Abstract
Healthy aging has been shown to modulate the neural circuitry underlying simple declarative memory; however, the functional impact of negative stimulus valence on these changes has not been fully investigated. Using BOLD fMRI, we explored the effects of aging on behavioral performance, neural activity, and functional coupling during the encoding and retrieval of novel aversive and neutral scenes. Behaviorally, there was a main effect of valence with better recognition performance for aversive greater than neutral stimuli in both age groups. There was also a main effect of age with better recognition performance in younger participants compared to older participants. At the imaging level, there was a main effect of valence with increased activity in the medial-temporal lobe (amygdala and hippocampus) during both encoding and retrieval of aversive relative to neutral stimuli. There was also a main effect of age with older participants showing decreased engagement of medial-temporal lobe structures and increased engagement of prefrontal structures during both encoding and retrieval sessions. Interestingly, older participants presented with relatively decreased amygdalar–hippocampal coupling and increased amygdalar– prefrontal coupling when compared to younger participants. Furthermore, older participants showed increased activation in prefrontal cortices and decreased activation in the amygdala when contrasting the retrieval of aversive and neutral scenes. These results suggest that although normal aging is associated with a decline in declarative memory with alterations in the neural activity and connectivity of brain regions underlying simple declarative memory, memory for aversive stimuli is relatively better preserved than for neutral stimuli, possibly through greater compensatory prefrontal cortical activity.
INTRODUCTION
Senescence is associated with alterations in the structureand function of neural circuits underlying sensorimotor, cognitive, and emotional processes. Converging evidence indicates that prominent changes occur across the human life span in declarative memory as well as in emotional information processing. However, the impact of an interaction between these two systems on age-related changes in memory function has been relatively unexplored.
Senescence-related deficits in declarative memory have been well characterized. Older participants show decreased recollection accuracy and increased reaction time (RT) during the retrieval of previously encoded word lists, faces, and complex scenes (Park et al., 1996; Parkin & Walter, 1992; Bartlett, Leslie, Tubbs, & Fulton, 1989). This has been observed in the context of stimuli with neutral content. More recently, researchers have begun to explore the impact of aging on emotional memory. Although in younger participants, memory for previously encoded items with aversive content has consistently been shown to be higher than for those with neutral content (for review, see LaBar & Cabeza, 2006; Hamann, 2001), reports related to the impact of advancing age on emotional memory have been less consistent. Some studies show that similar to young participants, older participants maintain an enhancement in performance for stimuli with aversive content (Denburg, Buchanan, Tranel, & Adolphs, 2003), particularly to highly arousing aversive stimuli (Kensinger, 2008), and some studies indicate negligible or no effect of aversive stimulus content on recognition performance with advancing age (Comblain, D’Argembeau, Van der Linden, & Aldenhoff, 2004; Charles, Mather, & Carstenson, 2003).
Converging evidence from lesion studies (Adolphs, Cahill, Schul, & Babinsky, 1997) and neuroimaging studies in young healthy participants (Smith, Henson, Dolan, & Rugg, 2004; Hamann, 2001) implicates the amygdala and the hippocampus to be critical structures for emotional memory. Although numerous studies have explored the neurobiology underlying age-related changes in declarative memory and emotional information processing independent of each other, to date, the effects of aversive stimulus content on age-related changes in emotional memory remain relatively unexplored. Converging evidence indicates that the hippocampal formation, a key structure underlying declarative memory processing, undergoes both structural and physiological changes with advancing age, both in healthy as well as in pathological states such as mild cognitive impairment and Alzheimer’s disease (Celone et al., 2006; Buckner et al., 2005; Dickerson et al., 2004). The majority of neuroimaging studies investigating the effect of aging on hippocampal function during episodic memory show relatively decreased activity in healthy older participants when compared to younger participants during both the encoding and retrieval of neutral items (Daselaar, Fleck, Dobbins, Madden, & Cabeza, 2006; Dennis, Daselaar, & Cabeza, 2006; Grady, McIntosh, & Craik, 2005; Cabeza et al., 2004; Daselaar, Veltman, Rombouts, Raaijmakers, & Jonkers, 2003; Grady, McIntosh, Rajah, Beig, & Craik, 1999).
Studies related to the effect of aging on emotional information processing show results ranging from decreased activity in the amygdala and the hippocampus during the perceptual processing of aversive stimuli (Tessitore et al., 2005; Mather et al., 2004; Gunning-Dixon et al., 2003; Iidaka et al., 2002), to comparable levels of amygdala activity across the life span (St.Jacques, Dolcos, & Cabeza 2008; Wright, Wedig, Williams, Rauch, & Alberts, 2006), and increased amygdala activity for positive stimuli (Mather et al., 2004). At the structural level, whereas morphometric studies using high-resolution structural MRI techniques show significant age-related decreases in hippocampal volume, studies related to the effect of aging on structural changes in the amygdala have either shown nonsignificant or mild volumetric shrinking with normal aging (Wright et al., 2006; Allen, Bruss, Brown, & Damasio, 2005; Raz et al., 2004; Good et al., 2001; Jernigan et al., 2001). In general, evidence thus far indicates age-related functional and/or structural changes in brain regions underlying emotional memory.
Studies have also found that older participants tend to activate the prefrontal (both ventrolateral and dorsolateral) cortex to a greater extent than young participants during episodic memory. This has been posited as a compensatory shift in processing to overcome a deficit in hippocampal function (Grady et al., 2005; Cabeza et al., 2004). The support for a compensatory role of prefrontal cortices also comes from studies that explored correlations between neural activity and performance as well as functional connectivity between the hippocampus and the prefrontal cortices. Grady, Bernstein, Beig, and Siegenthaler (2002) and Grady et al. (1995) showed that dorsolateral prefrontal cortex (DLPFC) activity in older adults significantly predicted retrieval performance, whereas in younger participants this behavior was predicted primarily by hippocampal activity. Functional connectivity approaches further support a compensatory role of the DLPFC by illustrating greater connectivity between the hippocampus and the DLPFC in older participants compared to younger participants during both the encoding and retrieval phases (Daselaar et al., 2006; Gutchess et al., 2005; Grady, McIntosh, & Craik, 2003; Cabeza et al., 1997). It should be noted that these age-related neurophysiological changes were observed for memory of items with neutral content, and whether a similar mechanism extends to emotional memory as well is yet to be explored.
Although there is growing evidence related to the effect of advancing age on emotional memory at the behavioral level as well as morphometric and functional changes in the neural structures underlying this cognitive process (vide supra), to date, the effect of advancing age on functional activation and connectivity of the brain regions within this network during both memory encoding as well as retrieval has been relatively unexplored. To this end, in the current study we investigated age-related differences in both the behavior and neural circuitry of declarative memory using neutral and aversive stimuli. Based on evidence from prior behavioral and neuroimaging studies of healthy aging (vide supra), we make the following hypotheses: (1) older participants will show decreased memory performance compared to younger participants; (2) similar to younger participants and consistent with prior behavioral reports related to the effect of aging on emotional memory (Kensinger, 2008; Denburg et al., 2003; Kensinger, Brierley, Medford, Growdon, & Corkin, 2002), older participants will maintain better memory for highly arousing, aversive stimuli; (3) older participants will show decreased activation and altered functional connectivity between regions critical to emotional memory processing including the hippocampus and the amygdala in response to aversive stimuli; and (4) older participants will engage in alternative strategies such as greater DLPFC recruitment and greater functional coupling between the amygdala and the DLPFC to maintain better memory for aversive stimuli. To test these hypotheses, we will (1) compare recognition performance for aversive and neutral pictures between older and younger participants; (2) compare hippocampal, amygdalar, and prefrontal activity during encoding and retrieval of both neutral and aversive stimuli between young and older participants; (3) evaluate the effects of aging on the difference in neural response between aversive and neutral stimuli; (4) assess functional coupling of the hippocampus with the other regions posited to play a role in emotional processing, namely, the amygdala and the DLPFC; and (5) assess the relationship between age-related differences in performance and changes in functional activation and connectivity of the key regions within this network.
METHODS
Participants
Thirty young healthy volunteers [mean age ± standard deviation (SD) = 25.6 ± 3.5 years] and 30 older healthy volunteers (mean age ± SD = 61.2 ± 4.6 years) who had undergone extensive clinical evaluations participated in this study. Recruitment evaluation included a complete history and physical examination, a detailed neurological exam, the Structured Clinical Interview for DSM-IV (SCID; First, Spitzer, Gibbon, & Williams, 1994), WAIS-R, a neuropsychological assessment, and a clinical brain MRI scan. Exclusion criteria included a current or past history of neurological or psychiatric disorders, medical treatment pertaining to cerebral metabolism or blood flow, or history of drug abuse. The two groups were matched for handedness (25 right-handers in each group as measured by the Edinburgh Handedness Inventory; Oldfield, 1971), sex (16 men in each group), race (29 Caucasians, 1 Asian in each group), and intelligence quotient (IQ) [obtained using the Weschler Adult Intelligence Scale; older group, mean ± SD = 116 ± 8.1; younggroup, mean ± SD = 116.0 ± 7.4;F(1,59) = 0.08,p = .78]. Older participants also underwent a thorough neuropsychological assessment to evaluate cognitive status and exclude pathologic cognitive decline (see Table 1). A secondary analysis was performed in participants that were also matched for performance in addition to the above demographics across both groups. This analysis consisted of 32 participants (16 young and 16 older) from the original 60 that were matched for sex (8 men in each group), handedness (13 right-handers in each group), race (1 Asian in each group), and IQ [older group, mean ± SD = 117.9 ± 7.2; young group, mean ± SD = 116.0 ± 7.5;F(1, 31) = 0.55; p = .46].
Table 1.
Neuropsychological Status of Older Participants
| Neuropsychology/Neurological Test | M (SD) | n |
|---|---|---|
| Cognitive Status | ||
| Mini-Mental State Examination (MMSE) | 30.0 (0.2)a | 22 |
| Executive Composite | ||
| Trail Making Test B (sec) | 72.1 (30.6)b | 30 |
| Word Fluency Test | 48.5 (11.9)c | 29 |
| Category Fluency Test | 54.3 (11.1)c | 29 |
| Letter and Number Sequencing | 11.9 (2.4)b | 30 |
| WAIS-IQ | 116.6 (8.1)b | 30 |
| Memory Composite | ||
| WMS-R Logical Memory Immediate Recall | 12.4 (2.6)d | 26 |
| WMS-R Logical Memory Delayed Recall | 13.8 (2.5)d | 26 |
| Processing Seed Composite | ||
| Trail Making Test A (sec) | 32.2 (13.6)b | 30 |
22 participants.
30 participants.
29 participants.
27 participants.
All participants underwent fMRI while performing an incidental encoding and memory retrieval task. All participants gave written informed consent, which was approved by the National Institute of Mental Health Institutional Review Board.
Experimental Paradigm
Each participant underwent BOLD fMRI during the encoding and retrieval of aversive and neutral scenes selected from the International Affective Picture System (Lang, Bradley, & Cuthbert, 2005). For both the encoding and retrieval sessions, the scenes were presented in a blocked fashion with two blocks of aversive/neutral scenes alternating with blocks of resting state. During experimental blocks, six scenes of similar valence (neutral or aversive) were presented serially to participants for 3 sec each. A Student’s t test revealed that the selected aversive scenes were rated significantly less pleasant and more arousing than the selected neutral scenes as determined by standardized ratings described in Lang et al. (2005) [pleasure (mean ± SD; aversive = 3.1 ± 0.9; neutral = 5.8 ± 1.1); arousal (mean ± SD; aversive = 5.9.1 ± 0.7; neutral = 3.03 ± 0.8); p < .0001 for each measure]. In a recent study, Backs, da Silva, and Han (2005) reported that there was no significant difference in the ratings of older participants (mean age ± SD: 66.3 ± 5.6 years) compared to younger participants (mean age ± SD: 20.0 ± 2.3 years) when rating for negatively valenced stimuli from the International Affective Picture System picture set obtained by Lang et al. (2005). During resting blocks, participants were asked to attend to a fixation cross presented in the center of the screen for 18 sec. These fixation blocks were treated as a baseline in the fMRI analyses. During the encoding session, participants were instructed to determine whether each picture depicted an “indoor” or “outdoor” scene. During the retrieval session, participants were instructed to determine whether the scene presented was seen during the encoding session; the participants were instructed to press the right button for scenes seen before during the encoding session (i.e., “old”) or press the left button for scenes not seen during the encoding session (i.e., “new”). In each retrieval session, half the scenes were old (i.e., presented during the encoding session), whereas the other half were new (i.e., not presented during the encoding session). Each session (encoding or retrieval) consisted of 17 blocks (four aversive, four neutral, and nine rest conditions). Participants completed the entire encoding session before beginning the retrieval session after a brief delay (about 2 min). Before each session, participants were given verbal instructions, and each run was preceded by a brief 2-sec instruction screen with a total scan time of 5 min 40 sec. For the encoding session, the presentation of “indoor” and “outdoor” scenes, and for the retrieval session, the presentation of “old” and “new” scenes, was counterbalanced within each block. In addition, the presentation order of aversive and neutral blocks was counterbalanced across participants. All participants responded with button presses using their dominant hand. Behavioral accuracy and RTs were recorded. This task has been shown to reliably engage the hippocampus as well as inferotemporal, parietal, and frontal cortices in healthy volunteers (Bertolino et al., 2006; Meyer-Lindenberg et al., 2006; Hariri et al., 2003).
fMRI Acquisition
BOLD fMRI was performed on a General Electric 3-Tesla Signa scanner (Milwaukee, WI) using a gradient-echo, echo-planar imaging sequence. Twenty-four axial slices covering the whole cerebrum and the majority of the cerebellum were acquired in an interleaved sequence with 4 mm thickness and a 1-mm gap (TR/TE = 2000/ 28 msec, FOV = 24 cm, matrix = 64 * 64). Scanning parameters were selected to optimize the quality of the BOLD signal while maintaining a sufficient number of slices to acquire whole-brain data.
Data Analysis
Behavioral Analysis
One-way factorial analyses of variance (ANOVAs) were performed on the behavioral data to explore the effects of age and stimulus valence on accuracy (ACC) and RT for both encoding and retrieval sessions. Two-way ANOVAs were also performed to assess an Age by Valence interaction on these measures. Statistical thresholds for significance were set at p <.05.
Functional Imaging Analysis
Image analysis was completed using SPM2 (www.fil.ion.ucl.ac.uk/spm). For each session (encoding and retrieval), subsequent images were realigned to the first image in the series to correct for head motion. These images were then spatially normalized to the MNI template using a fourth degree B-spline interpolation. Then the images were smoothed using an isotropic 8-mm3 full-width half-maximum kernel. Each individual dataset was then carefully screened for data quality using a variety of parameters including visual inspection for image artifacts, estimating indices for ghosting artifacts, signal-to-noise ratio across the time series, signal variance across individual sessions, and head motion (data from participants with head motion greater than 3 mm and/or head rotation greater than 2° were excluded).
For both the encoding and retrieval sessions, fMRI responses were modeled using the General Linear Model (GLM) with a canonical hemodynamic response function convolved to a boxcar function for the length of the block, normalized to the global signal across the whole brain, and temporally filtered to remove low-frequency signals (<84 Hz). Regressors were modeled for conditions of interest (for encoding session: aversive encoding and neutral encoding; for retrieval session: aversive retrieval and neutral retrieval) as well as six head motion regressors of no interest. Using this GLM model, individual t contrast maps were generated for contrasts of interest: aversive encoding > baseline, neutral encoding > baseline, aversive encoding > neutral encoding, aversive retrieval > baseline, neutral retrieval > baseline, and aversive retrieval > neutral retrieval.
Second-level random effects analyses were performed using one-sample t tests to explore the main effect of task for the encoding aversive, encoding neutral, retrieval aversive, and retrieval neutral conditions. For the encoding session, the t contrast option under an ANOVA in SPM2 was performed to assess the main effect of stimulus valence [(older aversive + young aversive) > (older neutral + young neutral)], the effect of age [(young aversive +young neutral) > (older aversive + older neutral) and (older aversive + older neutral) > (young aversive + young neutral)], and the effect of Age by Valence [young (aversive > neutral) > older (aversive > neutral); older (aversive > neutral) > young (aversive > neutral)]. For the retrieval session, to control for a significant difference in performance, the t contrast option under an analysis of covariance (ANCOVAs) in SPM2 using ACC and RT as covariates of no interest was performed to assess the effect of stimulus valence, the effect of age, and an effect of Age by Valence. All above ANOVAs were inclusively masked with conjunction maps of the effect of interest at p < .05, uncorrected.
Given the strong evidence for an important role of the amygdala during emotional memory processing (Dolcos, LaBar, & Cabeza, 2004b, 2005), a measure of functional connectivity was estimated to assess for residual brain connectivity between the amygdala and other brain regions after adjusting for task-related activity (Bertolino et al., 2006; Pezawas et al., 2005; Meyer-Lindenberg et al., 2001). This measure quantifies the covariation of neural activity between median activity (after mean signal and drift correction) of a seed in the amygdala and the rest of the voxels in the brain across the time series. Seed regions in the amygdala were constructed using a two-step process. First, a mask of significantly active voxels (p < .05, FDR-corrected) for the main effect of task was created separately for encoding and retrieval sessions across all participants. Then, seeds were constructed by determining each individual’s functionally active voxels (p < .05) within the above mask. Following this, individual connectivity maps (covariance maps) were created by correlating the time series of the amygdala with the time series of the voxels in the rest of the brain. Error, namely, the residual term in the GLM model, was used after adjusting for task effects and confounds (e.g., global signal and realignment parameters) to estimate functional coupling across brain regions (see Caclin & Fonlupt, 2006; Pezawas et al., 2005 for more details on this approach). Functional coupling estimated in this manner is thought to reflect the inherent connectivity between brain regions rather than correlations mediated by the task. This analysis was performed separately for both the encoding and retrieval sessions.
To assess correlations between functional data and behavior, simple regressions were performed using individual participant’s first-level contrast maps from the GLM and accuracy. For behavior–functional connectivity correlations, each individual’s connectivity values were normalized to the sample of the mean using a Fisher r to z transform before entering into the regression. Estimates of the weighted beta parameters and functional connectivity values were extracted from significant voxels (p < .05, uncorrected) within ROIs using MARSBAR toolbox (http://marsbar.sourceforge.net) and exported into STATISTICA 6 (www.statsoft.com) to calculate Pearson’s r for one-tailed analysis.
Statistical thresholds for all imaging analyses were set at p < .005 (uncorrected) within anatomical ROIs (see below) and p < .001 for all other regions. Results that survived p < .05, corrected for multiple comparisons (FDR-corrected, as described by Genovese, Lazar, & Nichols, 2002) are indicated within tables. All reported data were held to a cluster extent threshold of k > 5.
Given prior evidence of age-related changes in the circuits underlying episodic memory, ROIs of the hippo-campal formation (hippocampus/parahippocampus) and the amygdala were created using the Wake Forest University PICKATLAS.
RESULTS
Behavioral Results
During the encoding session, both older and young participants performed well with accuracy (young: ACC ± SD = 90.9 ± 7.9; older: ACC ± SD = 86.8 ± 9.7) (Figure 1). Although there was a significant difference [F(1, 59) = 6.6, p < .02] with young adults discriminating better than older adults, it should be noted that this was an incidental encoding task and the discrimination of indoor versus outdoor scenes was built into the task primarily to ensure that the participants were attending to the presented stimuli. There was no significant interaction between age and valence on encoding ACC [F(1, 58) = 3.5, p > .05]. There was a significant effect of valence with RTs for aversive stimuli being longer than for neutral stimuli [neutral: RT ± SD = 1266 ± 128; aversive: RT ± SD = 1576 ± 182; F(1, 59) = 116.0, p < .001]. Although not significant, older adults tended to have longer RTs [young: RT ± SD = 1387± 214 msec; older: RT ± SD = 1455 ± 224 msec; F(1, 59) = 2.875, p = .09]. There was no significant interaction between age and valence [F(1, 58) = 1.2, p > .28].
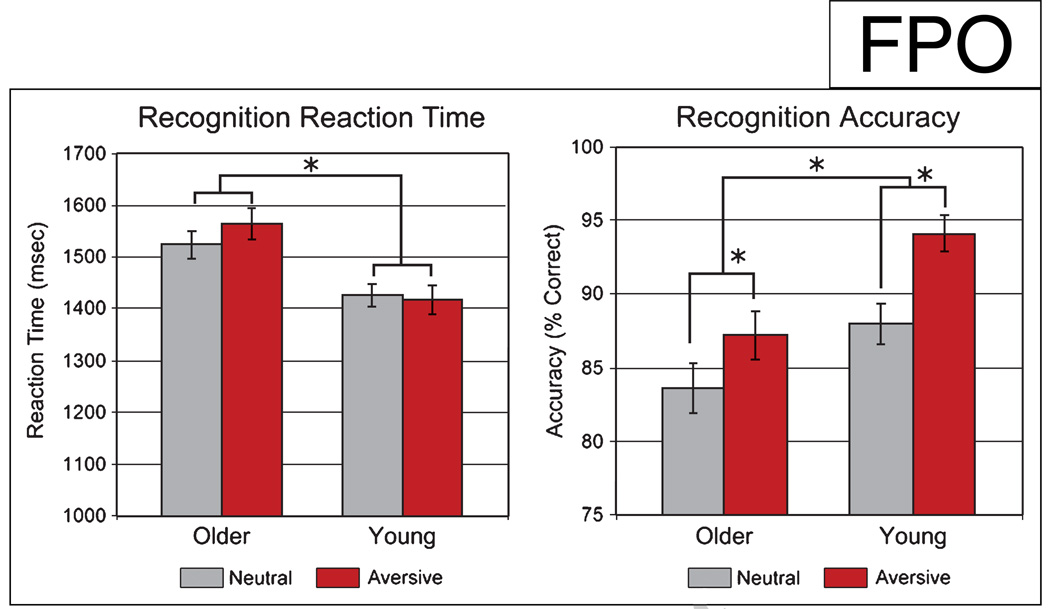
Figure 1.
Significantly lower performance as measured by RT and accuracy was seen in older participants when compared to the young participants. Aversive scene content increased subsequent recognition accuracy in both the young and older groups. Charts above depict RT (mean ± standard error) and ACC (% correct ± standard error) during scene recognition (*p < .001).
During the retrieval session, younger participants performed significantly better with greater accuracy [F(1, 59) = 14.2, p < .001] and faster RT [F(1, 59) = 21.7, p < .001] when compared to the older participants for both aversive and neutral stimuli (young: ACC ± SD = 91.1 ± 7.9, RT ± SD = 1421 ± 129 msec; older: ACC ± SD = 85.4 ± 9.2, RT ± SD = 1544 ± 159 msec). In both groups, recollection accuracy for aversive scenes was greater than for neutral scenes [F(1, 59) = 10.6, p < .002]; there was no difference in RT [F(1, 59) = 0.4, p = .33; neutral: ACC ± SD = 87.2 ± 9.1, RT ± SD = 1565 ± 168 msec; aversive: ACC ± SD = 94.1 ± 6.6, RT ± SD = 1416 ± 139 msec]. There was no significant Age × Stimulus valence interaction between age groups and stimulus valence for either ACC or RT [ACC: F(1, 58) = 0.7, p = .40; RT: F(1, 58) = 0.9, p = .33].
Imaging Results
Effect of Task [Older Aversive + Older Neutral + Young Aversive + Young Neutral > Rest]
Consistent with previous studies (Hariri et al., 2003; Schacter & Wagner, 1999), significant task-related activity was seen bilaterally in the hippocampal formation, the amygdala, the DLPFC (BA 46, 11, 30), the ventrolateral prefrontal cortex (VLPFC; BA 47, 45), the medial frontal cortex (BA 8), the visual cortices (BA 17, 18, 19), the parietal cortex (BA 7, 9), the basal ganglia and thalamus, and the right premotor/motor cortices (BA 4, 6) during the encoding session as well as the retrieval session for both aversive and neutral scenes.
Effect of Valence [(Older Aversive + Young Aversive) > (Older Neutral + Young Neutral)]
To investigate the effects of stimulus valence on the neural circuitry of simple declarative memory, aversive conditions were contrasted to the neutral conditions for both encoding and retrieval sessions (Table 2). This analysis revealed significantly greater activity in the amygdala bilaterally and the right hippocampus for aversive scenes when compared to neutral scenes during both the encoding and retrieval.
Table 2.
Comparison of Significant BOLD fMRI Responses between Aversive and Neutral Stimuli during Encoding and Retrieval Sessions
| Regions | Side | x | y | z | k | t Score |
|---|---|---|---|---|---|---|
| Encoding: Aversive > Neutral | ||||||
| Middle occipital gyrus | L | −49 | −68 | 9 | 189 | 10.2 |
| R | 53 | −72 | −8 | 313 | 11.6 | |
| Inferior frontal gyrus (VLPFC) |
L | −34 | 30 | −4 | 101 | 7.0 |
| R | 53 | 23 | −11 | 32 | 6.4 | |
| Superior frontal gyrus | L/R | 0 | 65 | 30 | 32 | 6.2 |
| L/R | 0 | 27 | 65 | 68 | 5.6 | |
| Amygdala* | L | −19 | 3 | −23 | 10 | 5.3 |
| R | 23 | 0 | −23 | 7 | 4.2 | |
| Hippocampus* | R | 27 | −15 | −19 | 25 | 4.4 |
| Retrieval: Aversive > Neutral | ||||||
| Middle temporal gyrus | L | −53 | −68 | 0 | 161 | 11.4 |
| Middle occipital gyrus | R | 53 | −72 | −8 | 250 | 11.1 |
| Middle frontal gyrus (DLPFC) |
R | 49 | 8 | 34 | 7 | 4 |
| Amygdala* | L | −19 | 0 | −23 | 13 | 6.2 |
| R | 23 | −4 | −19 | 12 | 5.4 | |
| Hippocampus* | R | 23 | −3 | −23 | 6 | 4.4 |
All listed clusters survive p < .05, FDR-correction (whole brain except regions denoted with an which represent regions that survived small-volume correction). Coordinates (x y z) are in Talairach space, and cluster size is represented by the value k.
Effect of Age [(Young Aversive + Young Neutral) > (Older Aversive + Older Neutral); (Older Aversive + Older Neutral) > (Young Aversive + Young Neutral)]
To investigate age-related changes in activity related to memory encoding and retrieval, functional activation maps for the encoding and retrieval sessions (including both aversive and neutral stimuli) were contrasted between the older and the young participants (Table 3A and B; Figure 2). During the encoding session, younger participants showed significantly greater activity bilaterally in the amygdala, the hippocampus, and the fusiform gyrus, whereas older participants showed significantly greater activity bilaterally in the parietal (BA 40, 7) and frontal cortices (BA 10, 11, 45, 46).
Table 3.
Comparison of Significant BOLD fMRI Responses between Young and Older Participants during (A) Encoding and (B) Retrieval
| Regions | Side | x | y | z | k | t Score |
|---|---|---|---|---|---|---|
| (A) | ||||||
| Encoding: Young > Older | ||||||
| Amygdala | L | −27 | 4 | −15 | 6 | 4.0 |
| R | 27 | 4 | −19 | 12 | 5.5 | |
| Hippocampus | L | −27 | −27 | −11 | 44 | 4.6 |
| R | 34 | −27 | −15 | 5 | 5.1 | |
| Lingual gyrus | L | −19 | −61 | 0 | 51 | 4.4 |
| Fusiform gyrus | R | 49 | 15 | −23 | 14 | 4.2 |
| R | 23 | −46 | −19 | 11 | 4.9 | |
| Superior temporal gyrus | L | −42 | 19 | −30 | 6 | 3.7 |
| R | 49 | 15 | −23 | 14 | 4.1 | |
| Encoding: Older > Young | ||||||
| Superior occipital gyrus | L | −34 | −84 | 27 | 12 | 4.2 |
| R | 48 | −84 | 30 | 48 | 5.6 | |
| Inferior parietal lobule | L | −38 | −53 | 45 | 120 | 5.4 |
| R | 38 | −61 | 42 | 14 | 3.7 | |
| Middle frontal gyrus (DLPFC) |
L | −42 | 0 | 61 | 34 | 3.7 |
| R | 46 | 42 | 23 | 16 | 4.2 | |
| R | 34 | 0 | 53 | 20 | 4.2 | |
| R | 53 | 0 | 42 | 5 | 3.0 | |
| Superior parietal lobule | L | −15 | −61 | 68 | 12 | 4 |
| R | 11 | −72 | 61 | 10 | 3.2 | |
| Superior frontal gyrus (VLPFC) |
R | 34 | 42 | −15 | 5 | 3.4 |
| Inferior frontal gyrus (VLPFC) |
L | −53 | 38 | 8 | 6 | 3 |
| (B) | ||||||
| Retrieval: Young > Older | ||||||
| Hippocampus | L | −27 | −30 | −8 | 74 | 6.0 |
| R | 34 | −19 | −19 | 51 | 6.0 | |
| Posterior cingulate | R | 11 | −61 | 8 | 8 | 5.1 |
| Cerebellum | R | 66 | 23 | −48 | 17 | 4.9 |
| Precuneus | R | 15 | −68 | 34 | 35 | 4.8 |
| Amygdala | R | 27 | 4 | −29 | 10 | 4.4 |
| Caudate | L | −15 | 0 | 19 | 8 | 4.3 |
| R | 11 | −4 | 19 | 5 | 3.5 | |
| Inferior frontal gyrus (VLPFC) |
L | −27 | 19 | − 11 | 20 | 4.2 |
| R | 34 | 23 | −23 | 15 | 4.0 | |
| Lingual gyrus | L | −19 | −61 | 0 | 45 | 4.0 |
| Retrieval: Older > Young | ||||||
| Precentral gyrus | R | 53 | 11 | 8 | 28 | 6.0 |
| Inferior parietal lobule | L | −38 | −57 | 46 | 189 | 5.2 |
| R | 53 | −38 | 53 | 44 | 5.0 | |
| Middle frontal gyrus (DLPFC) |
L | −38 | −4 | 49 | 89 | 5.0 |
| L | −34 | −4 | 30 | 24 | 4.8 | |
| R | 34 | −4 | 53 | 68 | 4.3 | |
| Superior frontal gyrus (DLPFC) |
R | 30 | 53 | 34 | 8 | 4.7 |
| R | 27 | −4 | 68 | 8 | 3.7 | |
| Superior occipital gyrus | R | 38 | −84 | 27 | 18 | 4.6 |
| Medial frontal gyrus | R | 8 | 0 | 53 | 5 | 4.4 |
| Superior parietal lobule | L | − 11 | −76 | 49 | 19 | 3.8 |
| R | 11 | −72 | 61 | 22 | 4.3 | |
| Inferior temporal gyrus | L | −53 | 57 | −11 | 19 | 4.2 |
| R | 57 | −57 | −15 | 5 | 3.8 | |
| Inferior frontal gyrus (VLPFC) |
L | −53 | 15 | 23 | 28 | 3.8 |
| L | −49 | 34 | 8 | 6 | 3.8 |
All listed clusters survive p < .05, FDR-correction. Coordinates (x y z) are in Talairach space and k represents cluster size.
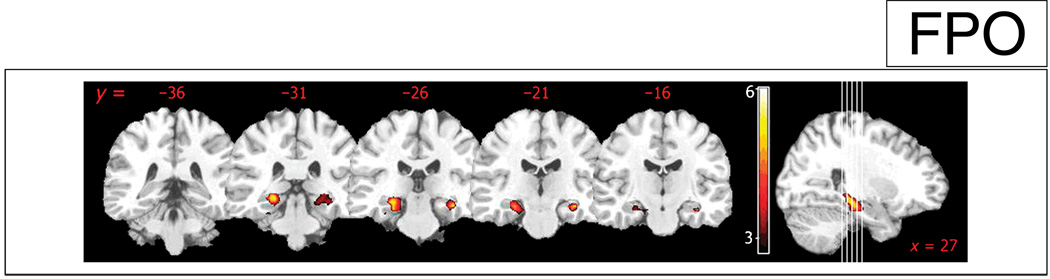
Figure 2.
Statistical parametric maps showing greater activity during retrieval in younger participants when compared to older participants in the hippocampal formation bilaterally ( p < .05, FDR-corrected). Age-related differences in activity are illustrated on coronal slices (left) and the sagittal slice (right) with respective statistical threshold (see color bar with t scores for reference). The numbers above the coronal slices and below the sagittal slice indicate the Talairach coordinate of the coronal planes and the sagittal plane of section, respectively.
Similarly, during the retrieval session, there was significantly greater activity in the hippocampus, the amygdala, and the caudate bilaterally in the younger participants when compared to the older participants (Figure 2). In contrast, older participants showed greater activity bilaterally in the frontal (BA 6, 9, 44, 45) and parietal cortices (BA 7, 40) when compared to the younger participants.
To ensure that the above findings were not driven by recognition accuracy differences between the two groups, we selected participants from each group that were matched for recognition accuracy as well as demographics [recognition accuracy (mean ± SD): older = 91.3 ± 4.8, young = 91.8 ± 1.9; F(1, 31) = .15, p = .70]. This analysis revealed similar results. During the encoding session, younger participants showed greater activity in the hippocampus and the amygdala during the encoding of scenes, whereas older participants showed greater activity compared to younger participants in prefrontal cortices (BA 6, 10, 11, 46), parietal cortices (BA 40), and regions in the ventral visual stream. During the retrieval session, similar to the encoding session, younger participants showed relatively greater activity in the hippocampus and the amygdala when compared to older participants (Table 4A and B). Again, older participants showed greater activity than younger participants in the parietal cortex (40), frontal cortices (BA 6), and brain regions in the ventral stream.
Table 4.
Comparison of Significant BOLD fMRI Responses during the (A) Encoding Session and (B) Retrieval Session between a Subset of Young and Older Participants Matched for Recognition Performance
| Regions | Side | x | y | z | k | t Score |
|---|---|---|---|---|---|---|
| (A) | ||||||
| Encoding: Young > Older | ||||||
| Middle temporal gyrus | R | 53 | −57 | 4 | 11 | 5.3 |
| Amygdala | L | 27 | 4 | −19 | 11 | 4.3 |
| R | −27 | 4 | −15 | 8 | 4.8 | |
| Hippocampus | L | 19 | −30 | −19 | 56 | 4.2 |
| R | −30 | −27 | −8 | 20 | 4.7 | |
| Cerebellum | R/L | 0 | −49 | −15 | 25 | 4.3 |
| Posterior cingulate | L | −19 | −57 | 4 | 16 | 3.4 |
| Fusiform gyrus | L | −23 | −53 | −15 | 7 | 3.2 |
| Encoding: Older > Young | ||||||
| Middle frontal gyrus (DLPFC) |
L | −46 | 49 | 8 | 20 | 5.2 |
| L | −38 | −4 | 57 | 50 | 4.9 | |
| R | 49 | 0 | 57 | 19 | 4.2 | |
| R | 49 | 38 | 27 | 5 | 3.7 | |
| R | 38 | 42 | −15 | 9 | 3.7 | |
| Fusiform gyrus | L | −49 | −61 | −19 | 7 | 3.8 |
| R | 52 | −52 | −17 | 10 | 4.7 | |
| Medial frontal gyrus | R | 4 | −8 | 57 | 27 | 4.6 |
| Visual cortex | L | −42 | −84 | −8 | 11 | 4. 5 |
| R | 34 | −72 | 27 | 65 | 4.5 | |
| R | 30 | −56 | −13 | 7 | 3.6 | |
| R | 46 | −84 | 0 | 9 | 3.5 | |
| Parietal cortex | L | −30 | −84 | 34 | 16 | 4.0 |
| L | −42 | −49 | 46 | 20 | 4.0 | |
| (B) | ||||||
| Retrieval: Young > Older | ||||||
| Hippocampus | L | −30 | −27 | −11 | 4 | 4.8 |
| R | 34 | −27 | −15 | 40 | 5.1 | |
| Amygdala | L | 30 | −4 | −19 | 21 | 4.5 |
| Inferior frontal gyrus (VLPFC) |
L | −38 | 27 | 11 | 12 | 4.6 |
| R | 38 | 15 | 17 | 8 | 4.0 | |
| Middle frontal gyrus | R | 23 | 15 | 68 | 12 | 4.3 |
| Superior temporal gyrus | R | 57 | 38 | −15 | 5 | 4.3 |
| Superior parietal lobule | R | 38 | −72 | 49 | 5 | 3.6 |
| Cerebellum | R | 19 | −42 | −27 | 11 | 3.5 |
| Retrieval: Older > Young | ||||||
| Medial frontal gyrus | R | 4 | 8 | 65 | 104 | 6.0 |
| Middle frontal gyrus (DLPFC) |
L | −34 | −4 | 53 | 77 | 5.8 |
| Middle temporal gyrus | R | 46 | −80 | 15 | 120 | 5.6 |
| Superior parietal gyrus | L | −34 | −61 | 52 | 93 | 5.4 |
| Precentral gyrus | R | 9 | −8 | 57 | 49 | 4.7 |
| Visual cortex | L | 11 | −84 | 30 | 67 | 4.6 |
| R | −30 | −89 | 49 | 25 | 4.6 | |
| Fusiform gyrus | L | −49 | −61 | −15 | 22 | 4.5 |
| R | 30 | −78 | −12 | 8 | 3.9 | |
| Parietal lobe | r | 46 | −42 | 57 | 31 | 4.3 |
All listed clusters survive p < .05, FDR-correction. Coordinates (x y z) are in Talairach space and k represents cluster size.
Effect of Valence on Age [Young (Aversive > Neutral) > Older (Aversive > Neutral); Older (Aversive > Neutral) > Young (Aversive > Neutral)]
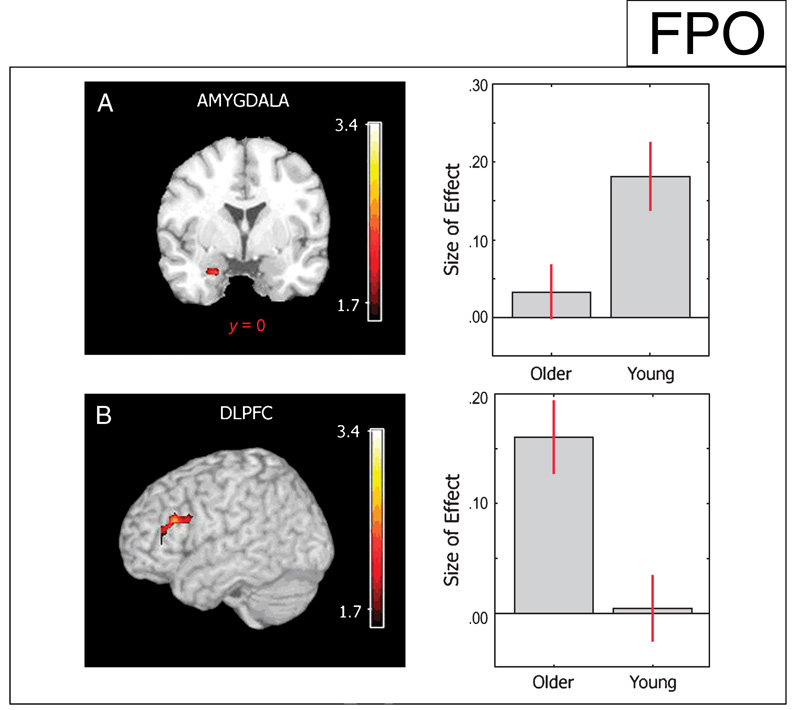
To characterize the effect of valence on age-related changes in memory, contrasts for the encoding and retrieval of aversive and neutral stimuli were compared between the full sample of young and older participants. During the encoding sessions, no significant clusters were seen in the young > older contrast, however, the reverse contrast [older (aversive > neutral) > young (aversive > neutral)] revealed a significant cluster in the left DLPFC (BA 10; Table 5). During the retrieval sessions, significant clusters were found in the left amygdala as well as a trend for significance in the left hippocampus ( p = .018, uncorrected; x = −27, y = −27, z = −15, k =5) that showed greater valence (aversive > neutral) related activity in the younger participants when compared to the older participants (Figure 3A; Table 5). Of note, both of these regions overlap with regions that were significant in the retrieval contrasts between young and older participants. The reverse contrast in the retrieval session [older (aversive > neutral) > young (aversive > neutral)] revealed significant activation in the right DLPFC (BA 45, 46; Figure 3B), the left parietal cortex (BA 7), and the cingulate cortex (BA 32, 34; Table 5).
Table 5.
Comparison of Significant BOLD fMRI Responses during the Encoding and Retrieval Session between Young and Older Participants in Contrasts of Aversive > Neutral Pictures
| Regions | Side | x | y | z | k | t Score |
|---|---|---|---|---|---|---|
|
Encoding: Older (Aversive > Neutral) > Young (Aversive > Neutral) | ||||||
| Middle frontal gyrus (DLPFC) | L | −27 | 56 | 7 | 12 | 3.2 |
|
Retrieval: Young (Aversive > Neutral) > Older (Aversive > Neutral) | ||||||
| Amygdala | L | −27 | −5 | −22 | 15 | 2.8 |
|
Retrieval: Older (Aversive > Neutral) > Young (Aversive > Neutral) | ||||||
| Middle frontal gyrus (DLPFC) | R | 49 | 30 | 20 | 61 | 3.4 |
| Parietal lobe | L | −27 | −71 | 79 | 7 | 3.6 |
| Cingulate cortex | R | 8 | 9 | 35 | 8 | 3.4 |
Coordinates (x y z) are in Talairach space and k represents cluster size. Please note that there were no regions that were significantly different in the young (aversive > neutral) > older (aversive > neutral) contrast during the encoding session.
Figure 3.
Effect of aversive stimuli on fMRI activity during emotional memory retrieval. t Maps of the contrast aversive > neutral were compared between older and young participants. (A) The left amygdala showed greater emotional activity in young participants as revealed by the greater effect size in this group (right). (B) A region in the DLPFC (middle frontal gyrus; depicted on the left with color bar of t scores) showed greater activity in older participants as seen in the effect size plots for each group (right). Beta-parameter estimates of the size of effect were calculated in voxels activated within an ROI showing an Age by Valence interaction during emotion processing ( p < .05).
Functional Connectivity
Functional connectivity between activity in the amygdala and other brain areas, particularly the hippocampus and the DLPFC, was assessed in both groups during the encoding and retrieval sessions. During the encoding session, greater functional coupling was seen between the DLPFC and the amygdala in older participants when compared to young participants (BA 47; right: x = 38, y = 15, z = −11, k = 43; left: x = −38, y = 19, z = −11, k = 53). On the reverse contrast (younger participants > older participants), although the differences did not reach the statistical threshold, there was a trend for significance toward greater functional coupling between the amygdala and the hippocampus (left: p = .02; x = −23, y = −19, z = −15, k = 5; right: p = .02; x = 27, y = −23, z = −15, k = 13) in the younger participants. During retrieval, significantly greater functional coupling was seen between the amygdala and the hippocampus in younger participants (right: x = 30, y = −15,z = −11, k = 20), and greater coupling between the amygdala and DLPFC regions in the older participants (BA 47/11; right: x = 59, y = 19, z = 0, k = 35).
Brain-Behavior Correlations
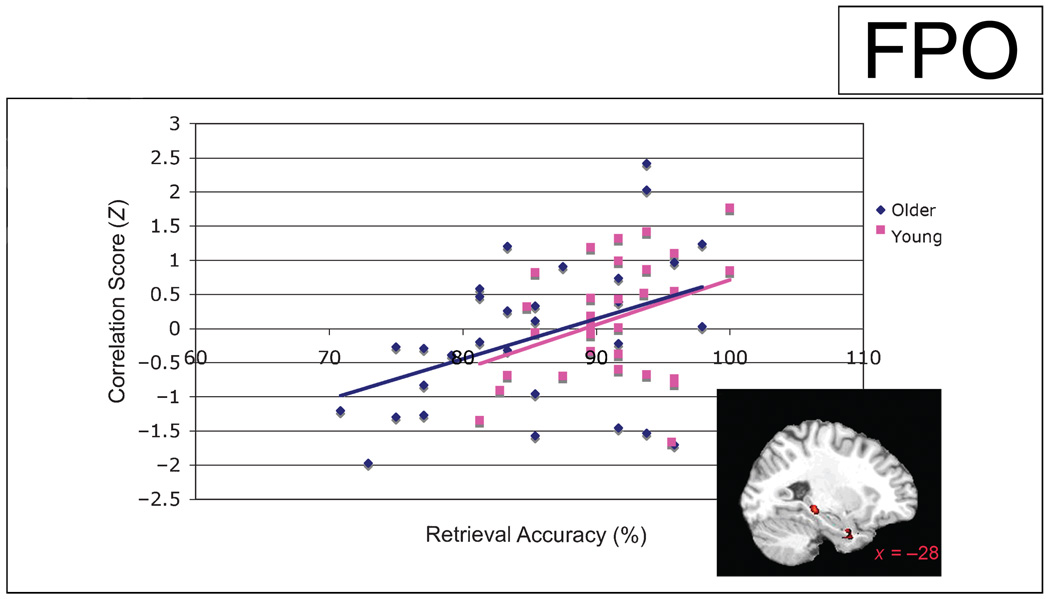
To assess which brain regions during encoding were important in predicting recognition accuracy, simple regressions were performed between each individual’sencoding activation maps and their recognition accuracy. This analysis revealed a significant positive correlation between amygdala activation and performance during recognition (r = .34; x = −19, y = −1, z = −16, k = 6). Most importantly, increased coupling between the amygdala and the hippocampus during the encoding session predicted better recognition performance (right: r = .41; x = 23, y = −30, z = −4, k = 33; Figure 4). Correlations between performance and the strength of the functional coupling between the right hippocampus and the amygdala were statistically significant within each group, however, no significant differences were seen between groups.
Figure 4.
Functional coupling during scene encoding between the amygdala and a cluster in the right posterior hippocampal formation (illustrated in the bottom right) significantly correlated with accuracy during the retrieval session (p < .001, r = .42, x = 23, y = −30, z = –4; all participants:p < .001, r = .41; older: p < .02; r = .41; younger: p < .05, r = .36). This regression reveals a positive relationship between the degree of coupling between these two limbic regions and behavioral performance. Older participants are represented in blue, whereas young participants are represented in pink. Correlation values are reported as normalized Pearson’s r scores.
A significant correlation was also found between activity in the right DLPFC during encoding (r = .34; x = 57, y = 23, z = 30, k = 11) and recognition performance. Interestingly, further analyses revealed that the effect size of this correlation, as determined by Cohen’s d, was larger in older participants (r = .37, medium effect size) than in younger participants (r = .15, small effect size). No significant brain–behavior correlations were seen between activity during retrieval and recognition performance. Furthermore, no significant differences were seen in the correlations above when divided by stimulus valence.
DISCUSSION
The results from this study provide further evidence for differences both at the level of behavior and brain activation between older and young participants during a declarative memory task with aversive and neutral stimuli. Although older participants performed relatively worse with decreased accuracy on scene recognition when compared to younger participants, similar to the younger participants, they maintained relatively higher accuracy scores for aversive stimuli when compared to neutral stimuli. Consistent with prior reports (Grady et al., 2005; Cabeza et al., 2004), our functional imaging data revealed relatively decreased hippocampal activity and increased DLPFC activity in the older participants during both the encoding and retrieval of both neutral and aversive scenes when compared to the younger participants. In addition, connectivity analyses revealed significantly decreased coupling between the amygdala and the hippocampus during the retrieval phase, and greater coupling between the amygdala and the DLPFC during both encoding as well as retrieval phases in older participants when compared to younger participants. Across all participants, correlation analysis revealed that the degree of activity in the amygdala and the DLPFC, and functional coupling between the amygdala and the hippocampus during encoding, correlated positively with recognition performance. Furthermore, during retrieval of aversive stimuli compared to neutral stimuli, younger participants presented with greater amygdala activity to aversive stimuli when compared to the older participants, whereas the older participants presented with greater DLPFC activity when compared to the younger participants. In summary, this study, although reconfirming age-related changes in the performance and activity of the brain regions’ underlying simple declarative memory, also addresses age-related alterations in the connectivity of brain regions underlying simple declarative memory for neutral and aversive stimuli.
Age-related Changes in Declarative Memory Performance and Circuitry
In line with recent neuroimaging studies (Dennis et al., 2006; Cabeza et al., 1997), our data revealed relatively decreased engagement of the hippocampus during both encoding and retrieval sessions in the older participants. In contrast, older participants showed increased activity in the parietal and frontal cortices, supporting a notion for increased cortical recruitment in older participants during declarative memory tasks as proposed by Grady etal. (2005). There was also greater coupling between the DLPFC, the VLPFC, and the amygdala in older participants during the encoding and retrieval of scenes, providing further support for an alternative strategy or a compensatory mechanism in the older participants to maintain performance.
Age-related Changes in the Encoding of Aversive Stimuli
The analyses of age-related changes in the encoding of aversive stimuli compared to neutral stimuli showed significantly greater DLPFC activity in the older participants in contrast to the younger participants. These findings are in line with prior reports related to aversive emotion perception in older participants, which suggest recruitment of an extended network of brain regions including the parietal and frontal cortices in older participants (Tessitore et al., 2005; Gunning-Dixon et al., 2003; Iidaka et al., 2002). Regression analyses revealed that activation in the amygdala and amygdala–hippocampus coupling during encoding correlated positively with subsequent scene recognition performance across the whole sample. These findings are consistent with those reported previously for successful encoding of aversive stimuli (Dolcos et al., 2004b). Interestingly, DLPFC activation during encoding also predicted retrieval performance, and this dependence on prefrontal systems for successful encoding was greater in older participants. In addition, in contrast to the tendency for relatively greater coupling between the amygdala and the hippocampus in young participants, there was greater functional coupling between the DLPFC and the amygdala in older participants. Taken together, these findings suggest a reorganization of the functional networks underlying picture encoding to compensate for the relatively decreased function of the amygdala and the hippocampus with advancing age.
Age-related Changes in the Recognition of Aversive Stimuli
Although the analysis of behavioral data revealed impairment in overall memory recognition with advancing age, both older and young participants showed higher recognition rates for aversive stimuli when compared to neutral stimuli. Consistent with recent behavioral studies (Denburg et al., 2003; Kensinger et al., 2002), these findings suggest that similar to what has been shown in younger participants, the aversive content of the stimulus tends to enhance subsequent recollection performance in older participants as well, even in the presence of age-related decreases in simple declarative memory.
Although both groups showed relatively better performance in the retrieval of aversive stimuli when compared to neutral stimuli, the functional imaging data suggest that the young and older participants use different neural circuitry to mediate this effect. Younger participants showed greater valence-mediated (aversive > neutral) activity in the amygdala and the hippocampus, and greater coupling between these two brain regions during scene retrieval when compared to older participants, whereas the older participants presented with greater DLPFC and parietal cortical activity and greater coupling between the DLPFC and the amygdala when compared to younger participants. The strength of the functional connectivity between the hippocampus and the amygdala has been proposed to reflect enhancement of recollection and reconsolidation processes in the hippocampus (Smith et al., 2006). However, given the decline of function within the amygdala with advancing age (Tessitore et al., 2005; Mather et al., 2004; Gunning-Dixon et al., 2003; Iidaka et al., 2002), it is likely that the preservation of emotional memory with advancing age is mediated by compensatory DLPFC activity. Findings of greater prefrontal recruitment in older participants during recognition processes have been seen during simple declarative memory of information with neutral content (e.g., Grady et al., 2005; Cabeza et al., 2004). Our data suggest that older participants use similar mechanisms to increase retrieval success in recollection of aversive memory as well.
Age-related Changes in Prefrontal Activity during Emotional Memory
Given the findings of this study, we suggest a compensatory role for the DLPFC in older adults during declarative memory for aversive scenes, which may explain the relatively better memory for aversive stimuli than for neutral stimuli despite a decreased function in the amygdala and the hippocampus with advancing age. This notion is further supported by our regression and functional connectivity analyses, which revealed greater recruitment of the DLPFC in older participants during both encoding and retrieval sessions in comparison to the younger participants who show greater activity and connectivity in the amygdala and hippocampal formation. An alternative interpretation of these findings would be that the relatively greater DLPFC engagement during memory of aversive stimuli in older adults could reflect increased top–down modulation of limbic activity to aversive stimuli. This could support a theory of greater emotional stability and regulation in older adults which is supported by both behavioral and neuroimaging studies (Williams et al., 2006; Mather & Carstensen, 2005), reflecting older participants’ dampened emotional response to aversive stimuli. However, whether these changes reflect a compensatory mechanism or enhanced top–down modulation of the limbic system in the older participants could not be discerned in the current study. Future studies using event-related designs and effective connectivity analysis approaches could better delineate these mechanisms.
Limitations
Some aspects of our current experimental design limited the scope and interpretation of our data. Although blocked-design paradigms provide reliable and robust activations of functional networks, they are not amenable to exploring the neurobiology underlying successful versus unsuccessful encoding and recognition. This limitation of the block-design approach, wherein both old and novel stimuli were interspersed within the same block, may explain one of the negative findings in our study, that is, a lack of correlation between recognition accuracy and strength of coupling between the hippocampus and the amygdala, which is in contrast to the observations of Dolcos et al. (2005), who used an event-related approach.
Also, in the current study, individual ratings of the stimuli by participants were not assessed, and therefore, a direct comparison of subjective ratings across older and young participants could not be performed. However, prior evidence suggests that there is no significant difference in the ratings of negative scenes between young and older adults (Backs et al., 2005). Furthermore, in the current study, age-related differences between arousal and valence could not be assessed as the two factors were collinear. Because recent studies have shown differential neural recruitment in the processing of these two factors (activity and connectivity between the medial-temporal lobe and the DLPFC) (Dolcos, LaBar, & Cabeza, 2004a; Kensinger & Corkin, 2004), investigating the effect of these factors independent of each other on age-related changes in emotional memory may be more informative.
Another shortcoming of our study is that we did not have structural imaging data in the older participants to explore the effect of age-related structural changes on the above-mentioned functional and behavioral processes. Structural imaging studies have generally shown age-related decreases in the volume and integrity of brain regions, particularly the hippocampal formation and the DLPFC, with increasing age (Schiltz et al., 2006; Raz, Gunning-Dixon, Head, Dupuis, & Acker, 1998). Although we and other groups have noted decreased hippocampal activity, we also observed increased DLPFC, , VLPFC, and parietal cortical activity. In addition, we also observed valence-related changes in activity of these brain regions as well as age-related alterations in the functional coupling of these brain regions with the amygdala. Therefore, it is unlikely that the above-mentioned age-related changes in activity of brain regions underlying simple declarative memory could be an artifact driven by age-related structural changes.
Conclusions
In summary, our findings support results from prior studies for a decline in simple declarative memory function with advancing age. Imaging data revealed decreased function in regions key to memory formation including the amygdala and the hippocampus with advancing age. Older participants, however, showed greater engagement of the DLPFC and parietal cortices during both memory encoding and retrieval, possibly providing a compensatory mechanism to maintain performance. In addition to characterizing age-related alterations in the activity and connectivity of brain regions underlying simple declarative memory, our findings also offer insight into how stimuli with aversive valence and high arousal can differentially modulate memory encoding and retrieval processes. The results from this study, while adding to evidence that normal aging is associated with a decline in simple declarative memory, also suggests that memory for aversive stimuli is relatively better preserved than for neutral stimuli.
REFERENCES
- Adolphs R, Cahill L, Schul R, Babinsky R. Impaired declarative memory for emotional material following bilateral amygdala damage in humans. Learning and Memory. 1997;4:291–300. doi: 10.1101/lm.4.3.291. [DOI] [PubMed] [Google Scholar]
- Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: The major lobes and a parcellation of the temporal region. Neurobiology of Aging. 2005;26:1245–1260. doi: 10.1016/j.neurobiolaging.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Backs RW, da Silva SP, Han K. A comparison of younger and older adults’ self-assessment manikin ratings of affective pictures. Experimental Aging Research. 2005;31:421–440. doi: 10.1080/03610730500206808. [DOI] [PubMed] [Google Scholar]
- Bartlett JC, Leslie JC, Tubbs A, Fulton A. Aging and memory for pictures of faces. Psychology and Aging. 1989;4:276–283. doi: 10.1037//0882-7974.4.3.276. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Rubino V, Sambataro F, Blasi G, Latorre V, Fazio L, et al. Prefrontal-hippocampal coupling during memory processing is modulated by COMT val158met genotype. Biological Psychiatry. 2006;60:1250–1258. doi: 10.1016/j.biopsych.2006.03.078. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, et al. Molecular, structural, and functional characterization of Alzheimer’s disease: Evidence for a relationship between default activity, amyloid, and memory. Journal of Neuroscience. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Daselaar M, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cerebral Cortex. 2004;14:364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Grady CL, Nyberg L, McIntosh AR, Tulving E, Kapur S, et al. Age-related differences in neural activity during memory encoding and retrieval: A positron emission tomography study. Journal of Neuroscience. 1997;17:391–400. doi: 10.1523/JNEUROSCI.17-01-00391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caclin A, Fonlupt P. Effect of initial fMRI data modeling on the connectivity reported brain areas. Neuroimage. 2006;33:515–521. doi: 10.1016/j.neuroimage.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Celone KA, Calhoun VD, Dickerson BC, Atri A, Chua EF, Miller SL, et al. Alterations in memory networks in mild cognitive impairment and Alzheimer’s disease: An independent component analysis. Journal of Neuroscience. 2006;26:10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles ST, Mather M, Carstenson LL. Aging and emotional memory: The forgettable nature of negative images for older adults. Journal of Experimental Psychology: General. 2003;132:310–324. doi: 10.1037/0096-3445.132.2.310. [DOI] [PubMed] [Google Scholar]
- Comblain C, D’Argembeau A, Van der Linden M, Aldenhoff L. The effect of ageing on the recollection of emotional and neutral pictures. Memory. 2004;12:673–684. doi: 10.1080/09658210344000477. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R. Effects of healthy aging on hippocampal and rhinal memory functions: An event-related fMRI study. Cerebral Cortex. 2006;16:1771–1782. doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Veltman DJ, Rombouts SA, Raaijmakers JG, Jonkers C. Neuroanatomical correlates of episodic encoding and retrieval in young and elderly subjects. Brain. 2003;126:43–56. doi: 10.1093/brain/awg005. [DOI] [PubMed] [Google Scholar]
- Denburg NL, Buchanan TW, Tranel D, Adolphs R. Evidence for preserved emotional memory in normal older persons. Emotion. 2003;3:239–253. doi: 10.1037/1528-3542.3.3.239. [DOI] [PubMed] [Google Scholar]
- Dennis NA, Daselaar S, Cabeza R. Effects of aging on transient and sustained successful memory encoding activity. Neurobiology of Aging. 2006;28:1749–1758. doi: 10.1016/j.neurobiolaging.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Bates JF, Atiya M, Killiany RJ, Greve DN, et al. Medial temporal lobe function and structure in mild cognitive impairment. Annals of Neurology. 2004;56:27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Dissociable effects of arousal and valence on prefrontal activity indexing emotional evaluation and subsequent memory: An event-related fMRI study. Neuroimage. 2004a;23:64–74. doi: 10.1016/j.neuroimage.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron. 2004b;42:855–863. doi: 10.1016/s0896-6273(04)00289-2. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Remembering one year later: Role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proceedings of the National Academy of Sciences, U.S.A. 2005;102:2626–2631. doi: 10.1073/pnas.0409848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV for Axis I Disorders, Patient Edition (SCID-P) New York: Biometrics Research Department; 1994. [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study for ageing in 464 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Grady CL, Bernstein LJ, Beig S, Siegenthaler AL. The effects of encoding task on age-related differences in the functional neuroanatomy of face memory. Psychology and Aging. 2002;17:7–23. doi: 10.1037//0882-7974.17.1.7. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Craik FI. Age-related differences in the functional connectivity of the hippocampus during memory encoding. Hippocampus. 2003;13:572–586. doi: 10.1002/hipo.10114. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Craik FI. Task-related activity in prefrontal cortex and its relation to recognition memory performance in young and old adults. Neuropsychologia. 2005;43:1466–1481. doi: 10.1016/j.neuropsychologia.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Horwitz B, Maisog JM, Ungerleider LG, Mentis MJ, et al. Age-related reductions in human recognition memory due to impaired encoding. Science. 1995;269:218–221. doi: 10.1126/science.7618082. [DOI] [PubMed] [Google Scholar]
- Grady CL, McIntosh AR, Rajah MN, Beig S, Craik FI. The effects of age on the neural correlates of episodic encoding. Cerebral Cortex. 1999;9:805–814. doi: 10.1093/cercor/9.8.805. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Gur RC, Perkins AC, Schroeder L, Turner T, Turetsky BI, et al. Age-related differences in brain activation during emotional face processing. Neurobiology of Aging. 2003;24:285–295. doi: 10.1016/s0197-4580(02)00099-4. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Welsh RC, Hedden T, Bangert A, Minear M, Liu LL, et al. Aging and the neural correlates of successful picture encoding: Frontal activations compensate for decreased medial-temporal lobe activity. Journal of Cognitive Neuroscience. 2005;17:84–96. doi: 10.1162/0898929052880048. [DOI] [PubMed] [Google Scholar]
- Hamann S. Cognitive and neural mechanisms of emotional memory. Trends in Cognitive Sciences. 2001;5:394–400. doi: 10.1016/s1364-6613(00)01707-1. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, et al. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. Journal of Neuroscience. 2003;23:6690–6694. doi: 10.1523/JNEUROSCI.23-17-06690.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iidaka T, Okada T, Murata T, Omori M, Kosaka H, Sadato N, et al. Age-related differences in the medial temporal lobe responses to emotional faces as revealed by fMRI. Hippocampus. 2002;12:352–362. doi: 10.1002/hipo.1113. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Garnst AC, Stout JC, Bonner J, et al. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiology of Aging. 2001;22:581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Kensinger EA. Age differences in memory for arousing and nonarousing emotional words. Journals of Gerontology: Series B, Psychological Sciences and Social Sciences. 2008;66:P13–P18. doi: 10.1093/geronb/63.1.p13. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Brierley B, Medford N, Growdon JH, Corkin S. Effects of normal aging and Alzheimer’s disease on emotional memory. Emotion. 2002;2:118–134. doi: 10.1037/1528-3542.2.2.118. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Corkin S. Two routes to emotional memory: Distinct neural processes for valence and arousal. Proceedings of the National Academy of Sciences, U.S.A. 2004;101:3310–3315. doi: 10.1073/pnas.0306408101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nature Reviews Neuroscience. 2006;7:54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-6. Gainesville, FL: University of Florida; 2005. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. [Google Scholar]
- Mather M, Canli T, English T, Whitfield S, Wais P, Oschner K, et al. Amygdala responses to emotionally valenced stimuli in older and younger adults. Psychological Science. 2004;15:259–263. doi: 10.1111/j.0956-7976.2004.00662.x. [DOI] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. Aging and motivated cognition: The positivity effect in attention and memory. Trends in Cognitive Sciences. 2005;9:496–502. doi: 10.1016/j.tics.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Buckholtz JW, Kolachana B, Hariri AR, Pezawas L, Blasi G, et al. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proceedings of the National Academy of Sciences, U.S.A. 2006;103:6269–6274. doi: 10.1073/pnas.0511311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Poline JB, Kohn PD, Holt JL, Egan MF, Weinberger DR, et al. Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. American Journal of Psychiatry. 2001;158:1809–1817. doi: 10.1176/appi.ajp.158.11.1809. [DOI] [PubMed] [Google Scholar]
- OldfieldC RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Park DC, Smith AD, Lautenschlager G, Earles JL, Frieske D, Zwahr M, et al. Mediators of long-term memory performance across the life span. Psychology and Aging. 1996;11:621–637. doi: 10.1037//0882-7974.11.4.621. [DOI] [PubMed] [Google Scholar]
- Parkin AJ, Walter BM. Recollective experience, normal aging, and frontal dysfunction. Psychology and Aging. 1992;7:290–298. doi: 10.1037//0882-7974.7.2.290. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, et al. 5-HTTLPR polymorphism impacts human cingulate–amygdala interactions: A genetic susceptibility mechanism for depression. Nature Neuroscience. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon FM, Head D, Dupuis JH, Acker JD. Neuroanatomical correlates of cognitive aging: Evidence from structural magnetic resonance imaging. Neuropsychology. 1998;12:95–114. doi: 10.1037//0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon FM, Head D, Rodriquez KM, Williamson A, Acker JD. Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: Replicability of regional differences. Neurobiology of Aging. 2004;25:377–396. doi: 10.1016/S0197-4580(03)00118-0. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Wagner AD. Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus. 1999;9:7–24. doi: 10.1002/(SICI)1098-1063(1999)9:1<7::AID-HIPO2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Schiltz K, Szentkuti A, Guderian S, Kaufmann J, Munte TF, Heinze HJ, et al. Relationship between hippocampal structure and memory function in elderly humans. Journal of Cognitive Neuroscience. 2006;18:990–1003. doi: 10.1162/jocn.2006.18.6.990. [DOI] [PubMed] [Google Scholar]
- Smith AP, Henson RN, Dolan RJ, Rugg MD. fMRI correlates of the episodic retrieval of emotional contexts. Neuroimage. 2004;22:868–878. doi: 10.1016/j.neuroimage.2004.01.049. [DOI] [PubMed] [Google Scholar]
- St. Jacques PS, Dolcos F, Cabeza R. Effects of aging on functional connectivity of the amygdala during negative evaluation: A network analysis of fMRI data. Neurobiology of Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.03.012. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessitore A, Hariri AR, Fera F, Smith WG, Das S, Weinberger DR, et al. Functional changes in the activity of brain regions underlying emotion processing in the elderly. Psychiatry Research. 2005;139:9–18. doi: 10.1016/j.pscychresns.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Williams LM, Brown KJ, Palmer D, Liddell BJ, Kemp AH, Olivieri G, et al. The mellow years?: Neural basis of improving emotional stability over age. Journal of Neuroscience. 2006;26:6422–6430. doi: 10.1523/JNEUROSCI.0022-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CI, Wedig MM, Williams D, Rauch SL, Alberts MS. Novel fearful faces activate the amygdala in healthy young and elderly adults. Neurobiology of Aging. 2006;27:361–374. doi: 10.1016/j.neurobiolaging.2005.01.014. [DOI] [PubMed] [Google Scholar]