Abstract
The newly discovered endocannabinoid system (ECS; comprising the endogenous lipid mediators endocannabinoids present in virtually all tissues, their G-protein-coupled cannabinoid receptors, biosynthetic pathways and metabolizing enzymes) has been implicated in multiple regulatory functions both in health and disease. Recent studies have intriguingly suggested the existence of a functional ECS in the skin and implicated it in various biological processes (e.g. proliferation, growth, differentiation, apoptosis and cytokine, mediator or hormone production of various cell types of the skin and appendages, such as the hair follicle and sebaceous gland). It seems that the main physiological function of the cutaneous ECS is to constitutively control the proper and well-balanced proliferation, differentiation and survival, as well as immune competence and/or tolerance, of skin cells. The disruption of this delicate balance might facilitate the development of multiple pathological conditions and diseases of the skin (e.g. acne, seborrhea, allergic dermatitis, itch and pain, psoriasis, hair growth disorders, systemic sclerosis and cancer).
The skin as an emerging neuro-immuno-endocrine organ
The skin and its appendages establish a ‘passive’ physicochemical barrier against constant environmental challenges. However, a plethora of recent research has defined that the skin and its adnexal components (i.e. hair follicles, sebaceous and sweat glands) also function as ‘active’ neuro-immuno-endocrine organs [1] with (i) well-defined neuronal networks and related functions; (ii) a wide-array of constantly remodeling non-neuronal cells and ‘mini-organs’ (i.e. hair follicle, sebaceous gland); (iii) orchestrated immunological machinery for inflammatory and immunological mechanisms; (iv) the synthesis and release of numerous growth factors, vasoactive substances and hormones (Box 1).
Introduction to skin biology
The skin is the largest organ of the integumentary system (the organ system that protects the body from damage) and is composed of multiple layers and cell types:
Epidermis
made of keratinocytes (which provide waterproofing and serve as a barrier to infection), Merkel cells (which function as mechanoreceptors for the sensation of touch and pressure), melanocytes (whose activity of melanogenesis defines skin color) and Langerhans cells (which function as professional antigen-presenting cells of the skin immune system). In addition, sensory nerve endings (recognizing e.g. touch, pressure, temperature as well as pain and itch) might also reach the lower layers of the epidermis.
Dermis
a dense connective tissue composed of collagen, elastic and reticular fibers produced mainly by dermal fibroblasts. In addition, the dermis is supplied by blood and lymphatic vessels and is highly innervated by both sensory afferent as well as motor efferent (which participate e.g. in vasoregulation) nerve fibers establishing a dense neuronal network. Of further importance, the dermis is the ‘home’ of the skin appendages such as the hair follicles as well as the sebaceous and sweat glands.
Hypodermis (or subcutis)
made of adipocytes, fibroblasts and macrophages (part of the skin immune system). In addition, the subcutis is well supplied by vessels and nerve fibers.
The skin layers and cell types form a complex, multicellular communications network, the proper function of which establish the physiological skin homeostasis. Selected functions of the skin involve:
Barrier functions
waterproof anatomical protection barrier against, for example, physical environmental challenges (e.g. UV, temperature), microbial invasion, allergens, chemical irritants and so on.
Sensory functions
sensation of heat and cold, touch, pressure, vibration as well as pain and itch (related to tissue injury); release of neuropeptides that regulate local vasoregulatory, immune-inflammatory and trophic functions.
Motor functions
vasoregulation (dilation or constriction of blood vessels) and piloerection.
Transport functions
transport of respiratory gases and nutrients between skin layers as well as from/to the skin surface; absorption of topically applied medications.
Exocrine functions
production and release (to the skin surface) of sweat and sebum, which exocrine products participate, for example, in thermoregulation, physical barrier formation, anti-microbial activity and so on.
Thermoregulatory functions
insulation by the subcuticular adipose tissue (actually, skin contains 50% of body fat); large cutaneous blood supply that enables precise control of direct heat losing mechanisms (i.e. radiation, convection and conduction); vasoregulation (vasodilation promotes heat loss whereas vasoconstriction preserves body heat); evaporation (both insensible via skin pores and sensible via sweating); piloerection to further support insulation.
Endocrine functions
synthesis of a wide-array of hormones (e.g. vitamin D, steroids and peptide hormones) in multiple cutaneous cells; functional expression of hormone receptors as well as enzymes involved in the synthesis and metabolism of hormones; immune and inflammatory functions (a wide array of cutaneous immune-competent cells); synthesis and release of pro- and anti-inflammatory mediators (e.g. cytokines, chemokines and trophic factors) in almost all skin populations; anti-microbial activity of the sebum.
Regenerative functions
well-orchestrated and balanced proliferation, differentiation, survival and death ‘programs’ of the cutaneous cells and appendage structures, which enable life-long regeneration and regeneration of the skin; stem cell supply; wound healing.
For the delicate execution of cutaneous neuro-immuno-endocrine functions, the aforementioned components establish a complex, multicellular communication network [2,3]. For example, activation of sensory neurons by various stimuli not only induces the antidromic (see Glossary) transmission of signals to the central nervous system but also results in the orthodromic release of certain neuropeptides (such as substance P and calcitonin-gene-related peptide) from the sensory afferents [4,5]. By contrast, these neuropeptides might then act on cutaneous non-neuronal cell types and exert local immuno-endocrine effects. Indeed, almost all skin cell populations (including those of the pilosebaceous unit) are capable of producing and releasing pro- and/or anti-inflammatory mediators that, by acting on neighboring cell types, can then fine-tune the overall immune response of the skin [1,3,5]. Similarly, production of numerous hormones by multiple cell types in the skin can exert local (paracrine or autocrine) regulation of cellular metabolism and functions of other cutaneous cell populations [1–5]. It is also important to note that the ‘passive’ (physico-chemical barrier) and ‘active’ (neuro-immuno-endocrine) functions of the skin and its appendages are strongly dependent on life-long regeneration and rejuvenation of cutaneous non-neuronal cells and mini-organs. These functions are defined by the well-orchestrated, delicate balance of cellular and organ proliferation and growth, survival and death, and regulated by a multitude of soluble mediators (e.g. growth and trophic factors, cytokines and chemokines) released from the skin cells [1–5].
Collectively, proper execution of the aforementioned mechanisms and the plasticity and pleiotropic nature of the cutaneous cells establish a solid base for physiological human skin homeostasis. Moreover, an appropriate equilibrium of cutaneous functions also enables the skin to protect the human body from constant environmental ‘stressor’ challenges such as microbial invasion, allergens, UV exposure and chemical irritation, among others. It is no wonder, therefore, that pathological alterations of cutaneous growth control and immuno-endocrine functions could lead to the development of multiple prevalent clinical conditions such as hyperproliferative skin diseases (e.g. psoriasis, tumors), hair growth disorders (e.g. alopecia, effluvium, hirsutism), acne vulgaris and atopic dermatitis [1–7].
In this article, the physiological regulatory function of the endocannabinoid system (ECS) in proliferation, differentiation, apoptosis and cytokine, mediator and hormone production of various cell types of the skin and appendages (e.g. hair follicle, sebaceous gland) are highlighted (Figure 1), and evidence on the putative involvement of the ECS in certain pathological conditions of the skin, such as allergic dermatitis, cutaneous itch and pain, and neoplastic cell growth, are discussed. Future preclinical and clinical research directions and strategies to therapeutically target ECS for the management of various skin diseases are also envisioned.
Figure 1.
Functions of the cutaneous ECS. Prototypic endocannabinoids such as anandamide (N-arachidonoylethanolamine; AEA) and 2-arachidonoylglycerol (2-AG) are produced locally in various cellular compartments of the skin (i.e. epidermis, sebaceous gland, hair follicle) (green arrows). These endocannabinoids, via binding to cannabinoid receptor subtypes 1 and/or 2 (CB1/CB2), constitutively control the proper and well-balanced cutaneous functions (e.g. sensation, growth, survival, immune competence and/or tolerance) (red arrows). For example, activation of CB1 and CB2 on epidermal keratinocytes by locally produced endocannabinoids results in the suppression of cellular proliferation, differentiation and the release of inflammatory mediators as well as the induction of apoptosis. Likewise, endocannabinoids, via CB1/CB2, inhibit inflammatory responses of resident and infiltrating immune cells. Furthermore, activation of CB1 in the hair follicle by AEA attenuates hair shaft elongation and intrafollicular proliferation, whereas it stimulates apoptosis and the development of catagen regression. On another member of the pilosebaceous unit (i.e. on the sebaceous gland-derived sebocytes), locally released endocannabinoids markedly enhance lipid production and apoptosis via CB2. Finally, skin-derived endocannabinoids inhibit various sensory phenomena (e.g. pain and itch) via CB1 expressed on sensory afferent nerves.
The ECS and the skin
Identification of the main cannabinoid receptors (CB1 and CB2), their endogenous lipid ligands (endocannabinoids), biosynthetic pathways and metabolizing enzymes (collectively termed the ECS) [8–10], coupled with the discovery and/or rational design of numerous exogenous ligands for CB receptors [11], has triggered an exponential growth in studies exploring the continuously growing regulatory functions of this newly discovered physiological system both in health and disease [12–14].
Excitingly, modulating the activity of the ECS has turned out to hold tremendous therapeutic potential for a multitude of diseases and pathological conditions affecting humans [13,15,16], ranging from inflammatory [17], neurodegenerative [18–20], gastrointestinal [21,22], liver [23,24], cardiovascular disorders [25,26] and obesity [27,28], to ischemia/reperfusion injury [29], cancer [30] and pain [31].
The most extensively studied endocannabinoids are anandamide (N-arachidonoylethanolamine,AEA) and 2-arachidonoylglycerol (2-AG) [8,32]. Multiple pathways are involved in synthesis and cellular uptake of these lipid mediators; these are described in several excellent recent reviews [10,33,34] and beyond the scope of this article. The most common degradation pathways for AEA and 2-AG are the fatty acid amid hydrolase (FAAH) and monoacylglycerol lipase (MAGL) enzymes [10]. Endocannabinoids, similar to Δ9-tetrahydrocannabinol (THC; the main active ingredient of the plant Cannabis sativa), predominantly exert their physiological effects via two main G-protein-coupled cannabinoid receptors; however, numerous additional signaling mechanisms and receptor systems (e.g. transient receptor potential cation channel, subfamily V, member 1; TRPV1) might also be involved [35]. Initially, the CB1-mediated effects were described centrally and CB1 receptors were thought to be restricted to the central nervous system, whereas CB2 was first identified at the periphery in immune cells. Excitingly, findings over the past decade have clearly demonstrated that functional ECS is present almost in all peripheral organ systems [13–15].
Indeed, components of the ECS have also been discovered in the skin recently (Figure 1). Both CB1 and CB2 immunoreactivities were observed on numerous human and murine skin cell populations in situ such as on cutaneous nerve fibers, mast cells, epidermal keratinocytes and cells of the adnexal tissues [36–42]. Similarly, both CB1 and CB2 have been identified (at protein and mRNA levels) on cultured human primary (NHEK) and HaCaT keratinocytes [43–45]. Interestingly, in organ-cultured human hair follicles, exclusive expression of CB1 was described [41], whereas CB2 expression (unlike CB1) was found on human sebaceous gland-derived SZ95 sebocytes [42]. AEA and 2-AG were detected in rodent skin [40,46], as well as in human organ-cultured hair follicles [41] and SZ95 sebocytes [42]. AEA, along with its transporter (AMT/EMT), synthetic and metabolizing enzymes (NAPE-PLD and FAAH) were also identified in cultured NHEK and HaCaT keratinocytes [43], and in murine epidermal cells/skin [40,47]. TRPV1, as key peripheral integrator of various sensory phenomena (e.g. pain, heat, itch), was originally described on nociceptive sensory neurons as a molecular target for capsaicin, the pungent vanilloid ingredient of hot chili peppers [48]. More recently, similar to CB1/2, TRPV1 was also found on numerous non-neuronal cells types including human skin epidermal keratinocytes, dermal mast cells, Langerhans cells, sebocytes, sweat gland epithelium and various keratinocyte populations of the hair follicle [49–52]. TRPV1 might have important roles in skin health and in certain skin disorders, especially in ones associated with inflammation, pain and itch (e.g. in various types of dermatitis) [3–5,7]. However, the involvement of TRPV1-coupled signaling in the cellular actions of AEA on cell growth, differentiation, proliferation and survival might exert marked cell-type specificity in the skin, and depending on the cell type it can be synergistic, antagonistic or independent from the CB1/2 receptor stimulation [41,42,45,51,52]. The discussion of these complex effects is beyond the scope of this brief synopsis.
Role of the cutaneous ECS in skin growth control, survival and differentiation
Recent intriguing data suggest that the cutaneous ECS is fully functional (Figure 1). Indeed, as described later, the ECS has been implicated in the regulation of skin cell proliferation, survival and differentiation, the delicate balance of which is a key determinant of proper cutaneous homeostasis. Furthermore, fine-tuning of the endocannabinoid tone appears to be a key factor in modulating cutaneous growth and differentiation (Table 1).
Table 1.
Functions of the cutaneous ECS
| Experimental system | Main findings | Pharmacological tools employed | Notes | Refs |
|---|---|---|---|---|
| In vitro cell and organ cultures | ||||
| Human epidermal keratinocytes (NHEK, HaCaT) |
CB1 and CB2 are expressed NAPE-PLD, AMT/EMT and FAAH are expressed AEA is produced AEA inhibits proliferation and induces apoptosis via CB1 Synthetic cannabinoids do not affect proliferation AEA inhibits differentiation via CB1 |
AEA, NADA: endocannabinoids WIN-55 212–2: mixed CB1/CB2 agonist JWH-133: selective CB2 agonist SR141716A: selective CB1 antagonist/inverse agonist SR144528: selective CB2 antagonist/inverse agonist |
CB1 and CB2 are expressed on human and mouse skin cell populations in situ |
[36–45] |
| Human and murine transformed (tumorigenic) epidermal keratinocytes |
Human: phyto- and synthetic cannabinoids inhibit proliferation (CB1/CB2 independent) Murine: synthetic cannabinoids inhibit proliferation and induce apoptosis via CB1 and CB2 |
HU210, WIN-55 212–2: mixed CB1/CB2 agonists JWH-015, JWH-133, BML-190: selective CB2 agonists THC, cannabidiol, cannabinol and cannabigerol: phytocannabinoids SR141716A: selective CB1 antagonist/inverse agonist SR144528: selective CB2 antagonist/inverse agonist |
Murine skin produces AEA and 2-AG, which express NAPE-PLD, AMT/EMT and FAAH |
[36,47,53] |
| Human organ-cultured hair follicles |
CB1 is expressed AEA and 2-AG are produced AEA (unlike 2-AG) and THC inhibit hair shaft elongation and proliferation, and induce intraepithelial apoptosis and catagen regression via CB1 |
AEA, 2-AG: endocannabinoids, mixed CB1/CB2 agonists THC: phytocannabinoid, mixed CB1/CB2 agonists AM-251: selective CB1 antagonist/inverse agonist |
CB1 and CB2 are expressed on human hair follicle in situ |
[36,37,41] |
| Human sebaceous-gland- derived SZ95 sebocytes |
CB2 is expressed AEA and 2-AG are produced AEA and 2-AG stimulate lipid/sebum production and apoptosis via CB2 |
AEA, 2-AG: mixed CB1/CB2 agonists ACEA: selective CB1 agonists JWH-015: selective CB2 agonists AM-251: selective CB1 antagonist/inverse agonist AM-630: selective CB2 antagonist/inverse agonist |
CB1 and CB2 are expressed on human sebaceous gland in situ |
[36,37,42] |
| In vivo animal models | ||||
| Tumor induction in mice | Synthetic CB1/CB2 agonists inhibit tumor growth, angiogenesis and metastasis, and induce apoptosis in non-melanoma tumors and melanomas |
THC: phytocannabinoid WIN-55 212–2: mixed CB1/CB2 agonist JWH-133: selective CB2 agonist SR141716A: selective CB1 antagonist/inverse agonist SR144528, AM-630: selective CB2 antagonists/inverse agonist |
Skin tumors (basal and squamous cell carcinomas, melanoma) express CB1 and CB2 |
[36,39] |
| Cutaneous contact allergic dermatitis |
CB1/2 double knockout mice display exacerbated allergic skin inflammation FAAH-deficient display reduced allergic response in the skin Locally administered CB antagonists exacerbate allergic inflammation Synthetic CB agonists and THC suppress inflammation |
THC: phytocannabinoid HU210: mixed CB1/CB2 agonist HU308: selective CB2 agonist SR141716A: selective CB1 antagonist/inverse agonist SR144528: selective CB2 antagonist/inverse agonist |
Skin levels of endocannabinoids increase in contact dermatitis |
[40] |
| IgE-induced cutaneous anaphylaxis |
Synthetic CB agonists and PEA suppress inflammation |
WIN-55 212–2, HU-210, CP 55 940: mixed CB1/CB2 agonists JWH-133: selective CB2 agonist SR141716A, AM-281: selective CB1 antagonists/inverse agonist SR144528, AM-630: selective CB2 antagonists/inverse agonist |
[57] | |
| Acute and chronic contact dermatitis |
CB2 antagonist attenuates inflammation | 2-AG: endocannabinoid, mixed CB1/CB2 agonists AM-251: selective CB1 antagonist/inverse agonist SR144528: selective CB2 antagonists/inverse agonist |
Skin level of 2-AG increases in contact dermatitis |
[61] |
| Allergic contact dermatitis | CB2 knockout mice display suppressed allergic skin inflammation Orally administered CB2 antagonist attenuates inflammation |
2-AG: endocannabinoid, mixed CB1/CB2 agonists HU308: selective CB2 agonist SR144528, JTE-907: selective CB2 antagonists/inverse agonists |
[62,63] | |
| UV-induced skin carcinogenesis and inflammation |
CB1/2 double knockout mice display attenuated UVB-induced skin carcinogenesis and inflammation |
WIN-55 212–2: mixed CB1/CB2 agonist |
[55] | |
| Bleomycin-induced dermal fibrosis |
CB2 knockout mice display increased dermal fibrosis and inflammation CB2 antagonist increased, agonist decreased the fibrosis and inflammation |
AM-630: selective CB2 antagonists/inverse agonist JWH-133: selective CB2 agonist |
Leukocyte expression of CB2 critically influences experimental fibrosis |
[64] |
Abbreviations: 2-AG, 2-arachidonoylglycerol; AEA, arachidonoylethanolamide; AMT/EMT, anadamide/endocannabinoid membrane transporter; CB1/2, type-1 and -2 cannabinoid receptor; FAAH, fatty acid amide hydrolase; IgE, immunoglobulin E; NADA, N-arachidonoyldopamine; NAPE, N-acylphosphatidylethanolamines; NAPE-PLD, NAPE-hydrolyzing phospholipase D; NHEK, normal human epidermal keratinocytes; PEA, N-palmitoylethanolamine; THC, Δ9-tetrahydrocannabinol; UV, ultraviolet.
Epidermis
Both phytocannabinoids and synthetic CB agonists inhibited proliferation of cultured transformed (HPV-16 E6/E7) human epidermal keratinocytes; yet, these effects were CB1- and CB2-independent [53]. On tumorigenic transformed murine keratinocytes (PDV.C57 and HaCa4), by contrast, the growth-inhibitory actions of synthetic CB agonists were prevented by both CB1 and CB2 antagonists [36]. It is also noteworthy that, on these latter mouse keratinocytes, the growth-inhibitory action of the cannabinoids was accompanied by CB1- and CB2-dependent apoptosis [36]. Interestingly, synthetic CB1 and CB2 agonists were ineffective in modulating cellular growth of both cultured NHEKs and non-tumorigenic human (HaCaT) and murine (MCA3D) keratinocytes [36].
By contrast, a recent study found that AEA markedly inhibited cellular growth and induced dose- and CB1-dependent apoptosis in human HaCaT keratinocytes [45]. Consistently with this report, the ECS also regulates human epidermal differentiation, probably via CB1-dependent mechanisms. Maccarrone et al. [43] have elegantly demonstrated that AEA, locally produced in the cells, inhibited the differentiation of cultured NHEK and HaCaT keratinocytes, as evidenced by the transcriptional downregulation of keratin 1, keratin 5, involucrin and transglutaminase-5 [44] and suppression of the formation of cornified envelopes. They have also shown that these effects were mediated by increasing DNA methylation through mitogen-activated protein kinase (MAPK)-dependent pathways (p38, p42/44) triggered by CB1 activation [44]. Involvement of CB1 in the regulation of epidermal differentiation is also suggested by the differential in situ expression of CB1 in the human epidermis, being higher in the more differentiated (granular and spinous) layers [36,37].
Skin appendages
The pilosebaceous unit of the human skin, comprising the intimately localized hair follicle (HF) and the sebaceous gland (SG), can be regarded as the ‘brain’ of the skin because it controls a wide-array of the biological functions of this organ (from stem-cell supply through immunomodulation to cytokine production) [1–3]. Recent studies have suggested that the ECS might also have a regulatory role in the human pilosebaceous unit. Both human organ-cultured HFs and human SG-derived SZ95 sebocytes have been reported to produce AEA and 2-AG [41,42]. Furthermore, AEA and THC (but not 2-AG) dose-dependently inhibited hair shaft elongation and the proliferation of hair matrix keratinocytes. Cannabinoids also induced intraepithelial apoptosis and premature HF regression (characteristic signs of catagen transformation in the HF), processes that could be inhibited by a selective CB1 antagonist. Because CB1, unlike CB2, is expressed in a hair-cycle-dependent manner in the human HF epithelium, these data support the idea that human HFs exploit a CB1-mediated endocannabinoid signaling system that might act as an autocrine–paracrine negative regulator of human hair growth. Consistently with this idea, a recent study has demonstrated that CB1 receptor antagonists do, indeed, induce hair growth in mice [54].
Interestingly, differential CB2-dependent regulation by endocannabinoids has been observed in human immortalized SZ95 sebocytes [42]. In accordance with these findings, SZ95 sebocytes predominantly express CB2, suggesting that CB2 is largely expressed in undifferentiated epithelial cells of the human SG in situ [37,42]. Both AEA and 2-AG enhanced lipid production and induced (chiefly apoptosis-driven) cell death, hallmarks of sebocyte differentiation and hence a model of holocrine sebum production [42] via CB2-coupled signaling involving the MAPK pathway. Moreover, endocannabinoids also upregulated the expression of key genes involved in lipid synthesis (e.g. peroxisome proliferator-activated receptor [PPAR] transcription factors and some of their target genes). Because cells with ‘silenced’ CB2 exhibited significantly suppressed basal lipid production, these results collectively suggest that human sebocytes utilize an autocrine–paracrine, endogenously (and probably constitutively) active, CB2-mediated endocannabinoid signaling system for positively regulating lipid production and cell death.
Skin tumorigenesis
Accumulating recent evidence also implicates the ECS in the regulation of growth of skin cells in vivo. Casanova et al. [36] have demonstrated that various human skin tumors (e.g. basal cell carcinoma, squamous cell carcinoma) express both CB1 and CB2. Local administration of synthetic CB1 and CB2 agonists induced growth inhibition of malignant skin tumors generated by intradermal inoculation of tumorigenic PDV.C57 mouse keratinocytes into nude mice. This growth inhibition was accompanied by enhanced intra-tumor apoptosis and impaired tumor vascularization (altered blood vessel morphology, decreased expression of pro-angiogenic factors such as VEGF, placental growth factor and angiopoietin 2). Consistently, cannabinoids were also reported to inhibit the in vivo growth of melanomas that express CB1 and CB2 by decreasing growth, proliferation, angiogenesis and metastasis formation, while increasing apoptosis [39]. By contrast, a recent study of Zheng et al.. [55] suggested that CB receptors and the related signaling pathways might be involved in the promotion of in vivo skin carcinogenesis. Using CB1/CB2 double gene-deficient mice, Zheng et al. [55] demonstrated that an absence of CB1 and CB2 receptors resulted in a marked decrease in UVB-induced skin carcinogenesis. They also found that a marked attenuation of UVB-induced activation of MAPKs and nuclear factor-κB was also associated with CB1 and CB2 deficiency.
Collectively, these studies suggest that the cutaneous ECS, as in other organs, might act to tonically modulate cell growth, proliferation and death [30,56] (Figure 1).
Role of the cutaneous ECS in allergic, inflammatory and fibrotic functions
Since the original discovery of the CB2 receptors in immune cells, much evidence using various CB receptor agonists and antagonists or compounds that enhance the levels of endocannabinoids by decreasing their metabolism suggest that the ECS has numerous important immune modulatory effects (e.g. suppression of production of various cytokines, chemokines, arachidonic acid-derived pro-inflammatory metabolites and nitric oxide) during inflammation [17]. Although some controversies do exist in the field, it is generally recognized that the ECS exerts protective functions in large number of acute and chronic inflammatory diseases [13,17].
A recent study by Karsak et al. [40] has suggested that the ECS exerts a protective role in allergic inflammation of the skin. Using an animal model for cutaneous contact (allergic) hypersensitivity, Karsak et al. [40] elegantly demonstrated that the skin level of endocannabinoids was increased in contact dermatitis. They also found that mice lacking both CB1 and CB2 (or treated with antagonists of these receptors) displayed exacerbated allergic inflammatory response. The existence of the ECS-mediated protection was also supported by a reduced allergic response in the skin of FAAH-deficient mice, which have increased levels of the endocannabinioid AEA. Moreover, the skin inflammation was suppressed by locally administered THC [40]. Similarly, in a murine model of passive IgE-induced cutaneous anaphylaxis, both synthetic non-selective CB agonists and saturated N-acylethanolamine derivatives (homologues of N-palmitoyl ethanolamine, PEA) exerted marked anti-inflammatory properties in vivo [57]. Notably, PEA does not act directly at CB1, CB2 or TRPV1, but it can markedly augment the effects of AEA at these receptors [58,59] as well as directly activate PPARα [60].
By contrast, using different animal models for acute and chronic contact dermatitis, Oka et al. [61] reported elevated 2-AG levels in the diseased skin. The symptoms of skin inflammation were markedly attenuated by CB2 (but not CB1) antagonists [61]. Likewise, others using different animal models (Table 1) to induce allergic contact dermatitis reported a decrease in the cutaneous inflammation of CB2-deficient mice [62], and similar suppression of the inflammatory response by orally administered CB2 antagonists was also observed [62,63]. Consistently, Zheng et al. [55], using CB1/CB2 double gene-deficient mice, recently reported that CB receptors are involved not only in the promotion of in vivo skin carcinogenesis (see earlier) but also in the UVB-induced cutaneous inflammatory processes. The reasons for the conflicting data on the role of CB1 and CB2 in cutaneous allergic responses and tumorigenesis are not clear, but they could, in part, be explained by the differences in the experimental models used (Table 1) and by an emerging scenario, according to which in some physiological functions ‘too much’ endocannabinoid tone can be as bad as ‘too little’, and both ‘enhancers’ and ‘reducers’ might be useful for the same type of disorder depending on its phase or exact cause [14]. The use of CB1 and/or CB2 antagonists, which are also inverse agonists [11] (Table 1), might further complicate the interpretation of some of these findings.
In a recent study Akhmetshina et al. [64] have demonstrated that CB2 knockout mice or controls treated with CB2 antagonist were more sensitive to bleomycin-induced dermal fibrosis compared with wild types and exhibited increased dermal thickness and leukocyte counts in the lesional skin. The phenotype of knockouts was mimicked by transplantation of knockout bone marrow into control mice, whereas CB2 knockouts transplanted with bone marrow from wide-type mice did not display an increased sensitivity to bleomycin-induced fibrosis, indicating that leukocyte expression of CB2 critically influences experimental fibrosis [64]. Decreased dermal fibrosis and inflammation was observed upon treatment with the CB2 agonist, suggesting a potential therapeutic utility of selective CB2 agonists for the treatment of early inflammatory stages of systemic sclerosis.
Role of the ECS in cutaneous sensory functions: pain and itch
The ECS has a crucial role in central and peripheral processing, and in the control of such skin-derived sensory phenomena as pain and itch. Synthetic CB agonists and/or endocannabinoids exert potent analgesic effects in both humans and animals by activation of CB1 and/or CB2 and possibly other receptors (e.g. TRPV1) at sensory nerve terminals and/or inflammatory cells. However, the detailed discussion of these effects is beyond the scope of this article and we would like to refer readers to overviews on this subject [31,65–67].
Perspectives in the ECS-targeted management of skin diseases
The aformentioned preclinical data encourage one to systematically explore whether ECS-modulating drugs can be exploited in the management of common skin disorders. However, the pleiotropic nature and strong cell-type dependence of the cutaneous ECS-mediated functions will require careful judgment for patient selection and indications. In this section, we review preliminary data and discuss the possible applications of ECS-targeted therapies (Figure 2; Table 2).
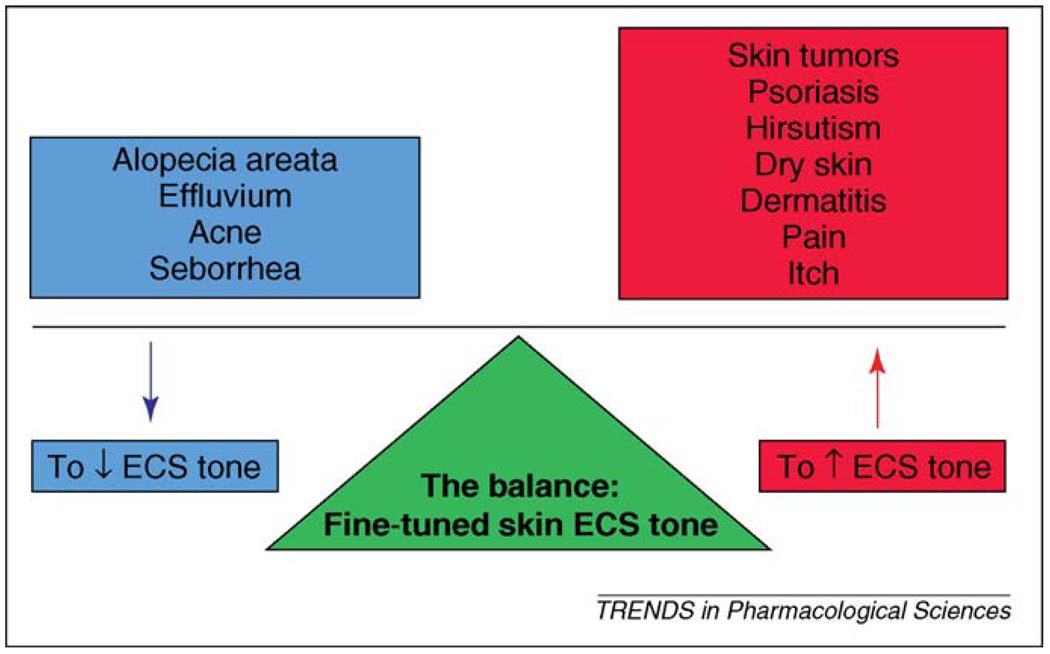
Figure 2.
ECS-targeted approaches in skin diseases. Modulations of the fine-tuned tone of the cutaneous endocannabinoid system (ECS) could have therapeutic values in the management of a large variety of human skin diseases. For example, suppression of the skin ECS tone (using e.g. CB antagonists and/or agents that attenuate the local production of endocannabinoids) could be used in the therapy of certain hair growth (e.g. forms of alopecia, effluvium) and sebaceous gland disorders (e.g. acne, seborrhea). Conversely, augmentation of the tone of the cutaneous ECS (using e.g. CB agonists and/or agents that stimulate the local production of endocannabinoids) could be beneficial in the treatment of various benign and malignant skin tumors, hyperproliferative skin diseases (e.g. psoriasis), excessive hair growth (e.g. hirsutism), different forms of dermatitis, dry skin conditions and sensory phenomena (e.g. pain, itch).
Table 2.
Possible ECS-targeted approaches in skin diseases
| Disease | Target cell population | Target receptor | Possible approach | Expected effects |
|---|---|---|---|---|
| Skin tumors | Transformed skin cell | CB1 and CB2 | CB agonists or agents that increase ECS tone |
Suppression of growth, angiogenesis and metastasis; induction of apoptosis |
| Psoriasis | Keratinocyte, immune cell | CB1 and CB2 | CB agonists or agents that increase ECS tone |
Suppression of keratinocyte proliferation and inflammation |
| Unwanted hair growth (e.g. hirsutism) |
Hair follicle epithelium | CB1 | CB1 agonists or agents that increase ECS tone |
Suppression of hair growth, induction of intrafollicular apoptosis and catagen regression |
| Alopecia areata, effluvium |
Hair follicle epithelium | CB1 | CB1 antagonists or agents that decrease ECS tone |
Stimulation of hair shaft elongation; suppression of intrafollicular apoptosis and catagen regression; induction of anagen |
| Acne, seborrhea | Sebaceous gland epithelium | CB2 | CB2 antagonists or agents that decrease ECS tone |
Inhibition of sebum/lipid production in the sebaceous gland |
| Dry skin | Sebaceous gland epithelium | CB2 | CB2 agonists or agents that increase ECS tone |
Stimulation of sebum/lipid production in the sebaceous gland |
| Dermatitis | Infiltrating immune cell, keratinocyte, sebocyte |
CB1 and CB2 | CB agonists or agents that increase ECS tone |
Suppression of immune/inflammatory processes |
| Systemic sclerosis (scleroderma) |
Infiltrating immune cells, fibroblasts |
CB2 | CB2 agonists or agents that increase ECS tone |
Suppression of immune/inflammatory processes and fibrosis |
| Pain | Sensory neuron, keratinocyte, other skin cells |
CB1 and CB2 | CB agonists or agents that increase ECS tone |
Suppression of release a algogenic substances; inhibition of transmission of signals in the nervous system |
| Itch | Sensory neurons, keratinocyte, sebocyte, other skin cells |
CB1 and CB2 | CB agonists or agents that increase ECS tone |
Suppression of release a pruritogenic substances; inhibition of transmission of signals in the nervous system |
CB1/2, type-1 and -2 cannabinoid receptor; ECS, endocannabinoid system.
Psoriasis and skin tumors: aiming to increase ECS tone
Data showing that the cutaneous ECS tonically inhibits cell growth and angiogenesis and induces apoptosis in most of the skin cell types, and that both human non-melanoma and melanoma tumors express considerable amounts of CB1 and CB2 [36,39,41,42,45,53], now warrant proof-of-principle studies to test the therapeutic value of cannabinoid agonists in the clinical management of hyperproliferative skin disease (e.g. psoriasis, which is characterized by a highly accelerated turnover of epidermal keratinocyte proliferation) and skin tumors of various cutaneous cell origins. Furthermore, these interventions (as detailed later) might also suppress skin inflammation seen in psoriasis.
Hair growth disorders: aiming to increase or decrease ECS tone
The novel concept that human HFs are both targets and sources of endocannabinoids, which, via CB1 establish an autocrine–paracrine system for negatively regulating hair growth, invites careful investigation of the growth-inhibitory effects of CB1 agonists in the putative management of unwanted hair growth such as hirsutism. Likewise, future exploitation of CB1-antagonist-based adjuvant treatment options in the clinical management of alopecia areata and effluvium is also of potential interest.
Acne and seborrhea
Acne and seborrhea, the most common dermatological diseases, are characterized by highly elevated lipid (sebum) production of the SGs. In light of the aforementioned data that CB2 activation in the SG by locally produced endocannabinoids markedly enhances lipid synthesis [42], it is envisaged that those agents that suppress the local production of endocannabinoids (NAPE-PLD and/or DAGL inhibitors) in the diseased SG and/or inhibit CB2 on the sebocytes (CB2 antagonists) might have therapeutic values. Furthermore, transdermal penetration of cannabinoids is well established [68,69], raising the possibility that these agents could be efficiently applied topically to the skin in the form of a cream.
Dry skin and related conditions
Conversely, applications of formulations containing cannabinoids that stimulate CB2 (CB2 agonists) in the SG, and/or augment the local production of endocannabinoids and/or inhibit their degradation (FAAH and/or MAGL inhibitors) in the SG might act as novel therapeutic tools in excessively dry skin by enhancing fat production in the SG (and, hence, might attract the interest of the cosmetics industry). It is important to note, however, that ideally these topical medications should contain such phyto- and/or synthetic ECS-acting substances that, on absorption to the blood, do not penetrate the brain and hence do not exert psychoactive effects. It is also noteworthy that skin dryness is a leading cause of and/or accompanied by other skin diseases and symptoms such as itching and dermatitis. Therefore, such cannabinoid-containing creams could also be beneficial under these conditions.
With respect to the possible treatment of itching, it is most promising that Stander et al. [65] have reported that topically applied emollient cream containing PEA markedly (>86%) reduced itching associated with dry skin. Therefore, it can be hypothesized that the fat-production-promoting actions of cannabinoids might, at least in part, contribute to the beneficial effects seen in these patients.
Dermatitis
Topical formulations that contain cannabinoid ligands (or that enhance the cutaneous ECS tone) could have therapeutic values in skin inflammations. Indeed, recently, a new drug containing PEA has been approved by the FDA for the treatment of dermatitis [70]. Moreover, a recent pilot study on 20 pediatric patients suffering from atopic dermatitis aimed to assess the efficacy and safety of the twice daily application of a topical emulsion containing 2% adelmidrol, a PEA analog. Excitingly, this study showed an 80% increase in symptom resolution [70,71].
Systemic sclerosis
A recent experimental study has suggested that CB2 agonists could represent a promising approach for the treatment of early inflammatory stages of systemic sclerosis (scleroderma) [64].
Pain and itch
As detailed elsewhere, various cannabinoid agonists in addition to agents that increase the cutaneous levels of endocannabinoids have been effectively used in various models of pain and itch [13,31,65–67].
Conclusions and future directions in experimental and clinical research
Collectively, it seems that the main physiological function of the cutaneous ECS is to constitutively control the proper and well-balanced proliferation, differentiation and survival, as well as immune competence and/or tolerance, of skin cells. Pathological alterations in the activity of the fine-tuned cutaneous ECS might promote or lead to the development of certain skin diseases. Therefore, it is envisaged (this is also strongly supported by pilot studies) that the targeted manipulation of the ECS (aiming to normalize the unwanted skin cell growth, sebum production and skin inflammation) might be beneficial in a multitude of human skin diseases. However, to predict the real therapeutic potential and translate the exciting preclinical observations discussed earlier into clinical practice, numerous important questions should carefully be addressed (Box 2). Nevertheless, targeting the cutaneous ECS for therapeutic gain remains an intriguing and provocative possibility warranting future studies.
Outstanding questions
Are all members of the ECS functionally expressed in the human skin and appendages?
How do various endogenous mechanisms (e.g. hormones, cytokines) that were shown to be involved in the control of human skin homeostasis regulate the activity of ECS?
Is there any crosstalk between the ECS and the endovanilloid system in the human skin?
Is there any alteration in the expression levels and patterns of elements of the ECS in various human skin diseases?
Acknowledgements
This publication was supported by Intramural Research Program of the National Institutes of Health/National Institute on Alcohol Abuse and Alcoholism (www.nih.gov; www.niaaa.nih.gov; to P.P.) and by the Hungarian Scientific Research Fund (www.otka.hu; OTKA 63153 to T.B.).
Glossary
- Acne vulgaris (or acne)
a common, multi-etiological skin condition characterized by increased sebum production and inflammation of the sebaceous glands; acne can be induced and/or aggravated, for example, by stress, endocrine conditions (adolescence), immune/inflammatory factors, bacterial infection of the skin, diet, and so on.
- a (N-arachidonoylethanolamine) and 2-AG (2-arachidonoylglycerol)
the two most studied endocannabinoids, which exert biological effects similar to marijuana via activation of two main cannabinoid receptors.
- Alopecia
a type of pathological hair loss affecting mostly the scalp; most common forms of alopecia: universalis, areata, androgenetic.
- Cannabinoid receptors
G-protein-coupled receptors that bind to and mediate the effects of cannabinoids.
- Cannabinoids
as a broader definition, cannabinoids refer to a group of substances that are structurally related to Δ9-tetrahydrocannabinol (THC), that bind to cannabinoid receptors, or that modulate the activity of the endocannabinoid system. Cannabinoids can be divided to various classes: ‘phytocannabinoids’ occuring in the cannabis plant; ‘endogenous cannabinoids’ produced in the body; and ‘synthetic cannabinoids’ chemically synthesized in a laboratory to target cannabinoid receptors and/or enzymes involved in the production or metabolism of endocannabinoids.
- Dermatitis
a universal term describing inflammation of the skin; as most skin diseases, dermatitis can be induced by various factors such as, for example, allergens (allergic dermatitis), infections, eczema (atopic dermatitis), external compounds (contact dermatitis) and so on.
- Effluvium (or telogen effluvium)
a form of alopecia characterized by diffuse hair shedding.
- Endocannabinoid system (ECS)
it includes endocannabinoids, the enzymes involved in the biosynthesis or metabolism, and their two G-protein-coupled cannabinoid receptors, CB1 and CB2, which are present in virtually all tissues.
- Endocannabinoids
bioactive lipid mediators produced in virtually all cell types and organs of the body, which exert biological effects similar to those of marijuana. The most extensively studied endocannabinoids are AEA and 2-AG.
- Hair cycle
a life-long regeneration program of the hair follicles controlled by various factors; the hair cycle can be divided to three major phases: anagen (growth), catagen (regression or involution) and telogen (resting or quiescence).
- Hirsutisms
is excessive and increased hair growth (especially in women) on body regions where the occurrence of hair normally is minimal or absent.
- Orthodromic, antidromic
in a neuron, an orthodromic impulse (i.e. an action potential) runs along an axon in its normal direction, that is, away from the soma towards the axon ending. An antidromic impulse in an axon refers to conduction of the action potentials opposite to the normal, orthodromic direction (i.e. from the axon terminal to the soma).
- Phytocannabinoids
cannabinoids that are isolated from the plant Cannabis sativa; the most known phytocannabinoid is THC and cannabidiol.
- Pilosebaceous unit
consists of the hair shaft, the hair follicle, the sebaceous gland and the erector pili muscle, which causes the hair to stand up when it contracts.
- Psoriasis
is a chronic, autoimmune skin disease that is characterized by epidermal hyperproliferation and skin inflammation.
- Seborrhea (or seborrhoeic dermatitis)
an inflammatory skin condition that particularly affects the sebaceous-gland-enriched areas of the skin; similar to acne, multiple factors are listed in its etiology.
- Sebum
a lipid-enriched, oily exocrine product of the sebaceous glands; sebum has various function such as waterproof-barrier formation, anti-microbial activity, transport, thermoregulation and so on.
- Systemic sclerosis (scleroderma)
a chronic autoimmune disease characterized by diffuse fibrosis (accumulation of connective tissue), degenerative changes, and vascular abnormalities in the skin, joints and internal organs.
- THC
the main active ingredient of the plant Cannabis sativa, which predominantly exerts its physiological effects via two main G-protein-coupled cannabinoid receptors.
- TRPV1
transient receptor potential cation channel, subfamily V, member 1; also referred as vanilloid receptor 1 (VR1).
References
- 1.Roosterman D, et al. Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol. Rev. 2006;86:1309–1379. doi: 10.1152/physrev.00026.2005. [DOI] [PubMed] [Google Scholar]
- 2.Paus R, et al. Frontiers in pruritus research: scratching the brain for more effective itch therapy. J. Clin. Invest. 2006;116:1174–1186. doi: 10.1172/JCI28553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paus R, et al. Neuroimmunoendocrine circuitry of the ‘brain-skin connection’. Trends Immunol. 2006;27:32–39. doi: 10.1016/j.it.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 4.Ansel JC, et al. Interactions of the skin and nervous system. J. Investig. Dermatol. Symp. Proc. 1997;2:23–26. doi: 10.1038/jidsymp.1997.6. [DOI] [PubMed] [Google Scholar]
- 5.Luger TA. Neuromediators–a crucial component of the skin immune system. J. Dermatol. Sci. 2002;30:87–93. doi: 10.1016/s0923-1811(02)00103-2. [DOI] [PubMed] [Google Scholar]
- 6.Steinhoff M, et al. Proteinase-activated receptors: transducers of proteinase-mediated signaling in inflammation and immune response. Endocr. Rev. 2005;26:1–43. doi: 10.1210/er.2003-0025. [DOI] [PubMed] [Google Scholar]
- 7.Steinhoff M, et al. Neurophysiological, neuroimmunological, and neuroendocrine basis of pruritus. J. Invest. Dermatol. 2006;126:1705–1718. doi: 10.1038/sj.jid.5700231. [DOI] [PubMed] [Google Scholar]
- 8.Mechoulam R, et al. Endocannabinoids. Eur. J. Pharmacol. 1998;359:1–18. doi: 10.1016/s0014-2999(98)00649-9. [DOI] [PubMed] [Google Scholar]
- 9.Howlett AC, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- 10.Di Marzo V. Endocannabinoids: synthesis and degradation. Rev. Physiol. Biochem. Pharmacol. 2008;160:1–24. doi: 10.1007/112_0505. [DOI] [PubMed] [Google Scholar]
- 11.Pertwee RG. Pharmacological actions of cannabinoids. Handb. Exp. Pharmacol. 2005:1–51. doi: 10.1007/3-540-26573-2_1. [DOI] [PubMed] [Google Scholar]
- 12.Mackie K. Cannabinoid receptors as therapeutic targets. Annu. Rev. Pharmacol. Toxicol. 2006;46:101–122. doi: 10.1146/annurev.pharmtox.46.120604.141254. [DOI] [PubMed] [Google Scholar]
- 13.Pacher P, et al. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol. Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Marzo V. Targeting the endocannabinoid system: to enhance or reduce? Nat. Rev. Drug Discov. 2008;7:438–455. doi: 10.1038/nrd2553. [DOI] [PubMed] [Google Scholar]
- 15.Pertwee RG. The therapeutic potential of drugs that target cannabinoid receptors or modulate the tissue levels or actions of endocannabinoids. AAPS J. 2005;7:E625–E654. doi: 10.1208/aapsj070364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pertwee RG. Emerging strategies for exploiting cannabinoid receptor agonists as medicines. Br. J. Pharmacol. 2009;156:397–411. doi: 10.1111/j.1476-5381.2008.00048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein TW. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat. Rev. Immunol. 2005;5:400–411. doi: 10.1038/nri1602. [DOI] [PubMed] [Google Scholar]
- 18.Mechoulam R, et al. Endocannabinoids and neuroprotection. Sci. STKE. 2002;2002:RE5. doi: 10.1126/stke.2002.129.re5. [DOI] [PubMed] [Google Scholar]
- 19.Centonze D, et al. The endocannabinoid system in targeting inflammatory neurodegenerative diseases. Trends Pharmacol. Sci. 2007;28:180–187. doi: 10.1016/j.tips.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 20.Baker D, Pryce G. The endocannabinoid system and multiple sclerosis. Curr. Pharm. Des. 2008;14:2326–2336. doi: 10.2174/138161208785740036. [DOI] [PubMed] [Google Scholar]
- 21.Izzo AA, Camilleri M. Emerging role of cannabinoids in gastrointestinal and liver diseases: basic and clinical aspects. Gut. 2008;57:1140–1155. doi: 10.1136/gut.2008.148791. [DOI] [PubMed] [Google Scholar]
- 22.Wright KL, et al. Cannabinoid CB2 receptors in the gastrointestinal tract: a regulatory system in states of inflammation. Br. J. Pharmacol. 2008;153:263–270. doi: 10.1038/sj.bjp.0707486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mallat A, Lotersztajn S. Endocannabinoids and liver disease. I. Endocannabinoids and their receptors in the liver. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;294:G9–G12. doi: 10.1152/ajpgi.00467.2007. [DOI] [PubMed] [Google Scholar]
- 24.Pacher P, Gao B. Endocannabinoids and liver disease. III. Endocannabinoid effects on immune cells: implications for inflammatory liver diseases. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;294:G850–G854. doi: 10.1152/ajpgi.00523.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mach F, et al. Cannabinoid receptors in acute and chronic complications of atherosclerosis. Br. J. Pharmacol. 2008;153:290–298. doi: 10.1038/sj.bjp.0707517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pacher P, et al. Modulation of the endocannabinoid system in cardiovascular disease: therapeutic potential and limitations. Hypertension. 2008;52:601–607. doi: 10.1161/HYPERTENSIONAHA.105.063651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Marzo V. The endocannabinoid system in obesity and type 2 diabetes. Diabetologia. 2008;51:1356–1367. doi: 10.1007/s00125-008-1048-2. [DOI] [PubMed] [Google Scholar]
- 28.Engeli S. Dysregulation of the endocannabinoid system in obesity. J. Neuroendocrinol. 2008;20 Suppl. 1:110–115. doi: 10.1111/j.1365-2826.2008.01683.x. [DOI] [PubMed] [Google Scholar]
- 29.Pacher P, Hasko G. Endocannabinoids and cannabinoid receptors in ischaemia-reperfusion injury and preconditioning. Br. J. Pharmacol. 2008;153:252–262. doi: 10.1038/sj.bjp.0707582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guzman M. Cannabinoids: potential anticancer agents. Nat. Rev. Cancer. 2003;3:745–755. doi: 10.1038/nrc1188. [DOI] [PubMed] [Google Scholar]
- 31.Walker JM, Hohmann AG. Cannabinoid mechanisms of pain suppression. Handb. Exp. Pharmacol. 2005:509–554. doi: 10.1007/3-540-26573-2_17. [DOI] [PubMed] [Google Scholar]
- 32.Mechoulam R, Hanus L. A historical overview of chemical research on cannabinoids. Chem. Phys. Lipids. 2000;108:1–13. doi: 10.1016/s0009-3084(00)00184-5. [DOI] [PubMed] [Google Scholar]
- 33.Ahn K, et al. Enzymatic pathways that regulate endocannabinoid signaling in the nervous system. Chem. Rev. 2008;108:1687–1707. doi: 10.1021/cr0782067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J, et al. Multiple pathways involved in the biosynthesis of anandamide. Neuropharmacology. 2008;54:1–7. doi: 10.1016/j.neuropharm.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howlett AC. Cannabinoid receptor signaling. Handb. Exp. Pharmacol. 2005:53–79. doi: 10.1007/3-540-26573-2_2. [DOI] [PubMed] [Google Scholar]
- 36.Casanova ML, et al. Inhibition of skin tumor growth and angiogenesis in vivo by activation of cannabinoid receptors. J. Clin. Invest. 2003;111:43–50. doi: 10.1172/JCI16116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stander S, et al. Distribution of cannabinoid receptor 1 (CB1) and 2 (CB2) on sensory nerve fibers and adnexal structures in human skin. J. Dermatol. Sci. 2005;38:177–188. doi: 10.1016/j.jdermsci.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 38.Ibrahim MM, et al. CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc. Natl. Acad. Sci. U. S. A. 2005;102:3093–3098. doi: 10.1073/pnas.0409888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blazquez C, et al. Cannabinoid receptors as novel targets for the treatment of melanoma. FASEB J. 2006;20:2633–2635. doi: 10.1096/fj.06-6638fje. [DOI] [PubMed] [Google Scholar]
- 40.Karsak M, et al. Attenuation of allergic contact dermatitis through the endocannabinoid system. Science. 2007;316:1494–1497. doi: 10.1126/science.1142265. [DOI] [PubMed] [Google Scholar]
- 41.Telek A, et al. Inhibition of human hair follicle growth by endo- and exocannabinoids. FASEB J. 2007;21:3534–3541. doi: 10.1096/fj.06-7689com. [DOI] [PubMed] [Google Scholar]
- 42.Dobrosi N, et al. Endocannabinoids enhance lipid synthesis and apoptosis of human sebocytes via cannabinoid receptor-2-mediated signaling. FASEB J. 2008;22:3685–3695. doi: 10.1096/fj.07-104877. [DOI] [PubMed] [Google Scholar]
- 43.Maccarrone M, et al. The endocannabinoid system in human keratinocytes. Evidence that anandamide inhibits epidermal differentiation through CB1 receptor-dependent inhibition of protein kinase C, activation protein-1, and transglutaminase. J. Biol. Chem. 2003;278:33896–33903. doi: 10.1074/jbc.M303994200. [DOI] [PubMed] [Google Scholar]
- 44.Paradisi A, et al. Anandamide regulates keratinocyte differentiation by inducing DNA methylation in a CB1 receptor-dependent manner. J. Biol. Chem. 2008;283:6005–6012. doi: 10.1074/jbc.M707964200. [DOI] [PubMed] [Google Scholar]
- 45.Biro T, et al. The endocannabinoid/endovanilloid anandamide regulates proliferation and apoptosis of human keratinocytes via novel signaling interactions between vanilloid receptor-1 (TRPV1) and cannabinoid receptor-1 (CB1) J. Invest. Dermatol. 2006;126:95. [Google Scholar]
- 46.Calignano A, et al. Control of pain initiation by endogenous cannabinoids. Nature. 1998;394:277–281. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- 47.Berdyshev EV, et al. Stress-induced generation of N-acylethanolamines in mouse epidermal JB6 P+ cells. Biochem. J. 2000;346:369–374. [PMC free article] [PubMed] [Google Scholar]
- 48.Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol. Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- 49.Stander S, et al. Expression of vanilloid receptor subtype 1 in cutaneous sensory nerve fibers, mast cells, and epithelial cells of appendage structures. Exp. Dermatol. 2004;13:129–139. doi: 10.1111/j.0906-6705.2004.0178.x. [DOI] [PubMed] [Google Scholar]
- 50.Denda M, et al. Immunoreactivity of VR1 on epidermal keratinocyte of human skin. Biochem. Biophys. Res. Commun. 2001;285:1250–1252. doi: 10.1006/bbrc.2001.5299. [DOI] [PubMed] [Google Scholar]
- 51.Bodo E, et al. A hot new twist to hair biology: involvement of vanilloid receptor-1 (VR1/TRPV1) signaling in human hair growth control. Am. J. Pathol. 2005;166:985–998. doi: 10.1016/S0002-9440(10)62320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Toth BI, et al. Transient receptor potential vanilloid-1 signaling inhibits differentiation and activation of human dendritic cells. FEBS Lett. 2009;583:1619–1624. doi: 10.1016/j.febslet.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 53.Wilkinson JD, Williamson EM. Cannabinoids inhibit human keratinocyte proliferation through a non-CB1/CB2 mechanism and have a potential therapeutic value in the treatment of psoriasis. J. Dermatol. Sci. 2007;45:87–92. doi: 10.1016/j.jdermsci.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 54.Srivastava BK, et al. Hair growth stimulator property of thienyl substituted pyrazole carboxamide derivatives as a cb1 receptor antagonist with in vivo antiobesity effect. Bioorg. Med. Chem. Lett. 2009;19:2546–2550. doi: 10.1016/j.bmcl.2009.03.046. [DOI] [PubMed] [Google Scholar]
- 55.Zheng D, et al. The cannabinoid receptors are required for ultraviolet-induced inflammation and skin cancer development. Cancer Res. 2008;68:3992–3998. doi: 10.1158/0008-5472.CAN-07-6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sarfaraz S, et al. Cannabinoids for cancer treatment: progress and promise. Cancer Res. 2008;68:339–342. doi: 10.1158/0008-5472.CAN-07-2785. [DOI] [PubMed] [Google Scholar]
- 57.Dalle Carbonare M, et al. A saturated N-acylethanolamine other than N-palmitoyl ethanolamine with anti-inflammatory properties: a neglected story. J. Neuroendocrinol. 2008;20 Suppl. 1:26–34. doi: 10.1111/j.1365-2826.2008.01689.x. [DOI] [PubMed] [Google Scholar]
- 58.Di Marzo V, et al. Palmitoylethanolamide inhibits the expression of fatty acid amide hydrolase and enhances the anti-proliferative effect of anandamide in human breast cancer cells. Biochem. J. 2001;358:249–255. doi: 10.1042/0264-6021:3580249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Costa B, et al. The endogenous fatty acid amide, palmitoylethanolamide, has anti-allodynic and anti-hyperalgesic effects in a murine model of neuropathic pain: involvement of CB1, TRPV1 and PPARgamma receptors and neurotrophic factors. Pain. 2008 doi: 10.1016/j.pain.2008.06.003. (in press) [DOI] [PubMed] [Google Scholar]
- 60.LoVerme J, et al. The search for the palmitoylethanolamide receptor. Life Sci. 2005;77:1685–1698. doi: 10.1016/j.lfs.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 61.Oka S, et al. Involvement of the cannabinoid CB2 receptor and its endogenous ligand 2-arachidonoylglycerol in oxazolone-induced contact dermatitis in mice. J. Immunol. 2006;177:8796–8805. doi: 10.4049/jimmunol.177.12.8796. [DOI] [PubMed] [Google Scholar]
- 62.Ueda Y, et al. Involvement of cannabinoid CB2 receptors in the IgE-mediated triphasic cutaneous reaction in mice. Life Sci. 2007;80:414–419. doi: 10.1016/j.lfs.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 63.Ueda Y, et al. Involvement of cannabinoid CB2 receptor-mediated response and efficacy of cannabinoid CB(2) receptor inverse agonist, JTE-907, in cutaneous inflammation in mice. Eur. J. Pharmacol. 2005;520:164–171. doi: 10.1016/j.ejphar.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 64.Akhmetshina A, et al. The cannabinoid receptor CB2 exerts antifibrotic effects in experimental dermal fibrosis. Arthritis Rheum. 2009;60:1129–1136. doi: 10.1002/art.24395. [DOI] [PubMed] [Google Scholar]
- 65.Stander S, et al. Hautarzt. 2006;57:801–807. doi: 10.1007/s00105-006-1180-1. [DOI] [PubMed] [Google Scholar]
- 66.Ashton JC, Milligan ED. Cannabinoids for the treatment of neuropathic pain: clinical evidence. Curr. Opin. Investig. Drugs. 2008;9:65–75. [PubMed] [Google Scholar]
- 67.Jhaveri MD, et al. Endocannabinoid metabolism and uptake: novel targets for neuropathic and inflammatory pain. Br. J. Pharmacol. 2007;152:624–632. doi: 10.1038/sj.bjp.0707433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Valiveti S, et al. Transdermal delivery of the synthetic cannabinoid WIN 55,212-2: in vitro/in vivo correlation. Pharm. Res. 2004;21:1137–1145. doi: 10.1023/b:pham.0000032999.31948.2e. [DOI] [PubMed] [Google Scholar]
- 69.Lodzki M, et al. Cannabidiol-transdermal delivery and anti-inflammatory effect in a murine model. J. Control. Release. 2003;93:377–387. doi: 10.1016/j.jconrel.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 70.Pulvirenti N, et al. Topical adelmidrol 2% emulsion, a novel aliamide, in the treatment of mild atopic dermatitis in pediatric subjects: a pilot study. Acta Dermatovenerol. Croat. 2007;15:80–83. [PubMed] [Google Scholar]
- 71.Lambert DM. Allergic contact dermatitis and the endocannabinoid system: from mechanisms to skin care. ChemMedChem. 2007;2:1701–1702. doi: 10.1002/cmdc.200700168. [DOI] [PubMed] [Google Scholar]