Abstract
Objective
Resistin is associated with inflammation and insulin resistance, and exerts direct effects on myocardial cells including hypertrophy and altered contraction. We investigated the association of serum resistin concentrations with risk for incident heart failure (HF) in humans.
Methods and Results
We studied 2902 older persons without prevalent HF (age, 73.6±2.9 years; 48.1% men; 58.8% white) enrolled in the Health ABC Study. Correlation between baseline serum resistin concentrations (20.3±10.0 ng/mL) and clinical variables, biochemistry panel, markers of inflammation and insulin resistance, adipocytokines, and measures of adiposity was weak (all rho<0.25). During a median follow-up of 9.4 years, 341 participants (11.8%) developed HF. Resistin was strongly associated with risk for incident HF in Cox proportional hazards models controlling for clinical variables, biomarkers, and measures of adiposity (HR, 1.15 per 10.0 ng/mL in adjusted model; 95%CI, 1.05–1.27; P=0.003). Results were comparable across sex, race, diabetes mellitus, and prevalent and incident coronary heart disease subgroups. In participants with available left ventricular ejection fraction at HF diagnosis (265 of 341; 77.7%), association of resistin with HF risk was comparable for cases with reduced vs. preserved ejection fraction.
Conclusions
Serum resistin concentrations are independently associated with risk for incident HF in older persons.
Keywords: heart failure, elderly, resistin
Resistin, a 12.5-kDa polypeptide, was initially discovered in the adipose tissue but now is known to be produced by other cell types e.g. macrophages.1–3 Resistin concentrations have been correlated with the risk for coronary heart disease (CHD),4, 5 renal dysfunction,6 and outcomes among stroke patients.7 Higher concentrations are found in subjects with prevalent heart failure (HF).8 Although the exact function of resistin is not known, it has been associated with insulin resistance and inflammation.1, 4 Several markers of inflammation e.g. C- reactive protein (CRP), interleukin-6 (IL-6), and tumor necrosis factor alpha (TNF-α) have been associated with incident HF.9, 10 Similarly, various measures of insulin resistance are associated with increased HF risk,11, 12 Thus, resistin may be related to HF risk through multiple pathways. Interestingly, several direct myocardial effects of resistin have also been demonstrated.13 In neonatal rats, adenovirus-mediated overexpression of resistin results in increased sarcomere organization, cell size, and protein synthesis in cardiomyocytes, and expression of atrial natriuretic factor and beta-myosin heavy chain. Overexpression of resistin in adult rat cardiomyocytes altered mechanics by depressing cell contractility as well as contraction and relaxation velocities. Thus it is possible that resistin may confer increased risk for HF through direct and indirect mechanisms. In this study, we sought to assess the association between serum resistin concentrations at baseline and development of new onset HF among older persons in the Health ABC Study.
METHODS
Study Population
The Health ABC Study enrolled 3075 well functioning, community-dwelling individuals aged 70 to 79 years between April 1997 and June 1998. Participants were identified from a random sample of white Medicare beneficiaries and all age-eligible black community residents in designated zip codes areas surrounding Pittsburgh and Memphis. Exclusion criteria included difficulties with activities of daily living, obvious cognitive impairment, inability to communicate, anticipated move within 3 years, or participation in a trial involving life-style intervention. The institutional review boards at both study sites approved the protocol.
Participants with HF or missing data on HF at baseline (n=140) were excluded from this investigation. Of the 2935 participants without prevalent HF, 2905 (99.0%) had available serum resistin concentrations. Three participants were excluded because of extreme outlying resistin values; the remaining 2902 participants were included in this study.
Serum Biomarker Measurements
In the Health ABC Study, blood samples were obtained after overnight fasting, frozen at −70°C, and shipped to the core laboratory at the University of Vermont. Serum resistin concentration was measured using a sandwich enzyme-linked immunosorbent assay (ELISA) (Linco Research Inc, St. Charles, MO). Intra- and inter-assay coefficients of variation for this assay are 4.5% and 7.4%, respectively.14 Cytokines were measured in duplicate by ELISA. The detectable limit for IL-6 was 0.10 pg/mL and for TNF-a 0.18 pg/mL. Serum concentrations of CRP were measured in duplicate by ELISA on the basis of purified protein and polyclonal anti-CRP antibodies. The CRP assay was standardized according to WHO First International Reference Standard with a sensitivity of 0.08 mg/L. Blind duplicate analyses (n=150) for IL-6, CRP, and TNF-α showed inter-assay coefficients of variation of 10.3%, 8.0%, and 15.8%, respectively. Serum leptin and adiponectin concentrations were measured in duplicate by radioimmunoassay (Linco Research Inc, St Charles, MO).
Measures of Adiposity
Total fat mass was determined with whole-body dual X-ray absorptiometry (DEXA) performed using the pencil beam technology (QDR 00, Hologic, Waltham, MA). Abdominal visceral and subcutaneous adipose tissue areas at the lumbar (L4–L5) level were measured with computed tomography (CT) using a Somatom Plus 4 (Siemens, Erlangen, Germany) or a Picker PQ 2000S (Marconi Medical Systems, Cleveland, OH) scanner in Memphis, TN, and a 9800 Advantage scanner (General Electric, Milwaukee, WI) in Pittsburgh, PA. Scans were conducted at 120 kVp, 200 to 250 mA sec, with 10mm slice thickness. Areas were calculated by multiplying the number of pixels of a given tissue type by the pixel area using imaging software (RSI Systems, Boulder, CO). Visceral fat was manually distinguished from subcutaneous fat by tracing along the fascial plane defining the internal abdominal wall.
Study Definitions
Race was self-defined by the participant. Diabetes mellitus was considered present if the participant reported a history of diabetes mellitus or use of anti-hyperglycemic medication. Smoking was defined as current, past (≥100 lifetime cigarettes), or never. Left ventricular hypertrophy was diagnosed based on electrocardiogram using the following voltage criteria; R amplitude > 26 mm in either V5 or V6, or R amplitude > 20 mm in any of leads I, II, III, aVF, or R amplitude > 12 mm in lead aVL or R amplitude in V5 or V6 plus S amplitude in V1 > 35 mm. Coronary heart disease was defined as; a) history of surgical or percutaneous revascularization; or b) electrocardiographic evidence of myocardial infarction; or c) self-reported history of myocardial infarction or angina accompanied by use of antianginal medications. Hypertension was defined as self-reported history of physician diagnosis accompanied by use of antihypertensive medications. Incident CHD was defined as hospitalization for myocardial infarction or angina pectoris, or elective revascularization.
Study Outcome
All participants were asked to report any hospitalizations and every 6 months were asked direct questions about interim cardiovascular events. Medical records for overnight hospitalizations were reviewed at each site. All first admissions with an overnight stay that was confirmed as related to HF were classified as incident HF. Local adjudicators classified HF, based on symptoms, signs, chest radiograph results, and echocardiographic findings, using criteria similar to those used in the Cardiovascular Health Study.15 The criteria required at least HF diagnosis from a physician and treatment for HF.16 All deaths were reviewed by the Health ABC Study diagnosis and disease ascertainment committee and underlying causes of death were determined by central adjudication. Information on ejection fraction post-HF development was abstracted from the hospital medical records during the index hospitalization and was derived from echocardiography or left ventriculography reports.
Statistical Analysis
The correlation between resistin concentrations with baseline participant characteristics and other biomarkers was evaluated by non-parametric tests (Spearman’s rank correlation for continuous and rank sum for binary variables). The univariable relation of resistin to HF risk was evaluated using Cox proportional hazards models with resistin entered as continuous variable. For comparison, we also evaluated the univariable relation of inflammatory markers, insulin resistance markers, adipose tissue hormones, and DEXA- and CT-derived measures of adiposity with incident HF risk.
In multivariable Cox models, we controlled for four sets of hierarchical, a-priori defined domains. First, we controlled for independent clinical predictors of incident HF as identified in the Health ABC HF model.12 Second, we additionally controlled for all baseline clinical variables with significant (p<0.05) correlation with resistin concentrations. Because resistin is associated with inflammation and glucose metabolism and possibly provides information that is collinear with other markers, the third set of variables included also baseline markers of inflammation (CRP, IL-6, and TNF-α) and insulin resistance (hemoglobin HbA1c and fasting insulin concentrations), and adipocytokines (leptin, adiponectin). Finally, because measures of adiposity might provide prognostic information that overlaps with that provided by resistin, the fourth set of variables included all previous variables and also CT- (abdominal total, visceral, and subcutaneous fat area) and DEXA- (total and trunk fat mass, percent body fat) derived measures of adiposity. In a secondary analysis, we evaluated the association between resistin and risk for HF with reduced (≤40%) vs. preserved (>40%) left ventricular ejection fraction. For these analyses, only incident HF cases with documented left ventricular ejection fraction were considered as events in separate (reduced vs. preserved) Cox models.
All continuous predictors were evaluated for non-linear associations with incident HF risk and appropriately transformed with fractional polynomials. The proportional hazards assumption was evaluated by examining the Schoenfeld residuals. For multivariable models, missing values of covariates were imputed using multiple imputation by chained equations as introduced by van Buuren et al.17, 18 Parameter estimates and confidence intervals were obtained by combining 10 imputed datasets using the method described by Barnard and Rubin to account for possible error in missing value analysis.19 A two-sided P<0.05 was accepted as statistically significant for all analyses. Analyses were performed with STATA 10 (StataCorp, College Station, TX).
RESULTS
Baseline Characteristics and Serum Resistin Concentrations
The mean age of participants was 73.6±2.9 years with 48.1% men and 58.8% white. Mean resistin concentration was 20.3±10.0 ng/mL (median, 18.0; interquartile range [IQR], 14.0 – 24.1; range, 3.0–101.1). Table 1 presents baseline population characteristics and their correlation with resistin concentrations. Multiple statistically significant but weak correlations were detected (all rho<0.25).
Table 1.
Baseline participant characteristics and correlation with resistin concentrations
| Variable | Value | rho* | P value |
|---|---|---|---|
| Age, years | 73 (71, 76) | 0.061 | 0.001 |
| Male sex, % | 48.1 | 0.040 | 0.030 |
| Black race, % | 41.2 | 0.039 | 0.035 |
| Body mass index, kg/m2 | 26.8 (24.1, 30.1) | 0.090 | <0.001 |
| Weight, kg | 75.0 (65.3, 85.0) | 0.104 | <0.001 |
| Waist circumference, cm | 99.2 (91.5, 107.0) | 0.096 | <0.001 |
| Thigh circumference, cm | 50.8 (47.1, 55.0) | 0.047 | 0.012 |
| Waist/thigh circumference ratio | 1.95 (1.80, 2.08) | 0.054 | 0.003 |
| Diabetes mellitus, % | 14.7 | 0.075 | <0.001 |
| Hypertension, % | 43.5 | 0.122 | <0.001 |
| Coronary heart disease, % | 16.5 | 0.056 | 0.003 |
| Left ventricular hypertrophy, % | 11.9 | 0.018 | 0.34 |
| Smoking, current %† | 10.4 | 0.022 | 0.23 |
| Alcohol consumption, % | −0.054‡ | 0.004 | |
| Never | 49.8 | ||
| Occasional | 21.2 | ||
| 1–7 drinks/week | 21.6 | ||
| 8 drinks/week | 7.4 | ||
| Physical activity, kcal/kg/week | 65.2 (38.5, 107.5) | −0.047 | 0.017 |
| Systolic blood pressure, mg/dl | 134 (122, 148) | 0.055 | 0.003 |
| Diastolic blood pressure, mg/dl | 71 (64, 79) | −0.006 | 0.73 |
| Heart rate, bpm | 64 (57, 72) | 0.050 | 0.006 |
| Fasting glucose, mg/dl | 94 (87, 105) | 0.066 | <0.001 |
| Albumin, gm/dl | 4.0 (3.8, 4.2) | −0.030 | 0.11 |
| Creatinine, mg/dl | 1.0 (0.9, 1.2) | 0.248 | <0.001 |
| Total cholesterol, mg/dl | 201 (177, 226) | −0.067 | <0.001 |
| Low density lipoprotein, mg/dl | 120 (99, 143) | −0.049 | 0.009 |
| High density lipoprotein, mg/dl | 52 (42, 63) | −0.098 | <0.001 |
| Triglycerides, mg/dl | 117 (88, 162) | 0.074 | <0.001 |
Values for continuous variables represent median (interquartile range).
Spearman’s rank correlation rho for continuous variables; for binary variables (present=1, absent=0), the z score of the rank sum test is transformed into the corresponding rho value for comparison purposes; rho=1 denotes perfect positive correlation, rho=−1 denotes perfect negative correlation, and rho=0 absent correlation.
Nonparametric trend converted to corresponding rho value
Past and never smoker categories were collapsed for this analysis
The serum concentrations of inflammatory markers (CRP, IL-6, TNF-a), insulin resistance (fasting insulin, hemoglobin A1c) markers, and adipocytokines (leptin, adiponectin) all had weak correlations with resistin concentrations (Supplementary Table A). Same was true for DEXA-derived (total fat mass, total fat-free mass, percent body fat, trunk fat mass, trunk fat-free mass) and CT-derived (total, visceral, and subcutaneous abdominal fat area) measures of adiposity (Supplementary Table B).
Resistin and Incident Heart Failure
During a median follow-up of 9.4 years (IQR, 7.0–9.4). 341 participants (11.8%) developed incident HF (14.9 per 1000 person-years). Mean resistin concentration was 24.0±12.7 ng/mL (median, 21.3; IQR, 15.9–27.7) among participants who developed HF as compared to 19.8±9.4 ng/mL (median, 17.7; IQR, 13.7–23.1) among those who did not (P<0.001). In univariable analysis, resistin concentrations had a strong, linear relation with risk for incident HF; HR was 1.37 (95% CI, 1.27–1.47; P<0.001) per standard deviation (10.0 ng/mL) of baseline resistin concentration. Figure 1 presents the observed HF incidence in the cohort in relation to baseline resistin concentrations. In a head-to-head, univariable comparison with inflammatory cytokines, insulin resistance markers, adipose tissue hormones, and adiposity parameters derived by imaging, resistin was a strong predictor of incident HF compared to all other markers, second only to interleukin-6 (Table 2).
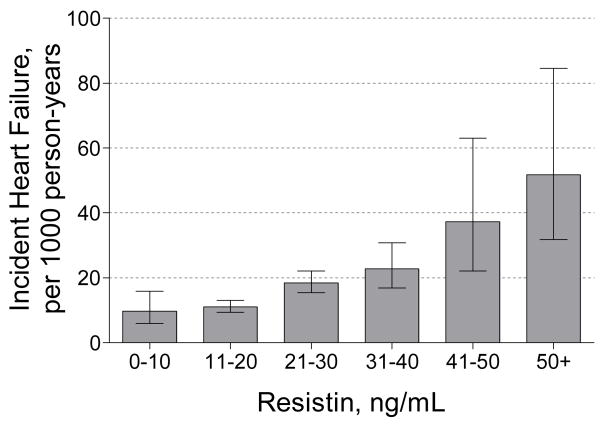
Figure 1.
Baseline serum resistin concentrations and incident heart failure rates (per 1000 person years) among participants without prevalent HF at baseline in the Health ABC Study. Error bars represent 95% confidence intervals.
Table 2.
Strength of association with incident heart failure risk
| Variable | Form* | LR X2† | P value | C |
|---|---|---|---|---|
| Resistin, μg/mL | Linear | 43.8 | <0.001 | 0.638 |
| Interleukin-6, pg/mL | 1/√x | 69.4 | <0.001 | 0.657 |
| Tumor necrosis factor α, pg/mL | Linear | 44.1 | <0.001 | 0.638 |
| C-reactive protein, μg/mL | 1/√x | 28.5 | <0.001 | 0.627 |
| Hemoglobin A1c, % | Linear | 18.0 | <0.001 | 0.614 |
| Fasting insulin, μIU/mL | Linear | 2.7 | 0.103 | 0.595 |
| Adiponectin, μg/mL | Linear | 1.0 | 0.320 | 0.593 |
| Leptin, ng/mL | Linear | 3.0 | 0.082 | 0.595 |
| DEXA – total fat mass, kg | Linear | 3.8 | 0.052 | 0.596 |
| DEXA – percent body fat, % | Linear | 2.0 | 0.156 | 0.593 |
| DEXA – trunk fat mass, kg | Linear | 7.4 | 0.007 | 0.600 |
| CT – abdominal total area, cm2 | Linear | 6.1 | 0.013 | 0.599 |
| CT – abdominal visceral fat area, cm2 | Linear | 12.5 | <0.001 | 0.606 |
| CT – abdominal subcutaneous fat area, cm2 | Linear | 0.4 | 0.507 | 0.592 |
Evaluated by first and second order fractional polynomial functions in univariable Cox models.
Adjusted for age, gender, and race.
CT: computed tomography; DEXA: dual X-ray absorptiometry; LR: likelihood ratio
Resistin concentrations were significantly associated with incident HF after adjustment for baseline clinical characteristics and predictors of incident HF (Table 3, models 1 & 2). This relation persisted after controlling for inflammatory cytokines, markers of insulin resistance, and adipose tissue hormones (model 3), and after controlling for adiposity parameters derived by DEXA and CT (model 4).
Table 3.
Serum resistin concentrations and incident heart failure risk in adjusted models
| Variable | HR (95% CI) | P value |
|---|---|---|
| Model 1 – Predictors of incident heart failure * | 1.22 (1.12–1.33) | <0.001 |
| Model 2 – Model 1 plus clinical correlates of resistin † | 1.22 (1.11–1.33) | <0.001 |
| Model 3 – Model 2 plus serum markers ‡ | 1.16 (1.06–1.27) | 0.002 |
| Model 4 – Model 3 plus adiposity parameters § | 1.15 (1.05–1.27) | 0.003 |
Hazard ratios are calculated per standard deviation (10.0 ng/mL) of resistin concentrations.
Includes age, history of coronary heart disease and smoking, systolic blood pressure and heart rate, electrocardiographic left ventricular hypertrophy, and serum levels of creatinine, fasting glucose, and albumin.12
Clinical parameters from Table 1 not included in model 1 with a significant (p<0.05) correlation with serum resistin concentrations.
Inflammatory markers (C reactive protein, interleukin-6, tumor necrosis factor-α), insulin resistance markers (HbA1c, insulin), and adipocytokines (leptin, adiponectin).
Computed tomography (abdominal total, visceral, and subcutaneous fat area) and dual X-ray absorptiometry (total and trunk fat, percent body fat) derived adiposity parameters.
The association between resistin concentrations and incident HF was similar in sex, prevalent CHD, and prevalent diabetes mellitus based subgroups (P=0.76, P=0.95, and P=0.97 for the interaction terms, respectively). Association with incident HF risk was stronger in white as compared with black participants; this interaction however did not reach statistical significance (P=0.20 for the interaction term).
Incident Coronary Heart Disease, Resistin, and Heart Failure
In participants without CHD at baseline (n=2383), the unadjusted association of resistin concentrations with incident HF risk was similar in those who developed CHD during follow-up (n=316; HR, 1.36; 95% CI, 1.20–1.55; P<0.001) and in those who remained free of CHD during follow up (n=2067; HR, 1.38; 95% CI, 1.20–1.57; P<0.001); P=0.95 for the interaction term.
Heart Failure with Reduced vs. Preserved Ejection Fraction
Data on left ventricular ejection fraction at time of HF diagnosis were available in 265 of 341 participants (77.7%). Mean ejection fraction was 42±16% (median: 42, IQR, 30–55); 129/265 participants (48.7%) had ejection fraction ≤40% while 136/265 (51.3%) had ejection fraction >40%. Baseline resistin concentrations were strongly predictive of incident HF in both participants with reduced (HR per 10ng/ml resistin concentration 1.35, 95% CI, 1.20–1.53, P<0.001) and preserved ejection fraction (HR 1.42, 95% CI, 1.27–1.58, P<0.001) in unadjusted analyses, P=0.58 for the comparison of HR.
Incremental value of Resistin for Incident Heart Failure Prediction
Clinical predictors of incident HF, as identified in the Health ABC HF model,12 had C index 0.718 (95% CI, 0.690–0.747) for HF prediction throughout the follow-up period. Addition of resistin to this model increased C index to 0.726 (95% CI, 0.697–0.754), P=0.035 for the increase.
DISCUSSION
In this population-based study, we report an independent association between resistin concentrations and risk for incident HF among older persons. This association persisted after controlling for baseline characteristics, predictors for incident HF, markers of inflammation and insulin resistance, adipocytokines, and multiple measures of adiposity. Our data adds to the growing literature on the role of serum resistin in modulating cardiovascular risk.
Beyond the statistical association, the true value of “novel” risk markers is their independent association with outcome. Many markers may provide collinear information affecting common pathophysiologic pathways. In our study, the correlation of resistin with baseline clinical variables and biomarkers, although statistically significant in many instances due to the large sample size, was overall weak. Most importantly, the association between resistin and incident HF risk persisted after extensive adjustment for possible confounders representing various disparate pathways. We also observed that resistin was a stronger predictor of incident HF when directly compared with other biomarkers, with the exception of interleukin-6. Our findings extend the results of the study by the Framingham investigators,20 which demonstrated that serum resistin concentrations were associated with risk for HF after controlling for clinical characteristics, inflammatory markers, and B-type natriuretic peptide levels in a younger population. Taken together, these findings signal to the strength of association between serum resistin and incident HF. Although resistin concentrations have been associated with diabetes mellitus21 and CHD,5 the degree of association with incident HF in our study was comparable among prevalent and incident CHD and diabetes mellitus subgroups, a finding that was also present in the Framingham report.20 Finally, this association was consistent across sex and race.
Initially it was felt that resistin was derived primarily from adipocytes. Beyond the originally described “passive” role of storing excess energy, the adipose tissue is an “active” endocrine organ.20–22 Adipocytokines affect the structure and function of various organ systems. Resistin expression and serum concentrations are related to obesity, insulin resistance, and inflammation in humans.23–25 These mechanisms are directly involved in the pathogenesis of CHD10, 26, 27 and the development and progression of both ischemic and non-ischemic cardiomyopathy.28, 29 Beyond the indirect effects, overexpression of resistin in cardiomyocytes has been associated with altered response to ischemia-reperfusion injury,30 depressed contractility,13 and hypertrophy.13 All these observations make our association between resistin and HF theoretically plausible. Interestingly, considering the weak correlations that we observed between multiple measures of adiposity and serum resistin concentrations, it raises the question whether resistin is primarily derived from the adipose tissue or elsewhere; and indeed multiple other cell types have now been associated with secretion of resistin e.g. macrophages.2, 3
Multiple reasons for this strong association between serum resistin and risk for incident HF can be hypothesized. A marker associated with multiple pathways of disease causation may be stronger in its association with outcomes than those associated with individual pathways. As discussed above, resistin is associated with inflammation and insulin resistance, and also direct effects of resistin on the myocardium have been demonstrated. Another possibility is a yet undefined direct pathophysiologic role of resistin. Finally, a recent experimental study showed that mechanical stretch enhances expression of resistin in cultured rat cardiomyocytes via TNF-α31 Thus, the possibility that resistin concentrations reflect cardiac load cannot be excluded.
What are the clinical implications of these results? First, considering the novelty of our data, these results will need to be replicated in other populations. If these results are consistent in other studies, then it is conceivable that serum resistin measurements may aid in improved identification of elderly subjects, beyond usual risk factors, at high risk for HF. Similarly, as the pathophysiology of new onset HF in general and the physiologic effects of resistin in specific are better understood, it is possible that serum resistin concentrations may help guide therapy. For example, neutralization of resistin activity by injection of antibodies against resistin decreases blood glucose levels and improves insulin sensitivity in obese, insulin-resistant mice.20 Most data on resistin currently however are derived from mice. Energy metabolism in human differs from mice and therefore it is not known how much of the resistin physiology in mice is applicable to humans. Thus, the role of resistin in modulating HF risk remains speculative at this point.
Our study has several limitations. Diagnosis of HF was based on HF hospitalization. Because some participants may have developed HF without hospitalization, HF rates are likely underestimated. Echocardiography was not performed at baseline. Thus, the association of resistin concentrations with structural characteristics of the heart could not be assessed. Resistin has been shown to modulate release of natriuretic peptides in experimental studies.13,30 However, baseline concentrations of B-type natriuretic peptide (BNP) were not available; thus, we could neither evaluate for possible correlation of resistin with BNP nor adjust for BNP in multivariable models. Ventricular function during hospitalization for HF was not prospectively assessed in Health ABC. The available data on ejection fraction were abstracted from medical records (i.e., the studies were not centrally read), and do not refer to a single modality since ejection fraction values were derived from echocardiography or left ventriclurography reports during hospitalization for HF. Therefore, the differential association of resistin with risk for HF with preserved vs. reduced ejection fraction should be interpreted with caution. Because our cohort included persons of age 70 or older, these findings might not apply in younger populations. Although every effort was taken to control for potential confounders, we cannot exclude the possibility of unmeasured confounding as an alternative explanation of the observed findings. Finally, temporal trends in the use of new classes of drugs or growing use of existing drugs to treat HF risk factors like hypertension or CHD may impact the risk of HF development related to those risk factors. Thus risk estimates for any given risk factors may change over time necessitating periodic reassessment in contemporary cohorts.
In conclusion, in this study we demonstrate that serum resistin concentrations independently predict risk for incident HF among older persons. The practical implications of this finding in terms of diagnostic and therapeutic uses need further study.
Supplementary Material
Acknowledgments
Sources of Funding: This research was supported in part by the Intramural Research Program of the National Institute of Aging, National Institutes of Health, Bethesda MD and by grants N01-AG-6-2101, N01-AG-6-2103, and N01-AG-6-2106. Dr. Vasan was supported by a research career award 2K24 HL04334.
The authors wish to acknowledge the careful review of manuscript and insightful comments provided by Mitchell A. Lazar, MD, PhD, Sylvan Eisman Professor of Medicine, Chief, Division of Endocrinology, Diabetes, and Metabolism, Institute for Diabetes, Obesity, and Metabolism, University of Pennsylvania School of Medicine, Philadelphia PA.
This research was supported in part by the Intramural Research Program of the National Institute of Aging, National Institutes of Health, Bethesda MD and by grants N01-AG-6-2101, N01-AG-6-2103, and N01-AG-6-2106. Dr. Vasan was supported by a research career award 2K24 HL04334.
Footnotes
Conflict of interest: None
Disclosures: The authors have no conflict of interest to disclose for this work.
References
- 1.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 2.Jung HS, Park KH, Cho YM, Chung SS, Cho HJ, Cho SY, Kim SJ, Kim SY, Lee HK, Park KS. Resistin is secreted from macrophages in atheromas and promotes atherosclerosis. Cardiovasc Res. 2006;69:76–85. doi: 10.1016/j.cardiores.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 3.Curat CA, Wegner V, Sengenes C, Miranville A, Tonus C, Busse R, Bouloumie A. Macrophages in human visceral adipose tissue: increased accumulation in obesity and a source of resistin and visfatin. Diabetologia. 2006;49:744–747. doi: 10.1007/s00125-006-0173-z. [DOI] [PubMed] [Google Scholar]
- 4.Reilly MP, Lehrke M, Wolfe ML, Rohatgi A, Lazar MA, Rader DJ. Resistin is an inflammatory marker of atherosclerosis in humans. Circulation. 2005;111:932–939. doi: 10.1161/01.CIR.0000155620.10387.43. [DOI] [PubMed] [Google Scholar]
- 5.Weikert C, Westphal S, Berger K, Dierkes J, Mohlig M, Spranger J, Rimm EB, Willich SN, Boeing H, Pischon T. Plasma resistin levels and risk of myocardial infarction and ischemic stroke. J Clin Endocrinol Metab. 2008;93:2647–2653. doi: 10.1210/jc.2007-2735. [DOI] [PubMed] [Google Scholar]
- 6.Ellington AA, Malik AR, Klee GG, Turner ST, Rule AD, Mosley TH, Jr, Kullo IJ. Association of plasma resistin with glomerular filtration rate and albuminuria in hypertensive adults. Hypertension. 2007;50:708–714. doi: 10.1161/HYPERTENSIONAHA.107.095257. [DOI] [PubMed] [Google Scholar]
- 7.Efstathiou SP, Tsiakou AG, Tsioulos DI, Panagiotou TN, Pefanis AV, Achimastos AD, Mountokalakis TD. Prognostic significance of plasma resistin levels in patients with atherothrombotic ischemic stroke. Clin Chim Acta. 2007;378:78–85. doi: 10.1016/j.cca.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 8.Takeishi Y, Niizeki T, Arimoto T, Nozaki N, Hirono O, Nitobe J, Watanabe T, Takabatake N, Kubota I. Serum resistin is associated with high risk in patients with congestive heart failure--a novel link between metabolic signals and heart failure. Circ J. 2007;71:460–464. doi: 10.1253/circj.71.460. [DOI] [PubMed] [Google Scholar]
- 9.Vasan RS, Sullivan LM, Roubenoff R, Dinarello CA, Harris T, Benjamin EJ, Sawyer DB, Levy D, Wilson PW, D’Agostino RB. Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham Heart Study. Circulation. 2003;107:1486–1491. doi: 10.1161/01.cir.0000057810.48709.f6. [DOI] [PubMed] [Google Scholar]
- 10.Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, Rubin SM, Ding J, Simonsick EM, Harris TB, Pahor M. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation. 2003;108:2317–2322. doi: 10.1161/01.CIR.0000097109.90783.FC. [DOI] [PubMed] [Google Scholar]
- 11.Ingelsson E, Sundstrom J, Arnlov J, Zethelius B, Lind L. Insulin resistance and risk of congestive heart failure. JAMA. 2005;294:334–341. doi: 10.1001/jama.294.3.334. [DOI] [PubMed] [Google Scholar]
- 12.Butler J, Kalogeropoulos A, Georgiopoulou V, Belue R, Rodondi N, Garcia M, Bauer DC, Satterfield S, Smith AL, Vaccarino V, Newman AB, Harris TB, Wilson PWF, Kritchevsky SB. Incident Heart Failure Prediction in the Elderly: The Health ABC Heart Failure Score. Circ Heart Fail. 2008;1:125–133. doi: 10.1161/CIRCHEARTFAILURE.108.768457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim M, Oh JK, Sakata S, Liang I, Park W, Hajjar RJ, Lebeche D. Role of resistin in cardiac contractility and hypertrophy. J Mol Cell Cardiol. 2008;45:270–280. doi: 10.1016/j.yjmcc.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linco Research, Inc. [Accessed Accessed: 02/20/2008];Human Resistin ELISA. Available at: www.millipore.com/catalogue/item/ezhr-95k.
- 15.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 16.Rodondi N, Newman AB, Vittinghoff E, de Rekeneire N, Satterfield S, Harris TB, Bauer DC. Subclinical hypothyroidism and the risk of heart failure, other cardiovascular events, and death. Arch Intern Med. 2005;165:2460–2466. doi: 10.1001/archinte.165.21.2460. [DOI] [PubMed] [Google Scholar]
- 17.van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med. 1999;18:681–694. doi: 10.1002/(sici)1097-0258(19990330)18:6<681::aid-sim71>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 18.Ambler G, Omar RZ, Royston P. A comparison of imputation techniques for handling missing predictor values in a risk model with a binary outcome. Stat Methods Med Res. 2007;16:277–298. doi: 10.1177/0962280206074466. [DOI] [PubMed] [Google Scholar]
- 19.Barnard J, Rubin B. Small-sample degrees of freedom with multiple imputation. Biometrika. 1999;86:948–955. [Google Scholar]
- 20.Shuldiner AR, Yang R, Gong DW. Resistin, obesity and insulin resistance--the emerging role of the adipocyte as an endocrine organ. N Engl J Med. 2001;345:1345–1346. doi: 10.1056/NEJM200111013451814. [DOI] [PubMed] [Google Scholar]
- 21.Yu YH, Ginsberg HN. Adipocyte signaling and lipid homeostasis: sequelae of insulin-resistant adipose tissue. Circ Res. 2005;96:1042–1052. doi: 10.1161/01.RES.0000165803.47776.38. [DOI] [PubMed] [Google Scholar]
- 22.Wang P, Mariman E, Renes J, Keijer J. The secretory function of adipocytes in the physiology of white adipose tissue. Journal of cellular physiology. 2008 doi: 10.1002/jcp.21386. [DOI] [PubMed] [Google Scholar]
- 23.Lehrke M, Reilly MP, Millington SC, Iqbal N, Rader DJ, Lazar MA. An inflammatory cascade leading to hyperresistinemia in humans. PLoS Med. 2004;1:e45. doi: 10.1371/journal.pmed.0010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petersen KF, Shulman GI. Etiology of insulin resistance. Am J Med. 2006;119:S10–16. doi: 10.1016/j.amjmed.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheng CH, Di J, Jin Y, Zhang YC, Wu M, Sun Y, Zhang GZ. Resistin is expressed in human hepatocytes and induces insulin resistance. Endocrine. 2008;33:135–143. doi: 10.1007/s12020-008-9065-y. [DOI] [PubMed] [Google Scholar]
- 26.Abbasi F, Brown BW, Jr, Lamendola C, McLaughlin T, Reaven GM. Relationship between obesity, insulin resistance, and coronary heart disease risk. J Am Coll Cardiol. 2002;40:937–943. doi: 10.1016/s0735-1097(02)02051-x. [DOI] [PubMed] [Google Scholar]
- 27.Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111:3481–3488. doi: 10.1161/CIRCULATIONAHA.105.537878. [DOI] [PubMed] [Google Scholar]
- 28.Nian M, Lee P, Khaper N, Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res. 2004;94:1543–1553. doi: 10.1161/01.RES.0000130526.20854.fa. [DOI] [PubMed] [Google Scholar]
- 29.Witteles RM, Fowler MB. Insulin-resistant cardiomyopathy clinical evidence, mechanisms, and treatment options. J Am Coll Cardiol. 2008;51:93–102. doi: 10.1016/j.jacc.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 30.Rothwell SE, Richards AM, Pemberton CJ. Resistin worsens cardiac ischaemia-reperfusion injury. Biochem Biophys Res Commun. 2006;349:400–407. doi: 10.1016/j.bbrc.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 31.Wang BW, Hung HF, Chang H, Kuan P, Shyu KG. Mechanical stretch enhances the expression of resistin gene in cultured cardiomyocytes via tumor necrosis factor-alpha. Am J Physiol Heart Circ Physiol. 2007;293:H2305–2312. doi: 10.1152/ajpheart.00361.2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.