Abstract
Pericytes, mural cells located on microvessels, are considered to play an important role in the formation of the vasculature and the regulation of local blood flow in some organs. Little is known about the physiology of cochlear pericytes. In order to investigate the function of cochlear pericytes, we developed a method to visualize cochlear pericytes using diaminofluorescein-2 diacetate (DAF-2DA) and intravital fluorescence microscopy. This method can permit the study of the effect of vasoactive agents on pericytes under the in vivo and normal physiological condition. The specificity of the labeling method was verified by the immunofluoresence labeling of pericyte maker proteins such as desmin, neural proteoglycan (NG2), and thymocyte differentiation antigen 1 (Thy-1). Superfused K+ and Ca2+ to the cochlear lateral wall resulted in localized constriction of capillaries at pericyte locations both in vivo and in vitro, while there was no obvious change in cochlear capillary diameters with application of the adrenergic neurotransmitter noradrenaline. The method could be an effective way to visualize cochlear pericytes and microvessels and study lateral wall vascular physiology. Moreover, we demonstrate for the first time that cochlear pericytes have contractility, which may be important for regulation of cochlear blood flow.
Keywords: cochlear pericyte, capillary of spiral ligament, diaminofluorescein-2 diacetate (DAF-2DA)
Introduction
In order to maintain the endocochlear potential and the function of the cochlea, cochlear blood flow is required to be tightly coupled to local metabolic needs (Nakashima et al., 2003; Wangemann, 2002). Impairment of cochlear microcirculation results in blood flow alterations, which accordingly affect the delivery of oxygen and metabolic substrates necessary for the physiologic function of the cochlea. Impaired cochlear blood flow has been considered to contribute to many otologic disorders including noise-induced hearing loss, presbycusis, endolymphatic hydrops, and sudden sensorineural hearing loss (Le Prell et al., 2007; Miller et al., 1995; Seidman et al., 1999). However, the understanding of the regulation of cochlear blood flow is far from complete. Although accumulated evidence has shown that the main regulation of cochlear blood flow (CBF) occurs in the spiral artery (Jiang et al., 2004; Wangemann et al., 2005) and radiating arterioles and far from the lateral wall, histological studies revealed that contractile proteins like tropomyosin and myosin are also expressed in the microvessels of the spiral ligament (Franz et al., 2004; Konishi et al., 1998), indicating regulation of blood flow may happen locally in the cochlear lateral wall.
Pericytes, which surround capillaries and contain contractile proteins (Herman et al., 1985), are thought to be an ideal regulator of blood flow in the capillary. In vitro experiments have reported that pericytes have the ability to control the capillary diameter in different organs such as the retina and the brain (Peppiatt et al., 2006; Puro, 2007; Yamanishi et al., 2006). Our research has demonstrated that pericytes are present on cochlear blood microvessels at a high density and they express some contractile proteins like α-smooth muscle actin and tropomyosin (Shi et al., 2008). Distribution of contractile proteins in the cochlear microvascular bed indicates that cochlear mural cells may have the potential to regulate the local blood flow.
It is difficult to visualize pericytes, endothelial cells, and capillary blood flow in vivo at the same time (Puro, 2007). This problem impedes the direct observation of the regulation of blood flow by pericytes. However, cochlear pericytes produce a large amount of nitric oxide (NO) (Shi et al., 2001; Shi et al., 2008). Here we report a method to visualize cochlear pericytes by using diaminofluorescein-2 diacetate (DAF-2DA), a sensitive detector of NO. The specificity of this method was confirmed by simultaneous immunohistochemical labeling of desmin, NG2, and Thy-1, marker proteins of pericytes (Armulik et al., 2005; Murfee et al., 2005). This novel approach offers new possibilities for the detailed examination of the physiological functions of cochlear pericytes and investigation of the regulation of cochlear blood flow in the capillaries of the lateral wall.
2. Materials and methods
2.1. Animals
Thirty albino guinea pigs from the Charles River Laboratories (both sexes, age four to five weeks, 300–450 g) were used in this study. Eight animals were used for observation of pericytes on the spiral ligament in situ and to study the physiological function of pericytes in vitro. Twenty-two animals were used to detect cochlear pericytes in vivo and to investigate the influence of vasoactive agents on spiral ligament microvessels. All procedures in this study were reviewed and approved by the Institutional Animal Care and Use Committee at Oregon Health & Science University.
2.2. Surgical preparation
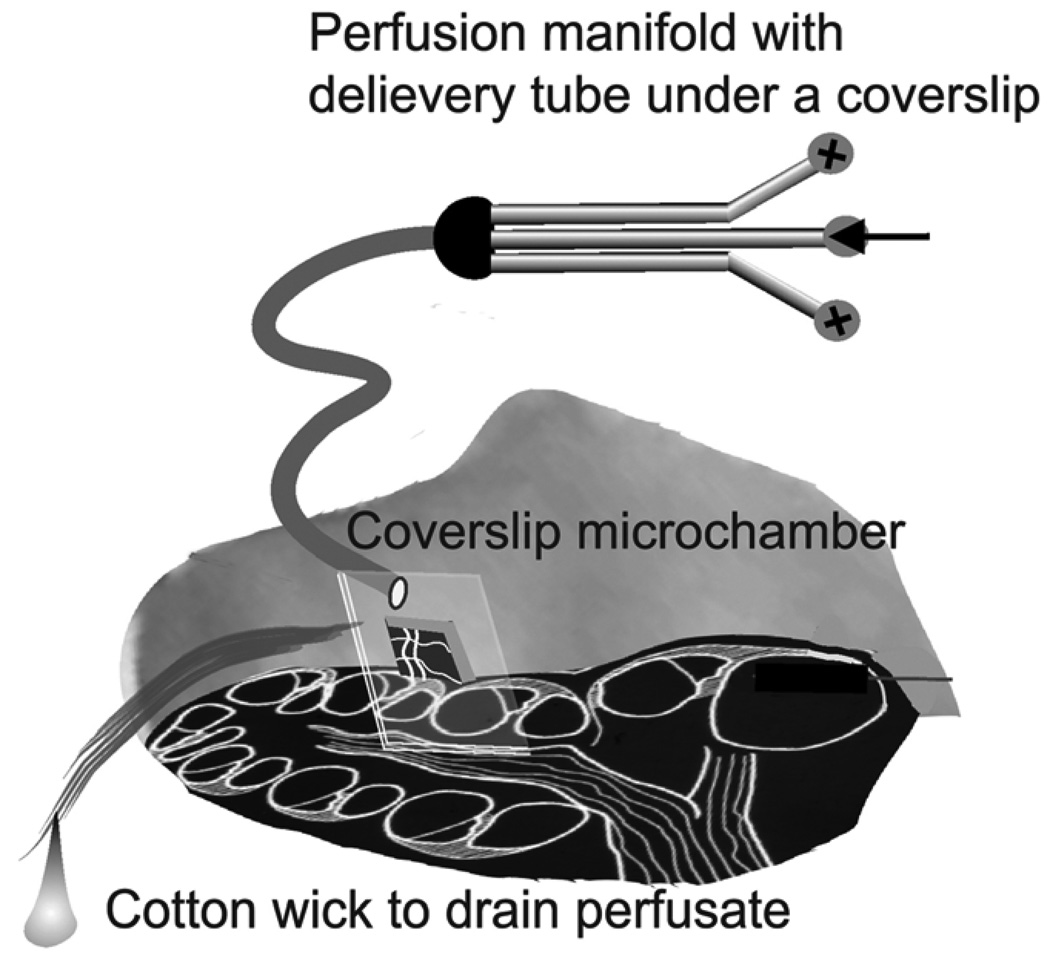
The animals were anesthetized with an injection of ketamine (40 mg/kg) and xylazine (10 mg/kg) (Abbott Laboratories, N. Chicago, IL) and were then wrapped in a heating pad with rectal temperature maintained at approximately 38°C. The head was fastened into a manipulator, which was heated in order to prevent conductive cooling. A ventrolateral surgical dissection was carried out to expose the bulla, which was then opened over its ventral surface. In order to observe the blood circulation in the vessels of the spiral ligament, we used a method that we have previously described (Nuttall, 1987; Shi et al., 2002). In brief, a rectangular fenestration (0.2 × 0.3 mm) into the cochlea was made by creating a window opening over the spiral ligament after scoring a rectangle of scratch marks into the bone of the fourth turn. The vessels of the cochlear lateral wall can be visualized under intravital microscope through this window opened on the cochlear lateral wall. We named the window “vessel-window” in this study. A thin cover slide (Fig. 1) was used to cover the vessel-window in order to preserve normal physiological conditions and also to have the best optical view for recording vessel images. By adjusting the optical focus, we visualized the vessels of the spiral ligament. The vessels within the window were monitored with intravital video-microscopy using a long-working-distance objective lens (20×, 0.4 NA).
Figure 1.
Illustrations of the guinea pig cochlea showing the vessel-window perfusion system. Fluids are delivered under the coverslip by a microtube that is connected to a manifold. This allows selection of the solution to be perfused without a long wait to clear tubing dead space. Perfusion is accomplished by a syringe pump. Fluid is wicked away from the cochlea with a cotton wick.
2.3. The detection of cochlear pericytes of the spiral ligament
2.3.1. Detection of cochlear pericytes in vivo
The animals were surgically prepared as described above. As shown in Fig. 1, a physiological solution containing 30 µM diaminofluorescein-2 diacetate (DAF-2DA) (Calbiochem Novabiochem, USA) was delivered under the coverslip by a microtube that was connected to a manifold. This allowed selection of the solution to be perfused without a long wait to clear tubing dead space. Perfusion was accomplished by a syringe pump and fluid was wicked away from the cochlea with a cotton wick. After 30 minutes (20 µl/min) superfusion, DAF-2DA loaded into the cells allowed the detection of the distribution of pericytes in the vessels of the spiral ligament. Intravital microscopy fluorescent images were obtained using an Olympus BXFM fluorescence microscope. DAF-2DA label was imaged with 488 nm wavelength excitation and 510 nm emission filters. Images were captured by a Hamamatsu Oca mode CCD camera and saved to a hard disk for later analysis.
To detect the specificity of this method, NG2 was used to verify that the high NO signal containing cells were pericytes. In brief, after we superfused DAF-2DA into the “vessel-window” in vivo, the animal was sacrificed. The tissue of the cochlear lateral wall within the “vessel-window” was carefully isolated from the bone under a dissection microscope and then fixed in 4% paraformaldehyde overnight, followed by an immunohistochemical procedure (see 2.5 Immunohistochemistry). The DAF-2DA labeled vessels were easily recognized under confocal fluorescent microscopy because there was no DAF-2DA fluorescent signal seen in the “no-vessel-window” area.
2.3.2. Detection of cochlear pericytes in vitro
The cochleae were dissected from the auditory bullae immediately after the animals were sacrificed by exsanguination under anesthesia. The lateral wall of the fourth cochlear turn was carefully removed from the cochleae. The stria vascularis of each lateral wall was detached from the spiral ligament. The tissues were then incubated in a Petri dish with a physiological solution (144 mM NaCl, 4 mM KCl, 0.9 mM MgCl2, 1.3 mM CaCl2, 10 mM Na2HPO4, 10mM glucose) containing 10 µM DAF-2DA for 30 minutes (Shi et al., 2001; Shi et al., 2008). After washing with the physiological solution for 30 minutes, segments of the spiral ligament were observed under a fluorescent microscope (Nikon Eclipse E600).
2.4. Time-lapse photography
After labeling with DAF-2DA, the vessels were observed under a microscope. The vessels which had normal blood flow were considered healthy and selected for the in vivo time-lapse experiments. A total of 41 vessels form 22 animals were used in this experiment. A physiological solution containing K+ (20 mM), Ca2+ (10 mM), and noradrenaline (10 µM) were superfused into the fenestration separately after the cochlear pericytes were visualized by DAF-2DA loading as described above. Capillaries of the spiral ligament were visualized using an Olympus BXFM fluorescence microscope equipped with a long distant objective (20×, 0.4 NA). The resolution of the in vivo imaging system defined by the size of a square pixel in the imaging CCD camera was 0.48 micrometers, which was sufficient for image analysis. To document changes in pericyte contractility and capillary diameter, time-lapse images were recorded at 1s intervals.
For the in vitro time-lapse experiments, the detached spiral ligament was positioned in a perfusion chamber, which was perfused by an 8 channel perfusion system (Bioscience tool, San Diego, CA, USA). Capillaries of the spiral ligament were visualized by differential interference contrast optics at ×400 magnification with a Nikon Eclipse E600 equipped with a water-immersion objective (40×, 0.8 NA). The resolution of the in vitro imaging system defined by the size of a square pixel in the imaging CCD camera was 0.175 micrometers, which was sufficient for image analysis. Pericytes were recognized by their shape and location and confirmed by the DAF-2DA labeling. Time-lapse images were recorded at 1s intervals using a Photometrics Sensys digital camera. Capillary diameter was measured after separate perfusion of extracellular K+ (20 mM), Ca2+ (10 mM), and noradrenaline (10 µM).
To analyze the effect of vasoactive agents on cochlear capillary diameters, the time-lapse movies were inspected visually to detect the constriction on pericyte-containing capillaries. Image J software was used to measure the lumen diameters at both pericyte and non-pericyte locations (15µm away from the pericyte soma). Lumen diameters near pericyte and non-pericyte locations were measured at the beginning of the perfusion and at the time when the changes in diameter were maximal.
2.5. Immunohistochemistry
After the animals were sacrificed, segments of the spiral ligament were removed and incubated in a Petri dish with a physiological solution containing 10 µM DAF-2DA for 30 minutes. The tissues were then fixed in 4% formaldehyde for 2 hours, washed in 0.02M phosphate-buffered saline (PBS; pH 7.4) for 30 minutes, permeabilized in 0.5% Triton x-100 (Sigma, St. Louis, MO) for 1 hour, then immunoblocked in a solution of 10% goat serum in 1% bovine albumin in 0.02M PBS for 30 minutes. The specimens were incubated overnight in anti-desmin solution (anti-desmin, rabbit monoclonal antibody, 1:500, Abcam Inc, USA), anti-NG2 solution (anti-NG2, rabbit monoclonal antibody, 1:200, Abcam Inc, USA) or anti-Thy-1 solution (anti-Thy-1, rabbit monoclonal antibody, 1:400, Abcam Inc, USA). Subsequently, the specimens were washed in 0.02M PBS for 30 minutes and then incubated in Alexa fluor® 568 anti-rabbit IgG (diluted 1:100 with 1% BSA–PBS) for 1h. After being washed in 0.02PBS for 30 minutes, the tissues were mounted and observed on an Olympus IX81 inverted microscope fitted with an Olympus Fluoview FV1000 confocal laser microscope system. Incubated tissues with 1% BSA–PBS to replace the primary antibody were used as negative controls.
2.6. Statistic
Data are presented as means ± SD. Significant differences between data sets were assessed with Student’s t-tests. Differences were considered significant at p < 0.05.
3. Results
3.1. Detection of cochlear pericytes in vivo
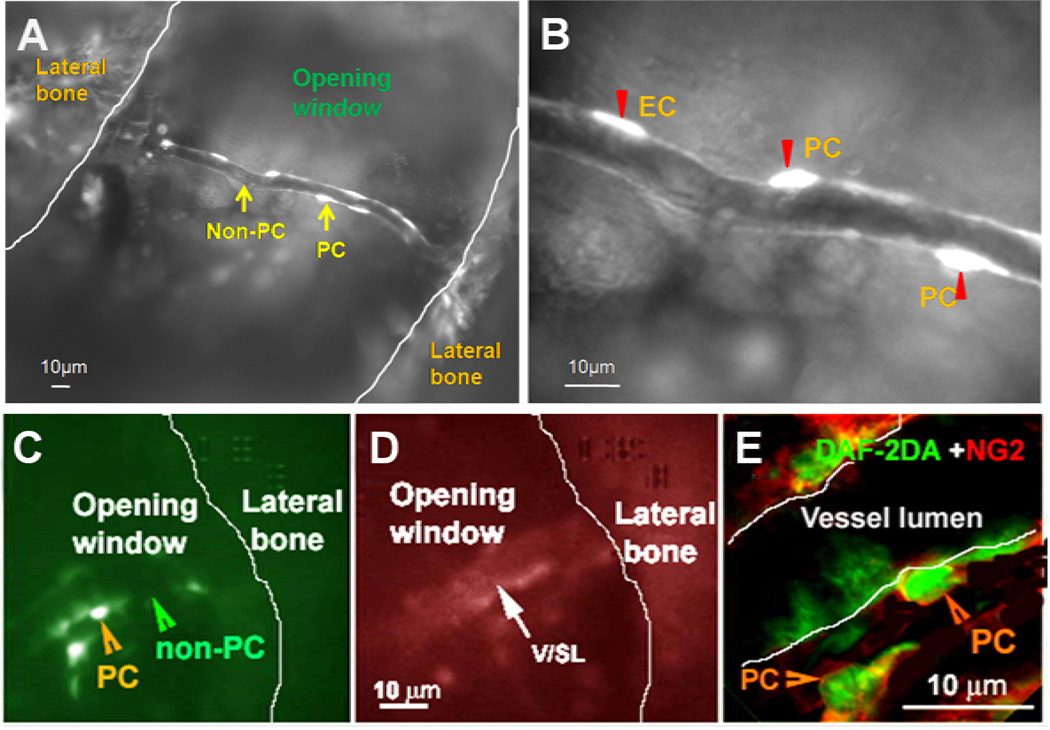
Our results show that pericytes can be visualized by DAF-2DA in living animals. After 30 minutes of superfusion of DAF-2DA, pericytes and endothelial cells of the spiral ligament can be visualized under the intravital fluorescence microscopy (Fig. 2A). Cochlear pericytes could be easily recognized in vivo by their higher fluorescence signals combined with their morphologically characteristic “bump on a log” shape (Kuwabara et al., 1960; Sakagami et al., 1999) (Fig. 2B). The specificity of the DAF-2DA labeling was confirmed by immunofluorescent labeling with NG2, a pericyte marker protein (Fig. 2E).
Figure 2.
Visualization and identification of cochlear pericytes in the cochlear spiral ligament in vivo. Panel A shows that cochlear vessels can be visualized in a cochlear wall “open window” by a fluorescence intravital microscopy with a long working distance lens. Both pericytes and endothelial cells in a vessel of the spiral ligament are labeled by DAF-2DA. Panel B, a high magnification image from Panel A, demonstrates that pericytes (PC and arrow head) could be easily recognized by their higher fluorescence signals combined with their morphologically characteristic “bump on a log” shape. Endothelial cells have elongated shapes (EC and arrow head). Panel C, in vivo imaging shows that pericytes on the capillaries of the spiral ligament are labeled with DAF-2DA (green); while Panel D shows that the same vessel is labeled with rhodamine B isothiocyanate-Dextrans (red). Panel E shows that the pericytes on the same vessel are further identified by immunolabeling with NG2 at a high magnification under confocal microscopy (Green: DAF-2DA; Red: NG2).
3.2. Detection of cochlear pericytes in vitro
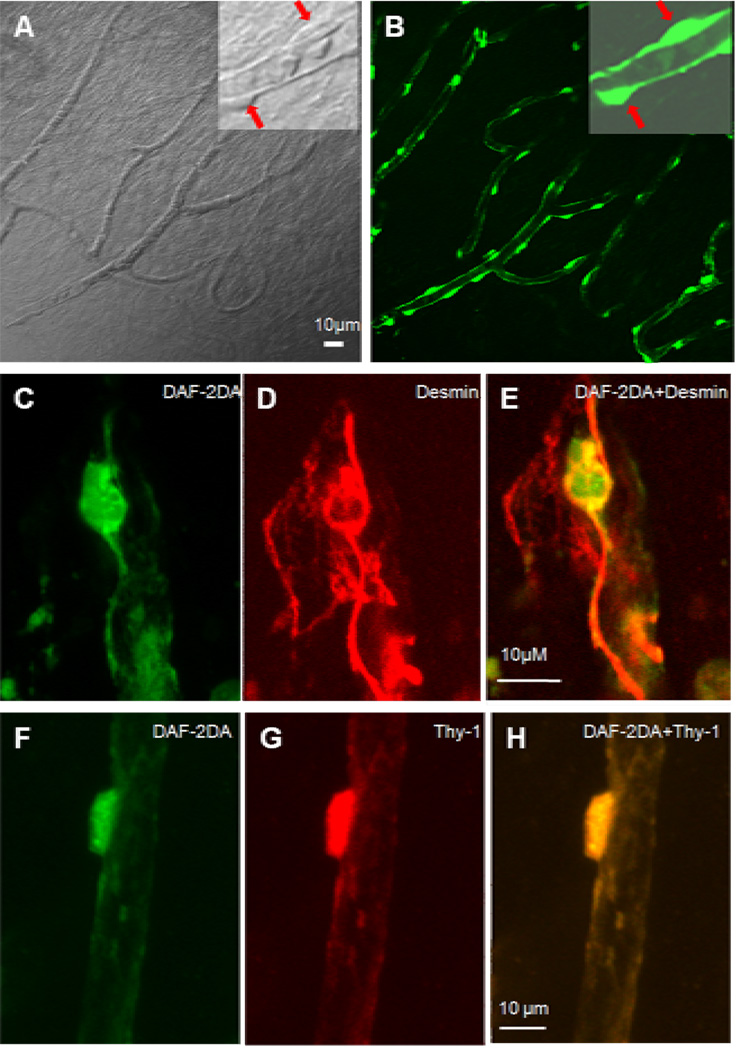
As shown in Fig. 3A, pericytes can be distinguished from other vascular cells under differential interference contrast (DIC) microscopy in situ based on their characteristic shape. After 30 minutes of incubation of DAF-2DA, cochlear pericytes can be visualized by a strong fluorescence signal (Fig. 3B). Although endothelial cells are also be labeled by DAF-2DA, pericytes can be easily distinguished from endothelial cells because of their higher intracellular fluorescent signals and their characteristic morphology and location. Furthermore, relatively high DAF-2DA fluorescent signal cells on the microvessels walls were verified by co-labeling with pericyte marker proteins desmin (Fig. 3D) and Thy-1 (Fig. 3G) with fluorescent immunohistochemistry.
Figure 3.
Visualization and identification of cochlear pericytes in the cochlear spiral ligament in vitro. Panel A (left), a differential interference contrast (DIC) image, shows the distribution of pericytes on the vessels of the cochlear spiral ligament. An inserted high magnification image in Panel A (right corner) shows two pericytes located on the vessel wall (identified by two red arrows). Panel B, a same image from Panel A, shows that pericytes are labeled by DAF-2DA (green). An inserted high magnification image shows two pericytes containing high NO fluorescence signals in comparison to endothelial cells (identified by two arrows). Panel C, a high magnification image, shows that a pericyte is labeled by DAF-2DA (green). Panel D shows the DAF-2DA labeled-pericyte in the Panel C is positive to a pericyte marker protein, desmin (red). Panel E, a merged image from Panels C & D. Panel F, a high magnification image, shows that a pericyte is labeled by DAF-2DA (green). Panel G shows the DAF-2DA labeled-pericyte in the Panel F is positive to another pericyte marker protein, Thy-1 (red). Panel H, a merged imaged from Panels F & G.
3.3. Responses of cochlear microvessels to vasoactive agents in vivo
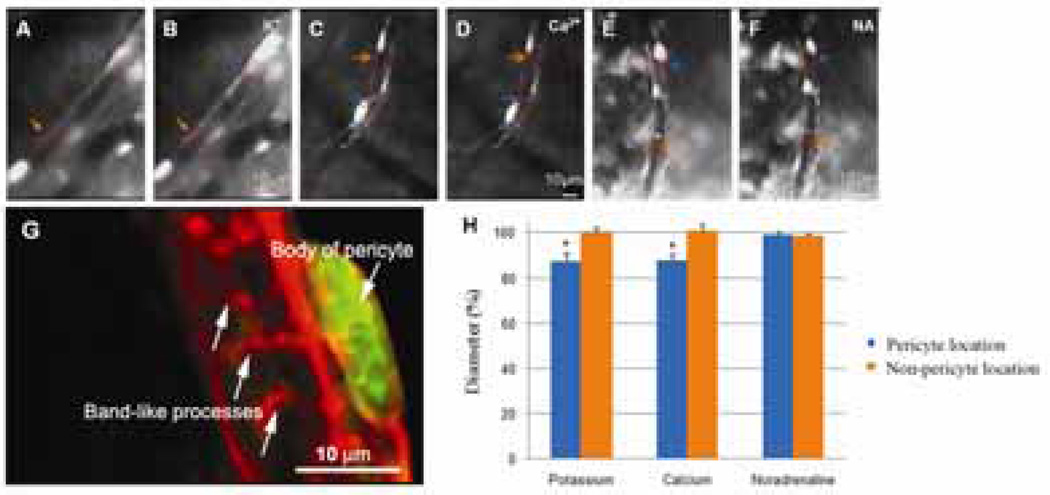
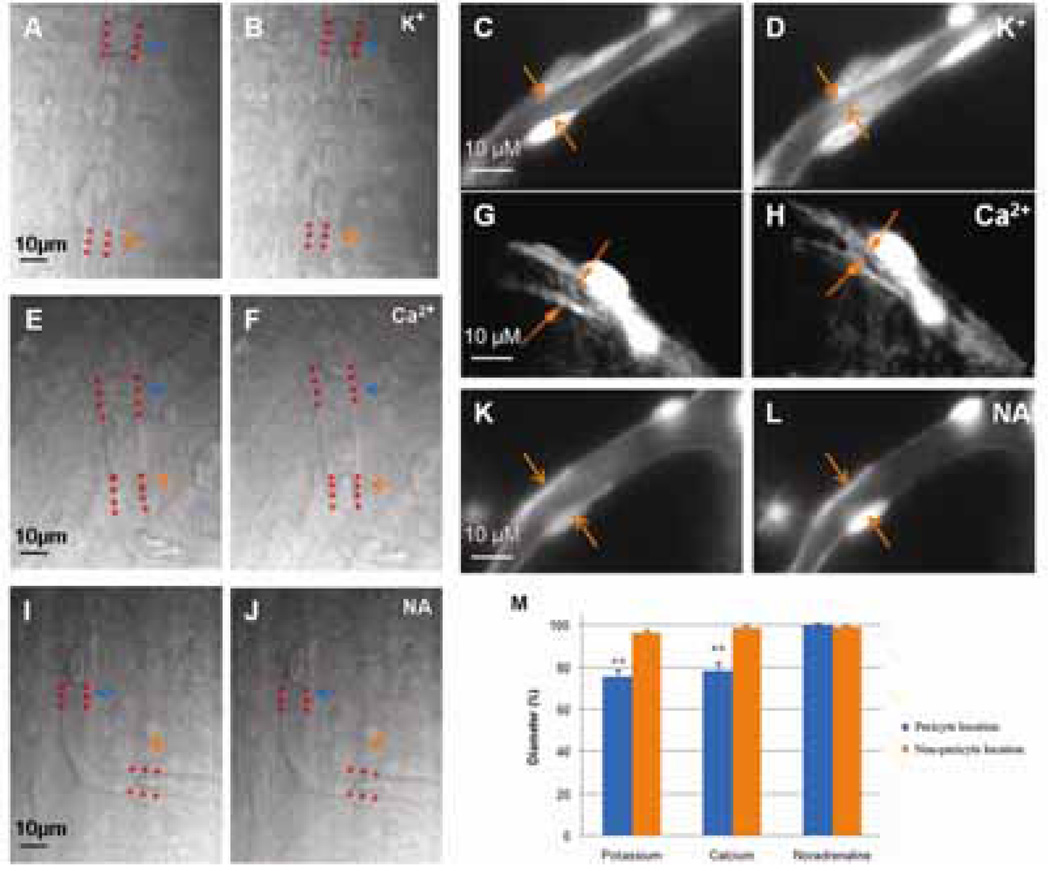
In order to investigate the contractility of the microvessels of the spiral ligament in vivo, timelapse photography was used to monitor the influence of high external K+ (20 mM), Ca2+ (10 mM), and noradrenaline (10 µM) on the cochlear pericytes and capillaries. External K+ caused 25% of the capillaries studied (n=16) to constrict at the pericyte location by 12.3 ± 3.6%. External Ca2+ also induced constriction of the lumen diameter in 20% of the capillaries studied (n=15) and the mean constriction was 11.8 ± 2.8% (Fig. 4). Noradrenaline (10 µM) did not affect the diameters of the cochlear capillaries (n=10), which is consistent with the concept that the adrenergic nerves do not innervate microvessels in the cochlear lateral wall (Nakashima et al., 2003).
Figure 4.
Time-lapse images of the effect of K+ (20 mM), Ca2+ (10 mM) and noradrenaline on capillary diameter change in vivo. Panels A & B show the capillaries from the spiral ligament are recorded before and after KCl superfusion of the tissue. KCl at 20 mM caused localized capillary constriction. Narrowing of the vessel lumen occurred at the pericyte-body region (or nearby regions, indicated by blue arrows and dotted lines), leaving non-pericyte locations unchanged (indicated by yellow arrows and dotted lines). Panels C & D show the changes of vessel diameter before (Panel C) and following the CaCl2 10 mM treatment (Panel D). Capillary contraction occurred at the pericyte-body region (or nearby regions, indicated by blue arrows and dotted lines), leaving the non-pericyte location unchanged (indicated by yellow arrows and dotted lines). Panels E & F show the changes of vessel diameter before (Panel E) and after noradrenaline (Panel F) treatments. No obvious capillary contraction at both pericyte (or nearby regions, indicated by blue arrows and dotted lines) and non-pericyte regions (indicated by yellow arrows and dotted lines) was noticed following noradrenaline treatment. Panel G, pericyte labeled with desmin (red) and DAF-2DA (green), shows that pericytes on the capillary have a “bump” body and large, circumferential band-like processes completely encircling the vessel (arrows). Panel H, a bar graph, shows the percentage changes of capillary diameter measured at both the pericyte and non-pericyte locations. KCl (* p=0.019<0.05; n=16) and CaCl2 (* p=0.024<0.05; n=15) treatment caused significant changes of capillary diameters at pericyte location. There is no statistical difference when treated with noradrenaline (p=0.35>0.05; n=10).
3.4. Responses of cochlear microvessels to vasoactive agents in vitro
To confirm the vasoactive ability of cochlear capillaries in vitro, the spiral ligament was carefully dissected and monitored by time-lapse photography when perfused with external K+ (20 mM), Ca2+ (10 mM), and noradrenaline (10 µM). Consistent with the results of the in vivo experiment, perfusion of the external K+ and Ca2+ to the freshly isolated spiral ligament also induced constriction of capillaries near the pericytes. The administration of external K+ and Ca2+ constricted 37% and 28% of microvessels monitored separately. Perfusion of external K+ caused 76.1 ± 2.4% constriction of the lumen diameter (n=43), while capillary diameter (n=36) decreased to 78.5 ± 2.6% when perfused by external Ca2+ (Fig. 5). The constriction always happened at the location near a pericyte soma, leaving non-pericyte regions unaffected. Although change of diameter could not be detected in some capillaries, movement of red blood cells can be observed in almost all of the capillaries studied, indicating that vasoconstriction happened out of the region of observation. Neither change of luminal diameters nor movement of red blood cells were induced in the capillaries monitored during exposure to 10 µM noradrenaline (n=10).
Figure 5.
Time-lapse images of the effect of K+ (20 mM), Ca2+ (10 mM) & noradrenaline on capillary diameter in vitro. Panels A, C & B, D show the capillaries from the spiral ligament are recorded before (Panel A, C) and after KCl treatments (Panel B, D). KCl at 20 mM causes capillary contraction. Narrowing of the vessel lumen occurs at the pericyte-body region (or nearby regions, indicated by yellow arrows and dotted lines). Panels E, F & G, H show the changes of vessel diameter before (Panel E, F) and following the 10 mM CaCl2 treatment (Panel F, H). Capillary contraction occurs at the pericyte-body region (indicated by yellow arrows and dotted lines). Panels I, K & J, L show the changes of vessel diameter before (Panel I, K) and after noradrenaline (Panel J, L) treatments. No obvious capillary contraction at both pericyte and non-pericyte regions is noticed following noradrenaline treatment. Panel M, a bar graph, shows the percentage changes of capillary diameter measured at both the pericyte and non-pericyte locations. KCl (** p=0.0004<0.01; n=43) and CaCl2 treatment (** p=0.0023<0.01; n=36) caused significant changes of capillary diameters at pericyte location. There is no statistic difference when treated with noradrenaline (p=0.24>0.05; n=10).
4. Discussion
4.1. Visualization of pericytes in vivo and in vitro
Recent studies show that pericytes have contractibility and can influence the capillary tone (Donoghue et al., 2006; Kawamura et al., 2004; Peppiatt et al., 2006; Yamanishi et al., 2006). However, most of these studies were carried out in vitro using the differential interference contrast microscopy. To explore the physiological function of pericytes in detail, methods to visualize the in vivo physiological pericytes are needed. One obstacle to investigating the physiological function of pericytes is that there is no specific dye to selectively label pericytes and visualize them in living tissue (Puro, 2007). Specific cell types are extremely difficult to observe in vivo without optical contrast enhancement. Hirase et al. describe a method to visualize brain pericytes by using dextran-conjugated fluorescent calcium indicator Calcium Green I combined with two-photon imaging (Hirase et al., 2004), but the labeling process is long and complicated. Moreover, not all pericytes can be labeled by this method.
In order to facilitate the study of cochlear pericytes, we developed a method to better visualize cochlear pericytes in vivo by labeling the pericytes with DAF-2DA. DAF-2DA is normally used to monitor intracellular NO production (Sugimoto et al., 2000). The mechanism that confers a high level of NO to cochlear pericytes is not clear at this moment. We hypothesized that the level of NO production in the pericytes is generated by neuronal NOS (nNOS) because our unpublished data indicated that cochlear pericytes are positive to nNOS. eNOS immunoactivity was detected in the endothelial cells. These results are consistent with reports from other groups (Heinrich et al., 2006; Heinrich et al., 2005). Relatively higher fluorescence signals for NO in pericytes compared to the signals in endothelial cells, combined with the pericyte’s morphological character, render pericytes easily distinguishable from the endothelial cells of the microvessels in the spiral ligament (Fig. 2B). Moreover, as short as 30 minutes after the superfusion of DAF-2DA, nearly all pericytes were labeled in the fourth cochlear turn. With a minimum level excitation light, pericytes can be clearly recognized. However, it should be noted that DAF-2DA fluorescence intensity is based on the cellular NO level, which is related to the activity of NO synthesis. Therefore, in order to avoid the change of NO level in pericytes influenced by age or diseases, healthy and non-aged animals should be selected. Furthermore, cautious surgical preparation is important for in vivo experiments and only the vessels with physical blood flow are thought to be suitable for experiments.
4.2. Do cochlear pericytes have contractile activity?
In the past decade, a number of elegant studies have provided substantial knowledge on the control of CBF by smooth muscle cells of the spiral modiolar artery (SMA), allowing the understanding of CBF at a macro-level (Herzog et al., 2003; Jiang et al., 2007; Miller et al., 2003; Nakashima et al., 2003; Seidman et al., 1992; Wangemann, 2002). The new challenge that we face is to understand how CBF is controlled at a micro-level, because microvascular structure and function are key aspects of tissue and organ health. Recent progress on the vascular capillaries of the brain and retina highlights the role of pericytes in the control of capillary blood flow, in the maintenance of microvascular homeostasis, and in various vascular diseases (Hughes et al., 2006; von Tell et al., 2006). Our previous work on pericyte morphology unexpectedly revealed a high density of pericytes in the cochlear microvascular network (Shi et al., 2008). This was surprising because pericyte distribution and location are related to the functional and metabolic needs in an organ-specific manner. Pericytes in the kidney, retina, liver, and lung play a role in regulating capillary blood flow (Allt et al., 2001; Betsholtz et al., 2006; Donoghue et al., 2006; Nehls et al., 1993; Pallone et al., 2001). For example, since there is no sphincter muscle in the retinal microvasculature, abundant pericytes are required for modulating retinal capillary perfusion (Quignard et al., 2003).
Pericytes are morphologically, biochemically, and physiologically heterogeneous (Sims, 2000). Virtually nothing is known about cochlear pericyte physiology and even their most basic character, their contractile capability. Therefore, in this study, we begin to determine the contractile ability of cochlear pericytes by perfusing them with K+, Ca2+, and noradrenaline, because it has been reported that pericytes have large conductance Ca2+-activated K+ channels and Ca2+ can enter into the cells via L-type voltage-dependent Ca2+ channels (Kamouchi et al., 2004). Pericytes have been proven to have a potential to contract in response to intercellular Ca2+ increase. Also it has been demonstrated that pericytes in other organs have significant response to high external K+ and noradrenaline (Herskovic et al., 2002; Zhang et al., 2002). Our results show that capillaries of the spiral ligament have the ability to constrict, indicating regulation of cochlear blood flow may happen at the capillaries, which is consistent with an earlier report by Wangemann et al (2002). Specifically, in this study, we found the greatest constriction always occurred at the location nearest to the pericyte soma, which supports that cochlear pericytes have the same function that pericytes in the brain do. The reason that we found the maximum contraction at the pericyte soma location is possibly due to its morphology. Cochlear pericytes have a body and numbers of short processes and a few slender long processes (Shi et al., 2008). The short processes near the body of the pericyte are band-like and encircle the vessels (Fig. 4G). The long processes of pericytes are parallel to the long axis of the capillary. The pericyte body where the band-like processes embrace the vessel wall is possibly the significant force generated constriction location, while the long processes of pericytes may have less contractile ability. Local application of noradrenaline has no significant effect on the diameter of the capillary or the cochlear pericyte. This result is different to that of the brain pericytes (Peppiatt et al., 2006). Peppiatt et al. (2006) reported that noradrenaline constricted 50% of capillaries in a cerebellar slice preparation. The different response of pericytes to noradrenaline is consistent with the notion that pericytes are heterogeneous between organs and is consistent with the lack of innervation to the lateral wall (Sims, 2000).
The fact that pericytes have contractile ability and can change capillary diameters has been mostly examined in vitro (Peppiatt et al., 2006; Yamanishi et al., 2006). In this study, we compared the cochlear contractibility in vitro and in vivo, and show that both external K+ and Ca2+ appear to cause a weaker constriction of cochlear capillaries in vivo compared to the results in vitro. K+ induced capillary constriction at pericytes location was 12.3% in vivo, while the constriction increased to 23.9% when treated in vitro. Blood flow related internal pressure in the capillaries may be attributed to the attenuation of the effect of pericyte contraction. However, it is worth noting that even small changes of capillary diameter in vivo induce significant changes of the blood flow resistance and local perfusion according to Poiseuille’s law ( Q: blood flow; P: blood pressure; r: vessel diameter; l: length of vessel).
Interestingly, only a part of the pericytes contracted in response to the vasoactive agents in both in vivo and in vitro experiments, which is in accordance with the reports on brain and retinal pericytes (Peppiatt et al., 2006; Yamanishi et al., 2006). We proposed that the different responses of the pericytes we studied were due to functional heterogeneity of retinal pericytes. Pericytes are morphologically and functionally heterogeneous from organ to organ and the pericyte function has a tight relationship with a specific local microenvironment. One possible explanation for this phenomenon is that only part of the pericytes contract in response to vasoactive signals is sufficient enough to regulate local blood flow, although presently the underlining mechanism is far from clear.
Taken together, our data show the visualization of cochlear pericytes by DAF-2DA is a feasible and efficient way to investigate physiological function of cochlear pericytes in both the in vivo and in vitro conditions. For the first time, we found that cochlear pericytes in the vessels of the spiral ligament have contractile activity. Diameter change of capillaries induced by pericytes is much greater in vitro than in vivo, suggesting that pericytes related capillary diameter change can be significantly influenced by experimental conditions.
Acknowledgement
This work was supported by National Institute of Deafness and Other Communications Disorders Grants NIDCD DC 00888, NIDCD DC 00105, and NIDCD DC 005983 (p30).
Abbreviations
- DAF-2DA
diaminofluorescein-2 diacetate
- CBF
cochlear blood flow
- NO
nitric oxide
- PBS
phosphate-buffered saline
- BSA
bovine serum albumin
- DIC
differential interference contrast
- NG2
neural proteoglycan
- Thy-1
thymocyte differentiation antigen 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allt G, Lawrenson JG. Pericytes: cell biology and pathology. Cells Tissues Organs. 2001;169:1–11. doi: 10.1159/000047855. [DOI] [PubMed] [Google Scholar]
- Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Cir. Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- Betsholtz C, Armulik A. Homeostatic functions of vascular endothelial growth factor in adult microvasculature. Am. J. Physiol. 2006;290:H509–H511. doi: 10.1152/ajpheart.01075.2005. [DOI] [PubMed] [Google Scholar]
- Donoghue L, Tyburski JG, Steffes CP, Wilson RF. Vascular endothelial growth factor modulates contractile response in microvascular lung pericytes. Am. J. Surg. 2006;191:349–352. doi: 10.1016/j.amjsurg.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Franz P, Helmreich M, Stach M, Franz-Italon C, Bock P. Distribution of actin and myosin in the cochlear microvascular bed. Acta Otolaryngol. 2004;124:481–485. doi: 10.1080/00016480410017206. [DOI] [PubMed] [Google Scholar]
- Heinrich UR, Selivanova O, Brieger J, Mann WJ. Endothelial nitric oxide synthase upregulation in the cochlea of the guinea pig after intratympanic gentamicin injection. Eur. Arch. Otorhinolaryngol. 2006;263:62–68. doi: 10.1007/s00405-005-0949-7. [DOI] [PubMed] [Google Scholar]
- Heinrich UR, Selivanova O, Feltens R, Brieger J, Mann W. Endothelial nitric oxide synthase upregulation in the guinea pig organ of Corti after acute noise trauma. Brain Res. 2005;1047:85–96. doi: 10.1016/j.brainres.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Herman IM, D'Amore PA. Microvascular pericytes contain muscle and nonmuscle actins. J. Cell Biol. 1985;101:43–52. doi: 10.1083/jcb.101.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskovic JJ, Speyer CL, Simples JE, Steffes CP, Ram JL. Lipopolysaccharide (LPS) enhancement of outward current in lung pericytes. Lung. 2002;180:215–220. doi: 10.1007/s004080000095. [DOI] [PubMed] [Google Scholar]
- Herzog M, Schon F, Muller J, Knaus C, Scholtz L, Helms J. Long term results after cochlear implantation in elderly patients. Laryngorhinootologie. 2003;82:490–493. doi: 10.1055/s-2003-40896. [DOI] [PubMed] [Google Scholar]
- Hirase H, Creso J, Singleton M, Bartho P, Buzsaki G. Two-photon imaging of brain pericytes in vivo using dextran-conjugated dyes. Glia. 2004;46:95–100. doi: 10.1002/glia.10295. [DOI] [PubMed] [Google Scholar]
- Hughes S, Gardiner T, Hu P, Baxter L, Rosinova E, Chan-Ling T. Altered pericyte-endothelial relations in the rat retina during aging: implications for vessel stability. Neurobiol. Aging. 2006;27:1838–1847. doi: 10.1016/j.neurobiolaging.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Jiang ZG, Shi X, Zhao H, Si JQ, Nuttall AL. Basal nitric oxide production contributes to membrane potential and vasotone regulation of guinea pig in vitro spiral modiolar artery. Hear. Res. 2004;189:92–100. doi: 10.1016/S0378-5955(03)00398-8. [DOI] [PubMed] [Google Scholar]
- Jiang ZG, Shi XR, Guan BC, Zhao H, Yang YQ. Dihydropyridines inhibit acetylcholine-induced hyperpolarization in cochlear artery via blockade of intermediate-conductance calcium-activated potassium channels. J. Pharmacol Exp. Ther. 2007;320:544–551. doi: 10.1124/jpet.106.115212. [DOI] [PubMed] [Google Scholar]
- Kamouchi M, Kitazono T, Ago T, Wakisaka M, Ooboshi H, Ibayashi S, Iida M. Calcium influx pathways in rat CNS pericytes. Brain Res. Mol. Brain Res. 2004;126:114–120. doi: 10.1016/j.molbrainres.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Kawamura H, Kobayashi M, Li Q, Yamanishi S, Katsumura K, Minami M, Wu DM, Puro DG. Effects of angiotensin II on the pericyte-containing microvasculature of the rat retina. J. Physiol. 2004;561:671–683. doi: 10.1113/jphysiol.2004.073098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi K, Yamane H, Iguchi H, Takayama M, Nakagawa T, Sunami K, Nakai Y. Local substances regulating cochlear blood flow. Acta Otolaryngol. 1998;538:40–46. [PubMed] [Google Scholar]
- Kuwabara T, Cogan DG. Studies of retinal vascular patterns. I. Normal architecture. Arch. Ophthalmol. 1960;64:904–911. doi: 10.1001/archopht.1960.01840010906012. [DOI] [PubMed] [Google Scholar]
- Le Prell CG, Yamashita D, Minami SB, Yamasoba T, Miller JM. Mechanisms of noise-induced hearing loss indicate multiple methods of prevention. Hear. Res. 2007;226:22–43. doi: 10.1016/j.heares.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JM, Ren TY, Nuttall AL. Studies of inner ear blood flow in animals and human beings. Otolaryngol. Head Neck Surg. 1995;112:101–113. doi: 10.1016/S0194-59989570308-X. [DOI] [PubMed] [Google Scholar]
- Miller JM, Brown JN, Schacht J. 8-iso-prostaglandin F(2alpha), a product of noise exposure, reduces inner ear blood flow. Audiol. Neurootol. 2003;8:207–221. doi: 10.1159/000071061. [DOI] [PubMed] [Google Scholar]
- Murfee WL, Skalak TC, Peirce SM. Differential arterial/venous expression of NG2 proteoglycan in perivascular cells along microvessels: identifying a venule-specific phenotype. Microcirculation. 2005;12:151–160. doi: 10.1080/10739680590904955. [DOI] [PubMed] [Google Scholar]
- Nakashima T, Naganawa S, Sone M, Tominaga M, Hayashi H, Yamamoto H, Liu X, Nuttall AL. Disorders of cochlear blood flow. Brain Res. 2003;43:17–28. doi: 10.1016/s0165-0173(03)00189-9. [DOI] [PubMed] [Google Scholar]
- Nehls V, Drenckhahn D. The versatility of microvascular pericytes: from mesenchyme to smooth muscle? Histochemistry. 1993;99:1–12. doi: 10.1007/BF00268014. [DOI] [PubMed] [Google Scholar]
- Nuttall AL. Techniques for the observation and measurement of red blood cell velocity in vessels of the guinea pig cochlea. Hear. Res. 1987;27:111–119. doi: 10.1016/0378-5955(87)90012-8. [DOI] [PubMed] [Google Scholar]
- Pallone TL, Silldorff EP. Pericyte regulation of renal medullary blood flow. Exp. Nephrol. 2001;9:165–170. doi: 10.1159/000052608. [DOI] [PubMed] [Google Scholar]
- Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 2006;443:700–704. doi: 10.1038/nature05193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puro DG. Physiology and pathobiology of the pericyte-containing retinal microvasculature: new developments. Microcirculation. 2007;14:1–10. doi: 10.1080/10739680601072099. [DOI] [PubMed] [Google Scholar]
- Quignard JF, Harley EA, Duhault J, Vanhoutte PM, Feletou M. K+ channels in cultured bovine retinal pericytes: effects of beta-adrenergic stimulation. J. Cardiovasc. Pharmacol. 2003;42:379–388. doi: 10.1097/00005344-200309000-00009. [DOI] [PubMed] [Google Scholar]
- Sakagami K, Wu DM, Puro DG. Physiology of rat retinal pericytes: modulation of ion channel activity by serum-derived molecules. J. Physiol. 1999;521(Pt 3):637–650. doi: 10.1111/j.1469-7793.1999.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman MD, Quirk WS. The anterior inferior cerebellar arterial network supplying the rat cochlea and its role in autoregulation of cochlear blood flow. Eur Arch Otorhinolaryngol. 1992;249:332–335. doi: 10.1007/BF00179384. [DOI] [PubMed] [Google Scholar]
- Seidman MD, Quirk WS, Shirwany NA. Mechanisms of alterations in the microcirculation of the cochlea. Ann. N. Y. Acad. Sci. 1999;884:226–232. doi: 10.1111/j.1749-6632.1999.tb08644.x. [DOI] [PubMed] [Google Scholar]
- Shi X, Nuttall AL. The demonstration of nitric oxide in cochlear blood vessels in vivo and in vitro: the role of endothelial nitric oxide in venular permeability. Hear. Res. 2002;172:73–80. doi: 10.1016/s0378-5955(02)00513-0. [DOI] [PubMed] [Google Scholar]
- Shi X, Ren T, Nuttall AL. Nitric oxide distribution and production in the guinea pig cochlea. Hear. Res. 2001;153:23–31. doi: 10.1016/s0378-5955(00)00254-9. [DOI] [PubMed] [Google Scholar]
- Shi X, Han W, Yamamoto H, Tang W, Lin X, Xiu R, Trune DR, Nuttall AL. The cochlear pericytes. Microcirculation. 2008;15:515–529. doi: 10.1080/10739680802047445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims DE. Diversity within pericytes. Clin. Exp. Pharmacol. Physiol. 2000;27:842–846. doi: 10.1046/j.1440-1681.2000.03343.x. [DOI] [PubMed] [Google Scholar]
- Sugimoto K, Fujii S, Takemasa T, Yamashita K. Detection of intracellular nitric oxide using a combination of aldehyde fixatives with 4,5-diaminofluorescein diacetate. Histochem. Cell Biol. 2000;113:341–347. doi: 10.1007/s004180000151. [DOI] [PubMed] [Google Scholar]
- von Tell D, Armulik A, Betsholtz C. Pericytes and vascular stability. Exp. Cell Res. 2006;312:623–629. doi: 10.1016/j.yexcr.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Wangemann P. Cochlear blood flow regulation. Adv. Otorhinolaryngol. 2002;59:51–57. doi: 10.1159/000059241. [DOI] [PubMed] [Google Scholar]
- Wangemann P, Wonneberger K. Neurogenic regulation of cochlear blood flow occurs along the basilar artery, the anterior inferior cerebellar artery and at branch points of the spiral modiolar artery. Hear. Res. 2005;209:91–96. doi: 10.1016/j.heares.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Yamanishi S, Katsumura K, Kobayashi T, Puro DG. Extracellular lactate as a dynamic vasoactive signal in the rat retinal microvasculature. Am. J. Physiol. 2006;290:H925–H934. doi: 10.1152/ajpheart.01012.2005. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Rhinehart K, Pallone TL. Membrane potential controls calcium entry into descending vasa recta pericytes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;283:R949–R957. doi: 10.1152/ajpregu.00251.2002. [DOI] [PubMed] [Google Scholar]