Abstract
Caspase-7 is an executioner caspase that plays a key role in apoptosis, cancer, and a number of neurodegenerative diseases. The mechanism of caspase-7 activation by granzyme B and caspase-3 has been well characterized. However, whether other proteases such as calpains activate or inactivate caspase-7 is not known. Here, we present that recombinant caspase-7 is directly cleaved by calpain-1 within the large subunit of caspase-7 to produce two novel products, large subunit p18 and p17. This new form of caspase-7 has a 6-fold increase in Vmax when compared with the previously characterized p20/p12 form. Zymography revealed that the smaller caspase-7 product (p17) is 18-fold more active than either the caspase-3-cleaved product (p20) or the larger calpain-1 product of caspase-7 (p18). Mass spectrometry and site-directed mutagenesis identified the calpain cleavage sites within the caspase-7 large subunit at amino acid 36 and 45/47. These proteolysis events occur in vivo as indicated by the accumulation of caspase-7 p18 and p17 subunits in cortical neurons undergoing Ca2+ dysregulation. Further, cleavage at amino acid 45/47 of caspase-7 by calpain results in a reduction in nuclear localization when compared with the caspase-3 cleavage product of caspase-7 (p20). Our studies suggest the calpain-activated form of caspase-7 has unique enzymatic activity, localization, and binding affinity when compared with the caspase-activated form.
Apoptosis is a well-defined cellular destruction pathway that primarily utilizes a family of cysteine proteases, the caspases (1, 2). This cell death program can be initiated by cell death receptor activation (extrinsic pathway) or a variety of drugs or cellular stresses (intrinsic pathway) leading to activation of apical caspase-8, -9, and/or -10 (1, 3, 4). These initiator caspases in turn directly activate the executioner caspases, caspase-3 and -7, which through proteolysis of defined substrates are responsible for the dismantling of the cell and subsequent death (3, 4). Granzyme B, released by cytotoxic T lymphocytes to protect the host from pathogens and tumor cells, can also initiate this apoptotic cascade and therefore is considered an apical caspase mimic (5–7). All caspases, as well as granzyme B, preferentially cleave after aspartic acid residues, with many having well-defined consensus sequences, making substrate cleavage sites easy to predict and establish (3, 4, 7, 8).
Caspases exist in a latent form prior to activation. Both the initiator and executioner caspases are synthesized as a single chain protein, which require proteolytic cleavage to become active. Procaspase-7 is expressed as a 303-amino acid residue polypeptide chain. The activation and regulation of executioner caspase-7 by caspases and granzyme B has been extensively studied. Caspase-7 requires cleavage by caspase-3 and caspase-8/-10 or granzyme B, for activation (6, 9). Current evidence suggests that caspase-3 initially cleaves off the first 23 amino acids (propeptide, 2 kDa), followed by caspase-8/-10 or granzyme B cleaving between the large (20 kDa) and small (12 kDa) subunit after amino acid 198 to activate the enzyme. The large subunit containing the catalytic His-237 and Cys-285 (caspase-1 numbering convention), and the small subunit are involved in the formation of the substrate-binding region. In vitro, granzyme B can also activate caspase-7 independently of caspase-3, but this does not appear to occur in vivo (5, 6). Currently, there is no evidence that other classes of proteases play a role in activating or modulating caspase-7 activity.
Changes in intracellular Ca2+ levels influence apoptosis in a number of cell types (10–13). Because in many of these apoptotic cell models the Ca2+-dependent cysteine proteases, calpains, are activated upstream of caspases (14–16), it is possible that calpains may activate and/or modulate caspase activity via direct cleavage. Studies directed at understanding calpains with respect to caspase activation are limited. Calpain-2 was shown to cleave procaspase-9, decreasing its activity (17). In the same study, calpain-2 treatment cleaved procaspase-7 to produce a single, novel fragment, but in this case the effect on enzymatic activity was not investigated (17). To improve our understanding of calpains and the role of calcium in cell death, we carried out studies directed at understanding how calpains activate or modulate caspase activity. We found that calpain treatment produced a large increase in caspase-7 activity. Calpain cleaves procaspase-7 to produce two large subunits of 18.5 and 17.2 kDa, the smaller of which has a robust increase in activity relative to the 20-kDa large subunit produced by caspase-3 cleavage of caspase-7. Both calpain cleavage sites in caspase-7 are identified using mass spectrometry. N-methyl-d-aspartate-induced Ca2+-dependent cell death in primary cortical neurons produced calpain-derived caspase-7 cleavage products in vivo. Lastly, the strictly cytosolic localization of the smaller calpain fragment confirms that a previously identified nuclear localization signal (18) is involved in caspase-7 cytosolic/nuclear distribution. Our data suggest that increases in Ca2+ leading to activation of calpains may significantly modulate caspase-7 activity and thus, apoptosis.
MATERIALS AND METHODS
Plasmids and Enzymes
Caspase-1 through caspase-3 and caspase-5 through-10 plasmids and active caspase-7, -8, -10, and -11 were gifts from Guy Salvesen. Caspase-4 plasmid was a gift from Jean-Louis Lalanne. Mouse caspase-12 and human caspase-13 plasmids were cloned into pcDNA3 by PCR amplification and sequenced. Procaspase-7, truncated caspase-7, active caspase-1 through caspase-6 and granzyme B were purchased from Biomol. Calpain-1 was obtained from Calbiochem, and calpain-2 was obtained from Sigma.
Mutagenesis of Caspase-7 Construct
Site-directed mutagenesis was used to identify and evaluate calpain cleavage of caspase-7. Amino acids 35–37, 44–46, and 46–48 were deleted individually in the pcDNA3-caspase-7-FLAG construct (9). Amino acids 43–46 were subsequently deleted from the pcDNA3-caspase-7 (Δ46–48)-FLAG construct to produce a Δ43–48 mutant. The aspartate at amino acid 23 was mutated to an asparagine in pcDNA3-caspase-7-FLAG construct to use as control. Sites were deleted or mutated by polymerase chain reaction mutagenesis using the following primers: caspase-7 Δ35–37, F 5′-GGTCCTCGTTTGTACCGTCCAAGAAGAAGAAAAATGTC-3′, R 5′-GACATTTTTTCTTCTTCTTGGACGGTACAAACGAGGACC-3′; caspase-7 Δ44–46, F 5′-GTAAGAAGAAGAAAAATGTCTCCATCAAGACCACCCGGG-3′, R 5′-CCCGGGTGGTCTTGATGGAGACATTTTTCTTCTTCTTAC-3′; caspase-7 Δ46–48, F 5′-GAAGAAAAATGTCACCATGAAGACCACCCGGGACCGAG-3′, R 5′-CTCGGTCCCGGGTGGTCTTCATGGTGACATTTTTCTTC-3′; caspase-7 Δ43–45, F 5′-CAGTAAGAAGAAG-AAAAATAAGACCACCCGGGACCGAG-3′, R 5′-CTCGGTCCCGGGTGGTCTTATTTTTCTTCTTCTTACTG-3′; D23N, F 5′-GCAAATGAAGATTCAGTGAATGCTAAGCCAGACCGGTCC-3′, R 5′-GGACCGGTCTGGCTTAGCATTCACTGAATCTTCATTTGC-3′. Polymerase chain reactions were performed using 100 ng of DNA, 5.0 μl of 10× Pfu buffer (Stratagene), 0.2 mm dNTPs (Promega), 125 ng each of forward and reverse primers (Integrated DNA Technologies), 5.0% Me2SO, and 1.0 μl Pfu polymerase (Stratagene) for 16 cycles at 96 °C for 1 min, 55 °C for 1 min, and 65 °C for 20 min, then 65 °C for 7 min. Plasmids were DpnI (Promega)-treated, transformed into XL1-Blue supercompetent cells (Stratagene), and purified using the QIAprep Spin Miniprep Kit (Qiagen). Mutations were confirmed by DNA sequencing.
Caspase-7 C-terminal Constructs
Caspase-7 constructs representing the C terminus of caspase-7 after cleavage with calpain (amino acids 37–303 and 48–303) or caspase-7 (amino acids 24–303) were made utilizing the pcDNA3-caspase-7-FLAG construct (9). The caspase-7 C-terminal products were amplified using polymerase chain reaction with the following primers: caspase-7 24–303, F 5′-AAACTAGGTACCTAGGCCATGGCTAAGCCAGAC-3′; Cs7 37–303, F 5′-AAACTAGGTACCTAGGCCATGAGTAAGAAGAAG-3′; Cs7 48–303, F 5′-AAACTAGGTACCTAGGCCATGATCAAGACCACC-3′; caspase-7-FLAG, R 5′-TGCATGCTCGAGCTACTTGTCATCGTCGTCCTT-3′. Polymerase chain reactions were performed using 100 ng of pcDNA3-caspase7-FLAG, 5.0 μl of 10X Pfu buffer (Stratagene), 0.2 mm dNTPs (Promega), 100 ng each of forward and reverse primers (Integrated DNA Technologies), 5.0% Me2SO, and 1.0 μl Pfu polymerase (Stratagene) for 30 cycles at 95 °C for 1 min, 56 °C-62 °C for 1 min, and 72 °C for 3 min, then 72 °C for 10 min. Samples were purified using the Qiaquick PCR Purification Kit and Qiaquick Gel Extraction Kit (Qiagen). DNA inserts were then cleaved with Xho1/KPN1 (Promega), re-purified, and ligated to an Xho1/KPN1-treated pcDNA3 plasmid using T4 DNA ligase (Promega) overnight at 16 °C. Final constructs were verified using DNA sequencing.
In Vitro Protein Synthesis and Cleavage
Caspase constructs were translated with 35S-labeled methionine (Promega coupled kit) and incubated with 9.0 units of calpain-1 in Nonidet P40 buffer (19) with 5 μm dithiothreitol (DTT)2 and 20 mm CaCl2 at 30 °C for 30 min. Reactions were terminated by addition of EDTA, SDS sample buffer, and boiling. One unit of calpain is defined as the amount of enzyme that will hydrolyze 1 pmol of Suc-LLVY-AMC in 1 min at 25 °C using the Fluorogenic Calpain Activity Assay kit (Calbiochem).
Caspase Activity Assays
Purified procaspase-7, ΔΝ caspase-7 (no prodomain) or active caspases were treated with calpain-1 (9.0 units) or calpain-2 (100 units) for 30 min at 30 °C in Nonidet P40 buffer with 5 μm DTT and 20 mm CaCl2. In some cases, samples were co-incubated with granzyme B (200 units). One unit of granzyme B is defined as the amount of enzyme that will hydrolyze 1 pmol of Ac-IEPD-pNA in 1 min at 30 °C. Following incubation, caspase activity was measured using appropriate fluorescently tagged substrates. Substrates used were: Ac-YVAD-AFC (Biomol) for caspase-1, -4, -5, and -11, Ac-VDVAD-AMC (Biomol) for caspase-2, (z-DEVD)2-R110 (Cell Technology, Inc) and Ac-DEVD-AMC (Biomol) for caspase-3 and -7, Ac-IETD-AMC (Biomol) for caspase-6 and -8 and Ac-LEHD-AFC (Biomol) for caspase-10. Change in fluorescence was measured in a caspase assay buffer (20 mm Pipes, pH 7.2, 100 mm NaCl, 1% CHAPS, 10% sucrose, 10 mm DTT) at 37 °C for 1 h in a SpectraMAX Gemini fluorimeter (Molecular Devices Corporation). A Student's t test or one-way analysis of variance with Tukey's multiple comparison test was used to determine statistical significance.
Western Analysis
Superfect reagent (Qiagen) was used for transient transfections in human embryonic kidney 293T cells with the pcDNA3-caspase7-FLAG constructs described above. Primary cortical cultures were made from ED16 mice and treated with AraC for 24 h at 3 DIV. Cultures were treated with 0, 100, or 250 μm N-methyl-d-aspartate for 3 h at 12 DIV. Cells were lysed with mammalian protein extraction reagent (MPER, Pierce) or fractionated into cytoplasmic and nuclear fractions with the NE-PER nuclear and cytoplasmic reagents (Pierce) in the presence of protease inhibitors (Complete mini, Roche Applied Science). Protein was determined using a BCA assay kit (Pierce). In some cases, samples were treated with 0.9 to 12.0 units of calpain-1 at 30 °C for 10 to 30 min with or without co-incubation with 200 units of granzyme B. Samples were boiled in NuPAGE sample buffer (Invitrogen) with 50 mm DTT, resolved on a 12% polyacrylamide gel and transferred to nitrocellulose. Blots were probed with the following antibodies: polyclonal caspase-7 9492 (1:200, Cell Signaling), polyclonal-cleaved caspase-7 9491S (1:200, Cell Signaling), polyclonal DYKDDDDK tag 2368 (1:200, Cell Signaling), polyclonal DYKDDDDK tag T503 (4.0 μg/ml, Signalway Antibody), monoclonal α-tubulin T-6119 (1.0 μg/ml, Sigma), polyclonal calreticulin (1:20,000, Stressgene), and polyclonal poly(ADP-ribose) polymerase SA-253 (PARP; 1:3000, Biomol). Proteins were subsequently visualized using an enhanced chemiluminescence kit (Thermo Scientific).
Mass Spectrometry
Purified active caspase-7 was incubated with calpain-1 (9.0 units) at 30 °C for 30 min in 50 mm Hepes, pH 7.4, 250 mm NaCl, 5 mm EDTA, 20 mm CaCl2, and 5 μm DTT. The samples were concentrated and purified using ZipTipC4® pipette tips (Millipore) prior to mass spectrometric analysis following manufacturer's instruction. Mass spectra of the samples were obtained by matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry on a Voyager DESTR plus instrument (Applied Biosystems) using 3.5-dimethoxy-4-hydroxycinnamic acid (sinapinic acid) as the matrix. Cytochrome c (Sigma) was used as external standard for calibration.
For the vMALDI experiment, ΔN caspase-7 was incubated with calpain-1 (9.0 units) at 30 °C for 30 min in 50 mm Hepes, pH 7.4, 250 mm NaCl, 5 mm EDTA, 20 mm CaCl2, and 5 μm DTT. The samples were then concentrated and purified using ZipTipC18® pipette tips (Millipore) prior to mass spectrometric analysis following manufacturer's instruction. Mass spectra of the samples were obtained on a vMALDI-linear trap quadrupole (LTQ) mass spectrometer (Thermo Fisher) using manual data acquisition. Threshold values of 1800 counts for MS and 80 counts for MS2 were applied to collect spectra. Parent ion isolation width is 3 m/z units. Fragmentation setting of activation Q = 0.25, activation time = 30 ms, and relative collision energy of 30% were used. vMALDI-LTQ data were searched against a custom data base containing full-length human caspase-7 using an in-house licensed bioinformatics data base search engine system Mascot version 2.2.04 (Matrix Sciences, London, UK) with the no enzyme option selected.
Caspase Zymography
Purified active caspase-7 was incubated with calpain-1 (9.0 units) and 20 mm CaCl2 at 30 °C for 30 min. Following addition of Tris-glycine SDS sample buffer (Invitrogen), samples were separated on a 14% Tris-glycine denaturing polyacrylamide gel (Invitrogen) and then renatured for 2 h at room temperature in Zymogram Renaturing Buffer (Invitrogen) supplemented with 1% CHAPS, 10% sucrose, and 10 mm DTT. Gel was then incubated for 1 h in a buffer containing 100 mm Tris, pH 8.0, 1% CHAPS, 10% sucrose, 10 mm DTT, and the fluorescent caspase-7 substrate Ac-Asp-Glu-Val-Asp-MCA (100 μm, Peptides International), and caspase activity was visualized at 365 nm using a light source (Alpha Innotech Corp.). Gels were subsequently fixed in a 10% MeOH, 7% acetic acid solution and stained overnight in SYPRO Ruby gel stain (Molecular Probes) to visualize total protein.
RESULTS
Activity of Caspase-7 Is Increased by Calpain-1 Cleavage
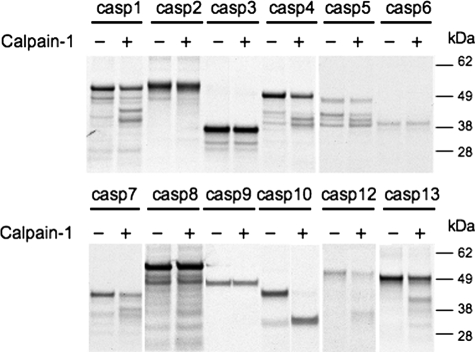
Previous studies have demonstrated that calpains are activated upstream of caspases in a variety of cell models (14–16). Therefore, we tested whether calpains can directly cleave caspases and the impact of cleavage on caspase activity. In vitro-translated 35S-labeled procaspases were treated with calpain-1 (Fig. 1). Calpain-1 is a ubiquitously expressed calpain family member requiring micromolar Ca2+ levels for activation. A large number of caspases were cleaved by calpain, although the number of cleavage products was distinct for each caspase. Some caspases (caspase-1 and -8) had numerous cleavage products while other caspases (caspase-4, -5, -7, -10, -12, and -13) had one or two defined cleavage products. The remaining caspases tested (caspase-2, -3, -6, and -9) were resistant to calpain cleavage (Fig. 1). Preincubation with calpain-2, which requires millimolar Ca2+ levels for activation, produced the same cleavage patterns (data not shown).
FIGURE 1.
Calpain-1 cleaves procaspases. In vitro-translated 35S-labeled procaspases were treated with active calpain-1 to determine which caspases are calpain substrates. Each caspase, with and without calpain treatment, was run on a single gel and developed by autoradiography. All of the caspases were run on four separate gels.
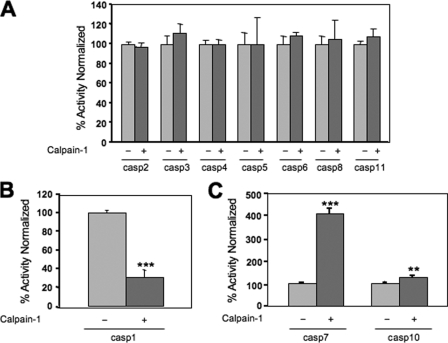
Active caspases were also preincubated with calpain-1 to see the effect on activity. The activities of the majority of caspases were unchanged by calpain-1 preincubation (Fig. 2A; n = 3). Interestingly, the activity of caspase-1, one of the caspases that had numerous calpain cleavage products, was reduced by 69% following calpain-1 treatment (Fig. 2B; n = 3; *, p < 0.001).
FIGURE 2.
Calpain-1 pretreatment affects the activity of a small number of active caspases. Active caspases were incubated with calpain-1 and then caspase activity was measured using a fluorescently labeled substrate with the peptide sequence relevant to each caspase. Results are presented as the % activity of the calpain-1-pretreated sample relative to the activity of the untreated active caspase. Calpain pretreatment resulted in no change in activity for the majority of caspases (A; n = 3), a dramatic reduction in caspase-1 activity (B; n = 3, ***, p < 0.001) and a significant increase in caspase-7 and -10 activities (C; n = 3, ***, p < 0.001, **, p < 0.01).
Surprisingly, the activity of active caspase-7 and active caspase-10 increased following calpain-1 pretreatment (Fig. 2C). Caspase-7 activity increased 4-fold (n = 3, ***, p < 0.001) and caspase-10 activity increased 27% (n = 3, **, p < 0.01) (Fig. 2C). A similar increase in caspase-7 activity was also observed following incubation with calpain-2 (data not shown). Additional experiments with active caspase-7 showed that variations in calpain-1 preincubation times (5 min to 1 h) and increases in the amount of calpain added did not further increase caspase-7 activity (data not shown).
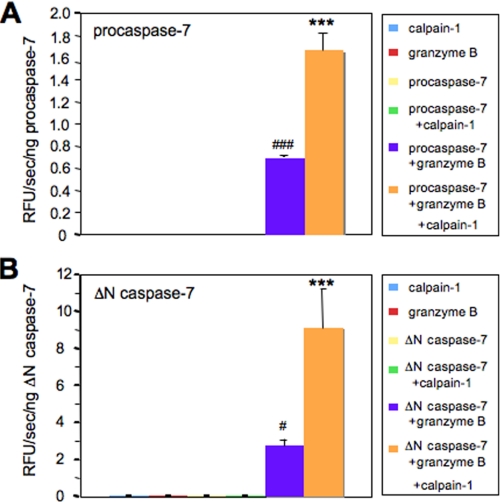
Calpain-1 pretreatment produced two defined cleavage products and a substantial increase in caspase-7 activity. Therefore, we tested whether calpain-1 could activate procaspase-7 alone, because granzyme B can independently activate procaspase-7 in vitro (5). When procaspase-7 was preincubated with calpain-1, caspase-7 was not activated as measured by a DEVD-rhodamine fluorescent substrate (Fig. 3A). As expected, granzyme B incubation alone activated procaspase-7 (Fig. 3A; n = 3, ###, p < 0.001) and calpain-1 and granzyme B co-incubation increased the activity by 2.4-fold (Fig. 3A; n = 3, ***, p < 0.001). When purified caspase-7 without the prodomain (ΔN caspase-7, first 23 amino acid removed) was preincubated with calpain-1 there was also no evidence of calpain-1 independently activating ΔN caspase-7 (Fig. 3B). As with procaspase-7, when ΔN caspase-7 was incubated with granzyme B there was a significant increase in activity (Fig. 3B; n = 3, #, p < 0.05) and co-incubation with granzyme B and calpain-1 led to a 3.3-fold increase in caspase-7 activity relative to preincubation with granzyme B alone (Fig. 3B; n = 3, ***, p < 0.001). From these results it is clear that calpain-1 can only increase the activity of caspase-7 following granzyme B cleavage and activation between the linker of the small and large subunit.
FIGURE 3.
Calpain-1 pretreatment increases the activity of granzyme B-activated caspase-7. Caspase-7 was incubated with calpain-1 alone, granzyme B alone, or calpain-1 and granzyme B together, and activity was measured using a rhodamine-DEVD substrate. Granzyme B pretreatment increased the activity of procaspase-7 (A; n = 3, ###, p < 0.001) and truncated (ΔN) caspase-7 (B; n = 3, #, p < 0.05). In addition, calpain-1 pretreatment increased the activity of granzyme B-activated procaspase-7 (A; n = 3, ***, p < 0.001) and granzyme B-activated ΔN caspase-7 (B; n = 3, ***, p < 0.001). RFU is the relative fluorescent units.
Calpain-1 Produces Two Caspase-7 Cleavage Products with the Smaller Fragment Displaying Increased Activity
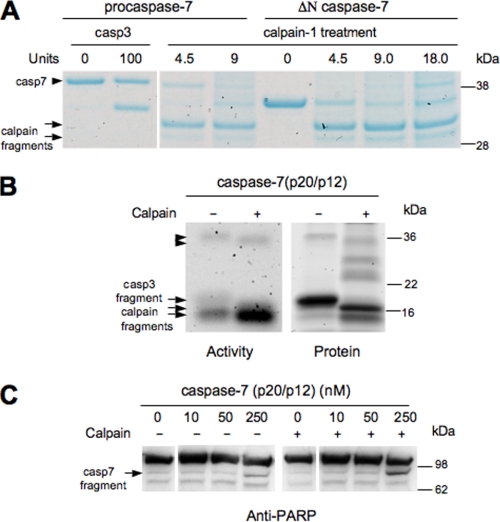
To identify the number and size of calpain cleavage products, purified procaspase-7 and ΔN caspase-7 was cleaved with increasing amounts of calpain-1, and protein was visualized with a Coomassie Blue stain (Fig. 4A). Two calpain cleavage products were produced, both smaller than ΔN caspase-7 and caspase-3-cleaved caspase-7 protein. Cleavage appeared to occur sequentially with lower amounts of calpain-1 producing the larger calpain fragment and higher amounts of calpain-1 producing more of the smaller calpain product. To determine which of the two calpain fragments produced are active, zymography of active caspase-7 with and without calpain-1 pretreatment was performed. After running samples on a denaturing gel, caspases were renatured and exposed to a DEVD fluorescently tagged substrate (Ac-DEVD-MCA) to visualize caspase-7 activity. Prior to calpain treatment, the caspase-3-cleaved active product of caspase-7 appeared as a blurry, faint activity band at ∼19 kDa (Fig. 4B). Following calpain-1 treatment, the faint 19-kDa activity band disappeared and a distinct, robust activity band appeared at ∼15 kDa (Fig. 4B). Subsequent SYPRO Ruby staining demonstrated that calpain treatment resulted in the disappearance of caspase-3-cleaved large subunit, p19 and the appearance of p16 and p15 (Fig. 4B, apparent migration on gel). While the larger calpain fragment, p16, appeared to have low activity, relatively equivalent to the activity observed for the caspase-3 fragment, the smaller calpain fragment had robust activity. Quantification of the change in caspase-7 activity intensity normalized to protein levels using densitometry demonstrates that the smaller calpain fragment is 18.4-fold more active than the caspase-3 fragment. Because the smaller, highly active calpain band was also present in the untreated active caspase-7 sample, this suggests that a calpain-like proteolysis may occur in the bacterial system used to make purified active caspase-7. Alternatively, production of a secondary translation product initiated at Met45 may be responsible for this product (9). Calpain-1 run alone does not have activity (data not shown). In addition, the caspase-3-cleaved fragment and calpain-1-cleaved fragment that was not processed between the large and small subunit was observed around 34 kDa in small quantities (Fig. 4B). Both proteins have low activity levels, suggesting that cleavage between the large and small subunit is necessary for the increase in activity observed with the calpain-1-cleaved large subunit.
FIGURE 4.
Calpain-1 cleavage of caspase-7 produces two novel cleavage products. A, purified procaspase-7 and truncated (ΔN) caspase-7 was treated with calpain-1 or caspase-3. Samples were run on a denaturing gel, and proteins were visualized using a Coomassie Blue stain. All samples were run on a single gel. B, activity of active caspase-7 ± calpain-1 treatment was analyzed by zymography, followed by protein quantitation using SYPRO Ruby Stain on the exact same gel. Top arrowhead indicates caspase-3-truncated caspase-7, and lower arrowhead indicates calpain-1-truncated caspase-7 (containing both large and small subunits). Upper arrow indicates caspase-3 derived caspase-7 large subunit and lower arrows indicate calpain-1-derived caspase-7 large subunits. C, calpain pretreatment of active caspase-7 increases the amount of caspase-7-mediated PARP cleavage. Arrow indicates caspase-7 cleavage product of PARP. Samples were run on two separate gels.
Next we evaluated if calpain-cleaved caspase-7 cleaves endogenous protein substrates. Cellular lysates were treated with active caspase-7 (0–250 nm) incubated with and without calpain-1. The amount of PARP cleavage, a well-characterized substrate of caspase-7, was quantified using Western blot analysis and densitometry. Calpain-activated caspase-7 resulted in increased levels of cleaved PARP (54.3%) when compared with caspase-7 untreated (29.8%) (Fig. 4C). Because calpain cleavage of caspase-7 increased PARP protein processing, this suggests that calpain-cleaved caspase-7 is active against known caspase-7 substrates.
Identification of Three Calpain Cleavage Sites in Caspase-7 by Mass Spectrometry
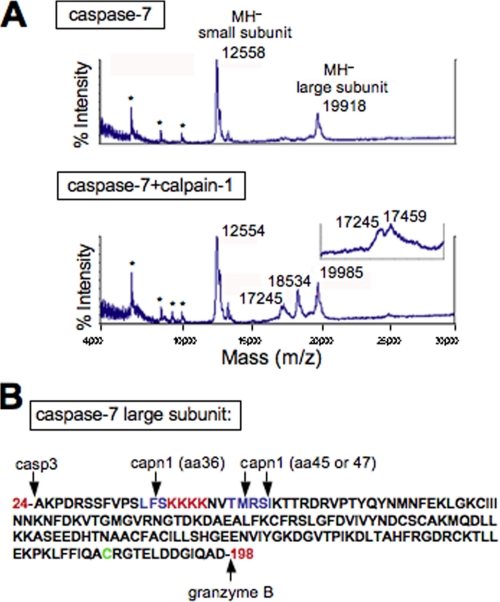
To identify the calpain cleavage sites in caspase-7, matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry was performed using active caspase-7 treated with and without calpain-1. Untreated active caspase-7 has two distinct peaks at 12,558 and 19,918 Da, representing the small and large subunit, respectively (Fig. 5A, top graph). When caspase-7 was cleaved with calpain-1, two new peaks at 18,534 and 17,245 Da were observed (Fig. 5A, bottom graph). Expansion of the smaller peak exposed an additional peak very close in size (17,245 and 17,459 Da, Fig. 5A, inset). When calpain-1 was run alone, no peaks below 30 kDa were observed (data not shown). Based on the assumption that cleavage occurs near the N terminus of the large subunit, because the active cysteine is close to the C terminus (Fig. 5B), mass spectrometry masses were used to accurately predict calpain cleavage sites in the large subunit near the N terminus. Protein calculation software predicted that cleavage after Phe-36 would produce an 18,535-Da protein, cleavage after Met-45 would produce a 17,490-Da protein and cleavage after Ser-47 would produce a 17,246-Da protein. The calculated mass of two of these fragments is within 1 Da of the measured mass and the third within 31 Da. Also, two of these cleavage sites are composed of three amino acid sequences, which are predicted calpain cleavage sites (Fig. 5B, LFS and TMR in blue) (20).
FIGURE 5.
Mass spectrometry predicts three calpain cleavage sites in caspase-7. A, active caspase-7 ± calpain-1 pretreatment was analyzed by MALDI-TOF mass spectrometry for protein fragmentation with the x axis representing protein size in Da. Insert above lower graph shows an expansion of the double peak observed at around 17,245 Da. The doubly charged molecular ions [MH2+] are indicated by asterisks, and a 1-point external calibration against cytochrome c was used. B, amino acid sequence of the caspase-7 large subunit with predicted calpain cleavage sites highlighted in blue, a putative nuclear localization signal highlighted in red and the active cysteine highlighted in green.
Sequencing the Calpain Cleavage Sites by vMALDI-LTQTM Linear Ion Trap
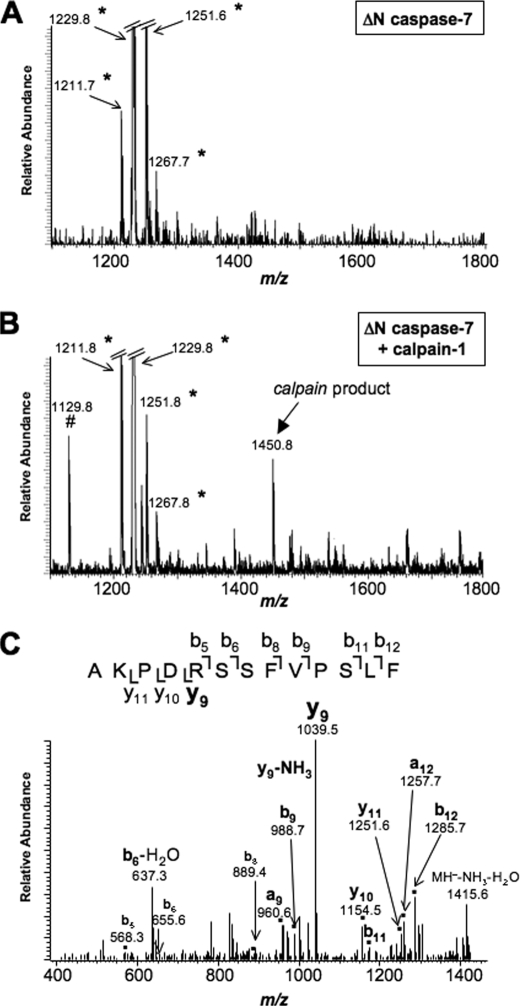
The MALDI-TOF data on calpain-treated caspase-7 samples suggest that cleavage occurs at Phe-36, Met-45, and Ser-47. However, this does not give sequence information. Therefore, analysis was performed on a vMALDI-LTQTM quadrupole linear ion trap of caspase-7 treated with and without calpain. Fig. 6A shows the MS spectra of untreated caspase-7. Upon treatment with calpain is the appearance of a peak at m/z 1450.83 (Fig. 6B). This peak was not present in the untreated caspase-7 sample (Fig. 6A). This peptide was selected for MS/MS analysis to determine the sequence of the cleavage product. As shown in Fig. 6C, the tandem mass spectra of peptide AKPDRSSFVPSLF (m/z 1450.83) corresponds to cleavage at amino acid Phe-36.
FIGURE 6.
Sequencing of the calpain-1 cleavage site in caspase-7. A, vMALDI-LTQ MS of ΔN caspase-7. B, vMALDI-LTQ MS of ΔN caspase-7 treated with calpain-1. A peptide resulting from calpain-1 cleavage appears at m/z 1450.83. C, vMALDI-LTQ MS/MS of the calpain-1 cleavage product, peptide AKPDRSSFVPSLF (m/z 1450.83). *, impurity in ΔN caspase-7 preparation. #, peak present in calpain-1 preparation.
Confirmation of the Three Calpain Cleavage Sites in Caspase-7 by Site-directed Mutagenesis
Calpain cleavage sites are not well conserved and recognition of the substrate depends on the three-dimensional structure of the substrate protein (20). We and others (17, 19) have previously shown that mutating a single amino acid is not adequate to prevent recognition of the substrate. Therefore we used deletion analysis in which we removed three amino acids in the region of cleavage. Controls for this approach are deletions in adjacent protein sequence, which are not recognized by calpains. We made deletions in caspase-7 at amino acids 35–37 (LFS site) and amino acids 46–48 (RSI site). Caspase-7, caspase-7 Δ35–37, and caspase-7 Δ46–48 C-terminal FLAG-tagged constructs were expressed in 293T cells. 293T lysates expressing wild-type or mutant caspase-7 proteins were treated with exogenous calpain-1. Calpain-1 treatment indicates that deletion of amino acids 35–37 eliminates production of the larger calpain fragment (supplemental Fig. S1A, top arrow) consistent with the mass spectrometry data mapping cleavage at amino acid Phe-36. Deletion of amino acids 46–48 significantly reduces production of the smaller calpain fragment suggesting cleavage occurs at Met-45 (supplemental Fig. S1A, bottom arrow). To confirm the calpain cleavage sites, C-terminal constructs of the predicted calpain fragments were made containing the C-terminal FLAG-tag (amino acids 37–303 and 48–303). The caspase-3-derived fragment was also made as a control (amino acids 24–303). When 293T lysates expressing these C-terminal calpain constructs were run on a gel with calpain-cleaved caspase-7, the calpain fragments appeared to be the same size as the C-terminal calpain constructs (supplemental Fig. S1B). We made deletion mutants at amino acids 44–46 (TMR site) as well as amino acids 43–48 (RSI and TMR site). Caspase-7, caspase-7 Δ35–37, caspase-7 Δ43–48, caspase-7 Δ44–46, and caspase-7 Δ46–48 C-terminal FLAG-tagged constructs were expressed in 293T cells and lysates and the deletion spanning both sites (Δ43–48) showed the greatest overall reduction in product (data not shown). These results suggest the smaller calpain product may be produced by calpain cleavage at both sites: Met-45 and Ser-47. These results could be influenced by altered conformation of the protein because of the deletion.
Calpain-1 Products of Caspase-7 Are Produced in Vivo
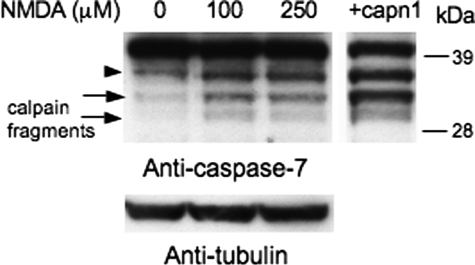
Because all of our experiments utilized in vitro systems, we investigated if calpain-derived caspase-7 products are produced in a cellular environment. Primary cortical neuronal cultures were treated with N-methyl-d-aspartate (NMDA; 0, 100, or 250 μm) for 3 h to increase Ca2+ levels and activate calpains (21–24). Treatment with NMDA resulted in an increase in both calpain-derived caspase-7 products (Fig. 7, arrows). Untreated lysates were also exogenously treated with calpain-1 as a positive control (Fig. 7). The caspase-3 product of caspase-7 was also increased (Fig. 7, arrowhead). These data support that calpain products of caspase-7 are produced in vivo.
FIGURE 7.
Calpain fragments of caspase-7 are observed in vivo in cortical neuronal cultures. Primary cortical cultures were treated with 0, 100, or 250 μm of NMDA for 3 h. Untreated lysates were exogenously treated with calpain-1 as a positive control. A caspase-7 antibody was used to detect full-length caspase-7, the caspase-3 fragment (arrowhead), and the calpain fragments (arrows). All samples were run on a single gel. Blots were reprobed with tubulin to demonstrate equal loading.
Smaller Calpain Fragment of Caspase-7 Has Decreased Nuclear Localization
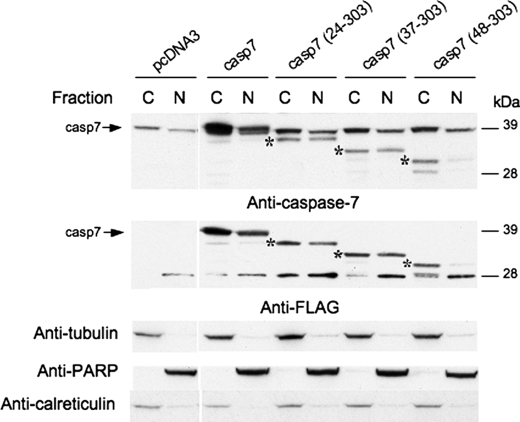
Caspase-7 has been reported to have cytoplasmic, endoplasmic reticulum, and nuclear localization depending upon the cell type and mode of apoptotic induction. Therefore, we evaluated the localization of caspase-7 cleavage products. Particularly relevant to this experiment is the putative nuclear localization signal (Fig. 5B, KKKK amino acids 38–41) that is removed upon calpain cleavage (18). Caspase-7 zymogen, caspase-3 fragment (amino acids 24–303) and calpain fragments (amino acids 37–303 and 48–303) were expressed in 293T cells, followed by cytosolic/nuclear fractionation. The caspase-7 24–303 fragment and caspase-7 37–303 fragment had an increased level of nuclear localization relative to procaspase-7. Consistent with deletion of a nuclear localization signal at amino acid 38–41, the caspase-7 48–303 fragment, the smaller calpain-derived fragment, was found in the cytoplasm (Fig. 8). This suggests that the putative nuclear localization signal may be necessary for caspase-7 to enter the nucleus and plays an important role in caspase-7 localization as the active protein is formed.
FIGURE 8.
Cleavage at the second calpain site prevents nuclear localization. Full-length caspase-7, the caspase-3 fragment (amino acids 24–303), the larger calpain-1 fragment (amino acids 37–303), and the smaller calpain-1 fragment (amino acids 48–303) were expressed in 293T cells. Cells were fractionated into cytoplasmic (C) and nuclear (N) components, and blots were probed with the caspase-7 antibody to visualize endogenous and expressed caspase-7 and a FLAG antibody to visualize expressed caspase-7 protein. Successful fractionation and equal loading were confirmed by reprobing the blots with tubulin, calreticulin, and PARP antibodies. Arrows indicate full-length caspase-7, and asterisks indicate the expressed C-terminal caspase-7 constructs. Control samples were run on a second gel.
The p17/p12 Caspase-7 Has Unique Substrate Specificity Compared with the p20/p12 Form
Our results suggest the calpain-activated form of caspase-7 may have unique catalytic activity. Therefore, we examined the substrate specificity of the caspase-3-activated form of caspase-7 relative to the calpain form of caspase-7. We hypothesized that the p17 large subunit may influence the turnover distinctly from the p20 large subunit. We examined substrates for all three classes of caspases and found that DEVD-AMC and VDVAD-AMC where good substrates for caspase-7 p17 or p20 (Table 1). Strikingly, the caspase-7 p17/p12 increased 6-fold in Vmax when compared with p20/p12 form for the DEVD-AMC. The affinity Km decreased 2.4-fold but the kcat increased 6.1-fold (Table 1). Interestingly when we examined a caspase-2 substrate, VDVAD-AMC, we found the calpain form of caspase-7 (p17/p12) has an increased Vmax (3-fold) and higher affinity for the substrate (lower Km) and increased kcat (3-fold). Our results suggest that the calpain-cleaved form of caspase-7 has a unique specificity.
TABLE 1.
Kinetic parameters of caspase-7 +/− calpain pretreatment
Calpain preincubation in calcium-containing buffer followed by caspase-7 activity measurement in caspase buffer (“Experimental Procedures”). kcat values are not corrected for enzyme purity and fractional activity.
| Substrate | Enzyme form | Vmax | Km | kcat |
|---|---|---|---|---|
| m·s−1 | μm | s−1 | ||
| DEVD-AMC | Caspase-7 | 3.6 × 10−8 | 68 | 0.49 |
| DEVD-AMC | Caspase-7 + calpain-1 | 2.3 × 10−7 | 163 | 3.0 |
| VDVAD-AMC | Caspase-7 | 3.9 × 10−8 | 241 | 0.53 |
| VDVAD-AMC | Caspase-7 + calpain-1 | 1.2 × 10−7 | 189 | 1.6 |
DISCUSSION
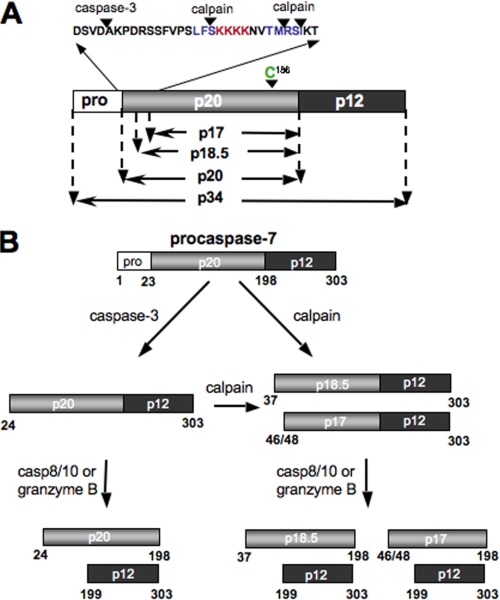
The major findings of our study are that (i) caspase-7 is cleaved at Phe-36, Met-45, and Ser-47 by calpains to form a p18.5 and p17 large subunit (see Fig. 9A); (ii) deletion analysis of caspase-7 at these three sites prevents processing by calpain; (iii) the p17/p12 form of caspase-7 is more active than the p20/p12; (iv) the catalytic parameters for the calpain activated form of caspase-7 are distinct; and (v) localization of caspase-7 is modulated by calpain cleavage. Therefore, our results indicate that the activation and regulation of executioner caspase-7 by calpains is distinct from the activation of this enzyme by caspases. The activation of caspase-7 by calpain is summarized in Fig. 9B.
FIGURE 9.
Diagram of the calpain cleavage sites of caspase-7. A, caspase propeptide is 34 kDa in length. Cleavage by caspase-3 after D23 and caspase-8/-10 or granzyme B after D198 releases the active large subunit (p20). Cleavage by calpains after Phe-36 produces an 18.5 kDa active large subunit. Further cleavage by calpains after Met-45 or Ser-47 produces a 17.4 or 17.2 kDa large subunit, respectively, which has increased activity and decreased nuclear localization. B, model of the activation process of caspase-7 by calpain and caspase-8/-10 or granzyme B.
Caspase-7 and caspase-3 are both executioner caspases that were originally thought to have redundant properties based on their close phylogenic relationship, structure, and sequence. While they have a number of similarities, such as identical small synthetic substrate specificity (25) and sensitivity to the inhibitor, XIAP (26, 27), they also have a number of distinct properties. Caspase-7 is catalytically less active than caspase-3 (28) and has recently been shown to have a unique set of protein substrates (29). While caspase-3 and caspase-7 have some common substrates such as PARP and ROCK I, caspase-3 preferentially cleaves Bid and caspase-9 and caspase-7 more efficiently cleaves cochaperone p23 (29). Caspase-3 knockouts further support their unique cellular roles, because a caspase-3-deficient mouse (30) and MCF-7 caspase-3-deficient cells (31) undergo delayed apoptosis. An important structural difference, which may explain these functional differences, lies in the amino acid sequence of their N-peptide region. While they both have an aspartate cleavage site close to the N terminus necessary for enzyme activation, caspase-7 has an extra 30 amino acid stretch that follows containing a putative nuclear localization signal (KKKK) (18). This KKKK sequence is a highly conserved, seen in both human and Xenopus caspase-7, suggesting it may play an important functional role, although its identification as a nuclear localization signal is controversial (9, 18, 32, 33). A further distinction for N terminus of caspase-7 is the calpain cleavage sites.
The caspase-7 N-terminal peptide is believed to play an important role in the regulation and localization of caspase-7. The peptide is highly conserved and removed during apoptosis by caspase-3. While removal of the N-terminal peptide does not appear to affect catalytic activity in vitro, it does appear to play an important role in initiating cell death in vivo showing an early increase in DEVDase activity and cell death relative to the caspase-7 zymogen (9). One hypothesis is that the caspase-7 zymogen may be physically sequestered from its activators in vivo (caspase-8, -9, and -10) and is only released and activated after N-peptide removal by caspase-3 (9). An alternative explanation is that the caspase-7 zymogen is primarily localized to the cytosol and caspase-3 cleavage results in increased nuclear compartmentalization (18), although this change in localization has not been observed in some studies (9).
We found that calpains cleave off a larger portion of the propeptide (45–47 amino acids) resulting in a caspase-7 large subunit that is much more active than its caspase-3-processed form. Cleavage by calpains may occur before caspase-3 cleavage, following caspase-3 cleavage or after caspase-8/-10 or granzyme B cleavage (Fig. 9B). While these three possibilities have not been fully explored in vivo, 293T cells expressing caspase-3-resistant caspase-7 (D23N) and calpain-1 do not show increases in caspase-3/7 activity relative to vector control, suggesting that caspase-3 removal of the propeptide precedes calpain cleavage (data not shown). Procaspase-7 is localized to both the cytosolic and nuclear compartment suggesting activation can occur in either compartment. This is consistent with the localization of caspases and calpains to both subcellular locations (19). It follows that calpain cleavage of caspase-7 can occur in either compartment. Caspase-7 can inactivate calpastatin, an endogenous inhibitor of calpains (34). Thus calpain activation of caspase-7 may initiate a vicious cycle of perpetually increasing calpain and caspase-7 activity.
Some studies indicate the KKKK motif of caspase-7 influences nuclear localization (18), while other studies suggest that the caspase-7 zymogen and its caspase-3 cleavage product are strictly localized to the cytosol (9, 32, 33). One recent study found that sumoylation of a lysine in the N-terminal region of caspase-7, possibly within the KKKK motif, promotes nuclear localization (35). Our data support its function as a nuclear localization signal. We found some of the procaspase-7 in the nucleus. Initial cleavage of caspase-7 by caspase-3 or calpain produces the same of amount caspase-7 in the nuclear fraction and further cleavage by calpains eliminates nuclear localization altogether. One hypothesis to explain this redistribution is that the caspase-3 N-terminal peptide (amino acids 1–23) physically blocks the KKKK site and that removal of this peptide by caspase-3 or calpain-1 increases exposure of this site, enhancing its ability to localize the protein to the nucleus. Subsequent removal of the KKKK motif by additional calpain cleavage eliminates the ability of the caspase-7 fragment to actively enter the nucleus, resulting in accumulation of this product in the cytosol. While our studies corroborate work done with Xenopus caspase-7 protein in a tadpole-derived myoblast cell line (XT-15–11), with the only clear difference being the amount of caspase-7 zymogen localized to the nucleus, they conflict with previous studies using the human caspase-7 protein in a monkey kidney cell line (COS-7). One potential explanation for differences in localization may be the cellular model used for these studies. One concern is that the caspase-7 constructs we used may be activated and induce apoptosis when expressed at high levels, potentially affecting their cellular distribution. Because the endogenous caspase-7 zymogen localized to the nucleus at the same levels as the overexpressed FLAG-tagged caspase-7 zymogen and the highly active smaller calpain fragment did not have any nuclear localization, this suggests that overexpression of caspase-7 did not affect localization.
Interestingly the catalytic parameters are distinct for the p17/p12 form of caspase-7 when compared with p20/p12 form. This suggests that the conformation of caspase-7 p20/p12 is altered when additional cleavage by calpains occurs after amino acid 45. The atomic structure has been solved for both procaspase-7 and the active form cleaved at amino acid 23 (36, 37). However, the structures do not reveal density for residues before Ser-47, and therefore we cannot make predictions on the nature of the structural changes that may occur and alter the caspase-7 catalytic specificity.
The ability of calpains to cleave and modulate caspase-7 activity is clearly unique. While calpain-1 cleaved a number of caspases, their activity did not increase. Because caspase-3 has a similar function and structure, one would expect it might also be modulated by calpain. Our data indicate this is not the case. The difference is due to the calpain cleavage sites being located in the 30 amino acid stretch that is absent in the caspase-3 zymogen. In our studies, mouse cortical cells treated with N-methyl-d-aspartate, produced the two caspase-7 calpain fragments indicating that mouse caspase-7 is calpain-sensitive in both regions. Here we have identified the unique role of caspase-7 as a coincident detector of Ca2+ dysregulation and caspase activation. This is the first study showing that the two cell death cascades can directly interact to influence each others activity and subcellular localization. Our data suggest that when cellular stressors activate the caspase cascade in the absence of cytosolic Ca2+ increases, caspase-7 is less active and degrades its specific targets in the cytosol and nucleus. Alternatively, when Ca2+ levels are up-regulated in the presence of caspase activation, the activity of caspase-7 dramatically increases, leading to caspase-7 degradation of an alternative set of target proteins exclusively in the cytosol. Further studies will need to be conducted to determine how calpain cleavage of caspase-7 affects the timing and progression of cellular destruction. Because both calpains and caspases are activated in a number of neurodegenerative diseases (24, 38–43), calpain proteolysis of caspase-7 may play an important role in disease progression. Specific calpain inhibitors may provide a useful tool in delaying apoptotic cell death in a number of these diseases.
Supplementary Material
Acknowledgment
We thank Guy Salvesen for recombinant caspases.
This work was supported, in whole or in part, by National Institutes of Health Grant NS40251A and Nathan Shock Grant P30 AG025708.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- DTT
- dithiothreitol
- CHAPS
- 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid
- MALDI-TOF
- matrix-assisted laser desorption/ionization-time of flight
- vMALDI
- vacuum matrix-assisted laser desorption/ionization
- LTQ
- linear trap quadrupole
- PARP
- poly(ADP-ribose) polymerase.
REFERENCES
- 1.Zhivotovsky B. (2004) Cell Cycle 3, 64–66 [PubMed] [Google Scholar]
- 2.Thornberry N. A. (1999) Cell Death Differ. 6, 1023–1027 [DOI] [PubMed] [Google Scholar]
- 3.Nicholson D. W. (1999) Cell Death Differ. 6, 1028–1042 [DOI] [PubMed] [Google Scholar]
- 4.Kumar S. (2007) Cell Death Differ. 14, 32–43 [DOI] [PubMed] [Google Scholar]
- 5.Zhou Q., Salvesen G. S. (1997) Biochem. J. 324, 361–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang X., Stennicke H. R., Wang B., Green D. R., Jänicke R. U., Srinivasan A., Seth P., Salvesen G. S., Froelich C. J. (1998) J. Biol. Chem. 273, 34278–34283 [DOI] [PubMed] [Google Scholar]
- 7.Talanian R. V., Yang X., Turbov J., Seth P., Ghayur T., Casiano C. A., Orth K., Froelich C. J. (1997) J. Exp. Med. 186, 1323–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Timmer J. C., Salvesen G. S. (2007) Cell Death Differ. 14, 66–72 [DOI] [PubMed] [Google Scholar]
- 9.Denault J. B., Salvesen G. S. (2003) J. Biol. Chem. 278, 34042–34050 [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez-Tarduchy G., Collins M., López-Rivas A. (1990) EMBO J. 9, 2997–3002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tong J. X., Eichler M. E., Rich K. M. (1996) Exp. Neurol. 138, 45–52 [DOI] [PubMed] [Google Scholar]
- 12.Zhivotovsky B., Cedervall B., Jiang S., Nicotera P., Orrenius S. (1994) Biochem. Biophys. Res. Commun. 202, 120–127 [DOI] [PubMed] [Google Scholar]
- 13.Squier M. K., Cohen J. J. (1997) J. Immunol. 158, 3690–3697 [PubMed] [Google Scholar]
- 14.Altznauer F., Conus S., Cavalli A., Folkers G., Simon H. U. (2004) J. Biol. Chem. 279, 5947–5957 [DOI] [PubMed] [Google Scholar]
- 15.Chaitanya G. V., Babu P. P. (2008) Neurochem. Res. 33, 2178–2186 [DOI] [PubMed] [Google Scholar]
- 16.Wu J., Liu T., Xie J., Xin F., Guo L. (2006) Cell Mol. Life Sci. 63, 949–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chua B. T., Guo K., Li P. (2000) J. Biol. Chem. 275, 5131–5135 [DOI] [PubMed] [Google Scholar]
- 18.Yaoita Y. (2002) Biochem. Biophys. Res. Commun. 291, 79–84 [DOI] [PubMed] [Google Scholar]
- 19.Gafni J., Hermel E., Young J. E., Wellington C. L., Hayden M. R., Ellerby L. M. (2004) J. Biol. Chem. 279, 20211–20220 [DOI] [PubMed] [Google Scholar]
- 20.Tompa P., Buzder-Lantos P., Tantos A., Farkas A., Szilágyi A., Bánóczi Z., Hudecz F., Friedrich P. (2004) J. Biol. Chem. 279, 20775–20785 [DOI] [PubMed] [Google Scholar]
- 21.Moore J. D., Rothwell N. J., Gibson R. M. (2002) Br. J. Pharmacol. 135, 1069–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Del Río P., Massieu L. (2008) Exp. Neurol. 212, 179–188 [DOI] [PubMed] [Google Scholar]
- 23.Del Rio P., Montiel T., Massieu L. (2008) Neurochem. Res. 33, 1475–1483 [DOI] [PubMed] [Google Scholar]
- 24.Lankiewicz S., Marc Luetjens C., Truc Bui N., Krohn A. J., Poppe M., Cole G. M., Saido T. C., Prehn J. H. (2000) J. Biol. Chem. 275, 17064–17071 [DOI] [PubMed] [Google Scholar]
- 25.Thornberry N. A., Rano T. A., Peterson E. P., Rasper D. M., Timkey T., Garcia-Calvo M., Houtzager V. M., Nordstrom P. A., Roy S., Vaillancourt J. P., Chapman K. T., Nicholson D. W. (1997) J. Biol. Chem. 272, 17907–17911 [DOI] [PubMed] [Google Scholar]
- 26.Scott F. L., Denault J. B., Riedl S. J., Shin H., Renatus M., Salvesen G. S. (2005) EMBO J. 24, 645–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deveraux Q. L., Takahashi R., Salvesen G. S., Reed J. C. (1997) Nature 388, 300–304 [DOI] [PubMed] [Google Scholar]
- 28.Stennicke H. R., Renatus M., Meldal M., Salvesen G. S. (2000) Biochem. J. 350, 563–568 [PMC free article] [PubMed] [Google Scholar]
- 29.Walsh J. G., Cullen S. P., Sheridan C., Lüthi A. U., Gerner C., Martin S. J. (2008) Proc. Natl. Acad. Sci. U. S. A. 105, 12815–12819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuida K., Zheng T. S., Na S., Kuan C., Yang D., Karasuyama H., Rakic P., Flavell R. A. (1996) Nature 384, 368–372 [DOI] [PubMed] [Google Scholar]
- 31.Jänicke R. U., Sprengart M. L., Wati M. R., Porter A. G. (1998) J. Biol. Chem. 273, 9357–9360 [DOI] [PubMed] [Google Scholar]
- 32.Shikama Y. U. M., Miyashita T., Yamada M. (2001) Exp. Cell Res. 264, 315–325 [DOI] [PubMed] [Google Scholar]
- 33.Zhivotovsky B., Samali A., Gahm A., Orrenius S. (1999) Cell Death Differ. 6, 644–651 [DOI] [PubMed] [Google Scholar]
- 34.Kato M., Nonaka T., Maki M., Kikuchi H., Imajoh-Ohmi S. (2000) J. Biochem. 127, 297–305 [DOI] [PubMed] [Google Scholar]
- 35.Hayashi N., Shirakura H., Uehara T., Nomura Y. (2006) Neurosci. Letts. 397, 5–9 [DOI] [PubMed] [Google Scholar]
- 36.Chai J., Wu Q., Shiozaki E., Srinivasula S. M., Alnemri E. S., Shi Y. (2001) Cell 107, 399–407 [DOI] [PubMed] [Google Scholar]
- 37.Riedl S. J., Fuentes-Prior P., Renatus M., Kairies N., Krapp S., Huber R., Salvesen G. S., Bode W. (2001) Proc. Natl. Acad. Sci. U. S. A. 98, 14790–14795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ray S. K., Karmakar S., Nowak M. W., Banik N. L. (2006) Neuroscience 139, 577–595 [DOI] [PubMed] [Google Scholar]
- 39.Gafni J., Ellerby L. M. (2002) J. Neurosci. 22, 4842–4849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wellington C. L., Singaraja R., Ellerby L., Savill J., Roy S., Leavitt B., Cattaneo E., Hackam A., Sharp A., Thornberry N., Nicholson D. W., Bredesen D. E., Hayden M. R. (2000) J. Biol. Chem. 275, 19831–19838 [DOI] [PubMed] [Google Scholar]
- 41.Galvan V., Gorostiza O. F., Banwait S., Ataie M., Logvinova A. V., Sitaraman S., Carlson E., Sagi S. A., Chevallier N., Jin K., Greenberg D. A., Bredesen D. E. (2006) Proc. Natl. Acad. Sci. U. S. A. 103, 7130–7135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amadoro G., Ciotti M. T., Costanzi M., Cestari V., Calissano P., Canu N. (2006) Proc. Natl. Acad. Sci. U. S. A. 103, 2892–2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hong S. C., Lanzino G., Goto Y., Kang S. K., Schottler F., Kassell N. F., Lee K. S. (1994) Brain Res. 661, 43–50 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.