Abstract
Background
Resistance to apoptosis is one of the key events that confer chemo-resistance and is mediated by over-expression of anti-apoptotic proteins, which inhibit caspase activation. The objective of this study was to evaluate whether the activation of an alternative cell death pathway (caspase-independent) could promote death in chemo-resistant ovarian cancer cells. We report the characterization of NV-128 as an inducer of cell death through a caspase-independent pathway.
Methods
Primary cultures of epithelial ovarian cancer cells (EOC) were treated with increasing concentration of NV-128 and GI50 was determined using Cell titer 96 assay. Apoptotic proteins were characterized by western blot analyses, Caspase Glo assays, immunohistochemistry, and flow cytometry. Protein-protein interactions were determined using immunoprecipitation. In vivo activity was measured in a xenograft mice model.
Results
NV-128 is able to induce significant cell death in both Paclitaxel- and Carboplatin- resistant EOC cells with GI50 between 1 and 5 ug/ml. Cell death was characterized by chromatin condensation but was caspase-independent. The activated pathway involved the down-regulation of phospho-AKT, phospho- mTOR, and phospho-S6 kinase, and the mitochondrial translocation of Beclin-1 followed by nuclear translocation of EndoG.
Conclusion
We have characterized a novel compound, which inhibits mTOR and promotes caspase-independent cell death. Our results indicate that inhibition of mTOR may represent a relevant pathway for induction of cell death in cells resistant to the classical caspase-dependent apoptosis. These findings demonstrate the possibility of using therapeutic drugs, such as NV-128, which could have beneficial effects in chemo-resistant ovarian cancer patients.
Keywords: ovarian cancer, chemo-resistance, caspase-independent apoptosis, mTOR
Introduction
Epithelial ovarian cancer (EOC) is the fourth leading cause of cancer related deaths in women in the U.S. and is the most lethal of the gynecologic malignancies, with 5-year survival approaching only 15% (1). A major challenge in the management of ovarian cancer is the development of chemo-resistance to most of the currently available agents. Consequently, there is an urgent need for better therapeutic options.
Although chemo-resistance is multifactor, it is well accepted that resistance to programmed cell death (PCD) is a major contributing cause (2). PCD is an evolutionary conserved pathway, the activation of which leads to an energy-dependent cell suicide mechanism. In general, two forms of PCD have been described, apoptosis or caspase-dependent cell death, and caspase-independent cell death (3).
Apoptosis is the better-characterized pathway of the two types of PCD such that the terms PCD and apoptosis have been used interchangeably throughout the literature. It involves the sequential activation of a group of proteases, the caspases, and can be activated either by ligation of death receptors like Fas and TNFR (extrinsic pathway), or by mitochondrial depolarization (intrinsic pathway) (4, 5). The Bcl2 family of proteins, which has both pro-apoptotic (Bak, Bax, etc.) and anti-apoptotic members (Bcl2, Bclx), controls mitochondrial integrity and the decision to engage the intrinsic pathway depends on the ratio of the pro- and anti-apoptotic members in the outer mitochondrial membrane (5). Numerous studies have shown that most chemotherapy agents induce cell death by activating the apoptotic pathway and that resistance to apoptosis, due to high intracellular levels of anti-apoptotic blockers like XIAP, is a major cause of chemo-resistance. Indeed, molecular or drug targeting of apoptotic blockers like XIAP results in the reversal of chemo-resistance (6-8).
Caspase-independent cell death encompasses all events that occur when cells die in the absence of caspase activation. Autophagy is the most characterized caspase-independent PCD pathway and hence is often called Type II PCD. It involves the controlled formation of autophagosomes, a double-membrane cytoplasmic vesicle, which can fuse with lysosomes thus leading to the digestion of molecules within the autophagosome (9). Autophagy is controlled by the Akt-mammalian target of rapamycin (mTOR) pathway and involves key proteins like Beclin-1 and Class III PI3 kinase (10). It is a pathway activated to promote cell survival, but due to the inherent mechanisms invoked can also lead to cell death (11).
Cancer cells in general, and EOC cells in particular, are very resistant to apoptotic cell death due mainly to high level expression of the apoptotic blockers XIAP and FLIP (12). Thus, we hypothesize that in addition to targeting specific apoptotic blockers, another way to overcome chemo-resistance is to circumvent apoptosis and exploit non-apoptotic or caspase-independent cell death pathways.
The phosphatidylinositol-3 kinase (PI3K)/AkT/mTOR signaling axis plays a central role in the regulation of multiple critical cellular functions including stress responses, cell growth and survival, and metabolism. In many human tumors, including EOC, the PI3K/AKT/mTOR signaling is deregulated by a variety of oncogenic events. Therefore, modulation of this pathway may provide alternative therapies for ovarian cancer patients.
NV-128 is a member of the phenyl-substituted isoflavone family of compounds similar to phenoxodiol (12, 13), which in preliminary screening showed to be effective on reducing viability in a series of cancer cells; suggestive of a potential anti-tumor activity.
In the present study we investigate the molecular mechanisms by which NV-128 induces cell death. We describe in this report the activation by NV-128 of a caspase-independent cell death pathway in EOC cells. This pathway involves the inhibition of mTOR phosphorylation, mitochondrial translocation of the autophagy molecule Beclin-1, and mitochondrial release and nuclear localization of the nuclease EndoG.
Materials and methods
Cell lines and culture conditions
Established human EOC cell lines, A2780 and A2780/CP70 (gifts from Dr. TC Hamilton) (14) were propagated in RPMI plus 10% fetal bovine serum (Gemini Bio-Products, Woodland, CA). Primary EOC cell lines were isolated from malignant ovarian ascites or explanted from ovarian tumors and cultured as previously described (6, 15).
Cell viability assay
Cell viability was determined as previously reported (6, 15) using CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega Corporation, Madison, WI). NV-128 (Novogen, Inc., NSW, Australia) was added to the medium from 10mg/ml stock to give various final concentrations as described in the results section. Each experiment was done in triplicate.
For experiments using the caspase inhibitor, Z-VAD-FMK (Sigma Aldrich, St. Louis, MO), the inhibitor was added to the cultures 30 mins prior to treatment to yield final concentration of 20μM. For experiments using the autophagy inhibitor, 3-methyladenine (Sigma Aldrich), the inhibitor was added 1h prior to treatment to yield final concentration of 10 mM.
Flow cytometry with Hoechst and Propidium iodide staining
Cells were stained with 5 μg/ml Hoechst 33342 (Invitrogen-Molecular Probes, Carlsbad, CA) and 1 μg/ml Propidium iodide (Sigma Aldrich) as previously described (12). Data was acquired using BD LSR II System and analyzed using FloJo FACS analysis software (Tree Star, Inc., Ashland, OR).
Protein preparation and cellular fractionation
Protein was extracted and measured as previously described (6, 15). For separation of the cytoplasmic/mitochondrial fractions, and cytoplasmic/nuclear fractions, cell pellets were processed using ApoAlert™ Cell Fractionation Kit (BD Biosciences, San Jose, CA) and NE-PER Nuclear and Cytoplasmic Extraction (Pierce Biotechnology, Inc., Rockford, IL), respectively.
Caspase-3/7, -8, and -9 activity assay
Caspase activity was measured using Caspase-Glo™ 3/7, 8, or 9 reagents (Promega) as previously described (6).
SDS-PAGE and Western blots
SDS-PAGE and western blots were performed as previously described (12).
Analysis of phoshpo-proteins using xMAP technology
After treatment, cell were lysed and lysates were used to measure the phosphorylation status of 8 proteins using the Beadlyte® 8-plex Multi-Pathway Signaling kit (Millipore, Billerica, MA) according to manufacturer's instructions. Data was acquired using the Bioplex System (Biorad, Hercules, CA).
Assay of mitochondrial depolarization using JC-1
Cells were trypsinized and stained with JC-1 dye using the Mitocapture™ mitochondrial apoptosis detection kit (BioVision Research Products, Mountain View,CA) according to manufacturer's instruction. Data was acquired using BD LSR II System and analyzed using BD FACSDiva Software™.
Immunoprecipitation
Beclin-1 was immunoprecipitated from the mitochondrial fraction of cells treated for 1h with 10 μg/ml NV-128 using the Catch and Release® v2.0 Reversible Immunoprecipitation System (Millipore, Billerica, MA) and anti-rabbit Beclin-1 (Abcam, Cambridge, MA) according to manufacturer's instruction.
Mice xenograft studies
Cells (1×106 cells) were resuspended in 200 μl total volume of 50% RPMI and 50% BD Matrigel Matrix (BD Biosciences, Bedford, MA) and injected subcutaneously onto the right flank of NCR nude mouse. Therapy commenced eight days post-inoculation as follows: Paclitaxel, 10 mg/kg q×3d i.p., Carboplatin in water (Sigma Aldrich), 40 mg/kg q×7d, and NV-128 in 20% HPBCD, 100 mg/kg every day. Control groups received 20% HPBCD in PBS. Mice were treated and observed for 3 weeks. Tumor size was determined by caliper measurements and anti-tumor activity was analyzed with respect to maximal tumor inhibition (treated/control, T/C) as described previously (12).
Immunohistochemistry
Immunohistochemistry was performed as described previously (15) using rabbit anti-EndoG (Lifespan Biosciences, Seattle,WA) at 1:100 dilution.
Results
NV-128-induced cell death involves caspase-independent chromatin condensation
NV-128 was found to decrease cell viability in a range of cancer cells including the CP70 EOC cell line (Table 1). Our first objective was to determine the effect of NV-128 on a panel of primary cultures of EOC cells isolated from either ascites or tumor tissue. This panel includes cultures that are Paclitaxel- and Carboplatin-resistant (Fig. 1A-B) (15) and express high levels of the anti-apoptotic proteins XIAP and FLIP (12). Sensitive, as well as resistant cultures, showed a significant reduction in the percentage of viable cells after treatment with NV-128, with GI50 between 5 and 10 μg/ml (Fig. 1C).
Table 1.
Anti-proliferative activity of NV-128 against a range of cancer cell lines.
| Cancer Indication | Cell line | Analogue (GI50 μM) |
|---|---|---|
| Mean */ Std. Dev. | ||
| Lung (NSCLC) | NCI-H460 | 1.15 */ 1.41 |
| NCI-H838 | 1.99 */ 1.00 | |
| Gastric | MKN1 | 0.61 */ 1.03 |
| NCI-N87 | 2.32 */ 1.00 | |
| Breast | MDA-MB-468 | 1.69 */ 3.18 |
| SK-BR-3 | 0.59 */ 1.00 | |
| Liver | JHH-1 | 1.86 */ 1.00 |
| SK-HEP-1 | 0.98 */ 1.00 | |
| Leukaemia | CCRF-CEM | 1.34 */ 1.76 |
| Ovarian | CP70 | 1.28 */ 1.56 |
| Glioma | HTB-138 | 1.16 */ 1.00 |
| Melanoma | IgR3 | 0.42 */ 1.09 |
| MM200 | 0.87 */ 1.55 | |
| Prostate | LNCaP | 21.09 */ 1.00 |
| PC3 | 1.43 */ 1.69 | |
| Pancreatic | MIA PaCa-2 | 0.40 */ 1.21 |
| PANC-1 | 2.69 */ 1.39 |
Figure 1. NV-128 decreases viability of Paclitaxel- and Carboplatin-resistant EOC cells.

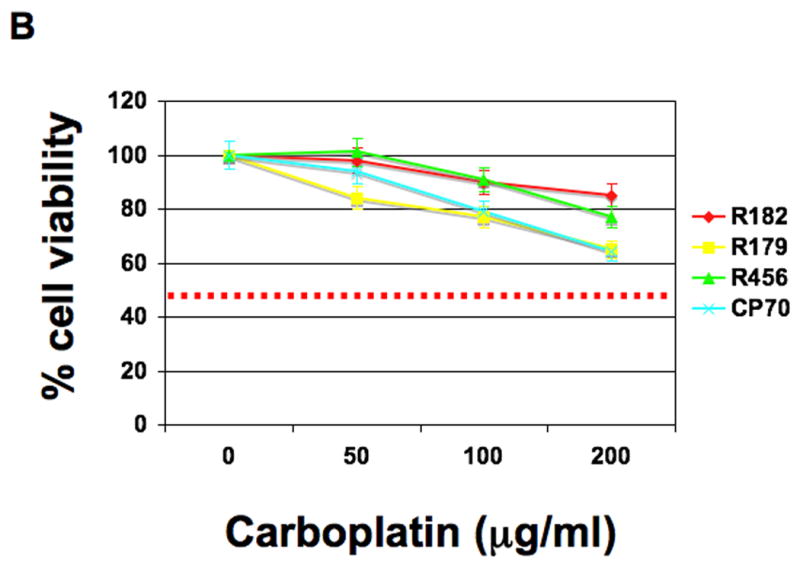
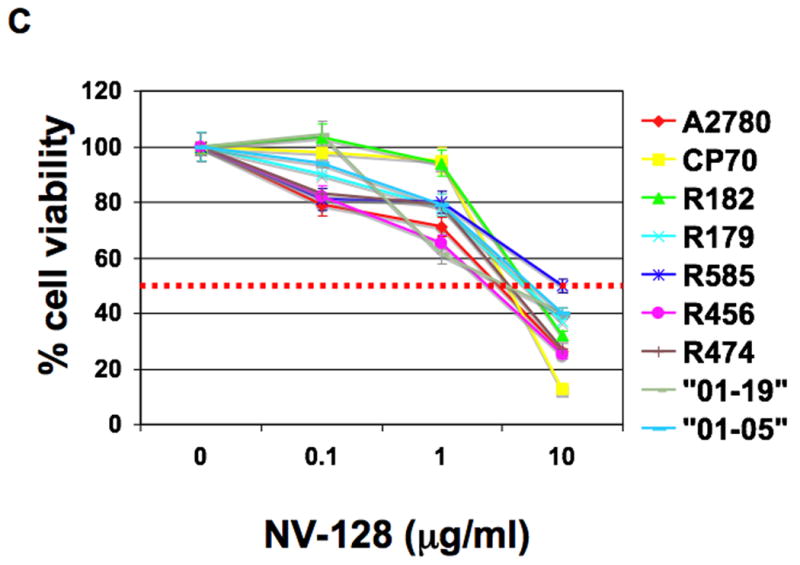
EOC cells were treated with increasing concentrations of (A) Paclitaxel, (B) Carboplatin, and (C) NV-128 for 24h and cell viability determined as described in the Materials and Methods section. Results shown are representative of three independent experiments. Note that GI50 was not reached for Paclitaxel and Carboplatin in lines that were considered resistant (dashed red line).
To determine if the decrease in cell viability was due to apoptosis, we measured the activity of caspases-3/7, -8, and -9 and determined the status of two anti-apoptotic molecules, XIAP and phosphorylated Akt (p-Akt). Interestingly, in contrast to the increase in caspase activity observed after treatment with the known apoptotic inducer, Paclitaxel, no changes in caspase-3/7, -8, and -9 activities were observed after treatment with NV-128 (Fig. 2A). In addition, no change in the status of XIAP was observed (Fig. 2B). However, a strong down-regulation of p-Akt was seen as early as 15 mins post-NV-128 treatment (Fig. 2C).
Figure 2. NV-128 does not induce caspase activation.



(A) Caspase activity was measured in cell lysates obtained from EOC cells treated for 24h with increasing concentrations of NV-128 or 2μM Paclitaxel. Results shown are for R179. Similar results were observed in other lines tested. (B,C) EOC cells were treated with 10 μg/ml NV-128 for the indicated time and whole cells lysates were analyzed by western blot for XIAP and phospho-Akt (p-Akt). β-actin and total Akt (t-Akt) blots demonstrate even loading. (D) No-treatment control cells, and cells treated with NV-128 (10 μg/ml) for 24h, were stained with Hoechst and PI and analyzed by flow cytometry. Results shown are for R182. Similar results were observed in other lines tested.
We then evaluated whether the decrease in cell viability was associated with changes in the DNA. Thus cells were stained with Hoecsht 33442 and Propidium iodide (PI) and analyzed by flow cytometry. Hoechst 33442 is a DNA binding dye that stains healthy DNA blue and fluoresce more intensely when bound to condensed chromatin. PI staining, on the other hand, is indicative of membrane permeabilization usually seen during the process of cell death. Flow cytometry analysis of NV-128 treated cells showed that cell death is accompanied by chromatin condensation. NV-128 induced a significant increase in double-positive cells with 95% of cells staining positively for both Hoechst and PI after 24h (Fig. 2D).
Chromatin condensation in the absence of caspase activation points to a caspase-independent pathway. To conclusively show that NV-128-induced cell death is caspase-independent, cells were treated with increasing concentrations of NV-128 in the presence or absence of the pan-caspase inhibitor Z-VAD-FMK. Inhibition of caspases had no impact on NV-128-induced cell death (Fig. 3). Taken together, these results suggest that NV-128-induced cell death proceeds via a caspase-independent-, but possibly p-Akt dependent-pathway culminating in chromatin condensation.
Figure 3. NV-128 induces caspase- and autophagy-independent cell death.
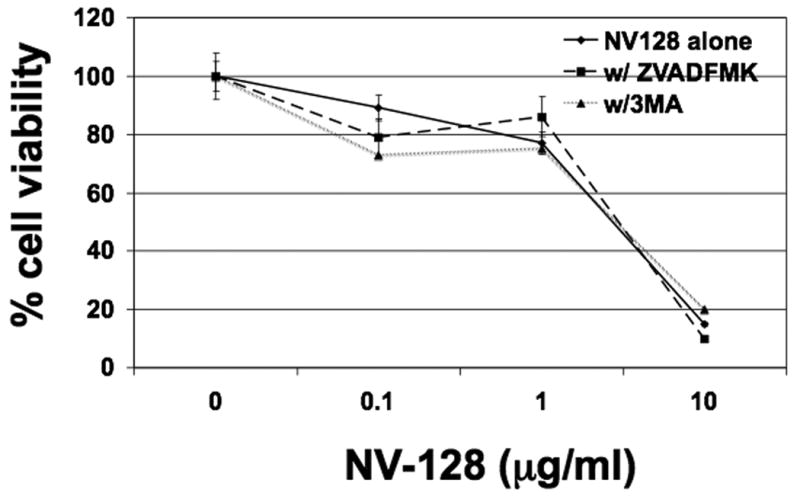
Cells were treated with increasing concentrations of NV-128 for 24h in the presence or absence of Z-VAD-FMK (20 μM) or 3-MA (10μM) and cell viability determined as described in the Materials and Methods section. Data shown are for R182, similar results were obtained with other lines analyzed.
NV-128 down-regulates the mTOR pathway
Our next objective was to identify and characterize the molecular pathways involved in NV-128-induced caspase-independent cell death. Thus, EOC cells were treated with NV-128 (10 μg/ml) and cell lysates were used to measure the levels of a panel of phospho-proteins as described in the Methods section. The only phospho-protein down regulated in the panel was phosphorylated-ribosomal p70 S6 kinase (p-S6k), suggesting that NV-128 might affect the mTOR pathway. NV-128 had minimal effect on pIκB, pSTAT3, and p38 and even induced up-regulation of pERK, pJNK, pSTAT5a/b, and pCREB (Fig. 4A), which probably represents a compensatory response.
Figure 4. NV-128 specifically down-regulates the mTOR pathway.



(A) Levels of 8 phospho-proteins were measured in the lysates as described in the Materials and Methods section. Data shown are for R182, similar results were obtained with other lines analyzed. (B,C) EOC cells were treated with 10 μg/ml NV-128 for the indicated time and lysates were analyzed by western blot for phospho-mTOR (p-mTOR), phospho- p70S6 kinase (p-S6k) and LC3-II.
To confirm the effect on the mTOR pathway, we evaluated the levels of p-S6k and phosphorylated mTOR (p-mTOR) using Western blot. As shown in figure 4B, p-mTOR and p-S6k were down-regulated 15 mins post-NV-128 treatment (Fig. 4B).
NV-128 induces autophagy
Morphologically, NV-128 treated EOC cells contained large intracellular vacuoles (Fig. 5B), which stained positively with acridine orange (data not shown) suggesting that NV-128 induces autophagic-like cell death. Autophagy is one of the best characterized caspase-independent form of cell death shown to be partly controlled by the Akt-mTOR pathway (16). To determine whether the process of autophagy is involved, we evaluated the autophagic marker LC3-II. Western blot analyses showed a significant increase in the level of LC3-II 8h-post NV-128 treatment (Fig. 4C) confirming the activation of the autophagic pathway.
Figure 5. NV-128 induces intracellular vacuole formation and mitochondrial depolarization.

Confocal microscope images of CP70 cells either unstimulated (A) or treated with 10 μg/ml NV-128 for 2h (B). Note the presence of intracellular vacuoles in B but not in A (red arrows). Fluorescent microscope images of cells stained with JC-1: unstimulated (C) or treated with 10 μg/ml NV-128 for 2h (D). Rounded arrow points to punctuate red staining in unstimulated cells; diamond-ending arrow points to a cell with some mitochondrial depolarization; and arrowhead points to a cell with bright green fluorescence suggesting most mitochondria have depolarized.
Autophagy is not required for NV-128-induced cell death
To determine if the process of autophagy is the primary mechanism of NV-128-induced cell death, cells were treated with NV-128 in the presence or absence of the well-characterized autophagy inhibitor, 3-methyladenine (3-MA), which inhibits the earliest step in the autophagosome formation (17). Cell viability studies employing 3-MA (10 μM) showed that the compound is not able to inhibit NV-128-induced cell death (Fig. 3). These data suggest that while some characteristics of autophagy were observed, it is not the primary mechanism of cell death induced by NV-128.
NV-128 induces mitochondrial depolarization
We next determined if NV-128-induced cell death involved the mitochondria since it contain nucleases that may be responsible for the observed chromatin condensation. Thus, control and treated cells were stained with JC-1 dye, a cationic fluorescent dye that stains intact mitochondria red and depolarized mitochondria green. Immunofluourescent images of control cells showed mostly red fluourescence representative of intact mitochondria (Fig. 5C), while images of NV-128 treated cells were mostly green (Fig. 5D), suggesting that NV-128 is able to induce mitochondrial depolarization. This spectral shift was confirmed by flow cytometry, which showed that NV-128 treated cultures had 59% and 84% depolarized cells (more than control) at 1 and 4h, respectively (Fig. 6).
Figure 6. Quantitative analysis of mitochondrial depolarization using flow cytometry.

EOC cells were treated with NV-128, stained with JC-1 dye, and analyzed by flow cytometry to quantify mitochondrial depolarization.
Beclin-1 mitochondrial translocation is associated with mitochondrial depolarization
Our next goal was to determine the upstream pathway responsible for NV-128-induced mitochondrial depolarization. The stability of the mitochondrial membrane is partly controlled by the Bcl2 family of proteins (5). Bid and Bax are cytoplasmic proteins that translocate to the mitochondria to initiate depolarization. In contrast, Bcl2 is a resident mitochondrial protein that stabilizes the membrane. Treatment with NV-128 induces mitochondrial translocation of Bax 2h post-treatment but does not induce the activation of Bid (Fig. 7A).
Figure 7. NV-128 induces Beclin-1 mitochondrial- and EndoG nuclear-translocation.



(A) Western blot analysis of cell lysates and mitochondrial fractions prepared from EOC cells treated with 10 μg/ml NV-128. Total cell lysates were analyzed for full-length Bid and mitochondrial fractions were analyzed for Beclin-1 and Bax. β-actin and VDAC are shown as loading controls. (B) Western blot analysis of anti-Beclin immunoprecipitates, derived from mitochondrial fractions of NV-128 (10 μg/ml, 1h) treated EOC cells, probed with anti-Bcl-2 and anti-Bak. (C) Western blot analysis of nuclear fractions for AIF and EndoG. Topoisomerase I (Topo-I) is shown as loading control.
Given that Bax translocation, normally one of the first mediators of mitochondrial membrane destabilization, occurs after the onset of NV-128-induced mitochondrial depolarization (seen 1h post-treatment), an alternative mechanism must be responsible for the initiation of mitochondrial instability. Beclin-1 is a tumor suppressor gene and has been shown to interact with Bcl-2 through its BH domain (18). Analysis of Beclin-1 mRNA by RT-PCR and Beclin-1 protein levels from whole cell lysates showed no changes in Beclin-1 message or protein expression after NV-128 treatment (data not shown). However, analysis of mitochondrial fractions showed that Beclin-1 translocates to the mitochondria as early as 1h post-NV-128 treatment (Fig. 7A), which correlates with the onset of mitochondrial depolarization.
Mitochondrial Beclin-1 associates with Bcl2
Apart from its role in the initiation of autophagy where it associates with Class III PI-3 kinase, it has been demonstrated that the BH3 domain of Beclin-1 is able to interact with both Bcl2 and Bclxl (18-20). In lieu of this, we hypothesized that Beclin-1 mitochondrial translocation can lead to its interaction with Bcl2 and therefore interfere with Bcl2's ability to stabilize the mitochondria. To show this association, we immunoprecipitated Beclin-1 from the mitochondrial fraction of untreated cells or cells treated for 1h with NV-128. The presence of Bcl2 and Bak in the immunecomplex was then determined by western blotting. We observed higher levels of Bcl2 associated with Beclin-1 after NV-128 treatment compared to no treatment control (Fig. 7B) demonstrating that NV-128-induced Beclin-1 mitochondrial translocation can lead to Beclin-1- Bcl2 interaction. On the other hand, Bak was not observed in the complex suggesting that this is a specific interaction between Beclin-1 and Bcl2. These findings imply that Beclin-1 may have the ability to initiate mitochondrial depolarization by translocating to the mitochondria where it binds to and inactivates Bcl2, potentially in a similar manner as those described for the Bid-Bak or Bid-Bax interaction.
NV-128 induces EndoG nuclear translocation
In the absence of caspase activation, our next goal was to determine how NV-128-induced mitochondrial depolarization and chromatin condensation are linked. Mitochondrial nucleases represent a family of proteins that, once released from the mitochondria, can translocate to the nucleus and cleave DNA. The observation that NV-128 is able to induce mitochondrial depolarization suggests that a mitochondrial nuclease may be responsible for the observed chromatin condensation. Thus, we used western blot analysis to quantify the levels of the mitochondrial nucleases, apoptosis-inducing factor (AIF) and endonuclease G (EndoG), in nuclear fractions of NV-128- treated cells. An increase in the level of nuclear EndoG, but not AIF, was observed after NV-128 treatment (Fig. 7C) suggesting that EndoG is released from the mitochondria, translocates to the nucleus and cleaves DNA resulting in chromatin condensation.
Anti-tumor activity of NV-128 on an EOC xenograft model
We next assessed the in vivo anti-tumor activity of NV-128. We established a nude mouse EOC xenograft model using EOC cells isolated from malignant ovarian cancer ascites. The anti-tumor activity of NV-128 was compared with that of Carboplatin and Paclitaxel as described in the Methods section. NV-128 induced a significant decrease in tumor proliferation kinetics and final tumor volume in a dose dependent manner compared to Carboplatin and Paclitaxel (Fig. 8A and 8B). T/C values were 30%, 58%, and 58% for NV-128, carboplatin, and paclitaxel, respectively.
Figure 8. In vivo activity of NV-128.



EOC tumors were established in s.c. in NCR nude mice and treatments were given as described in the Materials and Methods section. Tumor size was determined by caliper measurements. (A) EOC tumor proliferation kinetics; (B) excised tumors from representative mice dosed with vehicle control or NV-128; (C) representative mice from each treatment group – note that carbolatin-treated mice had significant weight loss; (D) Tumors from representative mice were lysed and analyzed by western blot analysis for p-S6K and t-S6k. (E) Paraffin-embedded sections of representative mouse tumors were analyzed for the localization of Endo by IHC.
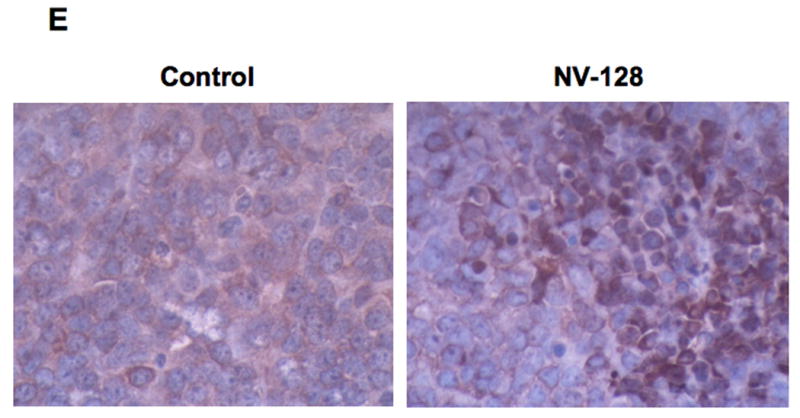
Finally, to demonstrate that the in vivo anti-tumor activity of NV-128 parallels the observed in vitro mechanisms, tumors were lysed and the level of p-S6k was determined by western blot analysis and paraffin-embedded sections of mouse tumors were immunostained for EndoG. As shown in Figure 8C, tumors taken from animals treated with NV-128 had a significant decrease on p-S6k, compared to the vehicle control. In addition, we observed the nuclear localization of EndoG in tumors form animals treated with NV-128, while tumors from control animals only had cytoplasmic staining (Fig. 8D).
Toxicology studies
Since NV-128 induced significant cell death in all cultures tested in vitro, a concern is its possible toxic side effects. Thus, we determined if NV-128 induce myelossuppresion or affect liver and kidney functions in the animals. Comparison of white blood cell and red blood cell counts, hematocrit, and haemoglobin levels in NV-128 treated and control animals showed no significant difference (Fig. 9A). Comparison of ALP and ALT activities, and creatinine and urea content in the serum also revealed no differences between the two groups (Fig. 9B). Moreover, histopathologic examination of H&E stained sections from liver, kidney, spleen, and stomach showed no histopathological changes that would suggest toxicity (data not shown). Taken together, these results indicate that NV-128 does not induce myelossuppresion and is not hepatotoxic nor nephrotoxic.
Figure 9. In vivo toxicology studies.


(A) Comparison of white blood cells (WBC WL×109/L), red blood cells (RBC × 1012/L), hematocrit (Hct), and hemoglobin (Hb g/L) levels in control and NV-128 treated animals. (B) Comparison of alkaline phosphatase (ALP U/L), alanine aminotransferase (ALT U/L), urea (mmol/L), and creatinine (Cre umol/L) in control and NV-128 treated animals.
Discussion
We describe in this study the anti-tumor activity of NV-128, which involved the inhibition of mTOR signaling both in vitro and in vivo. Furthermore, we describe the potential role of Beclin-1 as a regulator of mitochondrial membrane integrity through its interaction with Bcl-2.
Chemo-resistance, due to the blockade of the apoptotic pathway, is one of the major limitations in the successful treatment of EOC. Indeed, cancer cells in general are characterized by high levels of expression of anti-apoptotic proteins such as XIAP, Bcl2, and p-Akt. Although numerous in vitro studies have reported that knockdown of these proteins can sensitize cancer cells to apoptotic signals, the present state of gene therapy technology limits this approach.
The description of additional forms of cell death other than apoptosis suggests that an alternative approach to chemo-resistance may be to bypass apoptosis and specifically target alternative pathways that are independent of anti-apoptotic proteins.
In this study we used a panel of EOC cells including Paclitaxel-and Carboplatin-resistant cell lines and showed that treatment of these cells with NV-128 induces a caspase-independent cell death characterized by mitochondrial depolarization and chromatin condensation. We also showed that this pathway involved the early down-regulation of p-mTOR and p-AKT. An important function of mTOR is the control of translation via regulation of S6k and 4EBP1. This results in enhanced translation, enhanced cell mass, and cell cycle progression (21, 22). mTOR is an essential part of tumor progression capable of integrating proliferative, antiapoptotic, and angiogenic signaling by connecting VEGF, hypoxia-inducible factor 1 (HIF-1), and HER family receptors (23). In addition, signaling through mTOR is stimulated by survival pathways, including the PI3K-Akt and Ras/Raf/MAPK pathways (24). Therefore, inhibition of mTOR activity may have critical effects on many of these survival pathways.
EOC cells are resistant to Rapamycin (our unpublished data), the classic mTOR complex I inhibitor, which functions as a structural competitive inhibitor. Therefore, the down-regulation of p-mTOR may represent a more suitable target to block the pro-survival pathways activated by mTOR, more so than competitive inhibition.
In order to understand the downstream consequences of mTOR inhibition we evaluated proteins associated with autophagy and mitochondrial integrity. While we observed the activation of the autophagic pathway, the induction of cell death by NV-128 was not inhibited by the autophagy inhibitor, 3MA. We also observed changes in mitochondrial integrity and interestingly, we saw that the levels of Beclin-1, a main player in the autophagic pathway, significantly increased in the mitochondria post NV-128 treatment. Beclin-1 is a Bcl2-binding protein first identified using yeast two-hybrid systems (19). Its function as a pro-autophagic molecule has been extensively characterized. As part of a complex with Class III PI3-kinase, Beclin-1 is involved in autophagosome formation, which initiates the process of autophagy. Beclin-1 is monoallelically deleted in most cancers, which is suggestive of an anti-tumor function (25). Indeed, reports have shown that overexpression of Beclin-1 has pro-apoptotic effects. Furuya et al demonstrated that ectopic expression of Beclin-1 augments cisplatin-induced caspase-9 activation (26), while Levine's group showed that Beclin-1 gene transfer in breast cancer cells results in decreased tumorigenicity (25). Because of Beclin-1's role in autophagy, a process known initially as a cell survival mechanism, it was thus unclear how a molecule involved in a survival pathway would have anti-tumor activity.
Together with the description of Beclin-1's crystal structure and the demonstration that its BH3 domain associates with anti-apoptotic Bcl2 family members (20), our data, which shows the association between Beclin-1 mitochondrial translocation and mitochondrial depolarization, provides evidence of Beclin-1's possible pro-death function, which is independent of its role in autophagy. Exactly how Beclin-1 is preferentially shifted to the mitochondria and how Beclin-1-Bcl2 binding initiates mitochondrial depolarization remains to be elucidated. We can postulate that Beclin-1 binding to Bcl2 releases Bcl2's inhibitory effect on Bak, leading to Bak-induced mitochondrial depolarization.
Another interesting finding is the early down-regulation of p-Akt without any associated down-regulation of XIAP. XIAP is a substrate of Akt and has been shown to be ubiquitinated and degraded by the proteasome once de-phosphorylated (27). It was thus surprising to see stable levels of XIAP coupled with declining p-Akt. It is possible that several steps are required for proteosomal degradation of XIAP and that the ubiquitination of a de-phosphorylated XIAP requires other mechanisms. Another possibility is that phosho-XIAP may be quite stable, allowing it to maintain its phosphorylation status for an extended period of time even with declining levels of p-Akt. This stability in the level of XIAP may also be the reason for the absence of caspase-9 and caspase-3 activation.
We also showed that NV-128-induced mitochondrial depolarization leads to nuclear translocation of EndoG, but not AIF. This suggests that the mitochondrial insult induced by NV-128 is specific, allowing the release of only some mitochondrial proteins, and not a complete mitochondrial rupture, which would have released all resident mitochondrial proteins. Moreover, the demonstration that AIF is not found in the nucleus upon the induction of a caspase-independent pathway supports previous reports that caspase is required to cleave the attachment of AIF to the inner mitochondrial membrane prior to its release (28).
The anti-tumoral activity of NV-128 was further demonstrated in vivo studies. NV-128 is superior to Paclitaxel and Carboplatin and is well tolerated at its efficacious dose. It induced a significant decrease in tumor kinetics compared to Paclitaxel and Carboplatin without any evident decrease in animal weight and activity. In contrast, mice treated with Carboplatin lost more weight than control mice and exhibited severe cachexia. Moreover, we confirmed that the pathway described in vitro is likewise the main pathway that is activated in vivo.
Although, we have not identified the receptor/target of NV-128 in EOC cells we consider that the capacity of NV-128 to inhibit p-mTOR triggers a cascade of events leading to caspase-independent cell death in otherwise chemo-resistant EOC cells (Figs. 10A and B). This opens new possibilities for the use of NV-128 as a potential addition to conventional chemotherapy targeting EOC cells.
Figure 10. Proposed mechanism of NV-128-induced cell death.


(A) In unstimulated/healthy cells, the process of autophagy is inhibited by mTOR and anti-apoptotic proteins such as XIAP inhibit apoptosis. (B) NV-128 treatment induces down-regulation of p-mTOR and Beclin-1 mitochondrial translocation. Mitochondrial Beclin-1 inhibits Bcl2 and the resulting mitochondrial depolarization leads to EndoG nuclear translocation and chromatin condensation. Connection between p-mTOR and Beclin-1 mitochondrial translocation still remains to be determined.
The demonstration of a functional caspase-independent cell death pathway in apoptotic-resistant EOC cells is an important key step to the development of alternative targeted therapy for refractory patients. Further studies are currently ongoing to determine the main target of this compound.
Acknowledgments
Sources of support: This study was funded by NCI (RO1CA118678, RO1CA127913) and in part by Novogen, Inc.
Footnotes
Financial disclosure: David Brown is an employee of Novogen, Inc.
References
- 1.Schwartz PE. Current diagnosis and treatment modalities for ovarian cancer. Cancer Treat Res. 2002;107:99–118. doi: 10.1007/978-1-4757-3587-1_4. [DOI] [PubMed] [Google Scholar]
- 2.Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer. 2002;2:277–288. doi: 10.1038/nrc776. [DOI] [PubMed] [Google Scholar]
- 3.Edinger AL, Thompson CB. Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol. 2004;16:663–669. doi: 10.1016/j.ceb.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 5.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 6.Alvero AB, O'Malley D, Brown D, et al. Molecular mechanism of phenoxodiol-induced apoptosis in ovarian carcinoma cells. Cancer. 2006;106:599–608. doi: 10.1002/cncr.21633. [DOI] [PubMed] [Google Scholar]
- 7.Kluger HM, McCarthy MM, Alvero AB, et al. The X-linked inhibitor of apoptosis protein (XIAP) is up-regulated in metastatic melanoma, and XIAP cleavage by Phenoxodiol is associated with Carboplatin sensitization. J Transl Med. 2007;5:6. doi: 10.1186/1479-5876-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sapi E, Alvero AB, Chen W, et al. Resistance of ovarian carcinoma cells to docetaxel is XIAP dependent and reversible by phenoxodiol. Oncol Res. 2004;14:567–578. doi: 10.3727/0965040042707943. [DOI] [PubMed] [Google Scholar]
- 9.Ohsumi Y. Molecular dissection of autophagy: two ubiquitin-like systems. Nat Rev Mol Cell Biol. 2001;2:211–216. doi: 10.1038/35056522. [DOI] [PubMed] [Google Scholar]
- 10.Mizushima N, Ohsumi Y, Yoshimori T. Autophagosome formation in mammalian cells. Cell Struct Funct. 2002;27:421–429. doi: 10.1247/csf.27.421. [DOI] [PubMed] [Google Scholar]
- 11.Tsujimoto Y, Shimizu S. Another way to die: autophagic programmed cell death. Cell Death Differ. 2005;12 2:1528–1534. doi: 10.1038/sj.cdd.4401777. [DOI] [PubMed] [Google Scholar]
- 12.Kamsteeg M, Rutherford T, Sapi E, et al. Phenoxodiol--an isoflavone analog--induces apoptosis in chemoresistant ovarian cancer cells. Oncogene. 2003;22:2611–2620. doi: 10.1038/sj.onc.1206422. [DOI] [PubMed] [Google Scholar]
- 13.Alvero AB, Brown D, Montagna M, Matthews M, Mor G. Phenoxodiol-Topotecan Co-Administration Exhibit Significant Anti-Tumor Activity Without Major Adverse Side Effects. Cancer Biol Ther. 2007;6 doi: 10.4161/cbt.6.4.3891. [DOI] [PubMed] [Google Scholar]
- 14.Behrens BC, Hamilton TC, Masuda H, et al. Characterization of a cis-diamminedichloroplatinum(II)-resistant human ovarian cancer cell line and its use in evaluation of platinum analogues. Cancer Res. 1987;47:414–418. [PubMed] [Google Scholar]
- 15.Kelly MG, Alvero AB, Chen R, et al. TLR-4 signaling promotes tumor growth and paclitaxel chemoresistance in ovarian cancer. Cancer Res. 2006;66:3859–3868. doi: 10.1158/0008-5472.CAN-05-3948. [DOI] [PubMed] [Google Scholar]
- 16.Yang YP, Liang ZQ, Gu ZL, Qin ZH. Molecular mechanism and regulation of autophagy. Acta Pharmacol Sin. 2005;26:1421–1434. doi: 10.1111/j.1745-7254.2005.00235.x. [DOI] [PubMed] [Google Scholar]
- 17.Seglen PO, Bohley P. Autophagy and other vacuolar protein degradation mechanisms. Experientia. 1992;48:158–172. doi: 10.1007/BF01923509. [DOI] [PubMed] [Google Scholar]
- 18.Oberstein A, Jeffrey PD, Shi Y. Crystal structure of the Bcl-XL-Beclin 1 peptide complex: Beclin 1 is a novel BH3-only protein. J Biol Chem. 2007;282:13123–13132. doi: 10.1074/jbc.M700492200. [DOI] [PubMed] [Google Scholar]
- 19.Liang XH, Kleeman LK, Jiang HH, et al. Protection against fatal Sindbis virus encephalitis by beclin, a novel Bcl-2-interacting protein. J Virol. 1998;72:8586–8596. doi: 10.1128/jvi.72.11.8586-8596.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maiuri MC, Le Toumelin G, Criollo A, et al. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J. 2007;26:2527–2539. doi: 10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fingar DC, Richardson CJ, Tee AR, Cheatham L, Tsou C, Blenis J. mTOR controls cell cycle progression through its cell growth effectors S6K1 and 4E-BP1/eukaryotic translation initiation factor 4E. Mol Cell Biol. 2004;24:200–216. doi: 10.1128/MCB.24.1.200-216.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fingar DC, Salama S, Tsou C, Harlow E, Blenis J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 2002;16:1472–1487. doi: 10.1101/gad.995802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corradetti MN, Guan KL. Upstream of the mammalian target of rapamycin: do all roads pass through mTOR? Oncogene. 2006;25:6347–6360. doi: 10.1038/sj.onc.1209885. [DOI] [PubMed] [Google Scholar]
- 24.Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335–348. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 25.Qu X, Yu J, Bhagat G, et al. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest. 2003;112:1809–1820. doi: 10.1172/JCI20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furuya D, Tsuji N, Yagihashi A, Watanabe N. Beclin 1 augmented cis-diamminedichloroplatinum induced apoptosis via enhancing caspase-9 activity. Exp Cell Res. 2005;307:26–40. doi: 10.1016/j.yexcr.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 27.Dan HC, Sun M, Kaneko S, et al. Akt phosphorylation and stabilization of X-linked inhibitor of apoptosis protein (XIAP) J Biol Chem. 2004;279:5405–5412. doi: 10.1074/jbc.M312044200. [DOI] [PubMed] [Google Scholar]
- 28.Otera H, Ohsakaya S, Nagaura Z, Ishihara N, Mihara K. Export of mitochondrial AIF in response to proapoptotic stimuli depends on processing at the intermembrane space. EMBO J. 2005;24:1375–1386. doi: 10.1038/sj.emboj.7600614. [DOI] [PMC free article] [PubMed] [Google Scholar]


