Abstract
BACKGROUND
Significant cardiac neural and electrophysiologic remodeling occurs with hypercholesterolemia (HC). Whether simvastatin can reverse HC-induced remodeling is unclear.
OBJECTIVE
The purpose of this study was to determine the mechanisms underlying the antiarrhythmic effects of statins.
METHODS
Rabbits (N = 38) were fed HC chow (HC), standard chow (Control), HC chow followed by standard chow (Withdrawal), or HC chow and simvastatin (Statin) for 8 weeks. The hearts then were Langendorff-perfused for electrophysiologic studies. Nerves were identified by immunostaining of growth-associated protein-43 (GAP43) and tyrosine hydroxylase (TH). Action potential duration (APD) restitution in normal hearts with (N = 5) and without (N = 5) simvastatin therapy also was studied.
RESULTS
Serum cholesterol levels (mg/dL) were 1,855 ± 533 in HC, 50 ± 21 in Control, 570 ± 115 in Withdrawal, and 873 ± 112 in Statin groups (P <.001). Compared with HC (16,700 ± 5,342; 12,200 ± 3,878 µm2/mm2), the Statin group had significantly reduced GAP43-positive (10,289 ± 3,393 µm2/mm2, P = .03) and TH-positive (7,685 ± 2,959 µm2/mm2, P = .04) nerve density, respectively. APD was longer in HC rabbits than in controls (192 ± 20 ms vs 174 ± 17 ms; P <.03). Withdrawal and Statin groups had less APD prolongation than HC group. Statin group has less repolarization heterogeneity than HC group (P <.01). Statin therapy flattened the slope of APD restitution in normal hearts. Ventricular fibrillation was either induced or occurred spontaneously in 79% of hearts in HC, 20% in Control, and 66% in Withdrawal groups. However, there was no VF in hearts of Statin group (P <.001).
CONCLUSION
Simvastatin significantly reduced vulnerability to ventricular fibrillation via the mechanism of reduction of HC-induced neural and electrophysiologic remodeling.
Keywords: Arrhythmia, Statin, Lipids, Nervous system, Pathology
Introduction
Hypercholesterolemia (HC) in rabbits produces significant cardiac proarrhythmic neural and electrical remodeling.1–4 Dyslipidemia increases the incidence of ventricular tachycardia/ventricular fibrillation (VT/VF) after acute myocardial infarction.5 Lipid-lowering therapy using statins reduces VT/VF in patients with an implantable cardioverter-defibrillator (ICD).6,7 Furthermore, statin use was associated with a significant reduction in sudden cardiac death in addition to preventing VT/VF.7,8 Many other studies also found that statin use improved surrogate markers of arrhythmic risk in a population at certain risk for arrhythmic events.9,10 These findings support the notion that statin use is antiarrhythmic. However, the mechanisms by which statins are antiarrhythmic remain unclear. Our previous study indicated that HC in rabbits can induce significant proarrhythmic neural and electrophysiologic remodeling.1 Therefore, we hypothesize that statin is antiarrhythmic because it reverses the proarrhythmic remodeling induced by HC. The purpose of the present study was to test this hypothesis.
Methods
All animal study protocols were approved by the Animal Care and Use Committee and conformed to the guidelines of the American Heart Association. Three-month-old female New Zealand white rabbits were used for the study. Total serum cholesterol and triglyceride levels were determined before the animals were sacrificed.
Animals and diets
The rabbits were fed according to the following dietary protocols:
Control group: Ten rabbits were fed with standard chow (Purina 5321) for 8 weeks.
HC group: Fifteen rabbits were fed high fat and cholesterol chow (Purina 5321 + 0.5% cholesterol + 5% coconut oil) for 8 weeks.
Withdrawal group: Six rabbits were fed with high fat and cholesterol chow for 6 weeks, followed by standard chow for 2 weeks.
Statin group: Seven rabbits were fed with high fat and cholesterol chow and simvastatin 20 mg/day for 8 weeks.
To demonstrate the effects of statin on action potential duration (APD) restitution in rabbits without HC, two additional groups were included:
Control group: Five rabbits fed with standard chow for 8 weeks.
Statin group: Five rabbits fed with standard chow and simvastatin 20 mg/day for 8 weeks.
Immunocytochemical study
Ventricular tissues of rabbit hearts were fixed in 4% formalin for 1 hour, followed by 70% alcohol, and cross-sectioned from apex to base. Three sections of each heart were used for immunocytochemical studies. Details of the staining techniques have been reported elsewhere.1,11 In brief, anti–growth-associated protein-43 (GAP43) and anti-tyrosine hydroxylase (TH) antibodies (monoclonal mouse anti-GAP43 and anti-TH, respectively, 1:50 dilution; Chemicon International, Inc., Billerica, MA, USA) were used for immunocytochemical staining. GAP43 is a marker for nerve sprouting.12 TH is a marker of sympathetic nerves.13 Nerve density was determined by computerized morphometry (Image-Pro Plus 4.0, Media Cybernetics, Inc., Bethesda, MD, USA).1 The nerve density was the nerve area divided by the total area examined (µm2/mm2). Each slide was examined under a microscope with 20× objectives. For data analyses, the slides were divided into four quadrants. and one microscopic field with the highest nerve density from each quadrant was selected for nerve counting. Because three sections were taken for each heart, a total of 12 microscopic fields were counted. The average nerve density of all selected fields was used to represent the nerve density of that heart.
Isolated rabbit heart preparation and electrophysiologic study
The hearts were quickly removed during anesthesia and Langendorff-perfused with 37°C Tyrode’s solution of the following composition (in mM): 125 NaCl, 4.5 KCl, 0.25 MgCl2, 24 NaHCO3, 1.8 NaH2PO4, 1.8 CaCl2, and 5.5 glucose, equilibrated with 95% O2 and 5% CO2, pH 7.4. Coronary perfusion pressure was regulated between 80 and 95 mmHg. Pseudo-electrocardiogram (ECG) registered with a widely spaced bipole was used to monitor heart rhythm. Electrical stimuli of 2-ms duration and two to three times the diastolic threshold were delivered through a catheter in the right ventricle. Ventricular vulnerability was tested with single and double extrastimuli given after eight S1 at a pacing cycle length (PCL) of 400 ms. Premature coupling intervals initially were paced at 380 ms, then shortened successively until the effective refractory period was reached or fibrillation was induced. After baseline studies, 0.1 µM isoproterenol and repeated programmed stimulations were given to induce arrhythmia.
Optical mapping studies were performed using a setup similar to that reported in a previous study.14 The tissues were stained with 0.5 µM di-4-ANEPPS (Molecular Probes, Eugene, OR, USA). An electromechanical uncoupler (cytochalasin D 5 µM; Sigma Inc., St. Louis, MO, USA) was used to inhibit motion. Laser light of 532-nm wavelength (Verdi, Coherent Inc., Santa Clara, CA, USA) illuminated the tissues, and epifluorescence was collected through a long-pass filter with a cutoff wavelength of 600 nm (R60, Nikon, Melville, NY, USA) and a high-speed charge-coupled device (CCD) camera (420 frames/s, model CA D1-0128T, Dalsa Inc., Waterloo, Ontario, Canada). APD was measured at PCL of 400, 300, and 200 ms. One hundred points over the ventricular anterior wall were selected for APD analysis. A computer algorithm automatically determined APD80. The standard deviation (SD) and the difference (between the longest and shortest APD80) of APD80 were used to represent APD dispersion. APD restitution was determined by a dynamic pacing protocol.15 The ventricles initially were paced at a constant cycle length of 400 ms. PCL was shortened successively in steps of 20 ms for PCL >200 ms and in steps of 10 ms for PCL <200 ms until PCL = 100 ms or loss of 1:1 ventricular capture. Restitution curves were constructed from APD80 and the preceding diastolic intervals at nine evenly spaced sites on the left ventricular anterior wall using data obtained during pacing. The maximal slope of APD restitution curve was determined after curve fitting by first-order exponential fitting with Origin software (Micro-Cal, Northampton, MA, USA). Conduction velocity (CV) along three different directions from the S1 pacing site at PCL of 300 ms was measured. The mean of the three values was used as the CV (cm/s) for that heart.4,14
Statistical analysis
Values are expressed as mean ± SD. Analysis of variance for repeated measurements with Newman-Keuls test was used to determinate the significance of between-group comparisons. P ≤.05 was considered significant.
Results
Table 1 summarizes the serum cholesterol and triglyceride concentrations in the first four groups. Serum cholesterol levels were significantly higher in the HC group than in the Control group. Statin treatment resulted in a 53% reduction in serum cholesterol levels compared with the HC group (P <.001). The cholesterol levels in Statin group were significantly higher than in the Withdrawal group. There were no significant differences of serum triglyceride levels among the four groups.
Table 1.
Serum lipid profiles
| Cholesterol level (mg/dL) |
Triglyceride level (mg/dL) |
|
|---|---|---|
| Control group | 51 ± 26 | 39 ± 7 |
| Withdrawal group | 494 ± 227*†‡ | 26 ± 11 |
| Statin group | 873 ± 112*† | 63 ± 77 |
| Hypercholesterolemia group | 1,855 ± 533* | 105 ± 94 |
P <.001 vs control group.
P <.001 vs hypercholesterolemia group.
P <.01 vs statin group.
Nerve sprouting and sympathetic hyperinnervation
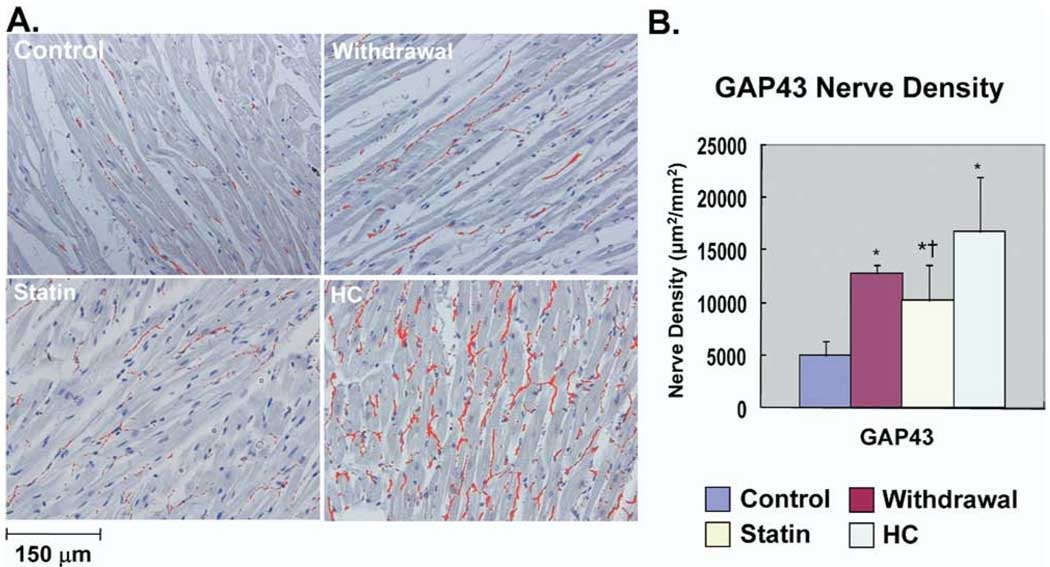
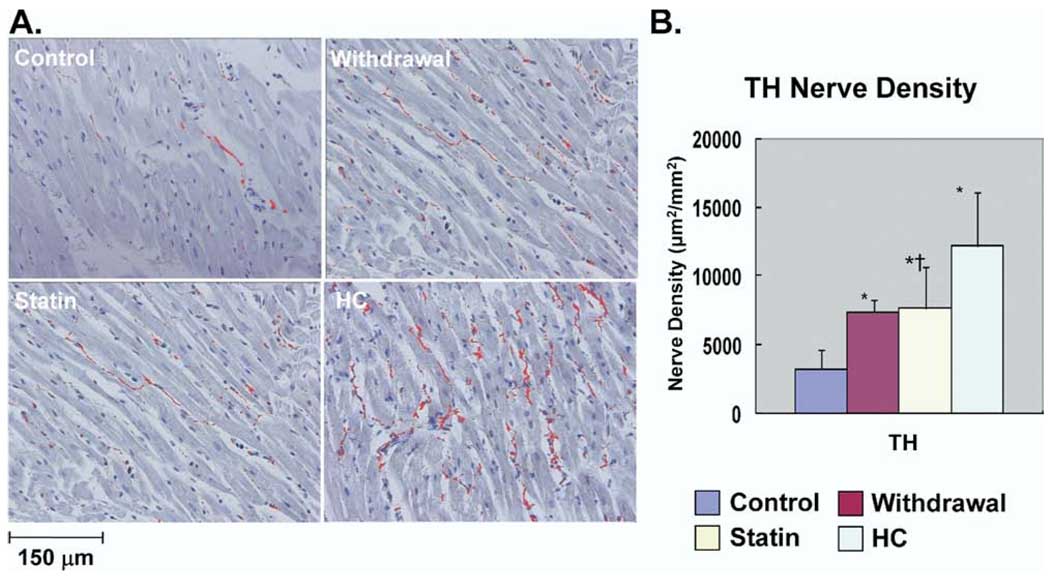
As we previously reported,1 significant neural remodeling occurred in HC rabbits. Figure 1A shows examples of GAP43 immunocytochemical staining. Nerve sprouting was much more abundant in HC group than in control group. Sprouting nerves were fewer in Withdrawal and Statin groups than in HC group. As shown in Figure 1B, GAP43(+) nerve density in HC (16,700 ± 5,342 µm2/mm2), Withdrawal (12,767 ± 832 µm2/mm2), and Statin (10,289 ± 3,393 µm2/mm2) groups all were significantly higher in than Control group (4,925 ± 1,452 µm2/mm2; P <.05). Compared with HC group, Statin group had a significant reduction in GAP43(+) nerve density (P = .03). Figure 2A shows examples of TH immunocytochemical staining. Sympathetic innervation was much more abundant in HC group than in Control group. TH(+) nerves were also fewer in Withdrawal and Statin groups than in HC group. As shown in Figure 2B, TH(+) nerve density in HC (12,200 ± 3,878 µm2/mm2), Withdrawal (7,400 ± 872 µm2/mm2), and Statin (7,685 ± 2,959 µm2/mm2) groups all were significantly higher than in Control group (3,250 ± 1,420 µm2/mm2; P <.05). Compared with HC group, Statin group had a significant reduction in TH(+) nerve density (P <.05). For all stained samples, there was a significant correlation between serum cholesterol level and nerve density of GAP43(+) nerves (r = 0.61, P = .005) and between serum cholesterol level and nerve density of TH(+) nerves (r = 0.68, P = .002).
Figure 1.
Immunohistologic studies of cardiac nerve sprouting. A: Examples of immunostaining results with anti– growth-associated protein-43 (GAP43) antibody in the four groups. Nerves are labeled red. B: Summary of nerve density (µm2/mm2) in the four groups. *P <.05 vs control group; †P <.05 vs hypercholesterolemia (HC) group.
Figure 2.
Immunohistologic studies of cardiac sympathetic nerve density. A: Examples of immunostaining results with anti- tyrosine hydroxylase (TH) antibody in the four groups. Nerves are labeled red. B: Summary of nerve density (µm2/mm2) in the four groups. * P <.05 vs control group; †P <.05 vs hypercholesterolemia (HC) group.
APD, APD dispersion, and APD restitution characteristics
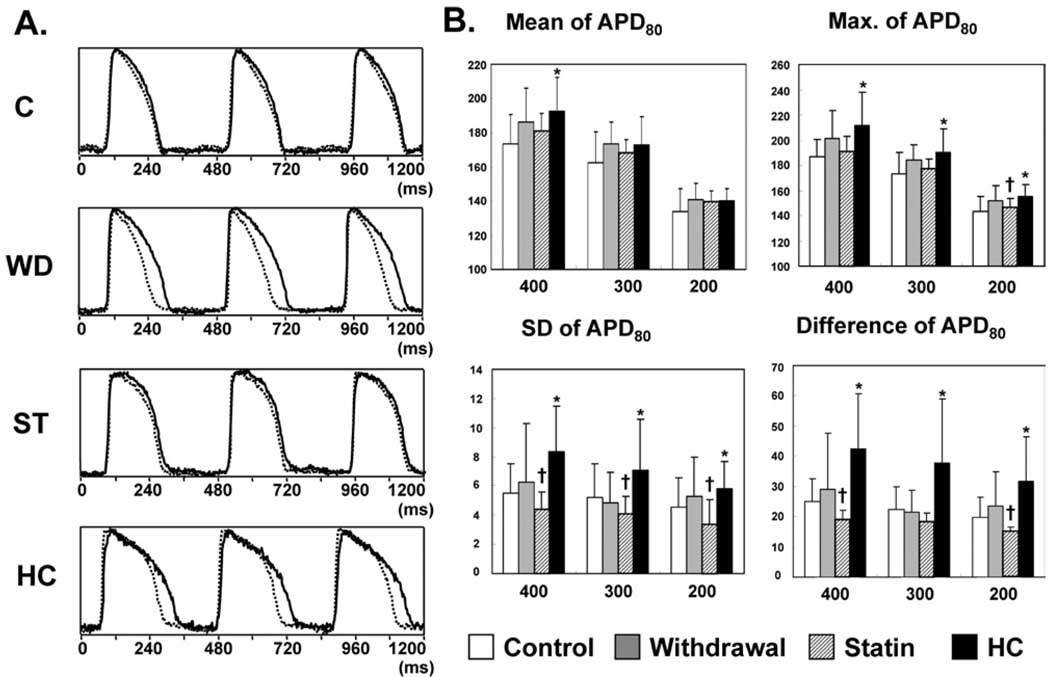
Figure 3A shows typical examples of action potentials in rabbits of the four groups at PCL of 400 ms at baseline. The HC group showed significant APD prolongation and increased APD dispersion after 8 weeks of feeding. APD was less prolonged in Withdrawal and Statin groups. However, there still was a large APD heterogeneity in Withdrawal group. The alterations in APD80 and APD80 dispersion are summarized in Figure 3B. Mean APD80 of the anterior wall at PCL of 400 ms was significantly longer in HC group than Statin group (APD80: 192 ± 20 ms vs 174 ± 17 ms; P <.03). There was a 34% and 59% reduction in mean APD prolongation in Withdrawal and Statin groups, respectively, compared with HC group at PCL of 400ms. The longest APD80 of the anterior wall in the HC group was significantly increased compared with Statin group at all three PCLs. There was a 42% and 85% reduction in longest APD prolongation in Withdrawal and Statin groups, respectively, compared with HC group at PCL of 400 ms. The spatial heterogeneity of APD, represented as either the SD of APD80 at 100 points or the difference between the longest and shortest APD80 over the anterior wall, also was significantly greater in HC group compared with Control group (PCL = 400 ms: SD: 8.3 ± 3.1 ms vs 5.5 ± 2.0 ms, P <.05; difference: 42.5 ± 18.2 ms vs 25.1 ± 7.3 ms, P <.01). The spatial heterogeneity of APD was significantly decreased in Statin group (SD: 4.4 ± 1.1 ms; difference: 19.2 ± 5.9 ms) than in HC group (P <.01). Note that the heterogeneity of Statin group at each PCL was less than in the Control group; however, the difference was not statistically significant.
Figure 3.
Effects of simvastatin on action potential duration (APD). A: Examples of action potentials at pacing cycle length of 400 ms in the four groups with shortest (dotted line) and longest (solid line) APD over anterior wall. B: Summary of mean, maximum (Max.), standard deviation (SD), and difference of APD80 (100 points) over the anterior wall in the four groups. Scale for both ordinate and abscissa of each graph is in milliseconds. C = Control; HC = Hypercholesterolemia; ST = Statin; WD = Withdrawal. *P <.05 vs control group; †P <.05 vs HC group.
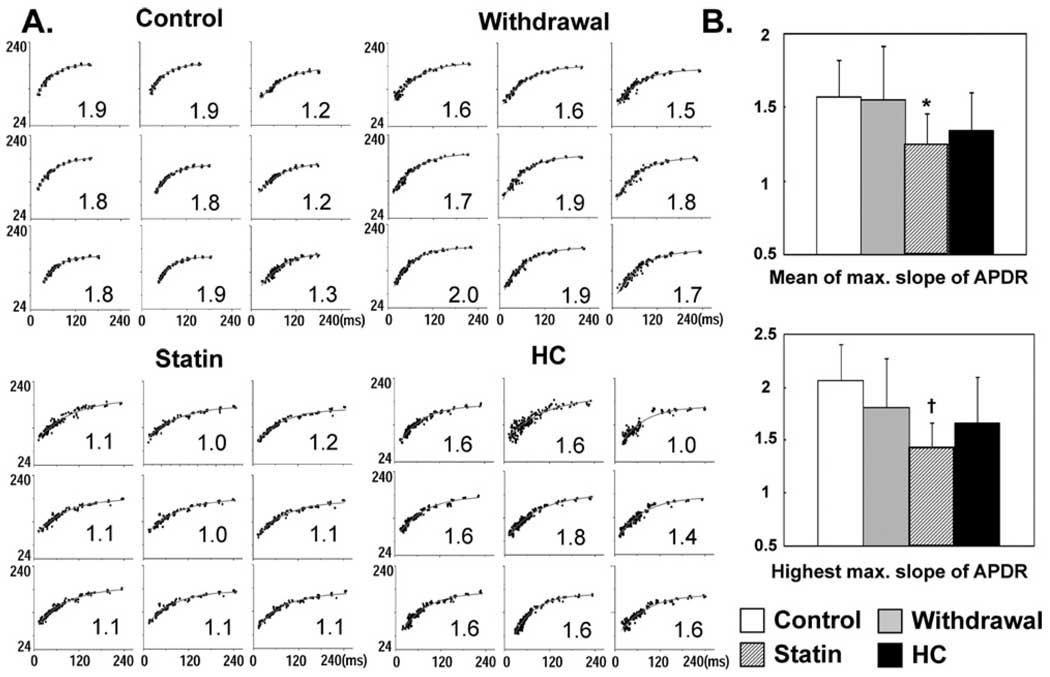
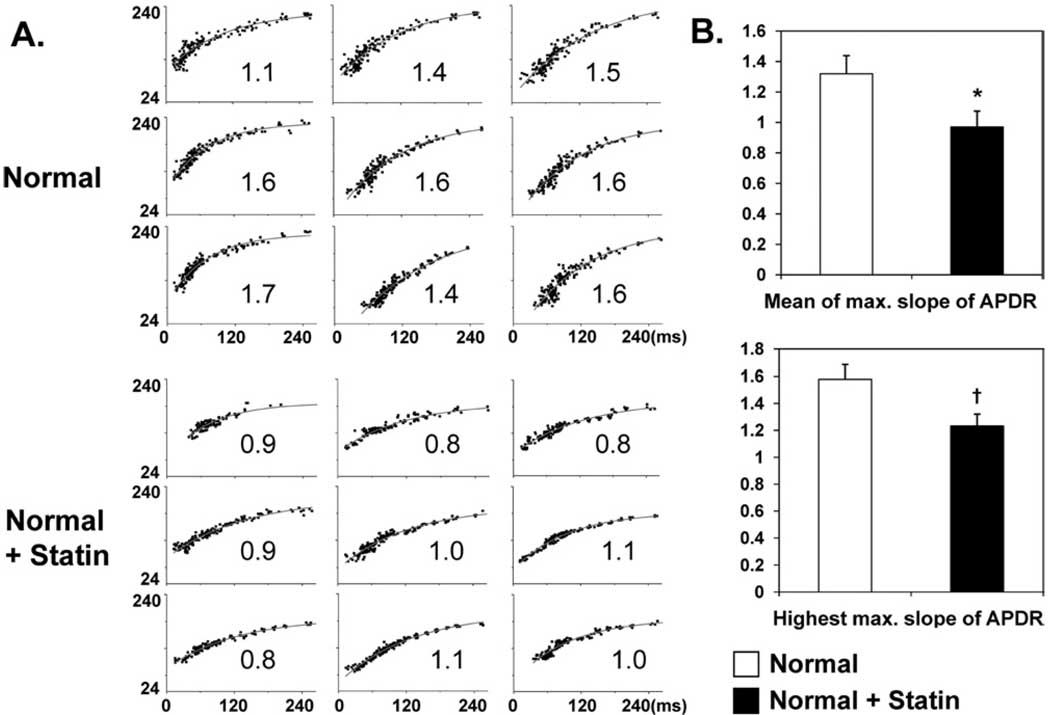
Figure 4 A shows typical examples of APD restitution curves in the four groups. Figure 4B summarizes the alterations in the maximum slope of APD restitution curves of all four groups. Both the mean and highest maximum slope of APD restitution (i.e., highest value of the entire mapped region) were significantly decreased in Statin group than in Control group (mean: 1.24 ± 0.21 vs 1.57 ± 0.24; P <.05; highest: 1.44 ± 0.23 vs 2.07 ± 0.33; P <.01). In normal hearts, 8-week simvastatin treatment significantly flattened the mean and highest maximum slope of APD restitution (statin vs control; mean: 0.97 ± 0. 1 vs 1.32 ± 0.12; P = .001; highest: 1.23 ± 0.09 vs 1.67 ± 0.11; P <.001). Figure 5 summarizes the effects of simvastatin on APD restitution in normal rabbits.
Figure 4.
Effects of simvastatin on action potential duration restitution (APDR). A: Examples of APD restitution curves at nine different sites in the left ventricular anterior wall in the four groups. The maximum slope of APD restitution is labeled in the lower right corner of each curve. The ordinate in each graph is APD in milliseconds; the abscissa is diastolic interval in milliseconds. The same scales are used in all graphs of each panel. B: Summary of the mean and highest values of maximum slope of APD restitution in the four groups. *P <.05 and †P <.001 vs control group.
Figure 5.
Effects of simvastatin on action potential duration restitution (APDR) in normal rabbits. A: Examples of APD restitution curves at nine different sites in the left ventricular anterior wall. The maximum slope of APD restitution is labeled in the lower right corner of each curve. The ordinate in each graph is APD; the abscissa is diastolic interval. The same scales are used in all graphs of each panel. B: Summary of the mean and highest values of maximum slope of APD restitution. *P = .001 and †P <.001 vs normal hearts not treated with simvastatin.
Conduction velocity
CV was significantly less in HC group (56.4 ± 6.2 cm/s) than Control group (63 ± 6.5 cm/s, P = 0.02). CV in Withdrawal (57.8 ± 6.6 cm/s) and Statin (61.2 ± 9 cm/s) groups were between these values, but these differences were not statistically significant.
Ventricular vulnerability to fibrillation
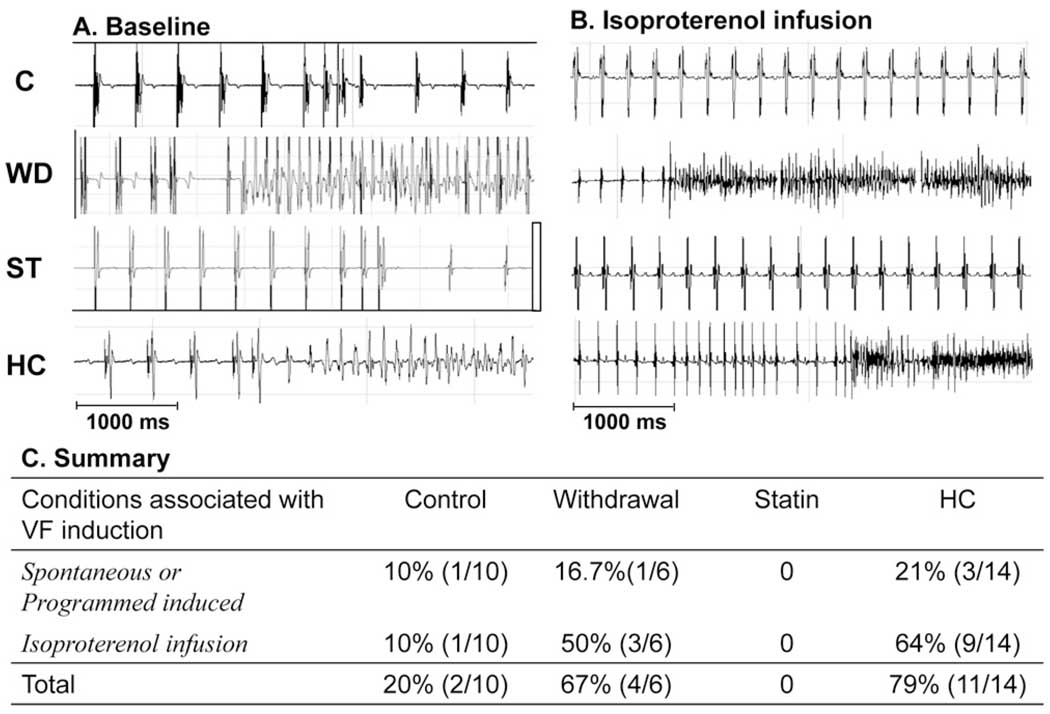
Figures 6A and 6B show examples of spontaneous or induced VF in HC and Withdrawal groups. VF was either induced or occurred spontaneously in 79% of hearts in HC, 20% in Control, and 66% in Withdrawal groups (Figure 6C). There was no VF in the Statin group (P <.001).
Figure 6.
Effects of hypercholesterolemia (HC) and simvastatin on ventricular arrhythmia. A: Examples of ventricular tachyarrhythmia induced by single and double ventricular extrastimuli in HC and Withdrawal groups. B: Under isoproterenol infusion, ventricular tachyarrhythmia occurred more frequently in HC and Withdrawal groups than in Control and Statin groups. C: Summary of occurrence of ventricular fibrillation (VF) in the four groups. C = Control; ST = Statin; WD = Withdrawal.
Discussion
This study shows that simvastatin significantly reduced neural and electrophysiologic remodeling induced by HC and reduced vulnerability to VF. Because the Withdrawal group had a lower cholesterol level than the Statin group but continued to have inducible VF, these data suggest that the antiarrhythmic effects of simvastatin may not be attributed to cholesterol-lowering effects alone.
Antiarrhythmic actions of statins in clinical trials
Lipid-lowering interventions have been shown to reduce coronary events and all-cause mortality.16 It is possible that some of the beneficial effects of lipid-lowering therapy can be attributed to the reduction of VT/VF and sudden death. In an observational study, DeSutter et al17 reported that in patients with coronary artery disease receiving an ICD, treatment with lipid-lowering drug therapy resulted in a substantial reduction in appropriate shocks. Mitchell et al6 sought to evaluate the antiarrhythmic effects of lipid-lowering therapy in patients enrolled in the Antiarrhythmics Versus Implantable Defibrillators (AVID) trial. In patients with an ICD for secondary prevention of sudden cardiac death, use of lipid-lowering drug therapy was associated with a 40% reduction in the relative hazard for VT/VF recurrence. In the Multicenter Automatic Defibrillator Implantation Trial II (MADIT-II), a primary prevention trial of sudden cardiac death, statin use was associated with a reduction in the risk of sudden cardiac death or VF.7 The antiarrhythmic properties of statin could also be demonstrated in patients with nonischemic dilated cardiomyopathy in that statin use significantly reduced arrhythmic sudden death.18 These secondary analyses of large clinical trials have provided strong supportive evidence for the beneficial effects of statins on life-threatening ventricular arrhythmias.
Effects of statins on electrophysiologic remodeling induced by HC
Vrtovec et al10 explored the potential antiarrhythmic benefit of atorvastatin in patients with advanced heart failure. They found that atorvastatin shortened the QTc interval and thereby may have reduced the risk of arrhythmias. In subjects with isolated hypercholesterolemia, simvastatin could normalize QTc dispersion and reduce ventricular premature complexes.9 These studies indicate that statins have significant effects on cardiac electrophysiologic properties. Consistent with these clinical findings, we found that simvastatin reduced HC-induced APD prolongation, decreased repolarization heterogeneity, and flattened APD restitution. Simvastatin also attenuated the effects of HC-induced conduction slowing.4 These findings suggest that statins achieve their antiarrhythmic drug action in part by preventing or reversing electrophysiologic remodeling induced by HC.
Effects of statins on neural remodeling induced by HC
Our previous studies showed that HC induced significant cardiac nerve sprouting and sympathetic hyperinnervation in both immunocytochemical studies in vitro and 123I-MIBG imaging in vivo in HC rabbits.1,2 Furthermore, there is an excellent dose–response relationship between serum cholesterol level and nerve density for GAP43(+) and TH(+) nerves.1 The present study demonstrated that simvastatin use in HC rabbits reduced nerve sprouting and sympathetic hyperinnervation induced by HC. Two-year atorvastatin treatment may increase heart rate variability in patients with hypercholesterolemia, with or without coronary artery diseases.19 Vrtovec et al10 also showed that atorvastatin therapy increases heart rate variability and decreases QT variability in patients with advanced heart failure. These human studies are compatible with a relative reduction of sympathetic tone versus parasympathetic tone after atorvastatin treatment. Putting together these findings, we propose that preventing or reversing neural remodeling might play a role in the antiarrhythmic drug action of statin therapy.
Lipid-lowering independent antiarrhythmic effects of statins
The results of the present study also suggest that simvastatin might have independent antiarrhythmic effects. Withdrawal group had lower cholesterol level than Statin group but continued to have inducible VF. In comparison, electrophysiologic and neural remodeling was less apparent in Statin group, despite higher cholesterol levels, compared with Withdrawal group. We also found that simvastatin therapy flattened the slope of APD restitution not only in HC hearts but also in normal hearts. Thus, the antiarrhythmic effects of simvastatin may not be attributable to lipid-lowering effects alone. Several plausible mechanisms might underlie the lipid-lowering independent antiarrhythmic actions of statin. First, atorvastatin appeared to desensitize neonatal rat cardiomyocyte to beta-adrenergic stimulation by a mechanism that involves the G-protein cellular content and cAMP accumulation.20 Second, simvastatin has been demonstrated to elevate the sarcoplasmic reticulum Ca2+ - ATPase and ryanodine receptor 2 in both gene and protein expression, suggesting increased sarcoplasmic reticulum Ca release and uptake during cardiac cycle.21 Third, statins also could interact with the lipid bilayer of the cell membrane,22 which may affect electrical stability and mitochondrial function. Lovastatin decreased sarcolemmal Na+/K+ ATPase pump density in cardiac myocyte, leading to increased sarcoplasmic Na+ concentration that might alter intracellular calcium dynamics through the Na+/Ca2+ exchanger.23 Statins appear to suppress intracellular calcium mobilization and membrane current induced by lysophosphatidylcholine in endothelial cells by preventing Rho kinase activation.24 Simvastatin increases mitochondrial NADH content and induced mitochondrial membrane depolarization and calcium efflux.25 Of note, these mechanisms are independent of statin HMG-CoA reductase activity. The lipid-lowering independent effects have also been documented by a study using pravastatin, which is water soluble and has a different biodistribution than other statins. In that study, hydrophilic pravastatin prevented reperfusion-induced lethal VF in normocholesterolemic rats.26
Study limitations
The electrophysiology studies were performed with Langendorff perfusion. It is possible that electrophysiologic parameters are different when the hearts were in situ and when the rabbits were conscious. Only one statin was statin was tested in this study. Whether the results can be generalized to all statin drugs is unclear. Because simvastatin reduced both electrophysiologic and neural remodeling, it is difficult to determine which of the two is primarily or solely responsible for the increased vulnerability.
Acknowledgments
This study was supported by National Science Foundation (Taiwan) Grant NSC 93-2314-B-002-204 and NSC 94-2314-B-002-204 to Dr. Liu; a Piansky endowment to Dr. Fishbein; an endowment from The Women’s Guild of Cedars-Sinai Medical Center, Los Angeles, California, to Dr. Bairey Merz; a Pauline and Harold Price endowment to Dr. Chen; a grant from Merck Inc.; NIH Grants P50 HL52319, HL66389, and HL71140; and the Ralph M. Parsons Foundation, Los Angeles, California. Merck Inc. donated the simvastatin and provided a research grant that partially supported this study.
We thank Tsai-Tzu Wu, Nina Wang, and Elaine Lebowitz for assistance.
References
- 1.Liu YB, Wu CC, Lu LS, et al. Sympathetic nerve sprouting, electrical remodeling, and increased vulnerability to ventricular fibrillation in hypercholesterolemic rabbits. Circ Res. 2003;92:1145–1152. doi: 10.1161/01.RES.0000072999.51484.92. [DOI] [PubMed] [Google Scholar]
- 2.Luo TY, Wu CC, Liu YB, et al. Dietary cholesterol affects sympathetic nerve function in rabbit hearts. J Biomed Sci. 2004;11:339–345. doi: 10.1007/BF02254438. [DOI] [PubMed] [Google Scholar]
- 3.Luo TY, Su MJ, Yang YF, et al. Effect of hypercholesterolemia on myocardial function in New Zealand white rabbits. J Biomed Sci. 2004;11:829–837. doi: 10.1007/BF02254368. [DOI] [PubMed] [Google Scholar]
- 4.Lin LC, Wu CC, Yeh HI, et al. Downregulated myocardial connexin 43 and suppressed contractility in rabbits subjected to a cholesterol-enriched diet. Lab Invest. 2005;85:1224–1237. doi: 10.1038/labinvest.3700324. [DOI] [PubMed] [Google Scholar]
- 5.Liu YB, Wu CC, Lee CM, et al. Dyslipidemia is associated with ventricular tachyarrhythmia in patients with acute ST-segment elevation myocardial infarction. J Formos Med Assoc. 2006;105:17–24. doi: 10.1016/S0929-6646(09)60104-2. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell LB, Powell JL, Gillis AM, et al. Are lipid-lowering drugs also antiarrhythmic drugs? An analysis of the antiarrhythmics versus implantable defibrillators (AVID) trial. J Am Coll Cardiol. 2003;42:81–87. doi: 10.1016/s0735-1097(03)00498-4. [DOI] [PubMed] [Google Scholar]
- 7.Vyas AK, Guo H, Moss AJ, et al. Reduction in ventricular tachyarrhythmias with statins in the Multicenter Automatic Defibrillator Implantation Trial (MADIT)-II. J Am Coll Cardiol. 2006;47:769–773. doi: 10.1016/j.jacc.2005.09.053. [DOI] [PubMed] [Google Scholar]
- 8.Goldberger JJ, Subacius H, Schaechter A, et al. Effects of statin therapy on arrhythmic events and survival in patients with nonischemic dilated cardiomyopathy. J Am Coll Cardiol. 2006;48:1228–1233. doi: 10.1016/j.jacc.2006.05.053. [DOI] [PubMed] [Google Scholar]
- 9.Gualdiero P, Esposito K, Ciotola M, et al. Simvastatin normalizes qtc dispersion and reduces ventricular electrical instability in isolated hypercholesterolemia. J Endocrinol Invest. 2002;25:RC16–RC18. doi: 10.1007/BF03345487. [DOI] [PubMed] [Google Scholar]
- 10.Vrtovec B, Okrajsek R, Golicnik A, et al. Atorvastatin therapy increases heart rate variability, decreases QT variability, and shortens QTc interval duration in patients with advanced chronic heart failure. J Card Fail. 2005;11:684–690. doi: 10.1016/j.cardfail.2005.06.439. [DOI] [PubMed] [Google Scholar]
- 11.Cao JM, Chen LS, KenKnight BH, et al. Nerve sprouting and sudden cardiac death. Circ Res. 2000;86:816–821. doi: 10.1161/01.res.86.7.816. [DOI] [PubMed] [Google Scholar]
- 12.Meiri KF, Pfenninger KH, Willard MB. Growth-associated protein, GAP-43, a polypeptide that is induced when neurons extend axons, is a component of growth cones and corresponds to pp46, a major polypeptide of a subcellular fraction enriched in growth cones [published erratum appears in Proc Natl Acad Sci U S A 1986;83:9274] Proc Natl Acad Sci U S A. 1986;83:3537–3541. doi: 10.1073/pnas.83.10.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen PS, Chen LS, Cao JM, et al. Sympathetic nerve sprouting, electrical remodeling and the mechanisms of sudden cardiac death. Cardiovasc Res. 2001;50:409–416. doi: 10.1016/s0008-6363(00)00308-4. [DOI] [PubMed] [Google Scholar]
- 14.Liu YB, Pak HN, Lamp ST, et al. Coexistence of two types of ventricular fibrillation during acute regional ischemia in rabbit ventricle. J Cardiovasc Electrophysiol. 2004;15:1433–1440. doi: 10.1046/j.1540-8167.2004.04337.x. [DOI] [PubMed] [Google Scholar]
- 15.Koller ML, Riccio ML, Gilmour RF., Jr Dynamic restitution of action potential duration during electrical alternans and ventricular fibrillation. Am J Physiol. 1998;275:H1635–H1642. doi: 10.1152/ajpheart.1998.275.5.H1635. [DOI] [PubMed] [Google Scholar]
- 16.Studer M, Briel M, Leimenstoll B, et al. Effect of different antilipidemic agents and diets on mortality: a systematic review. Arch Intern Med. 2005;165:725–730. doi: 10.1001/archinte.165.7.725. [DOI] [PubMed] [Google Scholar]
- 17.De Sutter J, Tavernier R, De Buyzere M, et al. Lipid lowering drugs and recurrences of life-threatening ventricular arrhythmias in high-risk patients. J Am Coll Cardiol. 2000;36:766–772. doi: 10.1016/s0735-1097(00)00787-7. [DOI] [PubMed] [Google Scholar]
- 18.Robin J, Weinberg K, Tiongson J, et al. Renal dialysis as a risk factor for appropriate therapies and mortality in implantable cardioverter-defibrillator recipients. Heart Rhythm. 2006;3:1196–1201. doi: 10.1016/j.hrthm.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 19.Pehlivanidis AN, Athyros VG, Demitriadis DS, et al. Heart rate variability after long-term treatment with atorvastatin in hypercholesterolaemic patients with or without coronary artery disease. Atherosclerosis. 2001;157:463–469. doi: 10.1016/s0021-9150(00)00746-2. [DOI] [PubMed] [Google Scholar]
- 20.Muhlhauser U, Zolk O, Rau T, et al. Atorvastatin desensitizes beta-adrenergic signaling in cardiac myocytes via reduced isoprenylation of G-protein gamma-subunits. FASEB J. 2006;20:785–787. doi: 10.1096/fj.05-5067fje. [DOI] [PubMed] [Google Scholar]
- 21.Zheng X, Hu SJ. Effects of simvastatin on cardiac performance and expression of sarcoplasmic reticular calcium regulatory proteins in rat heart. Acta Pharmacol Sin. 2005;26:696–704. doi: 10.1111/j.1745-7254.2005.00105.x. [DOI] [PubMed] [Google Scholar]
- 22.Siegl PK, Cragoe EJ, Jr, Trumble MJ, et al. Inhibition of Na+/Ca2+ exchange in membrane vesicle and papillary muscle preparations from guinea pig heart by analogs of amiloride. Proc Natl Acad Sci U S A. 1984;81:3238–3242. doi: 10.1073/pnas.81.10.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gray DF, Bundgaard H, Hansen PS, et al. HMG CoA reductase inhibition reduces sarcolemmal Na(+)-K(+) pump density. Cardiovasc Res. 2000;47:329–335. doi: 10.1016/s0008-6363(00)00106-1. [DOI] [PubMed] [Google Scholar]
- 24.Yokoyama K, Ishibashi T, Ohkawara H, et al. HMG-CoA reductase inhibitors suppress intracellular calcium mobilization and membrane current induced by lysophosphatidylcholine in endothelial cells. Circulation. 2002;105:962–967. doi: 10.1161/hc0802.104457. [DOI] [PubMed] [Google Scholar]
- 25.Sirvent P, Mercier J, Vassort G, et al. Simvastatin triggers mitochondria-induced Ca2+ signaling alteration in skeletal muscle. Biochem Biophys Res Commun. 2005;329:1067–1075. doi: 10.1016/j.bbrc.2005.02.070. [DOI] [PubMed] [Google Scholar]
- 26.Watano T, Harada Y, Harada K, et al. Effect of Na+/Ca2+ exchange inhibitor, KB-R7943 on ouabain-induced arrhythmias in guinea-pigs. Br J Pharmacol. 1999;127:1846–1850. doi: 10.1038/sj.bjp.0702740. [DOI] [PMC free article] [PubMed] [Google Scholar]