Abstract
The development of animal models of lung cancer is critical to our understanding and treatment of the human disease. Conditional mouse models provide new opportunities for testing novel chemopreventatives, therapeutics and screening methods that are not possible with cultured cell lines or xenograft models. This protocol describes how to initiate tumor formation in two conditional genetic models of human non-small cell lung cancer (NSCLC) utilizing the activation of oncogenic K-ras and the loss of function of p53. We discuss methods for sporadic expression of Cre in the lungs via engineered adenovirus or lentivirus and provide a detailed protocol for the administration of the virus by intranasal inhalation or intratracheal intubation. The protocol requires 1–5 minutes per mouse with an additional 30–45 minutes to set-up and allow for the recovery of mice from anesthesia. Mice may be analyzed for tumor formation and progression starting 2–3 weeks after infection.
INTRODUCTION
Lung cancer is the leading cause of cancer deaths worldwide with non-small cell lung cancer (NSCLC) being the most prevalent form of lung cancer1–3. Progress within the last decade has led to the sophisticated engineering and application of advanced preclinical models of human cancer in the mouse4,5. These models are critical to our understanding of the human disease because they shed light on events and processes that cannot be easily studied using transplantable or chemically-induced cancer models5–7. Several laboratories have constructed genetically-engineered mouse models of NSCLC that mimic the genetic and histopathological features of the human disease6,8–13. The development of Cre recombinase-controlled (Cre/LoxP) tumor models has allowed for the generation of autochthonous tumors derived from a limited number of somatic cells that become transformed in their natural location, surrounded by a normal tissue microenvironment5. By engineering LoxP DNA elements into the mouse genome that either surround (‘flox’) exons critical to a tumor suppressor gene’s function or surround a synthetic ‘stop’ element (‘LSL’) inserted in front of an oncogene, investigators can ‘turn-off’ tumor suppressors or ‘turn-on’ oncogenes with delivery of Cre recombinase to the appropriate cell types (Supp. Fig. 1 and see reference 5 for a review of Cre/LoxP-controlled genetically engineered mouse models of cancer). With this technology, investigators can not only recapitulate the genetic alterations found in the human disease, but also the timing of onset and potentially the cellular origin of the disease.
Common mutations in human NSCLC are activating mutations in K-RAS (10–30%) and loss of function point mutations in p53 (50–70%)2. Our laboratory has modeled an oncogenic mutation in K-ras by changing a glycine to aspartic acid at codon 12 in the gene’s endogenous locus. To control the expression of K-rasG12D, a lox-stop-lox (LSL) cassette was engineered into the first intron of the K-ras gene. The LSL cassette consists of transcriptional and translational stop elements flanked by LoxP sites that prevents the expression of the mutant allele until the stop elements are removed by the activity of Cre recombinase9,14. The LSL cassette thus creates a null version of the gene. It is important to note that K-ras null mice are embryonic lethal15; therefore, mice can only be heterozygous for the K-rasLSL-G12D allele. To mimic the loss of p53 function in our K-rasLSL-G12D-driven tumor model, we have utilized a conditional p53 allele from the laboratory of Anton Berns. This ‘floxed’ p53 allele (p53fl) has LoxP sites flanking exons two through ten of p53 that are deleted after Cre-mediated recombination, abolishing p53 function16 (Supp. Fig. 1). Prior to Cre-mediated recombination, the p53 locus is maintained in its wildtype state and p53 activity is normal. To more accurately recapitulate the p53 loss of function mutations commonly observed in human NSCLC, our laboratory has generated two conditional point mutant (mt) versions of p53 (R172H, R270H) that are engineered into the endogenous p53 locus, but silenced by a LSL cassette in the absence of Cre17. We use mice that are p53LSL-mt/fl or p53LSL-mt/+ to specifically express mutant p53 alone or with wildtype p53 (respectively) in tumors upon Cre-mediated recombination17,18. Importantly, as with the K-rasLSL-G12D allele, the LSL cassette in p53LSL-mt alleles blocks p53 expression in the absence of Cre, effectively creating a p53 null allele. Therefore, p53LSL-mt/fl or p53LSL-mt/+ mice are heterozygous for wildtype p53 and phenocopy p53+/− mice. To specifically express either of these mutant p53 alleles sporadically in cells of the lung, mice inhale viruses engineered to express Cre either by intranasal instillation or intratracheal intubation.
Additional Cre recombinase controlled animal models of lung cancer
In addition to the models of NSCLC utilized in this protocol, other conditional lung cancer models have been described which may be initiated with inhalation of viruses expressing Cre. The activation of oncogenic K-ras along with loss of p16Ink4a or Ink4a/Arf tumor suppressors, which are mutated in 20–50% of human cases, have been described11,19. In another mouse model, two other subtypes of NSCLC, squamous cell and large cell carcinoma, develop following the combined activation of oncogenic K-ras and loss of the LKB1 tumor suppressor, which is mutant in 10–30% of human cases19. NSCLC models driven by conditionally activated mutations in Braf or EGFR, mutated in 3% or 10–40% (respectively) of human lung cancers, have also been generated12,13 (and unpublished, K. Lane). A model of small cell lung cancer (SCLC) has been created in which tumors arise following the loss of both the p53 and Rb tumor suppressors20.
Limitations of viral delivery of Cre recombinase
Altough these Cre/LoxP models provide the most sophisticated means to sporadically control genetic events at their endogenous loci, they are also limited by the difficult requirement to introduce Cre into an initiating cancer cell. In this protocol, investigators cannot restrict the genetic events exclusively to initiating cells of the disease because of the inherently non-specific nature of the viruses used to deliver Cre to cells of the lung. However, this has in fact been beneficial in our laboratory because it does not require that investigators identify and target Cre specifically to the cell of origin of the disease. Perhaps the best system to deliver Cre specifically to the cells that give rise to the disease are next generation regulatable Cre alleles, such as the tamoxifen-inducible Cre-estrogen receptor (CreERT) fusion protein5. By expressing a CreERT transgene under the control of a cell type-specific promoter, investigators can induce Cre activity specifically in the cells of a given tissue by injecting animals with tamoxifen. With proper dosing of tamoxifen, investigators can in theory control the penetrance of Cre activity and therefore the multiplicity of the disease. However, various promoter-driven CreERT alleles are not yet available and CreERT models succumb to problems encountered with many transgenic models in that the expression and activity of these alleles are often leaky and do not provide tight control of tumor initiation in the organ of interest 5. Therefore, despite the drawback of using potentially hazardous viruses, this technique is the most effective means to sporadically deliver Cre to cells of the lung to initiate lung tumor formation.
Alternative animal models of lung cancer
There are several alternative lung cancer models to those described here that do not require viral delivery of Cre to the lungs to generate tumors (see reference 6 for a comprehensive review of mouse models of NSCLC). Transgenic models have been created that utilize lung tissue specific promoters to drive expression of viral oncoproteins, such as E6/E7 and large T antigen, or cellular oncogenes, such as c-myc, c-Raf-1 and v-H-ras6. However, these models are limited in recapitulating the human disease because the oncogenic transgene is expressed in all of the cells of a targeted organ beginning early in the organ’s development. To overcome this limitation, our laboratory has engineered a latent oncogenic allele of K-ras that is expressed spontaneously in only a limited number of cells in the lung to initiate lung tumor formation8. However, the stochastic nature of this allele does not allow investigators to control tumor onset or multiplicity. Double transgenic models that rely on doxycycline-regulated transcriptional transactivators have allowed for the induction of oncogene expression in adult animals, making it possible to control tumor initiation6. However, these models still fail to provide sporadic oncogene expression at physiological levels due to the use of doxycyline-regulated transcription factors. Furthermore, these transgenic systems make it difficult to control tumor multiplicity and require continuous doxycycline dosing to maintain oncogene expression.
Applications of intranasal and intratracheal delivery methods
The intranasal and intratracheal infection techniques described in this protocol are not restricted to tumor-initiating studies. Investigators may theoretically probe the role of any Cre/LoxP-controlled genes in various cells of the lung using this protocol to deliver Cre. Alternatively, the intranasal or intratracheal delivery methods can be used in applications other than the viral delivery of Cre recombinase. The viral delivery systems may be adapted to express cDNAs or shRNAs in cells of the lung by infection with lentiviruses21. Viruses, such as the influenza virus, can be delivered for pathological studies22. siRNAs can be delivered as therapeutic agents for disease23. While either the intranasal or intratracheal techniques can be utilized for the aforementioned studies, only intratracheal intubation is recommended for the orthotopic transplantation of lung tumor cells or lung tumor cell lines to the alveolar space of the lungs (unpublished, C. Kim and T. Jacks). Certain chemical injurants, such as bleomycin, can be delivered intratracheally to study repair mechanisms in the lung and the effect of injury on tumor development24. Therefore, there are a number of uses of this technique for a variety of studies involving the mouse lung. Here we describe the use of the intranasal and intratracheal delivery methods to introduce viruses expressing Cre to initiate the K-rasLSL-G12D/+ (K) and the K-rasLSL-G12D/+;p53fl/fl (KP) conditional mouse models of NSCLC (Supp. Fig. 1).
Experimental Design
After deciding to utilize autochthonous tumor models, investigators must consider the genetic tumor model, the viral system to express Cre recombinase, and the viral delivery method when initiating an experiment. Each option has certain advantages and limitations that are highlighted in the text and the Tables.
Genetic Model
Although both the K-rasLSL-G12D/+ (K) and the K-rasLSL-G12D/+;p53fl/fl (KP) NSCLC models induce tumors that resemble the human disease histopathologically, they have distinct features that make them more suitable for different applications (Table 1). In our laboratory’s mouse models of NSCLC, the activation of an oncogenic allele of K-ras is sufficient to initiate the tumorigenesis process, while the additional deletion or point mutation of p53 significantly enhances tumor progression, leading to a more rapid development of adenocarcinomas that have features of a more advanced disease. K-ras and p53 mutant tumors exhibit a greater incidence of cellular and nuclear pleomorphism, desmoplasia, and a high frequency of metastases to the mediastinal lymph nodes and the pleural spaces of the thoracic cavity, and less frequently to the liver and kidneys.
Table 1.
Choosing a tumor model
| K-rasLSL-G12D/+ (K) | K-rasLSL-G12D/+;p53fl/fl (KP) |
|---|---|
| Limited tumor progression (modifier screens) |
Rapid tumor progression |
| No metastases | Metastatic disease Local: draining lymph node, pleural cavity Distant: liver, kidney |
| Long survival | Short survival |
| Single allele (easy to breed) |
Multiple alleles |
| Small tumors (limited tumor tissue) |
Large tumors (more tumor tissue for DNA, RNA, protein) (easier to track with live imaging, microCT) |
Cre System
To generate tumors sporadically in the lungs of K-rasLSL-G12D/+ mice, our laboratory initially used replication-deficient adenoviruses expressing Cre (Ad-Cre) to deliver transient Cre expression to infected cells of the lung9,10. Prior to administration, Ad-Cre is precipitated with calcium phosphate (see procedure) to improve the delivery of Cre by increasing the efficiency of viral infection of the lung epithelium25. Recently, we have used lentiviruses to deliver Cre to the lung (Lenti-Cre)26. Lentiviruses are beneficial because they integrate into the genome of infected cells27, allowing for further modification of the tumors by simultaneously introducing Cre recombinase and stable expression of cDNAs to overexpress, or short-hairpin RNAs to silence, genes of interest (Table 2).
Table 2.
Choosing the Cre system
| Adenovirus Cre (Ad-Cre) | Lentivirus Cre (Lenti-Cre) |
|---|---|
| High, reproducible titer (typically produces a greater number of tumors, shorter survival) |
Variable titer |
| Titered virus is commercially available | In-house production may be required |
| Cannot introduce cDNAs or shRNAs with Cre recombinase |
Potential to modify tumors by introduction of cDNAs or shRNAs in lentivirus (gain-of-function or loss-of-function experiments) |
In order to control for the number of tumors generated, viruses are titered prior to use in experiments. Ad-Cre is titered at the University of Iowa, while Lenti-Cre is titered in our laboratory by assaying for Cre activity after infection of the 3TZ reporter cell line, a mouse fibroblast cell line modified to express β-galactosidase after Cre-mediated recombination28. We infect mice with 2.5×107 infectious particles of Ad-Cre (titered at Univ. Iowa) or approximately 104–105 infectious particles of Lenti-Cre (3TZ titered in-house). It is important to note that Ad-Cre and Lenti-Cre are titered differently, and as a result, the titers cannot be directly compared.
Viral Administration Method
While we initially delivered Cre to the lungs of anesthetized mice using intranasal (IN) instillation of the virus, we now prefer to deliver the virus to the lungs by intratracheal (IT) intubation. IT delivery provides the most direct and consistent method for the virus to reach the lungs. Reproducible delivery of the virus is critical because it directly affects the number of tumors generated in the mice. However, intratracheal intubation requires additional equipment and practice to perform it correctly and in a timely manner (Table 3). Therefore, it may be easier to begin with the IN delivery method to assess the tumor model and practice the IT method while continuing to breed animals for future experiments.
Table 3.
Choosing the viral administration method
| Intranasal (IN) | Intratracheal (IT) |
|---|---|
| No training required (can implement rapidly) |
Technique requires practice for proficiency |
| No additional equipment necessary | Requires catheters, light source, platform |
Indirect and variable delivery of virus to lungs
|
Direct delivery of virus to lungs |
Anesthesia
We recommend using avertin (2-2-2 Tribromoethanol) to anesthetize the mice. The amount of avertin administered to the mice is crucial to the success of the procedure. Mice administered too much avertin are more likely to stop breathing during the infection procedure, and recover poorly from the anesthesia. Conversely, mice administered too little avertin may struggle to inhale the virus and should be given more avertin before continuing. Therefore, we recommend using the smallest volume of avertin required to keep mice anesthetized during the procedure. Following the procedure, mice will recover better if they are kept warm to maintain their normal body temperature after anesthesia.
Age of Mice
Our laboratory has utilized mice between 6 and 12 weeks of age for tumor initiation by IN or IT delivery of viruses expressing Cre. Mice of this age are old enough to recover from the anesthetic, the volume of virus administered to the lung, and the intubation of the trachea with the catheter.
Volume of virus
Mice can be infected with a volume ranging from 50–125 µl per mouse, but we recommend using a total volume of 75 µl per mouse. Although a volume of 125 µl can be administered, it is not recommended for very young mice (6 weeks of age or younger). If 125 µl is administered, then the mice should receive two doses of 62.5 µl each, with a 1–5 minute break in between the doses, to allow the mice to recover a normal breathing pattern before receiving the second dose.
MATERIALS
REAGENTS
CRITICAL The following list of reagents represents our laboratory’s preference. All reagents, with the exception of Ad-Cre, can be modified according to investigator preference.
-
Mice: LSL-KrasG12D (Mouse Models of Human Cancers Consortium (MMHCC) Strain 01XJ6, Jackson Laboratory #008179 (B6), #008180 (129)); p53fl (MMHCC Strain 01XC2, Jackson Laboratory #008462 (B6)); p53 LSL-R270H (MMHCC Strain 01XM3, Jackson Laboratory #008651 (129svj)); p53LSL-R172H (MMHCC Strain 01XM2, Jackson Laboratory # 008652 (129svj)). Researchers in industry can obtain these mice by contacting the MIT Technology Licensing Office (http://web.mit.edu/tlo/www/industry/).
CAUTION All experiments should be done in accordance with protocols approved by the Institutional Animal Care and Use Committee.
-
K-rasLSL-G12D (LSL K-ras G12D) and p53 mutant genomic targeting vectors are available from Addgene (http://www.addgene.org/pgvec1?f=c&cmd=showcol&colid=171). Koch Institute Core Facilities can perform chemotherapeutic testing and small animal imaging (http://web.mit.edu/ki/facilities/core.html)
CAUTION Mice should be kept under specific pathogen-free conditions during experiments.
Protocols to genotype the mouse strains listed above can be found on the Jacks Lab website (http://web.mit.edu/jacks-lab/protocols_table.html)
Protocols to determine the recombination efficiency of the KrasLSL-G12D and p53fl alleles can be found on the Jacks Lab website (http://web.mit.edu/jacks-lab/protocols_table.html)
2-2-2 Tribromoethanol (Avertin, Sigma Aldrich T48402)
2-methyl-2-butanol (Tert-amyl alcohol, Sigma Aldrich 152463)
Phosphate Buffered Saline (PBS)
Minimal Essential Media (MEM, Sigma, M4655)
CaCl2 (Mallinckrodt, Catalog #4160)
Bleach
-
Adenovirus-Cre (University of Iowa, Gene Transfer Vector Core, http://www.uiowa.edu/~gene/; Jacks Lab website http://web.mit.edu/jacks-lab/protocols/AdenovirusCre.html)
CAUTION Preparation and administration of viruses should occur in a biosafety hood and follow all guidelines for biosafety level 2 research.
-
Lentivirus-Cre (Three plasmid transfection system of CMV-VSV-G (Addgene plasmid 8454), Δ8.2 (gag/pol) (Addgene plasmid 8455), and transfer vector expressing Cre (modified from Addgene plasmid 17408))26,27,29–32
CAUTION Preparation and administration of viruses should occur in a biosafety hood and follow all guidelines for biosafety level 2 research. Research with lentiviruses pseudotyped with VSV-G containing known or putative oncogenes or short hairpin RNAs to silence tumor suppressor genes should abide with all institute safety practices.
EQUIPMENT
Bottle top vacuum filter (.22 µM, 150 ml, Corning, cat. no. 430624)
Scale (Ohaus, cat. no. HH120D)
Needles (30 gauge, ½ inch, Becton Dickinson, cat. no. 305106)
Syringes (1 ml, Becton Dickinson, cat. no. 309602)
Flat forceps (Roboz, cat. no. RS-8260)
Exel Safelet IV catheters (22 gauge, 1 inch, Fisher, cat. no. 14-841-20)
Intubation platform (Steve Boukedes, labinventions@gmail.com)
Fiber-Lite Illuminator (Dolan-Jenner Industries, Inc., Model 3100-1)
Heat lamp (or latex gloves filled with warm water)
REAGENT SETUP
Avertin Stock (1.6 g ml−1)
Add 15.5 ml tert-amyl alcohol to 25 g of avertin. Stir overnight to dissolve. Stable at room temperature (18–25 °C) for approximately 1 year.
CRITICAL Seal bottle tightly and protect from light. Discard the solution if it yellows.
Avertin Working Solution (20 mg ml−1)
Dilute avertin stock in PBS, stir overnight, and protect from light. Sterilize by passing solution through a 0.22 µm filter. Aliquots may be safely stored at 4 °C in the dark for approximately 4 months.
2 M CaCl2
Dissolve in distilled water and store at 4 °C for up to 5 years.
Virus
Ad-Cre should be prepared fresh each time and used within one hour of preparation. For extended storage times, Lenti-Cre should be stored at −80 °C or alternatively, at 4 °C for periods of a few days. Keep viruses on ice prior to infection.
EQUIPMENT SET-UP
Biosafety hood
To set-up for either IN or IT administrations, arrange the virus, a heat lamp (or gloves filled with warm water), and a beaker filled with 50% bleach (to disinfect catheters and pipette tips that have contacted the virus) in a biosafety hood.
Intubation platform and light source
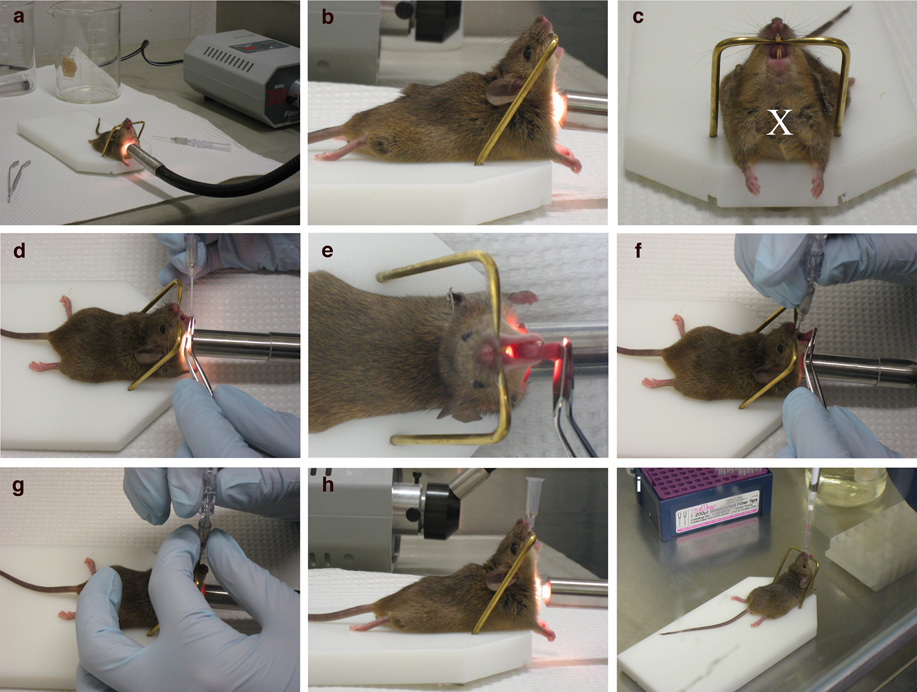
For IT administration, set up the platform and light source on a flat surface near the biosafety hood (Fig. 1a). Insert the catheter into the trachea outside of the hood, and then move the mouse into the biosafety hood to inhale the virus. A sharps waste container is also required for the proper disposal of the needles from the Exel Safelet IV Catheter.
Figure 1. Intratracheal infection technique.
Anesthetized mice are placed on the platform by their front teeth so that their chest hangs vertically beneath them (a, b). The light is directed on the mouse’s upper chest (a, c), on the spot marked by the ‘X’ (c). The mouth is opened using the Exel Safelet IV catheter (d), and the tongue is gently pulled out using the flat forceps. After locating the white light emitted from the trachea (e), the Exel Safelet IV catheter is slid into the trachea (f), and the needle is removed (g). The mouse with the inserted catheter (h) on the platform is moved into a biosafety hood, where the virus is dispensed into the opening of the catheter (i).
CAUTION All experiments should be done in accordance with protocols approved by the Institutional Animal Care and Use Committee.
PROCEDURE
Virus Preparation TIMING 30 min
-
1
For infection with Lenti-Cre, proceed to step 5. For Ad-Cre, prepare the adenovirus-precipitate mixture. Determine the number of mice that you will infect and pipette the appropriate volume of MEM into a tube. Refer to the Experimental Design Section to determine the volume of virus to administer per mouse.
CAUTION Preparation and administration of viruses should occur in a biosafety hood and follow all guidelines for biosafety level 2 research. Research with lentiviruses pseudotyped with VSV-G containing known or putative oncogenes or short hairpin RNAs to silence tumor suppressor genes should abide with all institute safety practices.
CAUTION All experiments should be done in accordance with protocols approved by the Institutional Animal Care and Use Committee.
CRITICAL STEP The adenovirus-precipitate mixture should be used within one hour of preparation. If many mice will be infected, precipitates should be prepared sequentially.
-
2
After the adenovirus thaws on ice, pipette the adenovirus directly into the MEM to obtain a final titer of 2.5×107 PFU per mouse. Flick the tube to mix.
CRITICAL STEP Do not freeze/thaw the adenovirus as this reduces the titer of the virus 10 fold with each thaw.
-
3
Add 2M CaCl2 into the MEM mix to obtain a final CaCl2 concentration of 10 mM. Flick the tube to mix.
-
4
Incubate at room temperature for 20 min to allow for the formation of calcium phosphate precipitates.
Delivery of Ad-Cre or Lenti-Cre using the intratracheal or intranasal infection method
-
5
Avertin Administration TIMING 5 min per mouse
Anesthetize mice via intra-peritoneal injection of room temperature 20 mg ml−1 avertin (use 0.4 mg g−1 body weight for females and 0.45 mg −1 body weight for males). Confirm the mice are fully anesthetized by ensuring that they lack a toe reflex.
CRITICAL STEP Administering the correct amount of avertin is crucial to successfully delivering the virus.
TROUBLESHOOTING
-
6
Delivery of Ad-Cre or Lenti-Cre can be carried out using Option A the intratracheal infection method or Option B the intranasal infection method
-
Intratracheal infection method (see Supplementary Video 1) TIMING 1–5 min per mouse
Place mouse on the platform so that it is hanging from its top front teeth on the bar (Fig. 1b, c).
Push the mouse towards the bar so that the chest is vertical underneath the bar (perpendicular to the platform) (Fig. 1b).
Direct the Fiber-Lite Illuminator to shine on the mouse’s chest, in between the front legs (Fig. 1c).
-
Prepare the Exel Safelet IV catheter for the infection procedure. To ensure that the needle does not become exposed and impale the mouse, hold the square part of the needle with one’s thumb and index finger, and using one’s middle finger, push the catheter over the end of the needle completely and continue to hold the catheter in place during the infection protocol (Supp. Fig. 2 a, b).
TROUBLESHOOTING
Using the Exel Safelet IV catheter, open the mouth and gently pull out the tongue with the flat forceps (Fig. 1d).
-
Locate the opening of the trachea by peering into the mouth and looking for the white light emitted from the trachea (Fig. 1e).
TROUBLESHOOTING
While holding the Exel Safelet IV catheter vertically, position the catheter over the white light emitted from the opening of the trachea, and allow the catheter to slide into the trachea until the top of the catheter reaches the mouse’s front teeth (Fig. 1f). There should be no resistance while inserting the catheter into the trachea.
-
While stabilizing the Exel Safelet IV catheter with one hand, remove the needle from the mouth (Fig. 1g).
CRITICAL STEP Prior to removing the needle, the mouse cannot breathe through the catheter. Once the Exel Safelet IV catheter has been inserted into the trachea, promptly remove the needle to allow the mouse to breathe through the catheter.
The proper placement of the catheter in the trachea can be confirmed by visualizing the white light shining through the opening of the catheter in the mouth (Fig. 1h).
Move the platform, mouse, and catheter into the biosafety hood.
Pipette the virus directly into the opening of the catheter to ensure the entire volume is inhaled (Fig. 1i).
-
If the catheter is correctly inserted into the trachea, the mouse will begin inhaling the virus immediately. Once the virus is no longer visible in the opening of the catheter, wait a few seconds for the entire volume to travel down the catheter before removing the catheter from the trachea and disposing of it in 50% bleach.
TROUBLESHOOTING
-
Delivery of Ad-Cre or Lenti-Cre using the intranasal delivery method TIMING 2–5 min per mouse
-
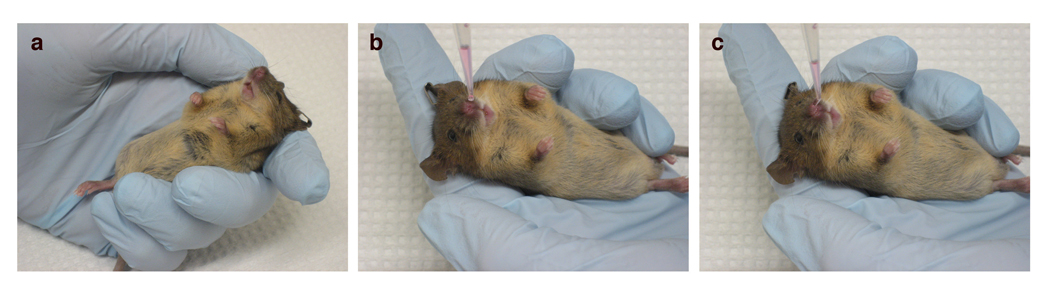
In the biosafety hood, lay the mouse in your hand, ventral side up. Tilt the mouse so that the head is positioned above its feet (Fig. 2a).
CRITICAL STEP Do not grasp the mouse tightly as this will inhibit the mouse’s breathing
-
Hold the end of a pipet tip over the opening of one nostril and dispense the virus dropwise until the entire volume of virus has been inhaled (Fig. 2b, c).
CRITICAL STEP Do not attempt to insert the pipet tip into the nostril.
TROUBLESHOOTING
-
-
Figure 2. Intranasal infection technique.
The anesthetized mouse lies gently in the hand of the investigator (a), and the virus is administered dropwise (b) into one nostril until the virus is completely inhaled (b, c).
CAUTION All experiments should be done in accordance with protocols approved by the Institutional Animal Care and Use Committee.
Animal Recovery TIMING 10–15 min
-
7
Place the mouse under a heat lamp (Supp. Fig. 3a) or on a latex glove filled with warm water (Supp. Fig. 3b) to recover in the biosafety hood.
TROUBLESHOOTING
TIMING
Steps 1–4 Virus Preparation (Ad-Cre only): 30 min
Step 5 Avertin Administration: 5 min per mouse
Step 6A Intratracheal Infection: 1–5 min per mouse after an initial training phase
Step 6B Intranasal Delivery: 2–5 min per mouse
Step 7 Animal Recovery: 10–15 min
TROUBLESHOOTING
Troubleshooting advice can be found in Table 4
Table 4.
Troubleshooting advice
| Step Number |
Problem | Possible Reason | Solution |
|---|---|---|---|
| 5 | Mouse is not falling asleep |
Not enough avertin administered |
Administer more avertin, 50–100 µl at a time |
| Mouse is over- anesthetized and breathing very slowly |
Too much avertin administered |
Wait until breathing becomes more regular but ensure the mouse still lacks a reflex response before attempting to infect the mouse |
|
| 6Aiv | Blood appears in mouth |
Catheter has slipped and exposed the needle |
Make sure that the catheter is held in place to cover the needle correctly (Supp. Fig. 2a, b) |
| 6Avi | Cannot visualize the white light |
Light is not in the correct position |
Re-direct the light on the upper chest |
| Not looking at the ventral surface of the throat |
Lean further over the mouth and push the tongue with the catheter towards the ventral surface of the throat |
||
| Saliva is covering the opening of the trachea |
Gently probe at the back of the throat with the Exel Safelet IV catheter to expose the trachea |
||
| 6Axii | Mouse does not inhale the virus (virus stays in the catheter) |
Catheter inserted into esophagus |
Pipette the virus out of the catheter for reuse, dispose of the catheter in 50% bleach, and begin the procedure again at step 6Aiv with a new catheter |
| 6B | Mouse is coughing and sputtering virus |
Too much virus placed on nostril before being inhaled |
Let coughing subside before continuing. Pipette the virus resting on nostril for reuse |
| Virus dispensed into both nostrils |
Only dispense virus into one nostril |
||
| 7 | Mouse does not recover well following anesthesia |
Mouse is too cold | Mouse should be kept warm until it starts to wake up |
| Mouse is too hot under heat lamp |
Turn off heat lamp temporarily or let mouse recover on a glove filled with warm water |
ANTICIPATED RESULTS
We have reported that recombination of K-rasLSL-G12D and p53fl alleles in tumors can be examined by PCR for the presence of a “1 lox”, or recombined, product (see Reagents in Materials section for the protocols)9. Although it is possible to perform PCR on DNA isolated from whole lung after infection to assess infection efficiency, this is not recommended. Typically very few cells in the lung have undergone recombination of these alleles, making it difficult to detect recombination. Instead, it is more informative to examine Cre expression after infection by using conditional reporter strains such as Rosa26LSL-LacZ or Rosa26LSL-eGFP and examining reporter expression by immunohistochemistry, immunofluorescence, or fluorescent activated cell sorting33. Polyclonal antibodies that can specifically detect the oncogenic K-rasG12D protein are no longer available; however, increased Ras-GTP levels in tumors or cells can be assessed using a Raf-GST pulldown assay14.
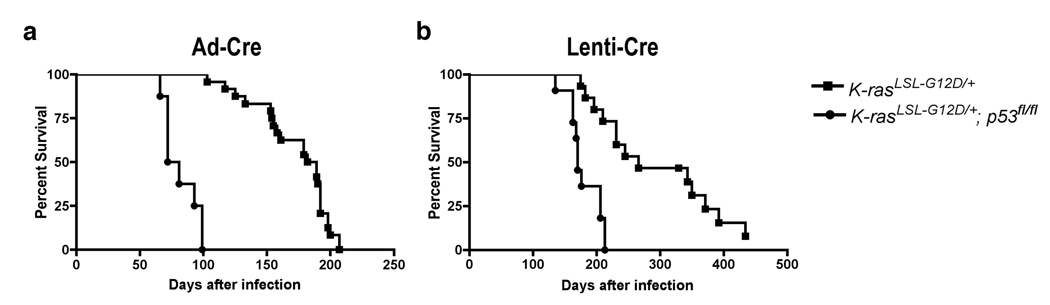
The time to tumor development and progression will vary depending upon the model chosen (K v. KP), while survival time will also depend on the amount and type of virus administered to the mice. The survival of mice is reduced approximately twofold in KP mice compared to K mice (median survival with Ad-Cre: K, 185 days; KP, 76 days, with Lenti-Cre: K, 266 days; KP, 170 days) (Fig. 3a, b). Decreased survival is due to a greater growth rate of tumors lacking p53, leading to the more rapid development of a tumor burden that disrupts the normal function of the lungs. Reduced survival after p53 loss is not due to an increased number of tumors or metastatic disease. Survival of mice is also reduced after infection with Ad-Cre as compared to Lenti-Cre (Fig. 3a, b). This is due to the higher titer of virus typically administered to mice with Ad-Cre but may also reflect the viral tropism or the efficiency of the virus to infect the cell of origin of the disease. Mice infected with 2.5×107 infectious particles of Ad-Cre (Univ. Iowa, Gene Transfer Vector Core) can generate greater than 200 tumors per mouse, whereas mice infected with roughly 104–105 infectious particles of Lenti-Cre (3TZ titered) can generate 10–100 tumors per mouse. Our laboratory has had success titrating the viruses to lower levels (Ad-Cre: 5×106 infectious particles/mouse, Lenti-Cre: 5×103 infectious particles/mouse) which reduce the number of primary tumors and increase the survival time of mice, allowing for a greater frequency of metastatic disease in the KP model.
Figure 3. Survival is reduced in KP model compared to K model.
Kaplan-Meier plot of KP mice (median survival 76 days) and K mice (median survival 185 days) infected with 2.5×107 PFU of Ad-Cre per mouse (provided by T. Oliver) (a). Kaplan-Meier plot of KP mice (median survival 170 days) and K mice (median survival 266 days) infected with 105 Lenti-Cre viruses per mouse (provided by P. Sandy and M. DuPage) (b).
CAUTION All experiments should be done in accordance with protocols approved by the Institutional Animal Care and Use Committee.
Monitoring tumor development and progression histologically is an important, though difficult, way to follow the disease, and it is especially useful in experiments where genetic events or modifications in addition to K-ras activation and/or p53 loss may be expected to impact tumor progression. We stress that the NSCLC model generates a multi-focal disease and therefore, investigators should expect some tumor heterogeneity; tumors do not always progress in exactly the same way or at the same time. To follow these changes objectively, we have employed a 4-stage grading system for tumor progression in our NSCLC models (adapted from ref. 18). The earliest lesions, designated as grade 1, are atypical adenomatous hyperpasias (AAH) or small adenomas that feature uniform nuclei and can appear as early as 2–3 weeks post-infection (Fig. 4a). These earliest lesions can only be identified through careful histological analysis. To see visible lesions on the surface of the lung, investigators should wait until 6–8 weeks after tumor initiation or later. Grade 2 tumors are larger adenomas that have slightly enlarged nuclei with prominent nucleoli and are observed 6–8 weeks post-infection (Fig. 4b, c). Adenocarcinomas are classified as grade 3; they have a great degree of cellular pleomorphism and nuclear atypia and can develop as early as 16 weeks post-infection (Fig. 4d, e). Grade 4 tumors are invasive adenocarcinomas (Fig. 4f) that harbor all the cellular characteristics of Grade 3 tumors but with a higher mitotic index - including irregular mitoses, a distinctive highly invasive stromal reaction (desmoplasia) (Fig. 4g), and invasive edges bordering lymphatic vessels, blood vessels, or the pleura (Fig. 4h). Grade 4 tumors may develop as early as 18 weeks post-infection in KP animals, but are not observed in K animals. Finally, locally metastatic disease to the mediastinal lymph nodes (Fig. 4i) or the pleural cavity develops in approximately 50% of KP mice as early as 18–20 weeks post-infection. In some mice, distant metastases can be found seeding the liver or the kidneys as early as 20 weeks post-infection (Fig. 4j). Although the time to progression in K and KP mice is described here to be similar up to Grade 3 lesions, mice with p53 deficient tumors often harbor cells that exhibit nuclear atypia at very early time points after tumor initiation. In addition, a greater proportion of tumors that lack p53 progress to higher grades during tumor development18. For example, at 6 weeks after infection the distribution of tumor grades in the K model is ~90% grade 1 and ~10 grade 2, whereas in the KP model it is ~40% grade 1, ~40% grade 2 and ~20% grade 3 18. At 19 weeks after infection in the KP model, the tumor distribution is ~5% grade 1, ~20% grade 2, ~70% grade 3 and ~5% grade 4 18. At 26 weeks after infection in the K model, ~30% of tumors are grade 1, ~40% are grade 2 and ~30% are grade 3 18. However, as with most autochthonous mouse tumor models, there is some variation in the results, such as tumor number and approximate time to progression, depending on the strain/ background of mice as well as other factors that vary between institutions.
Figure 4. Tumor progression and histopathological phenotype in KP model.
Haematoxylin and eosin stained (H&E) tumors from KP mice at various times after infection with Lenti-Cre: (a) Grade 1 lesion of an AAH progressing to a small adenoma. (b) Grade 2 adenoma. (c) Uniform nuclei in a grade 2 adenoma. (d) Pleomorphic nuclei in a Grade 3 adenocarcinoma. (e) Grade 3 adenocarcinoma displaying mixed cellular phenotypes. (f) Grade 4 invasive adenocarcinoma. (g) Grade 4 adenocarcinoma with glandular/acinar architecture and desmoplasia. (h) Adenocarcinoma from the lung (L) invading across the mesothelium into the pleural cavity (P). (i) Local lung tumor metastasis (T) to mediastinal lymph node (LN). (j) Distant lung tumor metastasis (T) to the kidney (K). Panels a, b, e, f, i and j are at 100X magnification. Panels c, d, g and h are at 400X magnification. Scale bar = 100 µm.
CAUTION All experiments should be done in accordance with protocols approved by the Institutional Animal Care and Use Committee.
Supplementary Material
Engineering of LoxP elements and a stop element allow for the controlled expression of oncogenic K-ras and the loss of p53 function after Cre expression.
Upon opening the Exel Safelet IV catheter, the needle is exposed (a). Slide the catheter over the end of the needle to completely cover the tip (b) and the Exel Safelet IV catheter is now ready to use.
Mice can be placed under a heat lamp (a) or on a glove filled with warm water (b) to recover following anesthesia in the biosafety hood.
CAUTION All experiments should be done in accordance with protocols approved by the Institutional Animal Care and Use Committee.
CAUTION All experiments should be done in accordance with protocols approved by the Institutional Animal Care and Use Committee.
ACKNOWLEDGMENTS
We would like to thank Carla Kim and Amber Woolfenden for originally training the authors to perform the intratracheal intubation technique. The technique was implemented in our lab with help from Kwok-Kin Wong and Samanthi Perera. We would like to thank Trudy Oliver and Peter Sandy for providing data to compile the Kaplan-Meier survival curves as well as Denise Crowley and Roderick Bronson for providing key histological and pathological advice. We also thank Keara Lane, Etienne Meylan, Eric Snyder, and Anne Deconinck for reviewing this protocol. This work was supported by funding from the Howard Hughes Medical Institute, the NCI (including a Cancer Center Support grant), and the Ludwig Center for Molecular Oncology at MIT. T.J. is the David H. Koch Professor of Biology and a Daniel K. Ludwig Scholar. Research was conducted in compliance with the Animal Welfare Act Regulations and other Federal statutes relating to animals and experiments involving animals and adheres to the principles set forth in the Guide for Care and Use of Laboratory Animals, National Research Council, 1996.
Footnotes
Competing financial interests
The authors declare that they have no competing financial interests.
Contributor Information
Michel DuPage, Email: dupage@mit.edu.
Alison L. Dooley, Email: ald32@mit.edu.
REFERENCES
- 1.Jemal A, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367–1380. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers--a different disease. Nat Rev Cancer. 2007;7:778–790. doi: 10.1038/nrc2190. [DOI] [PubMed] [Google Scholar]
- 4.Van Dyke T, Jacks T. Cancer modeling in the modern era: progress and challenges. Cell. 2002;108:135–144. doi: 10.1016/s0092-8674(02)00621-9. [DOI] [PubMed] [Google Scholar]
- 5.Frese KK, Tuveson DA. Maximizing mouse cancer models. Nat Rev Cancer. 2007;7:645–658. doi: 10.1038/nrc2192. [DOI] [PubMed] [Google Scholar]
- 6.Meuwissen R, Berns A. Mouse models for human lung cancer. Genes Dev. 2005;19:643–664. doi: 10.1101/gad.1284505. [DOI] [PubMed] [Google Scholar]
- 7.Shaw AT, Kirsch DG, Jacks T. Future of early detection of lung cancer: the role of mouse models. Clin Cancer Res. 2005;11:4999s–5003s. doi: 10.1158/1078-0432.CCR-05-9005. [DOI] [PubMed] [Google Scholar]
- 8.Johnson L, et al. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature. 2001;410:1111–1116. doi: 10.1038/35074129. [DOI] [PubMed] [Google Scholar]
- 9.Jackson EL, et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15:3243–3248. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meuwissen R, Linn SC, van der Valk M, Mooi WJ, Berns A. Mouse model for lung tumorigenesis through Cre/lox controlled sporadic activation of the K-Ras oncogene. Oncogene. 2001;20:6551–6558. doi: 10.1038/sj.onc.1204837. [DOI] [PubMed] [Google Scholar]
- 11.Fisher GH, et al. Induction and apoptotic regression of lung adenocarcinomas by regulation of a K-Ras transgene in the presence and absence of tumor suppressor genes. Genes Dev. 2001;15:3249–3262. doi: 10.1101/gad.947701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ji H, et al. The impact of human EGFR kinase domain mutations on lung tumorigenesis and in vivo sensitivity to EGFR-targeted therapies. Cancer Cell. 2006;9:485–495. doi: 10.1016/j.ccr.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 13.Dankort D, et al. A new mouse model to explore the initiation, progression, and therapy of BRAFV600E-induced lung tumors. Genes Dev. 2007;21:379–384. doi: 10.1101/gad.1516407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tuveson DA, et al. Endogenous oncogenic K-ras(G12D) stimulates proliferation and widespread neoplastic and developmental defects. Cancer Cell. 2004;5:375–387. doi: 10.1016/s1535-6108(04)00085-6. [DOI] [PubMed] [Google Scholar]
- 15.Johnson L, et al. K-ras is an essential gene in the mouse with partial functional overlap with N-ras. Genes Dev. 1997;11:2468–2481. doi: 10.1101/gad.11.19.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jonkers J, et al. Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nat Genet. 2001;29:418–425. doi: 10.1038/ng747. [DOI] [PubMed] [Google Scholar]
- 17.Olive KP, et al. Mutant p53 gain of function in two mouse models of Li-Fraumeni syndrome. Cell. 2004;119:847–860. doi: 10.1016/j.cell.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Jackson EL, et al. The differential effects of mutant p53 alleles on advanced murine lung cancer. Cancer Res. 2005;65:10280–10288. doi: 10.1158/0008-5472.CAN-05-2193. [DOI] [PubMed] [Google Scholar]
- 19.Ji H, et al. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448:807–810. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- 20.Meuwissen R, et al. Induction of small cell lung cancer by somatic inactivation of both Trp53 and Rb1 in a conditional mouse model. Cancer Cell. 2003;4:181–189. doi: 10.1016/s1535-6108(03)00220-4. [DOI] [PubMed] [Google Scholar]
- 21.Marumoto T, et al. Development of a novel mouse glioma model using lentiviral vectors. Nat Med. 2009;15:110–116. doi: 10.1038/nm.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen CH, et al. Loss of IL-7R and IL-15R expression is associated with disappearance of memory T cells in respiratory tract following influenza infection. J Immunol. 2008;180:171–178. doi: 10.4049/jimmunol.180.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ge Q, et al. Inhibition of influenza virus production in virus-infected mice by RNA interference. Proc Natl Acad Sci U S A. 2004;101:8676–8681. doi: 10.1073/pnas.0402486101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daly HE, Baecher-Allan CM, Barth RK, D'Angio CT, Finkelstein JN. Bleomycin induces strain-dependent alterations in the pattern of epithelial cell-specific marker expression in mouse lung. Toxicol Appl Pharmacol. 1997;142:303–310. doi: 10.1006/taap.1996.8056. [DOI] [PubMed] [Google Scholar]
- 25.Fasbender A, et al. Incorporation of adenovirus in calcium phosphate precipitates enhances gene transfer to airway epithelia in vitro and in vivo. J Clin Invest. 1998;102:184–193. doi: 10.1172/JCI2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar MS, et al. Suppression of non-small cell lung tumor development by the let-7 microRNA family. Proc Natl Acad Sci U S A. 2008;105:3903–3908. doi: 10.1073/pnas.0712321105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naldini L, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 28.Psarras S, et al. Gene transfer and genetic modification of embryonic stem cells by Cre-and Cre-PR-expressing MESV-based retroviral vectors. J Gene Med. 2004;6:32–42. doi: 10.1002/jgm.442. [DOI] [PubMed] [Google Scholar]
- 29.Pfeifer A, Brandon EP, Kootstra N, Gage FH, Verma IM. Delivery of the Cre recombinase by a self-deleting lentiviral vector: efficient gene targeting in vivo. Proc Natl Acad Sci U S A. 2001;98:11450–11455. doi: 10.1073/pnas.201415498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295:868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- 31.Tiscornia G, Singer O, Verma IM. Production and purification of lentiviral vectors. Nat Protoc. 2006;1:241–245. doi: 10.1038/nprot.2006.37. [DOI] [PubMed] [Google Scholar]
- 32.Stewart SA, et al. Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA. 2003;9:493–501. doi: 10.1261/rna.2192803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Engineering of LoxP elements and a stop element allow for the controlled expression of oncogenic K-ras and the loss of p53 function after Cre expression.
Upon opening the Exel Safelet IV catheter, the needle is exposed (a). Slide the catheter over the end of the needle to completely cover the tip (b) and the Exel Safelet IV catheter is now ready to use.
Mice can be placed under a heat lamp (a) or on a glove filled with warm water (b) to recover following anesthesia in the biosafety hood.
CAUTION All experiments should be done in accordance with protocols approved by the Institutional Animal Care and Use Committee.
CAUTION All experiments should be done in accordance with protocols approved by the Institutional Animal Care and Use Committee.