Abstract
The two current major staging systems in use for Lewy body disorders fail to classify up to 50% of subjects. Both systems do not allow for large numbers of subjects who have Lewy-type α-synucleinopathy (LTS) confined to the olfactory bulb or who pass through a limbic-predominant pathway that at least initially bypasses the brainstem. The results of the current study, based on examination of a standard set of 10 brain regions from 417 subjects stained immunohistochemically for α-synuclein, suggest a new staging system that, in this study, allows for the classification of all subjects with Lewy body disorders. The autopsied subjects included elderly subjects with Parkinson’s disease, dementia with Lewy bodies, incidental Lewy body disease and Alzheimer’s disease with Lewy bodies, as well as comparison groups without Lewy bodies. All subjects were classifiable into one of the following stages: I. Olfactory Bulb Only; IIa Brainstem Predominant; IIb Limbic Predominant; III Brainstem and Limbic; IV Neocortical. Progression of subjects through these stages was accompanied by a generally stepwise worsening in terms of striatal tyrosine hydroxylase concentration, substantia nigra pigmented neuron loss score, Mini Mental State Examination score and score on the Unified Parkinson’s Disease Rating Scale Part 3. Additionally there were significant correlations between these measures and LTS density scores. It is suggested that the proposed staging system would improve on its predecessors by allowing classification of a much greater proportion of cases.
Keywords: Parkinson’s disease, parkinsonism, dementia with Lewy bodies, Alzheimer’s disease, incidental Lewy bodies, α-synuclein, olfactory bulb, amgydala, limbic, brainstem, neocortex
It has been almost two centuries since the first description (73,74) of Parkinson’s disease (PD) and almost one century since the subsequent discovery of its characteristic microscopic lesion, the Lewy body (61,47,82). The intervening years have provided a wealth of detail on its clinical manifestations and pathology. The presenting syndromes are dementia, motor parkinsonism or both. Since Kosaka’s delineation of “diffuse Lewy body disease” associated with dementia in 1976(55), followed by the alternative concepts of “senile dementia of the Lewy body type” (78) and “Lewy body variant of Alzheimer’s disease” (41), those presenting with dementia are now termed dementia with Lewy bodies (DLB), the definition of which has undergone two major iterations (66,67). In both PD and DLB, aggregation, phosphorylation and nitration of α-synuclein, an abundant synaptic protein, have been suggested to be critical processes leading to Lewy body formation and clinical symptomatology (36,80,28,39,4) .
Investigations that have mapped the topographical distribution and density of Lewy bodies and their associated abnormal neurites have indicated that these are spread much more widely throughout the neuraxis than formerly appreciated (22,29,46,80,30,38,49,81,69,50,57). The SN, for so long believed to be the epicenter of PD, is neither the earliest nor the most severely affected region. More caudal brainstem structures are now recognized as involved prior to the SN while the amygdala and associated limbic structures may be struck first and hardest in some cases (12,64,85,84,76,40,65). Furthermore, it is also now more clearly apparent that Lewy body pathology frequently extends to the spinal cord and peripheral nervous system (16,24,44,72,17,86,54,10,11,13) .
Much effort has been expended by numerous investigators to devise staging systems that might be used, as in neoplastic disease, to define and categorize the progression of Lewy body disorders in terms of microscopic appearance and regional presence (67,66,15,65,81). These endeavors have not only provided more precise descriptions of disease extent, they have given an improved understanding of disease progression. It is hoped that this will lead to useful clues regarding disease pathogenesis.
However, it has become apparent that the current staging systems fall short of adequately describing the full histopathologic range and variability of Lewy body disorders. There are two major competing systems, both of which have been determined to some degree inadequate by several recent and comprehensive investigations and/or reviews (59,52,75,34,62,51). These two schemes have been differentially conceived, one for PD and the other for DLB (14,16,67,66). Both of these have been reported to provide insufficient guidance, especially when applied outside of their initial focus, so that up to 50% or more of cases are unclassifiable (51). The recent redefinition of DLB has left many cases, mainly those harboring Alzheimer’s disease (AD) and limbic lobe-restricted Lewy bodies, without a diagnostic label entirely (85,66). Undoubtedly contributing to the confusion are large differences between laboratories in the sensitivity of immunohistochemical methods for α-synuclein, along with variability in sampling sites, tissue processing and inter-observer assessment (20,70,3).
We undertook this investigation in an attempt to construct, from the valuable foundation already achieved, a unified staging system that could be applied to all of the major Lewy body disorders, including not only PD and DLB but also incidental Lewy body disease (ILBD) and Alzheimer’s disease with sparse, predominantly limbic Lewy bodies (ADLB). A sensitive immunohistochemical method for α-synuclein was used to stain sets of brain sections from more than 600 autopsied subjects. The densities and frequencies of occurrence of Lewy-type α-synucleinopathy (LTS) in 10 standard brain regions were tabulated and correlated with diagnostic category as well as measures of nigrostriatal degeneration, cognitive impairment and motor dysfunction. The results are presented in this manuscript.
MATERIALS AND METHODS
Human Subjects
Brain tissue was obtained from Sun Health Research Institute (SHRI), located in the Sun Cities retirement communities of northwest metropolitan Phoenix, Arizona. Brain necropsies were performed on elderly subjects who had volunteered for the SHRI Brain Donation Program (6). The majority of Brain Donation Program subjects are clinically characterized at SHRI with annual standardized test batteries that include functional, neuropsychological and neuromotor components, including the Mini Mental State Examination (MMSE) and Unified Parkinson’s Disease Rating Scale (UPDRS). Additionally, private medical records are requisitioned and reviewed for each subject and the postmortem Dementia Questionnaire (32) is administered to subject contacts to help determine the presence or absence of dementia for those subjects lacking standardized antemortem evaluations. The Brain Donation Program has been approved by the SHRI Institutional Review Board.
Subjects were chosen by searching the Brain Donation Program database for all cases with a completed neuropathologist’s examination, a full set of paraffin blocks (defined as blocks having all ten regions as defined in the histologic methods) and diagnoses of a Lewy body disorder, including PD, DLB, ILBD and ADLB. Comparison groups were composed of subjects without evidence of dementia or parkinsonism (normal elderly subjects) and subjects with AD but no Lewy body pathology (ADNLB).
Subjects received standardized neuropathological examinations. Specific diagnostic criteria were used for AD (1), PD (37) and DLB (67). For both AD and DLB, cases received the diagnosis if they were classified as “intermediate” or “high” probabilities in their respective classification schemes. Cases with LTS but not meeting these diagnostic criteria were designated as either ILBD, if they had no clinical history of parkinsonism or dementia, or ADLB if they had Alzheimer’s disease and Lewy bodies in any brain region but failed to meet criteria for DLB.
Gross and microscopic neuropathologic assessments were made by a single observer (TGB) without knowledge of the clinical history or clinical diagnosis; subsequently the clinical history was reviewed in order to make an appropriate clinicopathologic diagnosis. Subjects with Lewy body disorders were staged according to Braak (11) as well as the Dementia with Lewy Bodies Consortium (66). Apolipoprotein E genotyping was done on postmortem brain tissue using a standard technique (45).
Histologic Methods
Diagnostic histologic methods were performed on standard blocks of tissue that were fixed in 4% buffered formaldehyde and then either dehydrated and embedded in paraffin or cryoprotected and cut on a freezing, sliding microtome. Paraffin sections from the olfactory bulb and tract, anterior medulla (two levels anterior to the obex), anterior and mid-pons, mid-amygdala with adjacent transentorhinal area, anterior cingulate gyrus (1–3 cm posterior to the coronal slice containing the genu of the corpus callosum), middle temporal gyrus (at the level of the lateral geniculate nucleus), middle frontal gyrus (4–5 cm posterior to the frontal pole), and inferior parietal lobule were stained immunohistochemically for α-synuclein using a polyclonal antibody raised against an α-synuclein peptide fragment phosphorylated at serine 129, after epitope exposure with proteinase K. The process leading to the choice of immunohistochemical method, as well as details of the method, have been described in a previous publication (7). The density of α-synuclein-immunoreactive Lewy bodies and neurites in each of the above-mentioned brain regions was scored, for more than 90% of slides, by a single observer (TGB), without knowledge of diagnosis, as none, sparse, moderate, frequent and very frequent, using the templates provided by the Dementia with Lewy Bodies Consortium (66). The remaining slides were scored by trainees under the instruction of the primary observer. For the substantia nigra (SN), LTS was estimated using the same scoring method but on thioflavine-S-stained thick (40 micron) sections due to the standard laboratory practice of sectioning the SN in this manner for unbiased morphometric analysis.
Assessment of Nigrostriatal System Degeneration
The degree of nigrostriatal degeneration was assessed histologically and biochemically. Histologic evaluation of SN pigmented neuron loss was graded as none, mild, moderate or severe on H & E-stained microscopic sections taken at or close to the level of egress of the oculomotor nerve. The descriptive terms were converted to numerical scores from zero to three for statistical purposes.
Biochemical evaluation consisted of an enzyme-linked immunoassay (ELISA) for tyrosine hydroxylase (TH), the rate-limiting enzyme for the production of dopamine. The dissected sample was taken from frozen putamen at the level of the globus pallidus pars interna. This site has been reported to show the earliest loss of dopaminergic markers in PD (9). The ELISA method has been described in a prior publication (5).
Statistical Analysis
For statistical purposes, semi-quantitative microscopic lesion density estimates were converted to numerical scores from zero to three for CERAD neuritic plaque density, zero to four for LTS (DLB Consortium III templates) and zero to six for Braak neurofibrillary stage. For correlational analyses, the α-synuclein-immunoreactive density scores of the 10 evaluated brain regions were summed to give a single global score for each subject.
Statistical analyses consisted, for comparing group means, analysis of variance (ANOVA), or, for non-parametric data, Kruskall-Wallis ANOVA. Proportional measures were compared using chi-square tests. Correlations were performed using linear regression for continuous variables and Spearman’s rank correlation for discontinuous variables.
RESULTS
Basic Characteristics of the Study Subjects
Four-hundred and seventeen subjects had a full set of brain paraffin blocks (all ten regions specified in the methods section) as well as neuropathological diagnoses within the targeted groups. Descriptive measures of these groups are given in Table 1–Table 3. The subjects were all of advanced age (Table 1) and the group means differed significantly (p < 0.0001), with the youngest group (PD) having a mean age of 79.2 while the oldest group (ILBD) mean was 86.6. The diagnostic groups differed significantly (p = 0.003) in terms of disease duration (from first onset of symptoms until death), with DLB subjects having the shortest duration, at 6.9 years, while PD subjects had the longest duration, at 10.6 years. Subjects with AD were in between, with ADNLB subjects having a shorter duration than ADLB subjects. Subjects with PD, DLB and ILBD were more likely to be male (61–69% male) while normal subjects and subjects with AD (ADLB, ADNLB) were almost gender-neutral (46–51% male). Subjects with ADLB, ADNLB and DLB were much more likely to have one or more apoE-ɛ4 alleles (53–55%), in comparison with normal subjects, subjects with PD and subjects with ILBD (26–30%). The median postmortem intervals were uniformly short, ranging from 2.66 to 3.08 hours and the group means were not significantly different.
Table 1.
General characteristics of the study subjects, by neuropathologic diagnosis, age, gender, apoE genotype and postmortem interval (PMI). Means and standard deviations (SD) are given. Thirty-four of the DLB cases and 27 of the PD cases also met neuropathologic diagnostic criteria for AD.
| Diagnosis (N) | Age (SD)1 | Disease Duration, yrs (SD)2 | Gender (%M)3 | ApoE-ɛ4 (%)4 | PMI, hrs Median, mean (SD)5 |
|---|---|---|---|---|---|
| Normal (87) | 84.8 (6.9) | N/A | 51.7 | 26.4 | 2.7, 3.3 (2.8) |
| ILBD (26) | 86.6 (5.5) | N/A | 61.5 | 30.0 | 2.9, 3.1 (1.6) |
| PD (66) | 79.1 (6.9) | 10.6 (8.7) | 69.7 | 30.3 | 2.7, 3.8 (3.3) |
| DLB (40) | 80.7 (6.6) | 6.9 (4.7) | 65.0 | 55.0 | 3.1, 4.5 (4.7) |
| ADLB (85) | 83.4 (8.1) | 9.0 (4.7) | 50.6 | 55.3 | 3.0, 6.0 (8.9) |
| ADNLB (113) | 82.8 (9.5) | 7.3 (4.7) | 46.0 | 53.9 | 3.0, 6.3 (11.4) |
Group means were significantly different (p < 0.0001).
Group means were significantly different (p = 0.003).
Subjects with ADNLB differed in gender distribution from those with PD and DLB (P < 0.01 for the comparison with PD, p < 0.05 for the comparison with DLB); subjects with ADLB differed from those with PD (p < 0.05).
Normal subjects and ILBD subjects differed from those with DLB, ADLB and ADNLB in the proportion that were apoE-ɛ4 positive (p < 0.01 for comparisons with normal, p < 0.05 for comparisons with ILBD).
Group means were not significantly different. ILBD = incidental Lewy body disease; PD = Parkinson’s disease; DLB = dementia with Lewy bodies; ADLB = Alzheimer’s disease with Lewy bodies; ADNLB = Alzheimer’s disease with no Lewy bodies.
Table 3.
Neuropathological and neurochemical characteristics of the study subjects, by neuropathological diagnosis, CERAD neuritic plaque density, Braak neurofibrillary stage, substantia nigra (SN) pigmented neuron loss score and striatal tyrosine hydroxylase concentration (TH; ng/mg protein). Note that the lattermost was only available for a subset of the cases.
| Diagnosis (N) | Neuritic Plaque Density (SD)1 | Braak Neurofibrillary Stage (SD)1 | SN Pigmented Neuron Loss Score (SD)1 | Striatal TH (SD) N1 |
|---|---|---|---|---|
| Normal (87) | 1.26 (1.04) | 2.77 (0.95) | 0.39 (0.58) | 90.35 (92.75) 31 |
| ILBD (26) | 1.01 (0.93) | 2.85 (0.78) | 0.72 (1.02) | 45.86 (105.42) 13 |
| PD (66) | 1.29 (1.03) | 2.86 (0.97) | 2.71 (0.70) | 10.55 (14.41) 17 |
| DLB (40) | 2.20 (0.97) | 3.72 (0.85) | 2.15 (0.81) | - |
| ADLB (85) | 2.76 (0.47) | 5.22 (0.85) | 1.41 (0.96) | 99.7 (85.3) 14 |
| ADNLB (113) | 2.70 (0.48) | 4.71 (1.08) | 1.04 (0.94) | 75.53 (64.23) 13 |
Group means were significantly different (p < 0.0001). For striatal td concentrations, N for normal is 31, N for ILBD is 13, N for PD is 17, N for DLB is 2; N for ADLB is 14, N for ADNLB is 13. Abbreviations as given in Table 1.
Basic cognitive (MMSE) and neuromotor (Part 3 of the UPDRS) performance characteristics of the diagnostic groups are given in Table 2. The diagnostic groups differed significantly in their mean MMSE scores (p < 0.0001). Normal subjects and subjects with ILBD had higher mean MMSE scores (28.6 and 27.8, respectively) than all other subjects while subjects with ADLB, ADNLB and DLB had lower scores (9.9–12.9) than all other subjects. The diagnostic groups also differed significantly in their mean UPDRS scores (p < 0.0001). Normal subjects and subjects with ILBD had lower mean Part 3 UPDRS scores (9.8 and 7.8, respectively) than all other subjects while subjects with DLB had higher scores than all other subjects (mean score 52.1). Subjects with PD had higher UPDRS scores than normal subjects or subjects with ILBD. Subjects with AD had increased UPDRS scores compared to normal and ILBD subjects but ADLB subjects (mean score 31.5) did not differ appreciably from ADNLB subjects (mean score 28.9).
Table 2.
Cognitive and neuromotor screening test results for the study subjects, in terms of Mini Mental State Examination (MMSE) and motor scores (part 3) from the Unified Parkinson’s Disease Rating Scale (UPDRS). The time elapsed since the last clinical testing dates are given. Note that only a subset of the subjects listed in Table 1 had data available. Means and standard deviations are given where noted.
| Diagnosis | MMSE (SD) N1 | MMSE – Months Before Death (SD)2 | UPDRS (SD)3 | UPDRS - Months Before Death (SD)4 |
|---|---|---|---|---|
| Normal | 28.6 (1.5) 54 | 21.2 (16.7) | 9.8 (9.7) 55 | 14.3 (11.3) |
| ILBD | 27.8 (2.0) 15 | 16.7 (13.2) | 7.8 (4.2) 18 | 15.8 (12.3) |
| PD | 20.3 (8.7) 39 | 16.8 (13.8) | 41.0 (22.5) 27 | 15.1 (17.1) |
| DLB | 12.9 (8.9) 26 | 13.4 (14.7) | 52.1 (26.0) 9 | 14.2 (9.2) |
| ADLB | 9.9 (7.9) 54 | 15.0 (12.7) | 31.5 (28.6) 19 | 22.3 (26.0) |
| ADNLB | 11.1 (8.0) 68 | 15.1 (13.5) | 28.0 (23.4) 25 | 24.4 (20.8) |
Group means were significantly different (p < 0.0001).
Group means were not significantly different.
Group means were significantly different (p < 0.0001).
Group means were not significantly different.
Abbreviations as given in Table 1.
The elapsed times, for each diagnostic group, between the final clinical testing dates and death were compared (Table 2). For MMSE, the interval ranged, in the diagnostic groups, from 13 to 21 months but the group means were not significantly different. Similarly, for UPDRS, the interval between the final testing dates and death ranged from 14 to 24 months but the group means were not significantly different.
Relevant neuropathological characteristics of the study subjects are given in Table 3. The group means for all measures differed significantly (p < 0.0001). In terms of AD-related lesions, normal subjects and subjects with ILBD and PD had lower neuritic plaque scores and lower Braak neurofibrillary stages than subjects with DLB, ADLB and ADNLB. SN pigmented neuron loss was moderate to severe in PD and DLB. This was greater for PD subjects than for any other group. Subjects with ADLB had mild to moderate pigmented neuron loss, while subjects with ADNLB had only mild neuron loss, similar to that seen in normal and ILBD subjects. Subjects with DLB and PD had markedly decreased striatal TH concentrations, as compared with normal subjects (4% and 11%, respectively, compared to normal). For subjects with ILBD, TH was 50% depleted compared to normal subjects. Striatal TH correlated inversely with SN pigmented neuron loss score (R = −0.44, p = 0.002).
Regional Analysis of Lewy-Type LTS
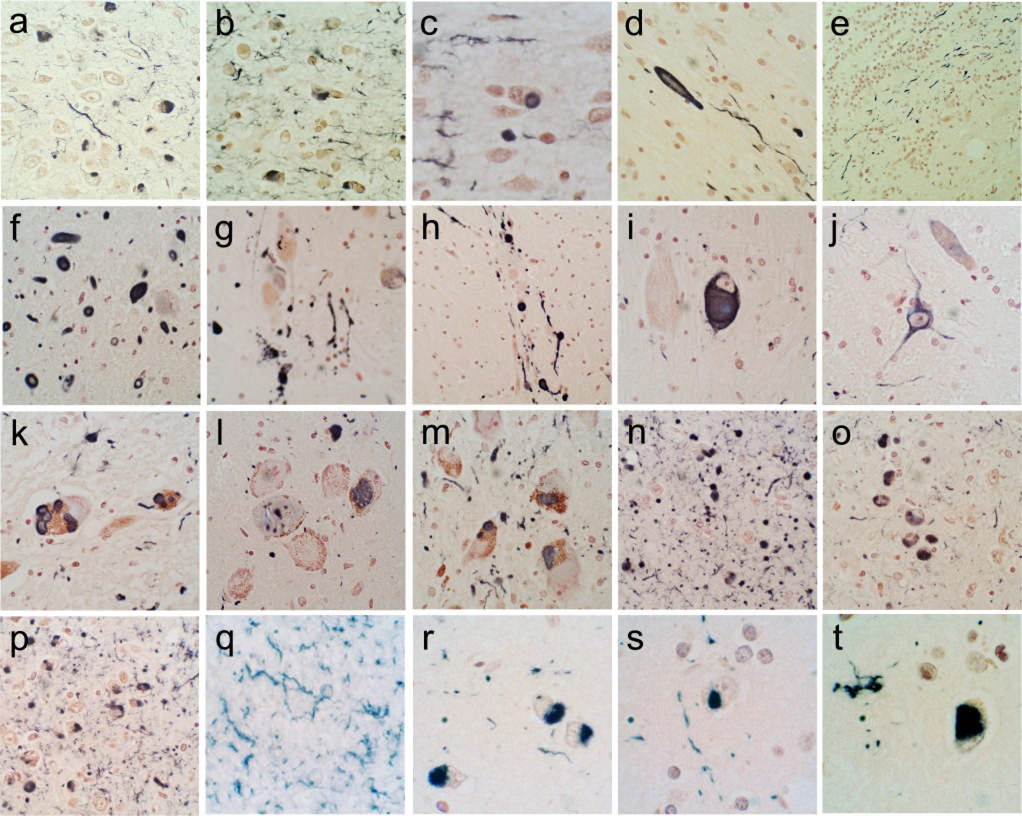
Figure 1 shows photomicrographs of the immunohistochemical staining for α-synuclein in the brain regions examined. Generally, sections with positive staining contained fibers, dot-like structures and neuronal perikaryal staining (a,b,c,g,k,l,m,n,o,p,q,s) but occasionally sections or regions contained just fibers or dots (d,e,f,h,r,t) or just perikaryal staining. The perikaryal staining was either diffusely distributed in the cytoplasm (i,j) or condensed into defined inclusions (a,b,c,g,k,l,m,n,o,p,q,s), a subset of which resembled classical Lewy bodies (c,k). The amygdala (n,o,p) was often the most densely stained region, especially those nuclei that lie superficially along the ambient and semilunar gyri. The cerebral cortex sections, other than the cingulate gyrus (r,s,t), were the least likely regions to be heavily stained but occasionally could display relatively dense arrays of immunoreactive structures (r) .
Figure 1.
Photomicrographs depicting immunohistochemical staining for α-synuclein in the brain regions investigated. Positive immunostaining is black; the counterstain is Neutral Red. (a–e) The olfactory bulb and tract. The anterior olfactory nucleus of the olfactory bulb is shown in a–c; both neuronal perikaryal inclusions as well as fibers are present. An enlarged, abnormal neurite within the olfactory tract is shown in d. Positive fibers coursing in parallel array through the internal plexiform layer are shown in e. (f–j) The anterior medulla. The dorsal motor nucleus of the vagus nerve is shown in f, the raphe nucleus in g and i, the internal tract of the IXth nerve in h and the lateral reticular nucleus in j. (k–m) The locus ceruleus in the pons. (n–p) The amgydala. (q) The cingulate gyrus. (r) The middle temporal gyrus. (s) The middle frontal gyrus. (t) The inferior parietal lobule.
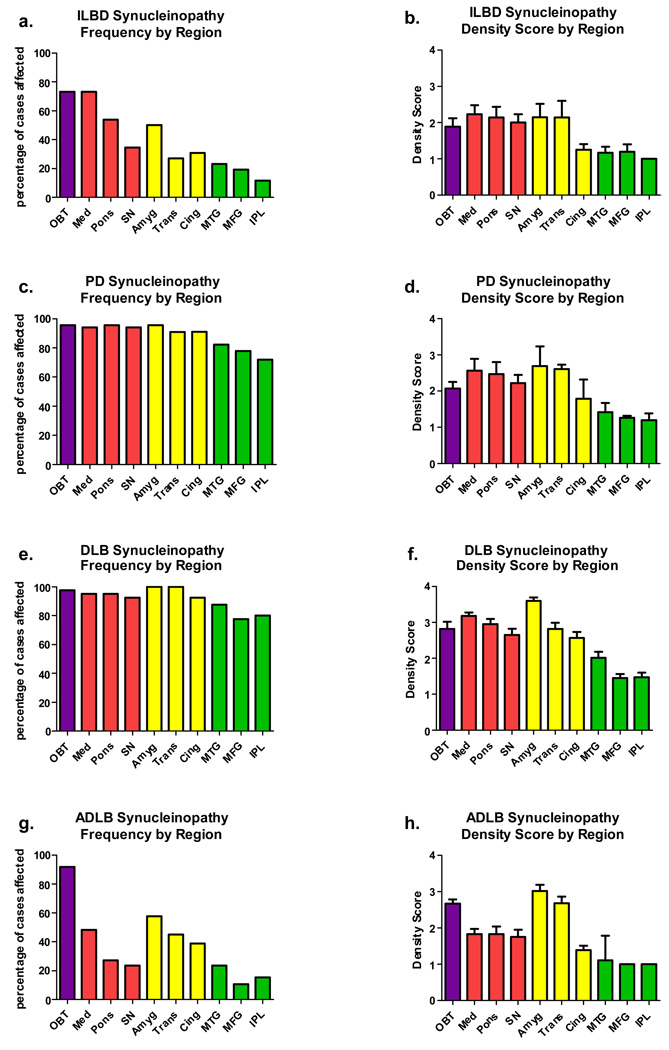
Figure 2 shows graphically the presence and density of Lewy bodies and related neurites in the ten selected brain areas and the four affected diagnostic groups (ILBD, PD, DLB, ADLB). The sub-regional distribution of LTS was not systematically examined but generally was consistent with that described previously in detail by others (16,59,48,85,80). It can be immediately appreciated from the frequency graphs in Figure 2 (a, c, e, g) that subjects with ILBD and ADLB have one pattern of involvement while those with PD and DLB have a second pattern. In ILBD and ADLB there are marked regional differences in the frequency of involvement (between 10% and 90%) while in PD and DLB all regions are frequently affected (70–90%). In both ILBD and ADLB, the regions most frequently affected are the olfactory bulb, medulla, pons and amygdala. Closer examination shows three major differences between ILBD and ADLB. The olfactory bulb is a most-affected region in both conditions but is the single most affected region in ADLB. While the medulla is frequently affected in both groups, it is much less frequently affected in ADLB than in ILBD. In ILBD, the brainstem regions, as a whole, are more frequently affected than the limbic regions, while in ADLB it is the reverse.
Figure 2.
Graphic depiction of Lewy-type synucleinopathy frequency (a,c,e,g) and density (b,d,f,h) by brain region. Purple bars are for olfactory bulb, red are for brainstem regions, yellow are for limbic regions and green are for neocortical regions. Error bars are standard error of the mean. Mean density scores differed significantly between regions for all diagnostic groups shown (p < 0.001). Abbreviations: OBT = olfactory bulb and tract; Med = medulla; SN = substantia nigra; Amyg = amygdala; Trans = transentorhinal cortex; Cing = cingulate gyrus; MTG = middle temporal gyrus; MFG = middle frontal gyrus; IPL = inferior parietal lobule.
The graphs depicting regional density scores (Figure 2b,d,f,h), only for those regions with a score of 1 or greater, are generally similar to the frequency graphs in that scores are uniformly higher in the PD and DLB groups and lower in the ILBD and ADLB groups. Again, the olfactory bulb is more heavily involved in ADLB than in ILBD, and again, in ADLB the limbic areas are, as a whole, more heavily affected than the brainstem while in ILBD there is a rough equivalency.
In all regions and diagnostic groups, the neocortical regions other than cingulate gyrus have the lowest frequencies of involvement and the lowest density scores when affected. Generally the temporal lobe section was affected more frequently and had higher density scores than the frontal or parietal sections.
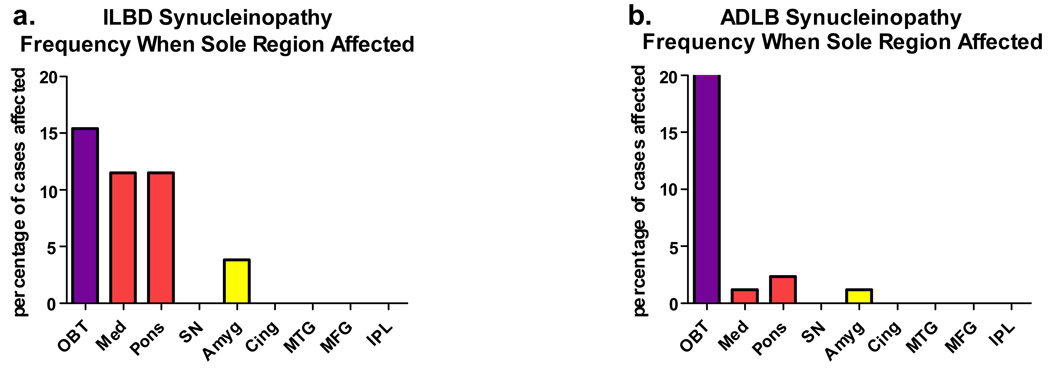
In an attempt to establish the brain region most likely to be initially involved by LTS, cases affected in only a single area were analyzed. The results are presented in Figure 3. There were no cases of PD or DLB that involved only a single brain region; for the latter condition, the diagnostic criteria preclude this possibility. For both ILBD and ADLB, the olfactory bulb was the brain region most frequently involved when only a single brain region was affected. For ILBD subjects, the medulla and pons lagged only slightly behind the olfactory bulb while for ADLB subjects, it was rare for another brain region to be the sole focus.
Figure 3.
Graphic depiction of the frequency with which brain regions were the sole region affected by Lewy-type synucleinopathy. (a) For ILBD subjects. (b) For ADLB subjects.
Correlations of LTS Density Scores with Nigrostriatal Degeneration, UPDRS and MMSE
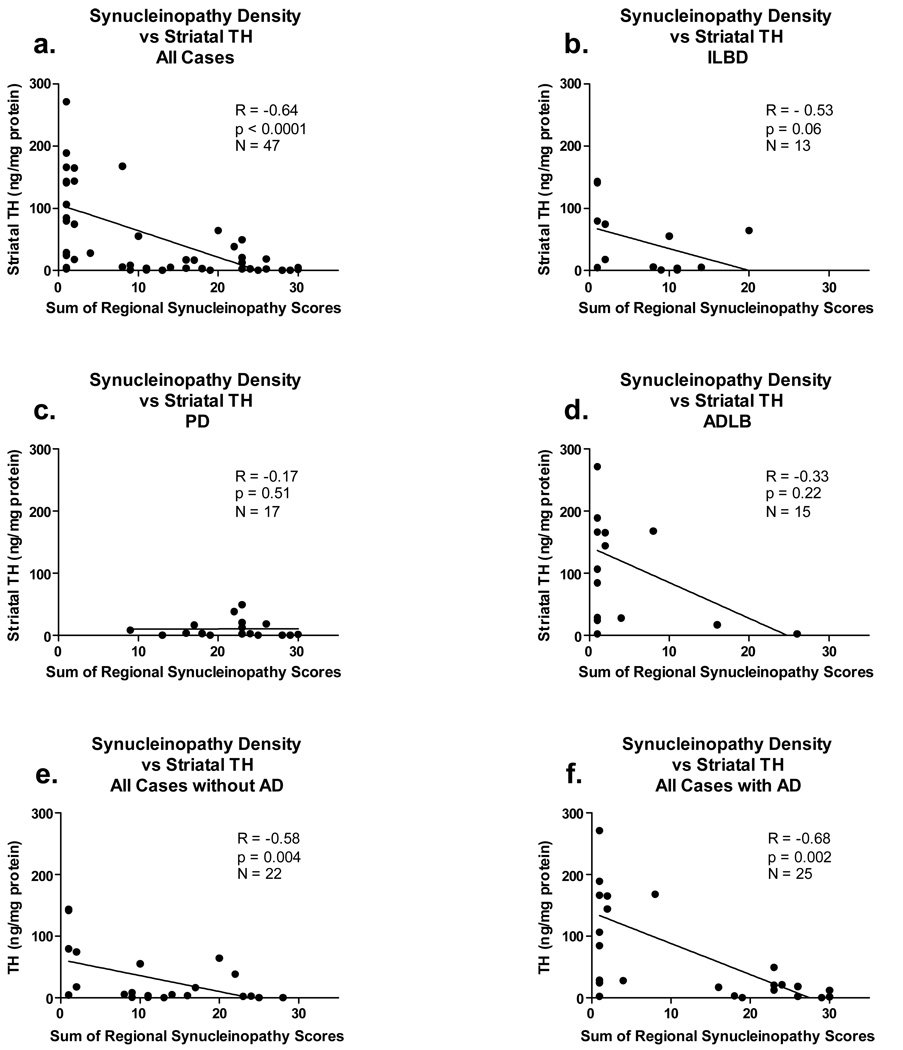
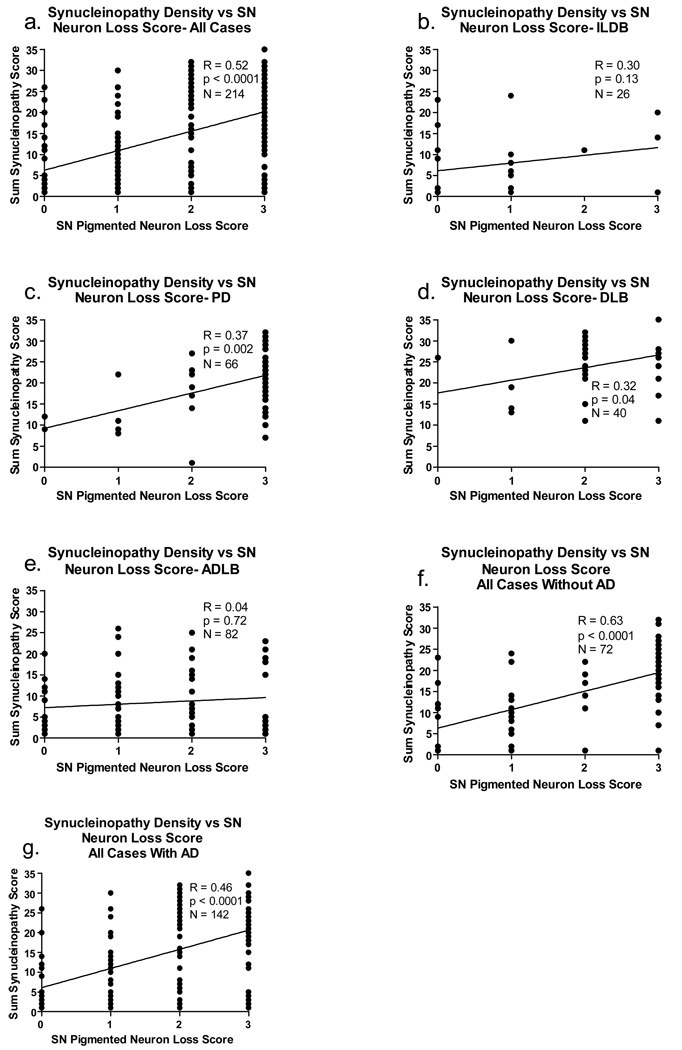
For these analyses, again only the subjects with LTS were included (ILBD, PD, DLB, ADLB). The global, sum-of-all-areas LTS density scores correlated significantly with both measures of nigrostriatal degeneration (correlations with striatal TH concentration shown in Figure 4, correlations with SN pigmented neuron loss score in Figure 5). The correlations were relatively strong and highly significant when all cases were considered together, regardless of diagnostic grouping (4a, 5a) as well as when all cases were subdivided by the presence (Figure 4f, Figure 5g) or absence (Figure 4e, Figure 5f) of diagnostic levels of AD histopathology (NIA-Reagan intermediate or high probability). For the ILDB group, there was a trend for LTS density scores to inversely correlate (R = - 0.53; p = 0.06) with striatal TH (Figure 4b) while the correlation with SN pigmented neuron loss score showed the same trend (Figure 5b; p = 0.13). For PD subjects, there was no significant correlation with striatal TH due to uniformly low TH values (Figure 4c) while the correlation with SN pigmented neuron loss score was significant (Figure 5c; p = 0.002). The DLB group had too few striatal TH measurements available for correlation analysis but the correlation with SN pigmented neuron loss score was significant (Figure 5d; p = 0.04). For the ADLB group there were no significant correlations (Figure 4d, Figure 5e).
Figure 4.
Graphs depicting correlations between the sum of brain regional synucleinopathy density scores and measures of striatal tyrosine hydroxylase (TH), in subjects divided or not divided by diagnostic group and presence or absence of AD. As there were only two DLB subjects with TH measurements available, there is no graph for this category, but the two subjects are included in graphs a and f. The graph titled “All Cases” included all subjects with Lewy body pathology and TH measurements available, regardless of diagnosis. The graph titled “with AD” included data from all subjects who also met NIA-Reagan “intermediate” or “high” criteria for AD, regardless of their other diagnoses. The graph titled “without AD” included data from all subjects who did not meet NIA-Reagan “intermediate” or “high” criteria for AD, regardless of their other diagnoses. R = Spearman Rho; N = number of subjects included in the analysis; p = probability. Abbreviations otherwise as for Figure 2.
Figure 5.
Graphs depicting correlations between the sum of brain regional synucleinopathy density scores and substantia nigra (SN) pigmented neuron loss score, in subjects divided or not divided by diagnostic group and presence or absence of AD. The graph titled “All Cases” included all subjects with Lewy body pathology regardless of diagnosis. The graph titled “with AD” included data from all subjects who also met NIA-Reagan “intermediate” or “high” criteria for AD, regardless of their other diagnoses. The graph titled “without AD” included data from all subjects who did not meet NIA-Reagan “intermediate” or “high” criteria for AD, regardless of their other diagnoses. R = Spearman Rho; N = number of subjects included in the analysis; p = probability. Abbreviations otherwise as for Figure 2.
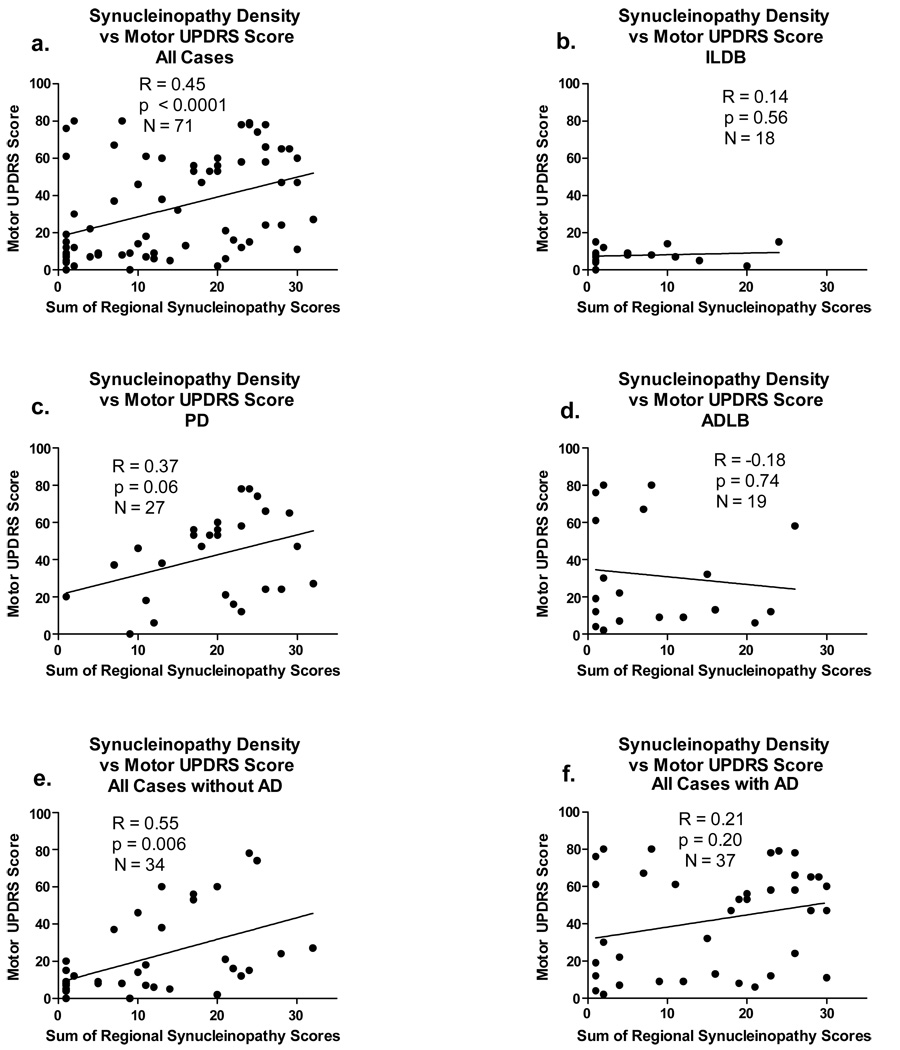
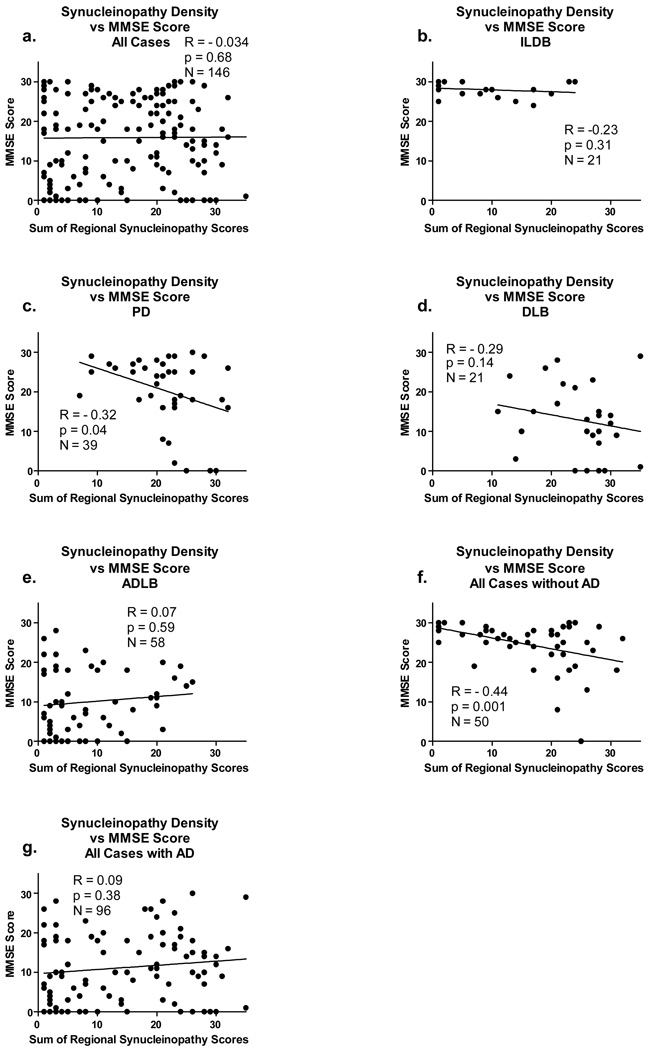
There is a relatively strong (R = 0.45) and significant (p < 0.0001) correlation between LST and UPDRS but not between LTS and MMSE when all subjects are considered together (Figure 6a and Figure 7a). In general, it appeared that significant correlations were obscured when subjects with diagnostic levels of AD were included. This is most dramatically illustrated for the correlation with MMSE (Figure 7 a, e, g), where there are no significant correlations when subjects with AD are included but a highly significant correlation when subjects with AD are excluded (Figure 7f). Similarly, but less dramatically, for UPDRS motor scores, when only subjects with AD are considered there are no significant correlations (Figure 6d, f). For subjects with PD and DLB, both of which have large fractions of subjects meeting diagnostic criteria for AD, the correlations for both UPDRS and MMSE are significant or near-significant for PD subjects, with a smaller fraction of co-existing AD (Figure 6c, Figure 7c) but not significant for DLB subjects, with a larger fraction of co-existing AD (only the correlation with MMSE is shown, due to too few DLB subjects with a motor UPDRS score). For ILBD subjects there are no significant correlations with either UPDRS and MMSE, consistent with the asymptomatic clinical status of this group.
Figure 6.
Graphs depicting correlations between the sum of brain regional synucleinopathy density scores and motor scores on the Unified Parkinson’s Disease Rating Scale (UPDRS), in subjects divided or not divided by diagnostic group and presence or absence of AD. As there were too few DLB subjects with UPDRS scores available, there is no graph for this category, but these subjects are included in graphs a, e and f. The graph titled “All Cases” included all subjects with Lewy body pathology regardless of diagnosis. The graph titled “with AD” included data from all subjects who also met NIA-Reagan “intermediate” or “high” criteria for AD, regardless of their other diagnoses. The graph titled “without AD” included data from all subjects who did not meet NIA-Reagan “intermediate” or “high” criteria for AD, regardless of their other diagnoses. R = Spearman Rho; N = number of subjects included in the analysis; p = probability. Abbreviations otherwise as for Figure 2.
Figure 7.
Graphs depicting correlations between the sum of brain regional synucleinopathy density scores and scores on the Mini Mental State Examination (MMSE), in subjects divided or not divided by diagnostic group and presence or absence of AD. The graph titled “All Cases” included all subjects with Lewy body pathology regardless of diagnosis. The graph titled “with AD” included data from all subjects who also met NIA-Reagan “intermediate” or “high” criteria for AD, regardless of their other diagnoses. The graph titled “without AD” included data from all subjects who did not meet NIA-Reagan “intermediate” or “high” criteria for AD, regardless of their other diagnoses. R = Spearman Rho; N = number of subjects included in the analysis; p = probability. Abbreviations otherwise as for Figure 2.
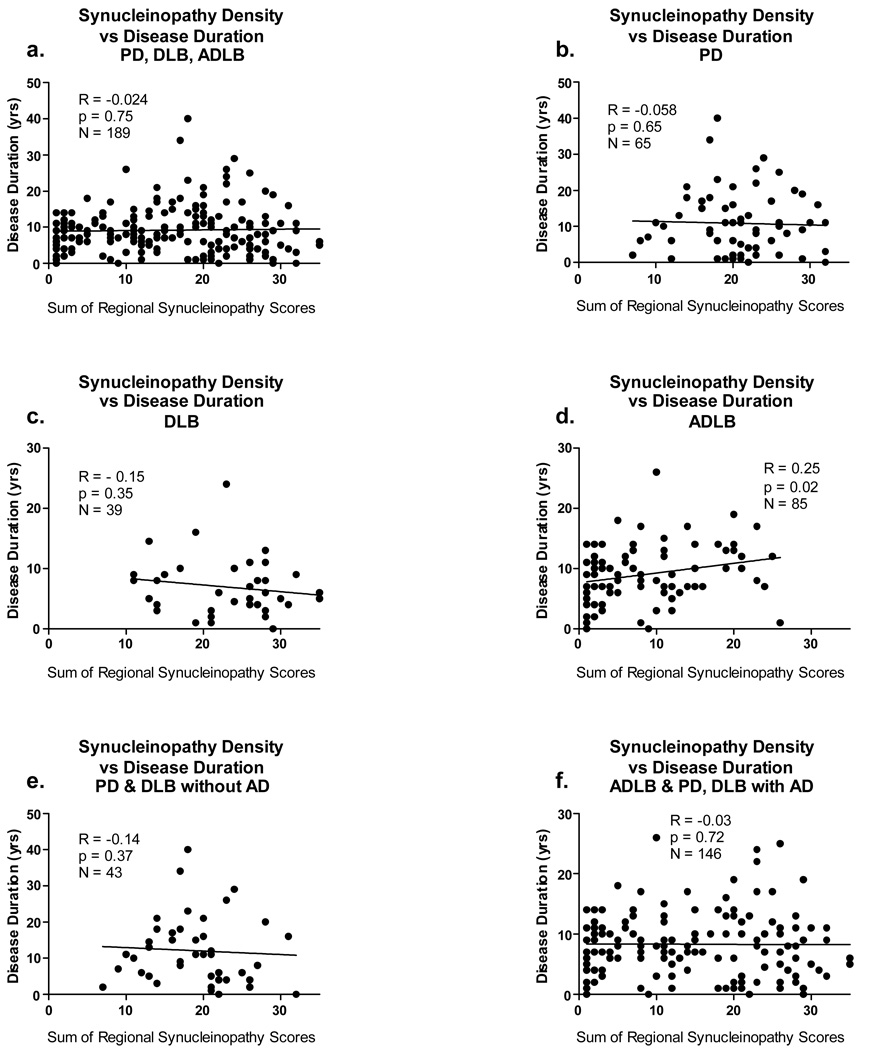
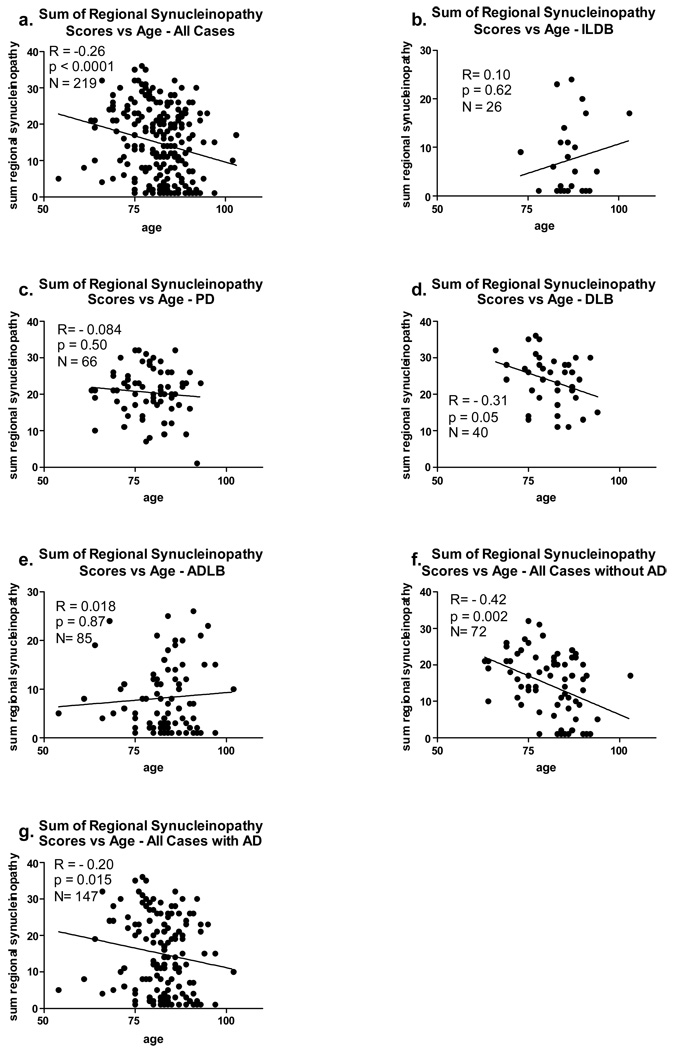
Correlations of LTS Density Scores With Disease Duration and Age
For the correlations with disease duration (Figure 8), all subjects with positive LTS density scores and a symptomatic disease condition (PD, DLB, ADLB) were included, while for the correlations with age (Figure 9), all subjects with a positive LTS score were included, regardless of clinical status (ILDB, PD, DLB, ADLB). The influence of co-existing AD was again explored by analyzing all subjects with and without AD.
Figure 8.
Graphs depicting correlations between the sum of brain regional synucleinopathy density scores and disease duration, in subjects divided or not divided by diagnostic group and presence or absence of AD. The graph titled “with AD” included data from all subjects who also met NIA-Reagan “intermediate” or “high” criteria for AD, regardless of their other diagnoses. The graph titled “without AD” included data from all subjects who did not meet NIA-Reagan “intermediate” or “high” criteria for AD, regardless of their other diagnoses. R = Spearman Rho; N = number of subjects included in the analysis; p = probability. Abbreviations otherwise as for Figure 2.
Figure 9.
Graphic depiction of the relationship between age and the sum of regional synucleinopathy densities, in subjects divided or not divided by diagnostic group and presence or absence of AD. The graph titled “with AD” included data from all subjects who also met NIA-Reagan “intermediate” or “high” criteria for AD, regardless of their other diagnoses. The graph titled “without AD” included data from all subjects who did not meet NIA-Reagan “intermediate” or “high” criteria for AD, regardless of their other diagnoses. R = Spearman Rho; N = number of subjects included in the analysis; p = probability. Abbreviations otherwise as for Figure 2.
The only significant correlation between LTS regional density scores and disease duration was within the ADLB group (Figure 8d; p = 0.02), where the sum of the regional LTS density scores increased mildly (R = 0.25) with increasing age. In contrast, there were significant correlations between age and LTS regional density scores when all subjects were considered together (Figure 9a; p < 0.0001) and also when all subjects with (Figure 9g; p = 0.015) or without a co-existing diagnosis of AD were considered (Figure 9f; p = 0.002). Additionally, there was an inverse correlation of age with LTS density within the DLB group (Figure 9d; p = 0.05). In contrast, the ILDB (Figure 9b)and ADLB (figure 9e) groups showed a trend, although non-significant, for a positive correlation with age.
LTS-Based Staging of Study Subjects
When study subjects were classified, in terms of their LTS regional involvement, by either the Braak system (11) or the DLB Consortium system (66), there were a high proportion of subjects that could not be assigned a stage (Table 4 and Table 5). For the Braak system, 42% of subjects were not classifiable while for the DLB Consortium system, 50% of subjects were unclassifiable. For both systems, approximately one-third of the unclassifiable subjects were due to olfactory bulb-only cases, while approximately two-thirds were due to limbic system involvement in the absence of brainstem pathology. Neither system provides for the latter pattern of involvement while the Braak system has been unclear as to the status of olfactory bulb-only cases.
Table 4.
Classification of study subjects (all diagnoses together) by the Braak Lewy body staging system. Twenty-six of the unclassifiable cases were due to involvement of the olfactory bulb only while 66 were due to non-sequential involvement of regions.
| I | II | III-IV | V | VI | Unclassifiable | |
|---|---|---|---|---|---|---|
| N (%) | 12 (5.5%) | 1 (0.5%) | 3 (3%) | 11 (5%) | 96 (44%) | 90 (42%) |
Table 5.
Classification of study subjects by Dementia with Lewy Body Consortium (III) system. Twenty-six of the unclassifiable cases were due to involvement of the olfactory bulb only while 83 were due to non-sequential involvement of regions.
| Brainstem Predominant | Limbic (Transitional) | Neocortical | Unclassifiable | |
|---|---|---|---|---|
| N (%) | 5 (2.3%) | 76 (35%) | 29 (13%) | 108 (50%) |
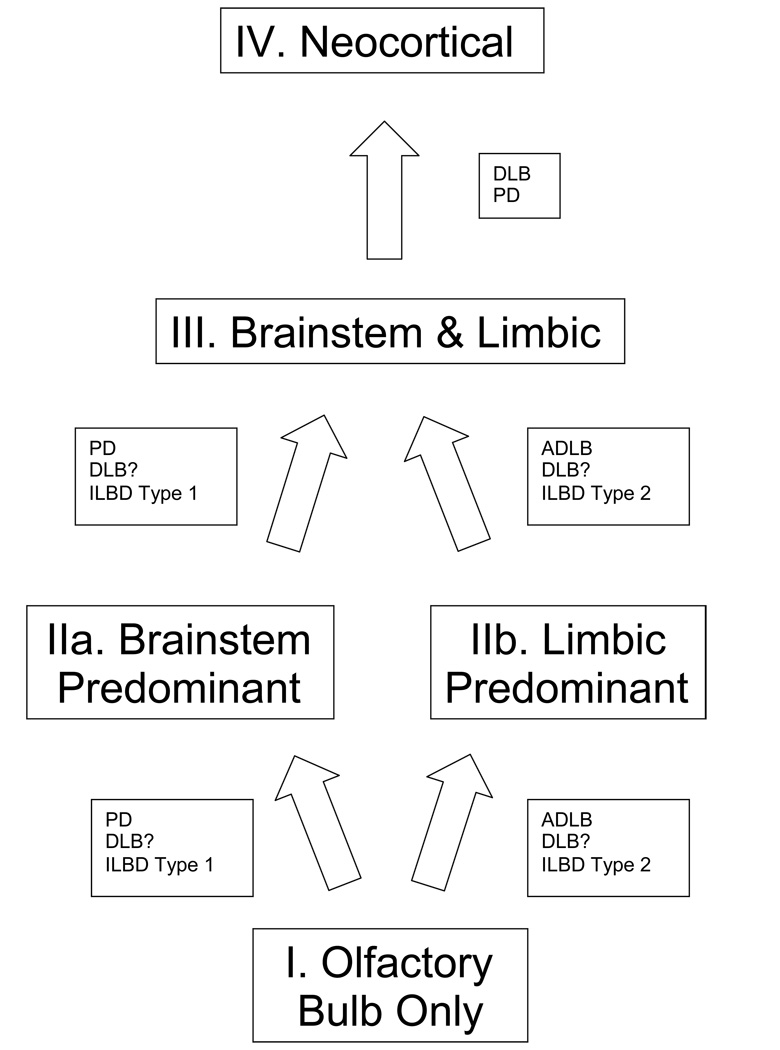
A new staging system was therefore devised so that all subjects could be assigned to a defined stage. The results of the regional frequency and density analyses (Figure 2a–h and Figure 3a–b) were used as the basis for constructing an hypothetical regional progression pathway and unified staging system for Lewy body disorders (Figure 10 and Table 6). As the olfactory bulb is the most frequently involved brain region, and the most frequent region to be solely involved, Stage I is defined as cases with pathology only in the olfactory bulb. However, as occasionally the olfactory bulb is not involved first, and rarely may not be involved at all, Stage I is not required and subjects may be classified as any higher stage without regard to the olfactory bulb score. From the olfactory bulb, the pathway diverges into one that has brainstem-predominant involvement (Stage IIa) and one that has involvement predominantly of the limbic system (Stage IIb). The basis for this distinction is made by comparing LTS density scores in brainstem and limbic regions, as detailed in Table 6; the distinction is made when scores in all areas of one region are clearly higher than the scores in all areas of the other region. Stage III (Brainstem and Limbic) subjects are those for whom LTS density scores in brainstem and limbic regions are roughly equivalent. The neocortical stage (Stage IV) is reached when any single neocortical region has an LTS density score of 2 or higher, signifying unequivocal involvement of the neocortex. Single or multiple neocortical LTS density scores of 1 do not affect staging.
Figure 10.
Schematic depiction of hypothetical progression pathways and stages for Lewy body disorders. The pathway for PD seems likely to proceed through Stage IIa (brainstem predominant) while that for ADLB passes through Stage IIb. The pathway followed by DLB is uncertain as the current definition of DLB largely excludes all but the neocortical stage. There may be two types of ILBD, one that leads to PD and another that leads to ADLB and possibly DLB. Only PD and DLB progress to the neocortical stage.
Table 6.
Classification rules for the proposed unified staging system for Lewy body disorders. Density scores are derived from the Third Dementia with Lewy Bodies Consortium conference. Scores refer to range of scores for all regions within a subdivision. The criteria for each stage are outlined in the columns and are dependent on the LTS density scores within the regions, ie. olfactory bulb, brainstem, limbic and neocortical. Scores given in the table are for any individual area within that region. For example, a subject would be scored as brainstem predominant if the highest brainstem area score range (1–2 or 3–4) was higher than the highest limbic score range (0–1 or 1–2, respectively). Additionally, to qualify as any stage less than neocortical, the highest neocortical area score would have to be 0 or 1. For olfactory bulb-only stage, all other regional scores must all be 0. Otherwise, the olfactory bulb score does not affect scoring for any other stage.
| Regional Score (DLB III) | I. Olfactory Bulb-Only | IIa. Brainstem Predominant | IIb. Limbic Predominant | III. Brainstem/Limbic | IV. Neocortical |
|---|---|---|---|---|---|
| Olfactory Bulb | Score 1–4 | Score 0–4 | Score 0–4 | Score 0–4 | Score 0–4 |
| Brainstem | Scores all 0 |
Either a or b a. Scores 1–2 b. Scores 3–4 |
Either a or b a. Scores 0 b. Scores 1–2 |
Either a or b a. Scores 1–2 b. Scores 3–4 |
Scores 0–4 |
| Limbic | Scores all 0 |
Match a & b with above a. Scores 0 b. Scores 1–2 |
Match a & b with above a. Scores 1–2 b. Scores 3–4 |
Match a & b with above a. Scores 1–2 b. Scores 3–4 |
Scores 0–4 |
| Neocortical | Scores all 0 | Scores 0–1 | Scores 0–1 | Scores 0–1 | Scores 2–4 |
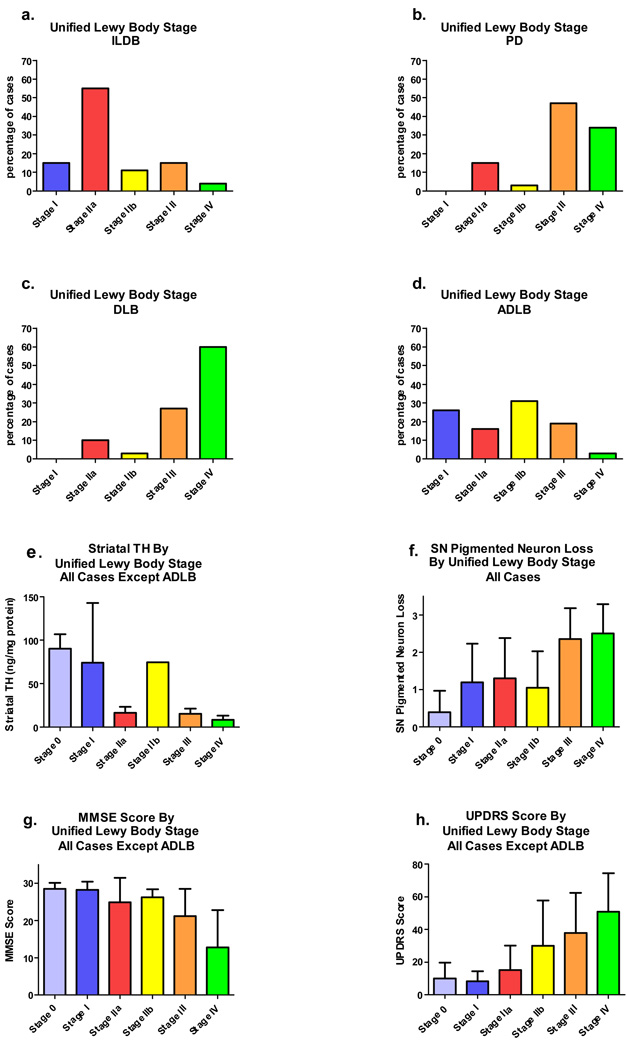
Figures 11a–d show the distribution of subjects within the proposed staging system, organized by disease entity. Most ILBD cases are classified as Stage IIa, Brainstem Predominant, most PD and DLB subjects are in Stages III, Brainstem and Limbic or IV, Neocortical, and most ADLB subjects are in stage I, Olfactory Bulb-Only or IIb Limbic Predominant. Table 7 and Table 8 show the proportions of PD subjects classified into the various stages after subdivision according to presence or absence of dementia, the latter further divided by the presence of absence of neuropathologically confirmed Alzheimer’s disease. Non-demented subjects with PD had a much higher percentage of Stage IIa Brainstem Predominant cases (30%) than the subjects with PD and dementia, with or without AD (3–12%), and many fewer subjects with Neocortical Stage IV (13% for non-demented PD vs 31–55% for PD with dementia), but still a large percentage (47%) with Stage III (Brainstem and Limbic). Surprisingly, PD/AD subjects had a much higher percentage of cases in the Neocortical Stage IV than the PDD subjects without AD (55% vs 31%).
Figure 11.
Graphic depiction of differential characteristics of the subjects classified by the proposed unified Lewy body staging system. For all graphs, the 0 stage data is from the normal control group. a) Incidental Lewy body disease (ILBD) subjects classified by Unified Lewy body stage. b) Parkinson’s disease (PD) subjects classified by Unified Lewy body stage. c) Dementia with Lewy bodies (DLB) subjects classified by Unified Lewy body stage. e) Unified Lewy body stage by striatal TH concentration. Subjects with ADLB were excluded from the analysis. Error bars show standard error of the mean. The means of the stages differed significantly (p < 0.001). f) Unified Lewy body stage by substantia nigra pigmented neuron loss score. All subjects with Lewy body pathology were included. Error bars indicate standard deviation. The means of the stages differed significantly (p < 0.001).
Table 7.
Basic characteristics of subjects with PD, subdivided by presence or absence of dementia and neuropathological Alzheimer’s disease. PDND = Parkinson’s disease, non-demented; PDD/AD = Parkinson’s disease, demented, Alzheimer’s disease; PDD/NAD = Parkinson’s disease, demented, no Alzheimer’s disease.
| Diagnosis (N) | Age (SD)1 | Gender (%M)2 | ApoE-ɛ4 +ve (%)3 | PMI, hrs4 Median, mean (SD) |
|---|---|---|---|---|
| PDND (23) | 80.5 (7.6) | 65.2 | 21.7 | 2.7; 3.8 (3.3) |
| PDD/AD (27) | 80.8 (5.6) | 70.4 | 37.0 | 2.5; 3.6 (3.0) |
| PDD/NAD (16) | 74.1 (6.2) | 70.6 | 31.2 | 3.0;3.9 (4.0) |
PDD/NAD subjects are significantly younger than other subjects (p < 0.05);
Gender distribution differences were not significant.
PDD/AD subjects have a significantly higher proportion that are positive for the ɛ4 allele, as compared with PDND subjects (p < 0.05).
Differences in PMI were not significant.
Table 8.
Classification of PD subjects into the proposed Lewy body staging system, after subdivision by presence or absence of dementia and neuropathological Alzheimer’s disease. Figures shown are number of subjects and percentage of category.
| I. Olfactory Bulb-Only | IIa. Brainstem Predominant | IIb. Limbic Predominant | III. Brainstem/Limbic | IV. Neocortical | |
|---|---|---|---|---|---|
| PD/ND N = 23 | 0 | 7 (30.4) | 2 (8.7) | 11 (47.8) | 3 (13.0) |
| PDD/AD N = 27 | 0 | 1 (3.7) | 0 (0) | 11 (40.7) | 15 (55.5) |
| PDD/NAD N = 16 | 0 | 2 (12.5) | 0 (0) | 9 (56.2) | 5 (31.2) |
Correlation of Proposed Unified Lewy Body Disorder Staging System with MMSE, UPDRS and Measures of Nigrostriatal Degeneration
Figures 11e–h graphically depict how the proposed unified Lewy body staging system relates to measures of cognitive impairment (MMSE; Figure 11g), motor dysfunction (UPDRS Part 3; Figure 11h) and nigrostriatal system degeneration (striatal TH; Figure 11e; SN pigmented neuron loss score; Figure 11f). As preliminary analyses indicated that inclusion of subjects with ADLB obscured relevant correlations with the other groups, ADLB subjects were excluded when this occurred . The results indicate that cognitive impairment and motor dysfunction generally progressively worsen with increasing stage. Similarly, measures of nigrostriatal degeneration become progressively greater passing from stage 0 to stage IV, with the exception of stage IIb (Limbic Predominant), which, predictably, has less nigrostriatal degeneration than stage IIa (Brainstem Predominant). The stepwise progression is most clearly seen with UPDRS scores (Figure 11h).
DISCUSSION
The usefulness of any disease staging system depends on the degree of its applicability to the intended population and on its ability to divide affected subjects into biologically significant stages. The two current major staging systems in use for PD and DLB, that of Braak (11) and the DLB Consortium (66), do not meet the first of these two criteria very well. Reports by several groups as well as the current study have indicated that both systems fail to classify up to 50% of subjects (59,52,75,34,62,51). Both systems have proposed stages based on a topographical progression that begins in the brainstem and then spreads progressively to the limbic lobe and the cerebral neocortex. Neither system allows for large numbers of subjects who have LTS confined to the olfactory bulb or who pass through a limbic-predominant pathway that at least initially bypasses the brainstem. A recent publication has acknowledged this difficulty, proposing a modification allowing a limbic-only stage (60). This study did not include the olfactory bulb, however, and did not therefore note the additional difficulties with classifying olfactory bulb-only cases. The results of the current study suggest a new, unified staging system that allows for the classification of all subjects with Lewy body disorders. The biological significance of these stages, as with the original staging systems, largely remains to be determined but the generally stagewise worsening of cognition, motor function and nigrostriatal degeneration provides initial support for its clinical and biological relevance. These correlations are generally not evident within the ADLB group, perhaps due to the loss of appreciation of a smaller effect due to the masking of a restricted and low-intensity LTS by a much more widespread and higher-intensity AD histopathology. In general, accompanying diagnostic-level AD histopathology tended to obscure LTS-related associations within all of the groups under consideration but within the DLB and PD groups correlations with LTS are generally still evident even with co-existing AD. It may be pertinent that some authors (71,35) have suggested that LTS and tauopathy, at least in some situations, may be generated by the same stimulus, with the outcome possibly having an inverse relationship dependent on genetic background or possibly environmental factors.
The current study suggests a new schematic for the temporal progression and topographical distribution of LTS (Figure 4), although the temporal aspect must be admittedly regarded as speculative. However, it seems likely that the earliest stages are most clearly seen in clinically normal elderly subjects, up to 25% of which harbor LTS in one or more brain regions and have been termed incidental Lewy body disease (ILBD). Early stages may also be exemplified by subjects with AD and Lewy body pathology insufficient to meet DLB Consortium criteria (ADLB). In both of these groups, the olfactory bulb is often the only brain region affected and for this reason such cases are assigned Stage I. From the olfactory bulb, subjects with ILBD and ADLB may diverge. Most subjects with ILBD pass into the Brainstem Predominant Stage IIa (ILBD Type 1) while most subjects with ADLB go directly to the limbic system, especially the amgydala, as the Limbic Predominant Stage IIb. A sizeable subset of ILBD cases are also classified as Stage IIb (ILBD Type 2). Following the two parts of Stage II, the pathways converge again into a single Brainstem and Limbic Stage III. The Neocortical Stage IV is reached virtually exclusively by subjects meeting diagnostic criteria for PD and DLB.
As subjects with clinically evident PD and DLB frequently have involvement of all of the regions under consideration, the pathways that these subjects initially followed become more of a matter of speculation. However, in both of these groups, more subjects were classified as Stage IIa (brainstem predominant) than stage IIb (limbic predominant), suggesting that most of these subjects pass through the brainstem prior to the limbic system, and are therefore unlike ADLB cases, most of which proceed to the limbic Stage IIb before significantly involving the brainstem. It seems unlikely that the differing patterns and densities of LTS are a result only of differing disease durations, as even though the greatest densities and distributions of LTS occur in PD and DLB, these have the longest and shortest disease durations, respectively, and disease duration did not correlate significantly with LTS in any diagnostic group except ADLB.
As ADLB has been defined almost accidentally by the most recent revision of the DLB Consortium, it seems at first consideration unlikely to be a condition completely separate from both PD and DLB. With respect to its accompanying AD pathology and high apoE-ɛ4 prevalence (Table 1), ADLB seems very similar to DLB, but the greater male gender distribution and greater nigrostriatal degeneration in DLB ally that condition more closely with PD. Although both ADLB and DLB have high neuritic plaque density scores (Table 3), the fact that DLB subjects have generally lower Braak neurofibrillary stages than ADLB cases, and that disease duration is much shorter in DLB than in ADLB, argue against the hypothesis that ADLB might represent early DLB. In some ways, ADLB resembles ILDB most closely, as with both there is a topographically-restricted LTS that increases gradually with age while both DLB and PD have widespread LTS that has an inverse correlation with age. At the present time it appears that ADLB, PD and DLB are sufficiently different from one another as to justify their separate existence while the LTS common to all is suggestive of at least some shared pathogenic pathways (63,27,85).
An important disclaimer is that the brain region frequency analysis has shown that it is possible that many cases may not follow the temporal progression pathway outlined in Figure 10. Some cases have brainstem and/or limbic involvement prior to the olfactory bulb and rare cases have isolated and/or sparse neocortical involvement before the preceding two stages are reached. Other cases, perhaps including PD and DLB, may begin with simultaneous and widespread but light involvement of many regions. This does not preclude these from classification, however, as the rules established here allow for non-sequential progression. The persistence of a subset of cases with an apparently random direction of pathological spread suggests, however, that progression through a specified topographical sequence is not an obligate form of disease advancement, but rather is indicative that the observed patterns of involvement are due to probabilistic rather than deterministic factors.
Prior published work has increasingly questioned the relevance of LTS for clinically relevant phenomena such as cognitive or motor dysfunction (19,75,51). The results presented here show that there are clear and significant correlations of LTS density scores with those for MMSE, UPDRS, SN pigmented neuron loss and striatal TH concentration. Also apparent, however, is the ability of co-existent AD histopathology to obscure these relationships, especially within the ADLB group. Inclusion of such cases in previous studies, which are probably relatively numerous in most populations, may have similarly prevented the appreciation of relevant correlations with LTS in these investigations. As ADLB is a condition with an LTS distribution and density that is much reduced compared to PD and DLB, and in fact resembling that seen in ILBD, it should not be surprising that this relatively light level of pathology has correspondingly little effect on clinical, biochemical or structural brain measures.
The results of this study are supportive of prior published evidence that subjects with ILBD have increased nigrostriatal degeneration relative to normal elderly subjects without LTS. Both in terms of SN pigmented neuron numbers (23,79,33) and striatal dopaminergic markers (23,25), subjects with ILBD have significant degeneration that places them intermediate between PD and age-similar normal elderly subjects. This important finding makes it very likely that ILBD is not a benign disorder and may represent the earliest stage of other Lewy body disorders. As the current study and other recent work (81,69,50) have shown that between 16 and 30% of the asymptomatic elderly have ILBD, this greatly expands the estimated prevalence of Lewy body disorders in the human population. Indeed, the clinically manifest Lewy body disorders may be regarded as truly the tip of the iceberg in terms of the numbers of people affected as it is now apparent that the most common Lewy body disorder by far is ILBD. Further study of this crucial group may identify clinical or laboratory biomarkers that would allow for presymptomatic detection and preventative therapy.
The present work indicates that the olfactory bulb has a special vulnerability for LTS. It has long been observed that subjects with Parkinson’s disease (PD) have decreased olfactory sensation (26,2,42,56). Attempts to use tests of olfaction for clinical diagnosis have had mixed results, however, due to loss of olfactory ability in other conditions as well (68,53). Lewy bodies and LTS have been reported to be present in the olfactory bulbs of subjects with PD, DLB, ILBD and ADLB (83,48,21,77,14,35), suggesting that olfactory bulb LTS is common to all Lewy body disorders and occurs at an early stage of disease. We have recently published results showing that the presence of LTS in the olfactory bulb is an excellent biomarker for the presence of a more widely-distributed Lewy body disorder, with sensitivity and specificity of close to or greater than 90% for subjects with PD, DLB or ADLB (8). It is now clear, from the large number of cases identified in the present study as having LTS exclusively in the olfactory bulb, that for many subjects this is the first area affected. Other authors have offered reasons for this special vulnerability, citing thinly myelinated and lengthy axons (17), an unusually high degree of neuronal plasticity (31,48) or the spatial proximity to the external environment, with its associated exposure to toxins and infectious agents (58,18,43). These remain speculative but it seems likely that further investigations of the olfactory bulb may yield new insights into Lewy body disorders. The virtually universal involvement of the olfactory bulb is a unifying feature of the Lewy body disorders, providing some support for the concept that they are all part of a continuum, with the variability coming from the varying genetic and environmental background of affected individuals.
The correlation analyses of LTS vs disease duration and LTS vs age showed a mild progression of LTS with age in ILBD and ADLB but an inverse correlation with age in DLB and PD. This is similar to what has been widely known about AD histopathology for many years, ie. younger cases have more severe pathology. While AD and Lewy body disorders are clearly age-associated, and their respective histopathologies are both more prevalent in the population with increasing age, it appears that individuals with more susceptibility to these conditions are affected earlier and more severely while those with less susceptibility are affected later and less severely. Individuals with ILDB and ADLB may contain disproportionate numbers of less-susceptible individuals.
It may be maintained that because the Lewy body disorders may differ biologically, they each should have their own unique staging systems. The failure, within ADLB subjects, to achieve a significant correlation between LTS density, MMSE scores and UPDRS scores, might suggest that these subjects are biologically different from the others. However, it is also possible that ADLB subjects, like ILDB subjects, have LTS that is too limited, in both distribution and density, to affect clinical measures. It is suggested here that there is insufficient data to answer the question of biological similarity. Supporting biological similarity is the frequent presence in subjects in all of the categories, ie. ILDB, PD, DLB and ADLB, of plaques, tangles and Lewy bodies. Another unifying feature is that, as discussed above, virtually all cases have olfactory bulb involvement. The overall similarities within the pairing of ILBD and ADLB, as well as within the pairing of PD and DLB, with respect to the topographical patterns of their LTS density and distribution frequencies, is supportive of two separate groups with two disorders in each, but these two groups may simply represent earlier and later stages of the same disorder. Justifiably, some researchers still argue that all of these conditions are part of a continuum.
Although the Braak and DLB Consortium systems work relatively well within their targeted diagnostic groups, it is difficult to decide which system to use for ILBD and ADLB, neither one of which is well served by either. The proposed unified staging system would be a simple way to quickly and efficiently describe the topographical distribution of Lewy bodies in any single subject, in any diagnostic category. Importantly this would also allow researchers to easily compare Lewy body stages across diagnostic categories, something that has not been easily possible with the usage of multiple staging systems.
Acknowledgements
This research is supported by grants to the Sun Health Research Institute Brain Donation Program and the Arizona Parkinson's Disease Consortium by the Michael J. Fox Foundation for Parkinson’s Research (The Prescott Family Initiative), the Arizona Biomedical Research Commission (contracts 4001, 0011 and 05-901) and the National Institute on Aging (P30 AG19610).
References
- 1.Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Neurobiol Aging. 1997;18:S1–S2. [PubMed] [Google Scholar]
- 2.Adler CH. Nonmotor complications in Parkinson's disease. Mov Disord. 2005;20 Suppl 11:S23–S29. doi: 10.1002/mds.20460. [DOI] [PubMed] [Google Scholar]
- 3.Alafuzoff I, Parkkinen L, Al-Sarraj S, Arzberger T, Bell J, Bodi I, Bogdanovic N, Budka H, Ferrer I, Gelpi E, Gentleman S, Giaccone G, Kamphorst W, King A, Korkolopoulou P, Kovacs GG, Larionov S, Meyronet D, Monoranu C, Morris J, Parchi P, Patsouris E, Roggendorf W, Seilhean D, Streichenberger N, Thal DR, Kretzschmar H. Assessment of alpha-synuclein pathology: a study of the BrainNet Europe Consortium. J Neuropathol Exp Neurol. 2008;67:125–143. doi: 10.1097/nen.0b013e3181633526. [DOI] [PubMed] [Google Scholar]
- 4.Baba M, Nakajo S, Tu PH, Tomita T, Nakaya K, Lee VM, Trojanowski JQ, Iwatsubo T. Aggregation of alpha-synuclein in Lewy bodies of sporadic Parkinson's disease and dementia with Lewy bodies. Am J Pathol. 1998;152:879–884. [PMC free article] [PubMed] [Google Scholar]
- 5.Beach TG, Adler CH, Sue LI, Peirce JB, Bachalakuri J, Dalsing-Hernandez JE, Lue LF, Caviness JN, Connor DJ, Sabbagh MN, Walker DG. Reduced striatal tyrosine hydroxylase in incidental Lewy body disease. Acta Neuropathol. 2008;115:445–451. doi: 10.1007/s00401-007-0313-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beach TG, Sue LI, Walker DG, Roher AE, Lue L, Vedders L, Connor DJ, Sabbagh MN, Rogers J. The Sun Health Research Institute Brain Donation Program: description and experience, 1987–2007. Cell Tissue Bank. 2008;9:229–245. doi: 10.1007/s10561-008-9067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beach TG, White CL, Hamilton RL, Duda JE, Iwatsubo T, Dickson DW, Roncaroli F, Buttini M, Hladik CL, Sue LI, Noorigian JV, Adler CH. Evaluation of alpha-synuclein immunohistochemical methods used by invited experts. Acta Neuropathol. 2008;116:277–288. doi: 10.1007/s00401-008-0409-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beach TG, White CL, III, Hladik CL, Sabbagh MN, Connor DJ, Shill HA, Sue LI, Sasse J, Bachalakuri J, Henry-Watson J, Akiyama H, Adler CH. Olfactory bulb alpha-LTS has high specificity and sensitivity for Lewy body disorders. Acta Neuropathol. 2009;117:169–174. doi: 10.1007/s00401-008-0450-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20:415–455. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- 10.Bloch A, Probst A, Bissig H, Adams H, Tolnay M. Alpha-synuclein pathology of the spinal and peripheral autonomic nervous system in neurologically unimpaired elderly subjects. Neuropathol Appl Neurobiol. 2006;32:284–295. doi: 10.1111/j.1365-2990.2006.00727.x. [DOI] [PubMed] [Google Scholar]
- 11.Braak H, Bohl JR, Muller CM, Rub U, de Vos RA, Del Tredici K. Stanley Fahn Lecture 2005: The staging procedure for the inclusion body pathology associated with sporadic Parkinson’s disease reconsidered. Mov Disord. 2006;21:2042–2051. doi: 10.1002/mds.21065. [DOI] [PubMed] [Google Scholar]
- 12.Braak H, Braak E, Yilmazer D, de Vos RA, Jansen EN, Bohl J, Jellinger K. Amygdala pathology in Parkinson's disease. Acta Neuropathol. 1994;88:493–500. doi: 10.1007/BF00296485. [DOI] [PubMed] [Google Scholar]
- 13.Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach's plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett. 2006;396:67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Braak H, Del Tredici K, Bratzke H, Hamm-Clement J, Sandmann-Keil D, Rub U. Staging of the intracerebral inclusion body pathology associated with idiopathic Parkinson's disease (preclinical and clinical stages) J Neurol. 2002;249 Suppl 3:III/1–III/5. doi: 10.1007/s00415-002-1301-4. [DOI] [PubMed] [Google Scholar]
- 15.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 16.Braak H, Del Tredici K. Invited Article: Nervous system pathology in sporadic Parkinson disease. Neurology. 2008;70:1916–1925. doi: 10.1212/01.wnl.0000312279.49272.9f. [DOI] [PubMed] [Google Scholar]
- 17.Braak H, Ghebremedhin E, Rub U, Bratzke H, Del TrediciK. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- 18.Braak H, Rub U, Gai WP, Del TK. Idiopathic Parkinson's disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm. 2003;110:517–536. doi: 10.1007/s00702-002-0808-2. [DOI] [PubMed] [Google Scholar]
- 19.Burke RE, Dauer WT, Vonsattel JP. A critical evaluation of the Braak staging scheme for Parkinson’s disease. Ann Neurol. 2008;64:485–491. doi: 10.1002/ana.21541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Croisier E, MRes DE, Deprez M, Goldring K, Dexter DT, Pearce RK, Graeber MB, Roncaroli F. Comparative study of commercially available anti-alpha-synuclein antibodies. Neuropathol Appl Neurobiol. 2006;32:351–356. doi: 10.1111/j.1365-2990.2006.00722.x. [DOI] [PubMed] [Google Scholar]
- 21.Daniel SE, Hawkes CH. Preliminary diagnosis of Parkinson's disease by olfactory bulb pathology. Lancet. 1992;340:186. doi: 10.1016/0140-6736(92)93275-r. [DOI] [PubMed] [Google Scholar]
- 22.Del Tredici K, Rub U, de Vos RA, Bohl JR, Braak H. Where does parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol. 2002;61:413–426. doi: 10.1093/jnen/61.5.413. [DOI] [PubMed] [Google Scholar]
- 23.Delledonne A, Klos KJ, Fujishiro H, Ahmed Z, Parisi JE, Josephs KA, Frigerio R, Burnett M, Wszolek ZK, Uitti RJ, Ahlskog JE, Dickson DW. Incidental Lewy body disease and preclinical Parkinson disease. Arch Neurol. 2008;65:1074–1080. doi: 10.1001/archneur.65.8.1074. [DOI] [PubMed] [Google Scholar]
- 24.den Hartog Jager WA, Bethlem The distribution of Lewy bodies in the central and autonomic nervous systems in idiopathic paralysis agitans. J Neurol Neurosurg Psychiatry. 1960;23:283–290. doi: 10.1136/jnnp.23.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dickson DW, Fujishiro H, DelleDonne A, Menke J, Ahmed Z, Klos KJ, Josephs KA, Frigerio R, Burnett M, Parisi JE, Ahlskog JE. Evidence that incidental Lewy body disease is pre-symptomatic Parkinson's disease. Acta Neuropathol. 2008;115:437–444. doi: 10.1007/s00401-008-0345-7. [DOI] [PubMed] [Google Scholar]
- 26.Doty RL, Deems DA, Stellar S. Olfactory dysfunction in parkinsonism: a general deficit unrelated to neurologic signs, disease stage, or disease duration. Neurology. 1988;38:1237–1244. doi: 10.1212/wnl.38.8.1237. [DOI] [PubMed] [Google Scholar]
- 27.Duda JE. Pathology and neurotransmitter abnormalities of dementia with Lewy bodies. Dement Geriatr Cogn Disord. 2004;17 Suppl. 1:3–14. doi: 10.1159/000074677. [DOI] [PubMed] [Google Scholar]
- 28.Duda JE, Giasson BI, Chen Q, Gur TL, Hurtig HI, Stern MB, Gollomp SM, Ischiropoulos H, Lee VM, Trojanowski JQ. Widespread nitration of pathological inclusions in neurodegenerative synucleinopathies. Am J Pathol. 2000;157:1439–1445. doi: 10.1016/S0002-9440(10)64781-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duda JE, Giasson BI, Mabon ME, Lee VM, Trojanowski JQ. Novel antibodies to synuclein show abundant striatal pathology in Lewy body diseases. Ann Neurol. 2002;52:205–210. doi: 10.1002/ana.10279. [DOI] [PubMed] [Google Scholar]
- 30.Duda JE, Giasson BI, Mabon ME, Miller DC, Golbe LI, Lee VM, Trojanowski JQ. Concurrence of alpha-synuclein and tau brain pathology in the Contursi kindred. Acta Neuropathol. 2002;104:7–11. doi: 10.1007/s00401-002-0563-3. [DOI] [PubMed] [Google Scholar]
- 31.Duda JE, Shah U, Arnold SE, Lee VM, Trojanowski JQ. The expression of alpha-, beta-, and gamma-synucleins in olfactory mucosa from patients with and without neurodegenerative diseases. Exp Neurol. 1999;160:515–522. doi: 10.1006/exnr.1999.7228. [DOI] [PubMed] [Google Scholar]
- 32.Ellis RJ, Jan K, Kawas C, Koller WC, Lyons KE, Jeste DV, Hansen LA, Thal LJ. Diagnostic validity of the dementia questionnaire for Alzheimer disease. Arch Neurol. 1998;55:360–365. doi: 10.1001/archneur.55.3.360. [DOI] [PubMed] [Google Scholar]
- 33.Fearnley JM, Lees AJ. Ageing and Parkinson's disease: SN regional selectivity. Brain. 1991;114(Pt 5):2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- 34.Fujimi K, Sasaki K, Noda K, Wakisaka Y, Tanizaki Y, Matsui Y, Sekita A, Iida M, Kiyohara Y, Kanba S, Iwaki T. Clinicopathological outline of dementia with Lewy bodies applying the revised criteria: the Hisayama study. Brain Pathol. 2008;18:317–325. doi: 10.1111/j.1750-3639.2008.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujishiro H, Tsuboi Y, Lin WL, Uchikado H, Dickson DW. Co-localization of tau and alpha-synuclein in the olfactory bulb in Alzheimer's disease with amygdala Lewy bodies. Acta Neuropathol. 2008;116:17–24. doi: 10.1007/s00401-008-0383-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, Shen J, Takio K, Iwatsubo T. alpha-Synuclein is phosphorylated in LTS lesions. Nat Cell Biol. 2002;4:160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 37.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56:33–39. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- 38.Giasson BI, Duda JE, Forman MS, Lee VM, Trojanowski JQ. Prominent perikaryal expression of alpha- and beta-synuclein in neurons of dorsal root ganglion and in medullary neurons. Exp Neurol. 2001;172:354–362. doi: 10.1006/exnr.2001.7805. [DOI] [PubMed] [Google Scholar]
- 39.Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VM. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in LTS lesions. Science. 2000;290:985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- 40.Hamilton RL. Lewy bodies in Alzheimer's disease: a neuropathological review of 145 cases using alpha-synuclein immunohistochemistry. Brain Pathol. 2000;10:378–384. doi: 10.1111/j.1750-3639.2000.tb00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hansen L, Salmon D, Galasko D, Masliah E, Katzman R, DeTeresa R, Thal L, Pay MM, Hofstetter R, Klauber M. The Lewy body variant of Alzheimer's disease: a clinical and pathologic entity. Neurology. 1990;40:1–8. doi: 10.1212/wnl.40.1.1. [DOI] [PubMed] [Google Scholar]
- 42.Hawkes C. Olfaction in neurodegenerative disorder. Adv Otorhinolaryngol. 2006;63:133–151. doi: 10.1159/000093759. [DOI] [PubMed] [Google Scholar]
- 43.Hawkes CH, Del Tredici K, Braak H. Parkinson's disease: a dual-hit hypothesis. Neuropathol Appl Neurobiol. 2007;33:599–614. doi: 10.1111/j.1365-2990.2007.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hishikawa N, Hashizume Y, Yoshida M, Sobue G. Clinical and neuropathological correlates of Lewy body disease. Acta Neuropathol. 2003;105:341–350. doi: 10.1007/s00401-002-0651-4. [DOI] [PubMed] [Google Scholar]
- 45.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with Hhal. J Lipid Res. 1990;31:545–548. [PubMed] [Google Scholar]
- 46.Hladik CL, White CL. Comparison of digestive enzyme and formic acid pretreatment for optimal immunohistochemical demonstration of alpha-synuclein-immunoreactive cerebral cortical Lewy neurites. J Neuropathol Exp Neurol. 2003;62:554. [Google Scholar]
- 47.Holdorff B. Friedrich Heinrich Lewy (1885–1950) and his work. J Hist Neurosci. 2002;11:19–28. doi: 10.1076/jhin.11.1.19.9106. [DOI] [PubMed] [Google Scholar]
- 48.Hubbard PS, Esiri MM, Reading M, McShane R, Nagy Z. Alpha-synuclein pathology in the olfactory pathways of dementia patients. J Anat. 2007;211:117–124. doi: 10.1111/j.1469-7580.2007.00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iseki E, Marui W, Kosaka K, Akiyama H, Ueda K, Iwatsubo T. Degenerative terminals of the perforant pathway are human alpha-synuclein-immunoreactive in the hippocampus of patients with diffuse Lewy body disease. Neurosci Lett. 1998;258:81–84. doi: 10.1016/s0304-3940(98)00856-8. [DOI] [PubMed] [Google Scholar]
- 50.Jellinger KA. Lewy body-related alpha-LTS in the aged human brain. J Neural Transm. 2004;111:1219–1235. doi: 10.1007/s00702-004-0138-7. [DOI] [PubMed] [Google Scholar]
- 51.Jellinger KA. A critical reappraisal of current staging of Lewy-related pathology in human brain. Acta Neuropathol. 2008;116:1–16. doi: 10.1007/s00401-008-0406-y. [DOI] [PubMed] [Google Scholar]
- 52.Kalaitzakis ME, Graeber MB, Gentleman SM, Pearce RK. The dorsal motor nucleus of the vagus is not an obligatory trigger site of Parkinson's disease: a critical analysis of alpha-synuclein staging. Neuropathol Appl Neurobiol. 2008;34:284–295. doi: 10.1111/j.1365-2990.2007.00923.x. [DOI] [PubMed] [Google Scholar]
- 53.Katzenschlager R, Lees AJ. Olfaction and Parkinson's syndromes: its role in differential diagnosis. Curr Opin Neurol. 2004;17:417–423. doi: 10.1097/01.wco.0000137531.76491.c2. [DOI] [PubMed] [Google Scholar]
- 54.Klos KJ, Ahlskog JE, Josephs KA, Apaydin H, Parisi JE, Boeve BF, DeLucia MW, Dickson DW. Alpha-synuclein pathology in the spinal cords of neurologically asymptomatic aged individuals. Neurology. 2006;66:1100–1102. doi: 10.1212/01.wnl.0000204179.88955.fa. [DOI] [PubMed] [Google Scholar]
- 55.Kosaka K. [Dementia with Lewy bodies--from its finding to the present, including the CDLB guideline-revised] Rinsho Shinkeigaku. 2007;47:703–707. [PubMed] [Google Scholar]
- 56.Kranick SM, Duda JE. Olfactory dysfunction in Parkinson's disease. Neurosignals. 2008;16:35–40. doi: 10.1159/000109757. [DOI] [PubMed] [Google Scholar]
- 57.Kuusisto E, Parkkinen L, Alafuzoff I. Morphogenesis of Lewy bodies: dissimilar incorporation of alpha-synuclein, ubiquitin, and p62. J Neuropathol Exp Neurol. 2003;62:1241–1253. doi: 10.1093/jnen/62.12.1241. [DOI] [PubMed] [Google Scholar]
- 58.Lerner A, Bagic A. Olfactory pathogenesis of idiopathic Parkinson disease revisited. Mov Disord. 2008;23:1076–1084. doi: 10.1002/mds.22066. [DOI] [PubMed] [Google Scholar]
- 59.Leverenz J, Hamilton R, Tsuang DW, Schantz A, Vavrek D, Kukull W, Lopez O, Galasko D, Masliah E, Kaye J, Nixon R, Clark C, Trojanowsk JQ, Montine TJ. Empiric refinement of the pathologic assessment of Lewy-related pathology in the dementia patient. Brain Pathol. 2008;1:1–2. doi: 10.1111/j.1750-3639.2007.00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leverenz JB, Hamilton R, Tsuang DW, Schantz A, Vavrek D, Larson EB, Kukull WA, Lopez O, Galasko D, Masliah E, Kaye J, Woltjer R, Clark C, Trojanowski JQ, Montine TJ. Empiric refinement of the pathologic assessment of Lewy-related pathology in the dementia patient. Brain Pathol. 2008;18:220–224. doi: 10.1111/j.1750-3639.2007.00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lewy FH. Handbuch der Neurologie III. Berlin: Springer-Verlag; 1912. Paralysis agitans 1. Pathologisch Anatomie; pp. 920–933. [Google Scholar]
- 62.Linazasoro G. Classical Parkinson disease versus Parkinson complex--reflections against staging and in favour of heterogeneity. Eur J Neurol. 2007;14:721–728. doi: 10.1111/j.1468-1331.2007.01853.x. [DOI] [PubMed] [Google Scholar]
- 63.Lippa CF, Duda JE, Grossman M, Hurtig HI, Aarsland D, Boeve BF, Brooks DJ, Dickson DW, Dubois B, Emre M, Fahn S, Farmer JM, Galasko D, Galvin JE, Goetz CG, Growdon JH, Gwinn-Hardy KA, Hardy J, Heutink P, Iwatsubo T, Kosaka K, Lee VM, Masliah E, McKeith IG, Nussbaum RL, Olanow CW, Ravina BM, Singleton AB, Tanner CM, Trojanowski JQ, Wszolek ZK. DLB and PDD boundary issues: diagnosis, treatment, molecular pathology, and biomarkers. Neurology. 2007;68:812–819. doi: 10.1212/01.wnl.0000256715.13907.d3. [DOI] [PubMed] [Google Scholar]
- 64.Lippa CF, Smith TW, Swearer JM. Alzheimer's disease and Lewy body disease: a comparative clinicopathological study. Ann Neurol. 1994;35:81–88. doi: 10.1002/ana.410350113. [DOI] [PubMed] [Google Scholar]
- 65.Marui W, Iseki E, Nakai T, Miura S, Kato M, Ueda K, Kosaka K. Progression and staging of Lewy pathology in brains from patients with dementia with Lewy bodies. J Neurol Sci. 2002;195:153–159. doi: 10.1016/s0022-510x(02)00006-0. [DOI] [PubMed] [Google Scholar]
- 66.McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del ST, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 67.McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, Salmon DP, Lowe J, Mirra SS, Byrne EJ, Lennox G, Quinn NP, Edwardson JA, Ince PG, Bergeron C, Burns A, Miller BL, Lovestone S, Collerton D, Jansen EN, Ballard C, de Vos RA, Wilcock GK, Jellinger KA, Perry RH. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 68.McKinnon JH, Demaerschalk BM, Caviness JN, Wellik KE, Adler CH, Wingerchuk DM. Sniffing out Parkinson disease: can olfactory testing differentiate parkinsonian disorders? Neurologist. 2007;13:382–385. doi: 10.1097/NRL.0b013e31815a351a. [DOI] [PubMed] [Google Scholar]
- 69.Minguez-Castellanos A, Chamorro CE, Escamilla-Sevilla F, Ortega-Moreno A, Rebollo AC, Gomez-Rio M, Concha A, Munoz DG. Do alpha-synuclein aggregates in autonomic plexuses predate Lewy body disorders?: a cohort study. Neurology. 2007;68:2012–2018. doi: 10.1212/01.wnl.0000264429.59379.d9. [DOI] [PubMed] [Google Scholar]
- 70.Müller CM, de Vos RA, Maurage CA, Thal DR, Tolnay M, Braak H. Staging of sporadic Parkinson disease-related alpha-synuclein pathology: inter- and intra-rater reliability. J Neuropathol Exp Neurol. 2005;64:623–628. doi: 10.1097/01.jnen.0000171652.40083.15. [DOI] [PubMed] [Google Scholar]
- 71.Obi K, Akiyama H, Kondo H, Shimomura Y, Hasegawa M, Iwatsubo T, Mizuno Y, Mochizuki H. Relationship of phosphorylated alpha-synuclein and tau accumulation to Abeta deposition in the cerebral cortex of dementia with Lewy bodies. Exp Neurol. 2008;210:409–420. doi: 10.1016/j.expneurol.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 72.Orimo S, Oka T, Miura H, Tsuchiya K, Mori F, Wakabayashi K, Nagao T, Yokochi M. Sympathetic cardiac denervation in Parkinson's disease and pure autonomic failure but not in multiple system atrophy. J Neurol Neurosurg Psychiatry. 2002;73:776–777. doi: 10.1136/jnnp.73.6.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Parkinson JD. London: Sherwood, Neely and Jones; 1817. The shaking palsy. [Google Scholar]
- 74.Parkinson JD. Mcmillan, London: Critchley M James Parkinson; 1955. An essay on the shaking palsy. [Google Scholar]
- 75.Parkkinen L, Pirttila T, Alafuzoff I. Applicability of current staging/categorization of alpha-synuclein pathology and their clinical relevance. Acta Neuropathol. 2008;115:399–407. doi: 10.1007/s00401-008-0346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Parkkinen L, Soininen H, Alafuzoff I. Regional distribution of alpha-synuclein pathology in unimpaired aging and Alzheimer disease. J Neuropathol Exp Neurol. 2003;62:363–367. doi: 10.1093/jnen/62.4.363. [DOI] [PubMed] [Google Scholar]
- 77.Pearce RK, Hawkes CH, Daniel SE. The anterior olfactory nucleus in Parkinson's disease. Mov Disord. 1995;10:283–287. doi: 10.1002/mds.870100309. [DOI] [PubMed] [Google Scholar]
- 78.Perry RH, Irving D, Blessed G, Fairbairn A, Perry EK. Senile dementia of Lewy body type. A clinically and neuropathologically distinct form of Lewy body dementia in the elderly. J Neurol Sci. 1990;95:119–139. doi: 10.1016/0022-510x(90)90236-g. [DOI] [PubMed] [Google Scholar]
- 79.Ross GW, Petrovitch H, Abbott RD, Nelson J, Markesbery W, Davis D, Hardman J, Launer L, Masaki K, Tanner CM, White LR. Parkinsonian signs and SN neuron density in decendents elders without PD. Ann Neurol. 2004;56:532–539. doi: 10.1002/ana.20226. [DOI] [PubMed] [Google Scholar]
- 80.Saito Y, Kawashima A, Ruberu NN, Fujiwara H, Koyama S, Sawabe M, Arai T, Nagura H, Yamanouchi H, Hasegawa M, Iwatsubo T, Murayama S. Accumulation of phosphorylated alpha-synuclein in aging human brain. J Neuropathol Exp Neurol. 2003;62:644–654. doi: 10.1093/jnen/62.6.644. [DOI] [PubMed] [Google Scholar]
- 81.Saito Y, Ruberu NN, Sawabe M, Arai T, Kazama H, Hosoi T, Yamanouchi H, Murayama S. Lewy body-related alpha-LTS in aging. J Neuropathol Exp Neurol. 2004;63:742–749. doi: 10.1093/jnen/63.7.742. [DOI] [PubMed] [Google Scholar]
- 82.Schiller F. Fritz Lewy and his bodies. J Hist Neurosci. 2000;9:148–151. doi: 10.1076/0964-704X(200008)9:2;1-Y;FT148. [DOI] [PubMed] [Google Scholar]
- 83.Sengoku R, Saito Y, Ikemura M, Hatsuta H, Sakiyama Y, Kanemaru K, Arai T, Sawabe M, Tanaka N, Mochizuki H, Inoue K, Murayama S. Incidence and extent of Lewy body-related alpha-LTS in aging human olfactory bulb. J Neuropathol Exp Neurol. 2008;67:1072–1083. doi: 10.1097/NEN.0b013e31818b4126. [DOI] [PubMed] [Google Scholar]
- 84.Tsuang DW, Riekse RG, Purganan KM, David AC, Montine TJ, Schellenberg GD, Steinbart EJ, Petrie EC, Bird TD. Lewy body pathology in late-onset familial Alzheimer's disease: a clinicopathological case series. J Alzheimers Dis. 2006;9:235–242. doi: 10.3233/jad-2006-9302. [DOI] [PubMed] [Google Scholar]
- 85.Uchikado H, Lin WL, DeLucia MW, Dickson DW. Alzheimer disease with amygdala Lewy bodies: a distinct form of alpha-LTS. J Neuropathol Exp Neurol. 2006;65:685–697. doi: 10.1097/01.jnen.0000225908.90052.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wakabayashi K, Takahashi H, Takeda S, Ohama E, Ikuta F. Parkinson's disease: the presence of Lewy bodies in Auerbach’s and Meissner’s plexuses. Acta Neuropathol. 1988;76:217–221. doi: 10.1007/BF00687767. [DOI] [PubMed] [Google Scholar]