Abstract
Background
In patients with cerebral malaria (CM), retinal angiography allows the study of infected central nervous system microvasculature in vivo. We aimed to examine retinal perfusion in children with CM by use of fluorescein angiography to investigate the pathophysiology of CM.
Methods
We performed fluorescein angiography on children with CM admitted to Queen Elizabeth Central Hospital, Malawi. We related angiograms to funduscopic findings.
Results
Fluorescein angiography was performed for 34 patients with CM, and impaired perfusion was identified in 28 (82%). Areas of capillary nonperfusion (CNP) were seen in 26 patients (76%). Multiple, scattered areas of CNP were typical and topographically matched to retinal whitening. Larger retinal vessels were occluded in 9 patients (26%) who had associated ischemia. These vessels appeared white on ophthalmoscopy. Intravascular abnormalities were seen in 9 patients (26%), including filling defects and mottling of the blood column. Limited fluorescein leakage occurred in 15 patients (44%) and was not related to angiographic intravascular abnormalities or visible vessel discoloration.
Conclusions
Impaired perfusion occurs in the retinal microvasculature of most children with CM. This is evidence for hypoxia and ischemia as important components in the pathogenesis of CM. Vessel occlusion and filling defects are likely to be due to sequestration of infected erythrocytes. Interventions which improve perfusion or limit hypoxic injury may be beneficial in CM.
Malaria is the third most common killer of children >5 years old in the world, and 94% of childhood malaria deaths occur in Africa [1]. Most die with cerebral malaria (CM) [2], a complication characterized by coma and convulsions. The pathogenesis of this condition remains poorly understood, but is thought to involve the sequestration of Plasmodium falciparum–infected erythrocytes in the microvasculature of many organs, including the brain and retina. Sequestration is the result of cytoadherence of parasitized erythrocytes to vascular endothelium [3-5].
Sequestration leads to the histological appearance of narrowed and blocked blood vessels, suggesting the possibility that impaired perfusion is a cause of coma and death in patients with CM. Some observers have disputed this conclusion, in part because of the rapid and apparently full neurological recovery of most survivors. The alternative hypothesis that coma and death in patients with CM are induced by cytokines and nitric oxide in locally high concentrations has been proposed [6]. These 2 mechanisms are not mutually exclusive.
Investigations of cerebral blood flow in patients with CM have used ultrasonography of large arteries [7, 8], but sequestration occurs predominantly in the microvasculature, where blood flow and perfusion have not been investigated. A study measuring global cerebral blood flow and metabolism indicated cerebral hypoxia and anaerobic metabolism in adults [9], findings supported by raised lactate levels in cerebrospinal fluid (CSF) samples from adults and children [10, 11]. The importance of systemic tissue hypoxia is underlined by the observation that elevated plasma lactate level and the ratio of lactate to pyruvate predict fatal outcome in children and adults [2, 10, 12].
CM in children is accompanied by a retinopathy that includes unique vessel abnormalities and patterns of retinal whitening [13-16]. These changes are commonly accompanied by retinal hemorrhages, cotton wool spots, and papilledema. The hemorrhages are predominately white-centered blot hemorrhages. Malarial retinopathy has diagnostic value, and in patients with CM its presence and severity predict the length of coma and risk of death [17, 18].
The retina is embryologically part of the central nervous system (CNS), with structures analogous to those of the brain, including neurons, glial cells, and a blood-tissue barrier. It is not surprising, therefore, that histological features of CM in the brain are mirrored in the retina [19, 20]. In particular, sequestration of mature parasites in retinal vessels occurs in eyes in which vessel whitening was demonstrated during life [4]. The study of malarial retinopathy provides a unique opportunity to visualize and investigate the infected CNS microvasculature in vivo.
We used fluorescein angiography of the retina to investigate blood flow, tissue perfusion, and the integrity of the blood-tissue barrier in the CNS of children with CM. Our aim was to improve our understanding of the pathogenesis of this disease.
METHODS
We studied children with CM who were admitted to the Pediatric Research Ward at Queen Elizabeth Central Hospital, Blantyre, Malawi, during 1 malaria season from December 2005 to May 2006. CM was defined as profound coma (Blantyre coma score 2 or less) with P. falciparum parasitemia, in the absence of any other identifiable explanation for altered consciousness (in particular, meningitis, hypoglycemia, or postictal state of ≤2 h).
All patients with CM were assessed for inclusion at the time of admission or shortly thereafter. Patients were excluded if, in the opinion of the attending clinicians, they were too ill to undergo angiography or if an improving level of consciousness made fundus imaging impossible. Written consent for the study was sought by a nurse in the language of the parent or guardian after antimalarial therapy and supportive care had been initiated and the patient's condition had stabilized.
Patients were treated for CM in accordance with protocols based on World Health Organization guidelines. All patients received intravenous quinine and fluids. Glucose, transfused blood, and anticonvulsants were given as required.
We performed direct and indirect ophthalmoscopy on patients for whom consent had been secured after the patient's pupils had been dilated with tropicamide and phenylephrine eye drops. We recorded and graded any malarial retinopathy on standardized charts [21, 22]. Fundus imaging was performed with a table-mounted Topcon 50-EX digital camera (Topcon). The unconscious patient was positioned on a raised bed with the head at the edge of the bed. We took color retinal photographs with the aim of a retinal survey of both eyes, although this objective was constrained by positioning and involuntary eye movements. Fluorescein angiography commenced with the intravenous injection of 10% sodium fluorescein (5 mL). We took images every few seconds for 2 min, continuing less frequently for 15–20 min. Imaging was performed by an ophthalmologist (N.A.V.B.), with a nurse and pediatric clinician present.
Clinical data on patients were entered on a database and angiograms were interpreted by the study ophthalmologists (N.A.V.B., S.P.H., and S.L.). Images were viewed using ImageNet software (Topcon) The study protocol was approved by the research ethics committees of the College of Medicine, Malawi, and the Liverpool School of Tropical Medicine, United Kingdom.
RESULTS
During the study period, 100 children with CM were admitted to the research ward, of whom 40 fulfilled the eligibility criteria for the study procedures. Six declined consent, and 34 underwent fundus fluorescein angiography.
The mean age was 39 months, and 18 subjects (53%) were male. There were 25 patients (74%) who were also severely anemic (hematocrit, ≤15%), and 15 patients (44%) had respiratory distress (usually attributable to metabolic acidosis in CM). The mean parasite density was 30 × 103 parasites/μL, and 9 patients (26%) had hypoglycemia (glucose level ≤2.2 mmol/L). There were 4 patients (12%) who died, and 5 (15%) had gross neurological deficits at discharge. There were no adverse effects attributable to the intravenous fluorescein.
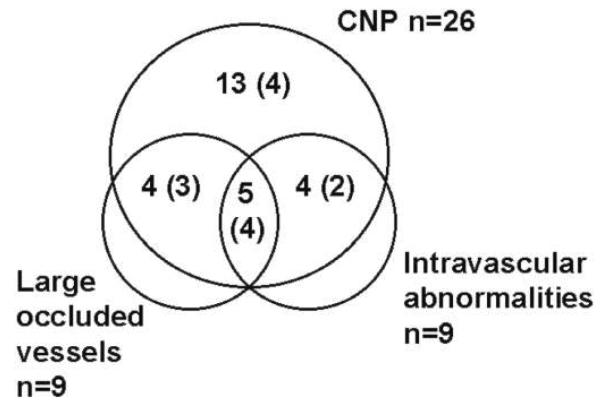
Perfusion abnormalities were present in the angiograms of 28 patients (82%). Abnormalities included capillary nonperfusion (CNP), blocked larger retinal vessels, areas of retinal ischemia, intravascular filling defects, and fluorescein leakage. Fluorescence was blocked by hemorrhages, where they occurred. There were 6 children (18%) with angiograms that were normal, apart from masking of fluorescence by hemorrhages; 2 of these children had mild retinal whitening. The number of patients with each of the fluorescein abnormalities is summarized in figure 1.
Figure 1.
Venn diagram summarizing results of fluorescein angiograms performed for 34 patients. There were 28 patients with perfusion abnormalities. Large occluded vessels and intravascular abnormalities only occurred in the presence of capillary nonperfusion (CNP). There were 6 patients with no vascular abnormalities, and 2 patients with fluorescein leakage alone. The number in parentheses is the number with fluorescein leakage in each group.
Capillary nonperfusion
Zones of CNP were seen in 26 patients (76%), affecting the macula (central retina) in 19 (56%) and the retinal periphery in 22 (65%) (figures 2-4 and figures 5 and 6, which are available only in the electronic version). These indicate the presence of occluded retinal capillaries and postcapillary venules. In the macula, zones of CNP topographically matched the retinal whitening visible in color photographs (figures 2 and 3). The size of zones of CNP varied considerably, with diameters ranging from 100 to 1000 μm. Other abnormalities of perfusion (see below) only occurred in patients who also had CNP.
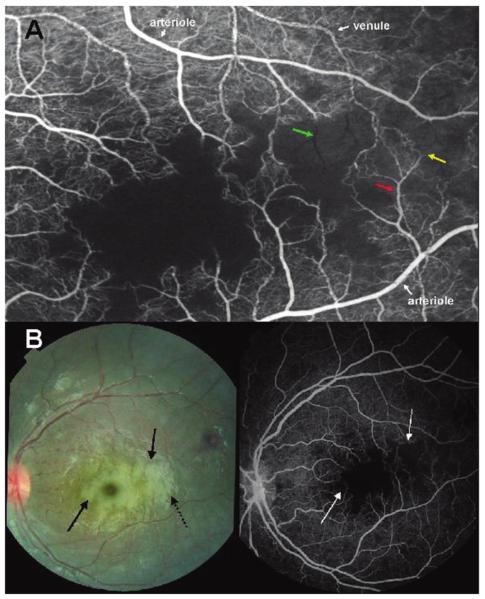
Figure 2.
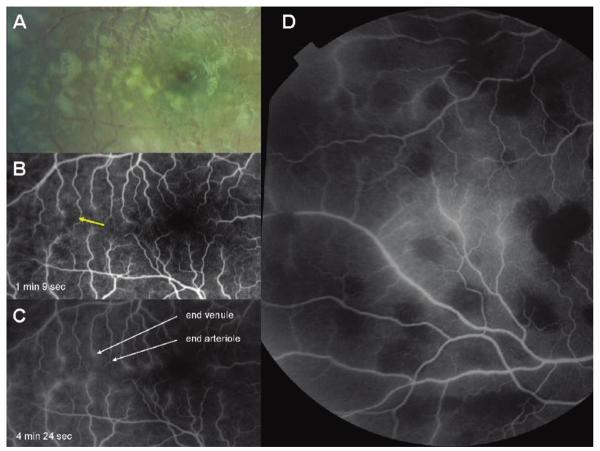
A, Fluorescein angiogram image of the macula of a child with cerebral malaria showing large areas of capillary nonperfusion (center of picture). Arteriolar and venous circulation have been labelled to illustrate mottling of the blood column, which is most dense in the venules, but also occurs in the arterioles (red arrow). Vessel occlusion is widespread on the border of areas of nonperfusion (resulting in “pruning,” yellow arrow). “Ghost vessels'”can be seen (green arrow); these are nonperfused vessels, the contents of which are blocking the background choroidal fluorescence. B, Paired color retinal photograph and fluorescein angiogram of the same macula. The paired images show the topographical matching between areas of retinal whitening (solid black arrows) and areas of capillary nonperfusion (white arrows). The dark disc at the center of the largest area of macular whitening is the unaffected foveola. Dashed black arrow, artefactual glare overlying retinal whitening.
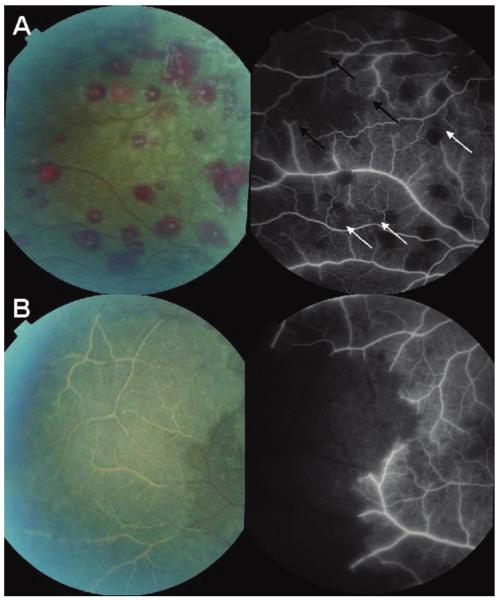
Figure 4.
A, Paired color photograph and angiogram of the retinal periphery of a child with cerebral malaria. The angiogram image shows occlusion of retinal vessels (“pruning,”black arrows) with large associated areas of nonperfusion. Smaller areas of capillary nonperfusion are also illustrated (white arrows). B, Paired color photograph and angiogram of the retinal periphery of a different child. The child has extensive white retinal vessels within an area of confluent retinal whitening. On the angiogram, these white vessels are occluded and the whole area of retinal whitening is nonperfused. Both venules and arterioles are affected and are a normal red color up to the point of occlusion.
Figure 5.
Paired color photograph and angiogram of retinal vessel affected by orange discoloration and tram-lining (i.e., whitening along the margin of the blood column) seen in malarial retinopathy.
The figure is available in its entirety in the online edition of the Journal of Infectious Diseases.
Figure 6.
Paired color photograph and angiogram of retinal vessels in child with cerebral malaria.
The figure is available in its entirety in the online edition of the Journal of Infectious Diseases.
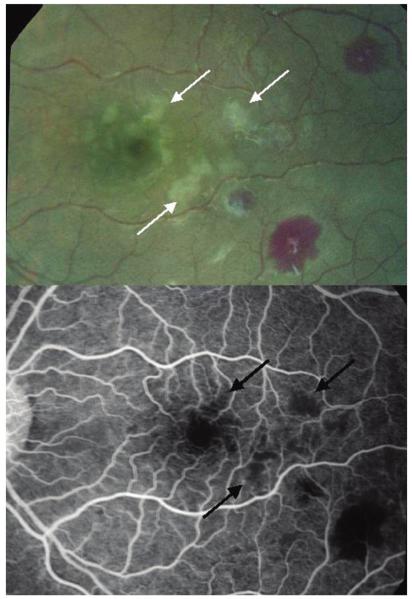
Figure 3.
Paired color photograph and angiogram of the macula of a child with cerebral malaria. The images show the topographical matching between areas of retinal whitening (white arrows) and areas of capillary nonperfusion (black arrows). A retinal hemorrhage (bottom right, both images) demonstrates fluorescein masking or blocked fluorescence in the angiogram.
In the periphery, 22 patients (65%) had angiograms that showed scattered zones of CNP (figure 4 and figure 5, which is available only in the electronic version), which ranged in number between 1 and 40 in a 50° field. These peripheral areas of CNP corresponded to areas of peripheral retinal whitening, which in some cases included visible white capillaries.
Macular retinal whitening occurred without matching zones of CNP at the time of the angiogram in 7 patients. This occurred to a mild or moderate degree in 6 patients and included 4 for whom the angiogram was delayed >24 h after admission. One patient had an area of severe macular whitening without CNP but with fluorescein leakage, as demonstrated in their angiogram, performed 4 days after admission.
Larger occluded vessels
There were 9 patients (26%) whose angiograms demonstrated larger occluded retinal vessels in the periphery. Both venules and arterioles were involved. These blocked retinal vessels appeared white on ophthalmoscopy and in color photographs. The territories of these vessels were not perfused, resulting in ischemic areas of peripheral retina (figure 4). In 4 patients, these ischemic areas were extensive, amounting to 5 optic disc areas in 3 patients and >30 disc areas in 1 patient with extensive white vessels (figure 4B).
In areas of ischemia, larger unperfused blood vessels could still be visualized on the angiograms as black “ghost vessels” against the normal background of fluorescence from the underlying choroid (figures 2 and 4). This indicates that areas of hypofluorescence were not the result of masking by retinal whitening and that the contents of these occluded ghost vessels blocked the background choroidal fluorescence.
Intravascular abnormalities
In 9 patients (26%), the angiograms demonstrated intravascular abnormalities, comprising filling defects and mottling within the blood column (figure 2 and figures 5 and 6, which are available only in the electronic version). The filling defects in larger vessels were along the margin, appearing to narrow the vessel lumen (figures 5 and 6, which are available only in the electronic version). In smaller vessels the density of filling defects was greater, affecting the width of the vessel and giving rise to a mottled appearance of the blood column (figure 2 and figure 6, which is available only in the electronic version). Intravascular abnormalities were seen in capillaries, venules and arterioles, but were more frequent and dense in venules. Postcapillary venules and capillaries were the most severely affected by mottling, but small arterioles were also affected to a lesser degree (figure 2). Mottling was seen in close association with areas of CNP (figure 2 and figure 6, which is available only in the electronic version).
Smaller vessels with intravascular mottling appeared orange on ophthalmoscopy (figures 5 and 6, which are available only in the electronic version) or exhibited tram-lining, whitening along the margin of the blood column (figure 5, which is available only in the electronic version). Larger vessels with fluorescein filling defects were not discolored. Orange discoloration of vessels is recognized as part of the spectrum of vessel whitening seen in malarial retinopathy. Where branch points of larger vessels with filling defects were imaged, these frequently marked a transition from marginal filling defects to mottling, corresponding to a change in the vessels' appearance from red to orange (figure 6, which is available only in the electronic version). Filling defects and mottling did not move or otherwise change during the course of the 15–20 min angiographic procedure.
Fluorescein leakage
Leakage of fluorescein from the retinal circulation indicates breakdown of the blood-retina barrier and was seen in 15 patients (44%). It was limited in extent and severity, with modest leakage from small venules and short segments of larger venules and without fluid accumulating in the tissue as retinal edema. It occurred particularly in terminal venules around the fovea and temporal macula, the watershed zone between the inferior and superior arcades (figure 7). It was also seen in sections of larger venules (figure 7D). The leaking vessels had no mottling or filling defects in the angiogram images and appeared normal in color images. No leakage was seen from within sites of hemorrhage.
Figure 7.
Color photograph (A) and early (B) and late (C) angiogram images of a macula showing leakage of fluorescein from terminal venules and not arterioles. This appears as fuzzy hyperfluorescence in C. The color image shows macular whitening around, and temporal to, the foveola (dark disc in the right of the image). There are corresponding areas of capillary nonperfusion (yellow arrow), which are associated with fluorescein leakage, indicating breakdown of the blood-retina barrier. D, Fluorescein image of peripheral retina showing leakage of fluorescein from veins in center of image. Round areas of hypofluorescence are all the result of fluorescence masking by retinal hemorrhages.
Angiograms with no perfusion abnormalities
In 6 patients, the angiograms were normal or only had fluorescence masking from hemorrhages. There was 1 death, and 2 patients had neurological sequelae in this group; none of these 3 had malaria-specific retinal abnormalities on funduscopy, but all had prolonged or multiple convulsions. In the 3 remaining patients with normal angiograms, prior funduscopy had revealed mild malaria-specific changes or retinal hemorrhages only.
DISCUSSION
The retina is the only part of the CNS in which the microvascular bed is visible to direct inspection. This makes it an ideal tissue to study in patients with CM, because the distinctive pathological process of sequestration occurs in the microvasculature. Using fundus fluorescein angiography, a routine ophthalmological investigation, we have found abnormalities of perfusion and intravascular filling in most children with CM.
The commonest abnormality, affecting 26 patients (76%), was capillary nonperfusion (CNP), the topography of which exactly matched that of the retinal whitening observed. Scattered zones of CNP in the temporal macula and retinal periphery were common. There were larger occluded vessels (arterioles and venules) and large areas of retinal ischemia in 9 patients (26%). Abnormalities that involved intravascular filling, or mottling, within the blood column of vessels were also seen in 9 patients (26%). These appearances are novel and not a feature of fluorescein angiography seen in other diseases. Dense mottling in retinal vessels corresponded to an abnormal orange appearance on ophthalmoscopy.
These findings have important implications for our understanding of the pathogenesis of CM. If the abnormalities seen on retinal angiography are mirrored in the brain, it is likely that hypoxia and ischemia are major contributors to the pathogenesis of CM. In most of the patients in our series, the ischemia was manifest as scattered zones of nonperfusion at a capillary level. In over a quarter of patients, larger venules and arterioles were occluded, leading to larger areas of ischemia. This picture of multiple small areas of microvascular occlusion suggests a model of cerebral hypoxia and infarcts that is consistent with studies indicating cerebral hypoxia [9] and a heterogeneity of microvascular sequestration. In this study, areas of CPN were demonstrated to occur next to areas of apparently normal perfusion. This heterogeneity has been demonstrated in postmortem cerebral tissue samples [3] and in vivo in other tissues [23]. It is also consistent with subtle cognitive and neuropsychological deficits observed during long-term follow-up of survivors [24-26]. Multiple small hypoxic areas or infarcts would still be compatible with the absence of gross neurological signs in most children at the time of hospital discharge.
A Kenyan study of arterial blood flow that used Doppler ultrasonography found normal velocities and pulsatilities in all children with CM on admission; abnormalities developed in 30%, mainly increased velocity in association with seizures [7]. However, ultrasonography measures flow in large cerebral arteries, not in the smaller vessels where sequestration is found. Orthogonal polarizing spectral (OPS) imaging has demonstrated severe disruption of capillary blood flow in the rectal mucosa in patients with severe malaria [23]. Dondorp et al. used OPS to demonstrate blocked capillary flow in 67% of adults with severe malaria and hyperdynamic flow in nearby capillaries [23]. Although fluorescein angiography does not demonstrate hyperdynamic flow, the proportion of patients with capillary blockage in the retina is remarkably similar to the proportion of patients with affected rectal mucosa.
We have found disruption of the blood-tissue barrier to occur in the retinas of some patients with CM, but observed this to be limited in distribution and severity. This corroborates a study of postmortem tissue samples from Malawian children that suggested limited and focal disruption of blood-brain barrier integrity in CM without leakage of plasma proteins [27]. The areas where breakdown took place in our study were not related to sequestration suggested by visible or angiographic vessel abnormalities. A study in Thai adults used film-based fluorescein angiography as one modality to investigate capillary permeability [28]. Davis et al. found fluorescein leakage in 5 of 18 adults with severe malaria which persisted till discharge in 2 [28]. They also found CNP in 5 patients in association with cotton wool spots. It is not clear whether the fluorescein leakage found in adults, and now in children, is specific to CM or is a nonspecific vascular response to severe infection. In 1 patient from the present study in whom it was possible to repeat the angiogram after 48 h, leakage developed at the site of previous CNP that had resolved. Our interpretation of these findings is that it is unlikely that breakdown of the blood-brain barrier is a pivotal pathological process in CM.
Studies of the blood-brain barrier in humans with CM have been limited to examining CSF composition and postmortem studies [29]. The results of these show some evidence of mild impairment of the barrier, but to a lesser extent than observed in other CNS infections [30]. Fluorescein angiography provides a unique method of assessing the tissue-blood barrier of an intact, infected vascular tree. The pattern of this barrier breakdown is limited to short venule segments and postcapillary venules adjacent to watershed zones. It does not occur in vessels demonstrating visible sequestration.
Our findings illuminate the pathogenesis of the unique retinopathy found in patients with severe malaria. Areas of CNP at the macula, the center of the retina where imaging was most consistent, were an exact topographical match to areas of retinal whitening. This substantiates the hypothesis that retinal whitening is a manifestation of hypoxia [31]. We postulate that retinal whitening is the result of oncotic cell swelling in response to hypoxic stress. Like other neural tissue, the neurons and photo-receptors in the retina are dependent on oxidative metabolism. The presence of glial cells (Müller cells) provides relative protection against hypoglycemia. The typical distribution of macular whitening—around the fovea and in the vascular watershed zone—also suggests hypoxic stress rather than blood-borne biochemical, cytokine, or toxic factors. The effects of these would be more evenly distributed and likely to be most severe adjacent to vessels.
Seven patients had macular whitening without corresponding CNP. In another patient, an additional angiogram performed 48 h after the first procedure demonstrated that CNP can resolve while retinal whitening persists. Depending on the stage in the course of the disease at which angiography is performed, whitening may remain without CNP but with leakage at corresponding sites. It is also possible that there may be relative hypoxia in a metabolically demanding area causing retinal whitening without frank small vessel occlusion.
It is likely that the intravascular filling defects or mottling we found are the result of sequestration, given that sequestration occurs in retinal vessels and has been demonstrated after death in eyes that had vessel abnormalities in life [4]. The filling defects appear to narrow the vessel lumen, and in smaller vessels, suggest severely disrupted blood flow. Sequestration, demonstrated by filling defects, occurred in venules, capillaries, and small arterioles simultaneously.
Occluded vessels occurred on both sides of the circulation in close proximity (figures 2 and 4). In occluded vessels, blockage is likely to be the result of sequestration, which may be accompanied by the platelet accumulation and fibrin deposition sometimes observed in cerebral vessels at autopsy. Larger vessel occlusions correspond to the white retinal vessels seen in ophthalmoscopy; these occlusions affected both arterioles and venules. The angiographic term for the appearance of blocked vessels, where fluorescein perfusion stops abruptly, is “pruning.” Retinal hemorrhages were not related to occluded or mottled vessels, and the points at which vessels were occluded were not accompanied by local hemorrhage. Figures 2, 3, 4B, and figure 6, which is available only in the electronic version, demonstrate scanty or no hemorrhages in relation to CNP or vessel abnormalities, whereas figure 7B demonstrates multiple hemorrhages in the absence of perfusion abnormalities. This suggests that another mechanism, possibly embolic or endothelial cell damage, is involved in the formation of hemorrhages.
A previous study that used fluorescein angiography to investigate retinal whitening in 12 Kenyan children with CM found no angiographic abnormalities to account for retinal whitening but did demonstrate small areas of CNP in 2 patients [32]. There was no breakdown of the blood-retina barrier. The differences between these findings and ours are most likely related to improved technical capacities and an evolving appreciation of the elements of malarial retinopathy. Like the Thai study [28], this Kenyan study used a film-based fundus camera with capabilities inferior to current models. Digital imaging also has the advantage that image quality can be monitored during acquisition. In addition, we now appreciate the importance of vessel abnormalities, which were not subject to scrutiny in the Kenyan study. The improved capabilities of modern digital fundus imaging and a greater appreciation of all lesions in malarial retinopathy have enabled this study to demonstrate CNP in a much higher proportion of patients and to demonstrate larger vessel obstruction and intravascular abnormalities for the first time.
In our study, image quality was generally good, and image analysis was not limited by technical deficiencies. However our findings do have a number of limitations. Peripheral images were difficult to obtain, except when random eye movements or gaze deviation allowed for them. We could only perform angiography on a subset of the population admitted with CM. However, patients were excluded at both ends of the spectrum of severity, and the fatality rate in patients who underwent an angiogram was similar to that of the whole group. Fluorescein angiograms are not normally performed on children with severe systemic infections, so there is no comparable study for another disease. It may be that focal fluorescein leakage occurs in other overwhelming infections, encephalopathies, or meningitis.
At present we have no data on visual outcomes in the children studied, although a previous study suggested no visual deficits attributable to malarial retinopathy [31]. It would be surprising if those with severe nonperfusion around the fovea did not have visual sequelae, but as occlusive vasculopathy is rare in infants, there may be previously unrecognized plasticity in the infant retina that allows recovery from hypoxic insult. Vision assessment is difficult in this age group, and further studies are planned to measure vision in survivors once they are older.
To our knowledge, this is the first study to demonstrate perfusion abnormalities in the microvasculature of a CNS tissue affected by CM. We have also found novel angiographic intravascular abnormalities which are likely to be manifestations of sequestration. This raises the possibility of direct study of sequestration of infected erythrocytes in the CNS in humans in vivo. The findings provide substantial evidence for the hypothesis that hypoxia and ischemia play a pivotal role in the pathogenesis of CM. Therapeutic interventions which improve perfusion and limit hypoxic injury may be of benefit to comatose children with CM.
Acknowledgments
We gratefully acknowledge the assistance of Prof. Elizabeth Molyneux, Head of the Department of Pediatrics, Queen Elizabeth Central Hospital, Malawi, as well as all the clinicians caring for the patients, in particular Drs. Rachel Bronzan, Kwame Chiwaza, Limangeni Mankhambo, Ajib Phiri, and Karl Seydel. We would like to thank all the parents, guardians, and patients for participating. We are also grateful to Prof. Peter Winstanley who ensured the safe delivery of a fundus fluorescein camera to Malawi.
Financial support: Wellcome Trust (project grant 074125/Z/04/Z to M.E.M.); British Eye Research Fund (BERF) (Iris fund award to N.A.V.B.). Enrolled patients were treated in a research ward jointly funded by the US National Institutes of Health (NIH) (AI34969) and The Wellcome Trust. The Wellcome Trust, NIH, and BERF had no influence over the conduct of the study, manuscript preparation, or decision to submit for publication.
Footnotes
Potential conflicts of interest: none reported.
Presented in part: American Society of Tropical Medicine and Hygiene Annual Congress, “Eye in cerebral malaria seminar,” 12–16 November 2006, Atlanta, Georgia; Royal College of Ophthalmologists Annual Congress, 22–24 May 2007, Birmingham, United Kingdom; Royal Society of Tropical Medicine and Hygiene Centenary Conference, 13–15 September 2007, London, United Kingdom (abstract 07).
References
- 1.Bryce J, Boschi-Pinto C, Shibuya K, Black RE. WHO estimates of the causes of death in children. Lancet. 2005;365:1147–52. doi: 10.1016/S0140-6736(05)71877-8. [DOI] [PubMed] [Google Scholar]
- 2.Marsh K, Forster D, Waruiru C, et al. Indicators of life-threatening malaria in African children. N Engl J Med. 1995;332:1399–404. doi: 10.1056/NEJM199505253322102. [DOI] [PubMed] [Google Scholar]
- 3.Silamut K, Phu NH, Whitty C, et al. A quantitative analysis of the microvascular sequestration of malaria parasites in the human brain. Am J Pathol. 1999;155:395–410. doi: 10.1016/S0002-9440(10)65136-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewallen S, White VA, Whitten RO, et al. Clinical-histopathological correlation of the abnormal retinal vessels in cerebral malaria. Arch Ophthalmol. 2000;118:924–8. [PubMed] [Google Scholar]
- 5.Seydel KB, Milner DA, Jr, Kamiza SB, Molyneux ME, Taylor TE. The distribution and intensity of parasite sequestration in comatose Malawian children. J Infect Dis. 2006;194:208–5. doi: 10.1086/505078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark IA, Budd AC, Alleva LM, Cowden WB. Human malarial disease: a consequence of inflammatory cytokine release. Malar J. 2006;5:85. doi: 10.1186/1475-2875-5-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newton CR, Marsh K, Peshu N, Kirkham FJ. Perturbations of cerebral hemodynamics in Kenyans with cerebral malaria. Pediatr Neurol. 1996;15:41–9. doi: 10.1016/0887-8994(96)00115-4. [DOI] [PubMed] [Google Scholar]
- 8.Clavier N, Rahimy C, Falanga P, Ayivi B, Payen D. No evidence for cerebral hypoperfusion during cerebral malaria. Crit Care Med. 1999;27:628–32. doi: 10.1097/00003246-199903000-00047. [DOI] [PubMed] [Google Scholar]
- 9.Warrell DA, Veall N, Chanthavanich P, et al. Cerebral anaerobic glycolysis and reduced cerebral oxygen transport in human cerebral malaria. Lancet. 1988;2:534–8. doi: 10.1016/s0140-6736(88)92658-x. [DOI] [PubMed] [Google Scholar]
- 10.Molyneux M, Taylor T, Wirima JJ, Borgstein A. Clinical features and prognostic indicators in paediatric cerebral malaria:a study of 131 comatose Malawian children. QJM. 1989;71:441–59. [PubMed] [Google Scholar]
- 11.White NJ, Warrell DA, Looareesuwan S, Chanthavanich P, Phillips RE, Pongpaew P. Pathophysiological and prognostic significance of cerebrospinal-fluid lactate in cerebral malaria. Lancet. 1985;1:776–8. doi: 10.1016/s0140-6736(85)91445-x. [DOI] [PubMed] [Google Scholar]
- 12.Day NP, Phu NH, Mai NT, et al. The pathophysiologic and prognostic significance of acidosis in severe adult malaria. Critical Care Medicine. 2000;28:1833–40. doi: 10.1097/00003246-200006000-00025. [DOI] [PubMed] [Google Scholar]
- 13.Lewallen S, Harding SP, Ajewole J, et al. A review of the spectrum of clinical ocular fundus findings in P. falciparum malaria in African children with a proposed classification and grading system. Trans R Soc Trop Med Hyg. 1999;93:619–22. doi: 10.1016/s0035-9203(99)90071-8. [DOI] [PubMed] [Google Scholar]
- 14.Idro R, Jenkins NE, Newton CR. Pathogenesis, clinical features, and neurological outcome of cerebral malaria. Lancet Neurol. 2005;4:827–40. doi: 10.1016/S1474-4422(05)70247-7. [DOI] [PubMed] [Google Scholar]
- 15.Lynn WA, Lightman S. The eye in systemic infection. Lancet. 2004;364:1439–50. doi: 10.1016/S0140-6736(04)17228-0. [DOI] [PubMed] [Google Scholar]
- 16.Beare NA, Taylor TE, Harding SP, Lewallen S, Molyneux ME. Malarial retinopathy: a newly established diagnostic sign in severe malaria. Am J Trop Med Hyg. 2006;75:790–7. [PMC free article] [PubMed] [Google Scholar]
- 17.Lewallen S, Bakker H, Taylor TE, Wills BA, Courtright P, Molyneux ME. Retinal findings predictive of outcome in cerebral malaria. Trans R Soc Trop Med Hyg. 1996;90:144–6. doi: 10.1016/s0035-9203(96)90116-9. [DOI] [PubMed] [Google Scholar]
- 18.Beare NA, Southern C, Chalira C, Taylor TE, Molyneux ME, Harding SP. Prognostic significance and course of retinopathy in children with severe malaria. Arch Ophthalmol. 2004;122:1141–7. doi: 10.1001/archopht.122.8.1141. [DOI] [PubMed] [Google Scholar]
- 19.White VA, Lewallen S, Beare N, Kayira K, Carr RA, Taylor TE. Correlation of retinal haemorrhages with brain haemorrhages in children dying of cerebral malaria in Malawi. Trans R Soc Trop Med Hyg. 2001;95:618–21. doi: 10.1016/s0035-9203(01)90097-5. [DOI] [PubMed] [Google Scholar]
- 20.Hidayat AA, Nalbandian RM, Sammons DW, Fleischman JA, Johnson TE. The diagnostic histopathologic features of ocular malaria. Ophthalmology. 1993;100:1183–6. doi: 10.1016/s0161-6420(93)31508-3. [DOI] [PubMed] [Google Scholar]
- 21.Beare NA, Southern C, Lochhead J, Molyneux ME, Lewallen S, Harding SP. Inter-observer concordance in grading retinopathy in cerebral malaria. Ann Trop Med Parasitol. 2002;96:105–8. doi: 10.1179/000349802125000565. [DOI] [PubMed] [Google Scholar]
- 22.Harding SP, Lewallen S, Beare NA, Smith A, Taylor TE, Molyneux ME. Classifying and grading retinal signs in severe malaria. Trop Doct. 2006;36(Suppl 1):1–13. doi: 10.1258/004947506776315781. [DOI] [PubMed] [Google Scholar]
- 23.Dondorp AM, Ince C, Charunwatthana P, et al. Direct in vivo assessment of microcirculatory dysfunction in severe falciparum malaria. J Infect Dis. 2008;197:79–84. doi: 10.1086/523762. [DOI] [PubMed] [Google Scholar]
- 24.Boivin MJ. Effects of early cerebral malaria on cognitive ability in Senegalese children. J Dev Behav Pediatr. 2002;23:353–64. doi: 10.1097/00004703-200210000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Carter JA, Ross AJ, Neville BG, et al. Developmental impairments following severe falciparum malaria in children. Trop Med Int Health. 2005;10:3–10. doi: 10.1111/j.1365-3156.2004.01345.x. [DOI] [PubMed] [Google Scholar]
- 26.Kihara M, Carter JA, Newton CR. The effect of Plasmodium falciparum on cognition: a systematic review. Trop Med Int Health. 2006;11:386–97. doi: 10.1111/j.1365-3156.2006.01579.x. [DOI] [PubMed] [Google Scholar]
- 27.Brown H, Rogerson S, Taylor T, et al. Blood-brain barrier function in cerebral malaria in Malawian children. Am J Trop Med Hyg. 2001;64:207–13. doi: 10.4269/ajtmh.2001.64.207. [DOI] [PubMed] [Google Scholar]
- 28.Davis TM, Suputtamongkol Y, Spencer JL, et al. Measures of capillary permeability in acute falciparum malaria: relation to severity of infection and treatment. Clin Infect Dis. 1992;15:256–66. doi: 10.1093/clinids/15.2.256. [DOI] [PubMed] [Google Scholar]
- 29.Gitau EN, Newton CR. Blood-brain barrier in falciparum malaria. Tropical Medicine and International Health. 2005;10:285–92. doi: 10.1111/j.1365-3156.2004.01366.x. [DOI] [PubMed] [Google Scholar]
- 30.Brown HC, Chau TT, Mai NT, et al. Blood-brain barrier function in cerebral malaria and CNS infections in Vietnam. Neurology. 2000;55:104–11. doi: 10.1212/wnl.55.1.104. [DOI] [PubMed] [Google Scholar]
- 31.Beare NA, Southern C, Kayira K, Taylor TE, Harding SP. Visual outcomes in children in Malawi following retinopathy of severe malaria. Br J Ophthalmol. 2004;88:321–4. doi: 10.1136/bjo.2003.025924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hero M, Harding SP, Riva CE, Winstanley PA, Peshu N, Marsh K. Photographic and angiographic characterization of the retina of Kenyan children with severe malaria. Arch Ophthalmol. 1997;115:997–1003. doi: 10.1001/archopht.1997.01100160167005. [DOI] [PubMed] [Google Scholar]