Abstract
Therapeutic options for patients with multiple myeloma whose disease has relapsed after a prior auto-SCT include novel biologic therapies, traditional chemotherapy or a second transplant, with no clear standard of care. Few published studies address the safety and efficacy of a second auto-SCT for relapsed disease. We reviewed the Abramson Cancer Center experience with salvage auto-SCT for relapsed multiple myeloma. Forty-one patients had received a salvage auto-SCT at our institution; the median time between transplants was 37 months (range 3–91). The overall response rate in assessable patients was 55%, and treatment-related mortality was 7%. With a median follow-up time of 15 months, the median PFS was 8.5 months and the median overall survival (OS) was 20.7 months. In a multivariate analysis of OS, independent prognostic factors were ≥5 prior lines of therapy and time to progression after initial auto-SCT of ≤12 months. We conclude that in well-selected patients, salvage auto-SCT is safe and effective for relapsed myeloma.
Keywords: multiple myeloma, auto-SCT, salvage therapy
Introduction
High dose melphalan and auto-SCT is currently a standard part of the initial line of therapy for eligible patients with multiple myeloma, based on two randomized trials demonstrating improved overall survival (OS) of auto-SCT versus conventional chemotherapy1,2 and a number of non-randomized comparative trials.3,4 Evidence also supports a tandem transplant approach, although the benefit of tandem transplantation may be restricted to those not achieving >90% cytoreduction after the first transplant.5 Unfortunately, despite this success, nearly all patients will relapse and require salvage therapy.
The number of options for patients with relapsed multiple myeloma has increased significantly in recent years. Novel therapies include thalidomide, lenalidomide and bortezomib, which have been studied extensively in a relapsed setting as single agents, in combination with dexamethasone, and in combination with cytotoxic agents or with each other.6 Salvage chemotherapy regimens such as vincristine, doxorubicin and dexamethasone (VAD) can also be an option for relapsed disease, though they have largely been replaced by novel agents in the front-line setting. Finally, a second transplant, whether autologous or reduced-intensity allogeneic, can also be used for relapsed disease. The efficacy of an auto-SCT in the salvage setting is unknown.
The available evidence regarding outcomes after salvage auto-SCT suggests that the procedure is safe and reasonably effective.7–15 Reports of PFS after second salvage auto-SCT are variable and range from a median of 6.8 months to 4.2 years.7,11 Most studies have found that time to progression (TTP) after the first transplant, or alternatively the interval between the first and second transplants, is predictive of outcomes after the second transplant. However, many of these studies contain small numbers of patients and have variable duration of follow-up; the true benefit of salvage auto-SCT for myeloma is therefore not well defined.
We reviewed the experience of our center with salvage auto-SCT for relapsed multiple myeloma, both to augment the available literature and to determine the value of TTP after the initial transplant as a prognostic factor for superior outcome after salvage auto-SCT.
Patients and methods
Patients
At the University of Pennsylvania Abramson Cancer Center, approximately 550 auto-SCTs have been performed for multiple myeloma between 1991 and 2008. From a prospectively collected database, 41 were identified as being a salvage auto-SCT. These transplants occurred between July 1998 and December 2007. Retrospective analysis of this group of patients was approved by the University of Pennsylvania Institutional Review Board.
Definitions
A transplant was defined as ‘salvage’ if the patient had received a prior auto-SCT, and underwent a second auto-SCT after evidence of disease progression regardless of the number of prior lines of therapy since the first transplant. Patients who received a second transplant as part of a planned tandem transplant were excluded.
Definitions of response and disease progression were used according to the updated European Group for Blood and Marrow Transplantation criteria.16 Response to transplant (whether initial or salvage) was defined as the change in disease burden as a result of the line of therapy, which could include both induction therapy as well as the transplant itself. A line of therapy was defined as one or more types or doses of treatment uninterrupted by disease progression, toxicity requiring change of therapy, or a period of observation. OS and PFS after transplant were measured from the day of infusion of stem cells (day 0).
Statistical analysis
Actuarial estimates of PFS and OS were estimated using the methods of Kaplan and Meier. The Cox proportional hazards model was used to perform univariate analyses of possible prognostic variables for PFS and OS, after confirming the proportionality of each variable using time-dependent covariates. To determine which variables were independently prognostic for both PFS and OS, multivariate Cox analysis was then performed using backward stepwise selection methods to build parsimonious models. The log-rank test for equality of survivor functions was used to detect differences across ordered categories (for groups of patients with 0, 1 or 2 risk factors derived from the multivariate analysis of OS). All analyses were performed using Stata version 10.0 (StataCorp LP, College Station, TX, USA).
Results
Patient characteristics
Forty-one patients were included in this analysis. Demographic and clinical characteristics at diagnosis and at the time of the first auto-SCT are shown in Table 1. The median age at diagnosis was 50 years (range 25–69). Patients received a median of 1 line of therapy prior to and including the initial auto-SCT (range 1–5); the median time from diagnosis to initial auto-SCT was 9 months (range 4–48).
Table 1.
Demographic and clinical characteristics at diagnosis and at the time of the initial auto-SCT
| Variable | Number (range or percent) or median (range) (n = 41) |
|---|---|
| Gender | 32 male, 9 female |
| Age at diagnosis | 50 years (25–69) |
| Ig subtype | |
| IgG | 25 (61%) |
| IgA | 5 (12%) |
| κ-Light chain | 6 (15%) |
| λ-Light chain | 4 (10%) |
| Non-secretory | 1 (2%) |
| Durie Salmon stage at diagnosis | |
| I | 8 (20%) |
| II | 10 (24%) |
| III | 14 (34%) |
| Missing | 9 (22%) |
| Age at initial auto-SCT | 51 years (25–70) |
| Conditioning | |
| Melphalan 200 mg/m2 | 32 (78%) |
| Reduced dose melphalan | 4 (10%) |
| BU/CY | 4 (10%) |
| CY/TBI | 1 (2%) |
| Response | |
| CR | 9 (22%) |
| VGPR | 3 (7%) |
| PR | 21 (51%) |
| SD | 6 (15%) |
| PD | 2 (5%) |
| TTP after initial auto-SCT | 21 months (3–73) |
Abbreviations: PD = progressive disease; PR = partial response; SD = stable disease; TTP = time to progression; VGPR = very good partial response.
Initial auto-SCT
All patients received the initial transplant using peripherally collected stem cells, which were mobilized using CY/G-CSF (29 of 41) or G-CSF alone (9 of 41). Twelve patients (29%) had achieved a CR/VGPR (very good partial response) to the initial auto-SCT, and the overall response rate was 80%. Eighteen patients (44%) received maintenance therapy after initial auto-SCT, with either IFN or thalidomide. The median TTP after initial auto-SCT was 21 months (range 3–73).
Salvage auto-SCT
Clinical characteristics of the patients at the time of salvage auto-SCT are shown in Table 2. Median age at the time of salvage auto-SCT was 54 years (range 28–73). The median time from diagnosis to second auto-SCT was 49 months (range 12–108), and the median time interval between the first and second auto-SCT was 37 months (range 3–91). The median number of prior lines of therapy was 3 (range 1–10). Prior to the salvage auto-SCT, 19 patients (46%) had been treated with thalidomide, 9 (22%) with lenalidomide and 24 (59%) with bortezomib; five patients (12%) had received all three novel agents. Only 15 of 41 patients (37%) had responsive disease at the time of salvage auto-SCT.
Table 2.
Salvage auto-SCT clinical characteristics and response
| Variable | Number (range or percent) or median (range) (n = 41) |
|---|---|
| Age at second auto-SCT | 54 years (28–73) |
| Cytogenetics | |
| Any result | 25 (61%) |
| Abnormal result | 9 (22%) |
| Time from first to second auto-SCT | 37 months (3–91) |
| Number of prior lines of therapy | 3 (1–10) |
| Specific therapies prior to second auto-SCT | |
| Prior thalidomide | 19 (46%) |
| Prior lenalidomide | 9 (22%) |
| Prior bortezomib | 24 (59%) |
| Responding disease prior to auto-SCT | 15 (37%) |
| Conditioning | |
| Melphalan 200 mg/m2 | 12 (29%) |
| Reduced dose melphalan | 11 (27%) |
| Melphalan/TBI | 14 (34%) |
| BU/CY | 3 (7%) |
| CY/TBI | 1 (2%) |
| Response | |
| CR | 2 (5%) |
| VGPR | 4 (10%) |
| PR | 15 (37%) |
| SD | 11 (27%) |
| PD | 6 (15%) |
| Could not be assessed | 3 (7%) |
| Treatment-related mortality | 3 (7%) |
Abbreviations: PD = progressive disease; PR = partial response; SD = stable disease; VGPR = very good partial response.
Forty of 41 salvage transplants were performed using peripherally collected stem cells. Thirty-five of these used stored peripheral stem cells from the patient’s initial collection, three were re-mobilized using G-CSF and two were re-mobilized using CY/G-CSF. Conditioning regimens used for the second auto-SCT included melphalan 200 mg/m2 (29%), reduced dose melphalan (22%; 80–180 mg/m2), TBI-based regimens (37%; 900–1200 cGy with melphalan 100–140 mg/m2 or CY 120 mg/m2 in one patient) and BU/CY (7%). Median creatinine was 1.0 (range 0.4–7.6), median albumin was 3.9 (range 2.5–5.0) and median β-2 microglobulin was 3.0 (range 1.6–12.2) in 16 patients for whom β-2 microglobulin was available.
Thirty-eight patients were assessable for response after the second auto-SCT. Of these, six (16%) achieved a CR/VGPR, and the overall response rate was 21 of 38 (55%). Four patients died during the first 100 days (one of disease progression, three of toxicity), for a treatment-related mortality of 7%. Five patients received maintenance therapy, with INF, thalidomide or lenalidomide.
Thirteen of 41 patients had a TTP inversion (PFS after second auto-SCT was longer than TTP after first auto- SCT); in these 13 patients, the median increase in TTP was 5 months. Conditioning regimens for these 13 patients were well balanced and included melphalan 200 mg/m2 (4 of 13), reduced dose melphalan (4 of 13), melphalan/TBI (4 of 13) and BU/CY (1 of 13). Only one of 13 patients who had a TTP inversion had received maintenance therapy after salvage auto-SCT, compared with four of 28 patients who did not have a remission inversion (P = 0.54). No other clinical or treatment factors were identified that were predictive of TTP inversion.
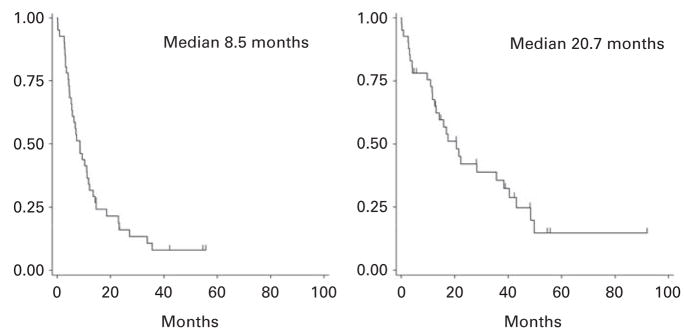
With a median follow-up for all subjects of 15 months (range 1–91) after second auto-SCT, the median PFS after the second salvage auto-SCT is 8.5 months (range <1–55), and the median OS is 20.7 months (range<1–91; Figure 1). For all patients, with a median follow-up of 6.1 years since diagnosis, the median OS since diagnosis was 6.8 years.
Figure 1.
Progression-free (left) and overall survival (right) for all patients after second salvage auto-SCT.
Prognostic variables
Prognostic variables prior to the second auto-SCT which were examined for significance in univariate analyses include: age, response to initial auto-SCT, TTP after initial auto-SCT, time interval between the first and second transplants, number of prior lines of therapy, prior receipt of specific therapies (thalidomide, lenalidomide, bortezomib), responsive disease at the time of salvage transplant, abnormal cytogenetics, conditioning regimen, and pre-transplant hemoglobin, creatinine, albumin and LDH. β-2 microglobulin was not informative because of a high percentage of missing values.
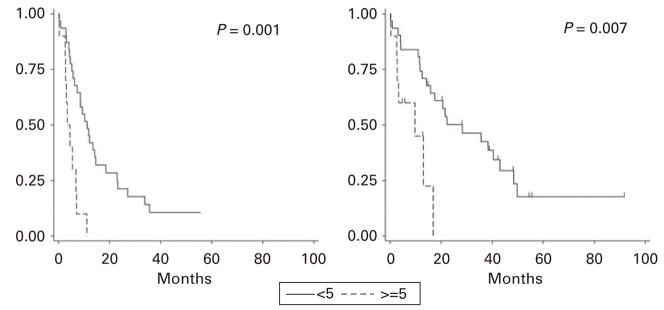
In univariate analysis, prior lines of therapy (≥5 versus <5; P = 0.001, Figure 2), prior thalidomide (P = 0.06), prior lenalidomide (P = 0.04), and lack of response to initial transplant (SD/PD versus CR/VGPR, P = 0.02) were each predictive of a shorter PFS. In a multivariate model, prior lines of therapy and lack of response to initial transplant retained significance, but prior thalidomide and lenalidomide did not do so (Table 3); these were likely surrogates for prior lines of therapy. Age ≥65 years was also found to be a significant prognostic factor for PFS when controlling for other significant prognostic factors.
Figure 2.
Progression-free (left) and overall survival (right) by number of prior lines of therapy (<5 versus ≥5).
Table 3.
Multivariate Cox regression models for PFS and OS after second auto-SCT
| Variable | PFS model |
OS model |
||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P-value | Hazard ratio (95% CI) | P-value | |
| Prior lines of therapy (≥5 lines n = 10 versus <5 n = 31) | 5.2 (2.2–12.5) | <0.001 | 3.9 (1.4–10.9) | 0.008 |
| Age (≥65 years n = 7 versus <65 n = 34) | 3.6 (1.1–12.1) | 0.04 | — | — |
| Response to initial ASCT (versus CR/VGPR n = 12) | ||||
| PR (n = 21) | 1.4 (0.5–3.9) | 0.57 | — | — |
| SD/PD (n = 8) | 7.4 (2.0–27.5) | 0.003 | — | — |
| TTP after initial ASCT (≤12 months n = 14 versus >12 n = 27) | — | — | 2.4 (1.1–5.5) | 0.04 |
Abbreviations: CI = confidence interval; OS = overall survival; PD = progressive disease; PR = partial response; SD = stable disease; TTP = time to progression; VGPR = very good partial response.
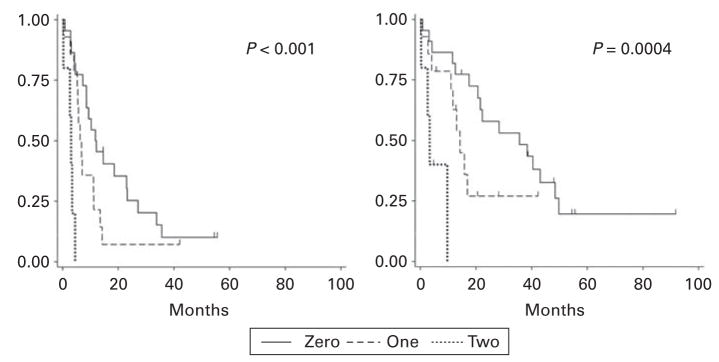
In univariate analysis of OS, only prior lines of therapy (P = 0.007, Figure 2) and TTP after initial transplant ≤12 months (P = 0.04) were predictive of poor outcome. Both variables retained independent significance in a multivariate model (Table 3). By categorizing patients into those who possessed zero, one or two of these adverse prognostic factors, three risk groups could be identified, with a median OS of 35, 14 and 3 months, respectively (P = 0.0004 by log-rank test), and a median PFS of 11, 6 and 3 months, respectively (P < 0.0001 by log-rank test; Figure 3).
Figure 3.
Progression-free (left) and overall survival (right) after second auto-SCT of patients with zero, one or two adverse prognostic factors (≥5 prior lines of therapy and time to progression after initial auto-SCT ≤12 months).
Discussion
In our single center experience of 41 patients, salvage auto-SCT appears to be a safe and effective treatment for relapsed multiple myeloma. The overall response rate of 55% in assessable patients and low rate of treatment-related mortality suggest a favorable risk/benefit profile.
After a median follow-up of 15 months, the median PFS for all patients after salvage auto-SCT was 8.5 months. Other studies have reported higher estimates,7,8,10,12 but in the setting of salvage auto-SCT at the time of first relapse10,12 or with no data provided on the number of prior therapies.7,8 Our data suggest that receipt of multiple prior lines of therapy is a strong prognostic factor for poor outcomes, which would explain the more durable responses seen when salvage auto-SCT is used in patients without significant pre-treatment. In a recent small study describing patients with a greater degree of pre-treatment than our patient cohort (median six prior therapies versus median three, respectively), the median PFS was 6.8 months.11
The number of prior lines of therapy (≥5 vs <5) was the strongest predictor in multivariate models of both PFS and OS after salvage auto-SCT. This is likely a surrogate marker for the presence of chemoresistant disease or the persistence of residual toxicities from prior therapies. It can be noted that the presence of cytogenetic abnormalities at any time prior to salvage transplant was not predictive of either PFS or OS, and neither was the presence of responsive disease prior to transplant. Our study is the first to investigate the significance of the number of prior lines of therapy as a prognostic factor in salvage auto-SCT for myeloma, and this bears confirmation in additional series from other centers.
Time to progression after initial transplant was also independently predictive of OS, which is consistent with other previous reports of salvage auto-SCT.7,9,10,12,15 Some studies have found that the interval between transplants is prognostic of OS.11,13,14 This factor is likely a suboptimal surrogate for TTP after initial transplant, which in our study was not significantly associated with either PFS or OS in univariate or multivariate analyses. TTP ≤12 months after initial auto-SCT has also been shown to be predictive of shorter OS in a large group of patients with myeloma, suggesting that this factor is highly prognostic irrespective of subsequent therapies given at relapse.17
A number of conditioning regimens were used for the salvage auto-SCT in our cohort: melphalan 200 mg/m2, reduced dose melphalan and TBI-based regimens were each used in 25–35% of patients in this cohort. (Only three patients received BU/CY.) The choice of conditioning regimen was made by the treating physician and was based in large part on age, comorbid disease, prior therapy and renal function. There was no difference in PFS or OS between patients who received reduced dose melphalan, full-dose melphalan or TBI-based conditioning, suggesting that salvage autologous transplant may still be effective in the setting of renal insufficiency or other comorbidities.
The limitations of this study include its retrospective nature, limited sample size and incomplete data on known prognostic factors such as β-2 microglobulin and cytogenetics. In the context of the available literature on salvage auto-SCT for multiple myeloma, however, this study does support the safety of salvage auto-SCT and confirms the importance of TTP after initial transplant as a prognostic factor.
The value of tandem transplantation when compared to single auto-SCT is controversial. Although a number of studies have shown improved PFS and OS after tandem transplantation, one recent study suggested superior outcome after single auto-SCT with thalidomide maintenance. 18 The results of that study are difficult to interpret, as has been discussed.5 The relative merits of salvage auto- SCT at relapse, as compared with an upfront tandem transplant, are unknown. Preliminary results from a randomized trial of these two approaches show no difference in OS; however, longer follow-up is needed.10 For patients who have already received a single auto-SCT, our data indicate that salvage auto-SCT is safe and may be reasonably effective.
We conclude that second salvage auto-SCT generally has a favorable risk/benefit profile in patients with relapsed multiple myeloma. Patients with two adverse prognostic factors (≥5 prior lines of therapy and a TTP after initial transplant of ≤12 months) are unlikely to benefit significantly. Salvage auto-SCT should therefore be considered for appropriate patients with relapsed multiple myeloma.
References
- 1.Attal M, Harousseau JL, Facon T, Guilhot F, Doyen C, Fuzibet JG, et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003;349:2495–2502. doi: 10.1056/NEJMoa032290. [DOI] [PubMed] [Google Scholar]
- 2.Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 3.Lenhoff S, Hjorth M, Holmberg E, Turesson I, Westin J, Nielsen JL, et al. Impact on survival of high-dose therapy with autologous stem cell support in patients younger than 60 years with newly diagnosed multiple myeloma: a population-based study. Nordic Myeloma Study Group. Blood. 2000;95:7–11. [PubMed] [Google Scholar]
- 4.Palumbo A, Triolo S, Argentino C, Bringhen S, Dominietto A, Rus C, et al. Dose-intensive melphalan with stem cell support (MEL100) is superior to standard treatment in elderly myeloma patients. Blood. 1999;94:1248–1253. [PubMed] [Google Scholar]
- 5.Mehta J. One or two autografts for myeloma? Blood. 2008;111:3899–3900. doi: 10.1182/blood-2007-12-127704. [DOI] [PubMed] [Google Scholar]
- 6.Reece DE. Management of multiple myeloma: the changing landscape. Blood Rev. 2007;21:301–314. doi: 10.1016/j.blre.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Mikhael JR, Zadeh S, Samiee S. Second autologous stem cell transplant (ASCT) as salvage therapy in patients with relased multiple myeloma: Improved outcomes in patients with longer disease free interval after first ASCT. Blood. 2007;110 (abstract 946) [Google Scholar]
- 8.Simpson L, Verma R, Kumar S. Outcome after second stem cell transplantation for relapsed multiple myeloma. J Clin Oncol. 2007;25 (abstract 8118) [Google Scholar]
- 9.Mehta J, Singhal S. High-dose chemotherapy and autologous hematopoietic stem cell transplantation in myeloma patients under the age of 65 years. Bone Marrow Transplant. 2007;40:1101–1114. doi: 10.1038/sj.bmt.1705799. [DOI] [PubMed] [Google Scholar]
- 10.Abdelke A, Ladeb S, Ben Othman T. Timing of second autologous transplantations in multiple myeloma: Results of a multicenter sequential randomized clinical trial. Blood. 2006;108 (abstract 59) [Google Scholar]
- 11.Qazilbash MH, Saliba R, De Lima M, Hosing C, Couriel D, Aleman A, et al. Second autologous or allogeneic transplantation after the failure of first autograft in patients with multiple myeloma. Cancer. 2006;106:1084–1089. doi: 10.1002/cncr.21700. [DOI] [PubMed] [Google Scholar]
- 12.Alvares CL, Davies FE, Horton C, Patel G, Powles R, Morgan GJ. The role of second autografts in the management of myeloma at first relapse. Haematologica. 2006;91:141–142. [PubMed] [Google Scholar]
- 13.Lee CK, Barlogie B, Fassas A. Third autotransplant for the management of 98 patients among 1358 who had received prior tandem auto-transplants: Benefit apparent when 2nd to 3rd transplant interval exceeds 3 years. Blood. 2004;104 (abstract 540) [Google Scholar]
- 14.Mehta J, Tricot G, Jagannath S, Ayers D, Singhal S, Siegel D, et al. Salvage autologous or allogeneic transplantation for multiple myeloma refractory to or relapsing after a first-line autograft? Bone Marrow Transplant. 1998;21:887–892. doi: 10.1038/sj.bmt.1701208. [DOI] [PubMed] [Google Scholar]
- 15.Tricot G, Jagannath S, Vesole DH, Crowley J, Barlogie B. Relapse of multiple myeloma after autologous transplantation: survival after salvage therapy. Bone Marrow Transplant. 1995;16:7–11. [PubMed] [Google Scholar]
- 16.Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 17.Kumar S, Mahmood ST, Lacy MQ, Dispenzieri A, Hayman SR, Buadi FK, et al. Impact of early relapse after auto-SCT for multiple myeloma. Bone Marrow Transplant. 2008;42:413–420. doi: 10.1038/bmt.2008.180. [DOI] [PubMed] [Google Scholar]
- 18.Abdelke A, Ladeb S, Torjman L, Othman TB, Lakhal A, Romdhane NB, et al. Single autologous stem-cell transplantation followed by maintenance therapy with thalidomide is superior to double autologous transplantation in multiple myeloma: results of a multicenter randomized clinical trial. Blood. 2008;111:1805–1810. doi: 10.1182/blood-2007-07-101212. [DOI] [PubMed] [Google Scholar]