Summary
MHC class II-expressing double positive thymocytes induce progression of CD4 T cell development, as efficiently as cortical thymic epithelial cells (cTEC). Because double positive thymocytes expressing CD1d select NKT cells, we investigated whether thymocyte-selected CD4 (T-CD4) T cells require the same signaling components as NKT cells. We therefore examined the role of SAP, Fyn, PKCθ, one of the SAP binding receptors Ly108, T-bet, and IL-15Rα in T-CD4 T cell development. Using bone marrow chimeras, we found that SAP, Fyn and PKCθ are essential for T-CD4 T cell generation, whereas mutations in Ly108, IL-15Rα or T-bet had a marginal effect. Furthermore, SAP is critical for IL-4 production by T-CD4 T cells, but the PKCθ deficiency did not alter the ability of T-CD4 T cells to produce cytokines. T-bet is necessary to produce the maximum amount of IFN-γ for CD4 T cells regardless of the selection pathway. We conclude that, in contrast to mainstream epithelial cell-selected CD4 T cells, the two distinct lineages of T cells selected by thymocytes, i.e. T-CD4 and NKT cells both utilize the SAP/Fyn/PKCθ pathway for their development and function.
Introduction
It is well accepted that MHC class II expressed on cortical thymic epithelial cells (cTEC) plays a critical and unique role for positive selection of conventional CD4 T cells, and BM-derived hematopoietic cells cannot mediate positive selection of CD4 T cells. Recently, however, we and others identified an alternate pathway for CD4 T cell development that is mediated by MHC class II-expressing thymocytes (Choi et al., 2005; Li et al., 2005). To distinguish between the two CD4 T cell populations, we named them E- and T-CD4 T cells to reflect the cell type mediating selection: epithelial cell-selected (E-CD4) and thymocyte-selected CD4 (T-CD4) T cells, respectively. Unlike E-CD4 T cells that require an appropriate signal to differentiate to effector Th1 or Th2 cells, T-CD4 T cells produce both Th1 and Th2 cytokines immediately after in vivo stimulation, which is very similar to NKT cells (Li et al., 2007). Surprisingly, IL-4 production by T-CD4 T cells does not require Stat6 that is also dispensable for NKT but not E-CD4 T cells in the generation of IL-4. Moreover, mice that had a T-CD4 population in vivo were protected from the development of allergen-induced allergic airway inflammation. This data, with another report showing that mice with T-CD4 T cells are protected from EAE (Park et al., 2004), suggest that T-CD4 cells have an important immunoregulatory role. A significant percentage of human fetal and neonatal thymocytes express MHC class II (Gilhus and Matre, 1983; Marinova et al., 2001; Park et al., 1992; Thulesen et al., 1999) and several observations support the presence of two selection pathways in humans (Godthelp et al., 2000; Klein et al., 1995; Markert et al., 2004; Markert et al., 2003; Rice et al., 2004). Moreover, human hematopoietic stem cells can generate CD4 single positive thymocytes on Delta-like 1-expressing OP9 bone marrow stromal cells in vitro (La Motte-Mohs et al., 2005), further supporting the co-existence of E- and T-CD4 T cells in humans.
NKT cells are a specialized subset of T lymphocytes that are positively selected by cortical thymocytes expressing non-classic MHC class I molecules CD1d. NKT cells express TCR/CD3 complexes, but their TCR repertoire is very restricted with most of them carrying the invariant Vα14-Jα18 α chain preferentially associated with Vβ8.2, Vβ7, or Vβ2. They co-express surface markers characteristic of both conventional T cells and several NK cell-associated receptors such as NK1.1, NK inhibitory and stimulatory receptors. Functionally, NKT cells resemble innate effector cells in that they can promptly produce large amounts of Th1- and Th2-type cytokines, such as IFN-γ and IL-4 upon TCR stimulation. The effect of these cytokines could bridge innate and adaptive immunity. Accumulating data demonstrated that NKT cells play a critical role in regulating anti-viral, anti-tumor immune responses, allergy, and autoimmunity (Bendelac et al., 2006; Kronenberg, 2005; Taniguchi et al., 2003).
Both mainstream E-CD4 T cells and NKT cells are derived from the same lymphocyte precursors and develop through the DP stage (Coles and Raulet, 2000; Dao et al., 2004; Egawa et al., 2005; Gapin et al., 2001; Hammond et al., 1998; Tilloy et al., 1999). Yet, the requirements for E-CD4 T cell and NKT cell development are fundamentally different. NKT cells are positively selected by CD1d on cortical DP thymocytes in the presence of self glycolipid ligands, such as iGb3 (Zhou et al., 2004), whereas E-CD4 T cells develop on self-peptide/MHC class II complexes on cTEC (Starr et al., 2003). Although NKT cells share some development requirements with T cells selected on cTEC, recent studies have demonstrated that several signaling molecules, transcription factors, and cytokines are specifically required for NKT ontology at distinct differentiation stages (Bendelac et al., 2006; Matsuda and Gapin, 2005).
SAP (SLAM-Associated Protein) is selectively required for NKT but not E-CD4 T cell development in humans and mice (Chung et al., 2005; Nichols et al., 2005a; Pasquier et al., 2005). SAP binds to the specific tyrosine-based motifs (TxYxxV/I/L) within the cytoplasmic tail of six SLAM related receptors and relays the SLAM-elicited signaling through the recruitment and activation of the Src protein tyrosine kinase Fyn (Chan et al., 2003; Latour et al., 2003; Sayos et al., 1998). The SLAM family of hematopoietic-surface receptors comprises nine hematopoietic cell surface receptors. Whilst CD150 (SLAM), CD84, CD229 (Ly9), CD244 (2B4), Ly108 and CRACC bind the single SH2-domain adapters SAP and EAT-2, CD48, BLAME and SLAMF9 do not (Engel et al., 2003). These receptors are differentially expressed in various immune cells and all of them (except CD244 and CD48) serve as self- ligand or homophilic receptors (Bhat et al., 2005; Engel et al., 2003; Nichols et al., 2005b; Veillette and Latour, 2003). The SLAM/SAP/Fyn signaling pathway plays a critical role in the development, differentiation, and effector function of several leukocyte lineages in the innate and adaptive immune systems (Ma et al., 2007; Veillette, 2006). During T cell activation, SLAM related receptors function as co-stimulatory molecules and the SLAM/SAP/Fyn pathway enhances TCR-mediated and PKCθ-dependent NF-κB activation (Cannons et al., 2004). PKCθ/BCL10/NF-κB activation by Fyn has been implicated in NKT cell generation (Schmidt-Supprian et al., 2004; Sivakumar et al., 2003; Stanic et al., 2004a; Stanic et al., 2004b). Because the SLAM/SAP/Fyn/PKCθ signaling cascade plays an important role in inducing TCR-mediated Th2 cytokine production (Cannons et al., 2004; Graham et al., 2006; Howie et al., 2005; Marsland et al., 2004; Wang et al., 2004; Wu et al., 2001), we have set out to test the role of this pathway in T-CD4 T cell selection and expansion in the thymus.
As T-bet, which due to its critical role in IFN-γ gene activation, is thought to regulate terminal maturation, survival, and effector function of NKT cells (Matsuda et al., 2006; Townsend et al., 2004) we examined its role in T-CD4 development. Similarly, as the IL-15/IL-15R signaling pathway is important for expansion/survival and functional maturation of NKT cells, but not required for NKT cell generation (Kennedy et al., 2000; Lodolce et al., 1998; Ohteki et al., 1997; Ranson et al., 2003; Schluns et al., 2004), the effect of a disruption in the IL-15Rα gene was evaluated.
The experiments in the current study are designed to test the hypothesis that selection and progression of T-CD4 T cells and NKT cells depend on similar signaling pathways. To investigate the role of SAP, Fyn, PKCθ, Ly108, T-bet, and IL-15Rα in regulating T-CD4 cell development, survival, expansion, terminal differentiation, or effector function, we utilized bone marrow chimeras and mice in which these genes had been disrupted.
Results
Essential role of SAP and Fyn in T-CD4 T cell development
SAP is expressed in T cells, NK cells, and several other hematopoietic cells (Ma et al., 2007; Veillette, 2006) and its expression in BM-driven cells has been shown to be necessary and sufficient to mediate NKT cell development in both humans and mice (Chung et al., 2005; Nichols et al., 2005a; Pasquier et al., 2005). Since both NKT and T-CD4 T cells are developed through interactions between thymocytes, we hypothesized that SAP might also be essential for T-CD4 T cell generation. To test this, we utilized transgenic mice expressing the MHC class II transactivator (CIITA) as a transgene (denoted as Tg from hereon) (Patel et al., 2005). Because CIITA can activate the expression of MHC class II and other molecules that participate in MHC class II-restricted antigen presentation (Reith et al., 2005), thymocytes in Tg mice express MHC class II (Patel et al., 2005). Using Tg mice, we have demonstrated that T-CD4 T cells can be developed efficiently by MHC class II-expressing thymocytes (Li et al., 2005).
To examine the potential role of signaling molecules in T-CD4 T cell development and function, we utilized the notion that MHC class II expressing (Tg) thymocytes can select non-MHC expressing thmocytes in trans (Choi et al., 2005). To demonstrate the ability of Tg thymocytes to mediate development of the other thymocytes and to assess the co-selection efficiency in the mixed BM chimeric mice, BM from Tg and non-transgenic (WT) mice were co-transferred into the MHC class II Aβ deficient hosts (Tg+WT→Aβ-/-). As shown in Figure 1A and 1B, the percentages of CD4 SP thymocytes originated from Tg and WT BM (5.5 ± 1.7 vs. 3.9 ± 1.3) were not significantly different. In the periphery, splenic T-CD4 cells accumulated to similar levels, although the percentage of LN T-CD4 T cells from Tg BM was slightly higher than that of the WT cells (Figure 1A and 1B). WT BM transferred to Aβ-/- host in the absence of Tg BM generated very few CD4 T cells, similar to Aβ-/- mice (Li et al., 2005). As we have reported previously (Patel et al., 1995), CD8 T cells originated from Tg BM were over- and under-represented in the thymus and the periphery, respectively (Figure 1A and 1B). This change seems to be due to elevated IL-4 in the CIITATg thymus since CIITATg mice deficient in IL-4 gene expression did not show the alteration in CD8 T cell development (Patel et al., 2005). The results shown here set the premise that T-CD4 T cells are generated in Aβ-/- recipients when Tg thymocytes are present. In all experiments, CD45 congenic markers were used to distinguish cells derived from different BM sources and the recipients.
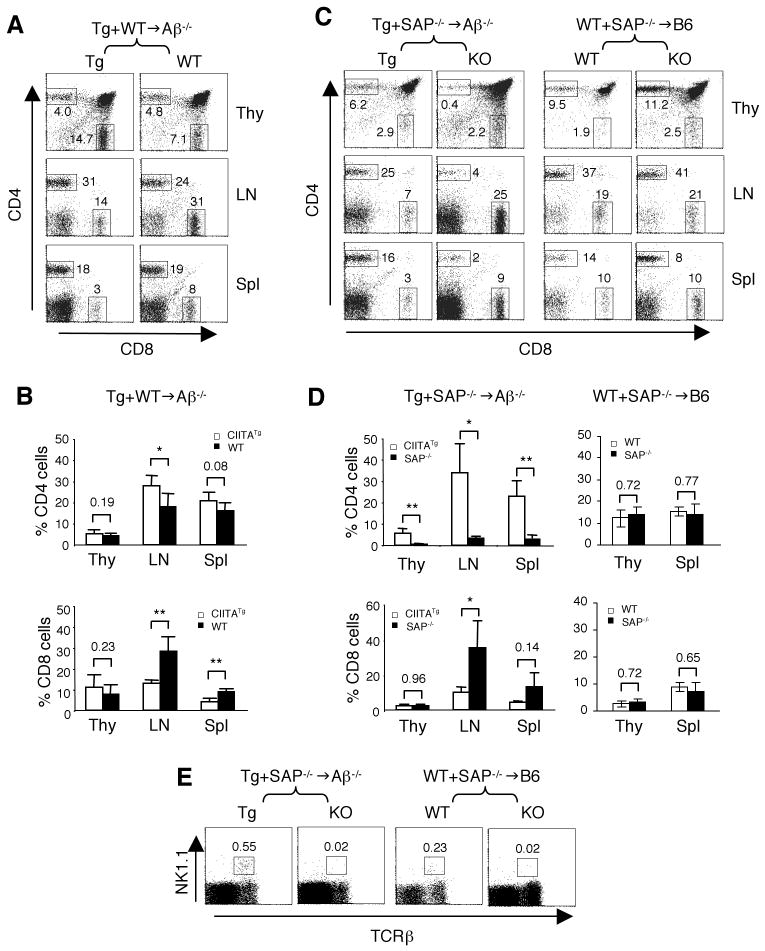
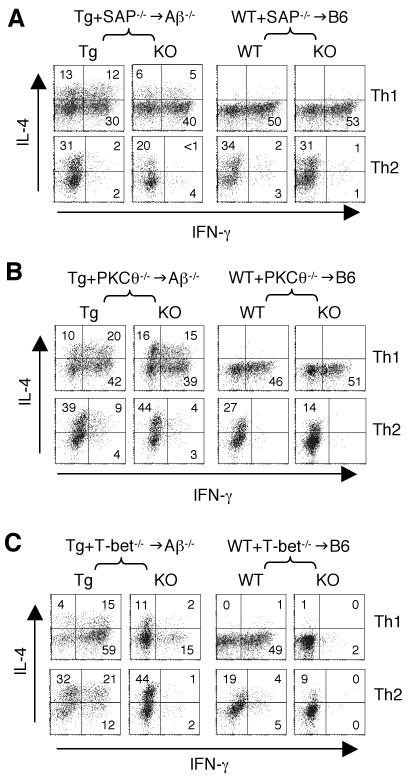
Figure 1. SAP plays a critical role in T-CD4 T cell development.
(A) CIITA-expressing thymocytes can efficiently select WT CD4 T cells. CIITATg (Tg) BM (CD45.1) were mixed with WT BM (CD45.1/2) and co-transferred to the MHC class II deficient Aβ-/- hosts (CD45.2). Thymocytes, LN and splenic cells from chimeric mice were analyzed for T cell repopulation by Tg and WT BM. The numbers in the dot plots indicate the percentages of gated CD4 and CD8 T cells of each BM driven cells. Data are representative of 5 mice.
(B) Percentages of CD4 (top graph) and CD8 (bottom graph) in Tg+WT-/-→Aβ-/- chimeric mice. Data are shown as mean ± SD from 5 mice. * and ** indicates p<0.05 and p<0.01, respectively.
(C) SAP is necessary for T-CD4 T cell selection. Tg (CD45.1/2) and SAP-/- (CD45.2) BM were co-transferred to Aβ-/- (CD45.1) recipients (left group). As a control, WT (CD45.1/2) and SAP-/- (CD45.2) BM were used to reconstitute B6 (CD45.1) hosts (right group). CD4 and CD8 populations in thymi, LN, and spleens by cells derived from the indicated BM source were shown. The numbers in the dot plots are the percentages of gated CD4 and CD8 cells of each BM driven cells.
(D) Percentages of CD4 (top panels) and CD8 (bottom panels) cells in Tg+SAP-/-→Aβ-/- (right groups) and WT+SAP-/-→WT (left groups) chimeric mice. Data are shown as mean ± SD from 4 Tg+SAP-/-→Aβ-/- mice and 2 WT+SAP-/-→WT mice.
(E) Percentage of thymic NK1.1+TCRβ+ NKT cells from different BM in Tg+SAP-/-→Aβ-/- (left two panels) and WT+SAP-/-→B6 chimeras (right two panels). Numbers indicate the percentages of NKT cells among total thymocytes.
We next tested the role of SAP in T-CD4 T cell development. Unlike the WT BM, generation of SAP-/- T-CD4 cells in Tg+SAP-/-→Aβ-/- mice was very poor in the thymus as well as in the peripheral lymphoid organs (Figure 1C, left group; 1D, top left graph). In the control chimeras (WT+SAP-/-→B6), both WT and SAP-/- E-CD4 T cells were selected equally well on MHC class II expressed in host cTEC (Figure 1C, right group; 1D, top right graph). The numbers of CD4 T cells were consistent with these results (Supplemental Table 1). In contrast, SAP did not influence the generation of CD8 SP cells in the thymus, and SAP-/- CD8 T cells tended to accumulate at a higher percentage than the Tg counterparts in the Aβ-/- hosts (Figure 1C, left group; 1D, bottom left graph). The percentages and the numbers of CD8 T cells in the thymus, spleen and the LN were summarized in Supplemental Table 2 and 3, respectively. In addition, we analyzed NKT cell development in Tg+SAP-/-→Aβ-/- and WT+SAP-/-→WT chimeric mice. The proportion of NKT cells was greatly diminished, if BM cells were from SAP-/- but not Tg or WT BM cells (Figure 1E). These results were consistent with the previous studies showing a critical role of SAP in NKT cell ontology but not in the generation of E-CD4 T cells. More importantly, SAP is essential for not only NKT but also T-CD4 T cell development.
Fyn and PKCθ are essential for T-CD4 T cell development
Fyn, the adaptor protein of SAP, is shown to be important for NKT but not E-CD4 T cell development (Eberl et al., 1999; Gadue et al., 1999). Since the deficiency of SAP dramatically reduced the T-CD4 T cell compartment, it is possible that Fyn is also necessary for T-CD4 T cell generation. In addition, TCR engagement activates several PKC isoforms that transduce signals to downstream events. Among them, PKCθ is selectively recruited to the immunological synapse upon TCR stimulation. Furthermore, this recruitment is enhanced by co-activation of TCR and SLAM (Cannons et al., 2004). Although PKCθ plays an essential role in T cell activation and survival (Cannons et al., 2004; Coudronniere et al., 2000; Manicassamy et al., 2006), it is dispensable for E-CD4 T cell development. However, a recent study revealed a critical role of PKCθ in the generation of functional NKT cells (Stanic et al., 2004b). Therefore, we investigated the role of Fyn and PKCθ in T-CD4 T cell development.
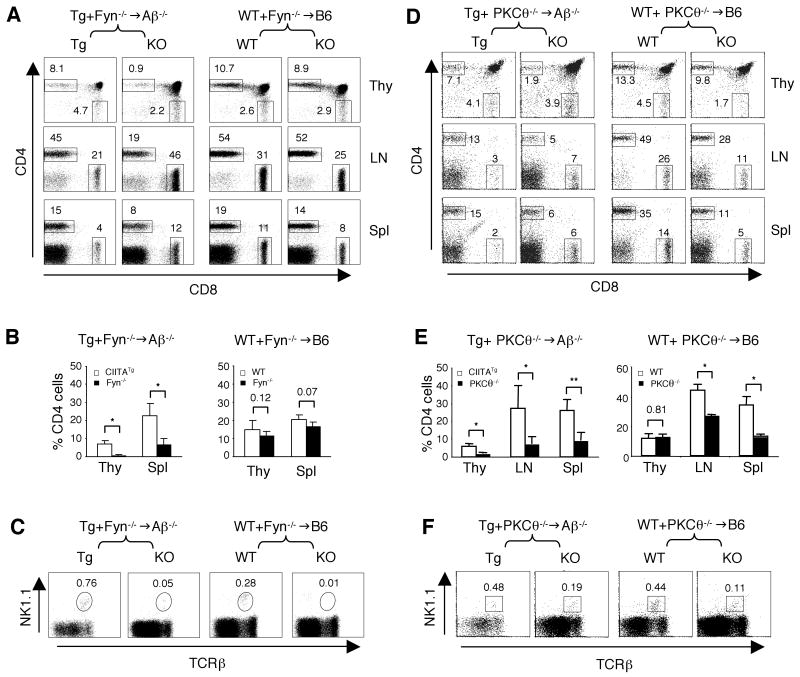
We constructed Tg+Fyn-/-→Aβ-/- and WT+Fyn-/-→WT chimeric mice and compared the development of E-, T-CD4 T cells, and NKT cells. Similar to SAP, the deficiency in Fyn also diminished T- but not E-CD4 T cell development (Figure 2A and 2B). In agreement with the published studies, Fyn deficient BM cells did not reconstitute NKT cells as efficient as wild type BM (Figure 2C). Likewise, in Tg+PKCθ-/-→Aβ-/- mice, Tg T-CD4 T cells were efficiently generated, but the reconstitution of PKCθ-/- T-CD4 T cells was significantly reduced (Figure 2D and 2E, left group). In contrast, the thymic generation of E-CD4 T cells was not impaired in the absence of PKCθ in comparison to the WT E-CD4 T cells shown in WT+PKCθ-/-→WT chimeric mice (Figure 2D and 2E, right group). However, there were fewer PKCθ-/- E-CD4 T cells than WT (p<0.05) in LN and spleens of WT+PKCθ-/-→WT chimeric mice, suggesting that PKCθ plays a role in the peripheral homeostasis of E-CD4 T cells (Figure 2D and 2E, right group). Thymic PKCθ-/- NKT cells were greatly reduced in both kinds of chimeric mice (Figure 2F). Taken together, both Fyn and PKCθ that functions downstream of SAP govern T-CD4 T cell as well as NKT cell development.
Figure 2. Important role of Fyn and PKCθ in T-CD4 T cell selection.
(A) CD4 and CD8 cell profile in thymocytes, LN and splenic cells from Tg+Fyn-/-→Aβ-/- (left group) and WT+Fyn-/-→B6 (right group). The numbers in the dot plots show the percentages of gated CD4 and CD8 SP thymocytes.
(B) Percentage of CD4 SP thymocytes LN and splenic CD4 T cells in Tg+Fyn-/-→Aβ-/- (left group) and WT+Fyn-/-→B6 chimeras (right proup). Data are shown as mean ± SD from 3 and 4 mice, respectively.
(C) Percentage of NK1.1+TCRβ+ NKT cells in Tg+Fyn-/-→Aβ-/- (left group) and WT+Fyn-/-→B6 chimeras (right proup). Numbers indicate the percentages of NKT cells among total thymocytes.
(D) CD4 and CD8 profiles of thymocytes, LN and splenic cells from Tg+PKCθ-/-→Aβ-/- (left group) and WT+PKCθ-/-→B6 (right group). The numbers in the dot plots show the percentages of gated CD4 and CD8 SP thymocytes.
(E) Percentage of CD4 SP thymocytes, CD4 T cells in LN and spleen from Tg+PKCθ-/-→Aβ-/- (left group) and WT+PKCθ-/-→WT (right group) chimeric mice. Data are shown as mean ± SD from 4 and 3 mice, respectively.
(F) A partial defect in PKCθ-/- NKT cell generation. The percentage of thymic NK1.1+TCRβ++ NKT cells in Tg+PKCθ-/-→Aβ-/- (left two panels) and WT+PKCθ-/-→B6 chimeras (right two panels).
Minimal involvement of Ly108, T-bet and IL-15Rα in regulating T-CD4 T cell ontology
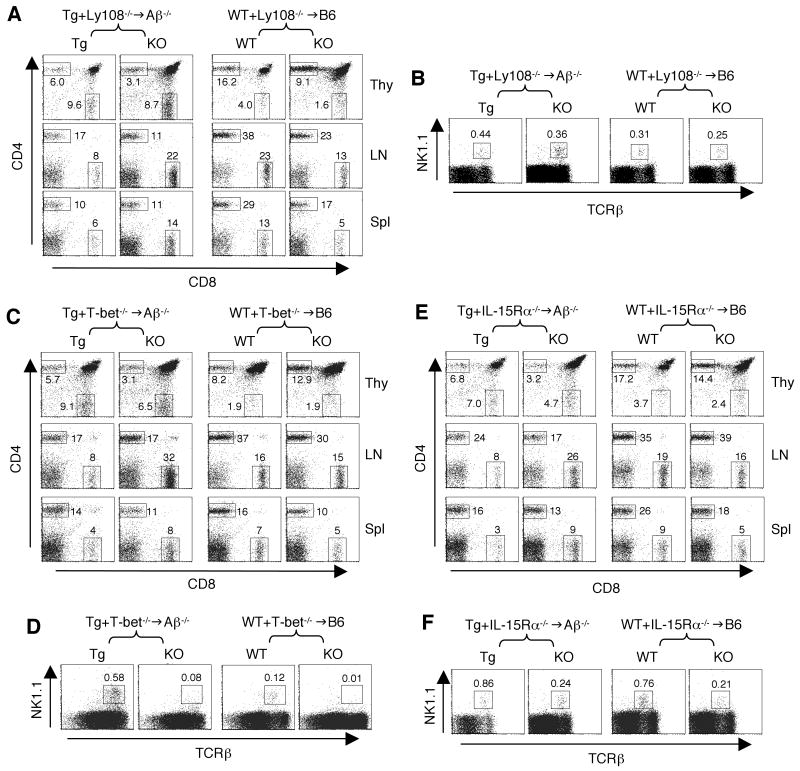
The SLAM related surface receptors recruit SAP and several family members, including CD84, CD150, CD229, and Ly108 ((Ma et al., 2007; Veillette, 2006) and the co-submitted manuscript)), are expressed on DP thymoyctes. Therefore, it is possible that they participate in the positive selection of T-CD4 T cells as well as NKT cells. The deficiency of CD229 did not cause the impairment of NKT cell development (Graham et al., 2006). However, the role of Ly108 has not been demonstrated in either NKT or CD4 T cell generation. To address this, we co-transferred Tg and Ly108-/- BM into Aβ-/- hosts and the chimeras were examined. We found a lower representation of Ly108-/- T-CD4 T cells than Tg T-CD4, but the differences were not significant (Figure 3A and Supplemental Figure 1A, left group). In addition, Ly108 was not required for E-CD4 development (Figure 3A and Supplemental Figure 1A, right group). Similarly, NKT cells were present in the absence of Ly108 although the NKT cell population was slightly reduced (Figure 3B). Thus, lack of Ly108 did not greatly affect the development of NKT or T-CD4 T cells, which was in contrast to the phenotype caused by the SAP deficiency. It is, however, likely that SAP controls cooperating signals initiated by several of the SLAM-family receptors (Engel et al., 2003; Graham et al., 2006; Howie et al., 2005), which are requisite for T-CD4 cell development.
Figure 3. T-CD4 T cell generation in the absence of Ly108, T-bet or IL-15Rα.
(A) CD4 and CD8 profiles of thymocytes, LN and splenic cells from Tg+Lyn-/-→Aβ-/- (left group) and of WT+Ly108-/-→B6 (right group). The numbers in the dot plots show the percentages of gated CD4 and CD8 T cells. The data shown here are one of three chimeras.
(B) A minor defect in Ly108-/- NKT cell generation. The percentage of thymic NK1.1+TCRβ+ NKT cells in Tg+Ly108-/-→Aβ-/- (left two panels) and WT+Ly108-/-→B6 chimeras (right two panels). Numbers indicate the percentages of NKT cells among total thymocytes.
(C) CD4 and CD8 cell populations in thymocytes, LN and splenic cells from Tg+T-bet-/-→Aβ-/- (left panels) and of WT+T-bet-/-→B6 (right panels). The numbers in the dot plots show the percentages of gated CD4 and CD8 T cells. The data shown here are one of three chimeras.
(D) A severe defect in T-bet-/- NKT cell generation in both Tg+T-bet-/-→Aβ-/- and WT+T-bet-/-→B6 chimeric animals. Numbers indicate the percentages of NKT cells among total thymocytes.
(E) CD4 and CD8 profiles from indicated organs in Tg+IL-15Rα-/-→Aβ-/- and WT+IL-15Rα-/-→B6 mice. The numbers in the dot plots show the percentages of gated CD4 and CD8 T cells driven from each BM source. The data shown here are one of three or four chimeras.
(F) IL-15Rα-/- BM had a reduction of the NKT cell population in the mixed BM chimeric mice. Data are representative of 4 individual chimeras. Numbers indicate the percentages of NKT cells among total thymocytes.
Using a similar scheme, we investigated the role of T-bet in T-CD4 T cell development. T-bet regulates terminal differentiation of NKT cells and its deficiency results in only a small number of immature NKT cells. If the maturation programs between NKT cells and T-CD4 T cells are similar, T-bet may play a similar role in T-CD4 cell development. The results shown in Figure 3C indicated otherwise. The absence of T-bet did not significantly affect thymic development or peripheral accumulation of T-CD4 as well as E-CD4 T cells, although in lymph nodes, T-bet-/- T-CD4 T cells were slightly decreased compared to Tg T-CD4 T cells (Figure 3C and Supplementary Figure 1B). As expected from the published results, T-bet-/- NKT cells were greatly under-represented, compared to the Tg or WT counterparts (Figure 3D).
IL-15 through its receptor IL-15R plays a critical role in the proliferation and expansion of mature NKT cells and CD8 T cells that constitutively express the IL-2/IL-15Rβ (CD122) chain. When we tested the significance of this signaling pathway, the absence of IL-15Rα had a marginal effect on development of T- or E-CD4 T cells (Figure 3E and Supplementary Figure 1C). Consistent with these data, T-CD4 T cells did not express a measurable level of CD122, whereas a significant proportion of NKT cells expressed CD122 (Supplemental Figure 2). However, maximum NKT cell generation seemed to require IL-15Rα since IL-15α-/- NKT cells were decreased in the chimeric mice in comparison to their Tg or WT controls (Figure 3F). Our results indicate that IL-15Rα is dispensable for both T- and E-CD4 T cell generation.
Differential role of signaling molecules on the cytokine production potential of T-CD4 T cells
Our data revealed that T-CD4 T cells did not completely overlap with NKT cells or E-CD4 T cells in their developmental requirements. Next, we investigated whether the effector function of T-CD4 T cells is altered by the deficiency of those signaling molecules. Although the absence of signaling molecules affected T-CD4 T cell development, the residual population of CD4 T cells in the periphery was detectable and hence was used to assess their cytokine production potential. The key feature of T-CD4 T cell effector function is the immediate secretion of cytokines (Li et al., 2007). Similar to NKT cells, T-CD4 cells produce both IFN-γ and IL-4 shortly after TCR stimulation without being skewed to either Th1 or Th2 lineage. In contrast, E-CD4 T cells express little cytokines under the same condition.
Previously, we have shown that cytokine producing T-CD4 T cells exhibit the effector/memory phenotype by expressing a high level of CD44 (Li et al., 2007) and that the majority of peripheral T-CD4 T cells are CD44 hi (Li et al., 2005). When we examined the expression of CD44, the deficiency of the above studied signaling molecules had no apparent effect on the CD44 phenotype of either E- or T-CD4 T cells in the mixed BM chimeric mice (Supplemental Figure 3A and 3B). To characterize the cytokine production, splenic CD4 T cells from the mixed BM chimeras shown in Figures 1-3 were purified and stimulated for 5 hours in the absence of exogenous cytokines. The cytokine production pattern of this type of cultures would show the propensity of CD4 T cells. Cells were then stained with the CD45.1, CD45.2, CD4, and NK1.1 antibodies followed by intracellular cytokine staining (ICS) to measure IFN-γ and IL-4 production in NK1.1- CD4 T cells.
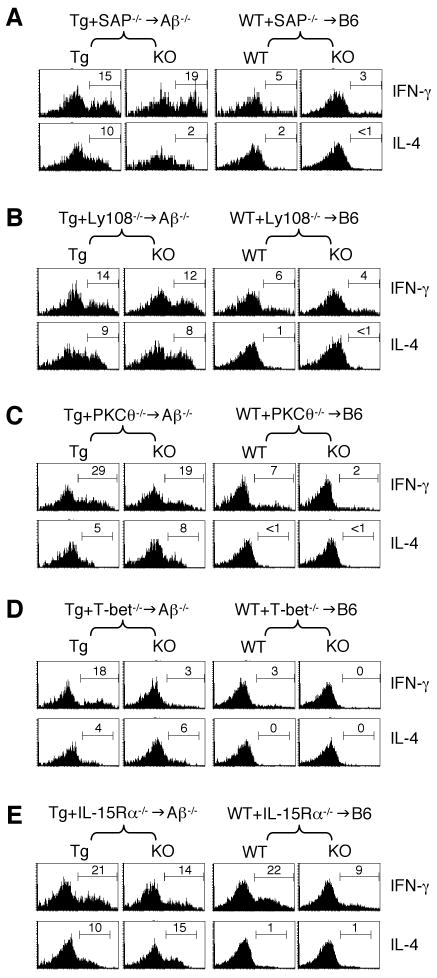
We first examined the role of SAP using CD4 T cells from Tg+SAP-/-→Aβ-/- and WT+ SAP-/-→WT mice. Although the number of SAP-/- T-CD4 T cells in the periphery was dramatically reduced in these chimeras, the residual SAP-/- T-CD4 T cells were able to produce both IFN-γ and IL-4 cytokines shortly after activation (Figure 4A, compare Tg vs. SAP-/- T-CD4 cells in the left chimera). The proportions of IFN-γ+ cells were comparable between SAP+/+ (Tg) vs. SAP-/- (KO) T-CD4 T cells, whereas percentages of IL-4+ cells were decreased in the absence of SAP. Nevertheless, E-CD4 T cells showed little IL-4 expression and small numbers of IFN-γ+ cells regardless of the SAP status (Figure 4A, right group). Similarly, Ly108 deficient T-CD4 T cells produced both IFN-γ and IL-4 at a comparable level to that of Ly108 sufficient cells (Figure 2B). Therefore, SAP and Ly108 are not essential for T-CD4 T cells to produce cytokines, but SAP appears to be required for optimal production of IL-4 by T-CD4 T cells.
Figure 4. Effect of signaling molecules on the cytokine production potential of T-CD4 T cells.
The same chimeric mice described in Figures 1-3 were used to prepare splenic CD4 T cells. Cells were then stimulated for 5 hours as described in Experimental Procedures in the absence of exogenous cytokines. IFN-γ (top row) and IL-4 (bottom row) production from SAP-/- (A), Ly108-/- (B), PKCθ-/- (C), T-bet-/- (D), and IL-15Rα-/- (E) was assessed by ICS.
The downstream T cell signaling events that are important for IL-4 production and also known to be affected by SAP deficiency include PKCθ recruitment to lipid rafts, downstream IκBα degradation, and NF-κB1 nuclear translocation (Cannons et al., 2004). However, PKCθ deficiency had little effect on IL-4 or IFN-γ production by T-CD4 T cells (Figure 4C, left group), which indicates other signaling pathways might compensate PKCθ deficiency in mediating SAP signal transduction to the IL-4 gene.
T-bet is a critical transcription factor for IFN-γ gene expression in E-CD4 T cells (Szabo et al., 2000). Similar to E-CD4 T cells (Figure 4D, right group), there was a reduction in IFN-γ+ cells that were from the T-bet-/- origin in Tg+T-bet-/-→Aβ-/- chimeras (Figure 4D, left group). However, when we compared the IFN-γ production between T-bet deficient E- vs. T-CD4 T cells, the two showed a difference. As reported, E-CD4 T cells generated no detectable IFN-γ-producing cells without T-bet, whereas T-bet-/- T-CD4 T cells expressed IFN-γ, albeit at a low level. Therefore, T-bet is important for IFN-γ production by both E- and T-CD4 T cells but less critical for T-CD4 T cells. Finally, we analyzed the cytokine production potential by IL-15Rα-/- T-CD4 T cells. IFN-γ production was reduced slightly in both IL-5Rα-/- T- and E-CD4 T cells, whereas the ability to produce IL-4 by T-CD4 T cells was not affected by IL-15Rα deficiency (Figure 4E).
The Role of the signaling molecules in Th1 and Th2 differentiation
The results shown in Figure 4 demonstrated the differential potential of CD4 T cells to produce cytokines in the absence of exogenous cytokines. We next examined Th1 or Th2 effector cells to ascertain the function of signaling molecules during Th cell differentiation of T-CD4 T cells. Among several signaling molecules, we chose to examine SAP, PKCθ and T-bet that are known to be critical for Th cell differentiation. Splenic CD4 T cells from the mixed BM chimeric mice that generate either E- or T-CD4 T cells were differentiated under Th1- and Th2-inducing conditions for 6 days as described in the Experimental Procedures and their cytokine production profiles were quantified by ICS.
Based on the published reports (Cannons et al., 2004; Wu et al., 2001), we expected that SAP-/- E-CD4 T cells would respond normally to the Th2-skewing conditions and express IL-4. Indeed, differentiation of SAP-/- E-CD4 T cells to either Th1 or Th2 cells was not affected (Figure 5A, right group). One of the characteristics of T-CD4 T cells is their ability to produce Th2 cytokines including IL-4 even under the Th1 skewing condition (Li et al., 2007; Patel et al., 2005). In fact, Th1 cells generated from SAP sufficient T-CD4 T cells produced IL-4 (Figure 5A, left group). In contrast, T-CD4 T cells lacking SAP produced a reduced amount of IL-4 under Th1 as well as Th2 conditions (Figure 5A). Nevertheless, SAP-/- Th1 cells produced IL-4 under the Th1 skewing conditions, if they were selected on thymocytes but not on epithelial cells (compare KO cells between the two chimeras).
Figure 5. Cytokine production by Th1 or Th2 differentiated T-CD4 T cells deficient in signaling molecules.
The same chimeric mice described in Figures 1-3 were used to prepare splenic CD4 T cells. Cells were cultured under the Th1 or Th2 skewing condition, restimulated for 5 hours, and analyzed for IFN-γ and IL-4 production as described in Experimental Procedures. Cytokine production by T-CD4 T cells deficient in SAP (A), PKCθ (B), and T-bet (C) are shown.
PKCθ has shown to be important for IL-4 production by E-CD4 T cells (Marsland et al., 2004). Indeed, when we assessed IL-4 production, E-CD4 T cells were not able to make the maximum amount of IL-4 without PKCθ (Figure 5B, right group). However, PKCθ seems to be dispensable for T-CD4 T cells to make IL-4 since T-CD4 T cells produced an equivalent level of IL-4 under either the Th1 or Th2 skewing condition (Figure 5B, left group).
T-bet is critical for Th1 differentiation of E-CD4 T cells (Szabo et al., 2000) and Th1 cells lacking T-bet barely expressed IFN-γ when they were selected on TEC (Figure 5C, right group). When T-CD4 T cells were examined, T-bet-/- Th1 cells could make IFN-γ but much less than T-bet sufficient T-CD4 T cells (Figure 5C, left group). Therefore, IFN-γ production is compromised in both E- and T-CD4 T cells, if T-bet is not expressed.
Discussion
T-CD4 T cells are positively selected by MHC class II-expressing thymocytes, similar to NKT cells that are selected by CD1d-expressing thymocytes. In this study, we investigated signaling requirements for the development and effector function of T-CD4 T cells. By analyzing several molecules known to be associated with NKT cell ontology, we showed that SAP, which is crucial for the NKT cell development in both humans and mice, is also required for the generation of T-CD4 T cells. Fyn and PKCθ were also involved in the development of both lineages. We observed that T-CD4 T cell development was more severely impaired by the deficiency of SAP than Fyn. SAP is known to regulate CD4 T cell-mediated help for humoral immunity through a Fyn-independent pathway (Cannons et al., 2006; McCausland et al., 2007). Perhaps, SAP could also activate Fyn-independent pathways that facilitate T-CD4 T cell development. Nonetheless, the SAP/Fyn/PKCθ signaling pathway plays an important role in the development of both thymocyte-selected NKT cells and T-CD4 T cells. On the other hand, T-bet and IL-15Rα necessary for maturation, expansion, and survival of NKT cells, were dispensable for E- or T-CD4 T cell development. Therefore, our data indicate that development of T-CD4 T cells and NKT cells share the requirement for some of the same molecules presumably due to the common selection pathway by thymocytes. Yet, the two distinct lineages of T cells seem to diverge at a certain point during their development and then require different sets of signaling molecules for further maturation (Figure 6).
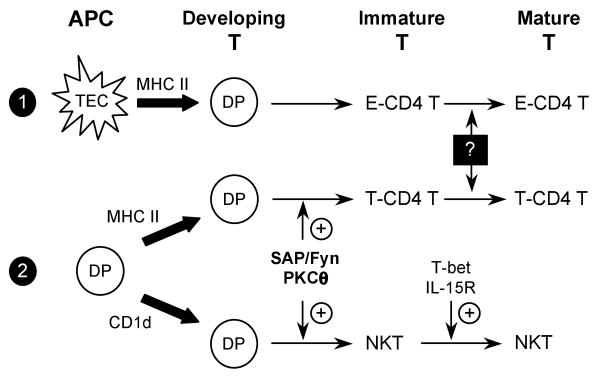
Figure 6. cTEC- and thymocyte-mediated T cell selection pathways.
E-CD4 T cells are positively selected through heterotypic interactions between DP thymocytes and MHC class II-expressing cTEC (pathway 1). Thymocyte-mediated T cell selection pathway select T-CD4 T cells and NKT cells through homotypic interactions between TCR on developing DP thymocytes with MHC class II or CD1d on DP thymocytes, respectively (pathway 2). Our studies indicate that the SAP/Fyn/PKCθ signaling cascade is a common pathway required for the generation of both T-CD4 T cells and immature NKT cells, while T-bet and IL-15R are selectively involved in the maturation of NKT cells. Whether E-CD4 T cells and T-CD4 T cells share similar molecular requirements for their maturation is currently unknown.
The SLAM/SAP/Fyn/PKCθ signaling pathway is considered to be specific for NKT cell generation. However, our current study implies that the same cascade is important for thymocyte-mediated CD4 T cell development. Positive selection of NKT and T-CD4 T cells requires the engagement of their TCR with the corresponding CD1d and MHC class II complexes on selecting thymocytes through homotypic T-T interactions. In contrast, E-CD4 T cell development is mediated by heterotypic cellular interactions between developing thymocytes and cTEC. Consequently, differences in the surface receptors and/or cytokines expressed by cTEC and thymocytes are likely to determine the activation of distinct signaling pathways that contribute to the development and function of different T cell subsets and NKT cells. In this regard, the co-stimulatory SLAM related family molecules expressed on thymocytes but not on cTEC could play an essential role in thymocyte-mediated positive selection but have a minimal effect on TEC-selected T cells. It is well established that SLAM-initiated signals are relayed by the SH2 domain-containing adaptor protein SAP to the Fyn kinase. Activated Fyn subsequently can enhance TCR-mediated PKCθ-dependent activation of NF-κB activity. Our study showed that SAP, Fyn and PKCθ, critical for NKT cell ontology, are the three of the important molecular requirements for the T-CD4 T cell lineage. Perhaps, H2-M3-restricted CD8 T cells that are selected on non-classic MHC class Ib expressing-hematopoietic cells (Urdahl et al., 2002) may also depend on the same signaling components.
Ly108, a member of the family of SALM related receptors had a modest role in both NKT and T-CD4 T cell development. Since several SLAM related receptors are expressed on thymocytes, it is conceivable that there may be a redundant role in NKT and T-CD4 cell development among these receptors. Indeed, mice with the deficiency in individual SLAM members such as CD229 do not show a defect in NKT cell generation (Graham et al., 2006). In the accompanying manuscript, however, homotypic self-interactions of both CD150 and Ly108 on thymocytes are shown to provide essential costimulatory signals to TCR signaling to drive NKT cell differentiation. In addition, a recent report also linked NKT cell defect in NOD mice to the CD150 and Ly108 loci (Jordan et al., 2007). Whether the same homotypic signals play a role during T-CD4 T cell development needs a further investigation.
Signaling mediated by the IL-15Rα chain was not required for T-CD4 development. The pleiotropic cytokine IL-15 acting through its receptor IL-15R can enhance survival, induce proliferation, and promote differentiation, which depend on cell types and their maturation status. After positive selection, immature NKT cells have to undergo a maturation process, which includes the up-regulation of CD122 expression, conferring their IL-15 responsiveness. Hence, NKT cells proliferation and homeostasis highly depend on IL-15 signaling (Matsuda et al., 2002; Ranson et al., 2003). IL-15, IL-15Rα, and IL-2/IL-15Rβ deficient mice all have reduced numbers of NKT cells (Kennedy et al., 2000; Lodolce et al., 1998; Ohteki et al., 1997; Ranson et al., 2003; Schluns et al., 2004). Recent studies have shown that IL-15Rα that binds IL-15 with high affinity can present IL-15 in trans to bystander IL-15Rα-/- cells (Dubois et al., 2002). Both parenchymal and BM-derived cells can trans-present IL-15 to support NKT cell development, although the later seem to be more important for NKT cell recovery within BM and the spleen (Schluns et al., 2004). However, as we have shown here (Figure 3F), IL-15Rα deficiency seems to affect NKT cell development. It is not clear at the moment what the mechanisms behind the reduction of NKT cells are. Nevertheless, signaling mediated by the IL-15Rα chain is not critical for T-CD4 development. As the majority of resting T-CD4 T cells do not express CD122, the nonessential role of IL-15Rα in T-CD4 generation may not be surprising. Similarly, the reason why T-bet is not required for T-CD4 T cell development could also be due to lack of CD122 expression on those cells. T-bet-/- NKT cells have a defect in the terminal maturation and homeostasis, which is associated with decreased expression of CD122 and several other genes normally expressed in mature NKT cells (Matsuda et al., 2006; Townsend et al., 2004).
We have reported the increase and the decrease of CD8 T cells in the thymus and the periphery of CIITATg mice, respectively, which is influenced by IL-4 production potential of T-CD4 T cells (Patel et al., 2005). In agreement of our observations, IL-4 transgenic mice also showed a similar pattern of CD8 T cell compartments (Lewis et al., 1991; Tepper et al., 1990). In several mixed BM chimeras presented here, we also observed differential reconstitutions of CD8 T cells originated from CIITATg BM. In addition, co-transferred BM cells deficient in each individual signaling molecule yielded a varying degree of thymic and peripheral CD8 T cells in the chimeras. One explanation could be that the thymic environment is influenced by the mixed population of thymocytes developing from the two BM sources, which could potentially affect the generation of CD8 SP thymocytes.
Besides the role in NKT cell development, the SLAM pathway also regulates Th2 cytokine production. In particular, SAP, Fyn, and PKCθ have been shown to affect TCR-mediated IL-4 production in E-CD4 T cells (Cannons et al., 2004; Graham et al., 2006; Howie et al., 2005; Marsland et al., 2004; Wang et al., 2004; Wu et al., 2001). The relative significance of this pathway in Th2 differentiation of T-CD4 T cells is not the same as we demonstrated here. We showed that T-CD4 T cells lacking SAP but not PKCθ have a defect in IL-4 production under Th2-skewing conditions, which is in contrast to the role of SAP or PKCθ in E-CD4 T cells. Therefore, CD4 T cells require a different set of signaling molecules for their development as well as function depending on the selection pathway in the thymus. Although molecular mechanisms governing the differences in shaping the effector function between E- and T-CD4 T cells are not yet clear, T-T interaction seems to be responsible for chromatin remodeling of the IL-4 locus in the absence of Th1 or Th2 differentiation signal (Li et al., 2007). We have demonstrated that histone acetylation of the IL-4 locus in CD4 SP and naïve CD4 T cells is increased when they are selected by thymocytes (Li et al., 2007). Perhaps, signals from TCR together with the SLAM/SAP/Fyn pathway set the intracellular environment that facilitates to activate and to maintain IL-4 gene transcription. If so, Stat6 would have no role in Th2 cytokine production in T-CD4 T cells or NKT cells. Moreover, when CD4 T cells are developed on cTEC, they may not receive the SLAM/SAP/Fyn-mediated signal necessary to enhance the accessibility of transcriptional machinery to the IL-4 locus. These CD4 T cells would require Th2 inducing factors, such as IL-4 and Stat6, to differentiate to IL-4 producing effector cells. Further investigations are warranted to have a better understanding of how the IL-4 locus is regulated differently in developing T-CD4 T cells upon receiving a signal from another thymocyte.
The similarities and differences among E-CD4 T, T-CD4 T, and NKT cells are summarized in Table 1. Notably, T-CD4 T cells are similar to NKT cells in that both are positively selected by thymocytes and they can promptly produce IL-4 in a Stat6-independent manner (Bendelac, 1995; Coles and Raulet, 2000; Kaplan et al., 1999; Li et al., 2007; Patel et al., 2005; Stetson et al., 2003). However, T-CD4 T cells are also distinct from NKT cells as they require MHC class II but not CD1d to develop; they do not express the NK1.1 marker; and they have a diverse TCR repertoire (Choi et al., 2005; Li et al., 2005). Our current study adds additional similarities and differences, demonstrating a unique T cell population bearing distinct developmental requirements and effector function.
Table 1.
Comparison of two types of CD4 T cells and NKT cells
| CD4 T | NKT | |||
|---|---|---|---|---|
| E-CD4 T | T-CD4 T | |||
| Selecting cell type | Epithelial cells | Thymocytes | Thymocytes | |
| Selecting element | MHC class II | MHC class II | CD1 | |
| Antigen | Peptides | ? | Glycolipids | |
| TCR repertoire | Diverse | Diversea | Limited | |
| NK1.1 expression | No | Nob | Yes | |
| Preformed IL-4 mRNA | No | Yesc | Yes | |
| Stat6 requirement to express IL-4 | Yes | Nod | No | |
| PKCθ requirement to express IL-4 | Yes | No | Yese | |
| Developmental requirement | SAP | No | ↓ | ↓ |
| Fyn | No | ↓ | ↓ | |
| PKCθ | No | ↓ | ↓ | |
| T-bet | No | NS | ↓ | |
| IL-15Rα | No | NS | ↓ | |
| Ly108 | No | NS | NS | |
↓, reduced; NS, not significant
References for a, b, c, d, and e are Choi et al. (2005), Li et al. (2005), Li et al. (2007), Patel et al. (2005), and Stanic et al. (2004), respectively.
The clinical significance of SAP has been illustrated in the inherited immunodeficiency X-linked lymphoproliferative syndrome (XLP). Many of XLP patients carry mutations in the SH2D1A locus that encodes SAP. They suffer from dysfunctional immune responses to the Epstein-Barr Virus (EBV) infection (the uncontrolled expansion of B cells and other leukocytes the production of inflammatory cytokines), hypogammaglobulinmia and lymphomas (Coffey et al., 1998; Nichols et al., 1998; Sayos et al., 1998). The pathogenesis has been linked to defective cell-mediated and humoral immunity to EBV infection (Ma et al., 2007). Given the multiple roles of NKT cells, the clinical manifestation in these patients could be primarily attributed to NKT cell deficiency. However, our study presented here suggests that XLP patients likely have the impairment in development and function of T-CD4 T cells as well. If so, defects in both NKT and T-CD4 T cells could contribute to the compound immune dysfunction in XLP patients.
Experimental Procedures
Mice
Mice carrying the human type III CIITA transgene (Tg) were described previously (Patel et al., 2005). CIITATg mice were bred to carry both the CD45.1 and CD45.2 congenic markers (CD45.1/2). Non-CIITATg littermate from heterozygous CIITATg breeding was used as wild type (WT) control. T-bet-/- mice on the B6 background were obtained from the Jackson Laboratory (Bar Harbor, ME). SAP-/-, Fyn-/-, PKCθ-/-, and IL-15Rα-/- mice on the B6 background mice were described previously (Eberl et al., 1999; Lodolce et al., 1998; Sun et al., 2000; Wu et al., 2001). Ly108-/- mice were generated by targeting exon 2 and 3 in Bruce 4 (C57BL6) stem cells and are described in detail elsewhere (Wang, Rietdijk and Terhorst, manuscript in preparation). CD45.1+ C57BL/6.SJL (B6) mice and the MHC class II Aβ deficient mice on the C57BL/6 or C57BL/6.SJL background (Aβ-/-) carrying the CD45.2 and CD45.1 congenic marker, respectively, were purchased from Taconic (Germantown, NY). All mice were housed in the animal facility at the Indiana University School of Medicine (IUSM) or The University of Michigan Medical School under SPF conditions and used at 6-12 weeks of age. All animal experiments were performed under protocols approved by the institutions.
Bone Marrow Chimeric Mice
For bone marrow transfer experiments, the recipient B6 or Aβ-/- mice were lethally irradiated with 950 rads 24 hr before receiving BM transfers. Total BM cells were harvested from the femurs and tibias of donor mice (2-3 months of age) and depleted of mature T cells, B cells, and MHC class II positive lymphocytes by using a cocktail of antibodies containing anti-CD4 (RL172) and anti-CD8 (TIB105, TIB210), anti-CD19 (1D3), and anti-MHC class II (M5/114), followed by complement-mediated lysis. T-depleted BM cells from two different types of donor mice were mixed at a ratio of 1:1 and each recipient mouse received 2-5×105 cells in 500 μl of 1× PBS via tail vein injection. All BM chimeras were reconstituted for at least 8 weeks before analysis of T and NKT cell development and function.
Flow Cytometry
All antibodies used for flow cytometry were purchased from BD Pharmingen (San Diego, CA). Cells were pre-incubated with the anti-FcγR mAb 2.4G2 to block non-specific antibody binding before stained with the following FITC-, PE-, PerCP-, CyChrome-, APC-or biotin-conjugated antibodies: TCRβ (H57), CD4 (L3T4), CD8 (53-6.7), NK1.1 (PK136), CD45.1 (A20), CD45.2 (104), anti-IL-4 (11B11), and anti-IFN-γ (XMG1.2). Flourochrome-conjugated streptavidin was used to visualize staining by biotinylated primary antibodies. Events were acquired on FACSCalibur, LSRII, or FACSCanto (Becton Dickinson) flow cytometer and the data were analyzed using the CELLQuest Pro or FlowJo software.
CD4 T Cell Preparation and Differentiation
CD4 cells were purified from single cell suspensions of splenocytes from chimeric mice with anti-mouse CD4 microbeads (Miltenyi Biotec, Auburn, CA). To induce Th differentiation, CD4 T cells (1×106/ml) were stimulated with 5 μg/ml plate-bound anti-CD3ε (145-2C11), 1 μg/ml anti-CD28 (37.51), and 50 U of IL-2 (Roche, Indianapolis, IN) for 5-7 days. For Th1 differentiation, 3.5 ng/ml of IL-12 and 10 μg/ml of anti-IL-4 (11B11) were added. Th2 cultures were supplemented with 10 ng/ml of IL-4 and 10 μg/ml of anti-IFN-γ (R4-6A2).
Cytokine Intracellular Staining
Freshly-isolated splenocytes depleted of red blood cells or differentiated Th1 and Th2 CD4 T cells were stimulated with 50 ng/ml phorbol myristyl acetate and 1.5 μM ionomycin (Calbiochem, San Diego, CA) for 5 hours. Monensin (Sigma, St. Louis, MO) at 3 μM was added during the last three hours of stimulation. Activated splenic cells were first stained with flurochrome-conjugated anti-CD45.1, anti-CD45.2, anti-CD4, and anti-NK1.1. Activated Th1 and Th2 cells were stained with anti-CD45.1, anti-CD45.2 antibodies. Cells were then fixed in 2-4% paraformaldehyde for 10 minutes at room temperature, permeabilized with 0.2% saponin (Sigma), followed by staining with anti-IL4 (11B11) and anti-IFN-γ (XMG1.2) for flow cytometry.
Statistic Analysis
The two-tailed Student's t-test was used to analyze the statistic significance of the difference in percentage of T cells between different groups. P value smaller than 0.05 was considered statistically significant. *= p<0.05, **= p<0.01.
Supplementary Material
Acknowledgments
We are grateful to Drs. Pam Schwartzberg (NIH), Dan Littman (New York University), Mark Kaplan (Indiana University), and Leo LeFrancois (U. Connecticut) for providing reagents. We also thank to Ms. Yu Qiao (U. Michigan) for contributing the CD122 data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bendelac A. Positive selection of mouse NK1+ T cells by CD1-expressing cortical thymocytes. J Exp Med. 1995;182:2091–2096. doi: 10.1084/jem.182.6.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendelac A, Savage PB, Teyton L. The Biology of NKT Cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- Bhat R, Eissmann P, Endt J, Hoffmann S, Watzl C. Fine-tuning of immune responses by SLAM-related receptors. J Leukoc Biol. 2006;79:417–424. doi: 10.1189/jlb.0905537. [DOI] [PubMed] [Google Scholar]
- Burkett PR, Koka R, Chien M, Chai S, Boone DL, Ma A. Coordinate expression and trans presentation of interleukin (IL)-15Ralpha and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis. J Exp Med. 2004;200:825–834. doi: 10.1084/jem.20041389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannons JL, Yu LJ, Jankovic D, Crotty S, Horai R, Kirby M, Anderson S, Cheever AW, Sher A, Schwartzberg PL. SAP regulates T cell-mediated help for humoral immunity by a mechanism distinct from cytokine regulation. J Exp Med. 2004;203:1551–1565. doi: 10.1084/jem.20052097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannons JL, Yu LJ, Hill B, Mijares LA, Dombroski D, Nichols KE, Antonellis A, Koretzky GA, Gardner K, Schwartzberg PL. SAP regulates T(H)2 differentiation and PKC-theta-mediated activation of NF-kappaB1. Immunity. 2004;21:693–706. doi: 10.1016/j.immuni.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Chan B, Lanyi A, Song HK, Griesbach J, Simarro-Grande M, Poy F, Howie D, Sumegi J, Terhorst C, Eck MJ. SAP couples Fyn to SLAM immune receptors. Nat Cell Biol. 2003;5:155–160. doi: 10.1038/ncb920. [DOI] [PubMed] [Google Scholar]
- Choi EY, Jung KC, Park HJ, Chung DH, Song JS, Yang SD, Simpson E, Park SH. Thymocyte-thymocyte interaction for efficient positive selection and maturation of CD4 T cells. Immunity. 2005;23:387–396. doi: 10.1016/j.immuni.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Chung B, Aoukaty A, Dutz J, Terhorst C, Tan R. Signaling lymphocytic activation molecule-associated protein controls NKT cell functions. J Immunol. 2005;174:3153–3157. doi: 10.4049/jimmunol.174.6.3153. [DOI] [PubMed] [Google Scholar]
- Coffey AJ, Brooksbank RA, Brandau O, Oohashi T, Howell GR, Bye JM, Cahn AP, Durham J, Heath P, Wray P, et al. Host response to EBV infection in X-linked lymphoproliferative disease results from mutations in an SH2-domain encoding gene. Nat Genet. 1998;20:129–135. doi: 10.1038/2424. [DOI] [PubMed] [Google Scholar]
- Coles MC, Raulet DH. NK1.1+ T cells in the liver arise in the thymus and are selected by interactions with class I molecules on CD4+CD8+ cells. J Immunol. 2000;164:2412–2418. doi: 10.4049/jimmunol.164.5.2412. [DOI] [PubMed] [Google Scholar]
- Coudronniere N, Villalba M, Englund N, Altman A. NF-kappa B activation induced by T cell receptor/CD28 costimulation is mediated by protein kinase C-theta. Proc Natl Acad Sci U S A. 2000;97:3394–3399. doi: 10.1073/pnas.060028097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao T, Guo D, Ploss A, Stolzer A, Saylor C, Boursalian TE, Im JS, Sant'Angelo DB. Development of CD1d-restricted NKT cells in the mouse thymus. Eur J Immunol. 2004;34:3542–3552. doi: 10.1002/eji.200425546. [DOI] [PubMed] [Google Scholar]
- Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17:537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- Eberl G, Lowin-Kropf B, MacDonald HR. Cutting edge: NKT cell development is selectively impaired in Fyn- deficient mice. J Immunol. 1999;163:4091–4094. [PubMed] [Google Scholar]
- Egawa T, Eberl G, Taniuchi I, Benlagha K, Geissmann F, Hennighausen L, Bendelac A, Littman DR. Genetic evidence supporting selection of the Valpha14i NKT cell lineage from double-positive thymocyte precursors. Immunity. 2005;22:705–716. doi: 10.1016/j.immuni.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Engel P, Eck MJ, Terhorst C. The SAP and SLAM families in immune responses and X-linked lymphoproliferative disease. Nat Rev Immunol. 2003;3:813–821. doi: 10.1038/nri1202. [DOI] [PubMed] [Google Scholar]
- Gadue P, Morton N, Stein PL. The Src family tyrosine kinase Fyn regulates natural killer T cell development. J Exp Med. 1999;190:1189–1196. doi: 10.1084/jem.190.8.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gapin L, Matsuda JL, Surh CD, Kronenberg M. NKT cells derive from double-positive thymocytes that are positively selected by CD1d. Nat Immunol. 2001;2:971–978. doi: 10.1038/ni710. [DOI] [PubMed] [Google Scholar]
- Gilhus NE, Matre R. Development and distribution of HLA-DR antigens and Fc gamma receptors in the human thymus. Acta Pathol Microbiol Immunol Scand [C] 1983;91:35–42. [PubMed] [Google Scholar]
- Godthelp BC, Van Eggermond MC, Van Tol MJ, Vossen JM, van den Elsen PJ. T cell immune reconstitution after allogeneic bone marrow transplantation in bare lymphocyte syndrome. Hum Immunol. 2000;61:898–907. doi: 10.1016/s0198-8859(00)00156-7. [DOI] [PubMed] [Google Scholar]
- Graham DB, Bell MP, McCausland MM, Huntoon CJ, van Deursen J, Faubion WA, Crotty S, McKean DJ. Ly9 (CD229)-deficient mice exhibit T cell defects yet do not share several phenotypic characteristics associated with SLAM- and SAP-deficient mice. J Immunol. 2006;176:291–300. doi: 10.4049/jimmunol.176.1.291. [DOI] [PubMed] [Google Scholar]
- Hammond K, Cain W, van Driel I, Godfrey D. Three day neonatal thymectomy selectively depletes NK1.1+ T cells. Int Immunol. 1998;10:1491–1499. doi: 10.1093/intimm/10.10.1491. [DOI] [PubMed] [Google Scholar]
- Howie D, Laroux FS, Morra M, Satoskar AR, Rosas LE, Faubion WA, Julien A, Rietdijk S, Coyle AJ, Fraser C, Terhorst C. Cutting edge: the SLAM family receptor Ly108 controls T cell and neutrophil functions. J Immunol. 2005;174:5931–5935. doi: 10.4049/jimmunol.174.10.5931. [DOI] [PubMed] [Google Scholar]
- Jordan MA, Fletcher JM, Pellicci D, Baxter AG. Slamf1, the NKT cell control gene nkt1. J Immunol. 2007;178:1618–1627. doi: 10.4049/jimmunol.178.3.1618. [DOI] [PubMed] [Google Scholar]
- Kaplan MH, Wurster AL, Smiley ST, Grusby MJ. Stat6-dependent and -independent pathways for IL-4 production. J Immunol. 1999;163:6536–6540. [PubMed] [Google Scholar]
- Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C, Cavazzana-Calvo M, Le Deist F, Jabado N, Benkerrou M, Blanche S, Lisowska-Grospierre B, Griscelli C, Fischer A. Bone marrow transplantation in major histocompatibility complex class II deficiency: a single-center study of 19 patients. Blood. 1995;85:580–587. [PubMed] [Google Scholar]
- Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- La Motte-Mohs RN, Herer E, Zuniga-Pflucker JC. Induction of T-cell development from human cord blood hematopoietic stem cells by Delta-like 1 in vitro. Blood. 2005;105:1431–1439. doi: 10.1182/blood-2004-04-1293. [DOI] [PubMed] [Google Scholar]
- Latour S, Roncagalli R, Chen R, Bakinowski M, Shi X, Schwartzberg PL, Davidson D, Veillette A. Binding of SAP SH2 domain to FynT SH3 domain reveals a novel mechanism of receptor signalling in immune regulation. Nat Cell Biol. 2003;5:149–154. doi: 10.1038/ncb919. [DOI] [PubMed] [Google Scholar]
- Lewis DB, Yu CC, Forbush KA, Carpenter J, Sato TA, Grossman A, Liggitt DH, Perlmutter RM. Interleukin 4 expressed in situ selectively alters thymocyte development. J Exp Med. 1991;173:89–100. doi: 10.1084/jem.173.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Kim MG, Gourley TS, McCarthy BP, Sant'angelo DB, Chang CH. An Alternate Pathway for CD4 T Cell Development: Thymocyte-Expressed MHC Class II Selects a Distinct T Cell Population. Immunity. 2005;23:375–386. doi: 10.1016/j.immuni.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Li W, Sofi MH, Yeh N, Sehra S, McCarthy BP, Patel DR, Brutkiewicz RR, Kaplan MH, Chang CH. Thymic selection pathway regulates the effector function of CD4 T cells. J Exp Med. 2007;204:2145–2157. doi: 10.1084/jem.20070321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- Ma CS, Nichols KE, Tangye SG. Regulation of Cellular and Humoral Immune Responses by the SLAM and SAP Families of Molecules. Annu Rev Immunol. 2007;25:337–379. doi: 10.1146/annurev.immunol.25.022106.141651. [DOI] [PubMed] [Google Scholar]
- Manicassamy S, Gupta S, Huang Z, Sun Z. Protein kinase C-theta-mediated signals enhance CD4+ T cell survival by up-regulating Bcl-xL. J Immunol. 2006;176:6709–6716. doi: 10.4049/jimmunol.176.11.6709. [DOI] [PubMed] [Google Scholar]
- Marinova T, Altankova I, Dimitrova D, Pomakov Y. Presence of HLA-DR immunopositive cells in human fetal thymus. Arch Physiol Biochem. 2001;109:74–79. doi: 10.1076/apab.109.1.74.4281. [DOI] [PubMed] [Google Scholar]
- Markert ML, Alexieff MJ, Li J, Sarzotti M, Ozaki DA, Devlin BH, Sedlak DA, Sempowski GD, Hale LP, Rice HE, et al. Postnatal thymus transplantation with immunosuppression as treatment for DiGeorge syndrome. Blood. 2004;104:2574–2581. doi: 10.1182/blood-2003-08-2984. [DOI] [PubMed] [Google Scholar]
- Markert ML, Sarzotti M, Ozaki DA, Sempowski GD, Rhein ME, Hale LP, Le Deist F, Alexieff MJ, Li J, Hauser ER, et al. Thymus transplantation in complete DiGeorge syndrome: immunologic and safety evaluations in 12 patients. Blood. 2003;102:1121–1130. doi: 10.1182/blood-2002-08-2545. [DOI] [PubMed] [Google Scholar]
- Marsland BJ, Soos TJ, Spath G, Littman DR, Kopf M. Protein kinase C theta is critical for the development of in vivo T helper (Th)2 cell but not Th1 cell responses. J Exp Med. 2004;200:181–189. doi: 10.1084/jem.20032229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda JL, Gapin L. Developmental program of mouse Valpha14i NKT cells. Curr Opin Immunol. 2005;17:122–130. doi: 10.1016/j.coi.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Matsuda JL, Gapin L, Sidobre S, Kieper WC, Tan JT, Ceredig R, Surh CD, Kronenberg M. Homeostasis of V alpha 14i NKT cells. Nat Immunol. 2002;3:966–974. doi: 10.1038/ni837. [DOI] [PubMed] [Google Scholar]
- Matsuda JL, Zhang Q, Ndonye R, Richardson SK, Howell AR, Gapin L, Matsuda JL, Gapin L, Townsend MJ, Weinmann AS, et al. T-bet concomitantly controls migration, survival, and effector functions during the development of Valpha14i NKT cells. Blood. 2006;107:2797–2805. doi: 10.1182/blood-2005-08-3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCausland MM, Yusuf I, Tran H, Ono N, Yanagi Y, Crotty S. SAP regulation of follicular helper CD4 T cell development and humoral immunity is independent of SLAM and Fyn kinase. J Immunol. 2007;178:817–828. doi: 10.4049/jimmunol.178.2.817. [DOI] [PubMed] [Google Scholar]
- Nichols KE, Harkin DP, Levitz S, Krainer M, Kolquist KA, Genovese C, Bernard A, Ferguson M, Zuo L, Snyder E, et al. Inactivating mutations in an SH2 domain-encoding gene in X-linked lymphoproliferative syndrome. Proc Natl Acad Sci U S A. 1998;95:13765–13770. doi: 10.1073/pnas.95.23.13765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols KE, Hom J, Gong SY, Ganguly A, Ma CS, Cannons JL, Tangye SG, Schwartzberg PL, Koretzky GA, Stein PL. Regulation of NKT cell development by SAP, the protein defective in XLP. Nat Med. 2005a;11:340–345. doi: 10.1038/nm1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols KE, Ma CS, Cannons JL, Schwartzberg PL, Tangye SG. Molecular and cellular pathogenesis of X-linked lymphoproliferative disease. Immunol Rev. 2005b;203:180–199. doi: 10.1111/j.0105-2896.2005.00230.x. [DOI] [PubMed] [Google Scholar]
- Ohteki T, Ho S, Suzuki H, Mak TW, Ohashi PS. Role for IL-15/IL-15 receptor beta-chain in natural killer 1.1+ T cell receptor-alpha beta+ cell development. J Immunol. 1997;159:5931–5935. [PubMed] [Google Scholar]
- Park SH, Bae YM, Kim TJ, Ha IS, Kim S, Chi JG, Lee SK. HLA-DR expression in human fetal thymocytes. Hum Immunol. 1992;33:294–298. doi: 10.1016/0198-8859(92)90338-n. [DOI] [PubMed] [Google Scholar]
- Park WS, Bae Y, Chung DH, Choi YL, Kim BK, Sung YC, Choi EY, Park SH, Jung KC. T cell expression of CIITA represses Th1 immunity. Int Immunol. 2004;16:1355–1364. doi: 10.1093/intimm/dxh132. [DOI] [PubMed] [Google Scholar]
- Pasquier B, Yin L, Fondaneche MC, Relouzat F, Bloch-Queyrat C, Lambert N, Fischer A, de Saint-Basile G, Latour S. Defective NKT cell development in mice and humans lacking the adapter SAP, the X-linked lymphoproliferative syndrome gene product. J Exp Med. 2005;201:695–701. doi: 10.1084/jem.20042432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel DR, Li W, Park JS, Sofi MH, Gourley TS, Hangoc G, Kaplan MH, Chang CH. Constitutive expression of CIITA directs CD4 T cells to produce Th2 cytokines in the thymus. Cell Immunol. 2005;233:30–40. doi: 10.1016/j.cellimm.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Ranson T, Vosshenrich CA, Corcuff E, Richard O, Laloux V, Lehuen A, Di Santo JP. IL-15 availability conditions homeostasis of peripheral natural killer T cells. Proc Natl Acad Sci U S A. 2003;100:2663–2668. doi: 10.1073/pnas.0535482100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith W, LeibundGut-Landmann S, Waldburger JM. Regulation of MHC class II gene expression by the class II transactivator. Nat Rev Immunol. 2005;5:793–806. doi: 10.1038/nri1708. [DOI] [PubMed] [Google Scholar]
- Rice HE, Skinner MA, Mahaffey SM, Oldham KT, Ing RJ, Hale LP, Markert ML. Thymic transplantation for complete DiGeorge syndrome: medical and surgical considerations. J Pediatr Surg. 2004;39:1607–1615. doi: 10.1016/j.jpedsurg.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Sayos J, Wu C, Morra M, Wang N, Zhang X, Allen D, van Schaik S, Notarangelo L, Geha R, Roncarolo MG, et al. The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM. Nature. 1998;395:462–469. doi: 10.1038/26683. [DOI] [PubMed] [Google Scholar]
- Schluns KS, Nowak EC, Cabrera-Hernandez A, Puddington L, Lefrancois L, Aguila HL. Distinct cell types control lymphoid subset development by means of IL-15 and IL-15 receptor alpha expression. Proc Natl Acad Sci U S A. 2004;101:5616–5621. doi: 10.1073/pnas.0307442101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Supprian M, Tian J, Grant EP, Pasparakis M, Maehr R, Ovaa H, Ploegh HL, Coyle AJ, Rajewsky K. Differential dependence of CD4+CD25+ regulatory and natural killer-like T cells on signals leading to NF-kappaB activation. Proc Natl Acad Sci U S A. 2004;101:4566–4571. doi: 10.1073/pnas.0400885101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivakumar V, Hammond KJ, Howells N, Pfeffer K, Weih F. Differential requirement for Rel/nuclear factor kappa B family members in natural killer T cell development. J Exp Med. 2003;197:1613–1621. doi: 10.1084/jem.20022234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanic AK, Bezbradica JS, Park JJ, Matsuki N, Mora AL, Van Kaer L, Boothby MR, Joyce S. NF-kappa B controls cell fate specification, survival, and molecular differentiation of immunoregulatory natural T lymphocytes. J Immunol. 2004a;172:2265–2273. doi: 10.4049/jimmunol.172.4.2265. [DOI] [PubMed] [Google Scholar]
- Stanic AK, Bezbradica JS, Park JJ, Van Kaer L, Boothby MR, Joyce S. Cutting edge: the ontogeny and function of Va14Ja18 natural T lymphocytes require signal processing by protein kinase C theta and NF-kappa B. J Immunol. 2004b;172:4667–4671. doi: 10.4049/jimmunol.172.8.4667. [DOI] [PubMed] [Google Scholar]
- Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- Stetson DB, Mohrs M, Reinhardt RL, Baron JL, Wang ZE, Gapin L, Kronenberg M, Locksley RM. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med. 2003;198:1069–1076. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Arendt CW, Ellmeier W, Schaeffer EM, Sunshine MJ, Gandhi L, Annes J, Petrzilka D, Kupfer A, Schwartzberg PL, Littman DR. PKC-theta is required for TCR-induced NF-kappaB activation in mature but not immature T lymphocytes. Nature. 2000;404:402–407. doi: 10.1038/35006090. [DOI] [PubMed] [Google Scholar]
- Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. The regulatory role of Valpha14 NKT cells in innate and acquired immune response. Annu Rev Immunol. 2003;21:483–513. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- Tepper RI, Levinson DA, Stanger BZ, Campos-Torres J, Abbas AK, Leder P. IL-4 induces allergic-like inflammatory disease and alters T cell development in transgenic mice. Cell. 1990;62:457–467. doi: 10.1016/0092-8674(90)90011-3. [DOI] [PubMed] [Google Scholar]
- Thulesen S, Jorgensen A, Gerwien J, Dohlsten M, Holst Nissen M, Odum N, Ropke C. Superantigens are presented by and activate thymocytes from infants. Exp Clin Immunogenet. 1999;16:226–233. doi: 10.1159/000019114. [DOI] [PubMed] [Google Scholar]
- Tilloy F, Di Santo JP, Bendelac A, Lantz O. Thymic dependence of invariant V alpha 14+ natural killer-T cell development. Eur J Immunol. 1999;29:3313–3318. doi: 10.1002/(SICI)1521-4141(199910)29:10<3313::AID-IMMU3313>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Townsend MJ, Weinmann AS, Matsuda JL, Salomon R, Farnham PJ, Biron CA, Gapin L, Glimcher LH. T-bet regulates the terminal maturation and homeostasis of NK and Valpha14i NKT cells. Immunity. 2004;20:477–494. doi: 10.1016/s1074-7613(04)00076-7. [DOI] [PubMed] [Google Scholar]
- Urdahl KB, Sun JC, Bevan MJ. Positive selection of MHC class Ib-restricted CD8(+) T cells on hematopoietic cells. Nat Immunol. 2002;3:772–779. doi: 10.1038/ni814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veillette A. NK cell regulation by SLAM family receptors and SAP-related adapters. Immunol Rev. 2006;214:22–34. doi: 10.1111/j.1600-065X.2006.00453.x. [DOI] [PubMed] [Google Scholar]
- Veillette A, Latour S. The SLAM family of immune-cell receptors. Curr Opin Immunol. 2003;15:277–285. doi: 10.1016/s0952-7915(03)00041-4. [DOI] [PubMed] [Google Scholar]
- Wang N, Satoskar A, Faubion W, Howie D, Okamoto S, Feske S, Gullo C, Clarke K, Sosa MR, Sharpe AH, Terhorst C. The cell surface receptor SLAM controls T cell and macrophage functions. J Exp Med. 2004;199:1255–1264. doi: 10.1084/jem.20031835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Nguyen KB, Pien GC, Wang N, Gullo C, Howie D, Sosa MR, Edwards MJ, Borrow P, Satoskar AR, et al. SAP controls T cell responses to virus and terminal differentiation of TH2 cells. Nat Immunol. 2001;2:410–414. doi: 10.1038/87713. [DOI] [PubMed] [Google Scholar]
- Zhou D, Mattner J, Cantu C, 3rd, Schrantz N, Yin N, Gao Y, Sagiv Y, Hudspeth K, Wu YP, Yamashita T, et al. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306:1786–1789. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.