Summary
Because microtubules perform many essential functions in neurons, delineating unique roles attributable to these organelles presents a formidable challenge. Microtubules endow neurons with shape and structure and are required for developmental processes including neurite outgrowth [1], intracellular transport [2], and synaptic formation and plasticity [3, 4]; microtubules in sensory neurons may be required for the above processes in addition to a specific sensory function. In Caenorhabditis elegans six touch receptor neurons (TRNs) sense gentle touch [5] and uniquely contain 15-protofilament microtubules [6]. Disruption of these microtubules by loss of either the MEC-7 β-tubulin [7] or MEC-12 a-tubulin [8] or by growth in 1 mM colchicine causes touch insensitivity [5, 6], altered distribution of the touch transduction channel, and a general reduction in protein levels. We show that the effect on touch sensitivity can be separated from the others; microtubule depolymerization in mature TRNs causes touch insensitivity but does not result in protein distribution and production defects. In addition, the mec-12(e1605) mutation selectively causes touch insensitivity without affecting microtubule formation and other cellular processes. Touching e1605 animals produces a reduced mechanoreceptor current that inactivates more rapidly than in wild type, suggesting a specific role of the microtubules in mechanotransduction.
Results and Discussion
Microtubules and Protein Localization
Mechanoreceptor channel complexes tranduce mechanical stimuli in the six TRNs (ALML/R, PLML/R, and AVM/PVM) [9]. Normally, these complexes are distributed in regular puncta along the TRN axons (Figure 1A and [10–12]). Previous work found that mec-7 and mec-12 mutations affect puncta distribution but not formation [13, 14].
Figure 1. TRN microtubules are required for intracellular transport.
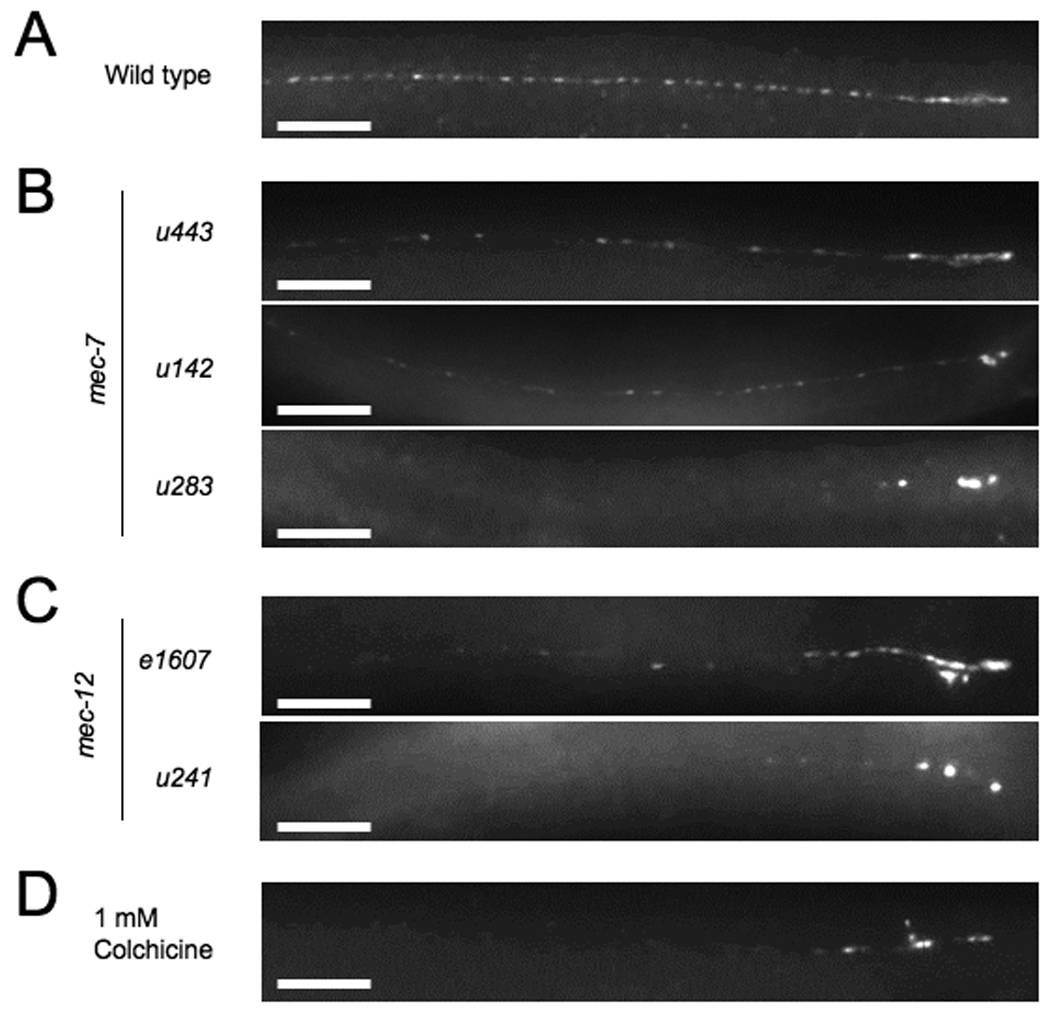
MEC-2 puncta distribution in the process of the ALM TRN in (A) wild type, (B) three mec-7 mutants, (C) two mec-12 mutants, and (D) an animal grown in 1 mM colchicine for several generations. Scale bars = 20 µm.
We reexamined the role of mec-7 and mec-12 on the localization of channel complex puncta using several more mutations in these genes and an antibody to the auxillary channel protein MEC-2 (Table S1). Recessive null mutations of mec-7(u142, u440, and u443), disrupt the distribution of MEC-2 punctata varying degrees. In some animals MEC-2 puncta appeared wild type, whereas in others the puncta were weaker and more dispersed, particularly in the middle of the neuronal process (Figure 1B). In contrast, dominant mec-7 mutations had a greater effect on the distribution of MEC-2 puncta. Specifically, mec-7(u18) and mec-7(u283) animals had MEC-2 puncta restricted to the most proximal part of the process (Figure 1B). These data suggest that wild-type MEC-7 has a modest role in the distribution of the puncta. Specific missense mutations in mec-7, however, can disrupt this distribution, perhaps by affecting the function of all TRN microtubules.
mec-12 mutations also affected MEC-2 distribution. Unlike mec-7, no early truncation or nonsense alleles are known for mec-12; all known alleles are missense mutations [8, 14]. One probable null allele is mec-12(e1607), a recessive missense mutation that eliminates the 15-protofilament microtubules in the TRNs [15]. MEC-2 distribution in e1607 animals is similar to that in mec-7 null mutants. In contrast, mec-12(u241), a dominant mutation that eliminates the large-diameter microtubules, results in a more severe phenotype similar to dominant mec-7 mutations (Figure 1C).
In summary, absence of the large-diameter microtubules due to loss of mec-7 or mec-12 results in a weak MEC-2 distribution phenotype. The nonresponsive TRNs in these animals, however, still develop and extend processes containing the 11-protofilament microtubules seen in other C. elegans cells [6]. The weak defect may be due to the ability of the 11-protofilament microtubules to allow transport to a modest degree. In contrast, dominant alleles of these genes produce more severe distribution phenotypes. These dominant mutations appear to cause the disruption of both types of microtubules, as evidenced by immunostaining with 6-11B-1, an antibody against acetylated ∝-tubulin, a marker for stable microtubules [16] (Supplemental Results and Figure S1). These results argue that a more complete loss of microtubules greatly restricts MEC-2 puncta distribution.
To examine the effects of total microtubule loss, we grew animals on 1 mM colchicine, which produces touch insensitivity by selectively depolymerizing all microtubules in the TRNs [6]. (Because the TRNs form in the embryo and colchicine does not appear to permeate the eggshell, the cells in treated animals have neuronal processes.) MEC-2 antibody staining is restricted to the most proximal parts of the TRN axon in adults that have been grown on colchicine from hatching (Figure 1D). This result supports the idea that all TRN microtubules must be eliminated or compromised for the severe distribution defects.
Microtubules and Touch Sensitivity
Elsewhere (Bounoutas et al., in preparation) we will describe the finding that loss of TRN microtubules also results in a reduction in overall protein levels; both TRN-specific and non-specific protein levels decrease in TRNs under these conditions. This reduction in protein levels and/or the disruption of protein localization described above may contribute to the resulting touch insensitivity caused by mutation of mec-7 and mec-12 or treatment with colchicine. A more direct role in mechanosensation for the TRN microtubules, however, is suggested by our finding of conditions under which defective microtubules cause touch insensitivity without demonstrably affecting these other activities.
An independent effect on mechanosensation can be seen when microtubules are depolymerized in adults, i.e., after their roles in transport and protein expression have presumably been fulfilled. Previous attempts to disrupt microtubules in adult TRNs failed because neither temperature shifts of temperature-sensitive alleles nor colchicine treatment affected adults, the latter presumably because the adult cuticle prevents absorption of the drug [6]. We have overcome these difficulties by using bus-17 animals, which lack a glycosyltransferase needed for cuticle integrity and are more permeable to drugs [17, 18].
Young bus-17 adults become touch insensitive when placed on 1 mM colchicine (Figure 2A). Adults treated for 24 hrs were partially touch insensitive, responding to 7.3 ± 0.3 out of 10 touches, compared to 9.1 ± 0.2 for untreated animals at the same age (mean ± S.E.M; n=20 for all conditions). Adults treated for 48 hrs were much more touch insensitive, responding to 3.3 ± 0.4 touches; untreated animals responded to 9.2+ 0.2. Microtubules were disrupted in the treated adults, since immunostaining against acetylated a-tubulin was reduced and fragmented (Figure 2B). In contrast, the intensity and distribution of MEC-2 puncta were unaffected by this late colchicine treatment (Figure 2B). The intensity of expression of the TRN-specific proteins MEC-18 and MEC-17::GFP (G. Gu, S. Zhang, and M. Chalfie, unpublished data and [19]) was unaffected in colchicine-treated bus-17 adults (Figure 2C and Figure S2). In some animals the expression of MEC-18 was not continuous (Figure 2C). We do not know what the breaks in the staining indicate, since the MEC-17∷GFP fluorescence showed that the processes were intact. Thus, late colchicine treatment produces touch insensitivity without a major impact on protein transport or expression, suggesting that the microtubules have a separable role in mechanosensation in these cells. An alternative hypothesis is that late colchicine treatment interferes with the expression or transport of a specific protein or proteins needed for touch sensitivity in adults, but these changes are obscured by earlier expression and transport.
Figure 2. Depolymerization of microtubules in adults causes touch insensitivity but does not affect protein localization or accumulation.
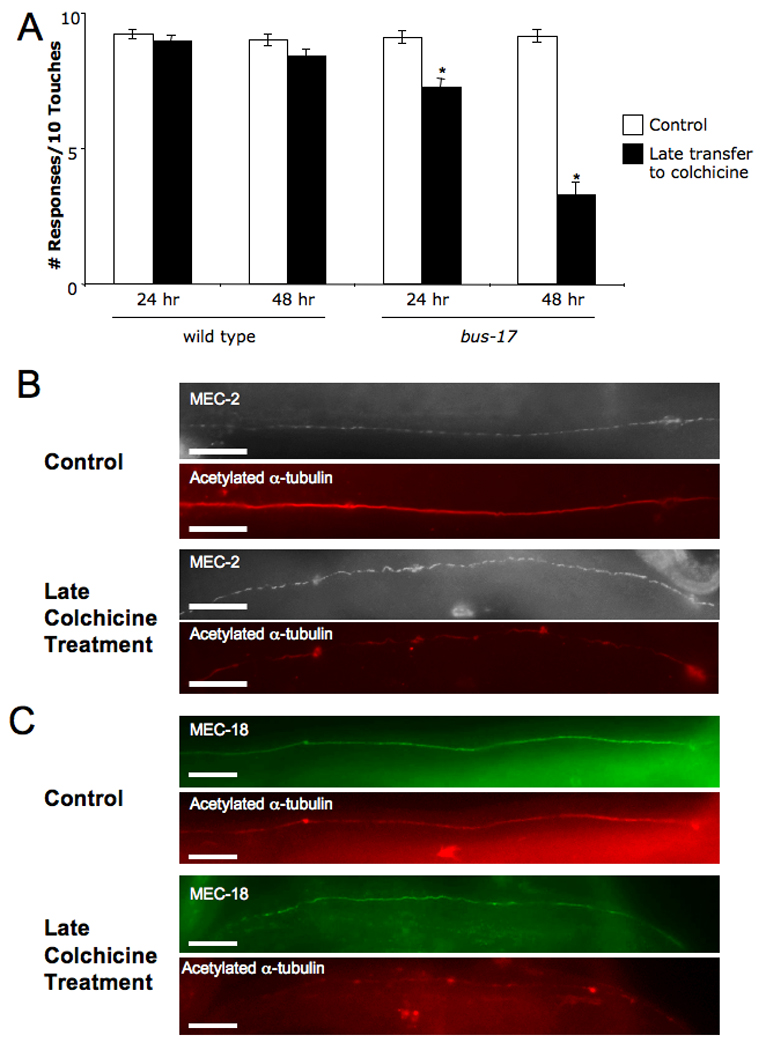
(A) Late colchicine treatment (black bars) reduces touch sensitivity of bus-17 adults compared to untreated animals (white bars). The mean ± S.E.M. is indicated; n=20. *Difference from wild type is statistically significant, p < 0.05. (B) Co-immunostaining against MEC-2 and anti-acetylated a-tubulin in ALM neurons 48 hrs after adults were transferred to colchicine-containing or control plates. (C) Co-immunostaining against MEC-18 and anti-acetylated a-tubulin in ALM neurons under same conditions. Scale bars = 20 µm.
We consider this latter hypothesis less likely given the touch-insensitive phenotype of mec-12’(e1 605) animals, e1 605 is a recessive, missense allele that produces touch insensitivity without disrupting the 15-protofilament microtubules in the TRNs [15]. mec-12(e1605) mutants immunostained normally for acetylated ∝-tubulin (Figure 3A), indicating that the microtubules retain wild-type stability. Unlike most mec-12 mutations, the e1605 allele did not disrupt MEC-2 distribution (Figure 3B and Figure S3). Another mec-12 mutation, u50, which has the identical molecular defect as mec-12 (e1 605) (see Experimental Procedures) and similarly did not disrupt the large-diameter microtubules [15], also failed to affect MEC-2 distribution (unpublished data). These mutations were the only mec-7 or mec-12 alleles tested that did not affect MEC-2 distribution. In addition, mec-12 (e1 605) animals had no demonstrable defects in TRN protein expression (Figure 3C and unpublished data). Since the e1605 animals are completely touch-insensitive, these mutations provide additional evidence separating the mechanosensory role of the microtubules from their functions in protein transport and production.
Figure 3. The mec-12(e1605) mutation does not affect microtubules formation, protein distribution, or protein levels.
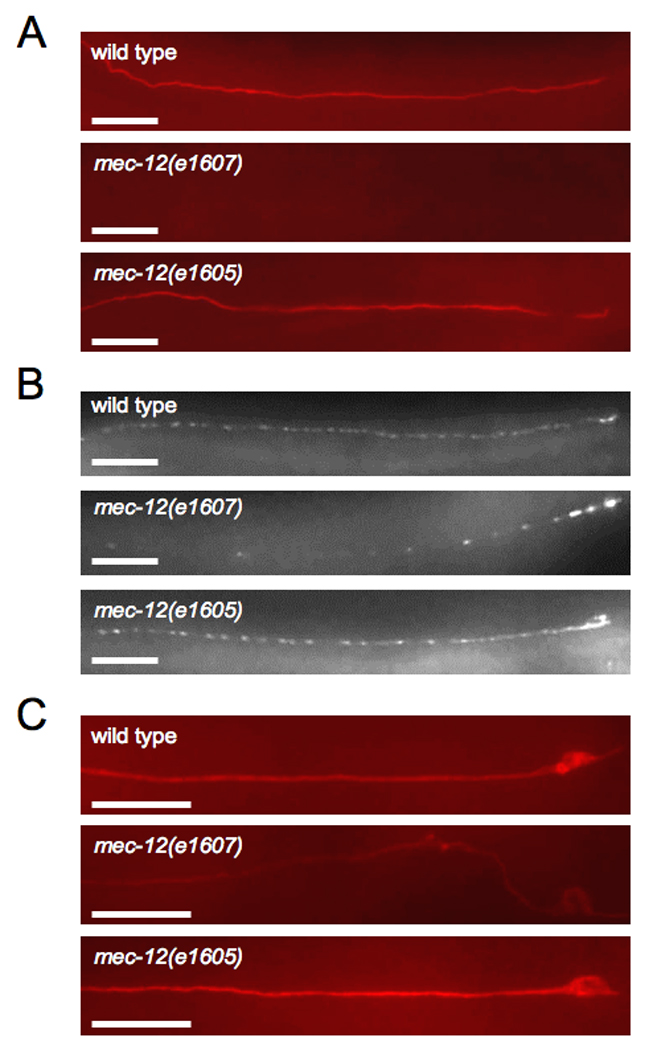
Immunostaining against (A) acetylated α-tubulin, (B) MEC-2, and (C) MEC-18 in ALM neurons of wild type, mec-12 (e1607), and mec-12(e1605) adult animals. Scale bars = 20 µm.
Electrophysiology of Tubulin Mutants
Touching the TRNs produces a very rapid mechanoreceptor current (MRC) that is reduced but not abolished in animals with the mec-7(u142) null mutation [9]. The presence of an MRC in these mutants indicates that 15-protofilament microtubules are not essential for channel gating.
To better characterize the mechanosensory phenotype produced by the e1 605 mutation, we recorded MRCs in TRNs from e1 605 and mec-12 (e1607) mutants and compared them to those in wild type and mec-7(u142) animals ([9] and Figure 4A). The average peak MRC amplitude in mec-12 (e1 605) animals was approximately one-fourth the size of MRCs in control animals (Figure 4B), indicating that wild-type microtubules are essential for optimal transduction. These MRCs, however, were significantly larger than those observed in mec-7 and mec-12 loss-of-function mutants (Figure 4B). Moreover, the pressure versus current relationships in wild type and mec-12 (e1 605) animals were essentially identical, whereas in mec-7(u142) animals more pressure was required to produce maximal responses (Figure 4C). Since the mec-7 and mec-12 loss-of-function mutations produce defects in protein localization and production (Figure 1 and Figure 3 and unpublished data), disrupting these processes likely contributes to the more severe phenotype in these animals.
Figure 4. Mutations in tubulin subunits reduce mechanoreceptor currents (MRC).
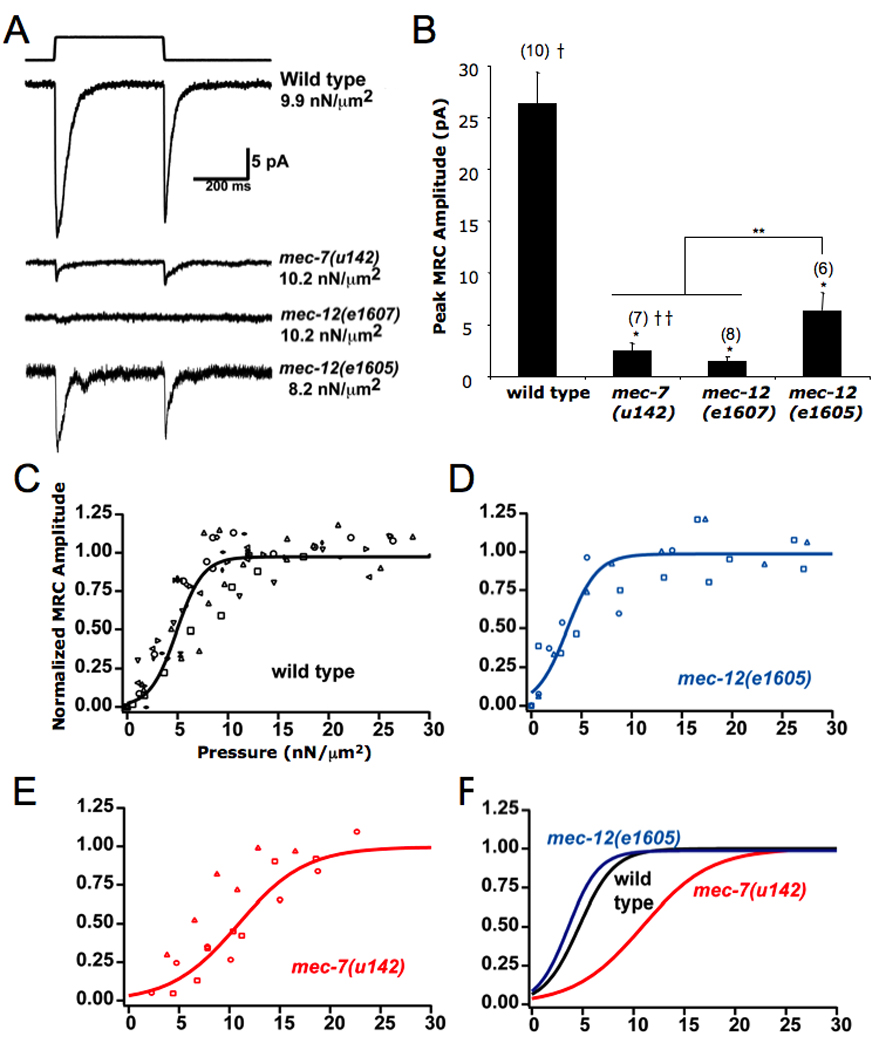
(A) Representative MRC traces from stimulated TRNs in wild type and tubulin mutant backgrounds. (B) Comparison of peak MRC amplitudes. The mean ± S.E.M is indicated; the number of animals tested is given in parenthesis above bar. *Difference from wild type is statistically significant, p < 0.05. **Significant difference between putative null alleles and mec-12(e1605), p<0.05. † Average of data from uls30 control animals (this study) and uIs31 control animals [9].†† ata from [9]. (C) – (E) Boltzman curves were fit to normalized maximum current responses as a function of stimulus pressure for wild-type and mutant PLM neurons. Data from different cells are depicted with different symbols. (C) Wild type; new data as well as data from [9] were included. Best fit parameters were P½ = 4.6 ± 0.2 nN/µm2, PsIope = 1.7 ± 0.08 nN/µm, n = 13. (D) mec-12(e1605); best fit parameters were P½= 3.6 ± 0.5 nN/fim, PsIope = 1.5 ± 0.4 nN/µm, n = 3. (E) mec-7(u142) from [9]; best fit parameters were P½ = 10.9 ± 0.8 nN/µm2, PsIope = 3.3 ± 0.8 nN/µm2, n = 3. (F) The curves are plotted together for comparison, mec-7(u142) is significantly different (p < 0.001) from both wild type and mec-12(e1605) using the F-test.0
Additionally, the time constant of adaptation (τ2)of MRCs was significantly shorter in e1605 TRNs (Table S2). A similar shortening was seen in the mec-7 null animals (Table S2 and [9]). (Small response amplitudes precluded accurate determination of the kinetics of MRCs in mec-12(el607) animals. We could not test adaptation in colchicine-treated bus-17 animals because we have been unable to record from TRNs in adults.) These results suggest that the microtubules are required to slow adaptation.
The Role of Microtubules in Mechanosensation
Our data indicate that the 15-protofilament microtubules have a separable, specific role in mechanosensation. These microtubules were once hypothesized to function as intracellular tethers to the mechanosensitive channels, but current evidence suggests that their contribution to mechanotransduction is more indirect [9, 11]. Because the large-diameter microtubules form interconnected bundles that fill the axonal processes and interact with the plasma membrane [11, 20], we believe they have a structural role. The microtubules might make the axon more rigid, allowing the membrane to respond more to touch and possibly conveying force over a much larger section of the process [5, 11]. However, the finding that the mec-12(e1605) animals are touch insensitive, yet have otherwise functional microtubules that form bundles, argues against the mere physical presence of the microtubules being essential for touch sensitivity.
We have suggested another hypothesis [21] based on the idea that adaptation results from changes in the shape of the plasma membrane after a touch stimulus. In this model, based in part on ideas suggested by Kung [22], deformation of the membrane caused by touch changes the forces in the bilayer on the touch channel complex, causing it to open. Adaptation results from the equilibration of the membrane after a touch has been administered. We envision that the microtubules, perhaps, through the many attachments they appear to make to the plasma membrane [11, 20], retard this equilibration, thus allowing the channels to be open longer. Removal or uncoupling of the microtubules would lead to faster adaptation (as seen in Table S2), which would result in smaller MRCs.
This faster adaptation (calculated as described in Supplemental Results), however, accounts for only 12–14% of the 75% reduction in peak MRCs in mec-12(e1605) cells (Figure 4B). Although e1 605 mutants have normal numbers of channel puncta (Figure S3), further reduction of MRC amplitude may be due to fewer channels that can be activated or inserted in the plasma membrane. This interpretation is consistent with the similarity of the normalized current versus pressure relationship in mec-12(e1605) and in wild type (Figure 4C). We attempted to determine the number of active channels in these mutants using noise analysis [9], but the amplitude of the responses was not sufficient compared to the background noise to make estimates of the unitary current or number of active channels.
Presumably the mec-12(e1605) mutation causes touch insensitivity by preventing an appropriate interaction between the microtubules and another protein. The mutation produces an H192Y substitution. Since MEC-12 is 93% identical to pig brain α-tubulin [23], we used the position of the histidine residue in the structure of dimeric pig brain tubulin [24] (Figure S4) to suggest possible consequences of the e1 605 mutation. H192 is located near the exterior of the α-tubulin and likely forms hydrogen bonds with residues in and directly preceding helix 12. A tyrosine substitution could alter the normal conformation of this helix, which has been hypothesized to interact with microtubule-associated proteins (MAPs; [25]). Such an interaction may be necessary for full touch sensation. Two MAPs have been implicated in touch sensitivity: the tau-like protein PTL-1 [26] and the EMAP protein ELP-1 [27]. The e1 605 mutation could disrupt binding of these or other MAPs, and such disruption may disassociate the large-diameter microtubules from the plasma membrane.
Our experiments suggest that the 15-protofilament microtubules of the C. elegans TRNs impact touch sensitivity in at least four ways. First, they are needed for the expression or abundance of components of the transduction machinery. Second, they are required for the transport of at least some mechanosensory channel subunits in the TRN processes. Third, the microtubules slow the adaptation rate of the transduction channel, producing a larger current. Finally, wild-type microtubules are needed for full activation of the transduction channel, perhaps via indirect interactions, or by attachments to the plasma membrane.
Experimental Procedures
Generation, Growth, and Maintenance of Nematode Strains
C. elegans strains were cultured at 20°C as previously described [28]. Isolation and initial characterization of mec-7 and mec-12 mutants have been described previously [5, 7, 8, 15, 29]. Defects in previously unreported mec-12 allele are found in the Supplemental Experimental Procedures. All the protein-coding sequences involved in this work were verified by PCR-based sequencing [14] at GeneWiz, Inc., North Brunswick, NJ. bus-17(e2800) animals [16] were a gift from Jonathan Hodgkin.
Colchicine Treatment
The effects of colchicine on C. elegans touch sensitivity were tested by growing animals for multiple generations on standard NMG agar plates [28] containing 1 mM colchicine [6]. To assess the effects of colchicine on microtubules in mature TRNs, we placed young adult wild-type and bus-17 animals on colchicine plates and observed them for several days.
Immunochemistry
Whole-mount immunochemistry was carried out as described previously [29]. Description of antibodies and dilutions used are included in the Supplemental Experimental Procedures.
Microscopy
For fluorescence microscopy, animals were anesthetized using 0.3M 2–3 butanedione monoxime in 10 mM HEPES (M. Goodman and M. Chalfie, unpub. data) and observed using a Zeiss Axiophot microscope. Images For Figure 1, Figure 2, Figure 3A–B, Figure S1 and S2 were taken with a Plan NEOFLUAR 40x objective; images for Figure 3C were taken with Plan-APOCHROMAT 63x objective. All images were taken with a Diagnostic Instruments Spot 2 camera at the same settings. To make images clearer for publication, all images in a set were treated equally to enhance contrast and brightness. Images for reported observations were made on at least 20 animals per mutation or condition.
Touch Sensitivity
Touch sensitivity of worms was tested by stroking the animals with an eyebrow hair attached to a toothpick [5]. Wild-type animals respond to touches to the anterior body by moving backwards and to posterior touches by accelerating forward. Each animal was tested 10 times by alternately touching the anterior and posterior; each strain and/or condition was tested blindly.
In vivo Electrical Recordings
Electrophysiological recordings from stimulated PLM TRNs were generated and analyzed as described previously [9]. Composition of solutions are included in the Supplemental Experimental Procedures. Previously published MRC data from wild type and mec-7(u142) animals expressing uIs31 as a cell marker [9] are included in this paper. Recordings from mec-12 alleles were performed using uIs30 as a marker [u1s30, like uIs31, contains an integrated mec-17::gfp transgene. The two alleles were generated simultaneously as previously described [9], differing only in location of chromosomal integration]. New MRCs from wild type animals expressing uIs30 were recorded and averaged with those of wild type animals expressing uIs31 (inclusive of data from [9]); all features of the electrophysiological recordings in the two strains were indistinguishable from one another. Statistical significance between peak MRC amplitudes and time course measurements was determined using a Student’s t-test. Two data sets were compared at a time, with each set treated as independent from one another and with unequal variance; P values < 0.05 were deemed statistically significant. Statistical significance between pressure sensitivity curves was determined using an F test.
Supplementary Material
Acknowledgements
We thank Shifang Zhang for the MEC-18 antibody, David Hall for electron microscopy of mec-7 mutants, and Michael Rudolph and Gabriele Amodeo for assistance with crystallography modeling programs. This research was supported by National Institute of Health grant GM30997 to MC and an HHMI Predoctoral Fellowship to RO.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
Online Supplemental Material
Supplemental Results and Discussion details general disruption of microtubules in dominant mutants and calculates the reduction of MRC amplitude attributable to faster adaptation in mec-12(e1605) mutants. Table S1 contains the genetic and molecular data of the mec-7 and mec-12 alleles used in this study. Figure S1 shows the extent of tubulin acetylation in wild type and mutant touch receptor neurons. Figure S2 demonstrates that levels of MEC-17::GFP are unaffected by late-colchicine treatment. Figure S3 compares the number of puncta in TRN processes between wild type animals and mec-12 mutants. Table S2 shows that wild-type microtubules are necessary for normal adaptation of the mechanoreceptor current (MRC). Figure S4 depicts the location of mec-12 (e1 605) amino acid substitution on the crystal structure of the pig brain α-tubulin β-tubulin dimer. Additional description of methods is included in Supplemental Experimental Procedures.
References
- 1.Gordon-Weeks PR. Microtubules and growth cone function. J. Neurobiol. 2004;58:70–83. doi: 10.1002/neu.10266. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein LS, Yang Z. Microtubule-based transport systems in neurons: the roles of kinesins and dyneins. Annu. Rev. Neurosci. 2000;23:39–71. doi: 10.1146/annurev.neuro.23.1.39. [DOI] [PubMed] [Google Scholar]
- 3.Chang Q, Balice-Gordon RJ. highwire, rpm-1, and futsch: balancing synaptic growth and stability. Neuron. 2000;26:287–290. doi: 10.1016/s0896-6273(00)81161-7. [DOI] [PubMed] [Google Scholar]
- 4.Bianchi M, Hagan JJ, Heidbreder CA. Neuronal plasticity, stress and depression: involvement of the cytoskeletal microtubular system? Curr. Drug Targets CNS Neurol. Disord. 2005;4:597–611. doi: 10.2174/156800705774322012. [DOI] [PubMed] [Google Scholar]
- 5.Chalfie M, Sulston J. Developmental genetics of the mechanosensory neurons of Caenorhabditis elegans. Dev. Biol. 1981;82:358–370. doi: 10.1016/0012-1606(81)90459-0. [DOI] [PubMed] [Google Scholar]
- 6.Chalfie M, Thomson JN. Structural and functional diversity in the neuronal microtubules of Caenorhabditis elegans. J. Cell. Biol. 1982;93:15–23. doi: 10.1083/jcb.93.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savage C, Hamelin M, Culotti JG, Coulson A, Albertson DG, Chalfie M. mec-7 is a beta-tubulin gene required for the production of 15-protofilament microtubules in Caenorhabditis elegans. Genes. Dev. 1989;3:870–881. doi: 10.1101/gad.3.6.870. [DOI] [PubMed] [Google Scholar]
- 8.Fukushige T, Siddiqui ZK, Chou M, Culotti JG, Gogonea CB, Siddiqui SS, Hamelin M. MEC-12, an alpha-tubulin required for touch sensitivity in C elegans. J. Cell. Sci. 1999;112(Pt 3):395–403. doi: 10.1242/jcs.112.3.395. [DOI] [PubMed] [Google Scholar]
- 9.O’Hagan R, Chalfie M, Goodman MB. The MEC-4 DEG/ENaC channel of Caenorhabditis elegans touch receptor neurons transduces mechanical signals. Nat. Neurosci. 2005;8:43–50. doi: 10.1038/nn1362. [DOI] [PubMed] [Google Scholar]
- 10.Chelur DS, Ernstrom GG, Goodman MB, Yao CA, Chen L, R OH, Chalfie M. The mechanosensory protein MEC-6 is a subunit of the C. elegans touch-cell degenerin channel. Nature. 2002;420:669–673. doi: 10.1038/nature01205. [DOI] [PubMed] [Google Scholar]
- 11.Cueva JG, Mulholland A, Goodman MB. Nanoscale organization of the MEC-4 DEG/ENaC sensory mechanotransduction channel in Caenorhabditis elegans touch receptor neurons. J. Neurosci. 2007;27:14089–14098. doi: 10.1523/JNEUROSCI.4179-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang S, Arnadottir J, Keller C, Caldwell GA, Yao CA, Chalfie M. MEC-2 is recruited to the putative mechanosensory complex in C. elegans touch receptor neurons through its stomatin-like domain. Curr. Biol. 2004;14:1888–1896. doi: 10.1016/j.cub.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 13.Emtage L, Gu G, Hartwieg E, Chalfie M. Extracellular proteins organize the mechanosensory channel complex in C. elegans touch receptor neurons. Neuron. 2004;44:795–807. doi: 10.1016/j.neuron.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Huang M, Gu G, Ferguson EX, Chalfie M. A stomatin-like protein necessary for mechanosensation in C. elegans. Nature. 1995;378:292–295. doi: 10.1038/378292a0. [DOI] [PubMed] [Google Scholar]
- 15.Chalfie M, Au M. Genetic control of differentiation of the Caenorhabditis elegans touch receptor neurons. Science. 1989;243:1027–1033. doi: 10.1126/science.2646709. [DOI] [PubMed] [Google Scholar]
- 16.Hammond JW, Cai D, Verhey KJ. Tubulin modifications and their cellular functions. Curr. Opin. Cell Biol. 2008;20:71–76. doi: 10.1016/j.ceb.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gravato-Nobre MJ, Nicholas HR, Nijland R, O’Rourke D, Whittington DE, Yook KJ, Hodgkin J. Multiple genes affect sensitivity of Caenorhabditis elegans to the bacterial pathogen Microbacterium nematophilum. Genetics. 2005;171:1033–1045. doi: 10.1534/genetics.105.045716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yook K, Hodgkin J. Mos1 mutagenesis reveals a diversity of mechanisms affecting response of Caenorhabditis elegans to the bacterial pathogen Microbacterium nematophilum. Genetics. 2007;175:681–697. doi: 10.1534/genetics.106.060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang S, Ma C, Chalfie M. Combinatorial marking of cells and organelles with reconstituted fluorescent proteins. Cell. 2004;119(1):137–144. doi: 10.1016/j.cell.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 20.Chalfie M, Thomson JN. Organization of neuronal microtubules in the nematode Caenorhabditis elegans. J. Cell. Biol. 1979;82:278–289. doi: 10.1083/jcb.82.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bounoutas A, Chalfie M. Touch sensitivity in Caenorhabditis elegans. Pflugers Arch. 2007;454:691–702. doi: 10.1007/s00424-006-0187-x. [DOI] [PubMed] [Google Scholar]
- 22.Kung C. A possible unifying principle for mechanosensation. Nature. 2005;436:647–654. doi: 10.1038/nature03896. [DOI] [PubMed] [Google Scholar]
- 23.Gogonea CB, Gogonea V, Ali YM, Merz KM, Jr, Siddiqui SS. Computational prediction of the three-dimensional structures for the Caenorhabditis elegans tubulin family. J Mol Graph Model. 1999;17(2):90–100. doi: 10.1016/s1093-3263(99)00025-x. [DOI] [PubMed] [Google Scholar]
- 24.Löwe J, Li H, Downing KH, Nogales E. Refined structure of alpha beta-tubulin at 3.5 A resolution. J. Mol. Biol. 2001;313(5):1045–1057. doi: 10.1006/jmbi.2001.5077. [DOI] [PubMed] [Google Scholar]
- 25.Nogales E, Wolf SG, Downing KH. Structure of the alpha beta tubulin dimer by electron crystallography. Nature. 1998;391(6663):199–203. doi: 10.1038/34465. [DOI] [PubMed] [Google Scholar]
- 26.Gordon P, Hingula L, Krasny ML, Swienckowski JL, Pokrywka NJ, Raley-Susman KM. The invertebrate microtubule-associated protein PTL-1 functions in mechanosensation and development in Caenorhabditis elegans. Dev. Genes Evol. 2008;218(10):541–551. doi: 10.1007/s00427-008-0250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hueston JL, Herren GP, Cueva JG, Buechner M, Lundquist EA, Goodman MB, Suprenant KA. The C. elegans EMAP-like protein, ELP-1 is required for touch sensation and associates with microtubules and adhesion complexes. BMC Dev. Biol. 2008;5:110. doi: 10.1186/1471-213X-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Savage C, Xue Y, Mitani S, Hall D, Zakhary R, Chalfie M. Mutations in the Caenorhabditis elegans beta-tubulin gene mec-7: effects on microtubule assembly and stability and on tubulin autoregulation. J. Cell. Sci. 1994;107(Pt 8):2165–2175. doi: 10.1242/jcs.107.8.2165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


