Abstract
Antiviral immunity in mammals involves several levels of surveillance and effector actions by host factors to detect viral pathogens, trigger α/β interferon production, and to mediate innate defenses within infected cells. Our studies have focused on understanding how these processes are regulated during infection by hepatitis C virus (HCV) and West Nile virus (WNV). Both viruses are members of the Flaviviridae and are human pathogens but they each mediate a very different disease and course of infection. Our results demonstrate common and unique innate immune interactions of each virus that govern antiviral immunity, and demonstrate the central role of α/β interferon immune defenses in controlling the outcome of infection.
Keywords: LGP2, IPS-1, IRF-3, MAVS, RIG-I
1. Introduction
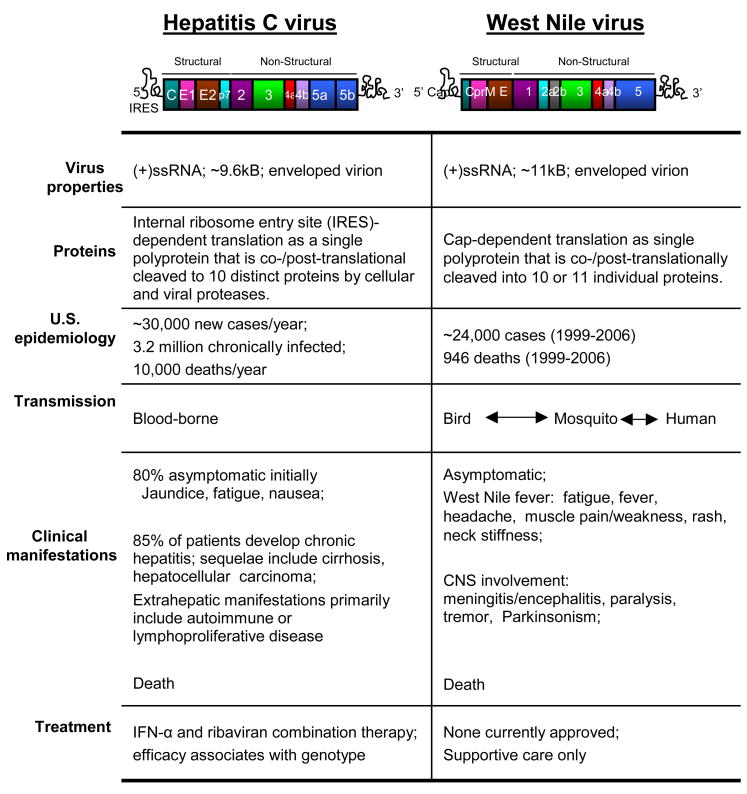
Viruses of the Flaviviridae are globally distributed and are responsible for major diseases of humans and other animals. Hepatitis C virus (HCV) and West Nile (WNV) are distantly related members of this family, and are enveloped viruses encoding a single-stand RNA genome of positive polarity. The viral genome serves as a template for the production of a large polyprotein that is post-translationally processed into the individual structural proteins that build new virus particles, and non-structural (NS) proteins that support virus replication. Virus replication takes place and in close association with intracellular membranes and through a double-stranded RNA (dsRNA) intermediate [1,2]. Despite their common replication strategy, HCV and WNV mediate a very different course of infection: wherein HCV is hepatotropic and typically causes a chronic infection, WNV is neurotropic and causes an acute infection (Fig. 1) [3–5]. Chronic HCV and acute WNV infection are each linked with viral strategies to control α/β interferon (IFN) immune defenses [6,7].
Figure 1. Characteristics of HCV and WNV.
The virologic, epidemiologic, and clinic features of each are indicated. Upper: structural representation of the viral genome and polyprotein coding region (shown in color). The viral proteins of the final processed polyprotein for HCV (left) are denoted as core (c), envelope 1 (E1) and envelope 2 (E2), p7, and NS2-5B proteins. For WNV the protein products are shown as shown as core (C), preM/M (preM), and NS1-5.
Innate immune defense programs induced by α/β IFNs represent an essential first line of protection against virus infection. The α/β IFNs are typically produced by cells during the early stages of virus infection when the virus is recognized by specific pathogen recognition receptor (PRR) molecules of the host (see Fig. 2). These include the cytoplasmic RNA helicases retinoic inducible gene-I (RIG-I) and melanoma differentiation antigen 5 (MDA5) and cell surface or endosomal Toll-like receptors (TLRs). PRRs bind to viral products and subsequently signal downstream activation of interferon regulatory factors (IRFs) and NF-κB transcription factors to trigger the production of α/β IFNs and other proinflammatory cytokines [8]. Binding of the α/β IFN receptor on cells within the local tissue triggers the receptor-mediated signaling of the Jak-Stat pathway by the secreted IFNs to induce the tissue-wide expression of hundreds of interferon-stimulated genes (ISGs) [9]. ISG products impart antiviral and immunomodulatory activities that limit virus replication and spread [10,11]. The expression of α/β IFNs has been differentially observed between HCV and WNV infection in vivo. α/β IFN transcripts are present but not highly expressed in hepatic tissue during chronic HCV infection but are induced to high levels in vivo during WNV infection [12,13]. These observations underscore the distinct strategies used by HCV and WNV to disrupt host innate immune defenses. Here, we provide an overview of our studies aimed at understanding how HCV and WNV evade antiviral defenses during infection.
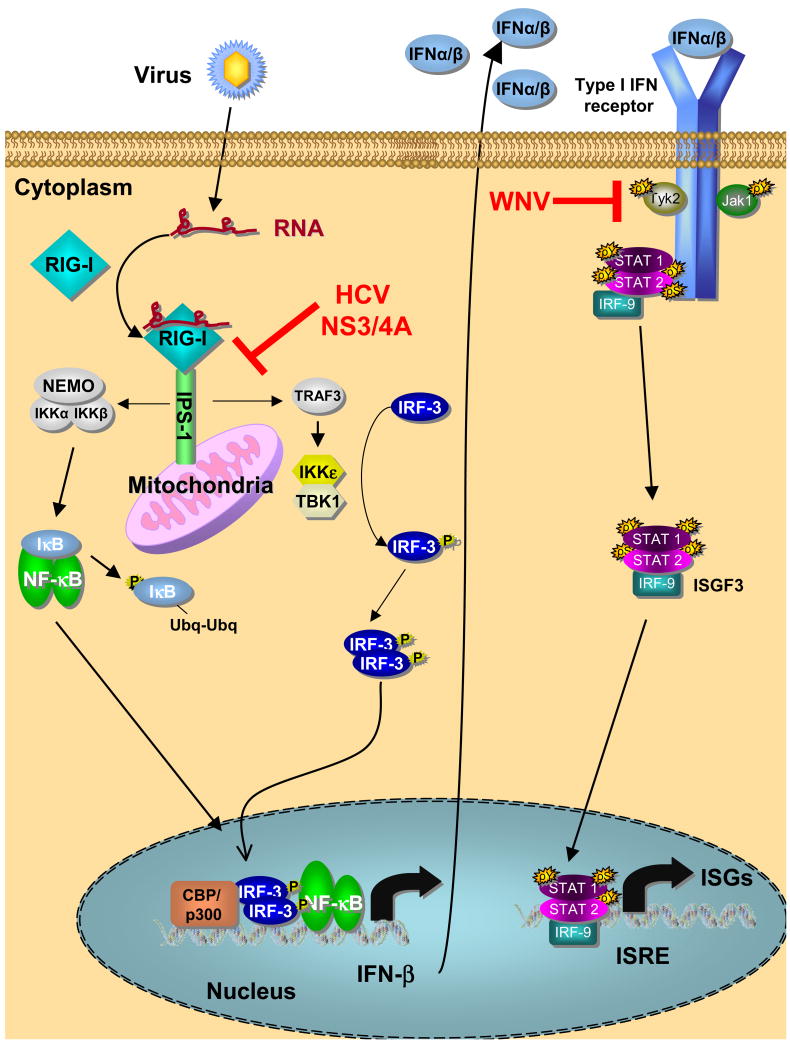
Figure 2. α/β IFN interactions with HCV and WNV.
Virus infection and replication produces replication products, such as dsRNA, that are recognized and bound by RIG-I. This drives a conformation change in RIG-I that promotes its interaction with IPS-1 anchored on the mitochondria outer membrane and results in recruitment and activation of signaling factors that activate IRF-3 and NF-κB. Transcription factor translocation to the nucleus results in induction of α/β IFN expression and secretion from the infected cell. α/β IFN binding to the IFN receptor triggers activation of receptor bound protein kinases and downstream Jak-Stat pathway signaling of ISG expression. The HCV NS3/4A protease cleaves IPS-1 off of the mitochondria outer membrane to ablate α/β IFN expression, while WNV disrupts Jak-Stat signaling processes of the α/β IFN receptor to attenuate IFN actions. P and Ubq respectively denote protein modification by phosphorylation and ubiquitination events triggered during innate immune signaling.
1.1 HCV is a global health problem
HCV emerged largely after the second world war and has become endemic throughout the world. There are approximately 200 million people now with chronic HCV infection, making the virus a serious public health problem well into the foreseeable future [4]. HCV is transmitted parenterally, and transmission typically results in a chronic infection. HCV infection is a major cause of liver disease and is often associated with hepatic fibrosis, cirrhosis, and hepatocellular carcinoma, making it the main indication for liver transplantation [14] (Fig 1). Infection with HCV is currently treated by weekly injections of pegylated IFN-α in conjunction with oral ribavirin [15]. There are six HCV genotypes, with genotype 1 being widespread and the most difficult to treat. As described below, HCV can effectively block virus signaling of α/β IFN production. This process of immune evasion provides a foundation for chronic infection.
1.2 West Nile is an emerging infectious disease
WNV was first identified in Uganda in 1937, and has circulated endemically throughout Africa, the Middle East and Europe. In the mid-to-late-1990s incidences of WNV-associated outbreaks of severe disease including meningitis and encephalitis suddenly increased [16], and in 1999 the virus was detected in the Western Hemisphere for the first time [17]. WNV has rapidly spread throughout the continental United States, Canada, Mexico and the Caribbean. In the United States alone there have been nearly 24,000 diagnosed human cases of WNV infection with approximately 4% of these resulting in death [http://www.cdc.gov/ncidod/dvbid/westnile/index.htm]. The death rate climbs to nearly 10% when the virus crosses the blood-brain barrier [16] (Fig. 1). WNV differs from HCV in that it is an arthropod-borne virus that is transmitted primarily in a bird-mosquito-bird cycle with humans and other animals as dead-end hosts. The illness caused by WNV is generally an acute, febrile illness with a small percentage of human infections resulting in neuroinvasive disease [3]. Currently there is no treatment for infection with WNV. Alick Issacs, the co-discoverer of IFN [18], originally defined the antiviral potential of IFN against WNV infection [19]. More recently, off-label use of IFNα-2b has been tried as a therapeutic for WNV-infected patients with varying success, in which treatment initiated less than 10 days after the onset of neurological symptoms resulted in a better clinical prognosis [20,21]. Various studies have linked WNV evasion of α/β IFN actions with a pathogenic infection outcome [7].
2. Distinct mechanisms of innate immune control by HCV and WNV
In order to replicate and spread, viruses must evade or resist innate immune defenses of the host cell [11]. While chronic virus infection obviates a need for a virus to direct the constitutive attenuation of α/β IFN production, viruses that mediate an acute infection course only have to avoid α/β IFN actions during their window period of virus production. These parameters underscore the distinct processes by which HCV and WNV evade innate immune actions. As described in the following sections, HCV antagonizes RIG-I signaling to suppress α/β IFN production by the infected cell, thereby avoiding the limitation to virus replication imposed by endogenous IFN. On the other hand, WNV infection induces α/β IFN production through processes involving RIG-I, but it antagonizes Jak-Stat signaling through the α/β IFN receptor, thus regulating ISG expression and the antiviral actions of IFN.
2.1 HCV regulation of the RIG-I pathway
Studies of HCV infection in chimpanzees have demonstrated that acute virus challenge and infection resolution associated with a robust α/β IFN response in hepatic tissue [22]. In vitro studies attribute this response to PRR triggering through HCV RNA recognition by RIG-I and signaling through the RIG-I pathway [23] (Fig. 2). The HCV genome contains motifs of RNA secondary structure within it 5′ and 3′ nontranslated regions (NTRs) and in areas throughout the coding region [24]. When transfected into human Huh7 hepatoma cells, HCV RNA triggers the activation of IRF-3 and the expression of IFN-β, but this response was found to be defective in a subclone of these cells that were highly permissive for HCV RNA replication [23,25]. Biochemical studies revealed that the permissive cells had a defective innate immune response to synthetic dsRNA and to HCV RNA that mapped to a signaling lesion upstream of IRF-3 activation. Further cDNA complementation studies identified RIG-I as a critical factor of HCV RNA signaling that was defective in the permissive cells [23]. These observation supported earlier work from Yoneyama et al. identifying RIG-I as a novel PRR that recognizes dsRNA and signals IRF-3 activation during virus infection [26]. Thus, our observations revealed a role for RIG-I in binding to structured HCV RNA in a reaction that initiates innate immune defenses controlling cellular permissiveness for HCV. Biochemical studies demonstrate RIG-I can bind to the structured 3′ and 5′ NTRs of the HCV genome RNA but not a linear, nonstructured domain of the HCV genome nor to synthetic single-stranded RNA [23,27]. These observations support a model of innate immune signaling during acute HCV infection in which the viral RNA is recognized by RIG-I, thereby triggering RIG-I signaling of downstream IRF-3 activation, α/β IFN production, and ensuing hepatic ISG expression (Fig. 2).
2.2 RIG-I as an on/off switch to innate immunity against HCV
Structurally, RIG-I contains two tandem caspase activation and recruitment domains (CARDs) and a DExD/H box RNA helicase domain [26]. The helicase domain is thought to mediate binding of viral RNA whereas the CARDs confer downstream signaling of IRF-3 activation [23,26]. CARD signaling occurs through interaction with the interferon promoter stimulator-1 (IPS-1) adaptor protein [28], also known as Cardif, MAVS and VISA [29]. IPS-1 is localized on the outer mitochondrial membrane where it serves to recruit a macromolecular complex or “signalsome” that directs innate immune signaling in response to RIG-I binding [29]. When overexpressed in cells, RIG-I does not normally result in constitutive signaling to IRF-3, but instead it amplifies virus activation of this process [23,30]. These observations implicate an internal mechanism for repression of RIG-I signaling in otherwise resting cells in the absence of virus infection. Overexpression of RIG-I lacking the CARDs confers dominant-negative suppression of virus signaling. Dissecting this region further has identified a 190 amino acid carboxyl-terminal domain of RIG-I that serves as a repressor domain (RD) to hold RIG-I in a closed, non-signaling conformation in the absence of a dsRNA ligand [27]. Structure-function and biochemical studies demonstrated that 1) the RIG-I RD confers signaling repression by interacting with both the amino-terminal CARDs as well as the helicase domain, and 2) binding of a RNA ligand to RIG-I juxtaposes the RD to reveal an open conformation competent to signal downstream activation of IRF-3. RIG-I RD function studies demonstrated that ectopic expression of the RD alone was sufficient to suppress innate immune defenses within cultured cells thus conferring increased permissiveness to HCV infection [27]. Taken together, these data suggest a model for RIG-I activation wherein RIG-I exists as a monomer in resting cells, but self-associates upon virus infection or high level expression induced by α/β IFN. This multimerization is necessary but not sufficient for RIG-I CARD signaling, and a further viral RNA binding event by the helicase domain releases the CARDs from RD inhibition and results in an active RIG-I complex that can signal downstream through IPS-1 (Fig. 2). Thus, RIG-I mediates innate immune signaling during HCV infection through processes governed by the RD as an on-off switch for innate immunity.
2.3 Disruption of RIG-I signaling by the HCV NS3/4A protease
The α/β IFN genes are not highly expressed during chronic HCV infection [13], and ISG expression varies widely among patients [31], suggesting that that the innate immune response to HCV infection undergoes virus-directed regulation. Our in vitro studies of the HCV RNA replicon model and of cells infected with the JFH1 HCV 2A infectious clone demonstrated that HCV imposes a blockade to RIG-I signaling of α/β IFN production [32,33]. Analysis of viral protein function identified the HCV NS3/4A protein complex an antagonist of virus-induced IRF-3 activation [34]. NS3/4A is the essential HCV protease and RNA helicase [1]. Studies of mutant NS3 lacking protease or helicase activity revealed that the protease activity and not the RNA helicase activity of NS3/4A was responsible for the IRF-3 activation blockade [35]. These observation were validated by treatment of cells with peptidomimetic active site inhibitors of the NS3 protease, in which inhibitor treatment restored virus activation of IRF-3 and ISG expression even in the presence of high levels of NS3/4A [34,35]. Studies to address the RIG-I pathway interactions with NS3/4A and HCV RNA replication defined this pathway as the target of the NS3/4A signaling blockade, and demonstrated that the protease actions of NS3/4A imposed the block of RIG-I signaling to prevent the downstream activation of both IRF-3 and NF-κB. Thus, this regulation impacts the expression of IRF-3 target genes and NF-κB target genes in parallel. The parallel disruption of IRF-3 and NF-κB activation by NS3/4A allows HCV to suppress the expression of innate immune effector and proinflammatory response genes that may otherwise control infection [32].
The identification of the HCV NS3/4A protease as an antagonist of RIG-I signaling presented the hypothesis that the HCV protease was targeting, cleaving, and inactivating an essential signaling protein within the RIG-I pathway. However, biochemical studies showed us that NS3/4A did not cleave any of the known components of the RIG-I pathway, thereby indicating that an undefined and essential cofactor of RIG-I signaling was the likely target of NS3/4A [23]. Thus, our studies inspired a global hunt for this factor, which was subsequently identified by several research groups, including our own, using functional or interactive cloning strategies and bioinformatics approaches. These efforts identified IPS-1/Cardif/MAVS/VISA, as an essential adaptor protein of RIG-I signaling [reviewed in reference 29]. Studies of HCV infection demonstrated that IPS-1 was targeted and cleaved by the NS3/4A protease during virus replication [33,36]. We found that NS3/4A targets and cleaves endogenous IPS-1 in vitro and in vivo [33]. Mechanistically, this cleavage event occurs at cysteine 508 of IPS-1 to release it from its membrane anchor. As a result, IPS-1 comes off the mitochondria and cannot recruit the signalsome that mediates downstream activation of IRF-3 and NF-κB during HCV infection (Fig. 2). Protein function studies now demonstrate that NS3/4A targeting of IPS-1 occurs through the protease domain and its minimal NS4A cofactor alone, and does not involve the NS3 helicase domain [35]. Moreover, NS3/4A protease inhibitors can effectively prevent proteolysis of IPS-1 during HCV infection, and treatment of infected cells actually restores the innate immune response to infection even in the presence of NS3/4A [33,35]. Thus, IPS-1 is an essential signal transducer of the RIG-I pathway that is targeted and cleaved by NS3/4A during infection (Fig 2). In effect, the proteolysis of IPS-1 attenuates both the production of α/β IFN and disrupts a critical amplification loop of α/β IFN signaling, thus suppressing innate immune defenses to HCV infection. Our studies reveal an immunomodulatory potential of NS3/4A protease inhibitors toward the therapeutic restoration of innate immune defenses against HCV [35,37].
2.4 MDA5 and LGP2: RIG-I family helicases
MDA5 is cytoplasmic RNA helicase with CARDs and is structurally similar to RIG-I [38]. MDA5 is an ISG and also serves as a PRR to initiate signaling of innate immune defenses during virus infection [8]. RIG-I and MDA5 exhibit remarkable distinctions of PRR function and recognition of viruses. In fibroblasts and tissue parenchymal cells RIG-I is a requisite PRR for different negative-strand RNA viruses and HCV, while MDA5 is an essential PRR of encephalomyocarditis virus infection (a positive-strand RNA virus) [23,27,39,40]. Both factors can bind and respond to dsRNA in vitro and in transfected cells, and single stranded RNA with exposed 5′ triphosphates (which are present in HCV RNA) has also been defined as a RIG-I ligand [41]. In contrast to RIG-I, MDA5 does not contain a functional RD, thus it constitutively activates the IFN-β promoter when expressed in cells [27]. While RIG-I has been shown to bind to regions of the HCV genome with high degrees of secondary structure, MDA5 does not efficiently bind to HCV RNA. Moreover, MDA5 is not required for signaling of innate defenses by the HCV genomic RNA [27], thereby indicating that RIG-I is the essential PRR for HCV.
LGP2 is a third member of the RIG-I-like helicase family [38], sharing homology with RIG-I and MDA5 but lacking CARDs. LGP2 has been shown to negatively regulate virus activation of RIG-I [38], and this occurs through the actions of a carboxyl-terminal RD with homology to the RD of RIG-I [27]. LGP2 has also been shown to displace IKK-ε (a component of the IPS-1 signalsome) from IPS-1 to thereby block signaling [42]. Like RIG-I, LGP2 is an RNA-binding protein, and this activity to bind RNA presents another possible mechanism by which it may inhibit RIG-I signaling through sequestration of RNA ligands [43]. It is important to note that LGP2 does not inhibit MDA5 signaling [27] even though RIG-I and MDA5 signal through IPS-1 as a common downstream adaptor protein [28]. As an ISG itself, LGP2 expression is indirectly subject to control through NS3/4A proteolysis of IPS-1. LGP2 thus defines an autoregulatory mechanism to control RIG-I signaling and innate immune programs [27]. However, the true function of LGP2 during virus infection and a possible role as a negative or positive effector of PRR signaling remain to be defined.
3. WNV regulation of IFN signaling
Unlike HCV, WNV does not actively inhibit the RIG-I pathway leading to the production of α/β IFN. Rather, WNV delays activation of PRR signaling long enough to give the virus a replicative advantage within the infected cell [44]. The delayed activation of IRF-3 during WNV infection results in a robust α/β IFN response that slows cell to cell virus spread but this response is largely ineffective at limiting infection by the emergent strain [45]. The exact mechanism by which WNV evades PRR detection is not understood. Studies using cells from gene knockout mice revealed that WNV signals innate defenses through RIG-I-dependent mechanisms as well as through processes independent of RIG-I likely involving MDA5 [44]. These studies revealed that efficient viral replication is dependent upon the virus delaying the activation of innate defenses inasmuch as ectopic activation of the RIG-I pathway results in a severe limitation of virus replication [44]. The delay in PRR detection of WNV provides the virus with a window of opportunity to essentially replicate unimpeded during the early stages of infection. Virus replication during this window period supports an accumulation of viral proteins that exert effects on α/β IFN actions.
3.1 WNV disruption of α/β IFN receptor signaling is a pathogenesis determinant
WNV replication in the face of a potent albeit delayed innate immune response suggests that it can effectively evade or control the ISG response signaled by IFNs [45–47]. Several groups have recently reported that WNV is capable of inhibiting activation of JAK-STAT signaling components [48–51]. However, the exact mechanism of this inhibition is not clear, as it has been proposed that the NS2A, NS2B3, NS4A and NS4B viral proteins each have inhibitory activity against IFN signaling. Further work using viral genetic approaches is needed to define the precise mechanisms by which WNV antagonizes α/β IFN signaling. It is clear, however, that ISG induction still occurs during WNV infection, suggesting that viral control of α/β IFN signaling is not complete, and that continuous induction of α/β IFN expression may occur through PRR signaling processes triggered during asynchronous cell to cell virus spread [12,45,51,52]. Thus, WNV may attenuate or “fine-tune” α/β IFN signaling sufficiently to support virus replication. The importance of this fine tuning of JAK-Stat signaling was demonstrated by comparing a highly pathogenic WNV strain (WNV-TX02) and a traditionally nonpathogenic strain (WNV-MAD78) during infection of wild type cells or cells recovered from mice lacking a functional α/β IFN receptor. In cells from wild type animals the nonpathogenic WNV-MAD78 strain was attenuated in its ability to antagonize IFN signaling compared to pathogenic WNV-TX02 [51]. This phenotype correlated with a completely avirulent phenotype of the nonpathogenic virus in vivo during infection of wild type mice. Importantly, virulence of the normally nonpathogenic WNV-MAD78 virus was unmasked upon infection of mice lacking a functional α/β IFN receptor. All WNV strains thus far shown by other groups to antagonize α/β IFN receptor signaling were derived from pathogenic isolates of the virus [49,50,53]. These studies demonstrate that the antiviral actions of α/β IFNs are essential for immunity and protection against WNV infection, and define viral suppression of JAK-Stat signaling through the α/β IFN receptor as a major determinant of WNV pathogenesis.
3.2 α/β IFN limits peripheral dissemination of WNV and protects neurons against lethal infection
In vivo studies have revealed an important role of α/β IFNs in controlling tissue tropism of WNV infection. Normally, WNV is not detected in peripheral organs such as the heart, kidney, liver, lung, or muscle, yet in mice lacking a function α/β IFN receptor high viral load was detected in each of these organs [12]. Furthermore, α/β IFN receptor deficient mice exhibited higher viral load in serum and in the central nervous system (CNS) that associated with a significant reduction in the survival of neurons infected with WNV. These results demonstrate the critical role α/β IFN plays in not only controlling WNV replication at the site of inoculation but also in protecting non-renewable neurons in the CNS from the damaging effects of infection. The effector molecules responsible for the control of WNV replication within infected cells are only incompletely defined. The identification of these important antiviral components could lead to new therapeutics effective not only against WNV but also against other viruses. Current evidence indicates pathogenic and non-pathogenic WNV strains induce distinct transcriptional profiles in infected cells [46]. Understanding the genes that are differentially regulated and therefore potentially responsible for control of viral replication, between pathogenic and nonpathogenic strains is critical to understanding the underlying biology of these viruses.
4. ISGs control HCV and WNV replication
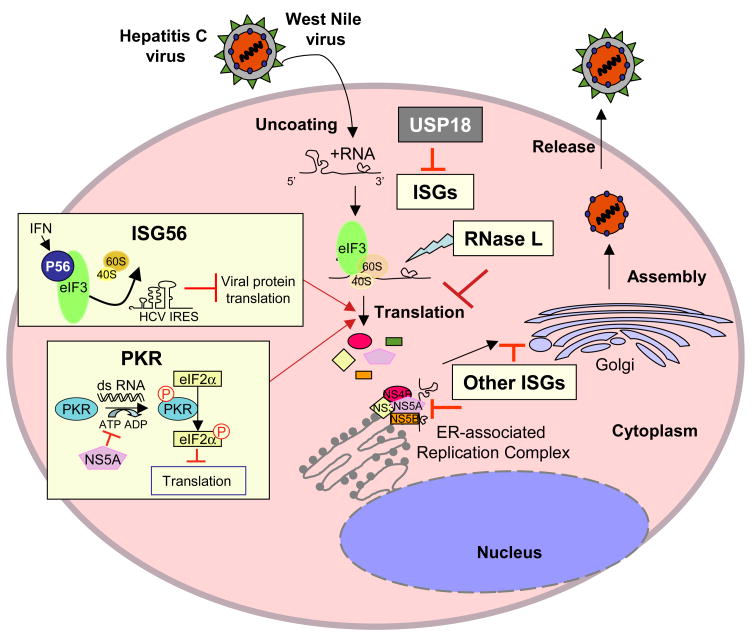
Our studies demonstrate an important role of α/β IFN immune defenses in controlling HCV or WNV infection outcome, and further imply important roles of ISGs in controlling hepatic spread of HCV or systemic and CNS dissemination of WNV. The spectrum of ISGs involved in these processes number in the hundreds and the functions of most are not known. However, studies of α/β IFN actions have revealed important insights into the antiviral functions of specific ISGs against HCV and WNV. Our work has demonstrated that α/β IFN exerts a dominant effect on HCV RNA translation that serves to suppress viral replication [54]. Biochemical studies defined PKR and ISG56 as ISG effectors of α/β IFN-induced translational control programs in cultured hepatoma cells [54,55]. PKR and ISG56 were shown to operate at different levels of translation initiation to respectively block eukaryotic initiation factor (eIF) 2 recycling and ribosome recruitment by eIF3, thereby attenuating HCV protein synthesis. α/β IFN has also been shown to suppress the production of the negative-strand intermediate of HCV RNA replication in association with a general reduction of viral RNA translation [56], and effective inhibition of HCV replication in vitro has corresponded with high level expression of ISG6-16, though the mechanisms of this control are not known [57]. Various studies to assess hepatic ISG expression in human patients have provided mixed results of expression or suppression of specific ISGs during chronic HCV infection. However, a recent study identified USP18 as a possible factor whose expression associated with a poor response rate of HCV infected patients undergoing IFN therapy [58]. In vitro studies have now demonstrated a possible role for USP18 as a negative regulator of ISG expression (Fig. 3) [59]. USP18 counters the specific antiviral actions of ISG15 [59] but its impact on other ISGs such as PKR and ISG56 has not been defined nor is the role of ISG15 in HCV infection known. The translational suppressive action of α/β IFN of HCV replication may contribute to the acute reduction of viral levels observed in vivo during the first hours and days of therapy, but it is clear that HCV can resist these actions to persist in the course of therapy, in part through viral countermeasures of IFN action [6]. Further studies are required to understand the nature of ISG expression control and function during HCV infection. Such efforts hold continued importance for understanding and improving current therapy for HCV.
Figure 3. ISG action against HCV or WNV replication.
HCV and WNV replication involves viral mRNA translation, membrane-associated replication of the viral genome, and a maturation/assembly process that involves secretion of enveloped virus particles from the infected cell. α/β IFN induced the expression of ISGs that suppress HCV or WNV RNA translational and replication. In addition, other ISGs likely impart antiviral actions against the various steps of virus replication. USP18 has been identified as a negative regulator of the ISG response against HCV.
Recent work is beginning to elucidate roles for specific ISGs in controlling WNV infection outcome. Analysis of gene expression following acute WNV infection of a human embryonic kidney cell line revealed the induction of several ISGs including PKR, ISG56, and ISG6-16. ISG56 is a direct IRF-3 target gene while PKR and ISG6-16 are induced through α/β IFN signaling actions [60]. Thus, WNV infection triggers an innate immune response in the host involving both the IRF-3 and α/β IFN signaling pathways [45]. After intranasal infection of mice with WNV, expression of ISG56 and its gene family members, ISG49 and ISG54, was significantly increased throughout the brain as compared to non-infected control mice [52]. Importantly, ISG56 was expressed in infected and non-infected cells within the brain of animals with WNV infection, suggesting it may contribute to protection from virus spread during a response induced by endogenous α/β IFN. Other studies have demonstrated that PKR and RNaseL modulate WNV pathogenesis in mice by controlling infection in peripheral tissues and neurons [61]. Like PKR, RNaseL modulates mRNA translation but does so by cleaving target RNA substrates [62]. PKR and RNaseL deficient mice were significantly more susceptible to subcutaneous WNV infection than wild type mice, and exhibited increased viremia and viral burden in peripheral tissues in association with earlier entry of the virus into the brain and CNS [61]. Thus, PKR and RNAseL contribute to the control of WNV dissemination and protection of peripheral tissues from infection. However, despite the role of PKR and RNaseL in controlling virus dissemination, the pathogenic WNV exhibits a less severe virulence phenotype in mice lacking either of these factors compared to mice lacking a functional α/β IFN receptor [12]. This observation underscores the complexity of function within the many ISGs induced by α/β IFNs, and indicate that additional ISGs are involved in α/β IFN mediated protection against WNV infection [7].
Conclusion
IFN was discovered 50 years ago as an antiviral agent secreted by infected cells [18]. This discovery and the many exciting studies of α/β IFN biology that have followed continue to be a driving force and constant inspiration to our work. HCV and WNV are important human pathogens, and our studies have defined intimate relationships of both with α/β IFN. HCV and WNV have evolved strategies of innate immune control that support virus replication and spread. Our studies of HCV have defined RIG-I and IPS-1 respectively as important PRR and signaling proteins involved in initiation of innate defenses to infection, and we have identified the viral NS3/4A protease as a major feature of innate immune control by HCV. Our studies define the NS3/4A-IPS-1 interface as a novel target of antiviral therapy by NS3 protease inhibitors that function to restore innate immune signaling to HCV infected cells. Examination of WNV/IFN interactions have defined viral processes controlling the α/β IFN response as a determinant of pathogenesis and infection control. A full understanding of α/β IFN biology and antiviral actions against HCV, WNV and other viral pathogens will require careful functional analysis of the PRR pathways and their signaling factors that induce α/β IFN expression in different cells and tissues, and definition of the specific actions of antiviral effector ISGs. Defining these processes will provide direction for future studies aimed at exploiting PRR signaling and ISG function in antiviral vaccine and therapeutic strategies of virus control.
Acknowledgments
Research in the Gale laboratory is supported by grants to MG from the NIH (AI060389, AI040035 Project 4, AI48235, and AI057568), The Ellison Medical Foundation, the Burroughs Wellcome Fund, and a gift from Mr. and Mrs. R. Batcheldor. BK and CJ were supported in part by a training grant to the UT Southwestern Medical Scientist Training Program. We are grateful to our collaborators, and we thank past and present members of our laboratory for their insightful contributions to this work.
Biographies
 Brian Keller received his Bachelor of Science Degree in Microbiology from Kansas State University in 2001. He is a student in the Medical Scientist training program, at the University of Texas Southwestern Medical Center, where he is currently completing his Ph.D. His dissertation research is focused on understanding how WNV evades α/β IFN actions.
Brian Keller received his Bachelor of Science Degree in Microbiology from Kansas State University in 2001. He is a student in the Medical Scientist training program, at the University of Texas Southwestern Medical Center, where he is currently completing his Ph.D. His dissertation research is focused on understanding how WNV evades α/β IFN actions.
Cynthia Johnson graduated from Texas Tech University with dual bachelor’s degrees in Microbiology and French. After a one year fellowship at the National Institutes of Health in Bethesda, Maryland, she entered the Medical Scientist Training Program at the University of Texas Southwestern Medical Center, where her Ph.D. work focuses on innate immune antiviral pathways subverted by the HCV NS3/4A protease. She plans to complete her Ph.D. training this year and return for the last two years of medical school.
Michael Gale, Jr. is a Professor in the Department of Immunology at the University of Washington School of Medicine. His research is focused on understanding how viruses trigger and control immunity to infection.
 Andrea Kaup Erickson has a degree in Biochemistry from The University of Texas at Austin. She is a 4th year Ph.D. student at the University of Texas Southwestern Medical Center. The main focus of her research is to define the biological actions of α/β IFNs against HCV.
Andrea Kaup Erickson has a degree in Biochemistry from The University of Texas at Austin. She is a 4th year Ph.D. student at the University of Texas Southwestern Medical Center. The main focus of her research is to define the biological actions of α/β IFNs against HCV.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lindenbach BD, Rice CM. Unravelling hepatitis C virus replication from genome to function. Nature. 2005;436:933–938. doi: 10.1038/nature04077. [DOI] [PubMed] [Google Scholar]
- 2.Brinton MA. The molecular biology of West Nile Virus: a new invader of the western hemisphere. Annu Rev Microbiol. 2002;56:371–402. doi: 10.1146/annurev.micro.56.012302.160654. [DOI] [PubMed] [Google Scholar]
- 3.Campbell GL, Marfin AA, Lanciotti RS, Gubler DJ. West Nile virus. Lancet Infect Dis. 2002;2:519–529. doi: 10.1016/s1473-3099(02)00368-7. [DOI] [PubMed] [Google Scholar]
- 4.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 5.Bialek SR, Terrault NA. The changing epidemiology and natural history of hepatitis C virus infection. Clin Liver Dis. 2006;10:697–715. doi: 10.1016/j.cld.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Gale M, Jr, Foy EM. Evasion of intracellular host defense by hepatitis C virus. Nature. 2005;436:939–945. doi: 10.1038/nature04078. [DOI] [PubMed] [Google Scholar]
- 7.Samuel MA, Diamond MS. Pathogensis of West NIle virus infection: a balance between virulence, innate and adaptive immunity, and viral evasion. J Virol. 2006;80:9349–9360. doi: 10.1128/JVI.01122-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saito T, Gale M., Jr Principles of intracellular viral recognition. Curr Opin Immunol. 2006;19:17–23. doi: 10.1016/j.coi.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Horvath CM. The Jak-STAT pathway stimulated by interferon alpha or interferon beta. Sci STKE 2004. 2004:tr10. doi: 10.1126/stke.2602004tr10. [DOI] [PubMed] [Google Scholar]
- 10.Sen GC. Viruses and interferons. Annu Rev Microbiol. 2001;55:255–281. doi: 10.1146/annurev.micro.55.1.255. [DOI] [PubMed] [Google Scholar]
- 11.Katze MG, He Y, Gale M., Jr Viruses and interferon: a fight for supremacy. Nature Reviews Immunology. 2002;2:675–667. doi: 10.1038/nri888. [DOI] [PubMed] [Google Scholar]
- 12.Samuel MA, Diamond MS. Alpha/beta interferon protects against lethal West Nile virus infection by restricting cellular tropism and enhancing neuronal survival. J Virol. 2005;79:13350–13361. doi: 10.1128/JVI.79.21.13350-13361.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mihm S, Frese M, Meier V, Wietzke-Braun P, Scharf JG, Bartenschlager R, Ramadori G. Interferon type I gene expression in chronic hepatitis C. Lab Invest. 2004;84:1148–1159. doi: 10.1038/labinvest.3700135. [DOI] [PubMed] [Google Scholar]
- 14.Verna EC, Brown RS., Jr Hepatitis C virus and liver transplantation. Clin Liver Dis. 2006;10:919–940. doi: 10.1016/j.cld.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Feld JJ, Hoofnagle JH. Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature. 2005;436:967–972. doi: 10.1038/nature04082. [DOI] [PubMed] [Google Scholar]
- 16.Zeller HG, Schuffenecker I. West Nile virus: an overview of its spread in Europe and the Mediterranean basin in contrast to its spread in the Americas. Eur J Clin Microbiol Infect Dis. 2004;23:147–156. doi: 10.1007/s10096-003-1085-1. [DOI] [PubMed] [Google Scholar]
- 17.Lanciotti RS, Roehrig JT, Deubel V, Smith J, Parker M, Steele K, Crise B, Volpe KE, Crabtree MB, Scherret JH, Hall RA, Mackenzie JS, Cropp CB, Panigrahy B, Ostlund E, Schmitt B, Malkinson M, Banet C, Weissman J, Komar N, Savage HM, Stone W, McNamara T, Gubler DJ. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science. 1999;286:2333–2337. doi: 10.1126/science.286.5448.2333. [DOI] [PubMed] [Google Scholar]
- 18.Isaacs A, Lindenmann J. Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci. 1957;147:258–267. [PubMed] [Google Scholar]
- 19.Isaacs A, WESTWOOD MA. Duration of protective action of interferon against infection with West Nile virus. Nature. 1959;184(Suppl 16):1232–1233. doi: 10.1038/1841232a0. [DOI] [PubMed] [Google Scholar]
- 20.Choe J, Kelker MS, Wilson IA. Crystal structure of human toll-like receptor 3 (TLR3) ectodomain. Science. 2005;309:581–585. doi: 10.1126/science.1115253. [DOI] [PubMed] [Google Scholar]
- 21.Kalil AC, Devetten MP, Singh S, Lesiak B, Poage DP, Bargenquast K, Fayad P, Freifeld AG. Use of interferon-alpha in patients with West Nile encephalitis: report of 2 cases. Clin Infect Dis. 2005;40:764–766. doi: 10.1086/427945. [DOI] [PubMed] [Google Scholar]
- 22.Su AI, Pezacki JP, Wodicka L, Brideau AD, Supekova L, Thimme R, Wieland S, Bukh J, Purcell RH, Schultz PG, Chisari FV. Genomic analysis of the host response to hepatitis C virus infection. Proc Natl Acad Sci U S A. 2002;99:15669–15674. doi: 10.1073/pnas.202608199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sumpter R, Loo Y-M, Foy E, li K, Yoneyama M, Fujita T, Lemon SM, Gale MJ. Regulating intracellular anti-viral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J Virol. 2005;79:2689–2699. doi: 10.1128/JVI.79.5.2689-2699.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simmonds P, Tuplin A, Evans DJ. Detection of genome-scale ordered RNA structure (GORS) in genomes of positive-stranded RNA viruses: Implications for virus evolution and host persistence. RNA. 2004 doi: 10.1261/rna.7640104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blight KJ, McKeating JA, Rice CM. Highly permissive cell lines for subgenomic and genomic hepatitis C virus RNA replication. J Virol. 2002;76:13001–13014. doi: 10.1128/JVI.76.24.13001-13014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 27.Saito T, Hirai R, Loo YM, Owen D, Johnson CL, Sinha SC, Akira S, Fujita T, Gale M., Jr Regulation of innate antiviral defenses through a shared repressor domain in RIG-I and LGP2. Proc Natl Acad Sci U S A. 2007;104:582–587. doi: 10.1073/pnas.0606699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H, Ishii KJ, Takeuchi O, Akira S. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol. 2005;6:981–988. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- 29.Johnson CL, Gale M., Jr CARD games between virus and host get a new player. Trends Immunol. 2005;27:1–4. doi: 10.1016/j.it.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Kang DC, Gopalkrishnan RV, Lin L, Randolph A, Valerie K, Pestka S, Fisher PB. Expression analysis and genomic characterization of human melanoma differentiation associated gene-5, mda-5: a novel type I interferon-responsive apoptosis-inducing gene. Oncogene. 2004;23:1789–1800. doi: 10.1038/sj.onc.1207300. [DOI] [PubMed] [Google Scholar]
- 31.Smith MW, Yue ZN, Korth MJ, Do HA, Boix L, Fausto N, Bruix J, Carithers RL, Jr, Katze MG. Hepatitis C virus and liver disease: global transcriptional profiling and identification of potential markers. Hepatology. 2003;38:1458–1467. doi: 10.1016/j.hep.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 32.Foy E, li K, Sumpter R, Jr, Loo YM, Johnson CL, Wang C, Fish PM, Yoneyama M, Fujita T, Lemon SM, Gale M., Jr Control of antiviral defenses through hepatitis C virus disruption of retinoic acid-inducible gene-I signaling. Proc Natl Acad Sci U S A. 2005;102:2986–2991. doi: 10.1073/pnas.0408707102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loo YM, Owen DM, li K, Erickson AK, Johnson CL, Fish PM, Carney DS, Wang T, Ishida H, Yoneyama M, Fujita T, Saito T, Lee WM, Hagedorn CH, Lau DT, Weinman SA, Lemon SM, Gale M., Jr Viral and therapeutic control of IFN-beta promoter stimulator 1 during hepatitis C virus infection. Proc Natl Acad Sci U S A. 2006;103:6001–6006. doi: 10.1073/pnas.0601523103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foy E, li K, Wang C, Sumpter R, Ikeda M, Lemon SM, Gale M., Jr Regulation of interferon regulatory factor-3 by the hepatitis C virus serine protease. Science. 2003;300:1145–1148. doi: 10.1126/science.1082604. [DOI] [PubMed] [Google Scholar]
- 35.Johnson CL, Owen DM, Gale M., Jr Functional and therapeutic analysis of hepatitis C virus NS3/4A protease control of antiviral immune defense. J Biol Chem. 2007 doi: 10.1074/jbc.M610361200. E pub Feb 8; In Press. [DOI] [PubMed] [Google Scholar]
- 36.Meylan E, Curran J, Hofmann K, Moradpour D, Binder M, Bartenschlager R, Tschopp J. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 2005 doi: 10.1038/nature04193. [DOI] [PubMed] [Google Scholar]
- 37.Tan S-L, Pause A, Shi Y, Sonenberg N. Hepatitis C virus therapeutics: Current status and emerging strategies. Nature Reviews Drug Discovery. 2002;1:1–17. doi: 10.1038/nrd937. [DOI] [PubMed] [Google Scholar]
- 38.Yoneyama M, Kikuchi M, Matsumoto K, Imaizumi T, Miyagishi M, Taira K, Foy E, Loo YM, Gale M, Jr, Akira S, Yonehara S, Kato A, Fujita T. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J Immunol. 2005;175:2851–2858. doi: 10.4049/jimmunol.175.5.2851. [DOI] [PubMed] [Google Scholar]
- 39.Kato H, Sato S, Yoneyama M, Yamamoto M, Uematsu S, Matsui K, Tsujimura T, Takeda K, Fujita T, Takeuchi O, Akira S. Cell type-specific involvement of RIG-I in antiviral response. Immunity. 2005;23:19–28. doi: 10.1016/j.immuni.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 40.Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh CS, Reis e Sousa, Matsuura Y, Fujita T, Akira S. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 41.Hornung V, Ellegast J, Kim S, Brzozka K, Jung A, Kato H, Poeck H, Akira S, Conzelmann KK, Schlee M, Endres S, Hartmann G. 5′-Triphosphate RNA Is the Ligand for RIG-I. Science. 2006 doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- 42.Komuro A, Horvath CM. RNA and Virus-Independent Inhibition of Antiviral Signaling by RNA Helicase LGP2. J Virol. 2006;80:12332–12342. doi: 10.1128/JVI.01325-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rothenfusser S, Goutagny N, DiPerna G, Gong M, Monks BG, Schoenemeyer A, Yamamoto M, Akira S, Fitzgerald KA. The RNA helicase Lgp2 inhibits TLR-independent sensing of viral replication by retinoic acid-inducible gene-I. J Immunol. 2005;175:5260–5268. doi: 10.4049/jimmunol.175.8.5260. [DOI] [PubMed] [Google Scholar]
- 44.Fredericksen BL, Gale M., Jr West Nile virus evades activation of interferon regulatory factor 3 through RIG-I-dependent and -independent pathways without antagonizing host defense signaling. J Virol. 2006;80:2913–2923. doi: 10.1128/JVI.80.6.2913-2923.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fredericksen BL, Smith M, Katze MG, Shi PY, Gale M., Jr The host response to West Nile Virus infection limits viral spread through the activation of the interferon regulatory factor 3 pathway. J Virol. 2004;78:7737–7747. doi: 10.1128/JVI.78.14.7737-7747.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Venter M, Myers TG, Wilson MA, Kindt TJ, Paweska JT, Burt FJ, Leman PA, Swanepoel R. Gene expression in mice infected with West Nile virus strains of different neurovirulence. Virology. 2005;342:119–140. doi: 10.1016/j.virol.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 47.Koh WL, Ng ML. Molecular mechanisms of West Nile virus pathogenesis in brain cell. Emerg Infect Dis. 2005;11:629–632. doi: 10.3201/eid1104.041076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Munoz-Jordan JL, Laurent-Rolle M, Ashour J, Martinez-Sobrido L, Ashok M, Lipkin WI, Garcia-Sastre A. Inhibition of alpha/beta interferon signaling by the NS4B protein of flaviviruses. J Virol. 2005;79:8004–8013. doi: 10.1128/JVI.79.13.8004-8013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guo JT, Hayashi J, Seeger C. West Nile virus inhibits the signal transduction pathway of alpha interferon. J Virol. 2005;79:1343–1350. doi: 10.1128/JVI.79.3.1343-1350.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu WJ, Wang XJ, Clark DC, Lobigs M, Hall RA, Khromykh AA. A single amino acid substitution in the West Nile virus nonstructural protein NS2A disables its ability to inhibit alpha/beta interferon induction and attenuates virus virulence in mice. J Virol. 2006;80:2396–2404. doi: 10.1128/JVI.80.5.2396-2404.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keller BC, Fredericksen BL, Samuel MA, Mock RE, Mason PW, Diamond MS, Gale M., Jr Resistance to alpha/beta interferon is a determinant of west nile virus replication fitness and virulence. J Virol. 2006;80:9424–9434. doi: 10.1128/JVI.00768-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wacher C, Muller M, Hofer MJ, Getts DR, Zabaras R, Ousman SS, Terenzi F, Sen GC, King NJ, Campbell IL. Coordinated regulation and widespread cellular expression of interferon-stimulated genes (ISG) ISG-49, ISG-54, and ISG-56 in the central nervous system after infection with distinct viruses. J Virol. 2007;81:860–871. doi: 10.1128/JVI.01167-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morrey JD, Day CW, Julander JG, Blatt LM, Smee DF, Sidwell RW. Effect of interferon-alpha and interferon-inducers on West Nile virus in mouse and hamster animal models. Antivir Chem Chemother. 2004;15:101–109. doi: 10.1177/095632020401500202. [DOI] [PubMed] [Google Scholar]
- 54.Wang C, Pflugheber J, Sumpter R, Sodora D, Hui D, Sen GC, Gale M., Jr Alpha interferon induces distinct translational control programs to suppress hepatitis C virus RNA replication. J Virol. 2002;77:3898–3912. doi: 10.1128/JVI.77.7.3898-3912.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pflugheber J, Fredericksen B, Sumpter R, Wang C, Ware F, Sodora D, Gale MJ. Regulation of PKR and IRF-1 during hepatitis C virus RNA replication. Proc Natl Acad Sci U S A. 2002;99:4650–4655. doi: 10.1073/pnas.062055699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guo J, Bichko V, Seeger C. Effect of alpha interferon on the hepatitis C virus replication. J Virol. 2001;75:8516–8523. doi: 10.1128/JVI.75.18.8516-8523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhu H, Zhao H, Collins CD, Eckenrode SE, Run Q, McIndoe RA, Crawford JM, Nelson DR, She JX, Liu C. Gene expression associated with interferon alfa antiviral activity in an HCV replicon cell line. Hepatology. 2003;37:1180–1188. doi: 10.1053/jhep.2003.50184. [DOI] [PubMed] [Google Scholar]
- 58.Chen L, Borozan I, Feld J, Sun J, Tannis LL, Coltescu C, Heathcote J, Edwards AM, McGilvray ID. Hepatic gene expression discriminates responders and nonresponders in treatment of chronic hepatitis C viral infection. Gastroenterology. 2005;128:1437–1444. doi: 10.1053/j.gastro.2005.01.059. [DOI] [PubMed] [Google Scholar]
- 59.Randall G, Chen L, Panis M, Fischer AK, Lindenbach BD, Sun J, Heathcote J, Rice CM, Edwards AM, McGilvray ID. Silencing of USP18 potentiates the antiviral activity of interferon against hepatitis C virus infection. Gastroenterology. 2006;131:1584–1591. doi: 10.1053/j.gastro.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 60.Grandvaux N, Servant MJ, tenOever B, Sen GC, Balachandran S, Barber GN, Lin R, Hiscott J. Transcriptional profiling of interferon regulatory factor 3 target genes: direct involvement in the regulation of interferon-stimulated genes. J Virol. 2002;76:5532–5539. doi: 10.1128/JVI.76.11.5532-5539.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Samuel MA, Whitby K, Keller BC, Marri A, Barchet W, Williams BR, Silverman RH, Gale M, Jr, Diamond MS. PKR and RNase L contribute to protection against lethal West Nile Virus infection by controlling early viral spread in the periphery and replication in neurons. J Virol. 2006;80:7009–7019. doi: 10.1128/JVI.00489-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liang SL, Quirk D, Zhou A. RNase L: its biological roles and regulation. IUBMB Life. 2006;58:508–514. doi: 10.1080/15216540600838232. [DOI] [PubMed] [Google Scholar]