Hs) are key enzymes in the synthesis of cell wall components. These monomeric enzymes belong to the 2-oxoglutarate dependent superfamily of enzymes characterized by a conserved jelly-roll framework. This algal P4H has high sequence similarity to the catalytic domain of the vertebrate, tetrameric collagen P4Hs, whereas there are distinct sequence differences with the oxygen-sensing hypoxia-inducible factor P4H subfamily of enzymes. We present here a 1.98-Å crystal structure of the algal Chlamydomonas reinhardtii P4H-1 complexed with Zn2+ and a proline-rich (Ser-Pro)5 substrate. This ternary complex captures the competent mode of binding of the peptide substrate, being bound in a left-handed (poly)l-proline type II conformation in a tunnel shaped by two loops. These two loops are mostly disordered in the absence of the substrate. The importance of these loops for the function is confirmed by extensive mutagenesis, followed up by enzyme kinetic characterizations. These loops cover the central Ser-Pro-Ser tripeptide of the substrate such that the hydroxylation occurs in a highly buried space. This novel mode of binding does not depend on stacking interactions of the proline side chains with aromatic residues. Major conformational changes of the two peptide binding loops are predicted to be a key feature of the catalytic cycle. These conformational changes are probably triggered by the conformational switch of Tyr140, as induced by the hydroxylation of the proline residue. The importance of these findings for understanding the specific binding and hydroxylation of (X-Pro-Gly)n sequences by collagen P4Hs is also discussed.
4R-Hydroxyproline (4Hyp)2 is an uncommon amino acid produced by the addition of a hydroxyl group to the C4 carbon atom of the proline pyrrolidine ring. 4Hyp residues have an essential role in the extensive collagen family, where they are necessary for the formation of stable triple helical molecules (1–3). 4Hyp residues are also found in many plant and algal hydroxyproline-rich glycoproteins (HRGPs), such as extensins and arabinogalactan proteins, which are the major structural components of their cell walls (4, 5). In addition to these structural roles, 4Hyp has a key role in the regulation of gene expression in an oxygen-dependent manner via the hypoxia-inducible transcription factor (HIF) (6–8).
The formation of 4Hyp in the above proteins is catalyzed by the prolyl 4-hydroxylases (P4Hs), which are 2-oxoglutarate (2OG) dioxygenases and also require Fe2+ and O2 (Fig. 1A) (1–3, 9). Two P4H families, the collagen P4Hs (C-P4Hs) and the HIF-P4Hs, each having three isoenzymes, are responsible for the hydroxylation of collagens and HIF, respectively (1–3, 6–9). The vertebrate C-P4Hs are α2β2 tetramers in which the α-subunits are responsible for the hydroxylation and the β-subunits are identical to protein-disulfide isomerase. The tetrameric assembly is required for stability and full activity (1–3). In contrast, the plant P4Hs and probably also the HIF-P4Hs are monomers (2, 10). Plant P4Hs have a ∼30% sequence identity to the catalytic domain of the C-P4H α-subunits (11, 12), and they also resemble C-P4Hs in that they hydroxylate proline-rich polypeptides and are located in the lumen of the endoplasmic reticulum (2). HIF-P4Hs, on the other hand, are cytoplasmic and nuclear enzymes (8, 9) that act on proline residues in -Leu-X-X-Leu-Ala-Pro- motifs in HIF-α (13, 14). C-P4Hs hydroxylate the central proline of the -X-Pro-Gly- repeats of collagen polypeptides, typically generating about 100 4Hyp residues in polypeptides with a length of about 1000 amino acids. Plant P4Hs can also hydroxylate peptides with -X-Pro-Gly- repeats in vitro, but generally much less effectively than peptides representing their physiological substrates, the HRGPs, which are rich in serine and proline and can fold into a left-handed fibrous poly(l-proline) type II (PPII) helix conformation (15, 16). The only known exception is isoenzyme 1 of the large Arabidopsis thaliana P4H (At-P4H) family, which efficiently hydroxylates a collagen-like (Pro-Pro-Gly)10 peptide, for which its Km is only 3-fold higher than that of the human C-P4H-I (17). Plant P4Hs accept also poly(l-proline) as a substrate, which is not hydroxylated by any of the C-P4Hs, but instead acts as an efficient competitive inhibitor of C-P4H-I (2).
FIGURE 1.
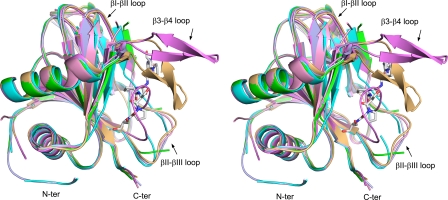
The reaction and the crystal structure of Cr-P4H-1. A, reaction catalyzed by P4Hs. The 2OG molecule coordinates the Fe2+ via C1-carboxylate and C2-keto oxygen atoms. The product is a trans-4-hydroxyprolyl residue. The two oxygen atoms of O2 (red) are used for the hydroxylation of the proline and the oxidation of 2OG, which is decarboxylated by releasing the C1-carboxylate group as CO2. B, schematic representation of the Cr-P4H-1 crystal structure. The jelly-roll fold is shown in darker brown the major sheet being in the front and the minor sheet at the back. The two substrate binding loops are shown with dashed lines. C, stereoview of the ribbon diagram showing the mode of (Ser-Pro)5 peptide substrate (in stick representation) binding to the jelly-roll motif of Cr-P4H-1. The zinc ion, indicating the active site region, is shown as a gray ball. The β-strands of the jelly-roll core are labeled using roman numerals as in B. The two major flexible loops, β3-β4 and βII-βIII, wrapping the peptide substrate are also indicated.
Several 2OG dioxygenases have now been crystallized and despite their low overall amino acid sequence identity the structures have revealed that the catalytic sites are always located at the same site of a common framework: a double-stranded β-helix (jelly-roll) fold that consists of 8 antiparallel β-strands (18, 19) (Fig. 1B). The catalytic sites of all P4Hs have the conserved -His-X-Asp-…-His- catalytic motif for Fe2+ coordination and a basic residue that binds the C5 carboxylate moiety of 2OG. The crystal structures of two P4Hs have been solved, namely those of human HIF-P4H-2 (10) and a plant P4H from Chlamydomonas reinhardtii (Cr-P4H-1) (12). The structures of these two enzymes share the jelly-roll core fold preceded by an N-terminal part that contains two long α-helices in both structures, and also three β-strands in Cr-P4H-1 and 2 β-strands in HIF-P4H-2 (10, 12). In each of these two structures the extra β-strands extend the major β-sheet of the jelly-roll fold (Fig. 1B), and the helices of the N-terminal part shield this major sheet from bulk solvent. The proximal histidine and the aspartate of the Fe2+ coordination motif are located in the βII-strand of the jelly-roll, the distal histidine of this motif is in the adjacent βVII-strand, whereas the basic 2OG-binding residue (lysine in Cr-P4H-1 and arginine in HIF-P4H-2) is in the βVIII-strand. Both enzymes have been crystallized in the presence of an active site metal ion and a 2OG analogue, but not with a peptide substrate (10, 12).
We present here the first crystal structure of a P4H complexed with a proline-rich peptide substrate. Cr-P4H-1 was cocrystallized with a 10-residue long peptide substrate (Ser-Pro)5 that adopts the PPII helix conformation. The structure reported here reveals an entirely novel binding mode for proline-rich peptide substrates that is also expected to be utilized in C-P4Hs but not in HIF-P4Hs.
EXPERIMENTAL PROCEDURES
Preparation of the Protein Samples and Activity Measurements
A truncated Cr-P4H-1 (Val29-His253) with an N-terminal His6SUMO fusion partner was expressed in Escherichia coli and purified to homogeneity as described previously (12). The tag was cleaved by digestion overnight with SUMO protease (12). Mutations were introduced using a QuikChangeTM site-directed mutagenesis kit (Stratagene). The wild-type and mutant variants of Cr-P4H-1 were purified for the kinetic analyses as above, with the exception that the His6SUMO fusion partner was not cleaved. The catalytic properties of the wild-type and mutant Cr-P4H-1 were measured using poly(l-proline), Mr 5000, (Ser-Pro)5 and (Pro-Pro-Gly)10 as substrates as described previously (11).
Crystallization
The purified and tagless Cr-P4H-1 was concentrated to 3 mg/ml in 0.01 m Tris-HCl, 0.1 m NaCl, 0.1 m glycine, pH 7.8, using a 10,000 molecular weight cutoff Amicon Ultra Centrifugal Filter Device (Millipore). The protein sample was supplemented with 5 mm ZnSO4, 2 mm pyridine 2,4-dicarboxylate (PDC) (Sigma), and 2.5 mm (Ser-Pro)5 (Innovagen). The microbatch under-oil method and 96-well hydrophilic vapor batch plates (Douglas Instruments) were used to co-crystallize Cr-P4H-1 with Zn2+, PDC, and (Ser-Pro)5. The crystallization drops and the covering 6-μl paraffin oil layer (Sigma) were pipetted by the Tecan Freedom Evo crystallization robot (Tecan) operated via the CrysScreen software (Tecan). The crystallization drops contained equal volumes (1.5 μl) of the protein sample and the precipitant solution. The precipitant solution contained a 5% (w/v) polyethylene glycol (PEG) mixture with equal amounts of PEG 400, PEG 1000, PEG 1500, PEG 4000, PEG 6000, PEG 8000, PEG monomethyl ether (MME) 550, PEG MME 750, and PEG MME 5000 (this PEG mixture is referred to as PEG Smear (20)) in 0.1 m acetate, pH 5.5, and 10 mm zinc acetate. The triangle-like plate crystals appeared in 1 week at +22 °C and grew to a final size of 350 × 350 × 100 μm in one month. The crystals were soaked for ∼1 min in a cryosolution of 17.5% (w/v) glycerol, 10% (w/v) PEG Smear, 10 mm zinc acetate, 0.1 m acetate, pH 5.5, and 1 mm (Ser-Pro)5, and were subsequently flash-frozen in liquid nitrogen.
Crystal Structure Determination and Validation
A dataset to 1.98-Å resolution was collected from a Cr-P4H-1 crystal using synchrotron radiation at the X12 beamline, EMBL, DESY, Hamburg at 100 K cryoconditions using a 0.932-Å wavelength. The data were collected using a strategy from BEST (21) based on two reference images collected 90 degrees apart and processed by MOSFLM (22). The final dataset was processed using XDS (23). The data collection statistics are summarized in Table 1. The structure was solved by molecular replacement using Phaser (24) and molecule A of the SeMet(apo) form of Cr-P4H-1 (PDB ID 2V4A) (12), excluding the flexible loop regions, as a model. The structures of Cr-P4H-1 in molecules A-D and of the (Ser-Pro)5 peptides at the active sites of molecules A and C were built with iterative cycles of manual building using COOT (25). A restrained refinement with translation, libration, and screw-rotation displacement (TLS) (26) was accomplished using Refmac5 (27) from the CCP4 package (28). The (Ser-Pro)5 at the active site of molecule A consists of residues Pro4-Ser9, whereas in molecule C the residues Pro2-Ser9 could be built in the electron density. A total of 14 zinc atoms were included in the model, one at each of the four active sites and the rest mostly at the molecule-molecule interfaces. There were strong densities close to the bound Zn2+ in each molecule in the 2OG-binding pocket, but these were not enough to build the PDC molecule. The acetate ions fitted perfectly in these densities and refined with normal B-values. Finally, 468 water molecules were added to the structure using the ARP/wARP (29) option in Refmac. The final refinement statistics are shown in Table 1. Noncrystallographic symmetry restraints were not used during the refinement. The TLSANL program (30) was used to obtain the isotropic and the anisotropic B-values for each individual atom. Ramachandran plot analysis showed 99.5% of the residues to be in favored regions and 0.5% in allowed regions according to MolProbity (31). The figures were prepared using Pymol (Delano Scientific LLC).
TABLE 1.
Data collection and refinement statistics regarding the Zn-peptide complex of Cr-P4H-1
Values in parentheses are for the highest resolution shell.
| Data collection | |
| Beamline | X12, EMBL DESY |
| Temperature (K) | 100 |
| Wavelength (Å) | 0.932 |
| Space group | C2 |
| Unit cell parameters (Å, °) | a = 177.3 |
| b = 58.8 | |
| c = 105.2 | |
| β = 102.2 | |
| Redundancy | 2.9 |
| Resolution range (Å) | 25–1.98 (2.04–1.98) |
| Completeness (%) | 93.4 (87.1) |
| I/σ(I) | 12.2 (4.3) |
| Rmerge (%) | 6.2 (20.1) |
| Vm (Å3/D) | 2.6 |
| Molecules/asymmetric unit | 4 |
| Wilson B (Å2) | 24.6 |
| Refinement | |
| Resolution (Å) | 20.6-1.98 |
| R (%) | 21.7 |
| Rfree (%) | 25.4 |
| Number of reflections | 65243 |
| Number of atoms | 6949 |
| Protein (molecules A-D) | 6356 |
| (Ser-Pro)5 in molecule A | 39 |
| (Ser-Pro)5 in molecule C | 52 |
| Waters | 468 |
| Zn2+ | 14 |
| Acetate | 20 |
| Geometry statistics | |
| R.m.s. deviations, bonds (Å) | 0.020 |
| R.m.s. deviations, angles (°) | 1.8 |
| Average B-factor (Å2) | 23.3 |
| Protein (molecules A-D) | |
| Main/side chain | 22.4/24.0 |
| (Ser-Pro)5 | |
| Main/side chain in A | 17.2/17.1 |
| Main/side chain in C | 24.9/25.2 |
| Waters | 24.6 |
| Zn2+ | 25.3 |
| Acetate | 15.5 |
| Ramachandran plot (Molprobity) | |
| Favoured (%) | 99.5 |
| Allowed (%) | 0.5 |
| PDB ID | 3GZE |
RESULTS AND DISCUSSION
Structure Determination
A truncated Cr-P4H-1 lacking the 29 N-terminal amino acids, was prepared as described (11, 12) and crystallized in the presence of the inhibitors Zn2+ and PDC, and the peptide substrate (Ser-Pro)5. PDC is a homologue of 2OG (Fig. 1A) having two identically placed carboxylate groups, and the (Ser-Pro)5 peptide is a shortened version of a (Ser-Pro)19 motif present in the GP1 protein of the C. reinhardtii cell wall, which is a potent substrate for Cr-P4H-1 (11, 16). Four independent molecules A–D are present in the resulting crystal form of Cr-P4H-1, and the final model was refined to 1.98-Å resolution (Table 1). Zn2+ is present at each of the four active sites, while the (Ser-Pro)5 peptide is found only in molecules A and C (Table 2). The Cr-P4H-1 molecules containing both Zn2+ and the peptide are referred to hereafter as the Zn-peptide complex. PDC is not found in any of the Cr-P4H-1 molecules, but instead an acetate molecule is placed at each active site close to the Zn2+ in the 2OG-binding pocket. The acidic crystallization conditions (pH 5.5) together with the presence of acetate ions in the buffer solution have apparently favored binding of the acetate ion in the 2OG-binding pocket instead of PDC. This is the second crystal form of Cr-P4H-1 obtained in the presence of Zn2+. A Cr-P4H-1 ternary complex with Zn2+ and PDC (referred to as the Zn-PDC complex) was crystallized earlier at pH 8.5 in Tris-HCl buffer (PDB entry 2JIG) (12). The new structural data on the Zn-peptide complex will first be described and compared with the previous Zn-PDC complex. This structure will subsequently be compared with the structures of corresponding ternary complexes of the other superfamily members and discussed also in the context of point mutation studies probing the functional importance of residues in the flexible loops.
TABLE 2.
The four known Zn2+ complex structures of Cr-P4H-1
| Complex structure | PDB ID/molecule | Metal ion | β3-β4 loop | β I-βII loop | Tyr140a | βII-βIII loop |
|---|---|---|---|---|---|---|
| Zn-peptide complex | 3GZE/A, C | Zn2+ | Ordered, closed | Ordered | In | Ordered, extended |
| Zn-PDC complex | 2JIG/A | Zn2+ | Ordered, open | Ordered | In | Ordered, compact |
| Zn-binary complex | 3GZE/B, D | Zn2+ | Disordered | Disordered | Out | Disordered |
| Zn-binary complex | 2JIG/B | Zn2+ | Disordered | Disordered | Out | Ordered, extended |
a “In” refers to the active conformation of Tyr140. In the “Out” conformation, the main chain atoms of Tyr140 also change. The βII strand that contains the metal binding -His-X-Asp- motif is always ordered in the Zn2+ complexes and adopts identical conformation in these four structures.
The Overall Structure
The (Ser-Pro)5 peptide adopts a typical PPII helix conformation in the Zn-peptide complex (Fig. 1C). Ser1 is disordered in molecule C, Ser1-Ser3 in molecule A and Pro10 in both molecules. Molecule C is therefore used as the reference molecule. The middle -Ser5-Pro6-Ser7- tripeptide region of the bound peptide has the lowest B-factors, and its geometry is well defined by the electron density map (Fig. 2). The Pro6 is deeply buried and points toward the catalytic site (Figs. 2 and 3A). The peptide is bound to the edge strands of the jelly-roll fold, namely the βII-strand of the minor sheet of the jelly-roll fold and the β5-strand that extends the major sheet (Fig. 1, B and C). The N terminus of the peptide is bound close to the minor sheet, which also provides the three conserved metal ion-binding residues, namely His143, Asp145 and His227 (Fig. 2). The peptide subsequently crosses over to the major sheet, where Ser9 interacts with the Trp99 of the β5 edge strand (Fig. 2). The bound peptide also interacts with the side chains of βVII (Leu226, near Pro2) of the minor sheet and of βI (Glu127 and Gly128, near Ser9) and βVIII (Trp243, near Pro6) of the major sheet (Fig. 3B). The -Ser5-Pro6-Ser7- region of the bound peptide is completely covered by two loops, the β3-β4 loop (Val75-Ser95) and the βII-βIII loop (Tyr146-Gly158), which protrude out of the jelly-roll fold (Figs. 1C, 3A, and 4). The β3-β4 loop in particular interacts tightly with the bound peptide via the residues Ser78-Val79-Val80 at the entrance to the loop and Ser87 and Arg93 at the exit (Fig. 3, A and B). These two loops, which are disordered in molecules B and D of this crystal form, are known to have large conformational flexibility (12) (Fig. 5 and Table 2). The β3-β4 loop, which was only seen in an ordered open conformation in the Zn-PDC complex (12), now adopts an ordered closed conformation in which its tip has moved 14 Å toward the tip of the βII-βIII loop (Fig. 5). The βII-βIII loop conformation of the Zn-peptide complex is referred to as an ordered-extended conformation (Table 2), previously also observed in the Zn-binary complex (12). In the other structures, this loop is partially disordered or observed in an ordered, compact conformation (Table 2). In the latter conformation the βII-βIII loop binds in the peptide-binding groove (Fig. 5).
FIGURE 2.
Stereoview of the (2Fo-Fc)αc omit density (countered at 1 σ) for the (Ser-Pro)5 in molecule C of the Cr-P4H-1 crystal structure. The active site with Zn2+, coordinated by His143, Asp145, His227 and an acetate molecule (green sticks), and some of the most important (Ser-Pro)5 binding residues are shown. Hydrogen bonds are indicated by dashed lines.
FIGURE 3.
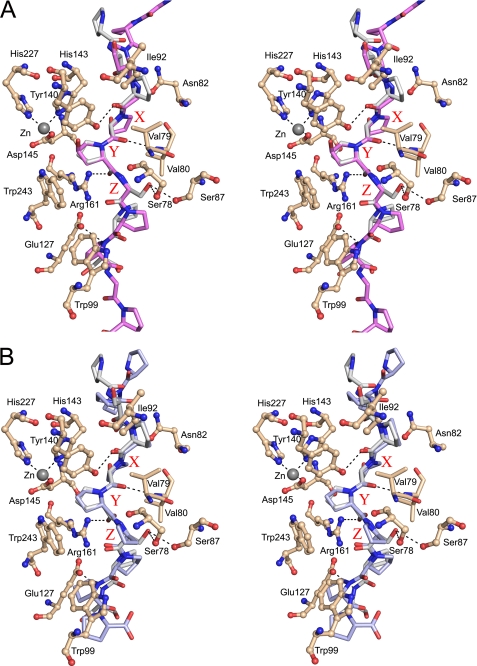
The hydrogen-bonding network between the (Ser-Pro)5 peptide substrate and the Cr-P4H-1. A, stereoview looking along the left-handed poly(l-proline) type II helix of the bound (Ser-Pro)5 molecule. The interactions between the flexible loops β3-β4, containing the -Asp/Asn-X-X-Ser/Thr-Gly- motif (upper loop), and βII-βIII, containing the -Asp/Glu-X-X-Asn/Asp- motif (lower loop), are also shown. Note that the side chain of the X-position amino acid of the central tripeptide of the (Ser-Pro)5, Ser5, points down toward the βII-βIII loop, whereas, the side chain in the Z-position, Ser7, points up toward the β3-β4 loop. Waters W37 and W40, binding between the two loops, are labeled. B, schematic view showing all the interactions between the (Ser-Pro)5 and the Cr-P4H-1 molecule. Water molecules are also shown. Note that the N terminus of the (Ser-Pro)5 is on the right side of the image. The X-Y-Z tripeptide motif is highlighted. Relevant van der Waals contacts between the hydrophobic Cr-P4H-1 side chains and each (Ser-Pro)5 residue are indicated by open arrows.
FIGURE 4.
Surface representation of Cr-P4H-1 with bound (Ser-Pro)5. The central -Ser5-Pro6-Ser7- tripeptide is covered by the two flexible loops β3-β4 and βII-βIII, while the rest of the peptide is bound in a shallow groove.
FIGURE 5.
Stereoview of the superimposed overall folds of the different conformational states of Cr-P4H-1. Altogether one Zn-peptide, one Zn-PDC, two Zn-binary complexes (Table 2) and two apo structures are included. The (Ser-Pro)5 ligand (stick representation) of the Zn-peptide complex is also shown. The largest deviations between the different states exist in the three loop regions labeled in the figure. The Zn-peptide complex (molecule A of PDB ID 3GZE, light brown) and the Zn-PDC complex (molecule A of PDB ID 2JIG, violet) are the only states where the β3-β4 loop is ordered being the “ordered closed” or “ordered open” conformation (Table 2), respectively. The βII-βIII loop exists in an extended conformation in all states except in the Zn-PDC complex, where it is in a “ordered compact” conformation. This loop is also partially disordered in the apo form (molecule D of PDB ID 2V4A, light blue) and in the Zn-binary complex of this study (molecule B of PDB ID 3GZE, green). The βI-βII loop is only ordered in the Zn-peptide (light brown) and Zn-PDC (violet) complexes. The N termini of the two apoforms are ordered and start at Met28 (molecule A of PDB ID 2JIJ, cyan; and molecule D of PDB ID 2V4A, light blue), whereas the rest of the molecules start at Glu37 or Trp38. The sixth Cα-trace is of the second Zn-binary complex (PDB ID 2JIG, pink).
In the Zn-peptide complex the tips of the β3-β4 and βII-βIII loops interact with each other, but not with the peptide substrate, as highlighted in Fig. 3A. Both tips adopt an α-helical-like conformation with a strong hydrogen bond between the main chain oxygen of Asp81 and nitrogen of Gly85 and a weak hydrogen bond between the main chain oxygen of Asp149 and nitrogen of Ala153. Two sequence motifs present in these Cr-P4H-1 loop tips are conserved in plant P4Hs and C-P4Hs, namely -Asp/Asn-X-X-Ser/Thr-Gly- (Asp81-Gly85) in the β3-β4 loop of Cr-P4H-1 and -Asp/Glu-X-X-Asn/Asp- (Asp149-Asn152) in the βII-βIII loop (Fig. 6). In these motifs, the side chains of Asp81 and Ser84 as well as those of Asp149 and Asn152 are hydrogen-bonded to each other (Fig. 3A). The side chains of Asp81 and Ser84 point into the bulk solvent, whereas the Asp149 and Asn152 side chains point to the partner loop (Fig. 3A). Nevertheless, only one direct hydrogen bond exists between the two loop tips, namely Asn152-Gly85 (Fig. 3A). The residue Gly85 is fully conserved in the plant P4H and C-P4H sequences (Fig. 6), the Phi/Psi values (82°/2°) not favoring a side chain in this position. In addition, there are water (W)-mediated hydrogen bonding interactions between the two loops via three well-defined water molecules W40, W37, and W81 (Fig. 3, A and B). W37, W40, and W81 are also hydrogen-bonded to the (Ser-Pro)5 peptide, in particular to the side chain oxygen of Ser5 (Fig. 3B). There is also a hydrophobic interaction between the two loops, mediated by Val80 and Phe147 (Fig. 3, A and B).
FIGURE 6.
Alignment of the amino acid sequences of Cr-P4H-1, At-P4H isoforms 1 and 2, and the catalytic α-subunits of various C-P4Hs corresponding to the Cr-P4H-1 β3-β4 (Val74-Ser95) and βII-βIII (Tyr146-Gly158) loop sequences. The residues discussed in the text are highlighted with black arrows, and the most flexible regions observed in the Cr-P4H-1 structures are shown with black lines below the alignments. The secondary structures of molecule C are indicated above the sequence alignment and the two sequence motifs, -Asp/Asn-X-X-Ser/Thr-Gly- and -Asp/Glu-X-X-Asn/Asp-, are highlighted with dashed lines. The residues conserved throughout all the sequences are indicated with a white letter on a black background, residues with 70–99% identity between the sequences with a white letter on a dark gray background and those with 40–69% identity with a black letter on a light gray background. The amino acid sequence of Cr-P4H-1 is aligned with the corresponding regions of the A. thaliana P4H-1 (At-P4H-1) and 2 (At-P4H-2), the catalytic α(I) and α(II) subunits of C-P4H isoenzymes I and II from human (Hs-α(I), Hs-α(II)), rat (Rn-α(I), Rn-α(II)), mouse (Mm-α(I), Mm-α(II)), Caenorhabtidis elegans (Ce-α(Phy1), Ce-α(Phy2) and Drosophila melanogaster (Dm-P4H).
The β3-β4 and βII-βIII loops have high conformational flexibility (Fig. 5), and the observed conformations will thus be affected by crystal contacts. For this reason a systematic mutagenesis study of the conserved motifs of the two loops was performed to confirm the importance of these two partner loops for the biocatalytic function of Cr-P4H-1 (Table 3). In each of these loop variants, i.e. the D81A, S84A, G85A, D149A, D149N, and N152A mutants, the Km values for the substrate poly(l-proline) were markedly increased and a 4–15-fold decrease was observed in the kcat values, although none of these residues directly interacts with the substrate. In the N152A variant the only hydrogen bond between the two loops is lost, causing the most drastic effect on the kinetic constants. The mutagenesis data show that modest residue changes, even at the tips of the loops (Table 3), which effect the loop-loop interactions, much reduce the catalytic efficiency.
TABLE 3.
Kinetic studies of wild-type and mutant variants of Cr-P4H-1 using poly(l-proline), Mr 5000, as a substrate
The Ser78 and Ser87 mutants were also analysed with the (Ser-Pro)5 and (Pro-Pro-Gly)10 peptide substrates. All the mutations are in flexible loop regions that are important for substrate binding, as discussed in the text.
| Mutation | Km (Poly)l-Proa | kcat (Poly)l-Proa | Km (Ser-Pro)5a | kcat (Ser-Pro)5a | Km (Pro-Pro-Gly)10a | kcat (Pro-Pro-Gly)10a |
|---|---|---|---|---|---|---|
| μm | s−1 | μm | s−1 | μm | s−1 | |
| Wild type | 290 ± 60 | 30 | 380 ± 130 | 19 ± 3 | 930 ± 170 | 7 ± 2 |
| Entrance and exit of the β3-β4 loop | ||||||
| S78T | 110 ± 20 | 20 ± 3 | 190 ± 50 | 14 ± 2 | 830 ± 120 | 9 ± 2 |
| S87L | 260 ± 50 | 8 ± 3 | 780 ± 100 | 18 ± 4 | 1050 ± 320 | 6 ± 1 |
| S78T/S87L | 350 ± 50 | 4 ± 0.1 | 140 ± 50 | 6 ± 2 | 1270 ± 230 | 5 ± 1 |
| Tip of the β3-β4 loop | ||||||
| D81A | 880 ± 120 | 7 ± 2 | ||||
| S84A | 770 ± 40 | 2 ± 0.1 | ||||
| G85A | 940 ± 50 | 4 ± 1.5 | ||||
| Tip of the βII-βIII loop | ||||||
| D149N | 560 ± 10 | 6 ± 2 | ||||
| D149A | 630 ± 60 | 2 ± 0.1 | ||||
| N152A | 1590 ± 240 | 2 ± 0.2 | ||||
| βI-βII loop | ||||||
| Y140F | 340 ± 80 | 3 ± 0.3 | ||||
a Values are means ± S.D. from at least 3–4 independent experiments.
The bound (Ser-Pro)5 peptide has adopted the PPII helix conformation, as generally observed in complexes of proline-rich peptides with proteins (32). The interactions between (Ser-Pro)5 and Cr-P4H-1 are unique in two other respects, however, i.e. (i) the absence of stacking interactions between the proline side chains of the bound peptide and aromatic residues of the protein, and (ii) the -Ser5-Pro6-Ser7- central tripeptide is completely buried in the complex, being shielded from the bulk solvent by the β3-β4 and βII-βIII loops (Fig. 4). This substrate-binding mode of Cr-P4H-1 is also unique within the 2OG dioxygenase superfamily. Most of the family members with known structures are microbial enzymes involved in antibiotic biosynthesis that use small molecule substrates (18). Only factor inhibiting HIF (FIH), which hydroxylates a specific asparagine residue in HIF-α, has been co-crystallized with its peptide substrate, but the substrate is not proline-rich and thus has no PPII helix conformation and is not bound in a tunnel (33). A loop region corresponding to the β3-β4 loop in Cr-P4H-1 also participates in the peptide binding in FIH (33), however, and it has been proposed that a topologically similar loop may be involved in the substrate binding of HIF-P4H-2 (34), the oxidative DNA/RNA repair enzyme AlkB from E. coli (35) and phytanoyl-CoA hydroxylase (36). However, in the corresponding loops of these homologues the characteristic -Asp/Asn-X-X-Ser/Thr-Gly- sequence motif is not conserved. Moreover, the βII-βIII loop is absent in FIH and in HIF-P4H-2. The fact that the latter loop is not present in any of the three HIF-P4H isoforms (6) indicates that this subfamily of P4H must have a different strategy for peptide substrate binding than plant P4Hs and C-P4Hs, which all have the elongated βII-βIII loop (Fig. 6).
Peptide Binding and Substrate Specificity
The interactions between (Ser-Pro)5 and Cr-P4H-1 are highlighted in a schematic way in Fig. 3B. The free main chain NH- and carbonyl oxygen groups of the peptide are hydrogen-bonded to the side chains and main chain of Cr-P4H-1, and well defined water molecules. The central proline, Pro6, of the peptide points to the active site and is surrounded by five hydrophobic residues, Val79 and Val80 of the β3-β4 loop, Tyr140 of βII, Phe147 of the βII-βIII loop and Trp243 of βVIII (Figs. 2 and 3, A and B). The side chains of the catalytic -His-X-Asp- motif, the Zn2+ ion and two arginines, Arg93 and Arg161, are also located nearby (Figs. 2 and 3). Arg93, which is stacked with the side chain of Tyr140, interacts with the (Ser-Pro)5 via a water molecule W174, whereas Arg161 forms a direct hydrogen bond with the backbone oxygen of Pro6 (Fig. 3). In addition, Tyr140 forms a hydrogen bond with the backbone oxygen of Pro4 of the peptide (Fig. 3, A and B). These three amino acids, Arg93, Tyr140, and Arg161, are fully conserved in all P4Hs, and mutation of any of these residues to alanine leads to complete inactivation of Cr-P4H-1 (12). Two main chain-main chain hydrogen bonds are formed between the -Ser78-Val79-Val80- region of the β3-β4 loop of Cr-P4H-1 and the -Ser5-Pro6-Ser7- region of the peptide (Fig. 3). In addition, the side chain oxygens of Ser78 and Ser87 of the β3-β4 loop are hydrogen-bonded to the side chain oxygen of the peptide Ser7 (Fig. 3). Although these four residues of the β3-β4 loop, Ser78, Val79, Val80, and Ser87, closely interact with (Ser-Pro)5, only Val79 is highly conserved in the animal C-P4Hs and plant P4Hs (Fig. 6).
The structural data obtained here provide a first rationale for understanding the substrate specificity of the animal C-P4Hs for (X-Pro-Gly)n sequences. Given the notable sequence identity between Cr-P4H-1 and the catalytic domain of C-P4Hs (12), it is predicted that the mode of binding of the peptide in Cr-P4H-1 and C-P4Hs is the same, implying that the bound collagen peptide in the C-P4H complex will also adopt the PPII conformation. As shown in Fig. 7, A and B, both a collagen peptide taken from the structure of a synthetic collagen triple helix and the poly(l-proline) peptide superimpose well with the bound (Ser-Pro)5 peptide substrate in Cr-P4H-1. In particular the residues of the central tripeptide region, referred to as being at the X, Y, and Z positions (Pro-Hyp-Gly in the collagen peptide and Pro-Pro-Pro in poly-l-proline), superimpose well with the corresponding -Ser5-Pro6-Ser7- tripeptide in both cases. This indicates that the hydrogen bonding interactions that exist between the main chain atoms of the central tripeptide of the (Ser-Pro)5 substrate and Cr-P4H-1 will also exist between the C-P4Hs and their -X-Pro-Gly- substrates.
FIGURE 7.
Comparison of the PPII helix conformation of the different P4H peptide substrates. Stereoview showing the bound (Ser-Pro)5 superimposed with (A) a collagenous (Pro-Pro-Gly)4-Pro-Hyp-Gly-(Pro-Pro-Gly)4 peptide from a synthetic collagen triple helix (PDB ID 2D3F) and (B) a poly(l-proline) peptide taken from a complex structure with human platelet profillin (PDB ID 1AWI). Some of the hydrogen bonds between the Cr-P4H-1 and the (Ser-Pro)5 peptide substrate are shown by dashed lines. Note that Val79, Trp99, Glu127, Tyr140, Arg161, and Trp243 are highly conserved in plant and C-P4Hs (12).
The structure presented here shows that each of the three positions of the central tripeptide of the bound (Ser-Pro)5 has unique features. Firstly, the serine in the Z-position, Ser7, points upwards and is hydrogen-bonded to the side chain oxygen atoms of Ser78 and Ser87 of the β3-β4 loop (Fig. 3B). The residues corresponding to these two serines in C-P4Hs are highly conserved threonine and leucine residues, respectively (Fig. 6). These more bulky and more hydrophobic residues, apparently favor a glycine in the Z-position of the tripeptide bound to C-P4Hs. This is fully consistent with the fact that these enzymes solely hydroxylate the proline in the Y position of the (Pro-Pro-Gly)10 peptide. This preference of C-P4Hs for a glycine also suggests that the peptide-protein main chain-main chain interaction between N(Ser7) and O(Ser78) is preserved in the C-P4H peptide substrate complexes, and this could be an important substrate specificity determinant in C-P4Hs.
The function of the hydrogen-bonding interactions between the two serines and the serine residue in the Z-position of the peptide substrate (Ser7) was probed by Cr-P4H-1 mutagenesis followed up by enzyme kinetic studies using three substrates, poly(l-proline), (Pro-Pro-Gly)10 and (Ser-Pro)5. Poly(l-proline) is used as a substrate by Cr-P4H-1 and At-P4H isoenzymes 1 and 2, their Km values for poly(l-proline), Mr 5000–10000, being 140, 2, and 30 μm, respectively (11, 17, 37, 38). Poly(l-proline) is not hydroxylated by C-P4Hs, but instead acts as an efficient competitive inhibitor of C-P4H-I with respect to the collagen substrate, the Ki being 0.5 μm (39). The (Pro-Pro-Gly)10 collagen peptide substrate has low affinity for Cr-P4H-1 and At-P4H isoenzyme type 2, the Km values being >1500 μm and 2800 μm, respectively, but binds tightly to At-P4H isoenzyme type 1 and C-P4Hs (11, 17, 37, 38). Ser78 and Ser87 were mutated to threonine and leucine, respectively, to mimic the C-P4H sequences (Fig. 6). Wild-type Cr-P4H-1 hydroxylates the (Ser-Pro)5 and (poly)l-Pro peptides with similar efficiency, whereas the (Pro-Pro-Gly)10 peptide is hydroxylated with lower catalytic efficiency (Table 3). The Cr-P4H-1 S78T, S87L and S78T/S87L mutations had no effect on the hydroxylation efficiency of (Pro-Pro-Gly)10 (Table 3). The S78T and S87L mutations reduced the kcat values by 1.5-fold and about 4-fold when poly(l-proline) was used as a substrate, while the effects were even milder, if any, with (Ser-Pro)5 (Table 3). The double mutation S78T/S87L reduced the kcat values obtained with poly(l-proline) and (Ser-Pro)5 10-fold and 3-fold, respectively (Table 3). These data indicate that the interactions between the Ser78 and Ser87 side chains and the peptide substrate provide only a moderate contribution to the substrate specificity of Cr-P4H-1.
The peptide-protein interactions in the X-position will also be important for the substrate specificity. The serine side chain in the X-position, Ser5, points downwards to the βII-βIII loop and makes two hydrogen bonds with main chain oxygen atoms (Tyr144 and Tyr146) of this loop (Fig. 3). Furthermore, Ser5 is hydrogen-bonded to water molecules involved in water-mediated loop-loop (β3-β4/βII-βIII) interactions (Fig. 3). As shown in Fig. 6, there are more amino acid differences between the Cr-P4H-1 and C-P4H βII-βIII loops than between their β3-β4 loops, the former also being 2 residues longer in the C-P4Hs. These differences correlate with different substrate specificities in the X-position of the central tripeptide, being preferably a proline in the C-P4Hs (1). As shown in Fig. 3, the side chains of the βII-βIII loop point upwards toward the β3-β4 loop, and they are therefore also critically important for the water structure between these two loops and therefore for the substrate specificity. For example, Asp149 of the βII-βIII loop is hydrogen bonded to water W40 and subsequently to water W37 and the main chain nitrogen of Ser5 (Fig. 3).
Active Site and Reaction Mechanism
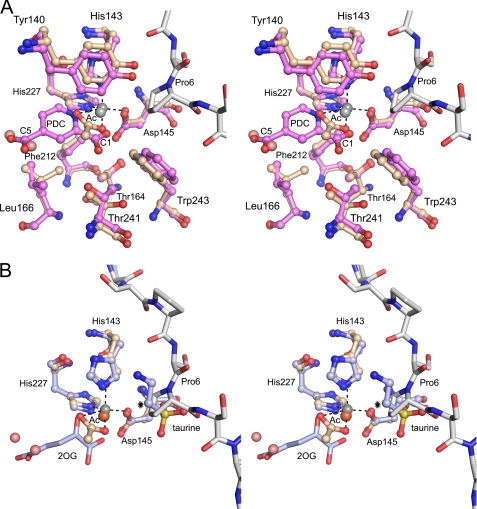
A characteristic feature of the structure of this competent complex is the “in” orientation of the side chain of Tyr140 (Table 2), pointing inwards into the 2OG binding cavity (Fig. 8A), and being sandwiched between the side chains of Arg93 and His143 (Fig. 3A). This geometry is observed in the Zn-peptide complex and in the Zn-PDC complex. The Zn2+ ion is complexed with a solvent molecule, acetate or PDC, respectively, in both structures. The acetate ion in the Zn-peptide complex mimics the binding of PDC in the Zn-PDC complex in that one acetate oxygen atom replaces the 1-carboxylate oxygen and the second acetate oxygen replaces the nitrogen atom of the inhibitor (Fig. 8A). In the Zn-peptide complex two water molecules are bound at the bottom of the 2OG binding cavity. These water molecules replace the two 5-carboxylate oxygens of PDC (Fig. 8A).
FIGURE 8.
The active site geometry of Cr-P4H-1. A, superimposed stereoview of the active sites of the Cr-P4H-1 Zn-peptide complex (light brown) and Zn-PDC complex (violet). The zinc ions at the active sites are shown as gray balls, and the dashed lines highlight the atoms directly interacting with the zinc ion in the Zn-peptide complex. The (Ser-Pro)5 is shown using gray bond colors. The amino acid side chain conformational differences at the proposed oxygen binding site opposite to the proximal histidine (below the zinc ions) are shown. The 1-carboxylate and 5-carboxylate ends of PDC are labeled. The acetate molecule (Ac) and two water molecules (light red) in the 2OG-binding pocket of the Zn-peptide complex are also shown. B, superimposed stereoview of the active sites of the Cr-P4H-1 Zn-peptide complex (light brown) and TauD (PDB ID 1OS7). The iron ion (brown) bound to TauD, and the taurine substrate is also shown. The hydroxylated atom of the taurine molecule is marked with an asterisk.
The geometry at the Zn2+ ion binding site of the Zn-PDC complex and the Zn-peptide complex shows the position of the three protein ligand atoms of the -His-X-Asp-…-His- motif and the two PDC/acetate atoms (Fig. 8A). The remaining sixth coordination position for molecular oxygen is predicted to be opposite the proximal histidine (12). In the Zn-PDC complex, a 31 Å3 cavity is calculated to occur in this region with ICM (MOLSOFT, LLC). This cavity is lined by the carboxylate moiety of Asp145, and by the hydrophobic side chain moieties of Thr164, Leu166, Phe212, Thr241, and Trp243 (Fig. 8A). Each of these residues protrudes out of the major sheet. In the Zn-peptide complex the side chains of Thr241, Trp243, and Leu166 have adopted slightly different orientations (Fig. 8A). Thr241 is hydrogen bonded to the inhibitor molecule in the Zn-PDC complex, but has rotated in the Zn-peptide complex, like the Leu166 and Trp241 side chains, causing the cavity to disappear in this complex. This finding is consistent with the fact that in the catalytic cycle molecular oxygen binds last to the active site (2), implying that both the substrate and the cofactor (2OG) are present when oxygen binds. It has been suggested that the O2 binding site may also be located opposite the proximal histidine in clavaminate synthase (40) and in the AlkB (35), but interestingly, the currently available structures of 2OG dioxygenases also suggests an alternative site for O2 binding, opposite to the distal histidine of the -His-X-Asp-…-His- motif (18).
The hydroxylation reaction is known to proceed via a FeIV(=O) ferryl intermediate which is formed after the activated molecular oxygen has reacted with 2OG, by which succinate is also formed (Fig. 1A) (2, 18, 19). The geometry of all available complexes suggest that the oxygen of the FeIV(=O) ferryl intermediate is bound to the metal ion in the position opposite the distal histidine, corresponding to the O1 position in the Cr-P4H-1 inhibitor complex (Fig. 8A). In this catalytic position the ferryl oxygen can abstract the C4 (Pro6) hydrogen atom, as predicted by spectroscopic studies on a P4H from Paramecium bursaria Chlorella virus 1, in which an FeIII(OH) radical and C4 (Pro6) radical are formed (41). Spectroscopic studies performed on taurine dioxygenase (TauD) from E. coli have provided very similar information on this mechanism (42). These mechanistic studies therefore suggest that the oxy-ferryl oxygen ligand migrates to its catalytic position after the decarboxylation of 2OG in the P4H reaction. In the next step of the P4H reaction cycle the OH-radical is donated back to the C4 (Pro6) radical, resulting in the hydroxylated product. The geometries of the Cr-P4H-1 Zn-peptide complex and the TauD Fe-2OG-taurine complex (PDB entry 1OS7) are compared in Fig. 8B. The TauD complex includes an Fe2+ ion, 2OG and the substrate taurine, but the reaction does not proceed, as the crystals have been grown under anaerobic conditions (43). In both TauD and the P4Hs O2 binding occurs after binding of the 2OG and the substrate (41, 42). The substrates are bound in topologically identical positions in the TauD and Cr-P4H-1 complexes (Fig. 8B). In Cr-P4H-1 the target C4 of the substrate Pro6 points to the acetate oxygen with a distance of 3.8 Å. This acetate oxygen superimposes on the O1-atoms of the PDC and 2OG molecules of the Cr-P4H-1 Zn-PDC complex and the TauD 2OG-taurine complex, respectively (Fig. 8, A and B). This geometry is suitable for the formation of the 4-R-OH product of the proline. The Pro6 is in the down-puckering (C4-endo) conformation (Fig. 9), which was recently shown to be the favored prolyl substrate conformation for C-P4Hs (44) and HIF-P4Hs (45). However, the preferred conformation of the 4-R-OH-proline is the up-puckering (C4-exo) conformation (see PDB entries: 1V4F, 1V6Q, 1V7H, 01YM, 1LM8) (44–48), and therefore it is expected that the 4Hyp will adopt the up-puckering conformation on completion of the chemical conversion. In this conformation it will clash with the side chain of Tyr140, as is visualized in the superimposition analysis (Fig. 9). Consequently, it can be predicted that the hydroxylation of Pro6 will cause Tyr140 to flip to its out conformation, as seen in the Zn-binary complexes of this study (Table 2) and in our previous study (12). As Tyr140 in the Zn-peptide complex is stacked with the Arg93 of the β3-β4 loop, the conformational change in Tyr140 will also directly affect the conformation of that loop. Concomitantly with the flipping out of Tyr140, causing the βI-βII loop to become disordered, the Glu141 side chain flips inwards and overlaps with the position of the β3-β4 loop in the Zn-peptide complex. Interestingly, the Y140A mutation completely inactivates Cr-P4H-1 (12), whereas the mutation to Phe has only minor effects on the kinetic constants (Table 3). Our model predicts that the Y140A mutation will disable the conformational switch of Tyr140, apparently causing the enzyme to become inactive, whereas the clash with the hydroxylated product in the Y140F variant will be the same as in the wild-type enzyme (Fig. 9). Evolutionary pressure has apparently generated the β3-β4 and βII-βIII loop-loop interactions to be sufficiently strong to stabilize the protein peptide interactions required for catalysis, while at the same time these loop-loop interactions are not too tight, allowing loop opening on completion of the hydroxylation step and thereby facilitating product release.
FIGURE 9.
The down-puckered Pro6 of (Ser-Pro)5 at the active site of the Zn-peptide complex with its (2Fo-Fc)αc omit density (countered at 1σ). The figure also includes a superimposed up-puckered 4-R-hydroxyproline residue (magenta bond colors), which is the product of the P4H reaction (PDB ID 2D3F). The side chain of Tyr140, which will clash with the product, is also shown.
Concluding Remarks
The structural and enzymological data on Cr-P4H-1 obtained here show that the β3-β4 and βII-βIII loops define the substrate binding tunnel. The bound peptide adopts the PPII conformation and the central -Ser-Pro-Ser- tripeptide is bound in this tunnel in such a manner that the first serine (in the X-position) points down toward the βII-βIII loop, the proline in the Y-position points toward the catalytic site and the serine in the Z-position points up toward the β3-β4 loop. The -Asp/Asn-X-X-Ser/Thr-Gly- and -Asp/Glu-X-X-Asn/Asp- motif at the tips of these loops are important for stabilizing the closed, liganded conformation, whereas the flanking regions of the loops participate in the determination of substrate specificity. It is proposed that the hydroxylation of the proline in the Y-position at the end of the catalytic cycle induces a conformational switch in which Tyr140 flips out, forcing Arg93 and the β3-β4 loop to adopt an unliganded, open (disordered) conformation. In this conformation the succinate produced by decarboxylation of 2OG can be released and replaced again by 2OG, and the hydroxylated peptide can translocate in the binding groove so that a new, unhydroxylated tripeptide is ready to be hydroxylated in the next catalytic cycle. The structure and sequence comparisons indicate that the PPII mode of binding of the central tripeptide is preserved in the C-P4H enzyme family and it is proposed that this complex enzymatic mechanism is also a common feature of these enzymes.
Acknowledgments
We thank Ville Ratas, Riitta Polojärvi, and Minna Siurua for technical assistance, the staff of the beamline X12 at the EMBL Hamburg for help during the data collection, and Dr. Ulrich Bergmann for the critical comments on the manuscript.
This work was supported by the Academy of Finland Grants 200966 (to R. K. W.), 115124 (to M. K. K.), and 1114344 and 1211128 (to J. M.) and by the Sigrid Juselius Foundation and Fibrogen Inc. (to J. M.).
The atomic coordinates and structure factors (code 3GZE) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
- 4Hyp
- 4R-hydroxyproline
- HIF
- hypoxia-inducible transcription factor
- P4H
- prolyl 4-hydroxylase
- 2OG
- 2-oxoglutarate
- C-P4H
- collagen prolyl 4-hydroxylase
- PPII
- poly(l-proline) type II
- At-P4H
- A. thaliana P4H
- Cr-P4H-1
- C. reinhardtii P4H type 1
- PDC
- pyridine 2,4-dicarboxylate
- PEG
- polyethylene glycol
- MME
- monomethyl ether
- FIH
- factor-inhibiting HIF
- TauD
- taurine dioxygenases
- R.m.s.
- root mean square
- PDB
- Protein Data Bank.
REFERENCES
- 1.Kivirikko K. I., Pihlajaniemi T. (1998) Adv. Enzymol. Relat. Areas Mol. Biol. 72, 325–398 [DOI] [PubMed] [Google Scholar]
- 2.Myllyharju J. (2003) Matrix Biol. 22, 15–24 [DOI] [PubMed] [Google Scholar]
- 3.Myllyharju J., Kivirikko K. I. (2004) Trends Genet. 20, 33–43 [DOI] [PubMed] [Google Scholar]
- 4.Kieliszewski M. J., Lamport D. T. A. (1994) Plant J. 5, 157–172 [DOI] [PubMed] [Google Scholar]
- 5.Cassab G. I. (1998) Annu. Rev. Plant Physiol. Plant Mol. Biol. 49, 281–309 [DOI] [PubMed] [Google Scholar]
- 6.Bruick R. K., McKnight S. L. A. (2001) Science 294, 1337–1340 [DOI] [PubMed] [Google Scholar]
- 7.Epstein A. C., Gleadle J. M., McNeill L. A., Hewitson K. S., O'Rourke J., Mole D. R., Mukherji M., Metzen E., Wilson M. I., Dhanda A., Tian Y. M., Masson N., Hamilton D. L., Jaakkola P., Barstead R., Hodgkin J., Maxwell P. H., Pugh C. W., Schofield C. J., Ratcliffe P. J. (2001) Cell 107, 43–54 [DOI] [PubMed] [Google Scholar]
- 8.Chowdhury R., Hardy A., Schofield C. J. (2008) Chem. Soc. Rev. 37, 1308–1319 [DOI] [PubMed] [Google Scholar]
- 9.Myllyharju J. (2008) Ann. Med. 40, 402–417 [DOI] [PubMed] [Google Scholar]
- 10.McDonough M. A., Li V., Flashman E., Chowdhury R., Mohr C., Liénard B. M., Zondlo J., Oldham N. J., Clifton I. J., Lewis J., McNeill L. A., Kurzeja R. J., Hewitson K. S., Yang E., Jordan S., Syed R. S., Schofield C. J. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 9814–9819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keskiaho K., Hieta R., Sormunen R., Myllyharju J. (2007) Plant Cell 19, 256–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koski M. K., Hieta R., Böllner C., Kivirikko K. I., Myllyharju J., Wierenga R. K. (2007) J. Biol. Chem. 282, 37112–37123 [DOI] [PubMed] [Google Scholar]
- 13.Jaakkola P., Mole D. R., Tian Y. M., Wilson M. I., Gielbert J., Gaskell S., J., Kriegsheim Av., Hebestreit H. F., Mukherji M., Schofield C. J., Maxwell P. H., Pugh C. W., Ratcliffe P. J. (2001) Science 292, 468–472 [DOI] [PubMed] [Google Scholar]
- 14.Ivan M., Kondo K., Yang H., Kim W., Valiando J., Ohh M., Salic A., Asara J. M., Lane W. S., Kaelin W. G., Jr. (2001) Science 292, 464–468 [DOI] [PubMed] [Google Scholar]
- 15.Adair W. S., Snell W. J. (1990) in Organization and Assembly of Plant and Animal Extracellular Matrix ( Adair W. S., Mecham R. P. eds) pp. 15–84, Academic Press, San Diego, CA [Google Scholar]
- 16.Ferris P. J., Woessner J. P., Waffenschmidt S., Kilz S., Drees J., Goodenough U. W. (2001) Biochemistry 40, 2978–2987 [DOI] [PubMed] [Google Scholar]
- 17.Hieta R., Myllyharju J. (2002) J. Biol. Chem. 277, 23965–23971 [DOI] [PubMed] [Google Scholar]
- 18.Clifton I. J., McDonough M. A., Ehrismann D., Kershaw N. J., Granatino N., Schofield C. J. (2006) J. Inorg. Biochem. 100, 644–669 [DOI] [PubMed] [Google Scholar]
- 19.Ozer A., Bruick R. K. (2007) Nat. Chem. Biol. 3, 144–153 [DOI] [PubMed] [Google Scholar]
- 20.Newman J., Egan D., Walter T. S., Meged R., Berry I., Ben Jelloul M., Sussman J. L., Stuart D. I., Perrakis A. (2005) Acta Crystallogr. D Biol. Crystallogr. 61, 1426–1431 [DOI] [PubMed] [Google Scholar]
- 21.Bourenkov G. P., Popov A. N. (2006) Acta Crystallogr. D Biol. Crystallogr. 62, 58–64 [DOI] [PubMed] [Google Scholar]
- 22.Leslie A. G. W. (1992) Joint CCP4 ESF-EACBM Newsl. Protein Crystallogr. 26, 22–33 [Google Scholar]
- 23.Kabsch W. (1993) J. Appl. Crystallogr. 26, 795–800 [Google Scholar]
- 24.McCoy A. J., Grosse-Kunstleve R. W., Storoni L. C., Read R. J. (2005) Acta Crystallogr. D Biol. Crystallogr. 61, 458–464 [DOI] [PubMed] [Google Scholar]
- 25.Emsley P., Cowtan K. (2004) Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 26.Winn M. D., Isupov M. N., Murshudov G. N. (2001) Acta Crystallogr. D Biol. Crystallogr. 57, 122–133 [DOI] [PubMed] [Google Scholar]
- 27.Murshudov G. N., Vagin A. A., Dodson E. J. (1997) Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 [DOI] [PubMed] [Google Scholar]
- 28.CCP4 ( 1994) Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 [DOI] [PubMed] [Google Scholar]
- 29.Evrard G. X., Langer G. G., Perrakis A., Lamzin V. S. (2007) Acta Crystallogr. D Biol. Crystallogr. 63, 108–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Howlin B., Butler S. A., Moss D. S., Harris G. W., Driessen H. P. C. (1993) J. Appl. Crystallogr. 26, 622–624 [Google Scholar]
- 31.Davis I. W., Leaver-Fay A., Chen V. B., Block J. N., Kapral G. J., Wang X., Murray L. W., Arendall W. B., 3rd, Snoeyink J., Richardson J. S., Richardson D. C. (2007) Nucleic Acids Res. 35, W375–W383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ball L. J., Kühne R., Schneider-Mergener J., Oschkinat H. (2005) Angew. Chem. Int. Ed. 44, 2852–2869 [DOI] [PubMed] [Google Scholar]
- 33.Elkins J. M., Hewitson K. S., McNeill L. A., Seibel J. F., Schlemminger I., Pugh C. W., Ratcliffe P. J., Schofield C. J. (2003) J. Biol. Chem. 278, 1802–1806 [DOI] [PubMed] [Google Scholar]
- 34.Flashman E., Bagg E. A., Chowdhury R., Mecinović J., Loenarz C., McDonough M. A., Hewitson K. S., Schofield C. J. (2008) J. Biol. Chem. 283, 3808–3815 [DOI] [PubMed] [Google Scholar]
- 35.Yu B., Edstrom W. C., Benach J., Hamuro Y., Weber P. C., Gibney B. R., Hunt J. F. (2006) Nature 439, 879–884 [DOI] [PubMed] [Google Scholar]
- 36.McDonough M. A., Kavanagh K. L., Butler D., Searls T., Oppermann U., Schofield C. J. (2005) J. Biol. Chem. 280, 41101–41110 [DOI] [PubMed] [Google Scholar]
- 37.Kaska D. D., Günzler V., Kivirikko K. I., Myllylä R. (1987) Biochem. J. 241, 483–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tiainen P., Myllyharju J., Koivunen P. (2005) J. Biol. Chem. 280, 1142–1148 [DOI] [PubMed] [Google Scholar]
- 39.Annunen P., Helaakoski T., Myllyharju J., Veijola J., Pihlajaniemi T., Kivirikko K. I. (1997) J. Biol. Chem. 272, 17342–17348 [DOI] [PubMed] [Google Scholar]
- 40.Zhang Z., Ren J., Harlos K., McKinnon C. H., Clifton I. J., Schofield C. J. (2002) FEBS Lett. 517, 7–12 [DOI] [PubMed] [Google Scholar]
- 41.Hoffart L. M., Barr E. W., Guyer R. B., Bollinger J. M., Jr., Krebs C. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 14738–14743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bollinger J. M., Jr., Price J. C., Hoffart L. M., Barr E. W., Krebs C. (2005) Eur. J. Inorg. Chem. 2005, 4245–4254 [Google Scholar]
- 43.O'Brien J. R., Schuller D. J., Yang V. S., Dillard B. D., Lanzilotta W. N. (2003) Biochemistry 42, 5547–5554 [DOI] [PubMed] [Google Scholar]
- 44.Gorres K. L., Edupuganti R., Krow G. R., Raines R. T. (2008) Biochemistry 47, 9447–9455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loenarz C., Mecinović J., Chowdhury R., McNeil L. A., Flashman E., Schofield C. J. (2009) Angew. Chem. Int. Ed. Engl. 48, 1784–1787 [DOI] [PubMed] [Google Scholar]
- 46.Okuyama K., Hongo C., Fukushima R., Wu G., Narita H., Noguchi K., Tanaka Y., Nishino N. (2004) Biopolymers 76, 367–377 [DOI] [PubMed] [Google Scholar]
- 47.Schumacher M., Mizuno K., Bächinger H. P. (2005) J. Biol. Chem. 280, 20397–20403 [DOI] [PubMed] [Google Scholar]
- 48.Hon W. C., Wilson M. I., Harlos K., Claridge T. D., Schofield C. J., Pugh C. W., Maxwell P. H., Ratcliffe P. J., Stuart D. I., Jones E. Y. (2002) Nature 417, 975–978 [DOI] [PubMed] [Google Scholar]