Abstract
Background
The combination of niacin and statin has proven value in hyperlipidemia management and heart disease prevention. However, the efficacy of the non-prescription time-release niacin, Slo-Niacin®, is little studied alone and not at all with atorvastatin. We gave Slo-Niacin® and atorvastatin, singly and together to determine efficacy on the combined abnormalities of triglyceride, LDL and HDL.
Methods
42 men and women with LDL-C>130mg/dL HDL-C <45 (men or 55mg/dL (women) were randomized to 3 months of atorvastatin 10 mg/day or incremental doses of Slo-Niacin® to 1500 mg/day. The alternate drug was added in the next 3-month segment. Lipid profiles and transaminases were measured monthly and other measures at baseline and the end of each treatment sequence.
Results
Mean entry lipids (mg/dL) were: TG 187, LDL-C 171, and HDL-C 39. Mean BMI was 32.6 Kg/m2. Monotherapy with Slo-Niacin® decreased median triglyceride 15%, mean LDL-C 12% and non-HDL-C 15% and increased HDL-C 8%. Atorvastatin decreased median triglyceride 26%, and mean LDL-C 36%, non-HDL-C 36% and increased HDL-C 6%. Combined therapy decreased median triglyceride 33% and mean LDL-C and non-HDL-C each 43%. HDL-C increased 10% (all p<0.001). Median remnant-like lipoprotein-C decreased 55%, mean apo-B 40%, median hsCRP 23% (all p<0.05), TNFa 12% and no change in IL-6. Mean LDL buoyancy increased 15%, apo-A-I 5% and median HDL2-C 20% (all p<0.05). ALT declined with Slo-Niacin® treatment alone compared to atorvastatin and also decreased when Slo-Niacin® was added to atorvastatin. Six subjects dropped out, 3 for niacin related symptoms.
Conclusions
Slo-Niacin® 1.5g/day with atorvastatin 10 mg/day improved lipoprotein lipids, apoproteins and inflammation markers without hepatotoxicity. Slo-Niacin® deserves further study as a cost-effective treatment of hyperlipidemia.
Keywords: niacin, statin, hyperlipidemia, hsCRP
Introduction
Niacin in various forms has demonstrated efficacy in cardiovascular disease prevention.1 Plain (immediate release (IR)) niacin reduced non-fatal myocardial infarction 27% in 5 years in the Coronary Drug Project and coronary mortality 11% in a 15-year follow-up.2,3 Plain niacin (± cholestyramine) reduced clinical coronary events and angiographic progression equal to statin (± bile acid binding resin) in the FATS study.4 Slo-Niacin® (± plain niacin) with simvastatin reduced angiographic disease progression and decreased clinical coronary events ∼90% vs. placebo in the HATS study.5 ER niacin (Niaspan ®) with simvastatin decreased carotid intima-media thickness, which progressed with statin alone in ARBITER II.6 A similar trend was seen when Niaspan was added to statin treatment in ARBITER III. 7
Combined statin-niacin treatment is especially suited to the management of combined (mixed) hyperlipidemia, characterized by high triglyceride, low HDL, small dense LDL and elevated LDL.1,8,9 This condition is a powerful risk predictor and is at least 3–4-fold more common than simple hypercholesterolemia in persons with coronary disease.10,11 Use of statin/niacin combinations allows lower doses of niacin to be used and helps avoid the skin flushing and hepatotoxicity of plain niacin.1,9,12–14 Time-release or extended release niacins further reduce skin flushing by delaying niacin dissolution in the gut.12–15
The lipid-lowering effect of the extended-release niacin formulation (Niaspan®) is well studied in combination with statins. 16–18 A time-release form of niacin, Slo-Niacin®, is available as a dietary supplement and also has potential value in lipid management. However, Slo-Niacin® has had little published study 5,19–21 and none with potent statins. To investigate these questions, we have compared the lipid and anti-inflammatory effects of Slo-Niacin® 1.5g/day to atorvastatin 10 mg/day, both given alone and then together in persons with features of combined hyperlipidemia.
Methods
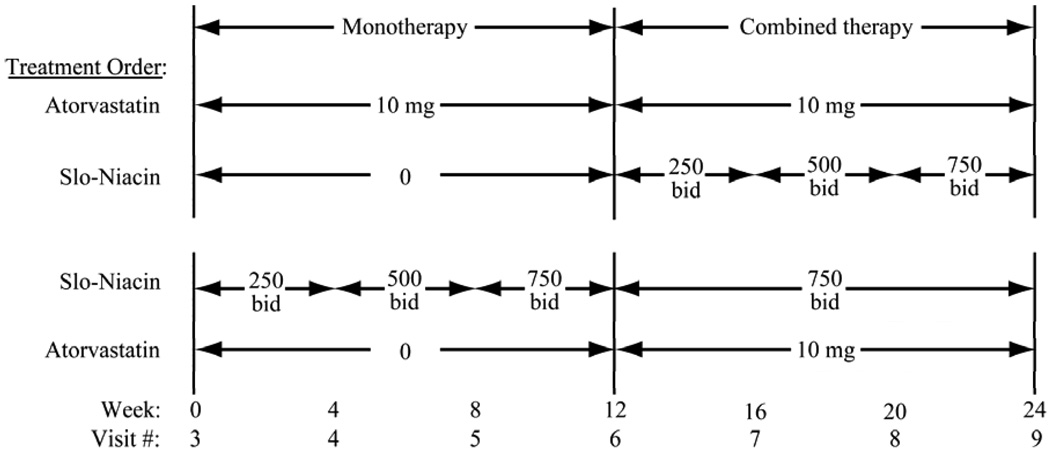
The SLIM study is an investigator-initiated, randomized study of Slo-Niacin® and atorvastatin given separately and together in an open label design. In the first 3-months, atorvastatin 10 mg is given alone and Slo-Niacin® also alone in a forced titration of 250 mg bid, 500 mg bid and 750 mg bid in monthly increments (Fig. 1). The alternate drug is added in the second 3-month cycle (Fig. 1). Men and women were recruited by public advertising. The study was approved by the University of Washington Human Subjects Review Committee.
Figure 1.
Randomization scheme for the order for drug administration with the monthly dose escalation of Slo-Niacin.
Slo-Niacin® was supplied by Upsher-Smith Pharmaceuticals, Inc. Slo-Niacin® is manufactured in an FDA monitored facility in Plymouth, MN, using a patented polygel® controlled-release system. The consistency and quality of each batch is assured by analytical testing prior to the release of each batch. Stability studies for Slo-Niacin® tablets demonstrate a shelf life of 36 months. Dissolution testing ensures the controlled release of niacin. The atorvastatin was purchased commercially.
Eligibility criteria were ages 21 to 75 years, LDL-C >130 mg/dL, HDL-C <45 mg/dL in men and <55 mg/dL in women. Women were >1year post-menopausal, surgically sterile or contracepting using an IUD, contraceptive hormones for >2 monthsbarrier contraception, or abstinence. Vasectomy in themale partner was also accepted as adequate contraception.
Exclusionary criteria were hypersensitivity to statin, niacin or aspirin; diabetes or fasting glucose >125 mg/dL, pregnancy or lactation, hyper- or hypothyroidism unless treatment stable for 3 months, use of lipid lowering medications within one month of screening, use of thiazides, beta blockers, lipid active nutritional supplements and medications contraindicated with atorvastatin use, active liver or gastrointestinal disease, SGOT >1.5 ULN, triglycerides >500 mg/dL, Frederickson’s Type III hyperlipidemia, BMI <19, blood pressure >150/95, more than 10 alcoholic drinks per week, history of gout and any medical condition within 30 days precluding participation in an investigational drug study. Stable sex hormone use was allowed.
First visit
medical history, vital signs, lipid and glucose assessment (Cholestech®, Medical Supplies Home, Salt Lake City, UT) and dipstick urinalysis were obtained. NCEP Step I Diet instructions were given by a research dietician (B.M.R.) and with advice to continue through the study.
Second visit
physical examination and EKG were performed and concurrent medications and metabolic panel obtained, including TSH, CBC and a lipoprotein profile to determine eligibility.
Third visit
subjects were randomized based on a computer generated sequential list of random numbers, with odd numbers assigned to one sequence and even numbers assigned to the other. Subjects were given atorvastatin 10 mg hs or Slo-Niacin® 250 mg bid with meals and 325 mg aspirin one hour before if experiencing symptoms of flushing. Both study drugs were supplied by Upsher-Smith Laboratories, Minneapolis, MN.
Subsequent visits
Compliance was checked by pill counts at return visits. If compliance was <80% at one visit the subject’s willingness to continue in the study was assessed. If <80% for 2 consecutive visits or 3 visits over the 6 on-drug visits, subjects were dropped and excluded from the analysis per protocol. If flu-like symptoms, nausea, fatigue or mucous membrane inflammation occurred, niacin was reduced to ½ of the previous dose. If the half-dose were tolerated at the next visit, the dose would be raised to the prior dose level. One subject required dose reduction.
Flushing symptoms were rated at each visit on a 1 to 6 scale: 1: just a “tingle”, 2: a little warmth for a few minutes, 3: warm and red for 15 minutes, 4: warm and red for 16 to 30 minutes, 5: hot and very flushed for 31 to 60 minutes and 6: red hot and swollen for 1 hour or more. The above symptoms were also categorized as: most frequent feeling, strongest feeling, occurring in the past 4 weeks, improved in the past 4 weeks and in relation to the use of aspirin.
A full lipoprotein quantification was obtained at weeks 0, 12 and 24 (visits 3, 6 and 9) and plasma total cholesterol, triglycerides, and HDL cholesterol with estimated LDL were obtained at follow-up weeks 4, 8, 16 and 20 (visits 4, 5, 7 and 8). Aspartate and alanine aminotransferases (AST/ALT), medical history, concomitant medications and vital signs were recorded at each visit. A questionnaire regarding flushing was completed at each visit. Blood glucose, insulin and hsCRP were assessedat baseline (Visit 3), and the end of each treatment sequence, visits 6 and 9.
Lipoprotein analyses were performed in the CDC standardized laboratories of the Northwest Lipid Research Clinic (T.N.) and the Northwest Lipid Research Laboratories (Santica Marcovina, PhD, Director), using enzymatic automated methods. Lipid profile The LDL cholesterol was estimated by the Friedewald equation.22 Full lipoprotein quantification was performed by ultracentrifugation at Visits 3, 6 and 9, providing direct VLDL-C and LDL-C measurements.22 IDL levels were determined in a subset of subjects by ultracentrifugation at density 1.019.22 HDL2 and HDL3 were separated by dextran sulfate precipitation.22 Apoprotein A-I and B assays were performed by turbidimetry using Roche reagents and a Cobas Mira analyzer.22 LDL buoyancy was determined by gradient ultracentrifugation.16 RLP was measured using an apo B/apo A-I immunoprecipitation method (JIMRO, Gunma, Japan).23 TNF alpha and IL-6 were measured using solid phase Enzyme Amplified Sensitivity Immunoassays with the first antibody directed against distinct cytokine epitopes and a second antibody labeled with horseradish peroxidase (BioSource Cytokines and Signaling, Camarillo, CA). Assays were performed in the Clinical Nutrition Research Unit Analytical Core, University of Washington. Glucose and insulin assays were performed in the Diabetes and Endocrinology Research Center by enzymatic and radio-immunoassay methods, respectively. hsCRP was performed by a 3 microliter assay (WAKO Chemical Diagnostics, Inc., Richmond VA.). AST and ALT levels and metabolic panel were performed in the Department of Laboratory Medicine, University of Washington.
Statistical analyses were performed using the SPSS statistical package (SPSS, Inc, Chicago, IL) in the Administrative Core of the Clinical Nutrition Research Unit (B.F.), University of Washington. The primary outcomes are the LDL-C change from baseline to end of intervention for atorvastatin 10 mg/day and Slo-Niacin® 1500 mg/day alone and the two together. Power estimates were based on 20 subjects in each individual drug group to detect a percent LDL-C reduction from baseline of 8.7% at p<0.05 with a power of 0.8 and 7.0% for a combined sample size of 40 for two-sided testing. All other statistical testing is exploratory. Comparisons were performed using two-sided Student’s t-test. Skewed values were tested non-parametrically, specifically percent changes in RLP, TG, HDL2, hsCRP and ALT. For the same reasons, median as well as mean±SD values are given for the mg/dL baseline and difference values for RLP, hsCRP, TNF alpha, IL-6 and ALT.
Results
Subjects
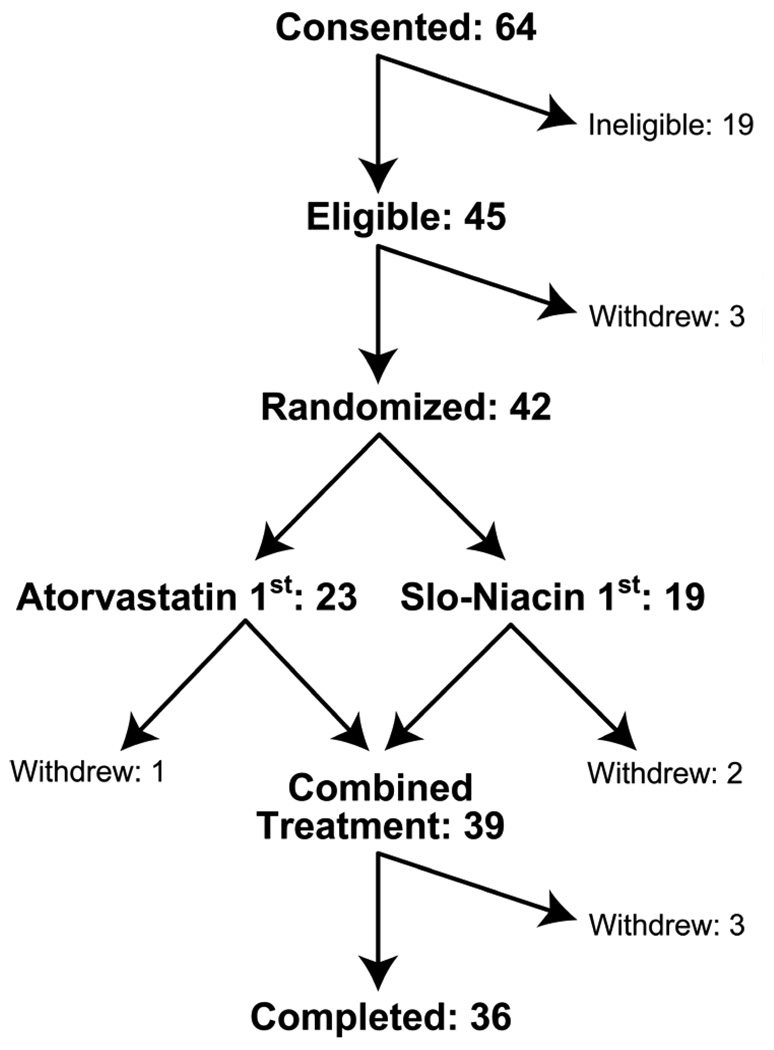
Sixty-four subjects were screened for the study, 19 were ineligible and 3 withdrew before randomization (Fig.2). Forty-two subjects were randomized and 36 completed. Six dropped out: two for lack of time, two for flushing symptoms on niacin, one for heat and sweating in warm weather after a niacin dose increase and one for fatigue, nausea and lightheadedness on atorvastatin.
Figure 2.
Depiction of the numbers of subjects consented, randomized and completed.
Baseline characteristics
Thirty-six subjects finished the study, 19 randomized to atorvastatin first and 17 randomized to Slo-Niacin® first (Table 1). The groups are similar in age, BMI, waist circumference, lipid levels and glucose and insulin levels. Menopausal status,race, smoking and hypertension history were similar for the two groups. Sixty-two% of the randomized cohort had glucose levels ≥100 mg/dL on two or three of three measurements in screening and 67% had triglycerides above 150 mg/dL at visit 2, 3 or 4.
Table 1.
Subject characteristics and baseline measurements
| Randomization Order |
||
|---|---|---|
| Atorvastatin First | Slo-Niacin First | |
| Number of Subjects | 19 | 17 |
| Men/Women | 13/6 | 10/7 |
| Post-menopausal | 5 | 4 |
| Caucasian/African-American | 19/0 | 16/1 |
| Smokers (No/Yes) | 19/0 | 14/3 |
| Hypertensive (No/Yes) | 16/3 | 12/5 |
| Mean±SD: | ||
| Age (years) | 57.1±10.3 | 52.2±9.4 |
| BMI (kg/m2) | 32.8±6.5 | 32.3±5.5 |
| Waist circumference (cm) | 105.5±10.4 | 108.8±11.7 |
| Lipoprotein lipids (mg/dL) | ||
| Triglyceride | 188±75 (177) | 185±93 (160) |
| Cholesterol (C) | 249±40 | 241±56 |
| LDL-C | 177±28 | 164±26 |
| Non HDL-C | 210±39 | 202±52 |
| Apo B | 116±26 | 104±28 |
| RLP-C | 21.5±12.4 (16.5) | 27.6±36.4 (13.3) |
| HDL-C | 38±4 | 39±6 |
| HDL2-C | 5.6±2.5 | 6.4±2.7 |
| Apo A-I | 129±12 | 128±14 |
| Glucose (mg/dL) | 100±11 | 98±7 |
| Insulin (µU/ml) | 24±14 | 27±13 |
| Median: | ||
| hsCRP (mg/dL) | 2.7 | 1.6 |
| TNFα (pg/mL) | 14.5 | 15.6 |
| IL-6 (pg/mL) | 18.9 | 31.2 |
Apo-B containing Lipoproteins
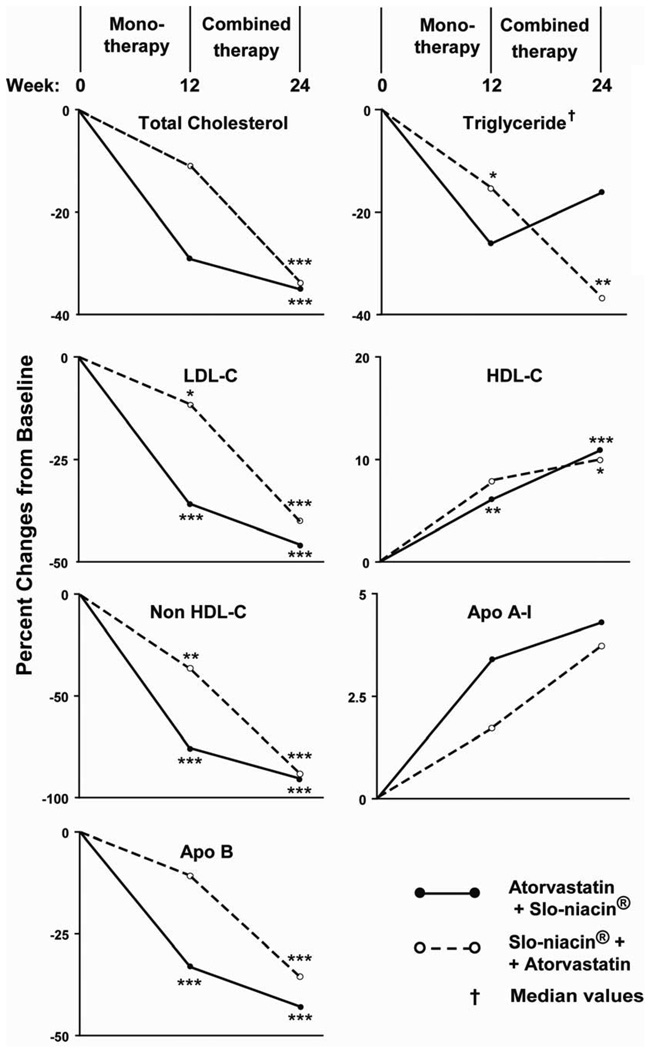
Percentage reductions in apo-B containing lipoproteins, LDL, non-HDL and apo-B respectively, were 36, 36 and 33% with atorvastatin and 12, 15 and 11% with Slo-Niacin®, (Fig. 3). The order of giving the two drugs made little difference as the respective reductions from baseline measures with combined treatment were 46, 43 and 43% in the atorvastatin first group and 40, 42 and 36% in the niacin first group. Percent reductions in total cholesterol (Fig. 3) parallel the other apo-B containing lipoproteins. Absolute reductions in mg/dL are shown in Table 2A and are consistent with percentage reductions.
Figure 3.
Percentage changes in lipoprotein levels from baseline on monotherapy (Week 12) and combined Therapy (Week 24). Primary end points are % change in LDL-C in single and combined drug treatment. ●--● Atorvastatin + Slo-niacin; ○- -○ Slo-Niacin + Atorvastatin *p<0.05, **p<0.01,***p<0.001
Table 2.
Changes from baseline or monotherapy in lipoproteins, apoproteins, glucose, insulin and inflammatory mediators
| Concentration changes: Mean±SD or Median† | |||||
|---|---|---|---|---|---|
|
Weeks 0–12 (1st drug) |
Weeks 12–24 (2nd drug) |
Weeks 0–24 (Both drugs) |
|||
| A) Apo B Lipoproteins (mg/dL) | |||||
| Cholesterol (C): | |||||
| Atorvastatin 1st | −74 ± 34*** | −12 ± 29 | −87 ± 42*** | ||
| Slo-Niacin 1st | −33 ± 58* | −51 ± 27*** | −84 ± 42*** | ||
| P | 0.013 | <0.001 | ns | ||
| LDL-C: | |||||
| Atorvastatin 1st | −65 ± 29*** | −16 ± 20** | −81 ± 25*** | ||
| Slo-Niacin 1st | −22 ± 36* | −45 ± 19*** | −67 ± 25*** | ||
| P | <0.001 | <0.001 | ns | ||
| Non HDL-C: | |||||
| Atorvastatin 1st | −77 ± 34*** | −14 ± 30 | −91 ± 44*** | ||
| Slo-Niacin 1st | −36 ± 61* | −52 ± 28*** | −88 ± 43*** | ||
| P | 0.027 | <0.001 | <0.001 | ||
| Apo B: | |||||
| Atorvastatin 1st | −39 ± 21*** | −9 ± 17* | −48 ± 15*** | ||
| Slo-Niacin 1st | −11 ± 22 | −26 ± 17*** | −37 ± 16*** | ||
| P | <0.001 | <0.005 | 0.031 | ||
| RLP: | |||||
| Atorvastatin 1st | −10.6 ± 9.7*** | −3.3 ± 18.7 | −7.2 ± 20.2** | ||
| Slo-Niacin 1st | −12.8 ± 24.7** | −4.8 ± 4.6** | −17.6 ±26.8*** | ||
| P | ns | ns | ns | ||
| B) Triglyceride, HDL, Apo A-I (mg/dL) | |||||
| Triglyceride: | |||||
| Atorvastatin 1st | −33 ± 75 | 3 ± 117 | −30 ±119 | ||
| Slo-Niacin 1st | −34 ±63* | −35 ±53* | −69 ±54*** | ||
| P | ns | ns | ns | ||
| HDL-C: | |||||
| Atorvastatin 1st | 2.2 ± 3.2** | 2.0 ± 4.7 | 4.2 ± 4.5*** | ||
| Slo-Niacin 1st | 2.9 ± 6.4 | 0.8 ± 4.4 | 3.6 ± 5.3* | ||
| P | ns | ns | ns | ||
| HDL2-C: | |||||
| Atorvastatin 1st | −0.2 ± 2.1 | 1.7 ± 2.6* | 1.5 ± 3.2 | ||
| Slo-Niacin 1st | 0.5 ± 3.2 | −0.2 ± 2.1 | 0.4 ± 2.1 | ||
| P | ns | <0.021 | ns | ||
| Apo A-I: | |||||
| Atorvastatin 1st | 3.6 ± 12.3 | 1.5 ± 12.4 | 5.1 ± 11.8 | ||
| Slo-Niacin 1st | 2.1 ± 16.4 | 2.6 ± 11.3 | 4.7 ± 13.0 | ||
| P | ns | ns | ns | ||
| C) Glycemic Measures | |||||
| Glucose (mg/dL): | |||||
| Atorvastatin 1st | 0.6 ± 7.8 | 3.6 ± 6.3* | 4.2 ± 8.0* | ||
| (n=18) | |||||
| Slo-Niacin 1st | 7.1 ± 10.2* | −1.1 ± 9.8 | 6.0 ± 10.2* | ||
| P | 0.04 | ns | ns | ||
| Insulin (µU/ml): | |||||
| Atorvastatin 1st | 0.1 ± 9.7 | 2.7 ± 6.8 | 2.7 ± 9.5 | ||
| (n=18) | |||||
| Slo-Niacin 1st | 3.4 ± 13.5 | 1.2 ±10.3 | 4.6 ± 13.5 | ||
| P | ns | ns | ns | ||
| D) Inflammatory Cytokines (Medians): | |||||
| hsCRP (mg/dL): | |||||
| Atorvastatin 1st | −0.40 | −0.003 | −0.20 | ||
| Slo-Niacin 1st | −0.13 | −0.29 | −0.27 | ||
| P | ns | ns | ns | ||
| TNFα(pg/mL): | |||||
| Atorvastatin 1st | −1.20 | −0.25 | −1.60* | ||
| Slo-Niacin 1st | −2.00 | −1.30 | −2.00 | ||
| P | ns | ns | ns | ||
| IL-6 (pg/mL): | |||||
| Atorvastatin 1st | −1.20 | 2.00 | −0.20 | ||
| Slo-Niacin 1st | −0.40 | 0.80 | 0.10 | ||
| P | ns | ns | ns | ||
| E) Transaminases (units): | |||||
| ALT:† | |||||
| Atorvastatin 1st | 2.0* | −4.0* | −1.0 | ||
| Slo-Niacin 1st | −5.0 | 3.0 | 4.0 | ||
| P | <0.038 | 0.057 | ns | ||
| AST:† | |||||
| Atorvastatin 1st | 0.2± 10.2 | 3.1± 18.2 | 3.3± 19.9 | ||
| Slo-Niacin 1st | −2.0± 10.1 | 1.3± 8.0 | −0.7± 8.1 | ||
| P | ns | ns | ns | ||
*,**,***: *P<0.05; ** P<0.01; ***P<0.001: Significance of differences in plasma concentrations between Weeks 0 and 12 (1st drug), Weeks 12 and 24 (2nd drug) and Weeks 0 and 24 (both drugs)
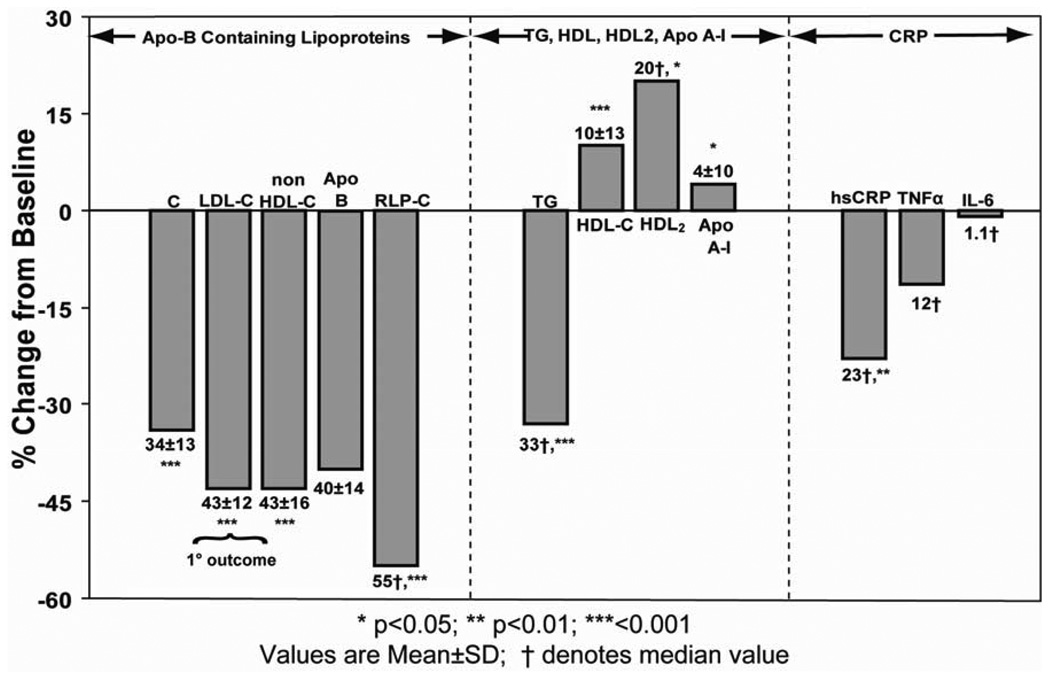
Results for the combined treatment groups are shown in Fig. 4. Mean LDL, non-HDL and apo-B reductions were 43, 43 and 40%, respectively (all p<0.001) (Fig. 4). The absolute LDL reductions attained with combination therapy were 81 mg/dL in the atorvastatin group 67mg/dL in the Slo-Niacin® group (Table 2A), yielding final LDL levels of 96 and 97 mg/dL respectively in the two groups.
Figure 4.
End of study measurements in the combined atorvastatin/Slo-Niacin and Slo-Niacin/ atorvastatin groups. The reduction in LDL-C is the primary end point. The 12.48% reduction in TNF-alpha is not significant but the concentration change between baseline and the end of combination therapy was statistically significant at 0.041 (Table 2D).
Remnant-like Lipoprotein: (RLP)
This apo B containing lipid fraction was reduced a median of 55% in the combined groups (Fig.4). Reductions in mg/dL are shown in Table 2A. The reduction in RLP was confirmed in four subjects by isolating intermediate-density lipoprotein (IDL) by ultracentrifugation. Mean values for RLP decreased 42.3% and IDL 40.0%.
Triglyceride, HDL-C, apo-A-I and LDL buoyancy
Median percent decreases in triglyceride were 26% with atorvastatin and 18% with Slo-Niacin® each given alone (Fig.3). When atorvastatin and Slo-Niacin® were given to those receiving the alternative drug as initial treatment, further (median percent) reductions were dissimilar: 14% in the atorvastatin/Slo-Niacin® group and 37% in Slo-Niacin®/atorvastatin group (Fig. 3). Baseline subject characteristics reveal no difference that might explain the disparate triglyceride reductions (Table 1). In the 36 subjects taken together, the median triglyceride reduction from baseline was 33% (p<0.001) (Fig. 4).
HDL-C increased with each drug given alone: 6.1% for atorvastatin and 7.9% for Slo-Niacin® (Fig. 3), 2.2 and 2.9 mg/dL respective absolute increases (Table 2B). Combined atorvastatin/Slo-Niacin® increased HDL-C 10.9 % in the atorvastatin first group and 10.0% in the Slo-Niacin® first group (Fig. 3), 4.2 and 3.6 mg/dL, respectively (Table 2B) and 10.4±13.0% for the two groups combined (Fig. 4) (p<0.001).
HDL2-C increased 0.5 mg/dL when Slo-Niacin® was given alone and 1.7 mg/dL when added to atorvastatin (p<0.01) (Table 2B). Administration of atorvastatin did not significantly change HDL2-C (−0.2 mg/dL from baseline, −0.2 mg/dL added to Slo-Niacin®) (Table 2B). The median HDL2-C increase with both treatment groups combined was 20% (p<0.032) (Fig. 4).
Apoprotein A-I levels generally followed the HDL-C increases (Table 2B), non-significantly in the two treatment groups individually (Fig. 3 and Table 2B), but 4% in the two treatment groups combined (p=0.019) (Fig. 4).
LDL buoyancy was measured in 24 subjects (data not shown in Table). Rf values represent the relative position of the LDL peak among 36 cholesterol containing fractions on gradient ultracentrifugation of whole plasma. From baseline values of 0.241±0.018 and 0.258±0.037 in the atorvastatin first and Slo-Niacin® first groups respectively, nonsignificant increases were observed to 0.250±0.025 and 0.267±0.035, respectively. Addition of the second drug yielded lesser increases of 0.011±0.021 and 0.008±0.020 units, respectively. The percent Rf increase in the combined treatment group was 15% (p=0.039).
Glucose and Insulin
Glucose levels increased 7.1 mg/dL from baseline when Slo-Niacin® was given first and 3.6 mg/dL when added to atorvastatin (both p< 0.05) (Table 2C). Glucose did not change significantly when atorvastatin was given alone or added to Slo-Niacin®. Glucose remained elevated at the end of combined drug treatment for each group (Table 2C) and in the combined treatment group, 5.2±8.9 mg/dL (p<0.001). Plasma insulin levels showed non-significant trends toward higher levels with the administration of Slo-Niacin®, whether given first or second (Table 2C).
Inflammation markers
Non-significant median decreases for both hsCRP and TNFα were observed in both the atorvastatin first and Slo-Niacin® first groups (Table 2D). In combined treatment, median hsCRP decreased significantly, 23.2% (Fig.4) and TNFa decreased significantly as the difference from baseline (p=0.014) and 12.5% (p=0.149). IL-6 levels were highly skewed, but no trends were seen in median values with Slo-Niacin® and atorvastatin given alone or given together.
Transaminases
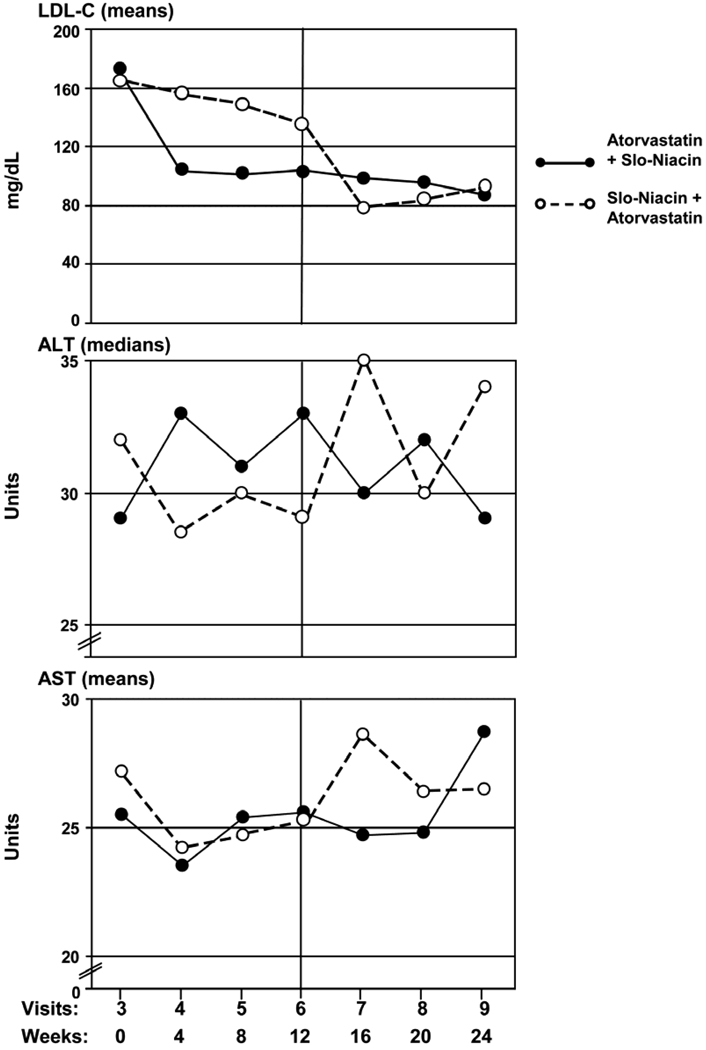
Median ALT levels increased 2 units from baseline with atorvastatin alone (p<0.05) (clinically non-significant), compared to a 5 unit decrease with Slo-Niacin® alone (Table 2E) (p=0.038). When Slo-Niacin® was added to atorvastatin, ALT decreased by 4 units (p<0.05) compared to an increase of 3 units when atorvastatin was added to Slo-Niacin® (p=0.057). The cross-over in ALT from higher values with atorvastatin to lower with Slo-Niacin® is seen in the monthly ALT plots (Fig. 5). Similar but lesser trends were seen in mean AST levels (Table 2E, Fig. 5).
Figure 5.
Serial monthly measurements of LDL, AST and ALT. ALT values are given as medians as the distribution is non-Gaussian. Monthly changes in LDL levels are shown for comparison.
Flushing Symptoms
Several parameters of flushing and flushing severity are reported in Table 3. Table 3A shows that about half of all subjects taking niacin had flushing, with about the same percentage of subjects reporting flushing at the lower doses of niacin as at the full dose of 1.5g. Table 3B shows that the most frequent level of flushing severity was distributed over the first 5 of the 6 levels of severity. Only one subject (6 %) in the Slo-Niacin®/atorvastatin group reported an intense flush lasting more than an hour, occurring at the 1g niacin dose. Table 3C shows the percentage of subjects experiencing the strongest level of flushing. The majority of these were at levels 3–5 in the first 3 months for both groups. One subject (5%) reported a level 6 flush. When taking Slo-Niacin® for 4–6 months, more subjects reported a lower level of flushing, at level two, than in the first 3 months in the Slo-Niacin®/atorvastatin group. Table 3D reports actions taken as a consequence of flushing. Two or three subjects in each group considered decreasing the niacin dose, and one did at the 1g level, but then progressed to the full dose. Five to 29% of subjects took aspirin at any given time. Most indicated that the aspirin helped the flushing symptoms.
Table 3.
Descriptions of Flushing Reported (% of Subjects)
| 3A. Any flushing in the past 4 weeks | ||||
|---|---|---|---|---|
| Atorvastatin/Niacin (n=19) |
Niacin/Atorvastatin (n=17) |
|||
| Study Visit: | Niacin Dose | Yes | Niacin Dose | Yes |
| 4 | 0 | - | 500 | 29 |
| 5 | 0 | - | 1000 | 59 |
| 6 | 0 | - | 1500 | 47 |
| 7 | 500 | 47 | 1500 | 41 |
| 8 | 1000 | 47 | 1500 | 59 |
| 9 | 1500 | 68 | 1500 | 41 |
| 3B. Most frequent level of flushing | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Severity: | 1 | 2 | 3 | 4 | 5 | 6 | 1 | 2 | 3 | 4 | 5 | 6 |
| Study Visit: | ||||||||||||
| 4 | - | - | - | - | - | - | 0 | 18 | 0 | 6 | 6 | 0 |
| 5 | - | - | - | - | - | - | 6 | 6 | 18 | 12 | 12 | 6 |
| 6 | - | - | - | - | - | - | 0 | 12 | 6 | 18 | 12 | 0 |
| 7 | 5 | 16 | 5 | 5 | 16 | 0 | 6 | 18 | 6 | 12 | 0 | 0 |
| 8 | 0 | 5 | 16 | 21 | 5 | 0 | 6 | 29 | 6 | 12 | 6 | 0 |
| 9 | 5 | 11 | 21 | 11 | 21 | 0 | 12 | 24 | 6 | 0 | 0 | 0 |
| 3C. Strongest flushing feeling | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Severity: | 1 | 2 | 3 | 4 | 5 | 6 | 1 | 2 | 3 | 4 | 5 | 6 |
| Study Visit: | ||||||||||||
| 4 | - | - | - | - | - | - | 0 | 6 | 12 | 0 | 12 | 0 |
| 5 | - | - | - | - | - | - | 0 | 6 | 12 | 18 | 6 | 0 |
| 6 | - | - | - | - | - | - | 0 | 12 | 0 | 12 | 18 | 6 |
| 7 | 5 | 5 | 0 | 11 | 26 | 0 | 0 | 18 | 6 | 6 | 12 | 0 |
| 8 | 0 | 5 | 11 | 21 | 11 | 0 | 0 | 29 | 0 | 12 | 24 | 0 |
| 9 | 5 | 11 | 11 | 16 | 21 | 5 | 6 | 18 | 6 | 12 | 0 | 0 |
| 3D. Actions related to flushing | ||||||||
|---|---|---|---|---|---|---|---|---|
| Action: | Consider Decrease | Decrease | Took ASA | ASA Helped | Consider Decrease | Decrease | Took ASA | ASA Helped |
| Study Visit: | ||||||||
| 4 | - | - | - | - | 0 | 0 | 6 | 6 |
| 5 | - | - | - | - | 0 | 0 | 18 | 18 |
| 6 | - | - | - | - | 18 | 0 | 24 | 24 |
| 7 | 0 | 0 | 5 | 5 | 0 | 0 | 18 | 18 |
| 8 | 11 | 5 | 11 | 11 | 0 | 0 | 29 | 18 |
| 9 | 11 | 0 | 21 | 16 | 0 | 0 | 18 | 18 |
Severity:
1 – Tingle
2 – Warm, few minutes
3 – Warm, red, 15 minutes
4 – Warm, red, 30 minutes
5 – Hot, flushed, 31–60 minutes
6 – Intense, > 60 minutes
Discussion
This study examined the effects of Slo-Niacin® and atorvastatin given separately and together on lipoproteins, glycemia, insulin, inflammatory markers and transaminases in subjects with combined hyperlipidemia and features of the metabolic syndrome. The principal outcome variable was LDL decrease with each individual drug and the two combined. This change was significant in all instances. Combined atorvastatin-Slo-Niacin® reductions in all apo-B lipoproteins were similar, 42–43% in LDL-C, non HDL-C and apo-B and 55% in remnant-like lipoproteins (RLP).
The LDL reduction of 43% in combined treatment was less than the sum of the separate effects of Slo-Niacin® and atorvastatin, 12 and 36%, respectively. However, the 43% LDL reduction with combined drug treatment equals that reported for 20 mg/day of atorvastatin in primary hypercholesterolemia and exceeds the 40.5% reduction with 80 mg/day of atorvastatin in persons with familial hypertriglyceridemia.24 In addition, 1.5g/day Slo-Niacin® and 10 mg/day atorvastatin/day attained reductions in LDL levels from 177 and 164 mg/dL to 96 and 98 mg/dL, respectively, within the LDL target of < 100mg/dL for persons with coronary artery disease or its equivalent.25
The LDL-C reduction of 12% with Slo-Niacin® alone is similar to LDL-C reductions of 12 and 13% observed with plain (immediate release (IR) niacin) and 11 to 13% with Niaspan®, both at 1500 mg/day.26 Likewise, an early study of Slo-Niacin® decreased LDL-C by 20% vs.7% in untreated control subjects (net decrease of 13%).21
HDL-C increased 8% with Slo-Niacin® given alone in the SLIM study. In an earlier study, Slo-Niacin® increased HDL-C 3% above control, also in a low-HDL population.21 On the other hand, Lavie et al found a 30 % HDL increase from baseline in a low-HDL population at an average Slo-Niacin dose of 2.4 g/day in 29 subjects. 20 When Slo- and plain (IR) niacins were compared in a crossover design, respective HDL-C increases were 15% and 25% above a 46.5 mg/dL baseline.19 When Niaspan® and plain niacin were compared at doses of 1500 mg/day, HDL-C increases were 17 and 19% above baseline levels of 43 to 45 mg/dL, respectively.26 The data suggest that the HDL-C response to niacin may depend in part on the baseline HDL level and the type of subjects studied. Further study is needed to determine if HDL raising with Slo-Niacin® is comparable or near comparable to the other forms of niacin.
Plasma triglyceride reductions were 15% with Slo-Niacin® alone, 26% with atorvastatin alone and 33% in combined treatment in the SLIM study. Previously reported triglyceride reductions with niacin monotherapy were 14.9% with Niaspan® and 12.9% with plain niacin.26 In that study, baseline triglyceride levels were 151 to 165 mg/dL, similar to the baseline values in the present study, indicating that the effect of Slo-Niacin® on triglycerides is similar to other niacins.
Remnant lipoprotein is a component of apo-B containing lipoproteins. The 55% reduction in the RLP measurement of this fraction with the combination of Slo-Niacin®/atorvastatin is notable, as remnant lipoproteins are considered to be highly atherogenic, possibly more so than LDL.27
Improvement in LDL buoyancy was also observed in this study with combined Slo-Niacin®/atorvastatin treatment consistent with previous work.28 LDL buoyancy is among the strongest predictors of CVD risk in persons with combined hyperlipidemia 29,30 and of coronary atherosclerosis regression determined angiographically with niacin, statin, and bile acid treatment.31
Previous studies of niacin/statin combinations report a range of LDL-C and HDL-C responses depending on statin type and dose. Two g/day Niaspan added to 40 mg/day simvastatin increased HDL 22% above that of simvastatin alone (baseline HDL-C 44 mg/dL) (SEACOAST Study).16 LDL did not decrease significantly.16 In the OCEANS Study, Niaspan® treatment incremented over 8 or 12 weeks to 2g/day, respectively increased HDL-C 21.6 and 19.5% from a baseline of 45.5 and 45 mg/dL and decreased LDL-C 21.9 and 20.1% on a 40 mg/dL simvastatin background.17 In the COMPELL Study, Niaspan® 2 g/atorvastatin 40 mg/day or Niaspan® 1g/rosuvastatin 20 mg/day increased HDL 15 and 17% above that of rosuvastatin 40 mg alone.18 In the SLIM Study, HDL-C increased 10% from a baseline of 38.5 mg/dL with combined Slo-Niacin® 1.5g/ atorvastatin 10mg treatment/day while LDL-C decreased 43%. Comparative studies are needed to address the comparability of HDL raising and LDL lowering among niacin products and niacin/statin combinations.
Glucose elevations observed with Slo-Niacin® alone or with atorvastatin ranged from 4 to7 %, typical of the elevations in non-diabetic persons in previous studies.32 Increases in insulin were 10 to 14%, consistent with the insulin resistance associated with niacin treatment.31 The insulin resistance may be clinically insignificant as niacin improves CVD risk as well as risk markers including adiponectin, brachial artery reactivity and carotid IMT.33 Even among participants in the HATS study with impaired fasting glucose, insulin resistance, metabolic syndrome or diabetes, the reduction in primary clinical events was 60%. 32
The median hsCRP reduction of 23% in the present study is consistent with improved vascular reactivity reported for niacin34 and statin 35 treatment. The greater decrease in CRP may reflect its hepatic origin compared to TNFα and IL-6 which reflect inflammation elsewhere, particularly adipose tissue. 36,37
Transaminase elevations are a typical concern in drug management of hyperlipidemia. Contrary to expectation, ALT decreased with Slo-Niacin® treatment and contrasts with the slight transaminase increase with atorvastatin. The transaminase reduction with Slo-Niacin® could be related to an improved hepatic triglyceride metabolism and reduced liver fat content. Previous studies of time-release niacin formulations with hepatotoxic potential have shown transaminase at niacin doses below 1.5g/day. At a dose of 500 mg/day of Goldline SR® time-release niacin, mean AST values increased from 23.8 to 27.9 units and ALT increased from 25.6 to 29.5 units and 40.4 and 36.3 units respectively at 1g/day.38 Enduracin®, another time release form was also associated with an increase in transaminase values at 1250 and 1500 mg/day 39 In our own study of Nicobid®, AST increased 14 to 21 units at a dose of 1.5g/day with no change at an equivalent dose of plain niacin and even higher at 3g/day, as also occurred with 3 g/day of plain niacin. 12 These comparisons illustrate that time-release formulations of niacin are not the same and that Slo-Niacin® has potential for use at 1.5 g without hepatotoxicity. Hepatotoxicity is common among at least 3 other time-release forms of time-release niacin as well as plain niacin at 3g/day.12,13,15,38,39
Regarding side effects, the dropout rate was 7.1% for niacin related complaints and 4.8% for flushing, similar to previous reports.17,18,40 Flushing itself was common, affecting 50% of subjects participating in the study and diminishing somewhat with time. Aspirin was taken in approximately 25% of cases and provided relief in 2/3rd’s of subjects. The main point is that the flushing symptoms were almost always tolerable, underscored by the fact that only 3 subjects dropped out for niacin-related complaints and there was no indication of hepatotoxicity. A randomized comparison of Slo-Niacin® with other forms of niacin is needed to determine if differences exist in the crucial parameters of flushing tolerance, propensity to hepatotoxicity and effects on lipoproteins and vascular response. Such a comparison is timely in light of the high priority given by the President’s Stimulus Plan on finding and testing cost-effective therapies in the management of prevalent diseases.41
In summary, Slo-Niacin® was well-tolerated alone and in combination with statin and benefitted all lipoprotein levels, lipoprotein composition and two of three inflammatory mediators, all as expected of niacin treatment. Notably, Slo-Niacin® extended LDL lowering in combination with atorvastatin 10 mg, approximating LDL lowering at 2–8 fold higher doses of atorvastatin given alone. The combination of Slo-Niacin® and atorvastatin modeled in the SLIM Study offers the possibility of a well-tolerated and cost-effective approach to managing combined hyperlipidemia 9 and its inherent cardiovascular disease burden. 1,13–15,42
Acknowledgement
We gratefully thank the study participants, and the staff of the Northwest Lipid Research Clinic and Kay Houser, Program Coordinator, all of whom made this study possible.
Support: Unrestricted Educational grant from Upsher-Smith Laboratories, Inc. Minneapolis, MN, the Clinical Nutrition Research Unit (CNRU) (NIH DK35816) and Diabetes and Endocrinology Research Center (NIH 5 P30 DK17047)
Abbreviations
- TG
Triglyceride
- C
cholesterol
- LDL
low-density lipoprotein
- HDL
high density lipoprotein
- hsCRP
highly sensitive C-reactive protein
- apo
apoprotein
- RLP
remnant-like lipoprotein
- IDL
intermediate density lipoprotein.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: Dr. Knopp has served as a consultant to Upsher-Smith Laboratories, Inc. and as a consultant and speaker for Abbott Laboratories
ClinicalTrials.gov Identifier: NCT00194402
References
- 1.Knopp R, Paramsothy P, Atkinson B, Dowdy A. Comprehensive lipid management vs.aggressive LDL lowering to reduce cardiovascular risk. American Journal of Cardiology. 2008;101(suppl) doi: 10.1016/j.amjcard.2008.02.038. 48B-57B. [DOI] [PubMed] [Google Scholar]
- 2.Clofibrate and niacin in coronary heart disease. Jama. 1975;231:360–381. [PubMed] [Google Scholar]
- 3.Canner PL, Berge KG, Wenger NK, Stamler J, Friedman L, Prineas RJ, Friedewald W. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol. 1986;8:1245–1255. doi: 10.1016/s0735-1097(86)80293-5. [DOI] [PubMed] [Google Scholar]
- 4.Brown G, Albers JJ, Fisher LD, Schaefer SM, Lin JT, Kaplan C, Zhao XQ, Bisson BD, Fitzpatrick VF, Dodge HT. Regression of coronary artery disease as a result of intensive lipid-lowering therapy in men with high levels of apolipoprotein B. N Engl J Med. 1990;323:1289–1298. doi: 10.1056/NEJM199011083231901. [DOI] [PubMed] [Google Scholar]
- 5.Brown BG, Zhao XQ, Chait A, Fisher LD, Cheung MC, Morse JS, Dowdy AA, Marino EK, Bolson EL, Alaupovic P, Frohlich J, Albers JJ. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345:1583–1592. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- 6.Taylor AJ, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation. 2004;110:3512–3517. doi: 10.1161/01.CIR.0000148955.19792.8D. [DOI] [PubMed] [Google Scholar]
- 7.Taylor AJ, Lee HJ, Sullenberger LE. The effect of 24 months of combination statin and extended-release niacin on carotid intima-media thickness: ARBITER 3. Curr Med Res Opin. 2006;22:2243–2250. doi: 10.1185/030079906x148508. [DOI] [PubMed] [Google Scholar]
- 8.Brunzell JD. Clinical practice Hypertriglyceridemia. N Engl J Med. 2007;357:1009–1017. doi: 10.1056/NEJMcp070061. [DOI] [PubMed] [Google Scholar]
- 9.Knopp RH. Drug treatment of lipid disorders. N Engl J Med. 1999;341:498–511. doi: 10.1056/NEJM199908123410707. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein JL, Schrott HG, Hazzard WR, Bierman EL, Motulsky AG. Hyperlipidemia incoronary heart disease. II. Genetic analysis of lipid levels in 176 families and delineation of a new inherited disorder, combined hyperlipidemia. J Clin Invest. 1973;52:1544–1568. doi: 10.1172/JCI107332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Genest JJ, Jr, Martin-Munley SS, McNamara JR, Ordovas JM, Jenner J, Myers RH, Silberman SR, Wilson PW, Salem DN, Schaefer EJ. Familial lipoprotein disorders in patients with premature coronary artery disease. Circulation. 1992;85:2025–2033. doi: 10.1161/01.cir.85.6.2025. [DOI] [PubMed] [Google Scholar]
- 12.Knopp RH, Ginsberg J, Albers JJ, Hoff C, Ogilvie JT, Warnick GR, Burrows E, Retzlaff B, Poole M. Contrasting effects of unmodified and time-release forms of niacin on lipoproteins in hyperlipidemic subjects: clues to mechanism of action of niacin. Metabolism. 1985;34:642–650. doi: 10.1016/0026-0495(85)90092-7. [DOI] [PubMed] [Google Scholar]
- 13.Vaccari CS, Hammoud RA, Nagamia SH, Ramasamy K, Dollar AL, Khan BV. Revisiting niacin: reviewing the evidence. J Clin Lipidol. 2007;1:248–255. doi: 10.1016/j.jacl.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Davidson MH. Niacin use and cutaneous flushing: mechanisms and strategies for prevention. Am J Cardiol. 2008;101 doi: 10.1016/j.amjcard.2008.02.028. 14B-19B. [DOI] [PubMed] [Google Scholar]
- 15.Guyton JR. Niacin in cardiovascular prevention: mechanisms, efficacy, and safety. CurrOpin Lipidol. 2007;18:415–420. doi: 10.1097/MOL.0b013e3282364add. [DOI] [PubMed] [Google Scholar]
- 16.Ballantyne CM, Davidson MH, McKenney J, Keller LH, Bajorunas DR, Karas RH. Comparison of the safety and efficacy of a combination tablet of niacin extended release and simvastatin vs simvastatin monotherapy in patients with increased non-HDL cholesterol (from the SEACOAST I study) Am J Cardiol. 2008;101:1428–1436. doi: 10.1016/j.amjcard.2008.02.092. [DOI] [PubMed] [Google Scholar]
- 17.Karas RH, Kashyap ML, Knopp RH, Keller LH, Bajorunas DR, Davidson MH. Long-term safety and efficacy of a combination of niacin extended release and simvastatin in patients with dyslipidemia: the OCEANS study. Am J Cardiovasc Drugs. 2008;8:69–81. doi: 10.2165/00129784-200808020-00001. [DOI] [PubMed] [Google Scholar]
- 18.McKenney JM, Jones PH, Bays HE, Knopp RH, Kashyap ML, Ruoff GE, McGovern ME. Comparative effects on lipid levels of combination therapy with a statin and extended-release niacin or ezetimibe versus a statin alone (the COMPELL study) Atherosclerosis. 2007;192:432–437. doi: 10.1016/j.atherosclerosis.2006.11.037. [DOI] [PubMed] [Google Scholar]
- 19.Brown BG, Bardsley J, Poulin D, Hillger LA, Dowdy A, Maher VM, Zhao XQ, Albers JJ, Knopp RH. Moderate dose, three-drug therapy with niacin, lovastatin, and colestipol to reduce low-density lipoprotein cholesterol <100 mg/dl in patients with hyperlipidemia and coronary artery disease. Am J Cardiol. 1997;80:111–115. doi: 10.1016/s0002-9149(97)00303-2. [DOI] [PubMed] [Google Scholar]
- 20.Lavie CJ, Mailander L, Milani RV. Marked benefit with sustained-release niacin therapy in patients with "isolated" very low levels of high-density lipoprotein cholesterol and coronary artery disease. Am J Cardiol. 1992;69:1083–1085. doi: 10.1016/0002-9149(92)90868-y. [DOI] [PubMed] [Google Scholar]
- 21.Squires RW, Allison TG, Gau GT, Miller TD, Kottke BA. Low-dose, time-release nicotinic acid: effects in selected patients with low concentrations of high-density lipoprotein cholesterol. Mayo Clin Proc. 1992;67:855–860. doi: 10.1016/s0025-6196(12)60824-6. [DOI] [PubMed] [Google Scholar]
- 22.Warnick GR. Enzymatic methods for quantification of lipoprotein lipids. In: Segrest JP, Albers JJ, editors. Methods in Enzymology, Part B: Characterization, Cell Biology, and Metabolism. New York: Academic Press; 1986. pp. 101–123. [DOI] [PubMed] [Google Scholar]
- 23.Nakajima K, Saito T, Tamura A, Suzuki M, Nakano T, Adachi M, Tanaka A, Tada N, Nakamura H, Campos E, et al. Cholesterol in remnant-like lipoproteins in human serum using monoclonal anti apo B-100 and anti apo A-I immunoaffinity mixed gels. Clin Chim Acta. 1993;223:53–71. doi: 10.1016/0009-8981(93)90062-9. [DOI] [PubMed] [Google Scholar]
- 24.Physicians'desk reference. Montvale, N.J: Thompson PDR; 2006. pp. 2495–2498. [Google Scholar]
- 25.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) Jama. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 26.Knopp RH, Alagona P, Davidson M, Goldberg AC, Kafonek SD, Kashyap M, Sprecher D, Superko HR, Jenkins S, Marcovina S. Equivalent efficacy of a time-release form of niacin (Niaspan) given once-a-night versus plain niacin in the management of hyperlipidemia. Metabolism. 1998;47:1097–1104. doi: 10.1016/s0026-0495(98)90284-0. [DOI] [PubMed] [Google Scholar]
- 27.Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 28.Jafri H, Alsheikh AA, Mooney P, Kimmelstiel CD, Karas RH, Kuvin JT. Extended-release niacin reduces LDL particle number without changing total LDL cholesterol in patients with stable CAD. J Clin Lipidol. 2009;3:45–50. doi: 10.1016/j.jacl.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Williams PT, Superko HR, Haskell WL, Alderman EL, Blanche PJ, Holl LG, Krauss RM. Smallest LDL particles are most strongly related to coronary disease progression in men. Arterioscler Thromb Vasc Biol. 2003;23:314–321. doi: 10.1161/01.atv.0000053385.64132.2d. [DOI] [PubMed] [Google Scholar]
- 30.St-Pierre AC, Cantin B, Dagenais GR, Mauriege P, Bernard PM, Despres JP, Lamarche B. Low-density lipoprotein subfractions and the long-term risk of ischemic heart disease in men: 13-year follow-up data from the Quebec Cardiovascular Study. Arterioscler Thromb Vasc Biol. 2005;25:553–559. doi: 10.1161/01.ATV.0000154144.73236.f4. [DOI] [PubMed] [Google Scholar]
- 31.Zambon A, Hokanson JE, Brown BG, Brunzell JD. Evidence for a new pathophysiological mechanism for coronary artery disease regression: hepatic lipase-mediated changes in LDL density. Circulation. 1999;99:1959–1964. doi: 10.1161/01.cir.99.15.1959. [DOI] [PubMed] [Google Scholar]
- 32.Vittone F, Chait A, Morse JS, Fish B, Brown BG, Zhao XQ. Niacin plus Simvastatin Reduces Coronary Stenosis Progression Among Patients with Metabolic Syndrome Despite a Modest Increase in Insulin Resistance: A Subgroup Analysis of the HDL-Atherosclerosis Treatment Study (HATS) J Clin Lipidol. 2007;1:203–210. doi: 10.1016/j.jacl.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poynten AM, Gan SK, Kriketos AD, O'Sullivan A, Kelly JJ, Ellis BA, Chisholm DJ, Campbell LV. Nicotinic acid-induced insulin resistance is related to increased circulating fatty acids and fat oxidation but not muscle lipid content. Metabolism. 2003;52:699–704. doi: 10.1016/s0026-0495(03)00030-1. [DOI] [PubMed] [Google Scholar]
- 34.Vaccari CS, Nagamia S, Thoenes M, Oguchi A, Hammoud R, Khan BV. Efficacy of controlled-release niacin in treatment of metabolic syndrome: correlation to surrogate markers of atherosclerosis, vascular reactivity and inflammation. J Clin Lipidol. 2007;1:605–613. doi: 10.1016/j.jacl.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Treasure CB, Klein JL, Weintraub WS, Talley JD, Stillabower ME, Kosinski AS, Zhang J, Boccuzzi SJ, Cedarholm JC, Alexander RW. Beneficial effects of cholesterol-lowering therapy on the coronary endothelium in patients with coronary artery disease. N Engl J Med. 1995;332:481–487. doi: 10.1056/NEJM199502233320801. [DOI] [PubMed] [Google Scholar]
- 36.Haus JM, Kashyap SR, Kasumov T, Zhang R, Kelly KR, Defronzo RA, Kirwan JP. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes. 2009;58:337–343. doi: 10.2337/db08-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nieto-Vazquez I, Fernandez-Veledo S, de Alvaro C, Lorenzo M. Dual role of interleukin-6 in regulating insulin sensitivity in murine skeletal muscle. Diabetes. 2008;57:3211–3221. doi: 10.2337/db07-1062. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.McKenney JM, Proctor JD, Harris S, Chinchili VM. A comparison of the efficacy and toxic effects of sustained- vs immediate-release niacin in hypercholesterolemic patients. Jama. 1994;271:672–677. [PubMed] [Google Scholar]
- 39.Keenan JM, Fontaine PL, Wenz JB, Myers S, Huang ZQ, Ripsin CM, Ripsin CM. Niacin revisited A randomized, controlled trial of wax-matrix sustained-release niacin in hypercholesterolemia. Arch Intern Med. 1991;151:1424–1432. doi: 10.1001/archinte.151.7.1424. [DOI] [PubMed] [Google Scholar]
- 40.Guyton JR, Simmons PD. Flushing and other dermatologic adverse events associated with extended-release niacin therapy. J Clin Lipidol. 2009;3:101–108. doi: 10.1016/j.jacl.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 41.Steinbrook R. Health care and the American Recovery and Reinvestment Act. N Engl J Med. 2009;360:1057–1060. doi: 10.1056/NEJMp0900665. [DOI] [PubMed] [Google Scholar]
- 42.Brown BG, Zhao XQ. Nicotinic acid, alone and in combinations, for reduction of cardiovascular risk. Am J Cardiol. 2008;101 doi: 10.1016/j.amjcard.2008.02.039. 58B-62B. [DOI] [PubMed] [Google Scholar]